Abstract
Maternal vascular dysfunction is a hallmark of preeclampsia. A recently described vascular phenotype of preeclampsia involves increased expression of matrix metalloproteinase-1 (MMP-1) in endothelial cells, vascular smooth muscle, and infiltrating neutrophils. In contrast, the expression of tissue inhibitor of metalloproteinases-1 (TIMP-1) and collagen type Iα 1 is either reduced or not changed in the vessels, suggesting an imbalance in vessel collagen degradation and synthesis in preeclampsia. In the present study, we explored the possible contribution of DNA methylation to the altered expression of genes involved in collagen metabolism. We assayed the differences in DNA methylation in omental arteries from normal pregnant and preeclamptic women, and determined whether reduced DNA methylation increases the expression of MMP-1 in cultured vascular smooth muscle cells and a neutrophil-like cell line, HL-60. Several MMP genes, including MMP1 and MMP8, were significantly less methylated in preeclamptic omental arteries, whereas TIMP and COL genes either were significantly more methylated or had no significant change in their DNA methylation status compared with normal pregnancy. Experimentally induced DNA hypomethylation increased MMP-1 expression in cultured vascular smooth muscle cells and MMP-1 cells. Our findings suggest that epigenetic regulation contributes to the imbalance in genes involved in collagen metabolism in blood vessels of preeclamptic women.
Preeclampsia is a multisystemic disorder that is diagnosed by new onset of hypertension and proteinuria, which occur after 20 weeks of gestation in pregnant women who are otherwise normal.1 It is also associated with pathological edema due to increased vascular permeability. Preeclampsia and its complications are among the leading causes of obstetrical mortality and morbidity.1 In the United States, preeclampsia occurs in approximately 6% to 8% of pregnant women, and is estimated to be responsible for 15% of obstetrical deaths.2 Recently, neutrophil infiltration associated with marked inflammation of maternal systemic blood vessels was reported as a characteristic phenotype in women with preeclampsia3–5
We recently reported that matrix metalloproteinase (MMP)-1 expression is increased in systemic blood vessels of preeclamptic women compared with normal pregnant women.6 The increased expression of MMP-1 in omental arteries was detected in vascular smooth muscle cells (VSMCs), endothelial cells, and infiltrating leukocytes. Plasma levels of MMP-1 were also increased in preeclamptic women. Interestingly, omental arteries collected from preeclamptic women showed no change in the expression of tissue inhibitor of metalloproteinases-1 (TIMP-1) and decreased expression of collagen type Iα 1 (COLIA1), which is the main component of type 1 collagen. Collectively, these observations suggest an imbalance in vascular collagen metabolism that favors degradation in preeclampsia. In addition, MMP-1 was a regulator of arterial vessel tone, implicating this metalloproteinase in the pathogenesis of systemic hypertension associated with preeclampsia.6
The expression level of several MMP genes, including MMPs 1, 2, 3, 7, 9, and 14, is regulated by DNA methylation,7 and MMP-9 has been shown to be regulated by DNA methylation in placentas of women with preeclampsia.8 DNA hypomethylation induced by 5-aza-2-deoxycytidine (5-Aza) treatment resulted in a significant increase in MMP-1 expression in cultured amnion fibroblasts.9 The increase in MMP-1 expression correlated with decreased DNA methylation at a particular location in the MMP1 promoter 1538 bp upstream from the transcription start site. DNA methylation at the same site was significantly reduced in amnion tissues collected from pregnancies with preterm premature rupture of the fetal membranes.9
This evidence for epigenetic regulation of MMP1, and other MMP genes, led us to hypothesize that reduced DNA methylation might contribute to the increased expression of MMPs in the systemic vasculature of preeclamptic women, leading to the imbalance in genes involved in collagen metabolism.6 In this study, we examined DNA methylation in the promoter regions of MMP, TIMP, and COL genes in omental arteries obtained from normal pregnant and preeclamptic women. We also studied the effect of DNA hypomethylation on the expression of MMP-1, TIMP-1, and COLIA1 in primary cultures of human VSMCs and the expression of MMP-1 and the neutrophil collagenase, MMP-8, in a neutrophil-like cell line.10 We examined the effect of hypomethylation in a neutrophil cell line because of the extensive vascular infiltration of neutrophils that occurs in women with preeclampsia.3–5
Materials and Methods
Study Subjects
Omental and s.c. fat samples of approximately 2 × 2 × 0.5 cm were obtained at cesarean section from normal pregnant women (n = 18) and women clinically diagnosed as having mild or severe preeclampsia (n = 21) at the Medical College of Virginia Hospital, Virginia Commonwealth University Medical Center (Richmond, VA). Mild preeclampsia was diagnosed when the blood pressure was >140 mmHg systolic or >90 mmHg diastolic and there was 0.3 g of urinary protein per 24 hours after 20 weeks of gestation. Severe preeclampsia was diagnosed when the blood pressure was >160 mmHg systolic or >110 mmHg diastolic, with 5 g of protein in the urine within 24 hours after 20 weeks of gestation.1 Omental fat arteries were used because they are a component of the maternal systemic vasculature, and they play a role in blood pressure regulation by contributing to the total peripheral vascular resistance. Women with chorioamnionitis, infections, active sexually transmitted diseases, lupus, or diabetes and women who were smokers or in labor were excluded because these conditions are associated with inflammatory changes. Patient clinical data are shown in Table 1. This study was approved by the Office of Research Subjects Protection, Virginia Commonwealth University. All subjects gave informed consent.
Table 1.
Clinical Characteristics of Patient Groups
| Variable | Normal pregnant women (n = 18) | Preeclamptic women (n = 21) |
|---|---|---|
| Maternal age (years) | 26.1 ± 1.3 | 24.6 ± 1.0 |
| Prepregnancy BMI (kg/m2) | 26.2 ± 1.2 | 30.7 ± 1.6⁎ |
| Systolic blood pressure (mmHg) | 117.1 ± 2.3 | 166.8 ± 2.4⁎⁎ |
| Diastolic blood pressure (mmHg) | 70.18 ± 1.8 | 93.5 ± 2.6⁎⁎ |
| Proteinuria (mg/24 hours) | ND | 286.8 ± 21.6 (n = 8) |
| Dipstick | ND | 2.7 ± 0.3 (n = 13) |
| Parity | ||
| Primiparous | 3 | 15 |
| Multiparous | 15 | 6 |
| Gestational age (weeks) | 39.2 ± 0.2 | 35.4 ± 0.9⁎⁎ |
| Infant birth weight (g) | 3503 ± 100 | 2452 ± 248⁎⁎ |
Values are presented as mean ± SEM unless otherwise indicated.
BMI, body mass index; ND, not determined.
P < 0.05,
P < 0.001 by t-test.
