Abstract
Chronic ulcerative colitis (CUC) is characterized by increased intestinal epithelial cell (IEC) apoptosis associated with elevated tumor necrosis factor (TNF), inducible nitric oxide synthase (iNOS), and p53. We previously showed that p53 is increased in crypt IECs in human colitis and is needed for IEC apoptosis in chronic dextran sulfate sodium-colitis. Herein, we examined the roles of TNF and iNOS in regulating p53-induced IEC apoptosis in CUC. The IEC TUNEL staining, caspases 3, 8, and 9, and p53 protein levels, induced by anti-CD3 monoclonal antibody (mAb) activation of T cells, were markedly reduced in TNF receptor 1 and 2 gene knockout mice. Induction of IEC apoptosis correlated with increased p53, which was attenuated in iNOS−/− mice. IEC p53 levels and apoptosis were reduced in IL-10−/− colitic mice treated with neutralizing TNF mAb and the iNOS inhibitor, aminoguanidine, further suggesting that TNF and iNOS are upstream of p53 during colitis-induced IEC apoptosis. IEC apoptosis and p53 levels were assessed in control versus untreated or anti-TNF–treated CUC patients with equivalent levels of inflammation. Data indicated that IEC apoptosis and p53 levels were clearly higher in untreated CUC but markedly reduced in patients treated with anti-TNF mAb. Therefore, TNF-induced iNOS activates a p53-dependent pathway of IEC apoptosis in CUC. The inhibition of IEC apoptosis may be an important mechanism for mucosal healing in anti-TNF–treated CUC patients.
Human inflammatory bowel diseases (IBDs) are characterized by excessive crypt epithelial apoptosis, surface ulceration, distorted crypt architecture, diarrhea, and bleeding. Barrier disruption is linked to epithelial apoptosis caused by aberrant activation of innate and adaptive immune responses.1–3 A hallmark of severe IBD is the overproduction of tumor necrosis factor (TNF) in mucosal tissue.2,4 The importance of TNF in disease pathogenesis is underlined by the pronounced clinical improvement induced when anti-TNF antibodies reduce diarrhea, weight loss, and bleeding.4,5 At the mucosal level, anti-TNF antibody treatment enhances mucosal healing with rapid re-epithelialization of ulcerated surfaces. Studies indicate some (eg, infliximab and adalimumab), but not all (eg, certolizumab), anti-TNF agents induce apoptosis of lamina propria cells, despite all three being able to enhance mucosal healing.6,7 However, it remains unclear whether the effect of anti-TNF on mucosal healing is related to reduced epithelial apoptosis and, if so, through what mechanism.
Overproduction of TNF in IBD has potent effects on mucosal adaptive and innate immune responses.8,9 TNF participates in macrophage activation by enhancing antimicrobial functions.10 In response to TNF, macrophages increase production of reactive nitrosative species, such as nitric oxide (NO−) and its metabolite, peroxynitrite (ONOO−).11 Inducible NO synthase (iNOS) blockade inhibits disease severity and epithelial apoptosis in animal models of IBD.12,13 Data from human IBD studies suggest that NO− and ONOO− stabilize p53 and activate response pathways.14,15 During tumorigenesis, NO−-induced mutations of p53 inactivate tumor suppressor function, with loss of protective effects.16 Thus, TNF-mediated activation of iNOS may be an important pathway for regulating epithelial cell apoptosis during colitis and colitis-induced dysplasia.
Understanding TNF receptor signaling is complex and difficult to apply to in vivo systems. TNF receptor 1 (TNFR1) associates with the TNF receptor–associated death domain, which activates the extrinsic, caspase 8–linked pathway of apoptosis.17,18 However, in some systems examined, TNF receptor–associated death domain is dispensable for TNF-induced apoptosis, and cross activation of TNFRl and TNFR2 converges unto common downstream signaling events, resulting in apoptosis mediated by intrinsic (mitochondrial) pathways.17,19 The proliferative zone for intestinal epithelial cells (IECs) resides in lower crypt regions. Cellular proliferation requires enhanced mitochondrial function. Given that epithelial apoptosis in IBD occurs in proliferative crypt epithelial cells, we suspected that pathways involving induction of mitochondrial pathways were used. In addition, a comprehensive understanding of the role of TNF receptor signaling within the mucosal microenvironment requires that receptor deficiency be restricted to distinct populations participating in mucosal immune responses.
Increased epithelial crypt cell apoptosis commonly occurs in ulcerative colitis (UC) and Crohn's disease.20,21 Numerous in vitro and in vivo model systems have studied this phenomenon, suggesting that TNF-mediated pathways play key roles in inducing programmed cell death in epithelial crypts. To model these pathways, a well-characterized model of T-cell activation was used that induces transient stem/progenitor cell activation, crypt IEC proliferation, and TNF-mediated diarrhea reminiscent of human IBD.22,23 The reproducible kinetics of the model permitted identification of the events in immune-mediated apoptosis and allowed application to relevant gene knockout models. We recently reported that p53 is the major mediator of colonic crypt IEC apoptosis in colitis.24 This article examines the upstream events leading up to p53 activation and IEC apoptosis. Results suggest a mechanism by which TNF signals, through both TNFR1 and TNFR2, stimulate iNOS-mediated p53-dependent apoptosis of crypt IECs. Studies in the IL-10−/− murine model of colitis confirmed that TNF-induced iNOS led to activation of p53 and induced IEC apoptosis. Finally, we confirm that TNF-induced p53-mediated apoptosis also occurs in vivo during human UC. Overall, the findings suggest that T-cell activation causes TNF and iNOS-mediated stabilization of p53, followed by p53-mediated crypt cell apoptosis in IBD. These data have direct relevance to mechanisms of barrier disruption, ulceration, and initiation of dysplasia seen in p53 mutant crypts.
Materials and Methods
Mice and Treatments
C57BL/6, TNFR1-knockout (TNFR1−/−), TNFR2-knockout (TNFR2−/−), combined TNFR1/2-knockout (TNFR1/2−/−), iNOS-knockout (iNOS−/−), p53-knockout (p53−/−), and IL-10–knockout (IL-10−/−) mice on the C57BL/6 background (>10 generations) were obtained from the Jackson Laboratory (Bar Harbor, ME) and screened for the absence of wild-type (WT) gene before use. Mice were maintained in barrier housing in the Northwestern University Center for Comparative Medicine (Chicago, IL), in accordance with guidelines of the Northwestern University Animal Care and Usage Committee. To model acute inflammation, mice were given i.p. injections of 0.2 mg hamster anti-CD3 monoclonal antibody (mAb; 145-2C11) or control hamster mAb (UC8-IB9) and sacrificed at different time points, as previously described.23 Anti-CD3 and control antibodies were purified from cell culture supernatant over a protein G column (GBioscience, St. Louis, MO). In some mice, the iNOS inhibitor, L-N6-(1-iminoethyl) lysine (L-NIL), was given i.p., 0.2 mg 2 hours before and at the time of anti-CD3 mAb treatment. IL-10−/− mice were moved to conventional housing 1 week before starting piroxicam chow feeding. To accelerate and synchronize the onset of colitis, IL-10−/− mice were fed 60 mg of piroxicam (Sigma, St Louis, MO)/250 g of rodent powdered chow for 1 week and then 80 mg of piroxicam/250 g of chow for another week. Controls were given powdered chow for only 2 weeks. Mice then resumed standard pelleted chow and were examined on days 28 and 46 after day 1 of piroxicam feeding. For chronic dextran sulfate sodium (DSS)-colitis, one cycle constituted giving the mice 2.0% DSS in their drinking water for 7 days, followed by 14 days of regular water. This was done three times, and the mice were sacrificed at the end of the third cycle. Aminoguanidine was administered at 200 μg/day for 2 days, and the mice were sacrificed 12 hours after the last injection. Anti-TNF, 0.2 mg, was injected into WT mice 24 hours before anti-CD3 treatment, and 0.5 mg of anti-TNF was administered at 36 hours or 1 week, as indicated, before the mice with colitis were sacrificed.
