Abstract
White adipose tissue (WAT) secretes adipokines, which critically regulate lipid metabolism. The present study investigated the effects of alcohol on adipokines and the mechanistic link between adipokine dysregulation and alcoholic fatty liver disease. Mice were fed alcohol for 2, 4, or 8 weeks to document changes in adipokines over time. Alcohol exposure reduced WAT mass and body weight in association with hepatic lipid accumulation. The plasma adiponectin concentration was increased at 2 weeks, but declined to normal at 4 and 8 weeks. Alcohol exposure suppressed leptin gene expression in WAT and reduced the plasma leptin concentration at all times measured. There is a highly positive correlation between plasma leptin concentration and WAT mass or body weight. To determine whether leptin deficiency mediates alcohol-induced hepatic lipid dyshomeostasis, mice were fed alcohol for 8 weeks with or without leptin administration for the last 2 weeks. Leptin administration normalized the plasma leptin concentration and reversed alcoholic fatty liver. Alcohol-perturbed genes involved in fatty acid β-oxidation, very low-density lipoprotein secretion, and transcriptional regulation were attenuated by leptin. Leptin also normalized alcohol-reduced phosphorylation levels of signal transducer Stat3 and adenosine monophosphate–activated protein kinase. These data demonstrated for the first time that leptin deficiency in association with WAT mass reduction contributes to the pathogenesis of alcoholic fatty liver disease.
Alcoholic liver disease is traditionally described as three progressive pathologic conditions: steatosis (fatty liver), hepatitis, and cirrhosis.1 Alcoholic fatty liver is characterized by lipid droplet accumulation in the cytoplasm of hepatocytes, and is one of the earliest pathologic alterations in the liver. Accumulation of lipids in the hepatocytes makes the liver susceptible to inflammatory mediators or other toxic agents, leading to further progression to hepatitis and eventually to fibrosis. Alcohol consumption may affect multiple pathways of hepatic lipid metabolism including de novo lipogenesis, fatty acid oxidation, lipid uptake, and lipid export in the form of very low-density lipoproteins.2,3 However, recent studies have suggested that extrahepatic factors such as adiponectin critically modulate hepatic lipid metabolism.4,5
White adipose tissue (WAT) is a major organ for body fat storage, and also functions as an endocrine organ.6 The hormones secreted by WAT are adipokines, and two of the most important adipokines related to energy homeostasis are adiponectin and leptin. Both adiponectin and leptin critically modulate hepatic lipid homeostasis toward reduction of lipid content in the liver. Adiponectin signaling in the liver leads to activation of the adenosine monophosphate-activated protein kinase (AMPK) pathway via adiponectin receptor.4–7 AMPK activation negatively regulates the hepatic lipid level by stimulating fatty acid oxidation and suppressing fatty acid influx and de novo lipogenesis. Leptin critically regulates whole-body energy homeostasis by inhibiting energy intake and stimulating energy expenditure. Leptin signaling in the liver via leptin receptor b (LepRb) activates AMPK and signal transducer Stat3 pathways.6,7 Mice lacking functional leptin develop not only obesity but also fatty liver. Therefore, adipose tissues via adipokine secretion significantly affect lipid homeostasis in the liver.
Alcohol exposure has been shown to affect adipose mass and adipokine secretion in both humans and animals. Patients with alcoholism have lower body mass index (BMI) and fat mass (FM) but higher liver fat levels.8–11 Studies in humans have also found that the serum leptin concentration was reduced by either chronic alcohol consumption or acute alcohol abuse.11–14 Chronic alcohol exposure in rodents reduced adipose tissue weight,15–17 and serum adiponectin18–23 and leptin24,25 concentrations in association with the development of fatty liver. Administration of exogenous adiponectin or stimulation of endogenous adiponectin production attenuated alcoholic fatty liver in mice.18,19,23,26 These studies indicate that adipokines critically regulate lipid homeostasis at the adipose tissue–liver axis. The present study reports that chronic alcohol exposure caused leptin deficiency, and leptin administration for 2 weeks reversed alcohol-induced fatty liver in mice previously exposed to alcohol for 6 weeks.
Materials and Methods
Animals and Alcohol Feeding Experiments
Male C57BL/6 mice were obtained from Harlan Laboratories, Inc. (Indianapolis, IN). All of the mice were treated according to the experimental procedures approved by the Institutional Animal Care and Use Committee. At age 4 months, mice were pair-fed a modified Lieber-DeCarli alcohol or isocaloric maltose dextrin control liquid diet for up to 8 weeks in a stepwise procedure. Caloric content of the alcohol diet was 16% protein, 12% carbohydrate, 34% fat, and 38% ethanol (Sigma-Aldrich Corp., St. Louis, MO). The ethanol content (w/v) in the diet was 4.8% (34% of total calories) for the first 2 weeks, and was increased by 0.2% every 2 weeks, reaching 5.4% (38% of total calories) for the last 2 weeks. On the basis of our observations, this stepwise feeding protocol improves the performance of the mouse model of alcohol feeding, as indicated by reduced mortality. Two sets of animal experiments were performed. Experiment 1 was performed to determine adipokine production in association with development of alcoholic liver disease, and mice were subjected to alcohol exposure for 2, 4, or 8 weeks. Experiment 2 was performed to determine the role of leptin in alcoholic liver disease because leptin deficiency was detected in experiment 1. Mice were subjected to alcohol exposure for 8 weeks, with leptin (purity >95%; ProSpec, East Brunswick, NJ) administration at 0.5 mg/d per kilogram body weight or saline solution as vehicle, via subcutaneous osmotic minipump (ALZET Osmotic Pump; DURECT Corp., Cupertino, CA) for the last 2 weeks.
Blood Parameters
Blood glucose concentration was measured using a OneTouch Ultra2 Blood Glucose Meter (LifeScan, Inc., Milpitas, CA), and ketone bodies using PTS PANELS Ketone Test Strips (Polymer Technology Systems, Inc., Indianapolis, IN). Plasma alanine aminotransferase activity and triglyceride and cholesterol concentrations were determined using Infinity Reagents (Thermo Fisher Scientific, Inc., Middletown, VA). Plasma free fatty acids (FFAs) were quantified using an FFA Quantification Kit (BioVision, Inc., Milpitas, CA). Plasma leptin and adiponectin concentrations were measured using commercial mouse enzyme-linked immunosorbent assay kits (Millipore Corp., Billerica, MA).
