Abstract
Thrombin generation is increased in patients with nonalcoholic fatty liver disease (NAFLD) and in mouse models of diet-induced obesity. Deficiency in the thrombin receptor protease activated receptor-1 reduces hepatic inflammation and steatosis in mice fed a Western diet. However, it is currently unclear whether thrombin inhibitors can modify the pathogenesis of established NAFLD. We tested the hypothesis that thrombin inhibition could reverse hepatic steatosis and inflammation in mice with established diet-induced NAFLD. Low-density lipoprotein receptor–deficient LDLr−/− mice were fed a control diet or a Western diet for 19 weeks. Mice were given the direct thrombin inhibitor argatroban ∼15 mg/kg/day or its vehicle via a miniosmotic pump for the final 4 weeks of the study. Argatroban administration significantly reduced hepatic proinflammatory cytokine expression and reduced macrophage and neutrophil accumulation in livers of mice fed a Western diet. Argatroban did not significantly impact hepatic steatosis, as indicated by histopathology, Oil Red O staining, and hepatic triglyceride levels. Argatroban reduced serum triglyceride and cholesterol levels in mice fed a Western diet. Argatroban reduced both α-smooth muscle actin expression and Type 1 collagen mRNA levels in livers of mice fed a Western diet, indicating reduced activation of hepatic stellate cells. This study indicates that therapeutic intervention with a thrombin inhibitor attenuates hepatic inflammation and several profibrogenic changes in mice fed a Western diet.
More than 70% of patients with abdominal obesity develop concurrent nonalcoholic fatty liver disease (NAFLD).1 NAFLD, the hepatic manifestation of metabolic syndrome, is characterized by excess accumulation of lipids in the liver (ie, hepatic steatosis)2,3 and affects approximately 25% of the Western population.4 Steatosis accompanied by marked histological inflammation is termed nonalcoholic steatohepatitis (NASH), which is the most severe form of NAFLD and a major cause of liver fibrosis and cirrhosis.5,6 Progression from simple steatosis to NASH is indicative of a poor clinical outcome and currently has no effective pharmacological treatment options. In addition, both obesity and NAFLD are associated with an increased risk of developing type 2 diabetes mellitus7 and cardiovascular disease.8,9 Therefore, there is an immediate need to identify novel pharmacological approaches to treat NAFLD.
A significant commonality among obesity-related diseases is inflammation. Obesity and hepatic steatosis are associated with increased expression of many inflammatory mediators in the liver.10 The expression of several of these mediators, particularly those involved in leukocyte recruitment, is further increased in patients with NASH.10 Several compelling studies have demonstrated that inflammatory chemokines such as monocyte chemoattractant protein-1 (MCP-1) and the subsequent recruitment and activation of hepatic macrophages (ie, Kupffer cells) are essential components of NAFLD pathogenesis.11–14 A systemic proinflammatory state, driven in part by hepatic inflammation, is associated with an increased risk of type 2 diabetes15,16 and adverse cardiovascular outcomes.17 In particular, systemic levels of high sensitivity C-reactive protein (hs-CRP), a biomarker of risk for acute cardiovascular events,18 are primarily dictated by the proinflammatory environment in the liver. Indeed, hs-CRP levels are independently associated with hepatic steatosis in patients with metabolic syndrome.8 These studies indicate that increased hepatic inflammation is a focal point of multiple diseases stemming from the metabolic syndrome. Of importance, the molecular triggers of hepatic inflammation in metabolic diseases such as obesity are not completely understood. To this end, understanding the cellular and molecular pathways coordinating hepatic inflammation in metabolic disease could lead to the development of clinical therapies that target inflammation as an underlying cause of multiple interrelated diseases.
Because the liver is the primary site of coagulation factor synthesis, liver diseases are often accompanied by a rebalancing of the hemostatic profile.19 Indeed, abdominal obesity, metabolic syndrome, and NAFLD are each associated with activation of the blood coagulation cascade, including increased generation of the serine protease thrombin.20–23 Moreover, thrombin generation is increased in mouse models of diet-induced obesity and hypercholesterolemia.24,25 Previous studies have shown that the induction of tissue factor on monocytes is essential for thrombin generation in mice fed a Western diet.26 Various hepatic manifestations of diet-induced obesity, including hepatic steatosis, are reduced in tissue factor–deficient mice.24 Moreover, we found previously that mice lacking a thrombin receptor, protease activated receptor-1 (PAR-1), did not develop hepatic steatosis when fed a Western diet.24 Although compelling, these genetic approaches do not directly address the question of whether intervention with pharmacological agents, perhaps anticoagulants, can reduce established liver disease. Indeed, it is currently unclear whether pharmacological inhibition of thrombin alters the course of established diet-induced fatty liver disease in mice.
To this end, we tested the hypothesis that pharmacological inhibition of thrombin could therapeutically reverse diet-induced hepatic inflammation and steatosis in hypercholesterolemic low density lipoprotein receptor–deficient (LDLr−/−) mice.
Materials and Methods
Mice and Experimental Diets
Six-week-old male LDLr−/− mice on a C57Bl/6 background purchased from the Jackson Laboratory (Bar Harbor, ME) were fed a control diet (AIN-93M, 10% kcal from fat; Dyets, Bethlehem, PA) or a Western diet (Diet #100244, 40% kcal from milk fat; Dyets) ad libitum for 15 weeks before implantation of a subcutaneous miniosmotic pump (see below). Mice fed each diet were then stratified to receive either vehicle or argatroban such that the body weights of each group of mice at the time of pump implantation were similar. Average food intake was measured weekly for each cage of mice, and mice were weighed weekly. All studies were approved by the Animal Care and Use Committee of the University of Kansas Medical Center and complied with National Institutes of Health guidelines.
Pump Preparation and Implantation
Alzet miniosmotic pumps (Model 2004, flow rate 0.25 μL/hour; Alzet, Cupertino, CA) were filled with vehicle or argatroban (AKScientific, Union City, CA) solution. Previous studies have demonstrated effective administration of argatroban to rodents via a micro-osmotic pump.27,28 The argatroban vehicle consisted of 10% glacial acetic acid, 2 mmol/L sodium acetate, 20% polyethylene glycol 400, and 10% propylene glycol in sterile water for injection. This solution is stated to stabilize argatroban for at least 4 weeks at 37°C.29 Argatroban was dissolved in the vehicle solution at 100 mg/mL, yielding a daily dose of approximately 15 mg/kg/day, a dose similar to previous studies using argatroban administered to rodents via a micro-osmotic pump.27 Pumps were filled in a sterile environment according to the manufacturer's protocol. Mice were anesthetized with 3% isoflurane and given an intraperitoneal injection of Buprenex (buprenorphine HCl, 1 mg/kg; Reckitt Benckiser Pharmaceuticals, Richmond, VA) before surgeries. Pumps were implanted subcutaneously slightly posterior to the scapulae, and the incision was closed with Syneture 4–0 chromic gut sutures (Roboz, Gaithersburg, MD). Sutures and incision sites were monitored daily. Mice were treated with topical zinc oxide for skin irritation as required and recommended by a clinical veterinarian. Mice were allowed ad libitum access to either control diet or Western diet (as specified above) for 28 days after pump implantation.