Sample Processing and Bisulfite Sequencing
Omental arteries from nine normal pregnant women and nine preeclamptic women (five mild and four severe) were dissected and cleaned of adhering fat. DNA was extracted from the arteries (approximately 10 mg by weight) using a QuickGene DNA tissue kit and a QuickGene-Mini80 system (AutoGen, Holliston, MA). DNA was treated with RNase A (Qiagen, Valencia, CA). DNA (1 μg) was bisulfite treated using a MethylEasy Exceed kit (Human Genetic Signatures, Randwick, Australia), and DNA sequencing was performed as previously described.9 Briefly, 100 ng of bisulfite-treated DNA was PCR amplified for two rounds using our previously published primers for the −1538 site in the MMP1 promoter. PCR products were purified with a QIAquick PCR Purification kit (Qiagen), and 1 μL of each product was cloned into pCR 2.1-TOPO vectors using the TOPO TA cloning kit (Invitrogen, Carlsbad, CA). After bacteria amplification, eight cloned PCR segments for each patient were sequenced in the Nucleic Acids Research Facilities at Virginia Commonwealth University. The −1538 site was selected because we previously demonstrated that its methylation correlates with the expression of MMP-1.9
Illumina Infinium HumanMethylation27 BeadChip Assay
Omental arteries from five normal pregnant and seven severe preeclamptic women were processed for DNA extraction. DNA was bisulfite treated and used in the high-throughput Illumina Infinium HumanMethylation27 BeadChip assay (Illumina, San Diego, CA) for global DNA methylation analysis. The BeadChip was run by the Nucleic Acids Research Facilities at Virginia Commonwealth University using the protocol provided by Illumina. Data analysis was performed using the beadarray package in the R programming environment.11 Methylation (β) values are expressed as a range from 0 to 1, where 0 means not methylated and 1 means fully methylated. Differences in methylation between normal pregnant and preeclamptic women were indicated by Δβ values.
IHC Data
Omental and s.c. fat samples from six normal pregnant and six preeclamptic women were cut into small pieces, fixed immediately in 10% neutral-buffered formalin, and embedded in paraffin. Tissues were cut into sections (8 μm thick), which were stained for rabbit anti-human polyclonal antibody specific for MMP-8, neutrophil collagenase (1:100 titer; catalog number 17874-1-AP; Proteintech, Chicago, IL), and mouse monoclonal IgM anti-human CD66b, a neutrophil antigen (1:50; catalog number 555723; BD BioSciences, San Diego, CA). Rabbit primary antibody isotype control (Invitrogen) was used as a negative control. A kit was used for immunohistochemical (IHC) staining [SuperPicTure Polymer Detection Kit Broad Spectrum (diaminobenzidine); Invitrogen]. To quench endogenous tissue peroxidase activity, slides were incubated in 3% hydrogen peroxide in methanol for 30 minutes. For antigen retrieval, slides were heat treated in 10 mmol/L citrate buffer for 5 minutes with a pressure cooker. Tissue slides were counterstained with a 1:5 dilution of Hematoxylin QS (Vector Laboratories, Burlingame, CA). Vessel staining for MMP-8 was evaluated using an intensity scale ranging from 0 to 4, where 0 was assigned for no staining and 4 was dark and extensive staining. Scoring was verified by a second investigator (S.W.W.) and by measuring the OD of staining using image analysis software (cellSens Imaging Software; Olympus America, Center Valley, PA), as previously described.3,4 The OD of staining for MMP-8 in vessels was normalized to the OD of the background. Slides were also analyzed for vessels with diffuse staining and leukocytes staining for MMP-8. Images were captured with cellSens Imaging Software.
Human VSMCs
Human VSMCs were cultured from chorionic plate arteries of placentas collected at cesarean section from healthy pregnant women at term deliveries, as previously described.12 Cells were cultured in T-25 flasks using Medium-199 (Gibco, Invitrogen) with 10% fetal bovine serum (Gibco, Invitrogen), 1% antibiotics, and antimycotics (100 U/mL penicillin, 100 μg/mL streptomycin, and 25 μg/mL amphotericin B; Gibco, Invitrogen). Cells were treated with 10 μmol/L of 5-Aza (Sigma-Aldrich, St. Louis, MO), an agent that inhibits DNA methylation when incorporated into DNA during cell division,13 for 48 hours. Treatments were refreshed daily. Cells were 50% confluent at treatment and nearly 100% confluent at harvesting. Conditioned media were collected and centrifuged to remove cell debris. Cells were rinsed in PBS and harvested for RNA or protein extraction.
HL-60 Cells
To examine the role of DNA methylation in regulating the expression of MMP-1 and MMP-8 in neutrophils, it was necessary to use a neutrophil-like cell line (HL-60) because neutrophils isolated from patients do not divide and, therefore, 5-Aza cannot be incorporated into the genomic DNA to induce hypomethylation. HL-60 cells (ATCC, Manassas, VA) were cultured in Iscove's modified Dulbecco's medium (ATCC) supplemented with 10% fetal bovine serum (Gibco, Invitrogen), 1% antibiotics, and antimycotics (100 U/mL penicillin, 100 μg/mL streptomycin, and 25 μg/mL amphotericin B; Gibco, Invitrogen), as recommended by ATCC. Approximately 500,000 cells/mL were seeded in 5 mL of media in a T-25 flask for treatments. Cell treatments were as follows: i) 10 μmol/L 5-Aza for 48 hours, followed by 10−8 mol/L of phorbol 12-myristate 13-acetate (PMA), a protein kinase C activator (Sigma-Aldrich) for 24 hours; ii) 10 μmol/L 5-Aza for 48 hours, followed by no treatment for 24 hours; iii) no treatment for 48 hours, followed by 10−8 mol/L PMA for 24 hours; or iv) no treatment for 72 hours to serve as a control. Cells were collected for RNA and protein extraction.
Quantitative Real-Time PCR
RNA was extracted using a QuickGene RNA cultured cell kit with a QuickGene Mini-80 system (AutoGen, Holliston, MA). DNase treatment was performed using a Turbo DNase kit (Ambion, Austin, TX). RNA (1 μg) was reverse transcribed to cDNA using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). Quantitative real-time PCRs were performed with RT2 SYBR Green qPCR Mastermix (SABiosciences, Frederick, MD), as recommended by the user manual, and 8 ng of cDNA was used per reaction. GAPDH was used as a housekeeping gene. Commercial primers were used for the MMP1 and MMP8 genes (SABiosciences). Primers for the GAPDH, TIMP1, and COLIA1 genes, shown in Table 2, were synthesized by Integrated DNA Technologies (Coralville, IA). Fold changes were calculated by the ΔΔCT method.
Table 2.
Quantitative RT-PCR Primers Used for Gene Expression
| Gene | Forward primer | Reverse primer |
|---|---|---|
| GAPDH | 5′-GATTCCACCCATGGCAAATT-3′ | 5′-AGATGGTGATGGGATTTCCATT-3′ |
| TIMP1 | 5′-CTGTGGCTCCCTGGAACA-3′ | 5′-CCAACAGTGTAGGTCTTGGTGAAG-3′ |
| COL1A1 | 5′-ACGAAGACATCCCACCAATCAC-3′ | 5′-CGTTGTCGCAGACGCAGAT-3′ |
Western Blot Analysis
Cells were lysed in radioimmunoprecipitation assay buffer containing 150 mmol/L sodium chloride, 1.0% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mmol/L Tris (pH 8.0), and one times Halt protease inhibitor (Thermo Scientific, Pittsburgh, PA) using a tip sonicator (Active Motif, Carlsbad, CA). Protein concentrations were measured using the bicinchoninic acid assay (Thermo Fisher Scientific, Rockford, IL). Denatured protein lysates (100 μg) were resolved by electrophoresis using 10% Tris-glycine SDS-PAGE electrotransferred to a polyvinylidene fluoride membrane (Immobilon-FL; Millipore, Billerica, MA). The primary antibodies used were as follows: goat anti-human MMP-1 (0.2 μg/mL; R&D Systems, Minneapolis, MN), goat anti-human COLIA1 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-human MMP-8 (1:250; Proteintech, Chicago, IL), rabbit anti-human β-actin (1:1000; Sigma-Aldrich), and mouse anti-human β-actin (1:1000; Sigma-Aldrich). Secondary antibodies used included Alexa Fluor 680 donkey anti-rabbit (1:20,000; Invitrogen) for the detection of primary antibodies raised in rabbit for β-actin or MMP-8; IRDye800 donkey anti-goat (1:20,000; Rockland Immunochemicals, Gilbertsville, PA) for the detection of primary antibodies raised in goat for MMP-1 or COLIA1; and IRDye800 goat anti-mouse (1:20,000; Rockland Immunochemicals) for the detection of primary antibody raised in mouse for β-actin. The LI-COR Odyssey Infrared Imaging System (Thermo Scientific, Pittsburgh) was used to detect the immunoreactive proteins.
