Abstract
This review focuses on recent advances in our understanding of how neural divergence and convergence give rise to complex encoding properties of retinal ganglion cells. We describe the apparent mismatch between the number of cone bipolar cell types, and the diversity of excitatory input to retinal ganglion cells, and outline two possible solutions. One proposal is for diversity in the excitatory pathways to be generated within axon terminals of cone bipolar cells, and the second invokes narrow-field glycinergic amacrine cells that can apparently act like bipolar cells by providing excitatory drive to ganglion cells. Finally we highlight two advances in technique that promise to provide future insights; automation of electron microscope data collection and analysis, and the use of the ideal observer to quantitatively compare neural performance at all levels.
Introduction
The retina represents an ideal system to study neural information processing, since the input, a two dimensional image focused onto a few million photoreceptors, can be defined very precisely, and the output, from several hundred thousand ganglion cell axons, can, in principle, be sampled completely. At the input, the photoreceptors show little diversity in structure or function, while the ganglion cells are widely diverse both in structure and function. The mammalian retina contains upwards of 20 different types of ganglion cell [1–4], each optimized in some sense to enable the organism to encode the visual world at a spatial and temporal resolution sufficient for effective behavioural responses and survival of the individual. These 20 parallel channels comprise arrays of essentially identical neurons that completely sample the visual field, and therefore, we need only to understand the coding properties of 20 output neurons to complete the analysis of retinal coding. However, the neural circuitry that generates the ganglion cell properties includes diverse arrays of bipolar cells and amacrine cells. In this review we will focus on excitatory pathways in the mammalian retina, and briefly outline recent insights into the properties of bipolar and amacrine cells that allow limited populations of neurons to perform multiple tasks. Finally, we will highlight two techniques that promise to advance our understanding of the anatomical and functional properties of neurons at all levels in the retina.
Counting neurons and defining circuits
At the first synaptic layer, the primary sensory neurons and inhibitory interneurons are known. Setting aside colour vision, achromatic form and motion vision, which represent the vast bulk of visual processing, are mediated by a single type of cone photoreceptor that provides synaptic input to all cone bipolar cells (CBCs), which in the mouse retina have been completely enumerated and comprise only 10 types [5•,6]. The axon terminals of cones are large and contain enough active zones to contact all 10 CBC types. It is generally assumed that these active zones are equivalent, and thus, the input to all CBCs is common.
These 10 types of bipolar cell provide the feed forward excitatory drive to the 20 arrays of ganglion cells, indicating that many ganglion cell types must share input from common types of bipolar cell [7,8]. Many of the retinal ganglion cell types have been documented physiologically, and have been labelled according to ‘trigger features’. For example in rabbit, there are local edge detectors, uniformity detectors, directional-selective ganglion cells, and orientation-selective ganglion cells to name a few. By contrast, somatic recordings, almost exclusively from retinal slice preparations, have revealed only two physiological types of CBC, On and Off, with no further subdivision generally accepted. Even allowing for possible heterogeneity in the response properties within the CBC types [9–13], the functional diversity at the output of the bipolar cells, as evident in the glutamatergic input to various GCs, exceeds the known anatomical types. This is obvious when one considers, for example, the On–Off direction-selective ganglion cells (DSGCs) and Off orientation- selective ganglion cells (Off-OSGCs) in the rabbit retina. There are four populations of DSGCs, each with a different preferred direction. Direction-selectivity in DSGCs is thought to be generated partly by directional glutamatergic input from On and Off CBCs, arising from GABAergic feedback onto the CBC terminals [14,15]. Similarly, vertically selective and horizontally selective Off-OSGCs receive strong orientation-selective glutamatergic input mediated by presynaptic GABAergic inhibition of Off bipolar cells [16••]. But there are only two or three types of Off CBCs with axonal arbors that stratify in the vicinity of these GCs, not enough to provide dedicated populations of Off-direction-selective and Off-orientation-selective CBCs. Similarly, other receptive field properties are impressed upon the output of bipolar cells, temporal and spatial tuning in local-edge-detectors, for example [17,18]. In the following we will discuss first how complex trigger features might arise at the output of the CBCs, and second, how small-field glycinergic amacrine cells might provide additional signalling pathways that increase the diversity in the excitatory drive to GCs.