Sample Collection
Mice were fasted overnight before sample collection. Under isoflurane anesthesia, blood was collected from the caudal vena cava into sodium citrate (final concentration, 0.38%) for the collection of plasma and into an empty syringe for the collection of serum. Sections of liver from the left lateral lobe were fixed in 10% neutral-buffered formalin for 48 hours and embedded in paraffin. The right medial lobe was affixed to a cork with optimal cutting temperature compound (Thermo Fisher Scientific, Waltham, MA) and immersed for approximately 3 minutes in liquid nitrogen–chilled isopentane. The remaining liver was snap frozen in liquid nitrogen.
Histopathology, Clinical Chemistry, and Determination of Lipid Levels in Liver
Paraffin-embedded livers were sectioned at 5 μm and stained with hematoxylin and eosin (H&E). Livers were scored by a board-certified pathologist (W.C.) for percent steatosis and the presence of inflammatory foci. The total number of foci of portal inflammation and lobular inflammation was counted for the entire section under ×200 magnification. The raw score of inflammatory foci per ×200 field was calculated by dividing the total number of inflammatory foci by the total number of fields. Formalin-fixed sections were also stained for collagen using a Chromaview Gomori Trichrome staining kit (Thermo Fisher Scientific) as described by the manufacturer's protocol. Oil Red O staining was performed as previously described.30 Quantification of collagen staining (blue) and Oil Red O staining was performed using Metamorph software (Molecular Devices, Sunnyvale, CA) and ImageJ version 1.45 (NIH, Bethesda, MD). Plasma thrombin-antithrombin levels were determined using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Siemens Healthcare Diagnostics, Tarrytown, NY). Lipids were extracted from 100 mg of snap-frozen liver as described,31 and hepatic and serum triglyceride and cholesterol levels were determined using commercially available reagents (Pointe Scientific, Canton, MI). Thrombin time was determined by clotting 50 μL of mouse plasma with 50 μL of Dade thrombin reagent (6.25 U/mL; Siemens Healthcare Diagnostics) using a Start4 coagulation analyzer (Diagnostica Stago, Parsippany, NJ).
RNA Isolation, cDNA Synthesis, and Real-Time PCR
Total RNA was isolated from approximately 100 mg of snap-frozen liver using TRI Reagent (Molecular Research Center, Cincinnati, OH) per the manufacturer's protocol. One microgram of RNA was used for the synthesis of cDNA using a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) and C1000 thermal cycler (Bio-Rad Laboratories, Hercules, CA). Levels of MCP-1, CD68, F4/80, intercellular adhesion molecule-1 (ICAM-1), macrophage inflammatory protein-2 (MIP-2), type 1 collagen (Col1a1), tissue inhibitor of metalloproteinases-1 (TIMP-1), transforming growth factor-β1 (TGFβ1), and 18S mRNA were determined using either TaqMan gene expression assays (Applied Biosystems) or PrimeTime qPCR Assays [Integrated DNA Technologies (IDT), Coralville, IA], iTaq Supermix with ROX (Bio-Rad), and a StepOnePlus thermal cycler (Applied Biosystems). Mouse MIP-2 (NM_009140) primer sequences were 5′-GAAGTCATAGCCACTCTCAAGG-3′ (forward primer), 5′-CTTCCGTTGAGGGACAGC-3′ (reverse primer), and 5′-/56-FAM/TCCTTTCCA/ZEN/GGTCAGTTAGCCTTGC/3IABkFQ/-3′ (probe). Mouse Col1a1 (NM_007742) primer sequences were 5′-CATAAAGGGTCATGGTGGCT-3′ (forward primer), 5′-TTGAGTCCGTCTTTGCCAG-3′ (reverse primer), and 5′-/56-FAM/TGGTGAACA/ZEN/AGGCCCCTCTGG/3IABkGQ/-3′ (probe). 18S (NM_003286) primer sequences were 5′-CTGTAGCCCTGTACTTCATCG-3′ (forward primer), 5′-CTACCACATATTCCTGACCATCC-3′ (reverse primer), and 5′-/56-FAM/CCTTCCTCC/ZEN/TTTTCATTGCCTGCTCT/3IABkFQ/-3′ (probe). MIP-2, Col1a1, and 18S primers and probes were purchased from IDT. All other gene expression assays were purchased from Applied Biosystems (MCP-1, Assay ID Mm00441242_m1; CD68, Assay ID Mm00839636_g1; F4/80, Assay ID Mm00802530_m1; ICAM-1, Assay ID Mm00516023_m1; TIMP-1, Assay ID Mm00441818_m1; and TGFβ1, Assay ID Mm00441724_m1). The expression of each gene was adjusted relative to 18S expression levels, and the relative expression level was determined using the comparative Ct method.
Macrophage and Neutrophil Staining
Frozen livers were sectioned at 8 μm for macrophage staining. Macrophages were identified in liver using CD68 and F4/80 antibodies as previously described.31 Paraffin-embedded livers were sectioned at 5 μm and stained for neutrophils by the Michigan State University Investigative HistoPathology Laboratory using a rabbit anti-neutrophil polyclonal antibody. The total number of neutrophils and the number of neutrophil foci (defined as three or more adjacent neutrophils) per 10 randomly selected ×200 fields were determined.
α-Smooth Muscle Actin Staining
Paraffin-embedded livers were sectioned at 5 μm, deparaffinized, and then boiled in citrate buffer. Sections were blocked with 5% goat serum and stained overnight with rabbit anti–α-smooth muscle actin (αSMA) antibody (Abcam, Cambridge, MA). Tissues were washed with Tris-buffered saline–Tween and stained with biotinylated anti-rabbit secondary antibody (Jackson ImmunoResearch, West Grove, PA) for 30 minutes. ABC reagent (Vector Laboratories, Burlingame, CA) was added for 30 minutes, and tissues were then incubated with ImmPACT DAB (Vector Laboratories) for 1 minute. Sections were counterstained with Gill's hematoxylin (Ricca Chemical Company, Arlington, TX) and Scott's Bluing Reagent (Ricca Chemical Company). Quantification of αSMA staining was performed using Metamorph software (Molecular Devices).
MCP-1 ELISA
Total protein was isolated from approximately 100 mg of snap-frozen liver using PBS containing 0.1% Triton X-100 and Halt protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific). Samples were rotated for 30 minutes at 4°C and then subjected to centrifugation at 12,000 × g for 15 minutes at 4°C. Protein concentrations were determined using a DC Protein Assay (Bio-Rad). The concentrations of MCP-1 in liver and plasma were determined using a commercially available ELISA kit (DuoSet ELISA; R&D Systems, Minneapolis, MN).
Statistics
Statistics were performed using SigmaPlot 11.0 software (Systat Software, San Jose, CA). Comparison of two groups was performed using Student's t-test. Comparison of three or more groups was performed using two-way analysis of variance followed by the Student-Newman-Keuls post hoc test for multiple comparisons. Data are expressed as mean ± SEM. The criterion for statistical significance was P < 0.05.