Liver Histopathologic and Lipid Concentrations
Liver tissues were fixed in 10% formalin and embedded in paraffin. Tissues were cut into 5-μm sections and processed for H&E staining and Sirus Red staining. Quantitative assay of lipids was conducted by measuring triglyceride, cholesterol, and FFA concentrations in the liver. Hepatic lipids are extracted by homogenizing liver tissue in chloroform using 1% Triton X-100. The organic extracts were air dried, vacuumed, and dissolved in 1% Triton X-100. Triglyceride, cholesterol, and FFA concentrations in extracts were determined using commercial kits (see Blood Parameters).
Quantitative RT-PCR
The total RNA was isolated from liver or WAT using the TRIzol method (Life Technologies Corp., Grand Island, NY), and reverse transcription was conducted using the TaqMan Reverse Transcription Reagents Kit (Applied Biosystems, Inc., Foster City, CA). The forward and reverse primers (Table 1) were designed using Primer Express Software v3.0.1 (Applied Biosystems), and quantitative RT-PCR (RT-qPCR) analysis with SYBR Green PCR master mix (Qiagen, Inc., Valencia, CA) was performed using a PRISM 7500 Sequence Detection System (Applied Biosystems). Data were normalized to β-actin expression, and are given as fold changes, setting the values of pair-fed mice at 1.
Table 1.
Primer Sequences for Real-Time RT-PCR
| Gene | GeneBank accession no. | Sequences (Forward/Reverse) |
|---|---|---|
| ACADL | NM_007381 | 5′-TCTTTTCCTCGGAGCATGACA-3′ |
| 5′-GACCTCTCTACTCACTTCTCCAG-3′ | ||
| ACOX1 | NM_015729 | 5′-TCCAGACTTCCAACATGAGGA-3′ |
| 5′-CTGGGCGTAGGTGCCAATTA-3′ | ||
| ApoB | NM_009693 | 5′-TTGGCAAACTGCATAGCATCC-3′ |
| 5′-TCAAATTGGGACTCTCCTTTAGC-3′ | ||
| C/EBP-α | NM_007678 | 5′-CAAGAACAGCAACGAGTACCG-3′ |
| 5′-GTCACTGGTCAACTCCAGCAC-3′ | ||
| FABP1 | NM_017399 | 5′-ATGAACTTCTCCGGCAAGTACC-3′ |
| 5′-CTGACACCCCCTTGATGTCC-3′ | ||
| FATP2 | NM_011978 | 5′-TCCTCCAAGATGTGCGGTACT-3′ |
| 5′-TAGGTGAGCGTCTCGTCTCG-3′ | ||
| FATP5 | NM_009512 | 5′-CTACGCTGGCTGCATATAGATG-3′ |
| 5′-CCACAAAGGTCTCTGGAGGAT-3′ | ||
| HNF-1α | NM_009327 | 5′-GACCTGACCGAGTTGCCTAAT-3′ |
| 5′-CCGGCTCTTTCAGAATGGGT-3′ | ||
| HNF-4α | NM_008261 | 5′-GCCTTCTGCGAACTCCTTCTG-3′ |
| 5′-GGGACGATGTAGTCATTGCCT-3′ | ||
| CPT1a | NM_013495 | 5′-CTCCGCCTGAGCCATGAAG-3′ |
| 5′-CACCAGTGATGATGCCATTCT-3′ | ||
| Leptin | NM_008493 | 5′-GAGACCCCTGTGTCGGTTC-3′ |
| 5′-CTGCGTGTGTGAAATGTCATTG-3′ | ||
| LepR-b | NM_146146 | 5′-TGGTCCCAGCAGCTATGGT-3′ |
| 5′-ACCCAGAGAAGTTAGCACTGT-3′ | ||
| MTTP | NM_008642 | 5′-TCAAGAGAGGCTTGGCTAGCTT-3′ |
| 5′-GCCTGGTAGGTCACTTTACAATCC-3′ | ||
| PPAR-α | NM_011144 | 5′-AGAGCCCCATCTGTCCTCTC-3′ |
| 5′-ACTGGTAGTCTGCAAAACCAAA-3′ | ||
| PPAR-γ | NM_011146 | 5′-TCGCTGATGCACTGCCTATG-3′ |
| 5′-GAGAGGTCCACAGAGCTGATT-3′ | ||
| β-Actin | NM_007393 | 5′-GGCTGTATTCCCCTCCATCG-3′ |
| 5′-CCAGTTGGTAACAATGCCATGT-3′ |
Immunoblot Analysis
Whole protein lysates of liver were extracted using 10% Nonidet P-40 lysis buffer supplemented with 1% protease inhibitor cocktail and 1% phenylmethylsulfonyl fluoride. Aliquots containing 60 μg proteins were loaded onto 10% SDS-PAGE, transblotted onto polyvinylidene difluoride membrane, blocked using 5% nonfat dry milk in Tris-buffered saline solution with 0.1% Tween-20, and incubated with rabbit anti-pStat3, Stat3, pAMPK, AMPK (Cell Signaling Technology, Inc., Danvers, MA), PPAR-α (peroxisome proliferator-activated receptor-α), HNF-1α (hepatocyte nuclear factor-1α), C/EBP-α (CCAAT/enhancer binding protein), or β-actin antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). The membrane was then incubated using horseradish peroxidase–conjugated anti-rabbit IgG. The bound complexes were detected via chemiluminescence. The immunoblot bands were quantified via densitometry analysis, and the ratio to β-actin was calculated and is given as fold changes, setting the values of pair-fed mice at 1.
Statistical Analysis
All data are given as mean ± SD. The results were analyzed using the one-sample t-test for two groups, and one-way analysis of variance with Turkey post hoc comparison for more than two groups. Correlation coefficients, analyzed using Prime 3, were used to determine linear association between plasma leptin concentration and WAT mass or body weight. In all statistical tests, P < 0.05 was considered significant.