Immunohistochemical Localization of Apoptotic Cells
Formalin-fixed, paraffin-embedded sections were stained for apoptotic cells using the TUNEL method for visualizing the 3′-OH ends of DNA fragments. After digestion in proteinase K, sections were rinsed and incubated with 0.3% H2O2 at room temperature for 20 minutes. Sections were incubated in a terminal deoxynucleotide mixture (Roche Diagnostics Corp., Indianapolis, IN), followed by anti-fluorescein mAb conjugated with horseradish peroxidase, then 3′,3′-diaminobenzidine as immunodetection substrate.25 Apoptotic indexes were calculated as the number of TUNEL-positive epithelial cells/total number of epithelial cells, multiplied by 100, to yield the apoptotic index. A total of five mice (four for IL-10−/− experiments) were analyzed in each group, and a minimum of 8 to 20 well-oriented crypts were counted for each mouse. The data are presented as the mean ± SEM.
Real-Time PCR and Primers
Total RNA from sonicated tissue was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany). The high-capacity cDNA RT kit (Applied Biosystems, Foster City, CA) was used to synthesize cDNA. Expression of genes was determined by real-time quantitative PCR using the ABI 7500 Real Time PCR system and the Power SYBR Green PCR master mix (Applied Biosystems). Primers were selected based on nucleotide sequences downloaded from the National Center for Biotechnology Information data bank for TNF-α (forward primer, 5′-CCCAGGGACCTCTCTCTAATCA-3′; reverse primer, 5′-GGTTTGCTACAACATGGGCTACA-3′) and iNOS (forward primer, 5′-CAAGTACGGCCGCTTCGA-3′; reverse primer, 5′-CACTCGTATTTGGGATGTTCCA-3′). For each sample assayed, the threshold value (CT) for target genes and glyceraldehyde-3-phosphate dehydrogenase (internal reference) was determined. All assays were performed in triplicate.
WB Analysis and Antibodies
Small-bowel (SB) epithelial cells were isolated by the EDTA method, depleted on sheep anti-rat IgG magnetic Dynabeads (Life Technologies, Grand Island, NY) preloaded with rat anti-mouse CD45 antibodies, and homogenized in radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitor cocktail (GBioscience).15 Homogenates were centrifuged at 15,700 × g for 30 minutes. Proteins were separated by SDS-PAGE using 8% to 16% precast gels (Lonza, Basel, Switzerland) and transferred to polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA). The membranes were blocked with protein-free T20 blocking buffer (Thermo Scientific, Rockford, IL) and incubated with primary antibodies specific for iNOS (BD Transduction Laboratories, San Jose, CA), p53 and nitrotyrosine (Cell Signaling), p-p53 (Ser 15; Santa Cruz Biotechnology Inc., Santa Cruz, CA), caspase 3 (cleaved fragment 17, 19 kDa; proform, 35 kDa; Cell Signaling, Danvers, MA), caspase 9 (37 kDa; Cell Signaling), and caspase 8 (20 kDa; Abcam, Cambridge, MA), followed by a corresponding anti-rabbit or anti-mouse IgG (Pierce Protein Research Products, Rockford, IL). The membranes were stripped and probed with β-actin (Sigma-Aldrich, St. Louis, MO) as a loading control. Proteins were detected by chemiluminescence (West Pico or West Dura kits; Pierce) on autoradiography film digitally scanned for quantification by densitometry using Adobe Photoshop (Adobe Systems Inc, San Jose, CA) analysis tools. Each Western blot (WB) was repeated at least three times.
Human Colonic Specimens and Histological Score
Biopsy specimens were obtained from human patients aged ≥18 years, undergoing diagnostic or surveillance colonoscopy for UC or therapeutic colonic resection, or healthy individuals undergoing routine colon cancer surveillance. Exclusion criteria were pregnant women, history of intestinal surgery, bleeding diathesis, or coagulopathy. Inflammation was scored by a blinded researcher (P.S.) on a scale from 0 to 8, based on mucosal leukocyte infiltration (0, no infiltration; 1, basolateral; 2, infiltration halfway up the crypt; 3, diffuse infiltration; and 4, crypt abscess) added to a crypt architecture score (0, no epithelial cell distortion; 1, crypt hyperplasia; 2, mild crypt distortion; 3, severe crypt distortion; and 4, complete loss of crypt structure). All untreated and anti-TNF–treated patients were inflamed and had a mean histological score greater than four. A total of six specimens from six patients were analyzed for each group. Collection of all patient materials for this study was approved by Northwestern University's Office for the Protection of Human Subjects.
Statistical Analysis
A two-tailed Student's t-test was used to evaluate differences between the groups. For any single experiment, up to five statistical comparisons were made. Bonferroni correction for multiple comparisons results in differences being considered statistically significant when P < 0.01. This would control the overall type I error rate for an experiment at 5%.
Results
TNF-Mediated Crypt Cell Apoptosis Is TNFR1 and TNFR2 Dependent
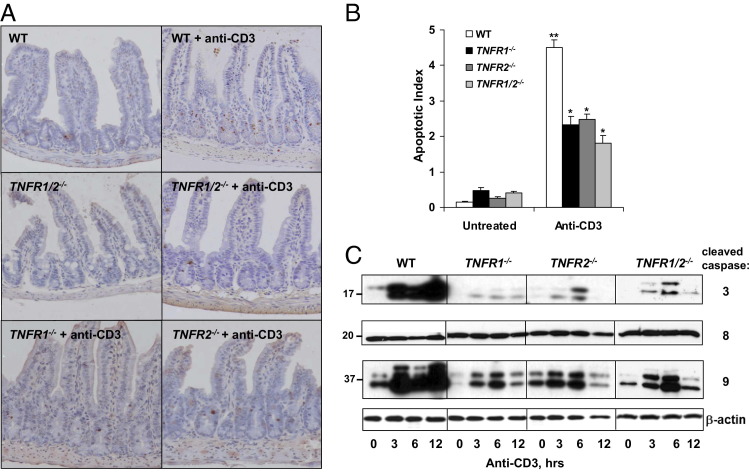
To examine the relative contribution of TNFR1 and TNFR2 signaling to T-cell–induced IEC apoptosis, WT, TNFR1−/−, TNFR2−/−, or TNFR1/2−/− mice, we stimulated with anti-CD3 mAb to activate T cells. Researchers reported that treating mice with anti-CD3 increases intestinal (epithelial and lamina propria) and serum levels of cytokines, including TNF.26–29 At 24 hours after injection, TUNEL staining of the SB of WT mice indicated that T-cell activation induced IEC apoptosis in lower to mid crypt regions (Figure 1A). By comparison, epithelial cell apoptosis was reduced in mice deficient for TNFR1 or TNFR2 (Figure 1A). Although individual TNFR1 or TNFR2 deletions reduced apoptotic responses by approximately 45%, mice with combined TNFR1 and TNFR2 deficiencies exhibited 60% less apoptosis (Figure 1B). Thus, both TNFR1 and TNFR2 contributed to T-cell–mediated IEC apoptosis in the SB.
Figure 1.
TNFR1 and TNFR2 signaling induces TNF-mediated crypt cell apoptosis. WT, TNFR1−/−, TNFR2−/−, and TNFR1/2−/− mice are injected with control or anti-CD3 antibody and sacrificed at the indicated time points. A: TUNEL staining of SB crypts from mice sacrificed 24 hours after anti-CD3 mAb injection. B: The apoptotic index is determined based on counting TUNEL-positive cells. ⁎P < 0.0001 compared with WT stimulated mice; ⁎⁎P < 0.0001 for anti-CD3-treated WT mice compared with untreated WT mice. C: WB of activated caspases 3, 8, and 9 from CD45-negative (data not shown) IEC lysates resolved by SDS-PAGE. β-Actin is used as a loading control.