Results
Effect of Argatroban Treatment on Weight Gain and Thrombin Generation in LDLr−/− Mice Fed a Western Diet
In agreement with our previous studies,24 plasma thrombin-antithrombin levels, a stable biomarker of thrombin generation, significantly increased in mice fed a Western diet compared to mice fed a control diet (data not shown). To confirm thrombin inhibition by argatroban, plasma thrombin time was determined. Argatroban treatment significantly prolonged thrombin time (43.3 ± 6.2 seconds) compared to mice treated with vehicle (27.9 ± 1.2 seconds) (P < 0.05). These studies suggest that thrombin generation is increased in mice fed a Western diet and that thrombin activity was reduced in mice treated with argatroban. Mice fed a control diet gained 42.1 ± 2.7% body weight, and mice fed a Western diet gained 97.1 ± 6.7% body weight in the 15 weeks before pump implantation (P < 0.05). Following pump implantation, argatroban treatment did not affect weight gain in mice fed either diet (data not shown). In addition, argatroban treatment did not significantly affect food intake (data not shown). No overt bleeding events were noted in mice treated with argatroban.
Effect of Argatroban Treatment on Inflammation in Livers of LDLr−/− Mice Fed a Western Diet
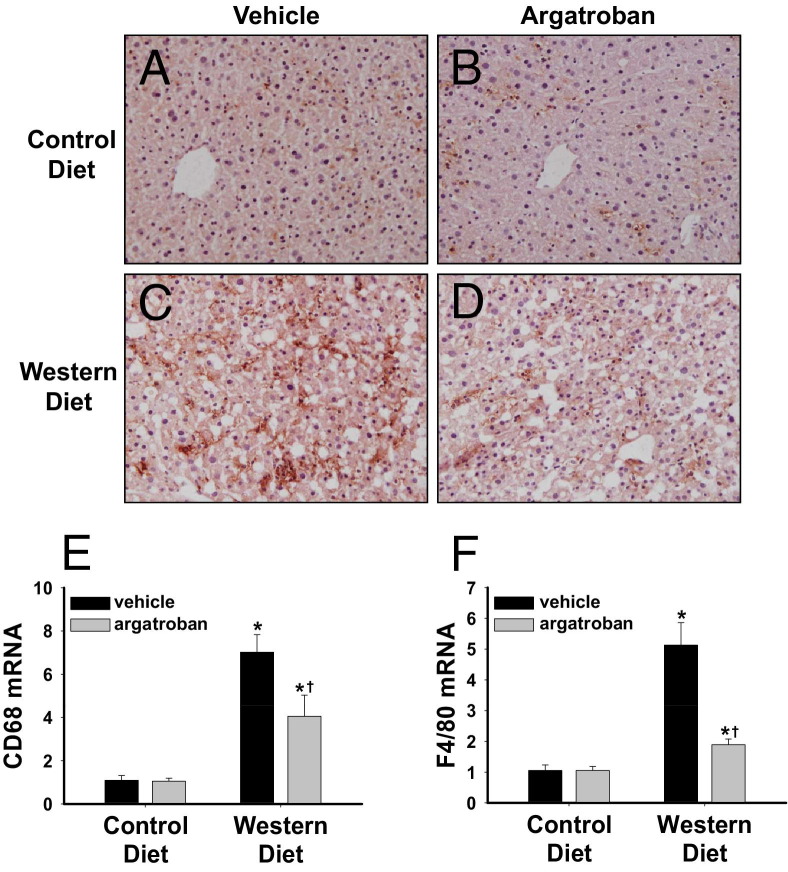
Argatroban administration significantly reduced hepatic macrophage accumulation in mice fed a Western diet (Figure 1, A–D). In agreement, hepatic expression of the mRNAs encoding CD68 and F4/80, two macrophage-selective genes, was significantly reduced by argatroban administration in mice fed a Western diet (Figure 1, E and F). MCP-1 is an inflammatory mediator that contributes to hepatic macrophage accumulation and the development of steatosis in mice fed a Western diet.11,12,32–34 Compared to LDLr−/− mice fed a control diet, hepatic MCP-1 mRNA, as well as hepatic and plasma MCP-1 protein levels, was increased in LDLr−/− mice fed a Western diet (Figure 2, A–C). Administration of argatroban for 4 weeks significantly reduced MCP-1 mRNA and protein expression in mice fed a Western diet (Figure 2, A–C).
Figure 1.
Effect of thrombin inhibition on macrophage accumulation in livers of mice fed a Western diet. LDLr−/− mice were fed a control diet or a Western diet for 19 weeks and were treated with vehicle or argatroban (15 mg/kg/day) via a miniosmotic pump for the final 4 weeks of the study. Representative photomicrographs of (A–D) CD68 and F4/80-stained (brown) liver sections in mice with (A and C) vehicle pumps and (B and D) argatroban pumps fed (A and B) control diet and (C and D) Western diet. Original magnification, ×200. Hepatic levels of (E) CD68 mRNA and (F) F4/80 mRNA were determined by real-time PCR. Data are expressed as mean ± SEM and as a fold change versus mice fed control diet with a vehicle pump. n = 5 to 7 mice per group. *P < 0.05 versus mice fed the control diet with the same drug treatment. †P < 0.05 versus mice fed the same diet with a vehicle pump.
Figure 2.

Effect of thrombin inhibition on MCP-1 induction in mice fed a Western diet. LDLr−/− mice were fed a control diet or a Western diet for 19 weeks and were treated with vehicle or argatroban (15 mg/kg/day) via a miniosmotic pump for the final 4 weeks of the study. A: Hepatic levels of MCP-1 mRNA were determined by real-time PCR. Data are expressed as mean ± SEM and as a fold change versus mice fed control diet with a vehicle pump. B: Hepatic levels and (C) plasma levels of MCP-1 protein were determined by ELISA. Data are expressed as mean ± SEM. n = 5 to 7 mice per group. *P < 0.05 versus mice fed the control diet with the same drug treatment. †P < 0.05 versus mice fed the same diet with a vehicle pump.
In addition to accumulation of macrophages, hepatic neutrophil accumulation and activation has been shown to mark the transition of simple steatosis to NASH.35 Hepatic neutrophil accumulation increased in livers of mice fed a Western diet, and argatroban treatment decreased neutrophil accumulation and clustering (Figure 3, A–F). Similarly, inflammatory foci were decreased in Western diet–fed mice after argatroban administration as scored by a pathologist (control diet, vehicle pump = 0.09 ± 0.04 foci/×200 field; control diet, argatroban pump = 0.05 ± 0.02 foci/×200 field; Western diet, vehicle pump = 1.69 ± 0.32 foci/×200 field; Western diet, argatroban pump = 0.70 ± 0.15 foci/×200 field, P < 0.05 compared to Western diet, vehicle pump). The expression of mRNAs encoding ICAM-1 and MIP-2, mediators contributing to neutrophil accumulation,36,37 increased in livers of mice fed a Western diet, and the expression of each gene was reduced by argatroban administration (Figure 4, A and B), although the reduction in MIP-2 expression did not achieve statistical significance (P = 0.07).
Figure 3.
Effect of thrombin inhibition on neutrophil accumulation in livers of mice fed a Western diet. LDLr−/− mice were fed a control diet or a Western diet for 19 weeks and were treated with vehicle or argatroban (15 mg/kg/day) via a miniosmotic pump for the final 4 weeks of the study. Representative photomicrographs of (A–D) liver sections stained for neutrophils in mice with (A and C) vehicle pumps and (B and D) argatroban pumps fed (A and B) control diet and (C and D) Western diet, and the quantification of (E) total hepatic neutrophil (PMN) accumulation, and (F) neutrophil foci as described in Materials and Methods. Original magnification: ×200 (A–D), ×800 (inset, C and D). *P < 0.05 versus mice fed the control diet with the same drug treatment. †P < 0.05 versus from mice fed the same diet with a vehicle pump. HPF, high-power field.