Results
Chronic Alcohol Exposure Induced Leptin Deficiency in Association With Fatty Liver Disease
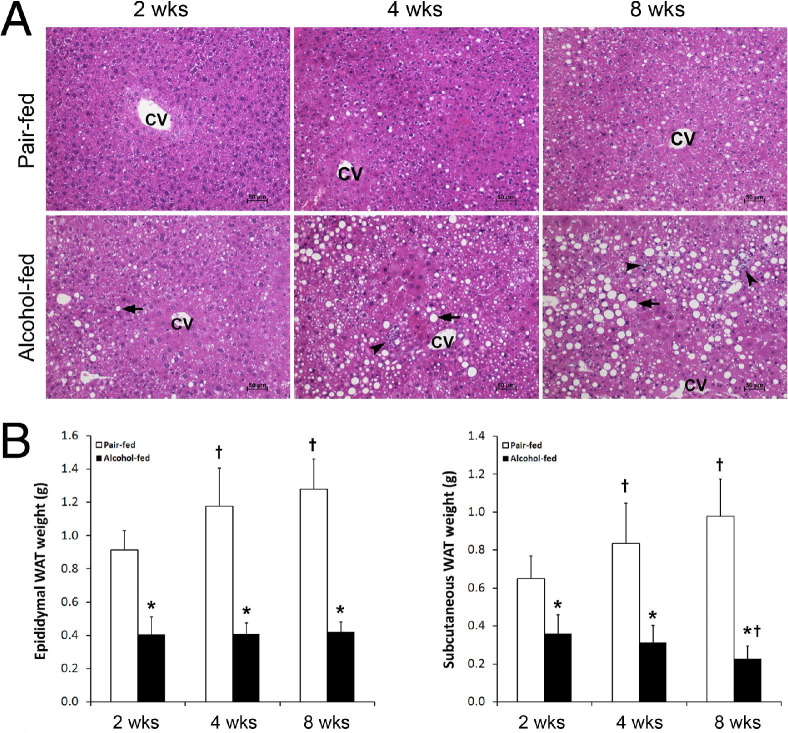
Alcohol exposure for 2, 4, and 8 weeks caused liver damage that was time-dependent (Figure 1A). Lipid droplet accumulation in the liver is the major pathologic change at 2 weeks. At 4 and 8 weeks, the number and size of the lipid droplets in the hepatocytes gradually increased, and inflammatory cell infiltration was frequently found. In contrast, alcohol exposure significantly reduced epididymal and subcutaneous WAT mass at all three times measured (Figure 1B). In comparison with a gradual increase in WAT weight in pair-fed mice, alcohol-fed mice maintained a low WAT weight. The body weight of alcohol-fed mice was lower than that of pair-fed mice at all three times measured (data not shown).
Figure 1.
Alcohol-induced adipose mass reduction in association with liver damage in mice fed with alcohol for 2, 4, or 8 weeks. A: Liver histopathologic analysis (H&E) revealed lipid droplets (arrows) and inflammatory cell infiltration (arrowheads). CV, central vein. B: WAT mass. Results are given as mean ± SD (n = 6 to 10). *P < 0.05 versus pair-fed mice (t-test); †P < 0.05 versus 2 weeks (analysis of variance).
To determine whether alcohol-induced lipodystrophy leads to alterations in adipokine secretion, plasma adiponectin and leptin levels were measured. Plasma adiponectin levels in pair-fed mice did not change at any times measured (Figure 2A). Alcohol exposure for 2 weeks increased the plasma adiponectin concentration, but it declined to normal at 4 and 8 weeks. The plasma leptin concentration in pair-fed mice showed a significant increase at 4 and 8 weeks in comparison with that at 0 and 2 weeks (Figure 2A). Alcohol exposure reduced the plasma leptin concentration by 40%, 20%, and 59% at 2, 4, and 8 weeks, respectively, compared with that at 0 week. RT-qPCR analysis demonstrated that alcohol exposure for 8 weeks dramatically down-regulated leptin gene expression in both epididymal and subcutaneous WATs (Figure 2B). Correlation analysis showed highly positive correlation coefficients between plasma leptin concentration and epididymal and subcutaneous WAT mass and body weight (Figure 2C).
Figure 2.
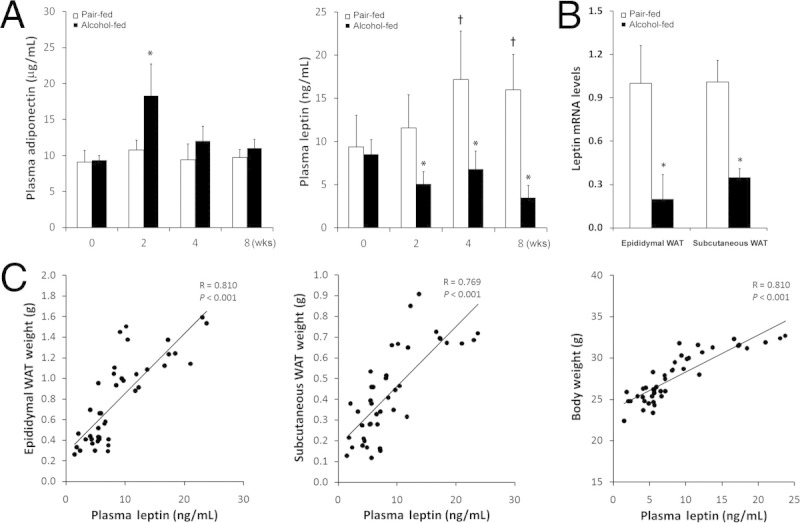
Effects of alcohol on plasma adiponectin and leptin levels in mice fed with alcohol for 2, 4, or 8 weeks. A: Plasma adiponectin and leptin concentrations. B: WAT leptin mRNA levels. C: Correlation of plasma leptin concentration with WAT mass or body weight. Correlation was analyzed using Prime 3. Results are given as mean ± SD (n = 6 to 10). *P < 0.05 versus pair-fed mice (t-test); †P < 0.05 versus 0 week (analysis of variance).
Effects of Leptin Administration on Body Weight, Liver Weight, Adipose Tissue Weight, and Blood Metabolites
To determine the role of leptin deficiency in the pathogenesis of alcoholic fatty liver, leptin was administered to alcohol-fed mice for the last 2 weeks of the study. Leptin administration normalized the alcohol-decreased plasma leptin concentration and stimulated hepatic LepRb gene expression (Figure 3A), although alcohol alone also up-regulated hepatic LepRb (Figure 3B). Effects of leptin administration on routine parameters are given in Table 2. Alcohol exposure with or without leptin administration reduced body weight, whereas liver weight was reduced only by alcohol plus leptin. As a consequence, the liver–body weight ratio was increased by alcohol exposure, which was partially reversed by leptin. Epididymal WAT weight and its ratio to body weight were reduced by alcohol, and the reduction was promoted by leptin. Alcohol exposure significantly elevated the plasma alanine aminotransferase concentration, which was normalized by leptin. Alcohol exposure decreased blood glucose and cholesterol concentrations, and increased the plasma ketone body concentration; these alterations were not affected by leptin. However, leptin administration normalized alcohol-reduced plasma FFA concentrations.
Figure 3.