ELISA
Commercial enzyme-linked immunosorbent assay (ELISA) kits were used to quantify total MMP-1 (GE Healthcare, Piscataway, NJ) and TIMP-1 (GE Healthcare) in VSMC culture medium. For normalization, the total amount of MMP-1 or TIMP-1 protein in 5 mL (volume of medium used in a T-25 flask) was divided by the total amount of DNA (μg) extracted from the cells of the same flask.
Statistical Analysis
The Mann-Whitney U-test was used to analyze the data from bisulfite sequencing and for visual intensity score data. A Student's t-test was used to make comparisons of parameters between two groups, and a one-way analysis of variance with a Newman-Keuls test was used to make comparisons of parameters between more than two groups for normally distributed data. Quantitative results were presented as mean ± SEM. We considered P < 0.05 as statistically significant. A statistical software program was used for data analysis (Prism 4 for Macintosh version 4.0a; GraphPad Software, Inc., San Diego, CA).
Results
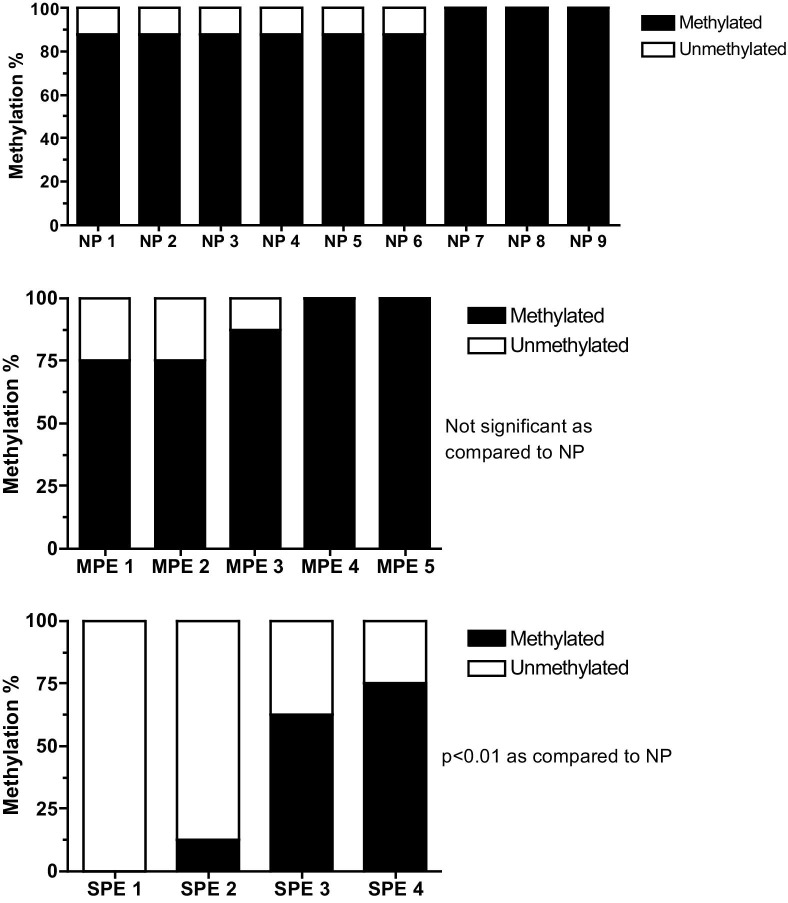
The Illumina BeadChip assay revealed a significant decrease in DNA methylation in the promoter regions of MMP1 and MMP8 genes in omental arteries from preeclamptic women compared with normal pregnant women. However, there were no significant changes in DNA methylation in the promoter regions of TIMP1 or COLIA1 genes. Other MMP, TIMP, and COL genes that were differentially methylated in preeclamptic arteries are shown in Table 3. All MMP genes, except two, were less methylated, whereas TIMP and COL genes were, in general, relatively more methylated. Bisulfite DNA sequencing revealed a significant decrease in methylation at the −1538 site in the promoter region of the MMP1 gene in omental arteries from women with severe preeclampsia compared with normal pregnant women (P < 0.01, Figure 1). Methylation at the same site was not significantly different in arteries from women with mild preeclampsia compared with arteries from normal pregnant women.
Table 3.
List of COL, MMP, and TIMP Genes that Were Significantly Differentially Methylated in Preeclamptic Omental Arteries
| Symbol | Name | Gene ID | Mean β⁎ |
Δβ⁎ | P value | |
|---|---|---|---|---|---|---|
| PE | NP | |||||
| COL8A1 | Collagen, type VIII, α 1 | 1295 | 0.078 | 0.054 | 0.024 | 0.02 |
| COL9A3 | Collagen, type IX, α 3 | 1299 | 0.536 | 0.480 | 0.056 | 0.03 |
| COL19A1 | Collagen, type XIX, α 1 | 1310 | 0.202 | 0.154 | 0.047 | 0.02 |
| MMP1 | Matrix metalloproteinase 1 | 4312 | 0.736 | 0.816 | −0.080 | 0.02 |
| MMP8 | Matrix metalloproteinase 8 | 4317 | 0.807 | 0.882 | −0.075 | 0.03 |
| MMP12 | Matrix metalloproteinase 12 | 4321 | 0.683 | 0.740 | −0.057 | 0.01 |
| MMP13 | Matrix metalloproteinase 13 | 4322 | 0.745 | 0.830 | −0.085 | 0.01 |
| MMP16 | Matrix metalloproteinase 16 | 4325 | 0.025 | 0.014 | 0.011 | 0.02 |
| MMP21 | Matrix metalloproteinase 21 | 118856 | 0.411 | 0.543 | −0.132 | 0.02 |
| MMP26 | Matrix metalloproteinase 26 | 56547 | 0.762 | 0.862 | −0.100 | 0.02 |
| MMP27 | Matrix metalloproteinase 27 | 64066 | 0.769 | 0.726 | 0.043 | 0.04 |
| TIMP3 | Tissue inhibitor of metalloproteinases 3 | 7078 | 0.437 | 0.338 | 0.098 | 0.02 |
NP, normal pregnant; PE, preeclamptic.
β indicates methylation values where 0 is no methylation and 1 is full methylation, and Δβ indicates the difference in methylation. Negative values indicate less methylation, and positive values indicate more methylation in PE arteries compared with NP arteries.