Bipolar cell channels
The diverse glutamatergic input to GCs could be generated from limited populations of CBCs, if trigger features, like orientation or direction selectivity, were imposed on boutons or axonal branches, rather than the whole CBC. Thus, a single CBC could generate multiple output pathways each comprising a few boutons or short segments of axon that receive local synaptic contacts with specific amacrine cells (CBC1, Figure 1a, see [19] for a review of inhibitory feedback to bipolar cells). However, bipolar cells are small, and are expected to be electrically compact [20]. Therefore GABAergic inhibitory feedback would need to be electrically silent so that it did not spread throughout the axonal arbor and modulate the output of all boutons. If the cell resting potential were at or near the chloride reversal potential, then GABA activated chloride conductance in a single bouton could shunt the depolarizing drive and reduce glutamate release without strongly affecting neighbouring boutons. The bouton selectivity of such a mechanism might be enhanced if combined with a strong nonlinearity, like action-potential generation [21,22], regenerative potentials due to T-type calcium channels [23], or a sharp release threshold due to activation of voltage-dependent calcium channels [24].
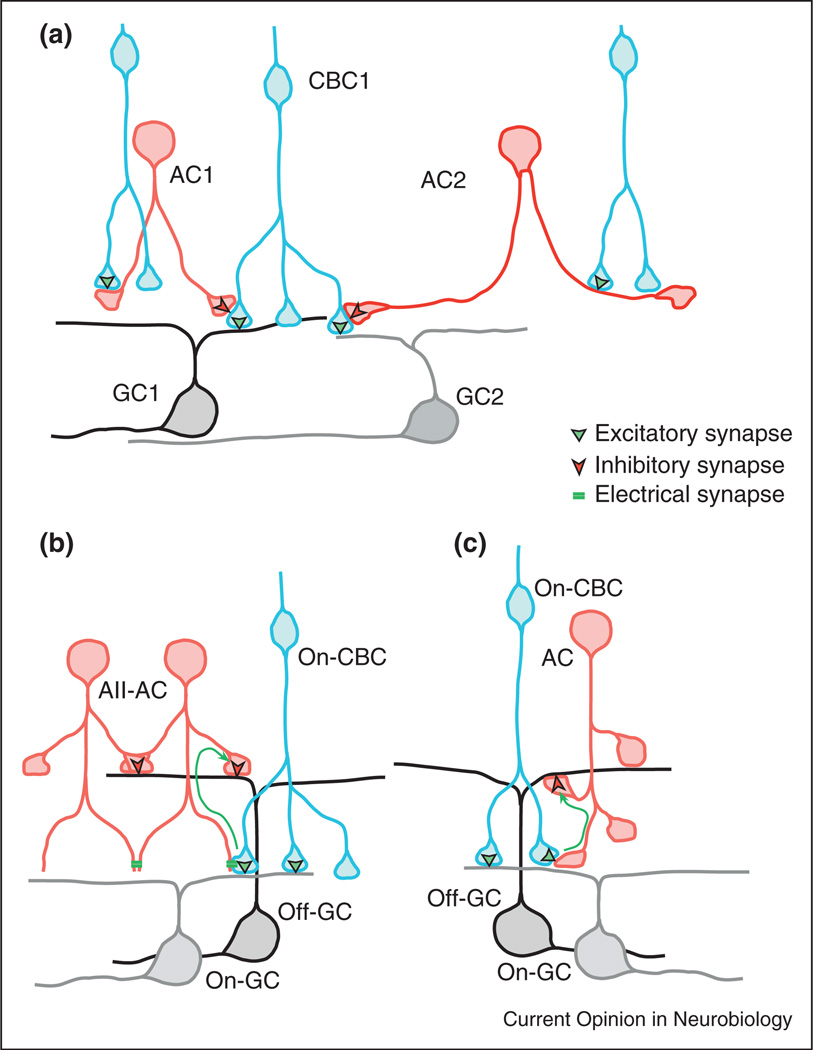
Figure 1.
Possible synaptic mechanisms that increase the functional diversity of CBC output. (a) A single CBC might provide output to multiple GCs through functionally independent axonal segments, involving specific connections with diverse amacrine cells. The amacrine cells are driven by glutamatergic CBC inputs. (b, c) Cross-over inhibition allows On CBCs to provide direct excitation of On-GCs, and indirect dis-inhibition to Off-GCs. Dis-inhibition requires the CBC and AC to be tonically active. ‘b’ shows cross-over inhibition mediated by the AII-AC, which is driven by gap-junction synapses with On-CBCs, and ‘c’ illustrates similar crossover mediated by glutamatergic synaptic connections from On-CBCs to other small-field ACs.
If the axonal boutons are to function independently, then they should be physically separated. For example, functionally similar boutons within CBC axonal arbors might be aligned along processes like beads on a string, or they might be isolated at the terminal ends of processes like a bunch of grapes. The latter structure is evident for the rod-bipolar axon terminals, where local micro-circuits involving GABAergic feedback from A17 amacrine cells has been documented [25]. However, it remains to be determined whether this feedback is local to individual boutons of the rod bipolar cells. The notion that single bipolar cells generate multiple output signals requires that diverse amacrine cell (AC) types contact each CBC, and moreover, that the contacts from each AC type should be clustered at functionally similar boutons on axonal segments. It also predicts that the axonal segments should make selective contacts with specific types of ganglion cells (Figure 1a). Large scale anatomical reconstruction of retinal circuits at submicron resolution will allow these ideas to be tested [26••,27••].