Next, caspase 3, 8, and 9 activation was assessed in IECs as an indicator of intrinsic and extrinsic apoptotic signaling pathways. The WB analysis of IEC isolates from control and anti-CD3-treated mice revealed that T-cell activation induced IEC caspase 3 cleavage 30-fold in WT mice (Figure 1C). Densitometry showed that caspase 3 cleavage persisted from 3 to 12 hours after treatment, consistent with times when proteins became nitrated (see Supplemental Figure S1A at http://ajp.amjpathol.org)26,30 and with times we reported that anti-CD3 induced TNF locally.26 By comparison, TNFR deficiency largely attenuated caspase 3 cleavage at every time point. The results of caspase 9 cleavage paralleled those of caspase 3 reductions seen in TNFR-deficient mice (Figure 1C; see also Supplemental Figure S1B at http://ajp.amjpathol.org). In contrast, IEC caspase 8 cleavage (p20 protein shown herein) was unaffected by T-cell activation. Together, these data indicated that both TNFR1 and TNFR2 signaling mediated IEC apoptosis via caspases 3 and 9. Not all apoptotic activity was abrogated by TNFR deficiency. These data suggested that, although TNFR signaling was important for IEC apoptosis, other pathways contributed as well (eg, Fas/FasL and perforin).31
TNF-Induced iNOS Mediated Immune-Mediated Crypt Cell Apoptosis
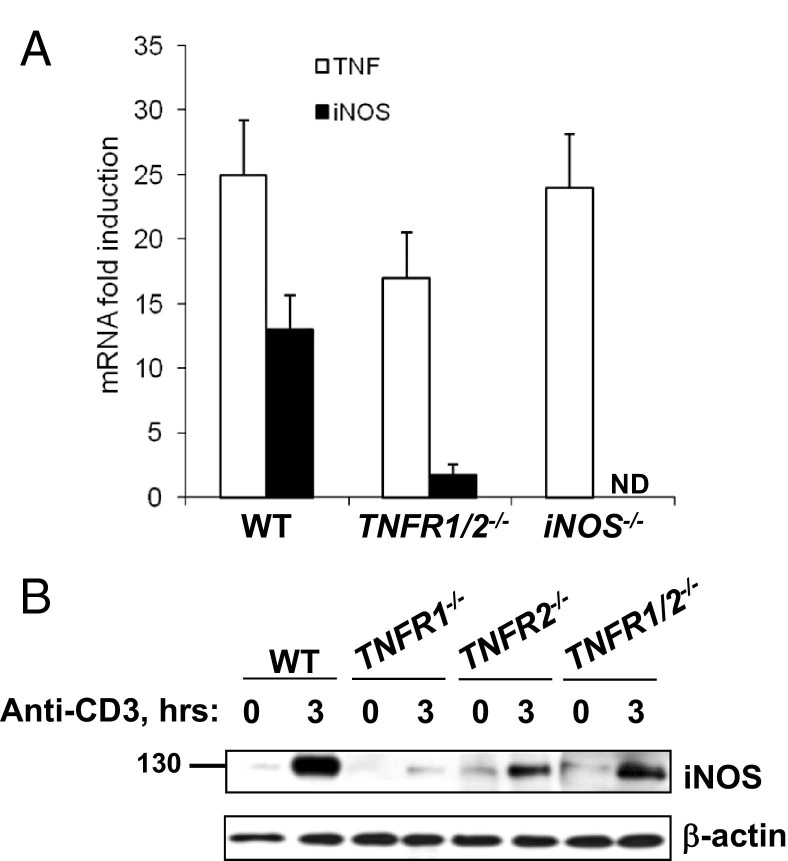
Next, we tested the hypothesis that TNF-induced iNOS contributed to T-cell–induced epithelial apoptosis. TNF and iNOS mRNA levels in anti-CD3-stimulated TNFR1/2−/− and iNOS−/− mice were assessed. The expression of iNOS was significantly reduced in TNFR1/2−/− mice relative to WT, whereas TNF levels were induced in WT, TNFR1/2−/−, and iNOS−/− mice (Figure 2A). WB analysis revealed that iNOS protein levels increased within 3 hours of T-cell activation in WT mice (Figure 2B). By comparison, iNOS induction was significantly reduced in the single and double TNF receptor knockout mice (Figure 2B). Therefore, TNFR1 and TNFR2 signaling induced iNOS after T-cell activation.
Figure 2.
iNOS mediates T-cell–induced crypt cell apoptosis downstream of TNFR1 and TNFR2. A: Fold induction of iNOS and TNF mRNA expression 3 hours after anti-CD3 injection determined by real-time RT-PCR from SB tissue from WT, TNFR1/2−/−, and iNOS−/− mice. B: WB analysis of iNOS protein expression in the indicated mice before, or 3 hours after, T-cell activation. β-Actin is used as a loading control. ND, not determined.
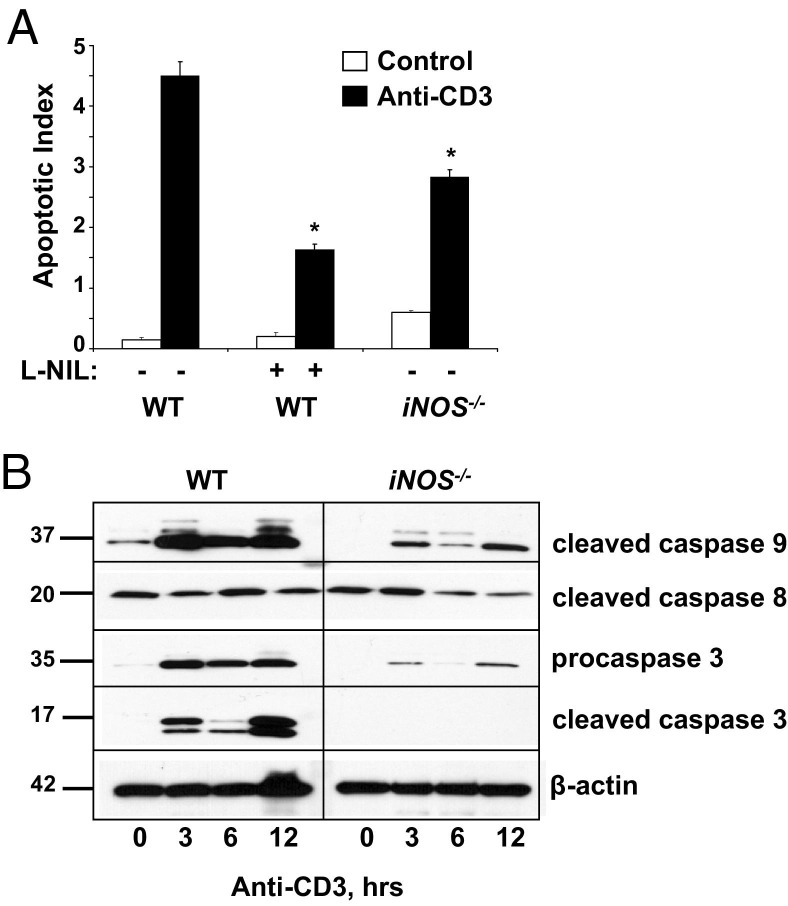
Next, we determined whether iNOS mediated T-cell–induced IEC apoptosis. Data shown in Figure 3A indicate that IEC apoptosis levels were significantly reduced when iNOS was blocked either pharmacologically with the selective inhibitor L-NIL or genetically using iNOS deficient relative to WT control mice. Furthermore, activation of caspases 3 and 9 was attenuated in iNOS−/− mice compared with anti-CD3-treated WT mice (Figure 3B). Procaspase 3 protein levels were induced by immune activation in WT mice and in iNOS−/− mice, albeit to lesser degrees over time. Therefore, procaspase 3 expression may be regulated by inflammatory mediators in IECs. Caspase 8 activation (p20 activated form) remained unchanged at every time point in IECs from both WT and iNOS−/− mice. Taken together, these data suggested that TNF-induced iNOS mediated IEC apoptosis after T-cell activation.
