Abstract
Glioblastomas (GBMs), the most common primary brain tumor in adults, are characterized by resistance to chemotherapy and radiotherapy. One of the defining characteristics of GBM is an abundant and aberrant vasculature. The processes of vascular co-option, angiogenesis, and vasculogenesis in gliomas have been extensively described. Recently, however, it has become clear that these three processes are not the only mechanisms by which neovascularization occurs in gliomas. Furthermore, it seems that these processes interact extensively, with potential overlap among them. At least five mechanisms by which gliomas achieve neovascularization have been described: vascular co-option, angiogenesis, vasculogenesis, vascular mimicry, and (the most recently described) glioblastoma-endothelial cell transdifferentiation. We review these mechanisms in glioma neovascularization, with a particular emphasis on the roles of hypoxia and glioma stem cells in each process. Although some of these processes are well established, others have been identified only recently and will need to be further investigated for complete validation. We also review strategies to target glioma neovascularization and the development of resistance to these therapeutic strategies. Finally, we describe how these complex processes interlink and overlap. A thorough understanding of the contributing molecular processes that control the five modalities reviewed here should help resolve the treatment resistance that characterizes GBMs.
Glioblastomas (GBMs) are the most common and aggressive primary brain tumors in adults, with a median survival of only 14 months despite the best available treatments. GBMs are characterized by their resistance to radiotherapy and chemotherapy, as well as their abundant and aberrant vasculature. Neovascularization has long been implicated as a salient feature of glioma progression. In fact, high-grade gliomas are among the most vascular of all solid tumors, and vascular proliferation is a pathological hallmark of GBMs.1 Tumor progression and resistance to both radiotherapy and chemotherapy lead to unfavorable clinical outcomes in glioma patients and are associated with the hypoxic tumor microenvironment known to exist within GBMs. Also contributing to resistance to traditional therapeutics are glioma stem cells (GSCs), which contain tumor-initiating functions and are thought to be responsible for replenishing and sustaining the glioma mass and promoting resistance to traditional cancer therapies. An increasing body of experimental evidence suggests that hypoxia and the hypoxia-inducible factors (HIFs) play a critical role in maintaining the stem-like fraction in gliomas by creating a microenvironment that provides the essential cellular interactions and environmental signals needed to prevent GSC differentiation and to support their survival and self-renewal.2 Although hypoxia is a well-known driver of neovascularization, there is also evidence demonstrating that non-hypoxia-driven mechanisms exist, including p53 and hypoxia-independent vascular endothelial growth factor (VEGF)-mediated pathways.
Glioma cells are able to sense and adapt to hypoxic environments. HIF-1 is a heterodimeric nuclear transcription factor3 that consists of two subunits, HIF-1α and HIF-1β. The HIF-1α subunit determines HIF-1 activity in response to changes in local O2 levels. Under normoxic conditions, the α subunit is rapidly degraded; under hypoxic conditions, however, this subunit remains intact and binds to the constitutively expressed β subunit to form HIF-1 in the cell nucleus, where it induces expression of many genes under the regulation of hypoxia response elements. This process triggers the up-regulation of multiple proangiogenic factors, the most studied and prominent of which is VEGF. The resulting migration and proliferation of endothelial cells are key events in the angiogenic cascade.