Small-field amacrine cells
Synaptic input from small-field glycinergic amacrine cells is a recently discovered source of functional diversity in the excitatory drive to some ganglion cells. To date the most extensively studied small-field amacrine cell, and also the most numerous, is the so-called AII amacrine cell (AII-AC). AII-ACs represent a remarkable example of neural parsimony since they perform several functions within retinal circuitry (reviewed recently [28]). In the current context, an excitatory role of AII-ACs seen at photopic (cone-driven) intensities is particularly relevant. Each AII and nearby On CBCs represent a local coupled network [29,30], with the central AII-AC providing indirect connection between all but one of the On CBC types [31–33]. Asynchronous background activity in the coupled On CBCs generates a tonic depolarization of the AII-ACs that in turn directly inhibits Off centre α-GCs. This tonic inhibition disappears during a center Off-stimulus, and the resulting dis-inhibitory input comprises a major component of the excitatory drive to Off α-GCs [34•,35•]. This has been termed crossover inhibition (for a discussion of possible roles see [36]). The Off α-GCs have On counter-parts, with largely similar receptive field properties except for inversion in the sign of the response to contrast. The inclusion of the glycinergic AC raises the possibility that the same set of On bipolar cells could directly and indirectly drive excitation in both On and Off α-GCs respectively (Figure 1b). Indeed the remarkable similarity in the kinetics of glutamatergic input to On α-GCs and glycinergic input to Off α-GCs is consistent with this hypothesis [35•]. More recently, other OFF-centre ganglion cells have been shown to receive dis-inhibitory excitation from narrow-field glycinergic amacrine cells that share the trigger feature of the direct CBC input, but are not AII-ACs [16••]. Finally, the so-called uniformity detector ganglion cells appear to receive no bipolar input whatsoever, but are intrinsically active, and continuously generate complex spikes under background illumination [37]. Light stimulation drives transient glycinergic inputs at onset and termination of a light flash, which briefly interrupts the spiking. Thus these cells are On–Off neurons. Owing to the similar temporal properties and close co-stratification of the On and Off dendritic arbors of the uniformity detectors and the ON–OFF direction-selective ganglion cells, it is tempting to speculate that they are driven by common bipolar cell signals, one direct, and one through an intervening glycinergic AC. The physiological roles of most small-field amacrine cells are unknown; perhaps many of these cells provide divergent excitatory pathways for the output of the bipolar cells via cross-over dis-inhibitory connections between On and Off CBCs and GCs (Figure 1c).
New approaches to circuit analysis
We have seen that diversity in the feed forward excitatory drive to ganglion cells arises from the connectivity from CBC types and small-field amacrine cells. The challenge is to trace the GC properties back to specific bipolar cell channels. For tracing information flow through neural circuits, we need to establish the anatomical and physiological connectivity. Large-scale electron microscopic reconstructions have already produced significant new findings and promise to greatly accelerate progress in mapping anatomical connections [26••,27••]. Advances in multi-neuronal imaging, if they can be adapted and applied to all neuronal layers in the retina, may accelerate progress in delineating the different physiological pathways [38,39]. Finally, the ideal observer method provides a powerful and general method to quantify and compare the performance of neurons at each stage in a neural pathway. For example, an ideal model of the stimulus defines the photon flux absorbed by the photoreceptors, and the corresponding Poisson noise distribution sets the maximum signal to noise ratio (SNR) available for the retina. Any neural processing can only reduce this maximum SNR because biological processes add noise [40]. Thus, given the noise distributions of the ideal model, the ideal observer can define the maximum performance possible by downstream retinal circuits. Measurements of the cone and bipolar cell responses will not reach this maximum, because both the cone visual transduction and the cone to bipolar cell synapse add noise to the signal [41••]. This lower SNR is ameliorated by the increased specialization of the CBC pathways, that is, subsequent to the output from the cones each parallel pathway loses sensitivity to non-relevant stimuli. The stimulus specificity in ganglion cell responses has been referred to here as the trigger feature, and at each stage of neural processing, trigger features appear or are further refined, at the cost of additional sources of noise. Thus, the signal processing performed by the inner retina can be seen as a compromise between specificity and sensitivity (Figure 2).