Figure 3.
iNOS is required for IEC apoptosis. A: Mice are sacrificed 24 hours after control or anti-CD3 mAb injection, and apoptosis is quantified in SB sections from WT mice, with or without L-NIL treatment, and from iNOS−/− mice. Apoptotic indexes are calculated based on counting TUNEL-positive cells. ⁎P < 0.001 compared with anti-CD3-treated WT mice. B: Activation of caspases 3, 8, and 9 is assessed by WB for the cleaved forms of the indicated protein in WT and iNOS−/− mice at 0, 3, 6, or 12 hours after T-cell activation. Procaspase 3 is also shown, and β-actin is used as a loading control.
TNFR1, TNFR2, and iNOS-Induced IEC Apoptosis Is p53 Dependent
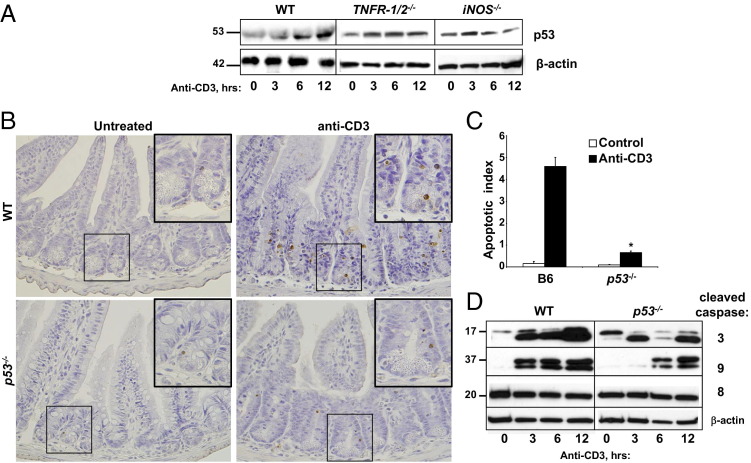
Data in other tissues, such as the brain, pancreas, and heart, suggested that iNOS-induced nitrosative oxygen species induced p53 protein stabilization, which then induced apoptosis.32 We recently reported that p53 was an important mediator of inflammation-induced apoptosis in the colon; however, the upstream mechanisms remained unknown.24 Data herein suggested that both TNFR1 and TNFR2 contributed to IEC apoptosis via the intrinsic pathway; thus, we examined p53 protein levels in IECs after anti-CD3 treatment. Data in Figure 4 showed that T-cell activation induced a progressive increase in p53 from 3 to 12 hours in the SB of WT mice. By comparison, p53 stabilization was severely attenuated in TNF receptor or iNOS-deficient mice (Figure 4A). These results were consistent with TNF and iNOS being upstream of p53 protein accumulation.
Figure 4.
p53 Plays a role in T-cell–induced crypt cell apoptosis downstream of TNFR1, TNFR2, and iNOS. A: WB analysis of p53 protein expression and stabilization in SB IECs from WT, TNFR1/2−/−, and iNOS−/− mice. B: Apoptosis is assessed in the SB crypts and villi in p53−/− mice by TUNEL staining 24 hours after stimulation with anti-CD3 mAb injection relative to WT mice. Insets: Areas magnified (original magnification, ×40) are demarcated by boxes in the ×10 picture. C: Results are quantified by counting TUNEL-positive cells to determine the apoptotic index. ⁎P < 0.0001. D: Caspase activation is determined by WB analysis at the indicated times after anti-CD3 injection. β-Actin is used as a loading control.
Because T-cell activation enhanced p53 levels, a role for p53 in IEC apoptosis was explored by TUNEL staining. Although IEC apoptosis increased in lower to mid crypts of tissue after T-cell activation, TUNEL staining of IECs was reduced approximately 89% in p53−/− mice (Figure 4, B and C). WB analysis confirmed p53-dependent IEC apoptosis because caspase 3 and 9 activation was significantly attenuated in p53−/− mice compared with WT (Figure 4D). Taken together, these data were consistent with the notion that p53 mediated IEC death in response to TNF and iNOS signaling.
TNF and iNOS Are Required for Colitis-Induced IEC Apoptosis
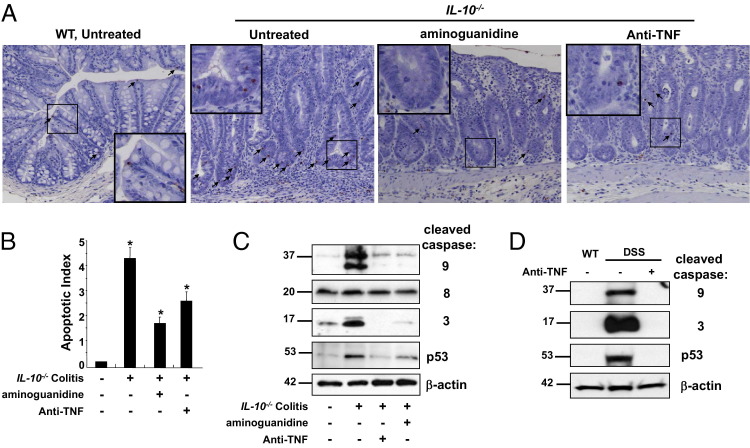
To determine the relevance of these findings to IBD colitis, IEC apoptosis was assessed in IL-10−/− mice. Colitis was induced by feeding mice piroxicam for 14 days.33,34 TUNEL staining revealed that, on the day of peak colitis (day 28), apoptosis increased within IECs localized to lower crypt regions (Figure 5A). To determine whether iNOS or TNF signaling contributed to colitis-induced apoptosis, mice were treated with either aminoguanidine (a specific iNOS inhibitor) or anti-TNF antibody, respectively. TUNEL staining revealed that apoptotic indexes decreased by 58% in aminoguanidine-treated mice and 37% in anti-TNF–treated mice, compared with untreated colitic mice (Figure 5, A and B). Short-term blocking of TNF by anti-TNF treatment was also effective at reducing apoptosis in the anti-CD3 model, as determined by TUNEL staining (see Supplemental Figure S2A at http://ajp.amjpathol.org). Analysis of cleaved caspases 3 and 9 in colitic mice confirmed that anti-TNF or aminoguanidine treatment decreased IEC apoptosis in colitic mice (Figure 5C). The expression of caspase 8 active polypeptide (p20) remained unchanged. Quantitative analysis of TNF-induced iNOS mRNA expression revealed that anti-TNF treatment of mice reduced downstream effects of TNF and, thus, colitis-induced epithelial iNOS gene transcription (see Supplemental Figure S2B at http://ajp.amjpathol.org). These data were also confirmed in another chronic colitis model. Mice that underwent three cycles of DSS in their drinking water revealed that anti-TNF reduced expression of relevant gene targets (TNF, mmp7, and iNOS; see Supplemental Figure S2C at http://ajp.amjpathol.org). Furthermore, anti-TNF abrogated elevated levels of epithelial cleaved caspases 3 and 9 in mice with chronic DSS-colitis (Figure 5D). Together, these data suggested that TNF and iNOS mediated IEC apoptosis in colitis.
Figure 5.
Inhibitors of TNF and iNOS decrease colitis-induced IEC apoptosis. Colitis is induced in IL-10−/− mice with piroxicam, and apoptosis is assessed at day 28. Mice are treated with aminoguanidine to block iNOS or anti-TNF mAb to inhibit TNF. Colonic IECs undergoing apoptosis are identified by TUNEL staining (A, arrows), and the apoptotic index is calculated based on cell counting of TUNEL-positive cells (B). ⁎P < 0.004 compared with colitic mice; ⁎⁎P < 0.0001 compared with noncolitic mice. High-power magnification (×40) is shown in the small boxes in the low-magnification (×10) pictures. C: WB analysis of colon IEC whole cell lysates for caspase 3, 8, and 9 activation and for p53 levels. Chronic colitis is also induced in WT mice after three cycles of DSS. D: Mice are either left untreated or treated with anti-TNF mAb, and levels of p53 and cleaved caspases 3 and 9 are analyzed. β-Actin is used as a loading control.