Figure 1.
Bisulfite sequencing of the −1538 site in the MMP1 promoter region in omental arteries from normal pregnant (NP), mild preeclamptic (MPE), and severe preeclamptic (SPE) women. Methylation of the −1538 site is significantly reduced in omental arteries from SPE women (P < 0.01), but not significantly different in omental arteries form MPE women compared with NP women.
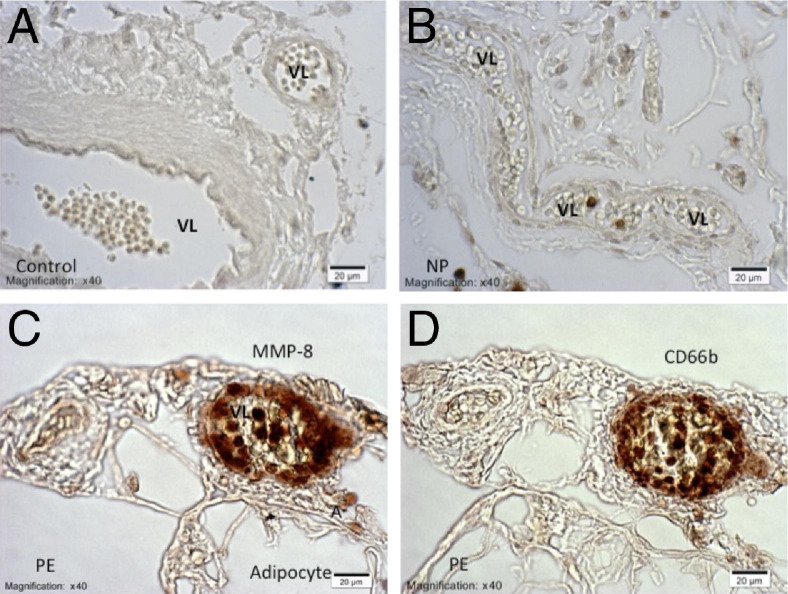
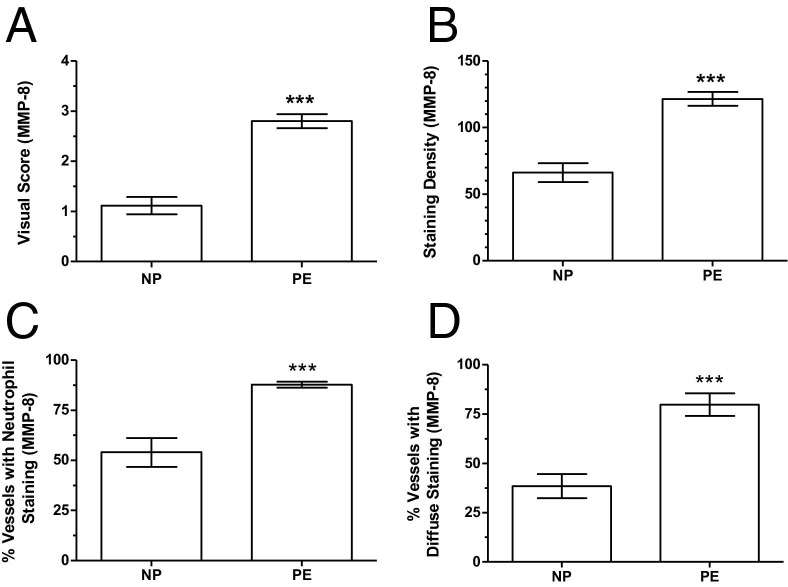
The IHC staining revealed increased expression of MMP-8 in omental and s.c. blood vessels of preeclamptic women compared with normal pregnant women (Figure 2, A–D), similar to what we previously showed for MMP-1.6 MMP-8 was mainly expressed in infiltrating neutrophils, as evidenced by similarity of staining for CD66b in serial sections, although diffuse staining was also observed in the vessels. Scoring for MMP-8 staining intensity was significantly higher in preeclamptic women compared with normal pregnant women (2.8 ± 0.1 versus 1.2 ± 0.2; P < 0.001; Figure 3A), as was the density of staining for MMP-8 (121.6 ± 5.2 versus 66.1 ± 7.0; P < 0.001; Figure 3B). Staining intensity scores and optical density of staining were highly correlated (r = 0.99). The percentage of vessels with neutrophils stained for MMP-8 was significantly higher in preeclamptic women compared with normal pregnant women (87.8% ± 1.5% versus 54.0% ± 7.2%; P < 0.001; Figure 3C). The percentage of vessels with diffuse staining for MMP-8 was also significantly higher in preeclamptic women compared with normal pregnant women (79.8% ± 5.7% versus 38.4% ± 6.1%; P < 0.001; Figure 3D).
Figure 2.
Representative sections of blood vessels in omental fat from normal pregnant (NP) and preeclamptic (PE) women immunostained for MMP-8 (A–C) and CD66b (D). Results for s.c. fat are similar. A: There is no brown staining for MMP-8 in negative control sections. B: Blood vessels of normal pregnant women show little or no staining for MMP-8. C and D: Vessels of preeclamptic women show significant brown staining for MMP-8 (C) and CD66b (D). Staining for MMP-8 in preeclamptic blood vessels is observed in neutrophils, which are in the lumen, adherent to the endothelium or infiltrated into the wall of the vessel. Diffused staining for MMP-8 is also present in the vessel wall. A, adipocyte; VL, vessel lumen. The magnification and scale bar are shown on each image.
Figure 3.
Quantitation of IHC staining for MMP-8 in omental and s.c. blood vessels from normal pregnant (NP; n = 5) and preeclamptic (PE; n = 5) women. A: The staining intensity score for MMP-8 is significantly higher in blood vessels of preeclamptic women compared with normal pregnant women. B: The OD of staining for MMP-8 is significantly higher in preeclamptic blood vessels. C and D: Preeclamptic women have a significantly higher percentage of blood vessels with neutrophils stained for MMP-8 and diffuse staining for MMP-8 compared with normal pregnant women. Data are presented as mean ± SEM. ***P < 0.001.