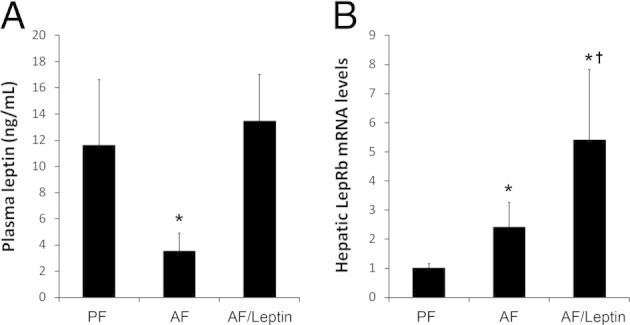
Plasma leptin concentration (A) and hepatic LepRb mRNA level (B) in mice fed with alcohol for 8 weeks with or without leptin administration for the last 2 weeks. Results are given as mean ± SD (n = 6 to 10 in A; n = 4 in B). *P < 0.05 versus pair-fed mice (PF) (analysis of variance); †P < 0.05 versus alcohol-fed mice (AF) (analysis of variance).
Table 2.
Effects of Chronic Alcohol Exposure and Leptin Administration on Body Weight, Liver Weight, Epididymal WAT Weight, and Blood Metabolites
| Measurements | PF | AF | AF/Leptin |
|---|---|---|---|
| Food intake (g/d per mouse) | 10.36 ± 0.83 | 11.02 ± 0.76 | 10.67 ± 1.47 |
| Alcohol intake (g/d per mouse) | NA | 0.59 ± 0.04 | 0.56 ± 0.07 |
| Body weight (g) | 29.77 ± 1.76 | 24.05 ± 1.40⁎ | 22.60 ± 0.52⁎ |
| Liver weight (g) | 1.25 ± 0.08 | 1.30 ± 0.07 | 1.09 ± 0.05⁎ |
| Epididymal WAT weight (g) | 1.28 ± 0.21 | 0.42 ± 0.04⁎ | 0.18 ± 0.04⁎† |
| Liver–body weight ratio | 4.17 ± 0.36 | 5.41 ± 0.14⁎ | 4.83 ± 0.14⁎ |
| Epididymal WAT–body weight ratio | 4.27 ± 0.59 | 1.74 ± 0.12⁎ | 0.78 ± 0.18⁎† |
| Plasma ALT (U/mL) | 24.36 ± 7.19 | 65.82 ± 9.60⁎ | 31.50 ± 11.18† |
| Blood glucose (mg/dL) | 277.0 ± 45.4 | 221.0 ± 19.1⁎ | 204.7 ± 26.1⁎ |
| Blood ketone bodies (mg/dL) | 4.90 ± 1.59 | 7.08 ± 1.65⁎ | 8.34 ± 1.25⁎ |
| Plasma triglycerides (mg/dL) | 75.45 ± 14.12 | 76.98 ± 21.32 | 57.83 ± 20.40 |
| Plasma cholesterol (mg/dL) | 91.29 ± 17.88 | 59.81 ± 11.24⁎ | 47.85 ± 9.66⁎ |
| Plasma free fatty acids (mmol/L) | 0.80 ± 0.15 | 0.43 ± 0.21⁎ | 0.72 ± 0.21† |
Mice were pair-fed alcohol or isocaloric maltose dextran for 8 weeks in the presence or absence of external leptin for the last 2 weeks.
Data are given as mean ± SD (n = 6–10).
AF, alcohol-fed; ALT, alanine aminotransferase; ANOVA, analysis of variance; PF, pair-fed; WAT, white adipose tissue.
P < 0.05 versus PF (ANOVA).
P < 0.05 versus AF (ANOVA).
Leptin Administration Reverses Alcohol Exposure-Induced Lipid Accumulation in the Liver
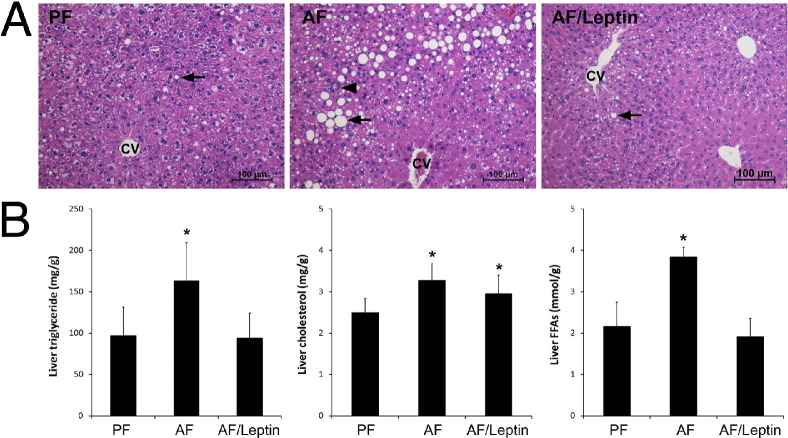
Alcohol exposure for 8 weeks caused lipid droplet accumulation in the liver, which was attenuated by leptin (Figure 4A). Quantitative assay of hepatic lipids demonstrated that alcohol exposure significantly increased the concentrations of triglycerides, cholesterol, and FFAs in the liver (Figure 4B). Leptin administration normalized the concentrations of hepatic triglycerides and FFAs, but did not affect the alcohol-elevated cholesterol concentration. To exclude the possibility that leptin may affect liver fibrogenesis, the hepatic collagen level was assessed using Sirius Red staining. Neither alcohol feeding alone nor alcohol feeding plus leptin administration caused significant collagen accumulation in the liver (see Supplemental Figure S1 at http://ajp.amjpathol.org).
Figure 4.
Liver histopathologic and lipid concentrations in mice fed with alcohol (AF) for 8 weeks with or without leptin administration for the last 2 weeks. A: Histopathologic analysis (H&E) revealed lipid droplets (arrows) and inflammatory cell infiltration (arrowhead). CV, central vein. B: Liver lipid concentrations. Results are given as mean ± SD (n = 6 to 10). *P < 0.05 versus pair-fed mice (PF) (analysis of variance).