To determine whether iNOS and TNF regulated IEC p53 levels in colitis, IEC lysates were analyzed by WB. Cytoplasmic p53 levels were much higher with colitis (Figure 5C). Significantly, anti-TNF and aminoguanidine treatment reduced p53 accumulation in the cytoplasm. These data were further supported by findings in three-cycle DSS-treated mice in which anti-TNF treatment abrogated colitis-induced IEC p53 stabilization (Figure 5D). In summary, these data illustrated that TNF-induced p53-mediated signaling accounted for IEC apoptosis in colitis.
IEC Apoptosis and p53 Expression Are TNF Dependent in Human UC
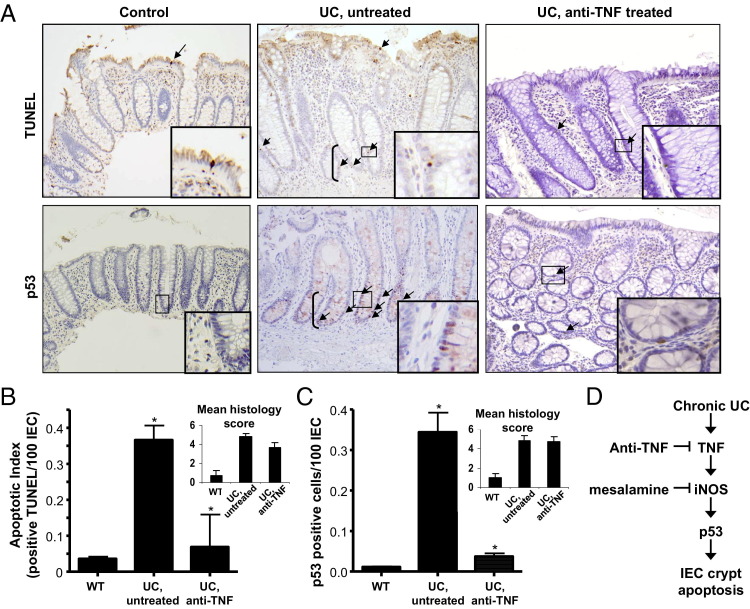
To examine a role for TNF to induce p53-mediated IEC apoptosis in human UC, IEC apoptosis and p53 staining were compared between control tissue and actively inflamed colitis tissue from untreated patients or those receiving anti-TNF therapy. All UC samples chosen for this study had moderately active inflammation, as determined by histological scores (Figure 6; see also Materials and Methods). Thus, levels of tissue inflammation were similar between untreated and anti-TNF–treated tissue. Patients undergoing routine surveillance colonoscopy rarely displayed TUNEL- or p53-positive cells (Figure 6). Apoptotic cells were seen primarily in plateau regions, whereas p53 was detected in lower crypts. Strikingly, colitis increased IEC apoptosis and p53 staining throughout lower and mid crypt regions (Figure 6). By comparison, IEC apoptosis and p53 staining were markedly reduced in patients treated with anti-TNF pharmacological agents. These data indicated that, despite equivalent levels of inflammation, anti-TNF Ab treatment reduced p53-mediated IEC apoptosis in human UC (Figure 6D).
Figure 6.
Apoptosis and p53 expression are induced by TNF in human UC. Colon tissue biopsy specimens from normal control, UC untreated, and UC anti-TNF–treated patients are analyzed for p53 and TUNEL staining (A, arrows) and then quantified by counting positive cells per 100 IECs (B and C). Insets: Areas magnified (×40) are demarcated by boxes. Untreated and anti-TNF–treated UC patients selected for study are inflamed, as indicated by mean histological scores (B and C). *P < 0.01 (B), P < 0.0001 (C) for UC untreated compared to anti-TNF–treated patients. The proposed paradigm for IEC crypt apoptosis, in which chronic inflammation from UC induces TNF production, which causes increased expression of iNOS. The effects of iNOS products (NO− and ONOO−) induce p53 protein in crypt epithelial cells. Points in the pathway where the therapeutic agents, anti-TNF and mesalamine, may inhibit IEC apoptosis are also shown.
Discussion
The intestinal epithelium provides essential roles important for host defense and homeostasis, including maintaining barrier function and participating in mucosal immune responses.1,2 Regulation of apoptosis of IECs is one way that the intestinal epithelium maintains or returns to homeostasis.35 During IBD, apoptosis is observed in acute inflammatory sites.35,36 Therapy with TNF-neutralizing antibody effectively reduces IEC apoptosis and increases mucosal repair; however, the mechanisms for TNF-mediated tissue injury in IBD remain unclear.37,38 Recent findings from our laboratory indicate that p53 is the predominant mediator of IEC apoptosis in IBD.24 The data presented herein suggest that TNF-induced iNOS stabilizes p53, which induces crypt IEC apoptosis (Figure 6D).
We show that p53 is a major mediator of inflammation-induced crypt IEC apoptosis. Previous reports showed elevated levels of p53 in patients with UC.15,16,24,39 Most significantly, we report a role for p53 in both an acute and chronic inflammatory model in mice that parallels these observations made from studying human disease and is, thus, clinically relevant. First, p53 levels were elevated in anti-CD3-treated and IL-10−/− mouse colitis models (Figures 1 and 5). Second, p53 deficiency reduced IEC apoptosis, as measured by TUNEL staining and WB of activated caspase 3 and 9 cleavage (Figure 4). Finally, regions of elevated p53 staining in patients with UC correlated with increased IEC apoptosis, and regions of reduced p53 paralleled reductions in IEC apoptosis after anti-TNF therapy (Figure 6). Taken together, these data further implicate p53 as a key player in IBD crypt IEC apoptosis.
Nitrosylated oxygen radicals produced by iNOS are major inducers of p53 activation and stabilization.40 In human IBD, iNOS is observed in inflamed areas of the colon in epithelial cells, lamina propria mononuclear cells, and neutrophils.14,15 Reductions of iNOS mRNA and protein in mice with T-cell–stimulated TNFR1/2−/− and anti-TNF–treated colitis suggest that TNF is a major mediator of iNOS in intestinal inflammation. Data presented herein in two mouse models directly implicate iNOS in inflammation-induced IEC apoptosis. IEC apoptosis was reduced in iNOS-deficient mice (Figure 3) and in mice treated with the specific inhibitors, L-NIL or aminoguanidine (Figure 5). Furthermore, p53 protein stabilization was attenuated in iNOS−/− mice treated with anti-CD3 and in colitic IL-10−/− mice treated with aminoguanidine. These data confirm that NO− and ONOO− actively participate in p53 stabilization. In fact, Singer et al14 previously demonstrated markedly elevated nitrotyrosine staining (a product of ONOO−) in IECs from patients with IBD. Thus, the effects of ONOO− likely directly affected p53 stabilization within IECs. Inhibition of p53 stabilization and caspase activation by L-NIL and aminoguanidine was incomplete. These data suggest that iNOS-independent mechanisms may also have some role in inflammation-induced apoptosis of IECs.
Our data indicate that inflammation-induced TNF is an important inducer of p53-dependent IEC apoptosis. Previously, expression of both TNFR1 and TNFR2 was increased on IECs during inflammatory conditions.41,42 Our analysis reveals that signaling through both receptors contributes to inflammation-induced IEC apoptosis because apoptosis was decreased in the single- and double-receptor knockouts after T-cell activation and by anti-TNF treatment in the IL-10−/− chronic colitis model. These data are consistent with reports showing that TNFR1 and TNFR2 cooperate in TNF-mediated apoptosis and also form functional heterocomplexes.19,43–45 Most significantly, TNF neutralization in patients with UC reduced IEC apoptosis and p53 staining (Figure 6, A–C). These data indicate a direct role for TNF in IEC apoptosis through p53-mediated pathways (Figure 6D).