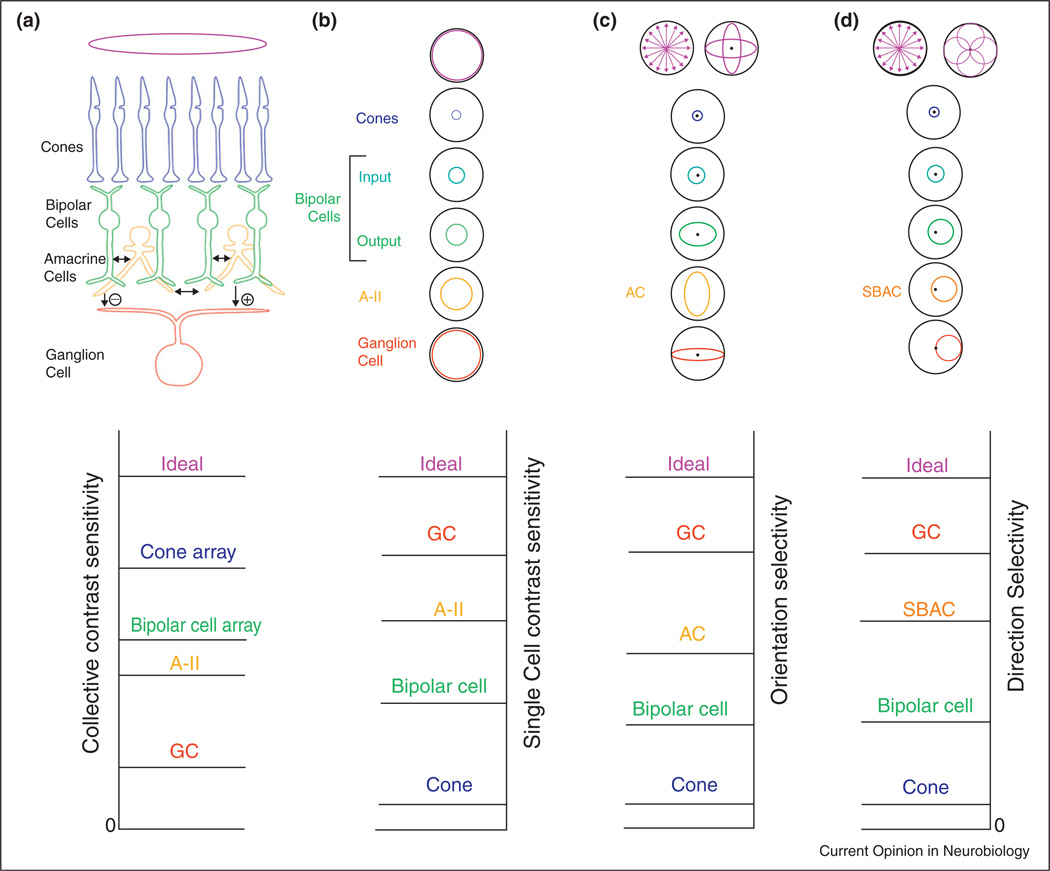
Figure 2.
Schematic showing how trigger features arise within retinal circuitry. Selectivity of higher order neurons requires several layers of synaptic connectivity, which adds synaptic noise. To remove the noise while maintaining sensitivity requires synaptic convergence. Top row: Diagrams showing correspondence of receptive fields from cones through to ganglion cells of different types. Bottom row: Conceptual plots showing relative sensitivity of the neurons to different stimulus dimensions. The ideal performance at the top of each plot represents the theoretical maximum possible for the noise distributions derived from the total photon flux. (a, b), Top: The receptive field of bipolar and ganglion cells increases through convergence. (a) Collective contrast sensitivity. Contrast sensitivity of a ganglion cell is less than ideal owing to addition of noise at intervening neural stages (bottom). However, the sensitivity of the bipolar cell and cone arrays as a whole are progressively higher than that of the ganglion cells. (b) For individual cells, however, the order of contrast sensitivity is reversed (bottom) because the synaptic noise added by successive stages is more than offset by the convergence and selective signal processing by the receptive field mechanisms. (c) Top: Orientation-selectivity might be encoded by many instances of narrow selectivity, or a few instances of overlapping and redundant selectivity. Polar plots of the receptive fields of individual cells in the 3 layers illustrate emergence of orientation selectivity. Bipolar cells have no orientation selectivity in their input, but generate orientation selectivity at their axon terminal output, from their interactions with amacrine cells in the inner retina [16••]. Compared with the concentric units in b, a ‘cost’ of orientation-selectively is the complete loss of sensitivity to stimulus edges in non-preferred orientations. (d) Similarly, direction of motion is encoded by a few broadly tuned populations. ON–OFF direction-selective ganglion cells do not provide information about the sign of stimulus contrast, and lack fine contrast sensitivity, but have excellent direction selectivity, which is developed at successive stages including the output from GABAergic ‘starburst’ amacrine cells (SBAC), and the output from bipolar cells [46,47].