Most studies have focused on the HIF-1α subunit, and less is known about the role of HIF-2α in tumor progression. Several studies have shown that HIF-2 may be involved in maintenance of GSCs. Both HIF-1α and HIF-2α are necessary for GSC maintenance. Furthermore, overexpression of HIF-2α promotes a cancer stem-cell like phenotype in preclinical models of GBM.4
Originally described simply as capillary sprouting from pre-existing host tissue capillaries (ie, angiogenesis), the process by which solid growing tumors generate an increasing blood supply to meet their ever-increasing nutrient and oxygen demand is now recognized as a highly complex spectrum of events. At least five distinct mechanisms of neovascularization in GBMs have been identified: i) vascular co-option, ii) angiogenesis, iii) vasculogenesis, iv) vascular mimicry, and v) glioblastoma-endothelial cell transdifferentiation. These mechanisms are not independent of one another, but rather are interlinked and are controlled, at least in part, by similar processes. Here, we review the evidence for and potential molecular mechanisms of each of these processes and discuss the experimental data for the roles of hypoxia and stem cells in each of the five mechanisms. In addition, we review the rationale for the targeting of neovascularization in gliomas (eg, antiangiogenic therapeutic strategies) and discuss the potential molecular mechanisms that could explain escape from antiangiogenic therapy. Finally, we describe how these complex mechanisms of vascularization might be interlinked. Although we focus our review on neovascularization in gliomas, there is evidence that many of these processes occur in a broad range of malignancies.
Our objective here is to provide a thorough and comprehensive review of the mechanisms of glioma-associated neovascularization. We discuss processes that have now become widely accepted by the scientific community, such as vascular co-option, angiogenesis, and vasculogenesis. We also describe vascular mimicry and transdifferentiation, processes that are only beginning to be explored. Because they have been identified only rather recently, the vascular mimicry and transdifferentiation processes will require confirmation and validation before they can be widely accepted. Furthermore, the contribution of these two mechanisms to the process of neovascularization on a whole-tumor scale may vary considerably among tumor types and for now remains largely unknown.
Vascular Co-Option
Temporally, vascular co-option is the first mechanism by which gliomas achieve their vasculature (Figure 1). This process involves organization of tumor cells into cuffs around normal microvessels. Holash et al5 were the first to definitively demonstrate vessel co-option, using a rat C6 glioma model. Early tumors were well vascularized through vessel co-option, and it was not until approximately 4 weeks after implantation that, after vascular regression, a robust angiogenic response was seen at the viable tumor periphery. In the interim, the majority of tumor vasculature was co-opted from normal brain vasculature. Co-opted vessels have been shown to express angiopoietin-2 (ANG-2).5,6
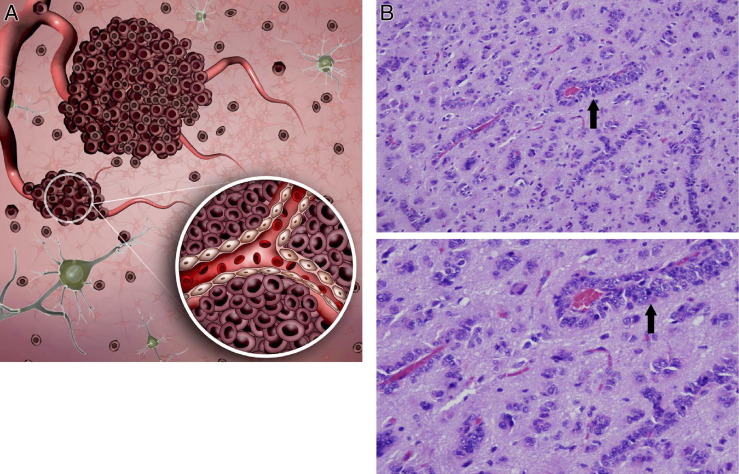
Figure 1.
Vascular co-option. A: Temporally, vascular co-option is the first process by which gliomas attain a vascular supply. The process involves organization of tumor cells into cuffs around normal microvessels (inset). Vascular co-option has been shown to precede angiogenesis in tumor models by up to 4 weeks. B: Photomicrographs of tumor cells in a sectioned human GBM specimen stained with H&E show vascular co-option as classic perivascular cuffs. Original magnification: ×50 (top); ×200 (bottom).