Leptin Administration Improves Alcohol Exposure–Dysregulated Hepatic Genes Related to Lipid Metabolism
The relative mRNA levels of hepatic genes related to lipid metabolism are given in Table 3. Among three fatty acid transport genes, FABP1 was down-regulated by alcohol regardless of leptin administration, whereas FATP2 was up-regulated by leptin. Alcohol exposure down-regulated all three genes related to fatty acid β-oxidation, CPT1a, ACADL, and ACOX1, which was normalized by leptin. Leptin administration also normalized alcohol-down-regulated very low-density lipoprotein assembly genes MTTP and ApoB. Among the five transcription factors related to lipid metabolism regulation, alcohol exposure down-regulated C/EBP-α, HNF-1α, PPAR-α, and PPAR-γ, and leptin normalized the expression levels of the first three but not PPAR-γ. The HNF-4α gene was not affected by either alcohol or leptin. Immunoblot analysis demonstrated that leptin administration reversed alcohol-reduced protein levels of PPAR-α and HNF-1α (Figure 5A). However, the protein level of C/EBP-α was not affected by alcohol regardless of leptin administration. Immunoblot analysis also showed that the phosphorylation levels of Stat3 and AMPK, the major molecules mediating leptin signaling, were reduced after alcohol exposure, but were normalized by leptin administration (Figure 5B).
Table 3.
Effects of Leptin Administration on Hepatic Genes Related to Lipid Metabolism
| Measurements | PF | AF | AF/Leptin |
|---|---|---|---|
| Fatty acid transport | |||
| FABP1 | 1.006 ± 0.13 | 0.41 ± 0.13⁎ | 0.33 ± 0.09⁎ |
| FATP2 | 1.005 ± 0.12 | 0.98 ± 0.29 | 1.50 ± 0.29⁎† |
| FATP5 | 1.003 ± 0.10 | 0.98 ± 0.10 | 1.23 ± 0.17 |
| Fatty acid β-oxidation | |||
| CPT1a | 1.001 ± 0.05 | 0.53 ± 0.02⁎ | 1.06 ± 0.27† |
| ACADL | 1.009 ± 0.16 | 0.70 ± 0.15⁎ | 1.007 ± 0.11† |
| ACOX1 | 1.001 ± 0.05 | 0.58 ± 0.14⁎ | 0.86 ± 0.26† |
| VLDL assembly | |||
| MTTP | 1.04 ± 0.35 | 0.64 ± 0.09⁎ | 0.71 ± 0.11 |
| ApoB | 1.003 ± 0.10 | 0.76 ± 0.02⁎ | 1.17 ± 0.20† |
| Transcription factors | |||
| C/EBP-α | 1.004 ± 0.11 | 0.67 ± 0.02⁎ | 0.93 ± 0.08† |
| HNF-1α | 1.008 ± 0.15 | 0.56 ± 0.12⁎ | 0.84 ± 0.12† |
| HNF-4α | 1.01 ± 0.21 | 1.07 ± 0.15 | 1.27 ± 0.27 |
| PPAR-α | 1.004 ± 0.11 | 0.62 ± 0.09⁎ | 0.93 ± 0.05† |
| PPAR-γ | 1.007 ± 0.14 | 0.27 ± 0.16⁎ | 0.16 ± 0.06⁎ |
Mice were pair-fed alcohol on isocaloric maltose dextran for 8 weeks in the presence or absence of external leptin for the last 2 weeks.
Gene expression was analyzed using quantitative RT-PCR.
Data are given as mean ± SD (n = 4).
AF, alcohol-fed; ANOVA, analysis of variance; PF, pair-fed; VLDL, very low-density lipoprotein.
P < 0.05 versus PF (ANOVA).
P < 0.05 versus AF (ANOVA).
Figure 5.
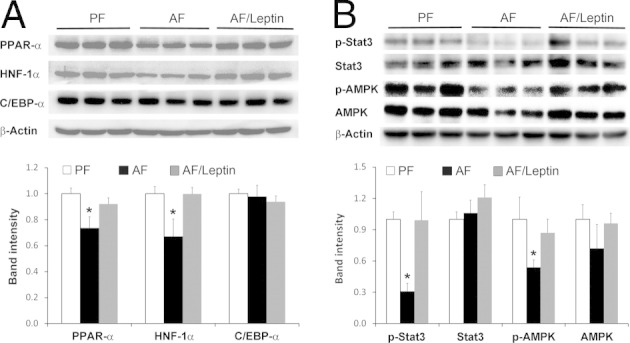
Protein levels of PPAR-α, HNF-1α, and C/EBP-α in the liver of mice fed with alcohol (AF) for 8 weeks with or without leptin administration for the last 2 weeks. A: Immunoblot analysis of PPAR-α, HNF-1α, and C/EBP-α. B: Immunoblot analysis of total and pStat3 and p-AMPK. Band intensity was quantified using densitometry analysis, and the ratio to β-actin was calculated by setting the value of pair-fed mice (PF) at 1. Results are given as mean ± SD (n = 3). *P < 0.05 versus PF mice (analysis of variance).
Discussion
Alcohol consumption has been shown to modulate plasma adipokine levels, in particular adiponectin. Alcohol exposure in mice decreased the serum adiponectin concentration in a time-dependent manner, whereas administration with mouse recombinant adiponectin attenuated alcohol-induced fatty liver and inflammation.18 The decrease in plasma adiponectin concentration was also found in other animal models including rats and micropigs.19–23 Elevation of the plasma adiponectin concentration was associated with protective effects of dietary supplementation with saturated fat, rosiglitazone, or resveratrol against alcoholic fatty liver.19,23,26 However, there are controversial reports on the effect of alcohol on adiponectin. A recent study demonstrated that high dosages of alcohol alone or plus overnutrition increased the plasma adiponectin concentration.27 Clinical studies have also demonstrated that the serum adiponectin concentration correlated well with daily alcohol consumption and liver injury, but declined during alcohol abstinence.28 The present study demonstrated that alcohol exposure did not reduce the plasma adiponectin concentration, although a dramatic reduction in adipose mass was found. A previous report showed that adipose tissue reduction in iNOS null mice was associated with hypoleptinemia, although the plasma adiponectin concentration was normal.29 These data suggest that the plasma adiponectin concentration does not correlate with WAT mass.
Alcohol abuse affected the serum leptin concentration in both human and animal studies. Serum leptin concentrations in patients with alcoholism correlate well with BMI and FM.11–14,24,25 Patients with alcoholism with lower BMI and FM had decreased serum leptin concentrations,11,12 whereas those with greater BMI and FM had increased serum leptin concentrations.30 Of importance, lower BMI and FM in patients with alcoholism were associated with lipid accumulation in the liver.11,12 An animal study demonstrated that acute alcohol exposure in rats reduced the plasma leptin concentration in a dose-dependent manner.24 Chronic alcohol exposure in rodents reduced the plasma leptin concentration in association with reduction of WAT mass and accumulation of lipids in the liver or bone marrow.16,17,25 The present study demonstrated that reduction of the plasma leptin concentration was association with alcoholic fatty liver, and highly positive correlations occur between the plasma leptin concentration and WAT mass and body weight. These findings suggest that hypoleptinemia is associated with WAT reduction and development of alcoholic fatty liver.