In our experiments, data suggest that the mitochondrial intrinsic pathway mediated inflammation-induced IEC apoptosis. We consistently saw, in murine models, that inflammation induced procaspase 3 and 9, but not 8, cleavage into their active forms. Caspase 8 primarily induces apoptotic death initiated by receptors containing death domains (eg, TNFR1, Fas, and DR5).46 Its activation, for example, mediates caspase 3 and BH3 interacting-domain death agonist cleavage, leading to the execution of apoptosis. Therefore, the absence of caspase 8 cleavage suggests that although TNFR1 was required, it did not induce caspases linked to the extrinsic pathway. Rather, our data indicate that TNFR-mediated IEC apoptosis required activation of the intrinsic or mitochondrial pathway. Mitochondrial dysfunction initiates apoptosis by the intrinsic apoptotic pathway and includes p53 translocation to the nucleus, where the protein modulates pro- and anti-apoptotic Bcl-2 family proteins.47 This pathway is tightly regulated by a balance between prosurvival and pro-apoptotic Bcl-2 family members.48 Some evidence suggests that p53 requires apoptotic protease activating factor 1, caspase-9, and cytochrome c release to perform apoptosis.49 Thus, the intrinsic pathway is vital for p53-dependent apoptosis and tumor suppression. It was previously reported that TNF-induced IEC apoptosis was only TNFR1 dependent and p53 independent, results that contradict those reported herein.50 It is possible that the epithelial populations undergoing apoptosis in these studies differed from those examined herein. Piguet et al50 induced villus IEC apoptosis and detachment by a relatively high-dose TNF injection; however, in our experiments, we observed the increase in apoptosis in IECs in the lower crypt regions, as seen in patients with human IBD. Because villus IEC apoptosis occurs after ischemia reperfusion, their studies likely present a valid model for this mode of IEC death. Conversely, the models used herein result in crypt IEC death and, therefore, more closely resemble mechanisms of IEC apoptosis in IBD.
One clinical implication of these studies is that commonly used therapeutic agents that block TNF (anti-TNF monoclonal antibody) or iNOS (mesalamine) may enhance mucosal healing by reducing IEC apoptosis.7,51 Our studies show that TNF induces iNOS, which activates p53 in epithelial cells (Figure 6D). These mediators, increased in IBD, induce IEC apoptosis by activating p53 in lower and mid crypt zones, where proliferation is induced. Thus, we strongly believe that IEC apoptosis in the lower and mid crypts is most relevant to the increased epithelial cell death seen in human IBD. It is, therefore, attractive to speculate that p53-mediated signaling induces apoptosis in proliferating progenitor populations. In biopsy specimens from patients with UC, we failed to see a correlation between p53 status (in a sample from anti-TNF–treated patients) and histological activity. In other reports,52,53 investigators found that mucosal inflammation was unaffected by p53 status; thus, the predominant effect of p53 function and IEC apoptosis may be on the development of dysplasia. In this scenario, a failure to induce apoptosis might allow for a long-lived progenitor cell population harboring DNA mutations to persist.
Chronic colitis induced in mice by DSS alone initiates the neoplastic process,54 as does colitis in IL-10−/− mice, suggesting that inflammation plays a key role in colitis-associated cancer development.54,55 Mutation of the tumor suppressor gene, p53, is an early event in the progression toward human colitis-associated cancer.16,56–59 We show herein that stabilization of epithelial p53 after immune activation is TNFR1/2 and iNOS dependent. Without p53 function, as would be the case after inflammation-induced p53 mutation, damaged proliferating cells would avoid apoptosis. Chen et al60 showed clonal expansion of p53-mutated epithelial cells in areas of colitis-induced dysplasia. The failure of p53-mutated IECs to undergo apoptosis may explain why IEC apoptosis is reduced in colonic tumor samples and carcinomas.35 In fact, loss of p53 enhanced cancer rates in mice with DSS colitis.52,61 Additional exploration of p53-deficient mice in chronic colitis models will further discern the role p53 plays in IBD-induced oncogenesis.
Footnotes
Supported by grants from the NIH (R01DK-054778 and R01AI-6171702 to T.A.B.).
Supplemental material for this article can be found at http://ajp.amjpathol.org or at http://dx.doi.org/10.1016/j.ajpath.2012.06.016.
Supplementary data
WT mice are injected with control or anti-CD3 antibody and sacrificed after 3 hours. A: WB of SB epithelial cell lysates reveals an increase in levels of nitrotyrosine phosphorylated proteins, which is confirmed by densitometry. B: Densitometry measurements are made for caspases 3 and 9 from WB data shown in Figure 1C. The average fold changes from three independent experiments are shown relative to untreated WT mice and normalized to actin protein levels.
Untreated and anti-TNF 24-hour pretreated WT mice are stimulated with anti-CD3, and the apoptotic index is determined 24 hours after T-cell activation by counting TUNEL-positive cells in the SB epithelium (A). Cytokine induction in colon IECs is analyzed in both the IL-10−/− (B) and three-cycle DSS (C) chronic colitis models, with or without anti-TNF treatment, and compared with WT untreated mice.
References
- 1.Shen L., Turner J.R. Role of epithelial cells in initiation and propagation of intestinal inflammation: eliminating the static: tight junction dynamics exposed. Am J Physiol Gastrointest Liver Physiol. 2006;290:G577–G582. doi: 10.1152/ajpgi.00439.2005. [DOI] [PubMed] [Google Scholar]
- 2.Groschwitz K.R., Hogan S.P. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124:3–20. doi: 10.1016/j.jaci.2009.05.038. quiz 21–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arseneau K.O., Tamagawa H., Pizarro T.T., Cominelli F. Innate and adaptive immune responses related to IBD pathogenesis. Curr Gastroenterol Rep. 2007;9:508–512. doi: 10.1007/s11894-007-0067-3. [DOI] [PubMed] [Google Scholar]
- 4.Bouma G., Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 5.Suenaert P., Bulteel V., Lemmens L., Noman M., Geypens B., Van Assche G., Geboes K., Ceuppens J.L., Rutgeerts P. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn's disease. Am J Gastroenterol. 2002;97:2000–2004. doi: 10.1111/j.1572-0241.2002.05914.x. [DOI] [PubMed] [Google Scholar]
- 6.Wong M., Ziring D., Korin Y., Desai S., Kim S., Lin J., Gjertson D., Braun J., Reed E., Singh R.R. TNFalpha blockade in human diseases: mechanisms and future directions. Clin Immunol. 2008;126:121–136. doi: 10.1016/j.clim.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilg H., Moschen A., Kaser A. Mode of function of biological anti-TNF agents in the treatment of inflammatory bowel diseases. Expert Opin Biol Ther. 2007;7:1051–1059. doi: 10.1517/14712598.7.7.1051. [DOI] [PubMed] [Google Scholar]
- 8.Lichtenberger G.S., Flavell R.A., Alexopoulou L. Innate immunity and apoptosis in IBD. Inflamm Bowel Dis. 2004;10(Suppl 1):S58–S62. doi: 10.1097/00054725-200402001-00012. [DOI] [PubMed] [Google Scholar]