Figure 4.

Effect of thrombin inhibition on ICAM-1 and MIP-2 mRNA expression in livers of mice fed a Western diet. LDLr−/− mice were fed a control diet or a Western diet for 19 weeks and were treated with vehicle or argatroban (15 mg/kg/day) via a miniosmotic pump for the final 4 weeks of the study. Hepatic levels of (A) ICAM-1 mRNA and (B) MIP-2 mRNA were determined by real-time PCR. Data are expressed as mean ± SEM and as a fold change versus mice fed control diet with a vehicle pump. n = 5 to 7 mice per group. *P < 0.05 versus mice fed the control diet with the same drug treatment. †P < 0.05 versus mice fed the same diet with a vehicle pump.
Effect of Argatroban Treatment on Serum Lipids and Hepatic Steatosis in LDLr−/− Mice Fed a Western Diet
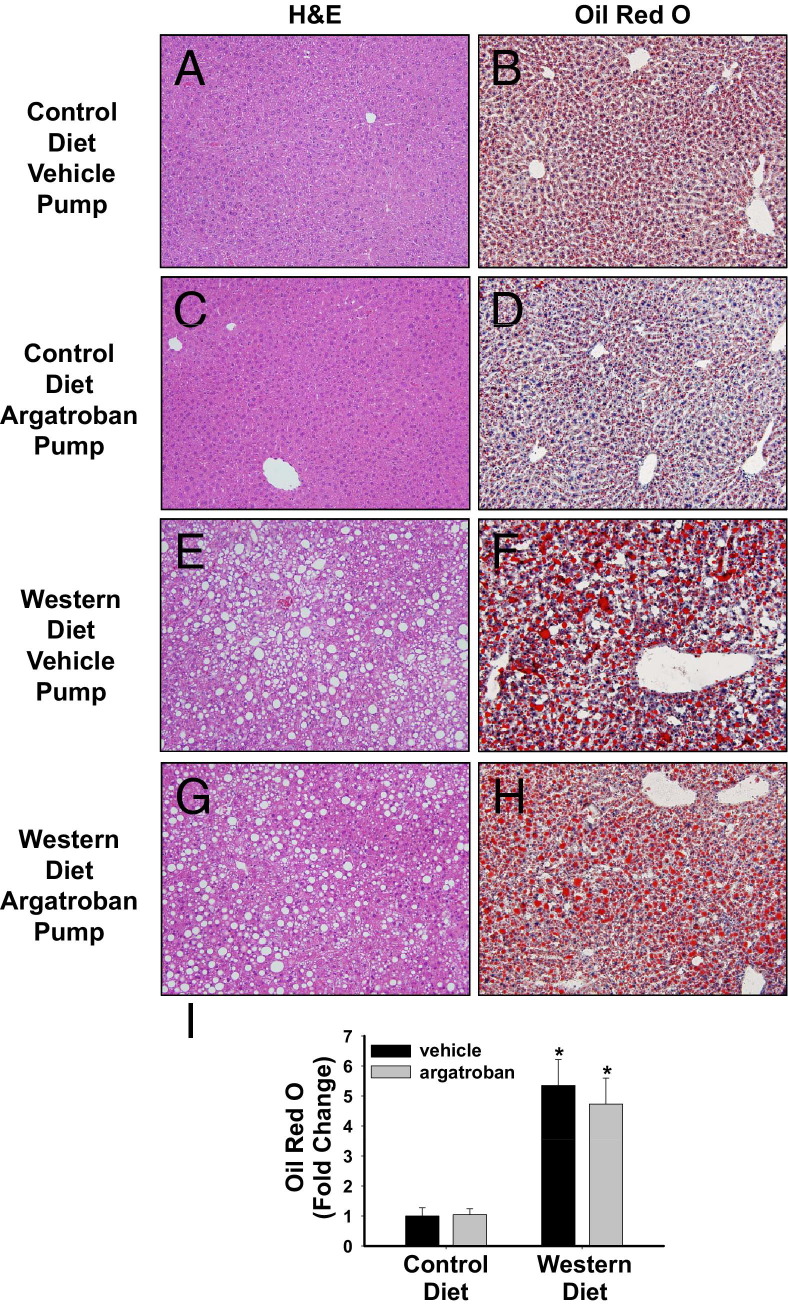
In agreement with a previous study,38 microvesicular steatosis was evident in livers of LDLr−/− mice fed a control diet (Figure 5B). Compared to LDLr−/− mice fed a control diet, marked macrovesicular steatosis indicated by histopathology, neutral lipid staining (Oil Red O), and increased hepatic triglyceride levels was evident in livers of mice fed a Western diet (Figures 5 and 6A). Hepatic triglyceride levels increased in mice fed a Western diet, although this increase did not achieve statistical significance in the argatroban group (Figure 6A, P = 0.1). Argatroban did not significantly affect hepatic steatosis elicited by a high-fat diet, as indicated by evaluation of liver histopathology, Oil Red O staining for neutral lipid, and quantification of hepatic triglycerides (Figures 5 and 6A). Hepatic cholesterol levels were increased similarly in both vehicle-treated and argatroban-treated mice fed a Western diet (Figure 6B). Serum triglyceride and cholesterol levels were significantly increased in LDLr−/− mice fed a Western diet compared to mice fed a control diet (Figure 6, C and D). Interestingly, 4 weeks of argatroban treatment was sufficient to completely reverse the increase in serum triglycerides and significantly reduce total cholesterol levels in serum of mice fed a Western diet (Figure 6, C and D).
Figure 5.
Effect of thrombin inhibition on lipid accumulation in mice fed a Western diet. LDLr−/− mice were fed a control diet or a Western diet for 19 weeks and were treated with vehicle or argatroban (15 mg/kg/day) via a miniosmotic pump for the final 4 weeks of the study. Representative photomicrographs of (A, C, E, and G) H&E-stained liver sections and (B, D, F, and H) Oil Red O–stained liver sections in mice with (A, B, E, and F) vehicle pumps and (C, D, G, and H) argatroban pumps fed (A–D) a control diet and (E–H) a Western diet. Original magnification, ×200. I: Quantification of Oil Red O staining expressed relative to mice fed control diet given vehicle. n = 5 to 7 mice per group. *P < 0.05 versus mice fed the control diet with the same drug treatment.
Figure 6.
Effect of thrombin inhibition on liver and serum triglyceride and cholesterol levels. LDLr−/− mice were fed a control diet or a Western diet for 19 weeks and were treated with vehicle or argatroban (15 mg/kg/day) via a miniosmotic pump for the final 4 weeks of the study. A: Hepatic triglyceride levels, (B) hepatic cholesterol levels, (C) serum triglyceride levels, and (D) serum cholesterol levels were determined. Data are expressed as mean ± SEM. n = 5 to 7 mice per group. *P < 0.05 versus mice fed the control diet with the same drug treatment. †P < 0.05 versus mice fed the same diet with a vehicle pump.