The angiopoietins constitute a family of factors that bind competitively on TIE-2. ANG-2 functions mainly as an antagonist of ANG-1, but both pro- and antiangiogenic functions have been described for both ANG-1 and ANG-2. Although the situation is more complex, it is thought that ANG-1 acts predominantly in pericyte recruitment and maintenance of vessel integrity and that up-regulation of ANG-2 expression leads to vessel destabilization. At this stage, and in the presence of VEGF, angiogenic vessel sprouting occurs. In the absence of VEGF, however, ANG-2 promotes endothelial cell apoptosis and vessel regression.5,7
A temporal study of experimental glioma vascularization identified vascular co-option as an initial step of a cascade of events in implanted murine GL261 gliomas.6 As early as 1 week after glioma cell implantation, vascular co-option was observed, with endothelial cell apoptosis appearing by week 3, resulting in vascular regression and regions of necrosis followed by angiogenesis. Involution of co-opted vessels resulted in tumor hypoxia, up-regulation of proangiogenic factors, and a shift toward an angiogenic phenotype. Rong et al8 reviewed pseudopalisading necrosis in GBM and described a similar sequence of events in human GBM.
Using in vivo multiphoton laser scanning microscopy and the same GL261 murine glioma cell line, Winkler et al9 described the invading potential of glioma cells when in close contact with brain microvessels. At the invasive border of the main tumor, vascularization occurred via co-option of pre-existing brain vessels, rather than by angiogenesis.
Possible molecular links between hypoxia and vascular co-option include the up-regulation of ANG-2 by hypoxia through HIF-1–dependent mechanisms and the presence of a HIF-1 binding hypoxia response element location identified in the first intron of the ANG-2 gene (ANGPT2).10 In addition, it has been shown that conditioned medium collected from neoplastic cells exposed to hypoxia promotes vascular co-option.11
Montana and Sontheimer12 recently described a potential role for bradykinin in chemotaxis during vascular co-option in primary brain tumors. In glioma biopsy specimens, they demonstrated increased expression of bradykinin receptors in regions of tumor, with the highest levels in perivascular regions. Using in vitro assays, they also demonstrated increased glioma cell motility and migration/invasion in both Transwell and brain slice invasion assays in response to bradykinin, which was mediated by bradykinin-induced Ca2+ oscillations.
Angiogenesis
Vascular co-option is followed by the development of new vessels from pre-existing ones (Figure 2), a process known as angiogenesis. This mechanism is integral to both physiological and pathological processes. Angiogenesis was described in GBM as early as 1976, when Brem13 observed intense neovascularization in rabbit corneas transplanted with GBMs, suggesting an in vivo production of a “vasoformative substance.”
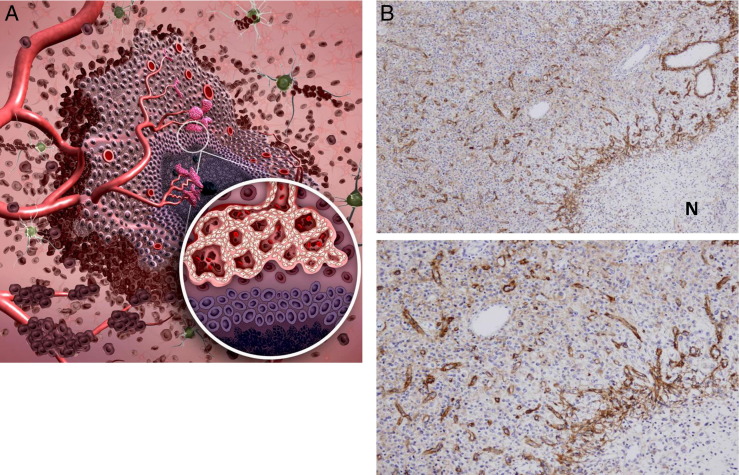
Figure 2.