- 9.Podolsky D.K. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 10.Parameswaran N., Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20:87–103. doi: 10.1615/critreveukargeneexpr.v20.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleinert H., Schwarz P.M., Förstermann U. Regulation of the expression of inducible nitric oxide synthase. Biol Chem. 2003;384:1343–1364. doi: 10.1515/BC.2003.152. [DOI] [PubMed] [Google Scholar]
- 12.Rachmilewitz D., Karmeli F., Okon E., Bursztyn M. Experimental colitis is ameliorated by inhibition of nitric oxide synthase activity. Gut. 1995;37:247–255. doi: 10.1136/gut.37.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krieglstein C.F., Cerwinka W.H., Laroux F.S., Salter J.W., Russell J.M., Schuermann G., Grisham M.B., Ross C.R., Granger D.N. Regulation of murine intestinal inflammation by reactive metabolites of oxygen and nitrogen: divergent roles of superoxide and nitric oxide. J Exp Med. 2001;194:1207–1218. doi: 10.1084/jem.194.9.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singer I.I., Kawka D.W., Scott S., Weidner J.R., Mumford R.A., Riehl T.E., Stenson W.F. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology. 1996;111:871–885. doi: 10.1016/s0016-5085(96)70055-0. [DOI] [PubMed] [Google Scholar]
- 15.Hofseth L.J., Saito S., Hussain S.P., Espey M.G., Miranda K.M., Araki Y., Jhappan C., Higashimoto Y., He P., Linke S.P., Quezado M.M., Zurer I., Rotter V., Wink D.A., Appella E., Harris C.C. Nitric oxide-induced cellular stress and p53 activation in chronic inflammation. Proc Natl Acad Sci U S A. 2003;100:143–148. doi: 10.1073/pnas.0237083100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussain S.P., Amstad P., Raja K., Ambs S., Nagashima M., Bennett W.P., Shields P.G., Ham A.J., Swenberg J.A., Marrogi A.J., Harris C.C. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-prone chronic inflammatory disease. Cancer Res. 2000;60:3333–3337. [PubMed] [Google Scholar]
- 17.Gupta S. Molecular steps of tumor necrosis factor receptor-mediated apoptosis. Curr Mol Med. 2001;1:317–324. doi: 10.2174/1566524013363780. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal B.B. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 19.Wallach D., Varfolomeev E.E., Malinin N.L., Goltsev Y.V., Kovalenko A.V., Boldin M.P. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 20.Iwamoto M., Koji T., Makiyama K., Kobayashi N., Nakane P.K. Apoptosis of crypt epithelial cells in ulcerative colitis. J Pathol. 1996;180:152–159. doi: 10.1002/(SICI)1096-9896(199610)180:2<152::AID-PATH649>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 21.Di Sabatino A., Ciccocioppo R., Luinetti O., Ricevuti L., Morera R., Cifone M.G., Solcia E., Corazza G.R. Increased enterocyte apoptosis in inflamed areas of Crohn's disease. Dis Colon Rectum. 2003;46:1498–1507. doi: 10.1007/s10350-004-6802-z. [DOI] [PubMed] [Google Scholar]
- 22.Lee G., Goretsky T., Managlia E., Dirisina R., Singh A.P., Brown J.B., May R., Yang G.Y., Ragheb J.W., Evers B.M., Weber C.R., Turner J.R., He X.C., Katzman R.B., Li L., Barrett T.A. Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology. 2010;139:869–881. doi: 10.1053/j.gastro.2010.05.037. 881.e1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musch M.W., Clarke L.L., Mamah D., Gawenis L.R., Zhang Z., Ellsworth W., Shalowitz D., Mittal N., Efthimiou P., Alnadjim Z., Hurst S.D., Chang E.B., Barrett T.A. T cell activation causes diarrhea by increasing intestinal permeability and inhibiting epithelial Na+/K+-ATPase. J Clin Invest. 2002;110:1739–1747. doi: 10.1172/JCI15695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dirisina R., Katzman R.B., Goretsky T., Managlia E., Mittal N., Williams D.B., Qiu W., Yu J., Chandel N.S., Zhang L., Barrett T.A. p53 and PUMA independently regulate apoptosis of intestinal epithelial cells in patients and mice with colitis. Gastroenterology. 2011;141:1036–1045. doi: 10.1053/j.gastro.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gavrieli Y., Sherman Y., Ben-Sasson S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang Y., Clayburgh D.R., Mittal N., Goretsky T., Dirisina R., Zhang Z., Kron M., Ivancic D., Katzman R.B., Grimm G., Lee G., Fryer J., Nusrat A., Turner J.R., Barrett T.A. Epithelial NF-kappaB enhances transmucosal fluid movement by altering tight junction protein composition after T cell activation. Am J Pathol. 2010;176:158–167. doi: 10.2353/ajpath.2010.090548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferran C., Sheehan K., Dy M., Schreiber R., Merite S., Landais P., Noel L.H., Grau G., Bluestone J., Bach J.F., Chatenoud L. Cytokine-related syndrome following injection of anti-CD3 monoclonal antibody: further evidence for transient in vivo T cell activation. Eur J Immunol. 1990;20:509–515. doi: 10.1002/eji.1830200308. [DOI] [PubMed] [Google Scholar]
- 28.Radojevic N., McKay D.M., Merger M., Vallance B.A., Collins S.M., Croitoru K. Characterization of enteric functional changes evoked by in vivo anti-CD3 T cell activation. Am J Physiol. 1999;276:R715–R723. doi: 10.1152/ajpregu.1999.276.3.R715. [DOI] [PubMed] [Google Scholar]
- 29.Clayburgh D.R., Barrett T.A., Tang Y., Meddings J.B., Van Eldik L.J., Watterson D.M., Clarke L.L., Mrsny R.J., Turner J.R. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merger M., Viney J.L., Borojevic R., Steele-Norwood D., Zhou P., Clark D.A., Riddell R., Maric R., Podack E.R., Croitoru K. Defining the roles of perforin, Fas/FasL, and tumour necrosis factor alpha in T cell induced mucosal damage in the mouse intestine. Gut. 2002;51:155–163. doi: 10.1136/gut.51.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu W., Liu L.Z., Loizidou M., Ahmed M., Charles I.G. The role of nitric oxide in cancer. Cell Res. 2002;12:311–320. doi: 10.1038/sj.cr.7290133. [DOI] [PubMed] [Google Scholar]
- 33.Berg D.J., Zhang J., Weinstock J.V., Ismail H.F., Earle K.A., Alila H., Pamukcu R., Moore S., Lynch R.G. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology. 2002;123:1527–1542. doi: 10.1053/gast.2002.1231527. [DOI] [PubMed] [Google Scholar]
- 34.Brown J.B., Lee G., Managlia E., Grimm G.R., Dirisina R., Goretsky T., Cheresh P., Blatner N.R., Khazaie K., Yang G.Y., Li L., Barrett T.A. Mesalamine inhibits epithelial beta-catenin activation in chronic ulcerative colitis. Gastroenterology. 2010;138:595–605. doi: 10.1053/j.gastro.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edelblum K.L., Yan F., Yamaoka T., Polk D.B. Regulation of apoptosis during homeostasis and disease in the intestinal epithelium. Inflamm Bowel Dis. 2006;12:413–424. doi: 10.1097/01.MIB.0000217334.30689.3e. [DOI] [PubMed] [Google Scholar]
- 36.Hagiwara C., Tanaka M., Kudo H. Increase in colorectal epithelial apoptotic cells in patients with ulcerative colitis ultimately requiring surgery. J Gastroenterol Hepatol. 2002;17:758–764. doi: 10.1046/j.1440-1746.2002.02791.x. [DOI] [PubMed] [Google Scholar]