Effect of Argatroban Treatment on Profibrogenic Changes in Livers of LDLr−/− Mice Fed a Western Diet
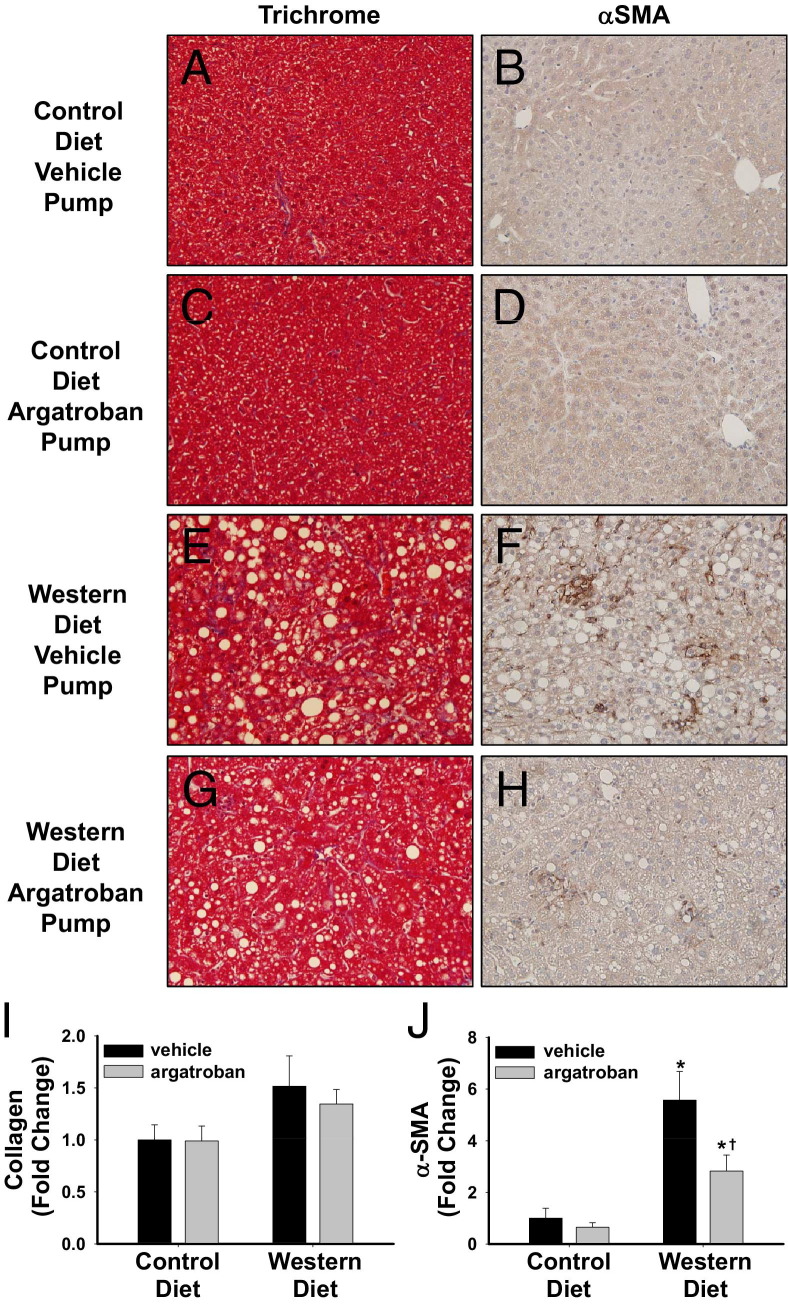
Compared to wild-type C57Bl/6 mice, LDLr−/− mice are more susceptible to the development of NASH, including the potential for increased progression to fibrosis.39 At this relatively early time in the disease course, trichrome staining did not reveal a significant increase in collagen deposition in livers of mice fed a Western diet (Figure 7I). To determine whether argatroban affected any early profibrogenic changes in livers of mice fed a Western diet, we evaluated expression of mRNAs encoding several profibrogenic genes, as well as hepatic α-SMA protein expression. Mice fed a Western diet exhibited an increase in hepatic α-smooth muscle actin protein expression, and this was decreased in mice treated with argatroban (Figure 7, F, H, and J). The expression of Col1a1, TIMP-1, and TGFβ1 mRNA was significantly increased in livers of LDLr−/− mice fed a Western diet compared to mice fed a control diet (Figure 8). Interestingly, argatroban treatment substantially decreased Col1a1 and TIMP-1 mRNA expression (Figure 8, A and B), but not TGFβ1 mRNA expression (Figure 8C). Overall, the data suggest that argatroban reduces several markers of profibrogenic changes in livers of mice fed a Western diet.
Figure 7.
Effect of thrombin inhibition on collagen deposition and αSMA expression in mice fed a Western diet. LDLr−/− mice were fed a control diet or a Western diet for 19 weeks and were treated with vehicle or argatroban (15 mg/kg/day) via a miniosmotic pump for the final 4 weeks of the study. A, C, E, and G: Representative photomicrographs of Trichrome-stained liver sections in mice with (A and E) vehicle pumps and (C and G) argatroban pumps fed (A and C) a control diet and (E and G) a Western diet. Original magnification, ×200. B, D, F, and H: Representative photomicrographs of α-SMA-stained liver sections in mice with (B and F) vehicle pumps and (D and H) argatroban pumps fed (B and D) a control diet and (F and H) a Western diet. Original magnification, ×200. Quantification of collagen (blue) staining (I) and αSMA staining (J) expressed relative to mice fed control diet given vehicle. n = 5 to 7 mice per group. *P < 0.05 versus mice fed the control diet with the same drug treatment. †P < 0.05 versus mice fed the same diet with a vehicle pump.
Figure 8.

Effect of thrombin inhibition on profibrogenic genes in livers of mice fed a Western diet. LDLr−/− mice were fed a control diet or a Western diet for 19 weeks and were treated with vehicle or argatroban (15 mg/kg/day) via a miniosmotic pump for the final 4 weeks of the study. Hepatic levels of (A) Col1a1 mRNA, (B) TIMP-1 mRNA, and (C) TGFβ1 mRNA were determined by real-time PCR. Data are expressed as mean ± SEM and as a fold change versus mice fed a control diet with a vehicle pump. n = 5 to 7 mice per group. *P < 0.05 versus mice fed the control diet with the same drug treatment. †P < 0.05 versus mice fed the same diet with a vehicle pump.
Discussion
Altered synthesis of coagulation factors by the fatty liver is associated with a procoagulant state in patients with metabolic syndrome.20,40,41 However, few studies have addressed the possibility that the procoagulant state associated with metabolic syndrome is a cause, rather than a consequence, of NAFLD. In this proof-of-principle study, we investigated the possibility that treatment with a direct thrombin inhibitor could be a potential therapeutic strategy to reduce inflammation and hepatic steatosis in mice fed a high-fat diet. We found that therapeutic administration of the thrombin inhibitor argatroban was sufficient to blunt hepatic inflammation, serum lipid levels, and profibrogenic gene expression in mice fed a Western diet. Data from this and previous studies in our laboratory indicate that the coagulation cascade and its downstream signaling pathways are critical triggers in the development of NAFLD,24 with thrombin being a central regulator of multiple components of the disease.