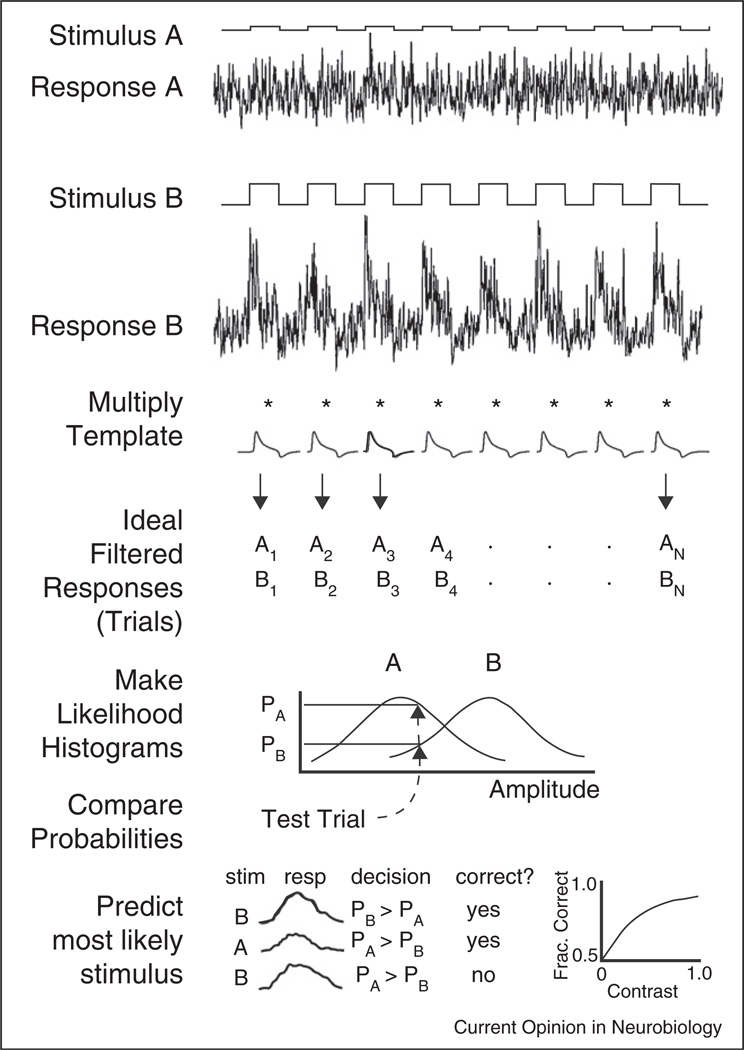
In practise, tracing signals through the retina is difficult because a ganglion cell can convey information about several stimulus dimensions (e.g. see [42]). The stimulus dimension for which SNR is greatest represents a good candidate for the trigger feature of the GC, and the number of discriminable levels in the response, obtained as the ratio of the dynamic range and the SNR, may provide a useful metric for comparing the relevance of different stimulus dimensions (reviewed in [43]). The simplest ideal observer method measures performance for a 2-alternative forced-choice discrimination task, in which a pair of stimuli that differ by a small amount in one or more dimensions are repeated, and the responses filtered with a correlation template ideal for the discrimination (Figure 3, top). The filtered responses are then inserted in probability density function histograms, which represent the likelihood (probability) that each stimulus evoked a given response (Figure 3, middle). The stimulus with the maximum likelihood is chosen and the fraction of correct choices is tabulated (Figure 3, bottom). A variety of templates can be constructed that are optimal or approach optimality for the given responses. This method can measure SNR and number of discriminable levels for different stimulus dimensions given the responses of individual cells, or collections of cells, regardless of whether the noise in their responses is correlated or uncorrelated. The ratios of the means to the standard deviations of the peaks of the PDFs provide measures of SNRs. When the peaks of a pair of responses differ by one standard deviation, they are defined to be discriminable. The sensitivity for discrimination is defined as the inverse of the stimulus dimension for the just-discriminable pair, for example, a discriminable contrast of 0.05 defines a contrast sensitivity of 20. By testing pairs of stimuli along one dimension in a model or a real experiment, one can measure the number of discriminable levels along that dimension. Thus, the ideal observer allows neural performance for different stimulus dimensions to be quantified, compared, and rated according to relevance. A comparison between different cell types of the number of discriminable levels for a stimulus dimension, for example, contrast or direction of motion, shows how much information about the stimulus is available to each cell type, and a comparison to the ideal model shows how much was lost. Moreover, by comparing the relevance of stimuli for neurons at different stages in a pathway, inferences can be made about the connectivity, and the signal processing roles of the circuit elements [43].