Angiogenesis. A: Angiogenesis follows vascular co-option during tumor vasculature development and is defined as the development of new vessels from pre-existing ones. Hypoxic pseudopalisading glioma cells around necrosis (inset) release proangiogenic factors. This results in the shift of the angiogenic balance toward a proangiogenic phenotype, inducing sprouting from pre-existing vessels. Hypoxia-independent mechanisms driving angiogenesis have also been described. B: Photomicrographs of a sectioned human GBM specimen stained for tenascin-C shows sprouting angiogenesis. Note the gradient from relatively poorly vascularized regions with little angiogenesis (upper left in each panel) to highly vascularized regions with hyperplastic vessels around necrosis (N) (lower right in each panel). Original magnification: ×20 (top); ×100 (bottom).
A detailed and comprehensive description of the molecular and cellular mechanisms and biology of angiogenesis is beyond the scope of this review. Briefly, glioma-associated sprouting angiogenesis begins with an angiopoietin-mediated breakdown of existing vessels. After vascular co-option, persistent up-regulation of ANG-2 and TIE-2 in endothelial and tumor cells promotes disruption of endothelial and perivascular cell junctions, resulting in vessel disruption.5,7,14 A key early event is the proteolysis of the basement membrane and extracellular matrix due to the activity of matrix metalloproteinases (MMPs). In the presence of ANG-2, VEGF promotes migration and proliferation of endothelial cells and stimulates sprouting of new blood vessels. Acquisition of the tip and stalk phenotypes among endothelial cells exposed to proangiogenic stimuli involves the delta-like 4 (DLL-4)/Notch pathway.15 Recently, ephrin-B2 has been shown to regulate VEGF-induced endothelial tip cell guidance during angiogenesis, similar to its role in axonal guidance.16
The final stages of angiogenesis involve capillary morphogenesis, mediated largely by integrins α3β1 and αvβ3, as well as by CD44.17 Activated endothelial cells secrete platelet-derived growth factor (PDGF), which recruits pericytes to the newly formed vessel,18 aided by the ANG/TIE pathway. Negative feedback by endogenous antiangiogenic factors, as well as accumulation of extracellular matrix, may modulate the process of vascular modeling.19 A role for DLL-4/Notch in differentiation of endothelial cells during the final stages of angiogenesis has also been described,20,21 and inhibition of that pathway has been proposed as a therapeutic target. Although intussusception, which does not require the steps described above, has been observed as a mechanism of angiogenesis in other tumor types, it has not been observed in glioma angiogenesis.
The end result of the neoplastic angiogenic process is a characteristically abnormal vascular network, with dilated and tortuous vessels and abnormal branching and arteriovenous shunts, which can also lead to abnormal perfusion. GBMs in particular have immature vasculature, with excessive leakiness, that can contribute to the breakdown of the blood-brain barrier. In addition to physical disruption of existing vessels by lifting and displacement of astrocytic foot processes by glioma cells,6 induction of leakiness by VEGF and vesiculovacuolar organelles22 contribute to an abnormal blood-brain barrier in the setting of glioma. The permeability of newly formed vascular channels is increased, compared with that of mature capillaries. Normal capillaries of the brain maintain the integrity of the blood-brain barrier, but the blood vessels of experimental and human brain tumors are structurally altered and have increased capillary permeability, in part due to lack of a basal lamina (resulting from persistent angiogenic stimuli leading to incomplete maturation).
Several key pathways have been identified in the process of glioma-associated angiogenesis, including erythropoietin and its receptor, DLL4 and its receptor Notch, macrophage migration inhibitory factor (MIF), neuropilin-2 (NRP2), placental growth factor (PlGF), and basic fibroblast growth factor (bFGF), among others. The most studied and best characterized factor is VEGF, which is discussed in more detail below.