LDLr−/− mice fed a high-fat diet develop more advanced hepatic inflammation than do C57Bl/6 mice.38,42 Similarly, in some patients with fatty liver disease, simple steatosis can progress to NASH, which is defined histopathologically by more severe inflammation combined with ballooning degeneration of hepatocytes.5 LDLr−/− mice also manifest marked hypercholesterolemia and atherosclerosis when fed a high-fat diet, features not present in wild-type C57Bl/6 mice. Utilization of LDLr−/− mice allows for the potential exploration into the mechanisms linking hepatic inflammation to these other disease processes. A central component of both NAFLD and NASH is inflammation. Patients with NAFLD have a low level of chronic inflammatory mediator expression.10 In particular, expression of the chemokine MCP-1 is involved in the recruitment of macrophages to the liver, a process essential to the development of steatosis in mouse models.43 In addition to macrophages, hepatic neutrophil accumulation is evident in livers of patients with NAFLD/NASH, particularly surrounding steatotic hepatocytes.35,44 Interestingly, the infiltration of neutrophils into the fatty liver correlates to disease severity and may contribute to the progression to NASH.35 Therapeutic administration of the thrombin inhibitor argatroban reduced multiple components of inflammation, including decreased hepatic accumulation of macrophages and neutrophils.
Deficiencies in select inflammatory mediators including MCP-1 and tumor necrosis factor-α (TNFα) significantly reduce hepatic steatosis in mice fed a high-fat diet.12,34 Exaggerated expression of these mediators likely contributes to the transition to NASH in patients with fatty liver disease.45 Of importance, within only 4 weeks, argatroban significantly reduced MCP-1 mRNA and protein expression, and reduced hepatic TNFα mRNA expression (data not shown). This duration of treatment with argatroban was not sufficient to reduce hepatic steatosis. By contrast, we have shown previously that PAR-1−/− mice, which have a significant defect in thrombin signaling, do not develop hepatic steatosis when fed a high-fat diet.46 This suggests that the reduction in hepatic inflammation by argatroban in the present study was not sufficient to correct hepatic steatosis. This may relate to the relatively short duration of argatroban administration (ie, 4 weeks), and a more prolonged administration of argatroban could lead to a reduction in steatosis.
Beneficial effects of reducing hepatic inflammation are likely to extend beyond limiting the transition to NASH. Hepatic inflammation in patients with NAFLD also increases the synthesis of hs-CRP, which is an important indicator of increased risk of acute cardiovascular events in patients.8 The mouse homologue of hs-CRP is the acute-phase protein serum amyloid A.47 Of interest, we found that argatroban dramatically suppressed expression of serum amyloid A1 mRNA in livers of mice fed a Western diet (data not shown). Moreover, other risk factors for the development of atherosclerosis and other cardiovascular diseases, including increased plasma MCP-1 expression and hypercholesterolemia and hypertriglyceridemia,48–50 were all reduced by argatroban administration in mice fed a Western diet. The mechanism of reduction of plasma lipid levels by thrombin inhibition is not known, but may relate to the correction of hepatic inflammation and restoration of hepatic lipid metabolism. Viewed in the context of previous studies identifying beneficial effects of thrombin and FXa inhibitors in preclinical models of atherosclerosis,51 these data suggest that thrombin inhibition could be beneficial for the treatment of atherosclerosis.
In a subset of patients with NASH, chronic liver injury and inflammation promotes excess deposition of extracellular matrix, particularly collagens, which can compromise liver function and increase the risk of developing fibrosis and cirrhosis. Indeed, NAFLD is associated with an increased incidence of cirrhosis and hepatocellular carcinoma in obese and diabetic patients.52 Collagen deposition is evident in mice that have been fed a high-fat diet for longer than 6 months or in mice that have been fed a high-fat, high-cholesterol diet.53,54 In the present study, we did not observe a significant increase in collagen deposition in livers of mice fed a Western diet. However, it is worth noting that thrombin activation of PAR-1 has been shown to elicit profibrogenic changes in hepatic stellate cells,55 which are responsible for liver fibrosis in fatty liver disease.56 We found that argatroban administration concurrently reduced the expression of α-SMA protein, a biomarker of stellate cell activation, and the expression of two profibrogenic genes, type 1 collagen and TIMP-1, in livers of LDLr−/− mice fed a Western diet. Argatroban administration did not significantly reduce TGF-β1 mRNA expression, indicating that thrombin inhibition did not universally attenuate all profibrogenic changes in this model. These studies suggest that treatment with a thrombin inhibitor could reduce the risk of developing fibrosis in patients with NAFLD by preventing early hepatic stellate cell activation.
These studies suggest that thrombin regulates multiple components of fatty liver disease, including inflammation, steatosis, and fibrosis. Thrombin inhibition with argatroban significantly reduced multiple facets of hepatic inflammation in LDLr−/− mice fed a Western diet, including decreasing macrophage and neutrophil accumulation, and decreasing chemokine expression. Argatroban treatment also significantly reduced serum triglyceride and cholesterol levels. In addition, argatroban significantly decreased profibrogenic gene expression and αSMA protein expression in the livers of LDLr−/− mice fed a Western diet. As the indications for anticoagulation increase, novel orally bioavailable direct inhibitors of thrombin (ie, dabigatran) and FXa (ie, apixaban, rivaroxaban)57 could represent a therapeutic approach to reduce systemic and hepatic inflammation in patients with obesity and metabolic disease. As with the use of anticoagulants in any disease, in-depth clinical evaluation of efficacy balanced with potential increased risk of bleeding would be required. Continued investigation of the mechanism whereby thrombin promotes hepatic inflammation may ultimately lead to therapeutic strategies to limit hepatic inflammation without affecting normal hemostasis.
Acknowledgments
We thank Dr. Judith Larson for her expert veterinary care of the mice in this study. We also thank Ruipeng Wang and Stephanie C. Bishop for technical assistance.
Footnotes
Supported by the American Heart Association grants 11POST7430043 (K.M.K.) and 0835121G (J.P.L.) and by the NIH grants DK073566 (B.L.C.) and R01 ES017537 (J.P.L.).
Current address of B.L.C, Department of Pharmacology and Toxicology, Michigan State University, East Lansing, MI; and of J.P.L., Department of Pathobiology and Diagnostic Investigation, Michigan State University, East Lansing, MI.