Figure 3.
Overview of the ideal observer method. Ideal observer single-interval two-alternative forced choice method for neural responses. Top, responses to a pair of stimuli (a, b) are multiplied by a template to produce a set of filtered responses (trials = A1…An, B1…Bn). Middle, PDF histograms are generated, then the probability of individual responses is looked up to find most likely stimulus. Bottom, repeat over all trials, tally correct responses, and plot as a neurometric function. From Smith and Dhingra (2009).
There are several advantages of this ideal observer metric. It is straightforward and can be independent of the underlying circuitry. Specific stimulus dimensions can be arbitrarily tested, making it easy to find which dimensions are most relevant. Moreover, the metric is relevant to behaviour, because neural performance can be directly compared to psychophysical performance. The 2-alternative choice paradigm precisely mimics a behavioural decision, and can readily be extended to multiple-choice paradigms. Another widely utilised method, Shannon information theory, can determine the information content of the same stimulus discrimination paradigms, but its results will differ from the maximum likelihood metric of the ideal observer, because information theory specifies a different task [44].
In summary, the cones are the least specialized but their signals are the most redundant, because their receptive fields are small and, owing to the structure of natural scenes, neighbours tend to be highly correlated. Within two synapses the ganglion cells represent a complex array of receptive field properties that are much more specific, and thus much less correlated. In attempting to delineate the neural pathways that generate this retinal code, it would help a great deal if, as Barlow pointed out [45], we knew what the GCs were doing. To this end, the ideal observer provides an objective method to determine what is important to a ganglion cell, and possibly where that information is extracted in the presynaptic circuitry.
Acknowledgments
This study was supported by NEI grants EY014888 (WRT) and EY016607 (RGS) and an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology, OHSU.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Rockhill RL, Euler T, Masland RH. Spatial order within but not between types of retinal neurons. Proc Natl Acad Sci USA. 2000;97:2303–2307. doi: 10.1073/pnas.030413497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troy JB, Shou T. The receptive fields of cat retinal ganglion cells in physiological and pathological states: where we are after half a century of research. Prog Retin Eye Res. 2002;21:263–302. doi: 10.1016/s1350-9462(02)00002-2. [DOI] [PubMed] [Google Scholar]
- 3.Sun W, Li N, He S. Large-scale morphological survey of mouse retinal ganglion cells. J Comp Neurol. 2002;451:115–126. doi: 10.1002/cne.10323. [DOI] [PubMed] [Google Scholar]
- 4.Dacey DM, Packer OS. Colour coding in the primate retina: diverse cell types and cone-specific circuitry. Curr Opin Neurobiol. 2003;13:421–427. doi: 10.1016/s0959-4388(03)00103-x. [DOI] [PubMed] [Google Scholar]
- 5. Wassle H, Puller C, Muller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009.. This paper provides the first complete enumeration of all bipolar cell types in a mammalian retina (mouse).
- 6.Strettoi E, Novelli E, Mazzoni F, Barone I, Damiani D. Complexity of retinal cone bipolar cells. Prog Retin Eye Res. 2010;29:272–283. doi: 10.1016/j.preteyeres.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGuire BA, Stevens JK, Sterling P. Microcircuitry of bipolar cells in cat retina. J Neurosci. 1984;4:2920–2938. doi: 10.1523/JNEUROSCI.04-12-02920.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen E, Sterling P. Demonstration of cell types among cone bipolar neurons of cat retina. Philos Trans R Soc Lond B Biol Sci. 1990;330:305–321. doi: 10.1098/rstb.1990.0201. [DOI] [PubMed] [Google Scholar]
- 9.Berntson A, Taylor WR. Response characteristics and receptive field widths of on-bipolar cells in the mouse retina. J Physiol. 2000;524(Pt 3):879–889. doi: 10.1111/j.1469-7793.2000.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pang JJ, Gao F, Wu SM. Light-evoked current responses in rod bipolar cells, cone depolarizing bipolar cells and AII amacrine cells in dark-adapted mouse retina. J Physiol. 2004;558:897–912. doi: 10.1113/jphysiol.2003.059543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Euler T, Masland RH. Light-evoked responses of bipolar cells in a mammalian retina. J Neurophysiol. 2000;83:1817–1829. doi: 10.1152/jn.2000.83.4.1817. [DOI] [PubMed] [Google Scholar]
- 12.DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;28:847–856. doi: 10.1016/s0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- 13.DeVries SH, Li W, Saszik S. Parallel processing in two transmitter microenvironments at the cone photoreceptor synapse. Neuron. 2006;50:735–748. doi: 10.1016/j.neuron.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 14.Taylor WR, Vaney DI. Diverse synaptic mechanisms generate direction selectivity in the rabbit retina. J Neurosci. 2002;22:7712–7720. doi: 10.1523/JNEUROSCI.22-17-07712.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fried SI, Munch TA, Werblin FS. Mechanisms and circuitry underlying directional selectivity in the retina. Nature. 2002;420:411–414. doi: 10.1038/nature01179. [DOI] [PubMed] [Google Scholar]
- 16. Venkataramani S, Taylor WR. Orientation selectivity in rabbit retinal ganglion cells is mediated by presynaptic inhibition. J Neurosci. 2010;30:15664–15676. doi: 10.1523/JNEUROSCI.2081-10.2010.. This paper shows that excitatory glutamatergic inputs from bipolar cells carry a strong orientation-selective signal that is mediated by GABAergic presynaptic inhitibion. The paper also shows that, at least for one type of OSGC, glycinergic dis-inhibition also provides an orientation-selective excitatory drive. Thus a small-field amacrine cell carries a similar signal as the glutamatergic inputs to provide push–pull excitatory drive.