Hypoxia-Induced Glioma Angiogenesis
Hypoxia has long been known as a major stimulator of angiogenesis in GBMs.3,23 The extensive list of angiogenic factors (many of which are up-regulated by hypoxia) and the various mechanisms of angiogenesis in gliomas have been described in detail previously and have been reviewed by Fischer et al.24 In particular, VEGF, which is up-regulated by hypoxia, stimulates vascularization during embryogenesis and in neoplastic tissues. The VEGF family consists of five members: VEGF-A (referred to here simply as VEGF), VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PlGF). VEGF exerts its effects on the vascular endothelium through binding to several high-affinity receptors, including VEGFR-1 (also known as FLT-1) and VEGFR-2 (also known as FLK-1 and KDR). The expression of VEGF and VEGFR correlates with the grade of diffuse astrocytomas, is crucial for glioma growth, and displays a temporal and spatial correlation with the angiogenesis seen in human gliomas.25 Hypoxia induces HIF-1α expression in GBMs and is the main molecular basis for the activation of VEGF gene transcription, leading to angiogenesis. The expression level of HIF-1α and VEGF in both human and murine gliomas is intense around areas of necrosis in pseudopalisading tumor cells,23,26 suggesting that this pattern of HIF-1α and VEGF expression is modulated by tumor oxygenation.
Abundant experimental evidence suggests that the C-X-C chemokine receptor type 4/stromal-derived factor-1α (CXCR4/SDF-1α) pathway is also a crucial component of neovascularization in gliomas.27 CXCR4 is normally expressed at low levels in resting endothelial cells, but is increased in response to VEGF stimulation.27 SDF-1α is a ligand of the chemokine receptor CXCR4. Like VEGF, CXCR4 and SDF-1α are up-regulated by hypoxia, as are several other molecules that play a critical role in the angiogenic cascade (eg, MMPs). Knockout mouse experiments demonstrated that CXCR4 and SDF-1α are required for normal embryonic development of the nervous system; importantly, CXCR4 is required for vascularization of the gastrointestinal tract. The angiogenic effects of SDF-1α have been shown both in vitro and in vivo.28 SDF-1α acts as a chemoattractant for endothelial cells and induces endothelial cell proliferation in vitro, and promotes capillary sprouting and branching in vivo. SDF-1α expression by gliomas and vascular endothelial cells has been correlated with survival of endothelial cells,29 whereas CXCR4 expression has been shown to promote high levels of VEGF production by human astrocytic glioma cells.30
Hypoxia-Independent Glioma Angiogenesis
Although hypoxia has been shown to play a critical role in glioma angiogenesis, some experimental evidence also indicates the existence of hypoxia-independent mechanisms. Vascular proliferation can occur near the leading/invading edge of GBM, often remote from the central necrotic and hypoxic core of the tumor.
For example, using fresh-frozen human tissue, Jubb et al31 showed in several tumor types that VEGF-A was up-regulated in the absence of markers of hypoxia. Similarly, Arany et al32 demonstrated induction of VEGF by peroxisome-proliferator-activated receptor-γ coactivator-1α (PGC-1-α) independently of HIF-1 through the estrogen-related receptor α (ERR-α). Hypoxia-independent mechanisms of HIF-1 stabilization have also been described. For example, several genetic mutations (including mutated genes encoding PDGFR, EGFR, p53, RB1, VHL, and PTEN) have been shown to result in HIF-1α stabilization3,23,33–35 resulting in increased angiogenesis due to the up-regulation of angiogenic factors. Some of these genetic mutations have been implicated in gliomas.