References
- 1.Bellentani S., Saccoccio G., Masutti F., Croce L.S., Brandi G., Sasso F., Cristanini G., Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 2.Tiniakos D.G., Vos M.B., Brunt E.M. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145–171. doi: 10.1146/annurev-pathol-121808-102132. [DOI] [PubMed] [Google Scholar]
- 3.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W., Ferrell L.D., Liu Y.C., Torbenson M.S., Unalp-Arida A., Yeh M., McCullough A.J., Sanyal A.J. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 4.Clark J.M., Brancati F.L., Diehl A.M. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 5.Farrell G.C., Larter C.Z. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 6.Friedman S.L. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotronen A., Yki-Jarvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27–38. doi: 10.1161/ATVBAHA.107.147538. [DOI] [PubMed] [Google Scholar]
- 8.Ndumele C.E., Nasir K., Conceicao R.D., Carvalho J.A., Blumenthal R.S., Santos R.D. Hepatic steatosis: obesity, and the metabolic syndrome are independently and additively associated with increased systemic inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1927–1932. doi: 10.1161/ATVBAHA.111.228262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Targher G., Bertolini L., Poli F., Rodella S., Scala L., Tessari R., Zenari L., Falezza G. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541–3546. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 10.Bertola A., Bonnafous S., Anty R., Patouraux S., Saint-Paul M.C., Iannelli A., Gugenheim J., Barr J., Mato J.M., Le Marchand-Brustel Y., Tran A., Gual P. Hepatic expression patterns of inflammatory and immune response genes associated with obesity and NASH in morbidly obese patients. PLoS One. 2010;5:e13577. doi: 10.1371/journal.pone.0013577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamura Y., Sugimoto M., Murayama T., Minami M., Nishikaze Y., Ariyasu H., Akamizu T., Kita T., Yokode M., Arai H. C-C chemokine receptor 2 inhibitor improves diet-induced development of insulin resistance and hepatic steatosis in mice. J Atheroscler Thromb. 2010;17:219–228. doi: 10.5551/jat.3368. [DOI] [PubMed] [Google Scholar]
- 12.Rull A., Rodriguez F., Aragones G., Marsillach J., Beltran R., Alonso-Villaverde C., Camps J., Joven J. Hepatic monocyte chemoattractant protein-1 is upregulated by dietary cholesterol and contributes to liver steatosis. Cytokine. 2009;48:273–279. doi: 10.1016/j.cyto.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Stienstra R., Saudale F., Duval C., Keshtkar S., Groener J.E., van Rooijen N., Staels B., Kersten S., Muller M. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology. 2010;51:511–522. doi: 10.1002/hep.23337. [DOI] [PubMed] [Google Scholar]
- 14.Huang W., Metlakunta A., Dedousis N., Zhang P., Sipula I., Dube J.J., Scott D.K., O'Doherty R.M. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010;59:347–357. doi: 10.2337/db09-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schenk S., Saberi M., Olefsky J.M. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarantino G., Colicchio P., Conca P., Finelli C., Di Minno M.N., Tarantino M., Capone D., Pasanisi F. Young adult obese subjects with and without insulin resistance: what is the role of chronic inflammation and how to weigh it non-invasively? J Inflamm (Lond) 2009;6:6. doi: 10.1186/1476-9255-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ridker P.M. High-sensitivity C-reactive protein and cardiovascular risk: rationale for screening and primary prevention. Am J Cardiol. 2003;92:17K–22K. doi: 10.1016/s0002-9149(03)00774-4. [DOI] [PubMed] [Google Scholar]
- 18.Calabro P., Golia E., Yeh E.T. CRP and the risk of atherosclerotic events. Semin Immunopathol. 2009;31:79–94. doi: 10.1007/s00281-009-0149-4. [DOI] [PubMed] [Google Scholar]
- 19.Lisman T., Porte R.J. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. 2010;116:878–885. doi: 10.1182/blood-2010-02-261891. [DOI] [PubMed] [Google Scholar]
- 20.Fritsch P., Kleber M., Rosenkranz A., Fritsch M., Muntean W., Mangge H., Reinehr T. Haemostatic alterations in overweight children: associations between metabolic syndrome, thrombin generation, and fibrinogen levels. Atherosclerosis. 2010;212:650–655. doi: 10.1016/j.atherosclerosis.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 21.Romano M., Guagnano M.T., Pacini G., Vigneri S., Falco A., Marinopiccoli M., Manigrasso M.R., Basili S., Davi G. Association of inflammation markers with impaired insulin sensitivity and coagulative activation in obese healthy women. J Clin Endocrinol Metab. 2003;88:5321–5326. doi: 10.1210/jc.2003-030508. [DOI] [PubMed] [Google Scholar]
- 22.Sola E., Navarro S., Medina P., Vaya A., Estelles A., Hernandez-Mijares A., Espana F. Activated protein C levels in obesity and weight loss influence. Thromb Res. 2009;123:697–700. doi: 10.1016/j.thromres.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Targher G., Zoppini G., Moghetti P., Day C.P. Disorders of coagulation and hemostasis in abdominal obesity: emerging role of fatty liver. Semin Thromb Hemost. 2010;36:41–48. doi: 10.1055/s-0030-1248723. [DOI] [PubMed] [Google Scholar]
- 24.Kassel K.M., Owens A.P., III, Rockwell C.E., Sullivan B.P., Wang R., Tawfik O., Li G., Guo G.L., Mackman N., Luyendyk J.P. Protease-activated receptor 1 and hematopoietic cell tissue factor are required for hepatic steatosis in mice fed a Western Diet. Am J Pathol. 2011;179:2278–2289. doi: 10.1016/j.ajpath.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwaki T., Sandoval-Cooper M.J., Brechmann M., Ploplis V.A., Castellino F.J. A fibrinogen deficiency accelerates the initiation of LDL cholesterol-driven atherosclerosis via thrombin generation and platelet activation in genetically predisposed mice. Blood. 2006;107:3883–3891. doi: 10.1182/blood-2005-09-3780. [DOI] [PubMed] [Google Scholar]
- 26.Owens A., III, Passam F., Antoniak S., Marshall S., McDaniel A., Rudel L., Williams J., Hubbard B., Dutton J., Wang J., Tobias P., Curtiss L., Daugherty A., Kirchhofer D., Luyendyk J., Moriarty P., Nagaraja S., Furie B., Furie B., Johns D., Temel R., Mackman N. Monocyte tissue factor-dependent activation of coagulation in hypercholesterolemic mice and monkeys is inhibited by simvastatin. J Clin Invest. 2012;122:558–568. doi: 10.1172/JCI58969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitaoka T., Hua Y., Xi G., Hoff J.T., Keep R.F. Delayed argatroban treatment reduces edema in a rat model of intracerebral hemorrhage. Stroke. 2002;33:3012–3018. doi: 10.1161/01.str.0000037673.17260.1b. [DOI] [PubMed] [Google Scholar]
- 28.Mihara M., Aihara K., Ikeda Y., Yoshida S., Kinouchi M., Kurahashi K., Fujinaka Y., Akaike M., Matsumoto T. Inhibition of thrombin action ameliorates insulin resistance in type 2 diabetic db/db mice. Endocrinology. 2010;151:513–519. doi: 10.1210/en.2009-0661. [DOI] [PubMed] [Google Scholar]
- 29.Owoo G, Burgos RA, inventors; Bayer Int, assignee. 2008 Feb 29. Argatroban formulations and methods for making and using same. United States patent US 7,915,290 B2
- 30.Tanaka Y., Aleksunes L.M., Yeager R.L., Gyamfi M.A., Esterly N., Guo G.L., Klaassen C.D. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J Pharmacol Exp Ther. 2008;325:655–664. doi: 10.1124/jpet.107.135822. [DOI] [PubMed] [Google Scholar]