Leptin critically regulates hepatic lipid homeostasis, and animals with either leptin mutation, as in ob/ob mice, or leptin receptor mutation, as in db/db mice and Zucker (fa/fa) rats, develop fatty liver in association with early onset of obesity and insulin resistance.31 Alcohol exposure caused more lipid accumulation and liver injury in Zucker (fa/fa) rats than in wild-type rats.32 Hypoleptinemia in patients with lipodystrophy contributes to fatty liver and insulin resistance.33–38 Leptin therapy for up to 18 months in these patients significantly reduced hepatic volume and triglyceride contents, and improved pathologic alterations in liver biopsy specimens including steatosis, ballooning injury, and parenchymal inflammation.33–35 Recent studies also have demonstrated that leptin therapy normalized hepatic triglyceride concentration and reversed deadly consequences in both types I and II diabetes.36–38 These findings suggest that leptin has potent antisteatotic and antidiabetic activities. The present study demonstrated that leptin administration ameliorated alcoholic fatty liver and corrected multiple lipid metabolic pathways. While up-regulating genes of fatty acid β-oxidation is likely a major mechanism underlying leptin action, leptin also attenuated alcohol-induced down-regulation of MTTP and ApoB. Furthermore, leptin normalized alcohol-induced down-regulation of transcription factors such as PPAR-α and HNF-1α. Knockout of either PPAR-α or HNF-1α causes fatty liver.39,40 The present study and other previously published reports have demonstrated that reactivation of PPAR-α via dietary supplementation with zinc or PPAR-α agonist attenuates alcoholic fatty liver.16,41 The present study also demonstrated that leptin administration abrogated the inhibitory effects of alcohol on AMPK and Stat3 signal pathways. Inhibition of AMPK has been reported to mediate alcohol action in the liver, and reactivation of AMPK was associated with prevention of alcoholic fatty liver by dietary supplementation with rosiglitazone or resveratrol.23,26 Recent studies also showed that activation of Stat3 mediates the protective effects of IL-6 and IL-22 on alcoholic liver injury.42,43 All of these studies suggest that AMPK and Stat3 are important molecular targets for therapeutic intervention in alcoholic fatty liver disease.
Leptin has been implicated in multiple cell functions including innate and adaptive immunity, liver regeneration, and fibrogenesis. Leptin treatment attenuates alcohol-induced apoptosis and production of proinflammatory cytokines and TGF-β in HepG2 cells.44,45 Leptin administration also attenuated TNF-α production and hepatocyte apoptosis in a mouse model of CCl4 hepatotoxicity.46 Inhibition of oxidative stress was associated with the protective effects of leptin in both in vivo and in vitro studies.47,48 Leptin-deficient rats have lower levels of metallothionein and zinc in the liver, which accounts for the susceptibility to alcohol-induced steatohepatitis.32 Indeed, our previous studies demonstrated that metallothionein and zinc suppress alcohol-induced oxidative stress, thereby preventing alcoholic liver injury.16,48,49 Leptin is also involved in liver fibrogenesis. Leptin-deficient ob/ob mice failed to develop liver fibrosis in either a methionine/choline deficiency or a CCl4 model.50 However, clinical studies with leptin therapy for 4 to 18 months improved the NASH (nonalcoholic steatohepatitis) score without affecting the fibrosis score in liver biopsy.35 It should be emphasized that leptin administration in the present study was performed on the basis of the pathophysiologic condition of hypoleptinemia. Normalizing the plasma leptin concentration in alcohol-fed mice reversed alcoholic fatty liver without affecting liver fibrogenesis.
In conclusion, leptin deficiency was associated with reduction of WAT mass in a mouse model of alcoholic fatty liver disease. Leptin administration attenuated alcohol-induced hypoleptinemia and hepatic lipid accumulation in association with normalization of alcohol-induced down-regulation of hepatic genes involved in lipid metabolism such as fatty acid oxidation, very low-density lipoprotein secretion, and related transcription factors. Leptin administration reversed alcohol-suppressed phosphorylation of AMPK and Stat3 in the liver. These findings suggest that leptin deficiency in association with WAT mass reduction may contribute to the pathogenesis of alcoholic fatty liver disease.
Acknowledgment
We thank Marion McClain for review of the manuscript.
Footnotes
Supported in part by grants R01AA018844 (Z.Z.), R01AA020212 (Z.Z.), R37AA010762 (C.J.M.), RC2AA019385 (C.J.M.), and P01AA017103 (C.J.M.) from the NIH and by the Veterans Administration (C.J.M.).
Supplemental material for this article can be found at http://ajp.amjpathol.org or at http://dx.doi.org/10.1016/j.ajpath.2012.06.013.
Supplementary data
Assessment of liver fibrogenesis in mice fed alcohol for 8 weeks with or without leptin administration for the last 2 weeks. Sirius Red staining of collagen on liver tissue sections. AF, alcohol-fed; PF, pair-fed, PV, portal vein.