Role of Glioma Stem Cells in Angiogenesis
Some experimental evidence suggests that GSCs play a critical role in tumor progression, at least in part, through their promotion of angiogenesis.36 Both VEGF and SDF-1α have been shown to be important angiogenic factors released by GSCs.36,37 This conception is thought to be valid because GSCs thrive under hypoxic conditions, proliferate, enhance the original tumor mass, and express distinctly high levels of VEGF. Furthermore, tumors with a high GSC content are highly angiogenic.36
Vasculogenesis
A third mechanism of tumor neovascularization, vasculogenesis, involves differentiation of circulating bone marrow-derived cells (BMDCs) known as endothelial progenitor cells (EPCs) (Figure 3A). Previous animal studies of tumor-associated vasculogenesis produced discordant findings. Although some studies have shown a significant role for vasculogenesis in tumor neovessel formation,38 others have not,39 and it is likely that the relative contribution of vasculogenesis to tumor neovascularization depends on the specific tumor type. Supporting a role for vasculogenesis in glioma neovascularization is the observation that impaired recruitment of BMDCs interferes with tumor growth.38,40–42
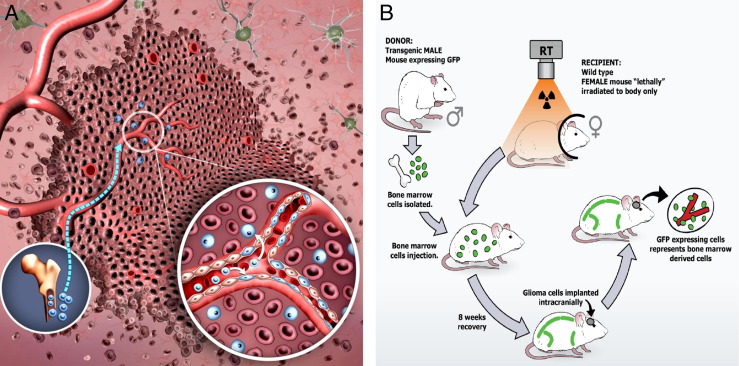
Figure 3.
Vasculogenesis. A: Vasculogenesis involves the mobilization, differentiation, and recruitment of BMDCs (blue). Similar to the angiogenic switch, vasculogenesis is induced largely by hypoxia-mediated release of various factors, the most studied of which are involved in the SDF-1α/CXCR4 pathway. Circulating precursor cells are not only incorporated into the neovasculature, but also can enter the tumor and become tumor-associated macrophages (inset). B: A transgenic murine model is used in our laboratory to study vasculogenesis in GL261 gliomas. When bone marrow cells from a donor male transgenic mouse are transplanted into a recipient female wild-type mouse, marrow-derived cells will express the Y chromosome and marrow-derived EPCs will express GFP detectable by fluorescence microscopy, immunohistochemistry, and flow cytometry. These marrow transplant mice can be implanted intracranially 8 weeks later with syngeneic GL261 glioma cells. Any contribution of GFP+ transplanted BMDCs from the male donor to the endothelium and/or intratumoral cellular milieu is easily detectable.
VEGF, which has been shown to play a critical role in angiogenesis, also contributes to EPC migration and proliferation. Injection of isolated EPCs in a U87 glioma xenograft model showed that EPCs make up approximately 18% of total vessels, suggesting a significant role for EPCs in tumor neovascularization. Although the molecular identity and differentiation lineage of EPCs is debated, these cells have been defined by their expression of progenitor (CD34, CD133) and endothelial (CD31, VEGFR-2) markers, as confirmed by oligonucleotide microarray analysis of cultured EPCs from human umbilical cord blood.43
Although vasculogenesis by definition refers to differentiation of EPCs, accumulating evidence suggests that in addition to bone marrow-derived EPCs, bone marrow-derived tumor-associated macrophages (TAMs), including TIE-2 expressing monocytes (TEMs), circulate in the blood and home to sites of pathological neovascularization and differentiate into endothelial cells or macrophages.44,45 The identity of TEMs has been shown as a subpopulation of tumor-infiltrating CD11b+ myeloid cells expressing TIE-2, F4/80, SCA-1 (also known as ataxin-1), and CD45.46 In mice, the circulating TIE-2+CD45+ hematopoietic cells are mostly CD11b+Gr-1low/neg, whereas in humans they express CD14, CD16, and CD11c.45,47 The surface marker profile of mouse and human TEMs is distinct from the classic profile of inflammatory monocytes and so-called resident monocytes.44,45 Interestingly enough, the vast majority of circulating TEMs lack expression of EPC markers, such as VEGFR-2, CD133, CD146, and CD34, but they do express pure hematopoietic markers, such as CD45.46 TEMs are important drivers of tumor angiogenesis and have been found in several mouse tumor models, including human GBMs grown in the mouse brain, and in spontaneous pancreatic tumors developing in RIP1-Tag2 transgenic mice.47
EPCs and TAMs express CXCR4 and migrate in response to an SDF-1α gradient.48–50 The importance of CXCR4/SDF-1α signaling in EPC and TAM mobilization and recruitment in the setting of central nervous system-specific malignancies has been emphasized. Consistent with this pathway, immunoreactivity for SDF-1α is strong in tumor-associated vessels of GBMs.51,52 SDF-1α produced by murine gliomas has been shown to contribute to vasculogenesis by incorporating EPCs into tumor endothelium.53 Moreover, Smadja et al54 found an SDF-1α/CXCR4–mediated increase in EPC migration, whereas inhibition of SDF-1α/CXCR4 signaling inhibited EPC migration, EPC differentiation, and tubule formation in Matrigel, highlighting the role of the SDF-1α/CXCR4 axis in both the migration and differentiation of EPCs.