- 31.Luyendyk J.P., Sullivan B.P., Guo G.L., Wang R. Tissue factor-deficiency and protease activated receptor-1-deficiency reduce inflammation elicited by diet-induced steatohepatitis in mice. Am J Pathol. 2010;176:177–186. doi: 10.2353/ajpath.2010.090672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clement S., Juge-Aubry C., Sgroi A., Conzelmann S., Pazienza V., Pittet-Cuenod B., Meier C.A., Negro F. Monocyte chemoattractant protein-1 secreted by adipose tissue induces direct lipid accumulation in hepatocytes. Hepatology. 2008;48:799–807. doi: 10.1002/hep.22404. [DOI] [PubMed] [Google Scholar]
- 33.Endo M., Masaki T., Seike M., Yoshimatsu H. TNF-alpha induces hepatic steatosis in mice by enhancing gene expression of sterol regulatory element binding protein-1c (SREBP-1c) Exp Biol Med (Maywood) 2007;232:614–621. [PubMed] [Google Scholar]
- 34.De Taeye B.M., Novitskaya T., McGuinness O.P., Gleaves L., Medda M., Covington J.W., Vaughan D.E. Macrophage TNF-alpha contributes to insulin resistance and hepatic steatosis in diet-induced obesity. Am J Physiol Endocrinol Metab. 2007;293:E713–E725. doi: 10.1152/ajpendo.00194.2007. [DOI] [PubMed] [Google Scholar]
- 35.Rensen S.S., Slaats Y., Nijhuis J., Jans A., Bieghs V., Driessen A., Malle E., Greve J.W., Buurman W.A. Increased hepatic myeloperoxidase activity in obese subjects with nonalcoholic steatohepatitis. Am J Pathol. 2009;175:1473–1482. doi: 10.2353/ajpath.2009.080999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang L., Froio R.M., Sciuto T.E., Dvorak A.M., Alon R., Luscinskas F.W. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106:584–592. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J., Cacalano G., Camerato T., Toy K., Moore M.W., Wood W.I. Chemokine binding and activities mediated by the mouse IL-8 receptor. J Immunol. 1995;155:2158–2164. [PubMed] [Google Scholar]
- 38.Gupte A.A., Liu J.Z., Ren Y., Minze L.J., Wiles J.R., Collins A.R., Lyon C.J., Pratico D., Finegold M.J., Wong S.T., Webb P., Baxter J.D., Moore D.D., Hsueh W.A. Rosiglitazone attenuates age- and diet-associated nonalcoholic steatohepatitis in male low-density lipoprotein receptor knockout mice. Hepatology. 2010;52:2001–2011. doi: 10.1002/hep.23941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subramanian S., Goodspeed L., Wang S., Kim J., Zeng L., Ioannou G.N., Haigh W.G., Yeh M.M., Kowdley K.V., O'Brien K.D., Pennathur S., Chait A. Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese LDL receptor-deficient mice. J Lipid Res. 2011;52:1626–1635. doi: 10.1194/jlr.M016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Targher G., Chonchol M., Miele L., Zoppini G., Pichiri I., Muggeo M. Nonalcoholic fatty liver disease as a contributor to hypercoagulation and thrombophilia in the metabolic syndrome. Semin Thromb Hemost. 2009;35:277–287. doi: 10.1055/s-0029-1222606. [DOI] [PubMed] [Google Scholar]
- 41.Cigolini M., Targher G., Agostino G., Tonoli M., Muggeo M., De Sandre G. Liver steatosis and its relation to plasma haemostatic factors in apparently healthy men: role of the metabolic syndrome. Thromb Haemost. 1996;76:69–73. [PubMed] [Google Scholar]
- 42.Bieghs V., Van Gorp P.J., Wouters K., Hendrikx T., Gijbels M.J., van B.M., Bakker J., Binder C.J., Lutjohann D., Staels B., Hofker M.H., Shiri-Sverdlov R. LDL receptor knock-out mice are a physiological model particularly vulnerable to study the onset of inflammation in non-alcoholic fatty liver disease. PLoS One. 2012;7:e30668. doi: 10.1371/journal.pone.0030668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obstfeld A.E., Sugaru E., Thearle M., Francisco A.M., Gayet C., Ginsberg H.N., Ables E.V., Ferrante A.W., Jr. C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes. 2010;59:916–925. doi: 10.2337/db09-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rensen S.S., Slaats Y., Driessen A., Peutz-Kootstra C.J., Nijhuis J., Steffensen R., Greve J.W., Buurman W.A. Activation of the complement system in human nonalcoholic fatty liver disease. Hepatology. 2009;50:1809–1817. doi: 10.1002/hep.23228. [DOI] [PubMed] [Google Scholar]
- 45.Haukeland J.W., Damas J.K., Konopski Z., Loberg E.M., Haaland T., Goverud I., Torjesen P.A., Birkeland K., Bjoro K., Aukrust P. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol. 2006;44:1167–1174. doi: 10.1016/j.jhep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 46.Kassel K.M., Owens A.P., III, Rockwell C.E., Sullivan B.P., Wang R., Tawfik O., Li G., Guo G.L., Mackman N., Luyendyk J.P. Protease-activated receptor 1 and hematopoietic cell tissue factor are required for hepatic steatosis in mice fed a Western diet. Am J Pathol. 2011;179:2278–2289. doi: 10.1016/j.ajpath.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vermeire S., Van Assche G., Rutgeerts P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:661–665. doi: 10.1097/00054725-200409000-00026. [DOI] [PubMed] [Google Scholar]
- 48.Boring L., Gosling J., Cleary M., Charo I.F. Decreased lesion formation in CCR2-/- mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 49.Namiki M., Kawashima S., Yamashita T., Ozaki M., Hirase T., Ishida T., Inoue N., Hirata K., Matsukawa A., Morishita R., Kaneda Y., Yokoyama M. Local overexpression of monocyte chemoattractant protein-1 at vessel wall induces infiltration of macrophages and formation of atherosclerotic lesion: synergism with hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2002;22:115–120. doi: 10.1161/hq0102.102278. [DOI] [PubMed] [Google Scholar]
- 50.Goldstein J.L., Hazzard W.R., Schrott H.G., Bierman E.L., Motulsky A.G. Hyperlipidemia in coronary heart disease: I. Lipid levels in 500 survivors of myocardial infarction. J Clin Invest. 1973;52:1533–1543. doi: 10.1172/JCI107331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ten Cate H. Tissue factor-driven thrombin generation and inflammation in atherosclerosis. Thromb Res. 2012;129(Suppl 2):S38–S40. doi: 10.1016/j.thromres.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 52.Sun B., Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 2012;56:704–713. doi: 10.1016/j.jhep.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kohli R., Kirby M., Xanthakos S.A., Softic S., Feldstein A.E., Saxena V., Tang P.H., Miles L., Miles M.V., Balistreri W.F., Woods S.C., Seeley R.J. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology. 2010;52:934–944. doi: 10.1002/hep.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeLeve L.D., Wang X., Kanel G.C., Atkinson R.D., McCuskey R.S. Prevention of hepatic fibrosis in a murine model of metabolic syndrome with nonalcoholic steatohepatitis. Am J Pathol. 2008;173:993–1001. doi: 10.2353/ajpath.2008.070720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fiorucci S., Antonelli E., Distrutti E., Severino B., Fiorentina R., Baldoni M., Caliendo G., Santagada V., Morelli A., Cirino G. PAR1 antagonism protects against experimental liver fibrosis: Role of proteinase receptors in stellate cell activation. Hepatology. 2004;39:365–375. doi: 10.1002/hep.20054. [DOI] [PubMed] [Google Scholar]
- 56.Hernandez-Gea V and Friedman S.L. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 57.Weitz J.I. Factor Xa and thrombin as targets for new oral anticoagulants. Thromb Res. 2011;127(Suppl 2):S5–S12. doi: 10.1016/S0049-3848(10)70147-X. [DOI] [PubMed] [Google Scholar]