References
- 1.Hall P., editor. Pathological spectrum of alcoholic liver disease: Alcoholic Liver Disease. ed 2. Edward Arnold; London: 1995. pp. 41–88. [Google Scholar]
- 2.Lakshman M.R. Some novel insights into the pathogenesis of alcoholic steatosis. Alcohol. 2004;34:45–48. doi: 10.1016/j.alcohol.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Gao B., Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers C.Q., Ajmo J.M., You M. Adiponectin and alcoholic fatty liver disease. IUBMB Life. 2008;60:790–797. doi: 10.1002/iub.124. [DOI] [PubMed] [Google Scholar]
- 5.You M., Rogers C.Q. Adiponectin: a key adipokine in alcoholic fatty liver. Exp Biol Med (Maywood) 2009;234:850–859. doi: 10.3181/0902-MR-61. [DOI] [PubMed] [Google Scholar]
- 6.Galic S., Oakhill J.S., Steinberg G.R. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Marra F., Bertolani C. Adipokines in liver disease. Hepatology. 2009;50:957–969. doi: 10.1002/hep.23046. [DOI] [PubMed] [Google Scholar]
- 8.Addolorato G., Capristo E., Greco A.V., Stefanini G.F., Gasbarrini G. Energy expenditure, substrate oxidation, and body composition in subjects with chronic alcoholism: new findings from metabolic assessment. Alcohol Clin Exp Res. 1997;21:962–967. [PubMed] [Google Scholar]
- 9.Addolorato G., Capristo E., Greco A.V., Stefanini G.F., Gasbarrini G. Influence of chronic alcohol abuse on body weight and energy metabolism: is excess ethanol consumption a risk factor for obesity or malnutrition? J Intern Med. 1998;244:387–395. doi: 10.1046/j.1365-2796.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- 10.Addolorato G., Capristo E., Marini M., Santini P., Scognamiglio U., Attilia M.L., Messineo D., Sasso G.F., Gasbarrini G., Ceccanti M. Body composition changes induced by chronic ethanol abuse: evaluation by dual energy X-ray absorptiometry. Am J Gastroenterol. 2000;95:2323–2327. doi: 10.1111/j.1572-0241.2000.02320.x. [DOI] [PubMed] [Google Scholar]
- 11.Greco A.V., Mingrone G., Favuzzi A., Capristo E., Gniuli D., Addolorato G., Brunani A., Cavagnin F., Gasbarrini G. Serum leptin levels in post-hepatitis liver cirrhosis. J Hepatol. 2000;33:38–42. doi: 10.1016/s0168-8278(00)80157-9. [DOI] [PubMed] [Google Scholar]
- 12.Santolaria F., Pérez-Cejas A., Alemán M.R., González-Reimers E., Milena A., de la Vega M.J., Martínez-Riera A., Gómez-Rodríguez M.A. Low serum leptin levels and malnutrition in chronic alcohol misusers hospitalized by somatic complications. Alcohol Alcohol. 2003;38:60–66. doi: 10.1093/alcalc/agg015. [DOI] [PubMed] [Google Scholar]
- 13.Calissendorff J., Brismar K., Röjdmark S. Is decreased leptin secretion after alcohol ingestion catecholamine-mediated? Alcohol Alcohol. 2004;39:281–286. doi: 10.1093/alcalc/agh054. [DOI] [PubMed] [Google Scholar]
- 14.Kalousová M., Zima T., Popov P., Spacek P., Braun M., Soukupová J., Pelinkova K., Kientsch-Engel R. Advanced glycation end-products in patients with chronic alcohol misuse. Alcohol Alcohol. 2004;39:316–320. doi: 10.1093/alcalc/agh058. [DOI] [PubMed] [Google Scholar]
- 15.Kang L., Chen X., Sebastian B.M., Pratt B.T., Bederman I.R., Alexander J.C., Previs S.F., Nagy L.E. Chronic ethanol and triglyceride turnover in white adipose tissue in rats: inhibition of the anti-lipolytic action of insulin after chronic ethanol contributes to increased triglyceride degradation. J Biol Chem. 2007;282:28465–28473. doi: 10.1074/jbc.M705503200. [DOI] [PubMed] [Google Scholar]
- 16.Kang X., Zhong W., Liu J., Song Z., McClain C.J., Kang Y.J., Zhou Z. Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4alpha and peroxisome proliferator-activated receptor-alpha. Hepatology. 2009;50:1241–1250. doi: 10.1002/hep.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong W., Zhao Y., Tang Y., Wei X., Shi X., Sun W., Sun X., Yin X., Sun X., Kim S., McClain C.J., Zhang X., Zhou Z. Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis. Am J Pathol. 2012;180:998–1007. doi: 10.1016/j.ajpath.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu A., Wang Y., Keshaw H., Xu L.Y., Lam K.S., Cooper G.J. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.You M., Considine R.V., Leone T.C., Kelly D.P., Crabb D.W. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 2005;42:568–577. doi: 10.1002/hep.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X., Sebastian B.M., Nagy L.E. Chronic ethanol feeding to rats decreases adiponectin secretion by subcutaneous adipocytes. Am J Physiol Endocrinol Metab. 2007;292:E621–E628. doi: 10.1152/ajpendo.00387.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esfandiari F., You M., Villanueva J.A., Wong D.H., French S.W., Halsted C.H. S-adenosylmethionine attenuates hepatic lipid synthesis in micropigs fed ethanol with a folate-deficient diet. Alcohol Clin Exp Res. 2007;31:1231–1239. doi: 10.1111/j.1530-0277.2007.00407.x. [DOI] [PubMed] [Google Scholar]
- 22.Song Z., Zhou Z., Deaciuc I., Chen T., McClain C.J. Inhibition of adiponectin production by homocysteine: potential mechanism for alcoholic liver disease. Hepatology. 2008;47:867–879. doi: 10.1002/hep.22074. [DOI] [PubMed] [Google Scholar]
- 23.Shen Z., Liang X., Rogers C.Q., Rideout D., You M. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G364–G374. doi: 10.1152/ajpgi.00456.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otaka M., Konishi N., Odashima M., Jin M., Wada I., Matsuhashi T., Ohba R., Watanabe S. Effect of alcohol consumption on leptin level in serum, adipose tissue, and gastric mucosa. Dig Dis Sci. 2007;52:3066–3069. doi: 10.1007/s10620-006-9635-x. [DOI] [PubMed] [Google Scholar]
- 25.Maddalozzo G.F., Turner R.T., Edwards C.H., Howe K.S., Widrick J.J., Rosen C.J., Iwaniec U.T. Alcohol alters whole body composition, inhibits bone formation, and increases bone marrow adiposity in rats. Osteoporos Int. 2009;20:1529–1538. doi: 10.1007/s00198-009-0836-y. [DOI] [PubMed] [Google Scholar]
- 26.Ajmo J.M., Liang X., Rogers C.Q., Pennock B., You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G833–G842. doi: 10.1152/ajpgi.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J., Lai K.K., Verlinsky A., Lugea A., French S.W., Cooper M.P., Ji C., Tsukamoto H. Synergistic steatohepatitis by moderate obesity and alcohol in mice despite increased adiponectin and p-AMPK. J Hepatol. 2011;55:673–682. doi: 10.1016/j.jhep.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buechler C., Schäffler A., Johann M., Neumeier M., Köhl P., Weiss T., Wodarz N., Kiefer P., Hellerbrand C. Elevated adiponectin serum levels in patients with chronic alcohol abuse rapidly decline during alcohol withdrawal. J Gastroenterol Hepatol. 2009;24:558–563. doi: 10.1111/j.1440-1746.2008.05693.x. [DOI] [PubMed] [Google Scholar]