- 17.van Wyk M, Taylor WR, Vaney DI. Local edge detectors: a substrate for fine spatial vision at low temporal frequencies in rabbit retina. J Neurosci. 2006;26:13250–13263. doi: 10.1523/JNEUROSCI.1991-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell TL, Werblin FS. Retinal synaptic pathways underlying the response of the rabbit local edge detector. J Neurophysiol. 2010;103:2757–2769. doi: 10.1152/jn.00987.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eggers ED, Lukasiewicz PD. Multiple pathways of inhibition shape bipolar cell responses in the retina. Vis Neurosci. 2011;28:95–108. doi: 10.1017/S0952523810000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oltedal L, Veruki ML, Hartveit E. Passive membrane properties and electrotonic signal processing in retinal rod bipolar cells. J Physiol. 2009;587:829–849. doi: 10.1113/jphysiol.2008.165415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Protti DA, Flores-Herr N, von Gersdorff H. Light evokes Ca2+ spikes in the axon terminal of a retinal bipolar cell. Neuron. 2000;25:215–227. doi: 10.1016/s0896-6273(00)80884-3. [DOI] [PubMed] [Google Scholar]
- 22.Dreosti E, Esposti F, Baden T, Lagnado L. In vivo evidence that retinal bipolar cells generate spikes modulated by light. Nat Neurosci. 2011;14:951–952. doi: 10.1038/nn.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui J, Pan ZH. Two types of cone bipolar cells express voltage-gated Na+ channels in the rat retina. Vis Neurosci. 2008;25:635–645. doi: 10.1017/S0952523808080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snellman J, Zenisek D, Nawy S. Switching between transient and sustained signalling at the rod bipolar-AII amacrine cell synapse of the mouse retina. J Physiol. 2009;587:2443–2455. doi: 10.1113/jphysiol.2008.165241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimes WN, Zhang J, Graydon CW, Kachar B, Diamond JS. Retinal parallel processors: more than 100 independent microcircuits operate within a single interneuron. Neuron. 2010;65:873–885. doi: 10.1016/j.neuron.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Briggman KL, Helmstaedter M, Denk W. Wiring specificity in the direction-selectivity circuit of the retina. Nature. 2011;471:183–188. doi: 10.1038/nature09818.. Scanning block face serial electron-microscopy is used to reconstruct a chunk of retina large enough to identify starburst amacrine cells and resolve their synaptic connections with direction-selective ganglion cells. Direction-selective cells are identified by prior calcium imaging. The study settles a long-standing question regarding the asymmetry of synaptic connectivity between the cells. The technique holds great promise for future elucidation of circuitry in the retina and brain.
- 27. Anderson JR, Jones BW, Watt CB, Shaw MV, Yang JH, Demill D, Lauritzen JS, Lin Y, Rapp KD, Mastronarde D, et al. Exploring the retinal connectome. Mol Vis. 2011;17:355–379.. A large, continuous series of conventional elecronmicroscopic sections are analyzed to reconstruct a column of rabbit retina about 0.25 mm in diameter. A particular advantage of the approach is the use of numerous immunohistochemical markers to label specific cell types, and transmitters within the tissue volume.