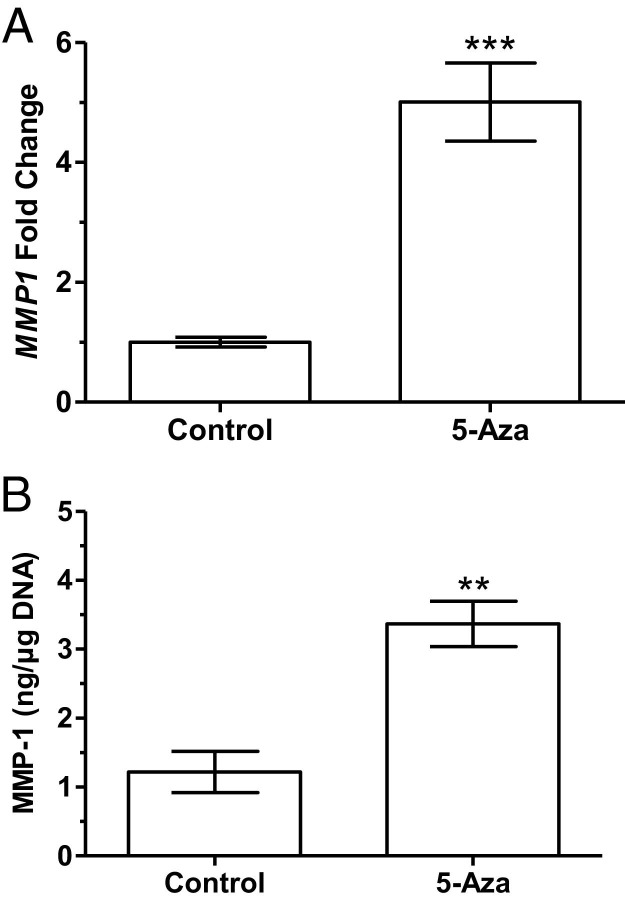
To determine whether DNA methylation could be involved in the regulation of MMP1 gene expression in VSMCs, 5-Aza was used to induce DNA hypomethylation in the cultured cells. Treatment with 5-Aza provoked a significant increase in MMP1 gene expression compared with control cells (5.0 ± 0.6-fold, P < 0.001, Figure 4A). To confirm that increased MMP1 gene expression was associated with an increase in protein production, ELISA was used to quantify MMP-1 secretion into the media. Treatment with 5-Aza significantly increased the production of MMP-1 protein (3.4 ± 0.3 versus 1.2 ± 0.3 ng/μg DNA; P < 0.01; Figure 4B). In contrast to MMP-1, 5-Aza treatment caused no significant change in gene expression of TIMP1 or COLIA1 in cultured VSMCs (Figure 5A) and did not affect protein levels of TIMP-1 in the media (Figure 5B) or COLIA1 content in cells (Figure 5C).
Figure 4.
MMP-1 expression in cultured human VSMCs treated with 5-Aza for 48 hours. A: Fold change in gene expression for MMP1 is significantly increased in treated cultured VSMCs compared with controls (P < 0.001, n = 8). B: Levels of MMP-1 protein in media are significantly increased in treated cells compared with controls (P < 0.01, n = 4). Data are shown as mean ± SEM. **P < 0.01, ***P < 0.001.
Figure 5.
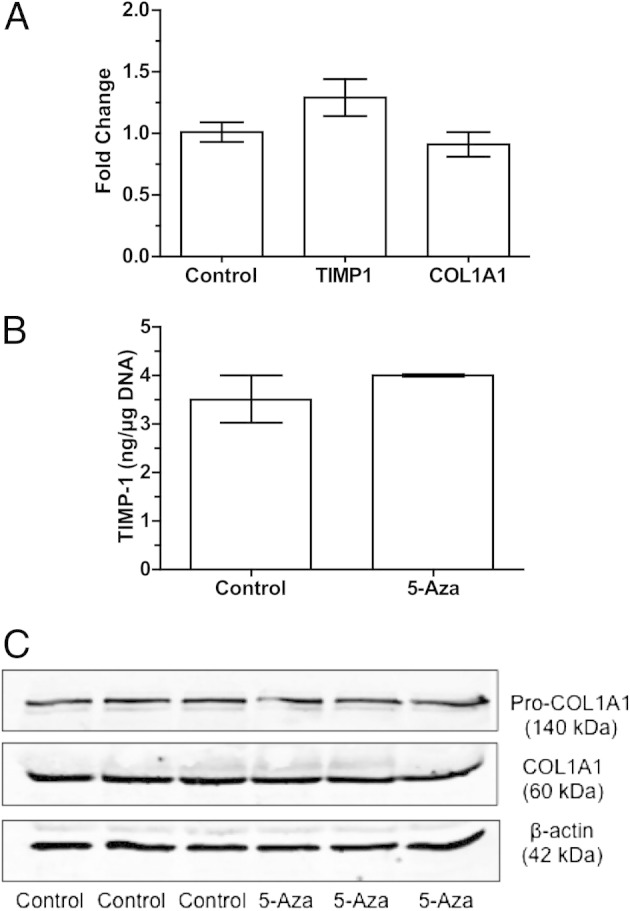
TIMP-1 and COLIA1 expression in cultured human VSMCs treated with 5-Aza for 48 hours. A: The fold change in the expression for TIMP1 and COLIA1 is not significantly changed by 5-Aza treatment compared with control (n = 8). B: TIMP-1 protein levels in media are not significantly changed compared with controls (n = 4). C: Western blot analysis for pro-COLIA1, and COLIA1 demonstrates no effect of 5-Aza treatment on the protein expression of COLIA1 (n = 3). Data are shown as mean ± SEM.
The effect of reduced DNA methylation on the expression of MMP-1 was also examined in a neutrophil-like cell line, HL-60 cells. The expression of MMP-1 in untreated HL-60 cells was low, but treatment with 5-Aza resulted in a significant increase in MMP1 gene expression compared with controls (62.4 ± 6.8-fold, P < 0.001). PMA treatment also resulted in a significant increase in MMP1 gene expression (19.6 ± 3.2-fold, P < 0.001). Treatment with PMA caused activation of the cells, as evidenced by cell adhesion to the culture flask surface. Combined treatment with 5-Aza and PMA resulted in a dramatic increase in MMP1 gene expression compared with controls (2622 ± 167-fold, P < 0.001), PMA alone (P < 0.001), or 5-Aza alone (P < 0.001) (Figure 6A). The gene expression profiles resulting from 5-Aza treatments correlated with protein expression, as indicated by using Western blot analysis (Figure 6, B and C). Treatment with 5-Aza significantly increased MMP-1 protein expression (164% ± 6% average density measurement of the Western blot compared with control, P < 0.001). PMA alone caused no significant change in MMP-1 protein expression compared with controls. However, combining 5-Aza with PMA significantly increased MMP-1 protein expression (261% ± 6% of control, P < 0.001), PMA alone (P < 0.001), or 5-Aza alone (P < 0.001).
Figure 6.