- 37.Zeissig S., Bojarski C., Buergel N., Mankertz J., Zeitz M., Fromm M., Schulzke J.D. Downregulation of epithelial apoptosis and barrier repair in active Crohn's disease by tumour necrosis factor alpha antibody treatment. Gut. 2004;53:1295–1302. doi: 10.1136/gut.2003.036632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marini M., Bamias G., Rivera-Nieves J., Moskaluk C.A., Hoang S.B., Ross W.G., Pizarro T.T., Cominelli F. TNF-alpha neutralization ameliorates the severity of murine Crohn's-like ileitis by abrogation of intestinal epithelial cell apoptosis. Proc Natl Acad Sci U S A. 2003;100:8366–8371. doi: 10.1073/pnas.1432897100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alkim C., Savas B., Ensari A., Alkim H., Dagli U., Parlak E., Ulker A., Sahin B. Expression of p53, VEGF, microvessel density, and cyclin-D1 in noncancerous tissue of inflammatory bowel disease. Dig Dis Sci. 2009;54:1979–1984. doi: 10.1007/s10620-008-0554-x. [DOI] [PubMed] [Google Scholar]
- 40.Goodman J.E., Hofseth L.J., Hussain S.P., Harris C.C. Nitric oxide and p53 in cancer-prone chronic inflammation and oxyradical overload disease. Environ Mol Mutagen. 2004;44:3–9. doi: 10.1002/em.20024. [DOI] [PubMed] [Google Scholar]
- 41.Kaiser G.C., Polk D.B. Tumor necrosis factor alpha regulates proliferation in a mouse intestinal cell line. Gastroenterology. 1997;112:1231–1240. doi: 10.1016/s0016-5085(97)70135-5. [DOI] [PubMed] [Google Scholar]
- 42.Mizoguchi E., Mizoguchi A., Takedatsu H., Cario E., de Jong Y.P., Ooi C.J., Xavier R.J., Terhorst C., Podolsky D.K., Bhan A.K. Role of tumor necrosis factor receptor 2 (TNFR2) in colonic epithelial hyperplasia and chronic intestinal inflammation in mice. Gastroenterology. 2002;122:134–144. doi: 10.1053/gast.2002.30347. [DOI] [PubMed] [Google Scholar]
- 43.Tartaglia L.A., Pennica D., Goeddel D.V. Ligand passing: the 75-kDa tumor necrosis factor (TNF) receptor recruits TNF for signaling by the 55-kDa TNF receptor. J Biol Chem. 1993;268:18542–18548. [PubMed] [Google Scholar]
- 44.Declercq W., Denecker G., Fiers W., Vandenabeele P. Cooperation of both TNF receptors in inducing apoptosis: involvement of the TNF receptor-associated factor binding domain of the TNF receptor 75. J Immunol. 1998;161:390–399. [PubMed] [Google Scholar]
- 45.Pinckard J.K., Sheehan K.C., Schreiber R.D. Ligand-induced formation of p55 and p75 tumor necrosis factor receptor heterocomplexes on intact cells. J Biol Chem. 1997;272:10784–10789. doi: 10.1074/jbc.272.16.10784. [DOI] [PubMed] [Google Scholar]
- 46.Taylor R.C., Cullen S.P., Martin S.J. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 47.Pietsch E.C., Sykes S.M., McMahon S.B., Murphy M.E. The p53 family and programmed cell death. Oncogene. 2008;27:6507–6521. doi: 10.1038/onc.2008.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adams J.M., Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soengas M.S., Alarcon R.M., Yoshida H., Giaccia A.J., Hakem R., Mak T.W., Lowe S.W. Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science. 1999;284:156–159. doi: 10.1126/science.284.5411.156. [DOI] [PubMed] [Google Scholar]
- 50.Piguet P.F., Vesin C., Guo J., Donati Y., Barazzone C. TNF-induced enterocyte apoptosis in mice is mediated by the TNF receptor 1 and does not require p53. Eur J Immunol. 1998;28:3499–3505. doi: 10.1002/(SICI)1521-4141(199811)28:11<3499::AID-IMMU3499>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 51.Kennedy M., Wilson L., Szabo C., Salzman A.L. 5-Aminosalicylic acid inhibits iNOS transcription in human intestinal epithelial cells. Int J Mol Med. 1999;4:437–443. doi: 10.3892/ijmm.4.4.437. [DOI] [PubMed] [Google Scholar]
- 52.Chang W.C., Coudry R.A., Clapper M.L., Zhang X., Williams K.L., Spittle C.S., Li T., Cooper H.S. Loss of p53 enhances the induction of colitis-associated neoplasia by dextran sulfate sodium. Carcinogenesis. 2007;28:2375–2381. doi: 10.1093/carcin/bgm134. [DOI] [PubMed] [Google Scholar]
- 53.Qiu W., Carson-Walter E.B., Liu H., Epperly M., Greenberger J.S., Zambetti G.P., Zhang L., Yu J. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell. 2008;2:576–583. doi: 10.1016/j.stem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berg D.J., Davidson N., Kuhn R., Muller W., Menon S., Holland G., Thompson-Snipes L., Leach M.W., Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okayasu I., Yamada M., Mikami T., Yoshida T., Kanno J., Ohkusa T. Dysplasia and carcinoma development in a repeated dextran sulfate sodium-induced colitis model. J Gastroenterol Hepatol. 2002;17:1078–1083. doi: 10.1046/j.1440-1746.2002.02853.x. [DOI] [PubMed] [Google Scholar]
- 56.Yoshida T., Mikami T., Mitomi H., Okayasu I. Diverse p53 alterations in ulcerative colitis-associated low-grade dysplasia: full-length gene sequencing in microdissected single crypts. J Pathol. 2003;199:166–175. doi: 10.1002/path.1264. [DOI] [PubMed] [Google Scholar]
- 57.Takaku H., Ajioka Y., Watanabe H., Hashidate H., Yamada S., Yokoyama J., Kazama S., Suda T., Hatakeyama K. Mutations of p53 in morphologically non-neoplastic mucosa of long-standing ulcerative colitis. Jpn J Cancer Res. 2001;92:119–126. doi: 10.1111/j.1349-7006.2001.tb01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fogt F., Vortmeyer A.O., Goldman H., Giordano T.J., Merino M.J., Zhuang Z. Comparison of genetic alterations in colonic adenoma and ulcerative colitis-associated dysplasia and carcinoma. Hum Pathol. 1998;29:131–136. doi: 10.1016/s0046-8177(98)90222-2. [DOI] [PubMed] [Google Scholar]
- 59.Kern S.E., Redston M., Seymour A.B., Caldas C., Powell S.M., Kornacki S., Kinzler K.W. Molecular genetic profiles of colitis-associated neoplasms. Gastroenterology. 1994;107:420–428. doi: 10.1016/0016-5085(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 60.Chen R., Rabinovitch P.S., Crispin D.A., Emond M.J., Bronner M.P., Brentnall T.A. The initiation of colon cancer in a chronic inflammatory setting. Carcinogenesis. 2005;26:1513–1519. doi: 10.1093/carcin/bgi106. [DOI] [PubMed] [Google Scholar]
- 61.Fujii S., Fujimori T., Kawamata H., Takeda J., Kitajima K., Omotehara F., Kaihara T., Kusaka T., Ichikawa K., Ohkura Y., Ono Y., Imura J., Yamaoka S., Sakamoto C., Ueda Y., Chiba T. Development of colonic neoplasia in p53 deficient mice with experimental colitis induced by dextran sulphate sodium. Gut. 2004;53:710–716. doi: 10.1136/gut.2003.028779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
WT mice are injected with control or anti-CD3 antibody and sacrificed after 3 hours. A: WB of SB epithelial cell lysates reveals an increase in levels of nitrotyrosine phosphorylated proteins, which is confirmed by densitometry. B: Densitometry measurements are made for caspases 3 and 9 from WB data shown in Figure 1C. The average fold changes from three independent experiments are shown relative to untreated WT mice and normalized to actin protein levels.
Untreated and anti-TNF 24-hour pretreated WT mice are stimulated with anti-CD3, and the apoptotic index is determined 24 hours after T-cell activation by counting TUNEL-positive cells in the SB epithelium (A). Cytokine induction in colon IECs is analyzed in both the IL-10−/− (B) and three-cycle DSS (C) chronic colitis models, with or without anti-TNF treatment, and compared with WT untreated mice.