- 29.Gómez-Ambrosi J., Becerril S., Oroz P., Zabalza S., Rodríguez A., Muruzábal F.J., Archanco M., Gil M.J., Burrell M.A., Frühbeck G. Reduced adipose tissue mass and hypoleptinemia in iNOS deficient mice: effect of LPS on plasma leptin and adiponectin concentrations. FEBS Lett. 2004;577:351–356. doi: 10.1016/j.febslet.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 30.Campillo B., Sherman E., Richardet J.P., Bories P.N. Serum leptin levels in alcoholic liver cirrhosis: relationship with gender, nutritional status, liver function and energy metabolism. Eur J Clin Nutr. 2001;55:980–988. doi: 10.1038/sj.ejcn.1601255. [DOI] [PubMed] [Google Scholar]
- 31.Anstee Q.M., Goldin R.D. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol. 2006;87:1–16. doi: 10.1111/j.0959-9673.2006.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomita K., Azuma T., Kitamura N., Tamiya G., Ando S., Nagata H., Kato S., Inokuchi S., Nishimura T., Ishii H., Hibi T. Leptin deficiency enhances sensitivity of rats to alcoholic steatohepatitis through suppression of metallothionein. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1078–G1085. doi: 10.1152/ajpgi.00107.2004. [DOI] [PubMed] [Google Scholar]
- 33.Oral E.A., Simha V., Ruiz E., Andewelt A., Premkumar A., Snell P., Wagner A.J., DePaoli A.M., Reitman M.L., Taylor S.I., Gorden P., Garg A. Leptin-administration therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 34.Petersen K.F., Oral E.A., Dufour S., Befroy D., Ariyan C., Yu C., Cline G.W., DePaoli A.M., Taylor S.I., Gorden P., Shulman G.I. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Javor E.D., Ghany M.G., Cochran E.K., Oral E.A., DePaoli A.M., Premkumar A., Kleiner D.E., Gorden P. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology. 2005;41:753–760. doi: 10.1002/hep.20672. [DOI] [PubMed] [Google Scholar]
- 36.Fujikawa T., Chuang J.C., Sakata I., Ramadori G., Coppari R. Leptin therapy improves insulin-deficient type 1 diabetes by CNS-dependent mechanisms in mice. Proc Natl Acad Sci USA. 2010;107:17391–17396. doi: 10.1073/pnas.1008025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang M.Y., Chen L., Clark G.O., Lee Y., Stevens R.D., Ilkayeva O.R., Wenner B.R., Bain J.R., Charron M.J., Newgard C.B., Unger R.H. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci USA. 2010;107:4813–4819. doi: 10.1073/pnas.0909422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cummings B.P., Bettaieb A., Graham J.L., Stanhope K.L., Dill R., Morton G.J., Haj F.G., Havel P.J. Subcutaneous administration of leptin normalizes fasting plasma glucose in obese type 2 diabetic UCD-T2DM rats. Proc Natl Acad Sci USA. 2011;108:14670–14675. doi: 10.1073/pnas.1107163108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugden M.C., Bulmer K., Gibbons G.F., Knight B.L., Holness M.J. Peroxisome-proliferator-activated receptor-alpha (PPARalpha) deficiency leads to dysregulation of hepatic lipid and carbohydrate metabolism by fatty acids and insulin. Biochem J. 2002;364:361–368. doi: 10.1042/BJ20011699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akiyama T.E., Ward J.M., Gonzalez F.J. Regulation of the liver fatty acid-binding protein gene by hepatocyte nuclear factor 1alpha (HNF1alpha): alterations in fatty acid homeostasis in HNF1alpha-deficient mice. J Biol Chem. 2000;275:27117–27122. doi: 10.1074/jbc.M004388200. [DOI] [PubMed] [Google Scholar]
- 41.Fischer M., You M., Matsumoto M., Crabb D.W. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonist treatment reverses PPARalpha dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem. 2003;278:27997–28004. doi: 10.1074/jbc.M302140200. [DOI] [PubMed] [Google Scholar]
- 42.Ki S.H., Park O., Zheng M., Morales-Ibanez O., Kolls J.K., Bataller R., Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller A.M., Wang H., Bertola A., Park O., Horiguchi N., Ki S.H., Yin S., Lafdil F., Gao B. Inflammation-associated interleukin-6/signal transducer and activator of transcription 3 activation ameliorates alcoholic and nonalcoholic fatty liver diseases in interleukin-10-deficient mice. Hepatology. 2011;54:846–856. doi: 10.1002/hep.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balasubramaniyan V., Murugaiyan G., Shukla R., Bhonde R.R., Nalini N. Leptin downregulates ethanol-induced secretion of proinflammatory cytokines and growth factor. Cytokine. 2007;37:96–100. doi: 10.1016/j.cyto.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 45.Balasubramaniyan V., Shukla R., Murugaiyan G., Bhonde R.R., Nalini N. Mouse recombinant leptin protects human hepatoma HepG2 against apoptosis: TNF-alpha response and oxidative stress induced by the hepatotoxin-ethanol. Biochim Biophys Acta. 2007;1770:1136–1144. doi: 10.1016/j.bbagen.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Serbetçi K., Uysal O., Erkasap N., Köken T., Baydemir C., Erkasap S. Anti-apoptotic and antioxidant effect of leptin on CCl(4)-induced acute liver injury in rats. Mol Biol Rep. 2012;39:1173–1180. doi: 10.1007/s11033-011-0847-6. [DOI] [PubMed] [Google Scholar]
- 47.Sailaja J.B., Balasubramaniyan V., Nalini N. Effect of exogenous leptin administration on high fat diet induced oxidative stress. Pharmazie. 2004;59:475–479. [PubMed] [Google Scholar]
- 48.Zhou Z., Wang L., Song Z., Saari J.T., McClain C.J., Kang Y.J. Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. Am J Pathol. 2005;166:1681–1690. doi: 10.1016/S0002-9440(10)62478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Z., Sun X., James Kang Y. Metallothionein protection against alcoholic liver injury through inhibition of oxidative stress. Exp Biol Med (Maywood) 2002;227:214–222. doi: 10.1177/153537020222700310. [DOI] [PubMed] [Google Scholar]
- 50.Leclercq I.A., Farrell G.C., Schriemer R., Robertson G.R. Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J Hepatol. 2002;37:206–213. doi: 10.1016/s0168-8278(02)00102-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Assessment of liver fibrogenesis in mice fed alcohol for 8 weeks with or without leptin administration for the last 2 weeks. Sirius Red staining of collagen on liver tissue sections. AF, alcohol-fed; PF, pair-fed, PV, portal vein.