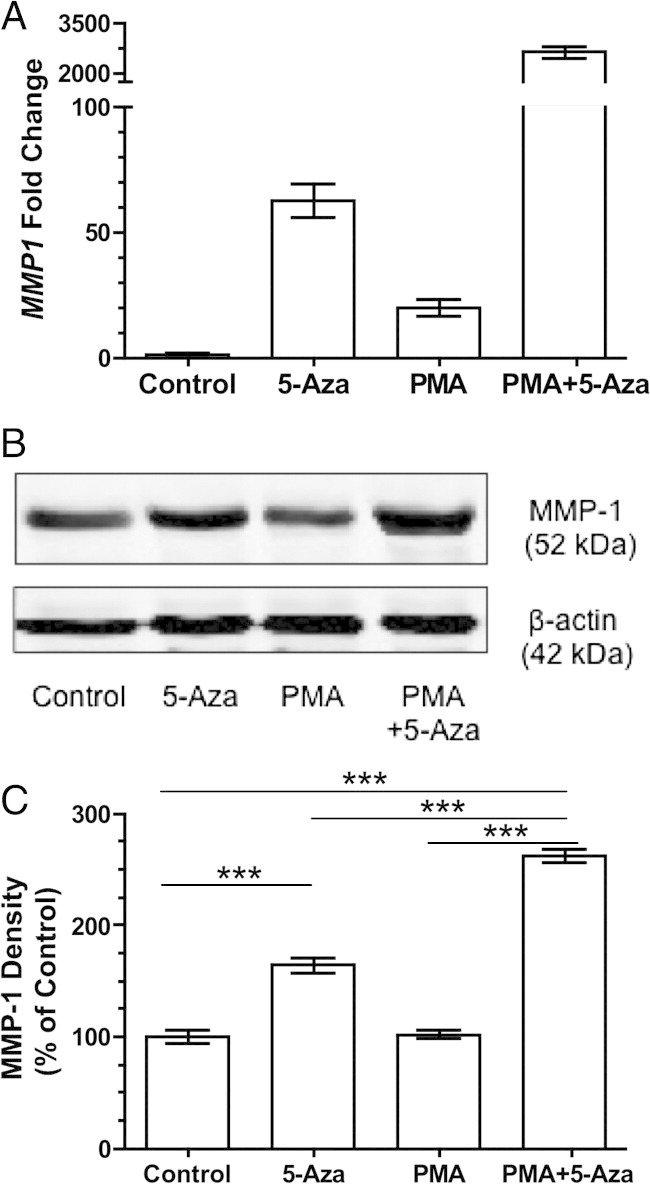
MMP-1 expression in HL-60 cells treated with 5-Aza for 48 hours, PMA for 24 hours, or 5-Aza for 48 hours, and then with PMA for 24 hours. A: The expression of the MMP1 gene is significantly increased in treated cells compared with control cells. Treatment with PMA after 5-Aza causes a remarkable increase in MMP1 gene expression compared with control, PMA alone, or 5-Aza alone (n = 8). P < 0.001 between all groups. Western blot analysis (B) and density measurements (C) of the Western blot showing treatment with 5-Aza significantly increased the expression of MMP-1 protein in HL-60 cells compared with control. Activation with PMA after treatment with 5-Aza results in a greater increase in MMP-1 expression compared with control, 5-Aza alone, or PMA alone (n = 4). Data are shown as mean ± SEM. ***P < 0.001.
The role of DNA methylation in the expression of MMP-8 was also examined in the neutrophil-like cell line. Treatment with 5-Aza caused a significant increase in MMP8 gene expression compared with the control (3.2 ± 0.2-fold, P < 0.001). In contrast to MMP1, PMA treatment down-regulated MMP8 gene expression, whereas combined treatment of PMA and 5-Aza partially restored MMP8 gene expression (Figure 7A). The changes in gene expression were associated with alterations in protein production, as confirmed by using Western blot analysis (Figure 7, B and C).
Figure 7.
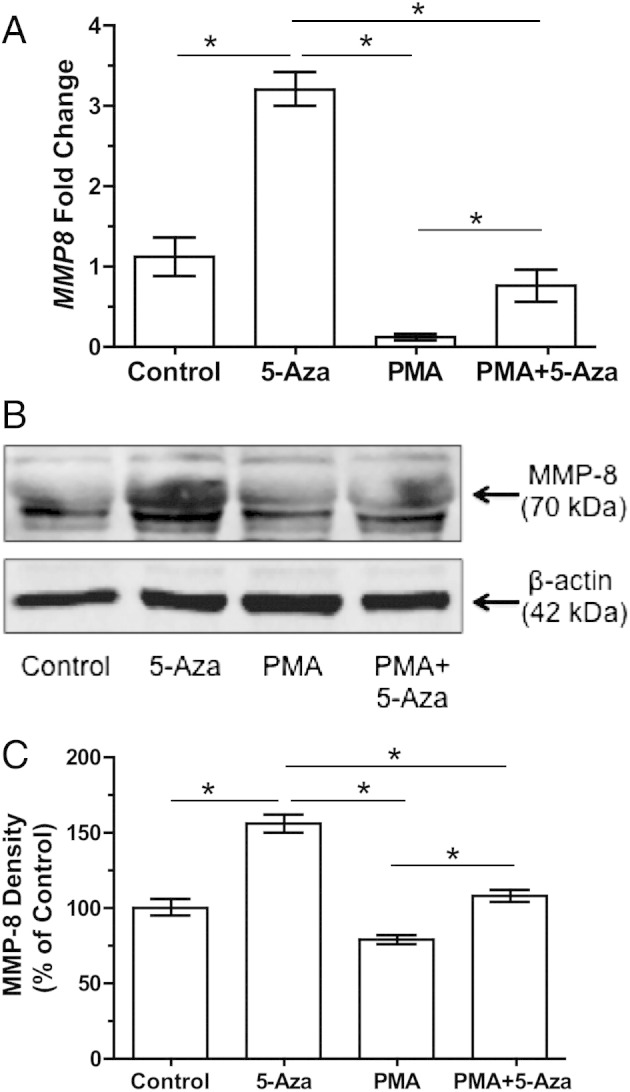
MMP-8 expression in HL-60 cells treated with 5-Aza for 48 hours, PMA for 24 hours, or 5-Aza for 48 hours and then with PMA for 24 hours. A: The fold change in the expression for MMP8 is significantly increased in 5-Aza–treated cells compared with control. PMA down-regulates MMP8 expression, but 5-Aza partially restores it (n = 8). Western blot analysis (B) and density measurements (C) of the Western blot showing 5-Aza significantly increases the expression of MMP-8. PMA down-regulates MMP-8 expression, but 5-Aza restores it (n = 5). More than one mol. wt. band is present for MMP-8 because it has multiple glycosylation sites. Data are shown as mean ± SEM. *P < 0.05.
Discussion
In our previous study,6 we demonstrated an imbalance in collagen-regulating genes, with increased expression of MMP-1 in VSMCs and infiltrating neutrophils of omental arteries of preeclamptic women. In this study, we explored the possible contributions of epigenetic regulation to this imbalance. We found that the promoter regions of the MMP1 and MMP8 genes had significantly reduced methylation in preeclamptic omental arteries compared with normal pregnant arteries, but there was no significant difference in the methylation status for TIMP1 or COLIA1. In a global assessment of methylation status for these gene families, we found reduced methylation in several of the MMP genes, whereas increased methylation, or no significant change, was present for TIMP and COL genes. The reason some genes are regulated by DNA methylation and others are not is not known, but it may have to do with their location. Five of the hypomethylated MMP genes in our study were located on chromosome 11, with four of them at the same position at 11q22.3. Perhaps certain areas of the chromosome are more susceptible to factors that can affect DNA methylation, such as oxidative stress.
In addition to global methylation assessment, we also examined a specific site (−1538) in the MMP1 promoter that we previously showed was hypomethylated in the amnion of pregnancies complicated by preterm premature rupture of membranes.9 DNA sequencing of that site demonstrated that it was significantly less methylated in omental arteries of women with severe preeclampsia, but not those with mild preeclampsia, compared with normal pregnant women. This may suggest a relationship between the severity of the preeclampsia and decreased DNA methylation. These studies demonstrate that, in both preterm premature rupture of membranes and preeclampsia, reduced DNA methylation is associated with increased expression of MMP-1 in tissues, specific to their pathological features.
Similar to the increased expression of MMP-1 we previously reported,6 the expression of MMP-8 was also increased in omental and s.c. blood vessels of preeclamptic women compared with normal pregnant women. MMP-8 was mainly expressed in neutrophils, but diffuse staining for MMP-8 was also present in the vessels, which likely represents MMP-8 secreted by the neutrophils.