- 28.Oesch NW, Wade Kothmann W, Diamond JS. Illuminating synapses and circuitry in the retina. Curr Opin Neurobiol. 2011;21:238–244. doi: 10.1016/j.conb.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills SL, Massey SC. Differential properties of two gap junctional pathways made by AII amacrine cells [see comments] Nature. 1995;377:734–737. doi: 10.1038/377734a0. [DOI] [PubMed] [Google Scholar]
- 30.Trexler EB, Li W, Mills SL, Massey SC. Coupling from AII amacrine cells to ON cone bipolar cells is bidirectional. J Comp Neurol. 2001;437:408–422. doi: 10.1002/cne.1292. [DOI] [PubMed] [Google Scholar]
- 31.Petrides A, Trexler EB. Differential output of the high-sensitivity rod photoreceptor: AII amacrine pathway. J Comp Neurol. 2008;507:1653–1662. doi: 10.1002/cne.21617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trexler EB, Li W, Massey SC. Simultaneous contribution of two rod pathways to AII amacrine and cone bipolar cell light responses. J Neurophysiol. 2005;93:1476–1485. doi: 10.1152/jn.00597.2004. [DOI] [PubMed] [Google Scholar]
- 33.Strettoi E, Raviola E, Dacheux RF. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol. 1992;325:152–168. doi: 10.1002/cne.903250203. [DOI] [PubMed] [Google Scholar]
- 34.Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, Demb JB. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. J Neurosci. 2008;28:4136–4150. doi: 10.1523/JNEUROSCI.4274-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Wyk M, Wassle H, Taylor WR. Receptive field properties of ON- and OFF-ganglion cells in the mouse retina. Vis Neurosci. 2009;26:297–308. doi: 10.1017/S0952523809990137.. These papers demonstrate the dual role of glycinergic dis-inhibition and excitation in generating the excitatory drive to specific types of ganglion cells in the rabbit and guinea pig retinas, and provide evidence for a role for the narrow-field AII amacrine cells. The second paper compares the homologous Off and On alpha cells in the mouse, and demonstrates the functional similarities in the kinetics of the glutamatergic excitatory drive to the On α-GCs, and the glycinergic dis-inhibition to the Off α-GCs.
- 36.Werblin FS. Six different roles for crossover inhibition in the retina: correcting the nonlinearities of synaptic transmission. Vis Neurosci. 2010;27:1–8. doi: 10.1017/S0952523810000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sivyer B, Taylor WR, Vaney DI. Uniformity detector retinal ganglion cells fire complex spikes and receive only light-evoked inhibition. Proc Natl Acad Sci USA. 2010;107:5628–5633. doi: 10.1073/pnas.0909621107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Briggman KL, Euler T. Bulk electroporation and population calcium imaging in the adult mammalian retina. J Neurophysiol. 2011;105:2601–2609. doi: 10.1152/jn.00722.2010. [DOI] [PubMed] [Google Scholar]
- 39.Baldridge WH. Optical recordings of the effects of cholinergic ligands on neurons in the ganglion cell layer of mammalian retina. J Neurosci. 1996;16:5060–5072. doi: 10.1523/JNEUROSCI.16-16-05060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faisal AA, Selen LP, Wolpert DM. Noise in the nervous system. Nat Rev Neurosci. 2008;9:292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Borghuis BG, Sterling P, Smith RG. Loss of sensitivity in an analog neural circuit. J Neurosci. 2009;29:3045–3058. doi: 10.1523/JNEUROSCI.5071-08.2009.. The contrast sensitivity of an alpha ganglion cell is compared to the output of the cones and to an ideal model of a flashed spot of light using an ideal observer. This study showed that each synaptic layer loses sensitivity by a factor of ~4-fold.
- 42.Nowak P, Dobbins AC, Gawne TJ, Grzywacz NM, Amthor FR. Separability of stimulus parameter encoding by on-off directionally selective rabbit retinal ganglion cells. J Neurophysiol. 2011;105:2083–2099. doi: 10.1152/jn.00941.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith RG, Dhingra NK. Ideal observer analysis of signal quality in retinal circuits. Prog Retin Eye Res. 2009;28:263–288. doi: 10.1016/j.preteyeres.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geisler WS, Albrecht DG, Salvi RJ, Saunders SS. Discrimination performance of single neurons: rate and temporal-pattern information. J Neurophysiol. 1991;66:334–362. doi: 10.1152/jn.1991.66.1.334. [DOI] [PubMed] [Google Scholar]
- 45.Barlow HB. Possible principles underlying the transformation of sensory messages. In: Rosenblith WA, editor. Sensory Communication, Contributions. MIT Press; 1961. pp. 217–234. [Google Scholar]
- 46.Demb JB. Cellular mechanisms for direction selectivity in the retina. Neuron. 2007;55:179–186. doi: 10.1016/j.neuron.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Taylor WR, Vaney DI. New directions in retinal research. Trends Neurosci. 2003;26:379–385. doi: 10.1016/S0166-2236(03)00167-X. [DOI] [PubMed] [Google Scholar]