Although our study was designed as an association study between DNA methylation and gene expression, enzyme activity is an important consideration. There is considerable evidence that increased levels of MMP-1 and MMP-8 in preeclamptic women represent active enzymes. We previously demonstrated active MMP-1 in lysates of omental arteries of preeclamptic women, but not normal pregnant women.6 In this study, diffuse staining of MMP-8 in endothelium and vascular smooth muscle likely represents active MMP-8 because reactive oxygen species released by activated neutrophils mediate conversion of pro-MMP-8 to the active form.14,15 We also previously reported that plasma of preeclamptic women contains significant amounts of active MMP-1, whereas plasma of normal pregnant women does not.6 Activation of MMPs is most likely related to the oxidative stress of preeclampsia16,17 and the release of reactive oxygen species by infiltrating neutrophils because reactive oxygen species are known activators of MMPs.14,15,18–20
To study the role of DNA methylation in the regulation of these genes in vascular tissue, we experimentally induced DNA hypomethylation in cultured human VSMCs. Hypomethylation resulted in a significant increase in MMP-1 expression but did not significantly affect TIMP-1 or COLIA1, which is similar to our findings for omental arteries of preeclamptic women.6 TIMP and COL genes are apparently regulated by mechanisms other than DNA methylation. Allelic variations in the COLIA1 gene have been associated with preeclampsia,21 which may explain its reduced expression in omental arteries of preeclamptic women.6
We also examined the effect of hypomethylation on MMP-1 and MMP-8 expression in a neutrophil cell line because neutrophils infiltrate maternal systemic blood vessels in preeclampsia and strongly express these MMPs. We found that DNA hypomethylation significantly increased both MMP-1 and MMP-8 expression in HL-60 cells.
Stimulation of HL-60 cells with PMA, a classic inducer of leukocyte activation, resulted in a significant increase in MMP-1, but not MMP-8, suggesting that MMP-1 and MMP-8 are differentially regulated in these cells. This is supported by the fact that when DNA hypomethylation was combined with PMA, there was a dramatic increase in the expression of MMP-1, but not MMP-8. On the other hand, results from 5-Aza treatment suggest that decreased DNA methylation in neutrophils contributes to the increased expression of both MMP-1 and MMP-8 in maternal systemic blood vessels of preeclamptic women by increasing the capacity of infiltrating neutrophils to produce these MMPs. A global decrease in DNA methylation in leukocytes was reported in other diseases involving the cardiovascular system, including atherosclerosis,22 ischemic heart disease, and stroke.23 Untreated HL-60 cells showed low expression of MMP-1, which is similar to unactivated neutrophils. This suggests that MMP-1 expression in unactivated cells is largely silenced by DNA methylation, and that reduced DNA methylation contributes to increased expression of MMP-1 in infiltrating neutrophils in preeclamptic women.
The imbalance in collagen-regulating proteins in preeclamptic vessels suggests increased collagenolytic activity, which could contribute to the development of edema and proteinuria by compromising the structural integrity of the vessels and increasing their permeability to blood proteins.
Increased expression of MMP-1 may contribute to the pathophysiological characteristics of preeclampsia in another, and unexpected, way. We found that activated MMP-1 is a potent vasoconstrictor acting via protease-activated receptor-1, which is overexpressed in omental arteries of preeclamptic women.6 We subsequently showed that activation of protease-activated receptor-1 resulted in release of endothelial endothelin-1, which mediated the vasoconstrictor effects of MMP-1.24 Important to this finding was that plasma levels of activated MMP-1 in preeclamptic women were within the dose-response range for vasoconstriction, whereas levels in normal pregnant women were not. Therefore, increased MMP-1 levels in blood vessels and plasma of preeclamptic women may play an important, but previously unrecognized, role in regulating vascular tone. It may seem counterintuitive that vessels with less collagen should have an increase in tone, but the function of collagen as a structural protein to maintain vessel integrity is independent of the function of vascular smooth muscle to control vessel tone. Because collagen is not a contractile protein, and imparts stiffness to vessels, the vessels may contract more extensively with less collagen present. Other investigators have shown that MMP-2 can cause vasoconstriction by activating vasoconstrictor peptides.25–27
Our study has several limitations in that our findings are correlative and do not prove a cause-and-effect relationship between reduced methylation and increased expression of MMP-1 and MMP-8. We also cannot prove that reduced methylation of the MMP1 or MMP8 gene promoters per se was responsible for increased expression, because methylation changes may have indirectly altered gene expression (eg, resulting in increased expression of critical transcription factors). In addition, we were not able to determine the cell types in which methylation changes were occurring in the omental arteries because of the cellular heterogeneity, which includes endothelial cells, VSMCs, and infiltrated neutrophils. However, by experimentally inducing hypomethylation in VSMCs and a neutrophil-like cell line, we were able to document that reduced methylation is strongly associated with increased expression of MMPs in both cell types.
In summary, we have shown that the promoter regions of the MMP1 and MMP8 genes have reduced methylation in omental arteries of preeclamptic women compared with those of normal pregnant women, whereas the promoter regions of the TIMP and COL genes tend to be more methylated or unchanged. By experimentally inducing DNA hypomethylation in cultured human VSMCs and a neutrophil-like cell line, we were able to mimic the imbalance in collagen-regulating genes present in systemic blood vessels of preeclamptic women. These findings suggest the possibility that epigenetic mechanisms directly involving the promoters of the target collagen metabolism genes, or possibly genes that regulate their expression (eg, transcription factors), play an important role in the vascular dysfunction associated with preeclampsia.
Our study may have clinical implications for the treatment or prevention of preeclampsia based on restoring methylation. Folate is an essential component in the folate-methionine cycle that synthesizes methyl donors used by methyltransferases to methylate DNA. Therefore, dietary supplementation with folate might restore reduced methylation in MMP genes and protect against the development of preeclampsia. Consistent with this notion is a recent large study composed of almost 3000 pregnant women, which found supplementation with multivitamins containing folic acid was associated with a reduced risk of preeclampsia.28
Acknowledgments
We thank the nurses, residents, and attending physicians of labor and delivery at Medical College of Virginia Hospital for their help in obtaining the omental fat samples and Sonya L. Washington for her participation in recruiting patients, obtaining the samples, maintaining cell cultures, and providing technical assistance.
Footnotes
Supported, in part, by grants from the National Heart, Lung, and Blood Institute (RO1 HL069851 to S.W.W.); the National Center on Minority Health and Health Disparities (P60 MD002256 to J.F.S.); the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, Department of Health and Human Services (N01 HD-2-3342 to J.F.S.); and NIH Fogarty grant (1D43 TW007692 to G.E.-G.). DNA sequencing and methylation analysis were performed at the Virginia Commonwealth University Nucleic Acids Research Facility core laboratory, which is supported, in part, by funding from NIH–National Cancer Institute Cancer Center Support Grant (P30 CA016059), and the National Center for Advancing Translational Sciences (CTSA award No. UL1TR000058).
References
- 1.Cunningham F.G., Leveno K.J., Bloom S.L., Gilstrap L.C., III, Hauth J.C., Wenstrom K.D. McGraw-Hill; New York: 2005. Williams Obstetrics. [Google Scholar]
- 2.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 3.Shah T.J., Walsh S.W. Activation of NF-kappaB and expression of COX-2 in association with neutrophil infiltration in systemic vascular tissue of women with preeclampsia. Am J Obstet Gynecol. 2007;196:48.e1–48.e8. doi: 10.1016/j.ajog.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 4.Leik C.E., Walsh S.W. Neutrophils infiltrate resistance-sized vessels of subcutaneous fat in women with preeclampsia. Hypertension. 2004;44:72–77. doi: 10.1161/01.HYP.0000130483.83154.37. [DOI] [PubMed] [Google Scholar]
- 5.Mishra N., Nugent W.H., Mahavadi S., Walsh S.W. Mechanisms of enhanced vascular reactivity in preeclampsia. Hypertension. 2011;58:867–873. doi: 10.1161/HYPERTENSIONAHA.111.176602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estrada-Gutierrez G., Cappello R.E., Mishra N., Romero R., Strauss J.F., 3rd, Walsh S.W. Increased expression of matrix metalloproteinase-1 in systemic vessels of preeclamptic women: a critical mediator of vascular dysfunction. Am J Pathol. 2011;178:451–460. doi: 10.1016/j.ajpath.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato N., Maehara N., Su G.H., Goggins M. Effects of 5-aza-2′-deoxycytidine on matrix metalloproteinase expression and pancreatic cancer cell invasiveness. J Natl Cancer Inst. 2003;95:327–330. doi: 10.1093/jnci/95.4.327. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z., Lu S., Liu C., Zhao B., Pei K., Tian L., Ma X. Expressional and epigenetic alterations of placental matrix metalloproteinase 9 in preeclampsia. Gynecol Endocrinol. 2009;26:96–102. doi: 10.3109/09513590903184100. [DOI] [PubMed] [Google Scholar]
- 9.Wang H., Ogawa M., Wood J.R., Bartolomei M.S., Sammel M.D., Kusanovic J.P., Walsh S.W., Romero R., Strauss J.F., 3rd Genetic and epigenetic mechanisms combine to control MMP1 expression and its association with preterm premature rupture of membranes. Hum Mol Genet. 2008;17:1087–1096. doi: 10.1093/hmg/ddm381. [DOI] [PubMed] [Google Scholar]
- 10.Gallagher R., Collins S., Trujillo J., McCredie K., Ahearn M., Tsai S., Metzgar R., Aulakh G., Ting R., Ruscetti F., Gallo R. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979;54:713–733. [PubMed] [Google Scholar]
- 11.Dunning M.J., Smith M.L., Ritchie M.E., Tavaré S. beadarray: R classes and methods for Illumina bead-based data. Bioinformatics. 2007;23:2183–2184. doi: 10.1093/bioinformatics/btm311. [DOI] [PubMed] [Google Scholar]
- 12.Leik C.E., Willey A., Graham M.F., Walsh S.W. Isolation and culture of arterial smooth muscle cells from human placenta. Hypertension. 2004;43:837–840. doi: 10.1161/01.HYP.0000119191.33112.9c. [DOI] [PubMed] [Google Scholar]
- 13.Kendrew J.C. Blackwell Science; Cambridge: 1994. The Encyclopedia of Molecular Biology. [Google Scholar]
- 14.Okamoto T., Akaike T., Nagano T., Miyajima S., Suga M., Ando M., Ichimori K., Maeda H. Activation of human neutrophil procollagenase by nitrogen dioxide and peroxynitrite: a novel mechanism for procollagenase activation involving nitric oxide. Arch Biochem Biophys. 1997;342:261–274. doi: 10.1006/abbi.1997.0127. [DOI] [PubMed] [Google Scholar]
- 15.Van Lint P., Libert C. Matrix metalloproteinase-8: cleavage can be decisive. Cytokine Growth Factor Rev. 2006;17:217–223. doi: 10.1016/j.cytogfr.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Hubel C.A. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med. 1999;222:222–235. doi: 10.1177/153537029922200305. [DOI] [PubMed] [Google Scholar]
- 17.Walsh S.W. Maternal-placental interactions of oxidative stress and antioxidants in preeclampsia. Semin Reprod Endocrinol. 1998;16:93–104. doi: 10.1055/s-2007-1016256. [DOI] [PubMed] [Google Scholar]
- 18.Galis Z.S., Khatri J.J. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- 19.Rajagopalan S., Meng X.P., Ramasamy S., Harrison D.G., Galis Z.S. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro: implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulz R. Intracellular targets of matrix metalloproteinase-2 in cardiac disease: rationale and therapeutic approaches. Annu Rev Pharmacol Toxicol. 2007;47:211–242. doi: 10.1146/annurev.pharmtox.47.120505.105230. [DOI] [PubMed] [Google Scholar]
- 21.Goddard K.A., Tromp G., Romero R., Olson J.M., Lu Q., Xu Z., Parimi N., Nien J.K., Gomez R., Behnke E., Solari M., Espinoza J., Santolaya J., Chaiworapongsa T., Lenk G.M., Volkenant K., Anant M.K., Salisbury B.A., Carr J., Lee M.S., Vovis G.F., Kuivaniemi H. Candidate-gene association study of mothers with pre-eclampsia, and their infants, analyzing 775 SNPs in 190 genes. Hum Hered. 2007;63:1–16. doi: 10.1159/000097926. [DOI] [PubMed] [Google Scholar]
- 22.Castro R., Rivera I., Struys E.A., Jansen E.E., Ravasco P., Camilo M.E., Blom H.J., Jakobs C., Tavares de Almeida I. Increased homocysteine and S-adenosylhomocysteine concentrations and DNA hypomethylation in vascular disease. Clin Chem. 2003;49:1292–1296. doi: 10.1373/49.8.1292. [DOI] [PubMed] [Google Scholar]
- 23.Baccarelli A., Wright R., Bollati V., Litonjua A., Zanobetti A., Tarantini L., Sparrow D., Vokonas P., Schwartz J. Ischemic heart disease and stroke in relation to blood DNA methylation. Epidemiology. 2010;21:819–828. doi: 10.1097/EDE.0b013e3181f20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra N.V., Strauss J.F., III, Walsh S.W. Matrix metalloproteinase-1 causes dose dependent vasoconstriction and enhances vessel reactivity to angiotensin II via protease activated receptor-1 and endothelin-1. Reprod Sci (Suppl) 2010;17:126A–127A. doi: 10.1177/1933719115607998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez-Patron C., Radomski M.W., Davidge S.T. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res. 1999;85:906–911. doi: 10.1161/01.res.85.10.906. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Patron C., Stewart K.G., Zhang Y., Koivunen E., Radomski M.W., Davidge S.T. Vascular matrix metalloproteinase-2-dependent cleavage of calcitonin gene-related peptide promotes vasoconstriction. Circ Res. 2000;87:670–676. doi: 10.1161/01.res.87.8.670. [DOI] [PubMed] [Google Scholar]
- 27.Martinez A., Oh H.R., Unsworth E.J., Bregonzio C., Saavedra J.M., Stetler-Stevenson W.G., Cuttitta F. Matrix metalloproteinase-2 cleavage of adrenomedullin produces a vasoconstrictor out of a vasodilator. Biochem J. 2004;383:413–418. doi: 10.1042/BJ20040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen S.W., Chen X.K., Rodger M., White R.R., Yang Q., Smith G.N., Sigal R.J., Perkins S.L., Walker M.C. Folic acid supplementation in early second trimester and the risk of preeclampsia. Am J Obstet Gynecol. 2008;198:45.e1–45.e7. doi: 10.1016/j.ajog.2007.06.067. [DOI] [PubMed] [Google Scholar]