Like the SDF-1α/CXCR4 pathway, the ANG-2/TIE-2 pathway is important for vasculogenesis, and ANG-2 has been linked to the recruitment of EPCs and TEMs in tumors.44,55–57 Although some studies suggest a direct incorporation of BMDCs into tumor vasculature during the process of vasculogenesis, others point to a more supportive role. Previous animal studies of tumor-associated vasculogenesis have produced discordant findings, with possible explanations ranging from insufficient marrow reconstitution, nonoptimized GFP detection based on endogenous fluorescence levels, and undefined time points. Thus, the exact mechanism of contribution of EPCs and TEMs to the neovascularization of brain tumors has yet to be fully elucidated. A transgenic murine glioma model that can be used to study vasculogenesis is presented in Figure 3B.
Role of Hypoxia in Vasculogenesis
It is thought that a molecular switch largely induced by hypoxic conditions also promotes the recruitment of circulating BMDCs, including EPCs and TEMs.47,50,57 For example, the recruitment and retention of BMDCs into gliomas has been shown to be, at least in part, controlled by hypoxia-induced molecular mechanisms, including the SDF-1/CXCR4 pathway.50,53,58 Moreover, hypoxia up-regulates TIE-2 expression on TEMs.47,57
Role of Glioma Stem Cells in Vasculogenesis
Folkins et al36 recently described increased microvessel density and tumor perfusion, as well as mobilization and homing of EPCs, in tumors rich in cancer stem cells (CSCs), generated by neurosphere culture, compared with CSC-poor tumors in the C6 glioma xenograft model. Moreover, VEGF and SDF-1α were preferentially overexpressed by CSC-rich tumor cells in culture. In addition, blockade of either VEGF or SDF-1α signaling pathways was sufficient to decrease CSC-rich vascularization to CSC-poor levels. Finally, endothelial cell proliferation, tubule formation, and EPC mobilization were significantly decreased with this inhibition. Thus, the molecular mechanisms controlling vasculogenesis and leading to the mobilization and recruitment of EPCs and TEMs to the neovascularization of brain tumors have been partially elucidated and appear to be similar, at least in part, to those described for angiogenesis.
Vascular Mimicry
A fourth mechanism of glioma vascularization, vascular mimicry, is defined as the ability of tumor cells to form functional vessel-like networks (Figure 4) and was first described in human melanoma models. Within tissue sections of aggressive intraocular and metastatic cutaneous melanomas, red blood cell-containing vascular channel networks were found to be devoid of endothelial cells. Furthermore, in vitro, invasive and metastatic melanoma cells were able to form vascular channels in three dimensional cultures. Using a three-dimensional culture and microdissection, Demou and Hendrix59 showed that networks of aggressive melanoma cells displayed a strong angiogenic gene expression signature, suggesting an underlying genetic basis for this process.
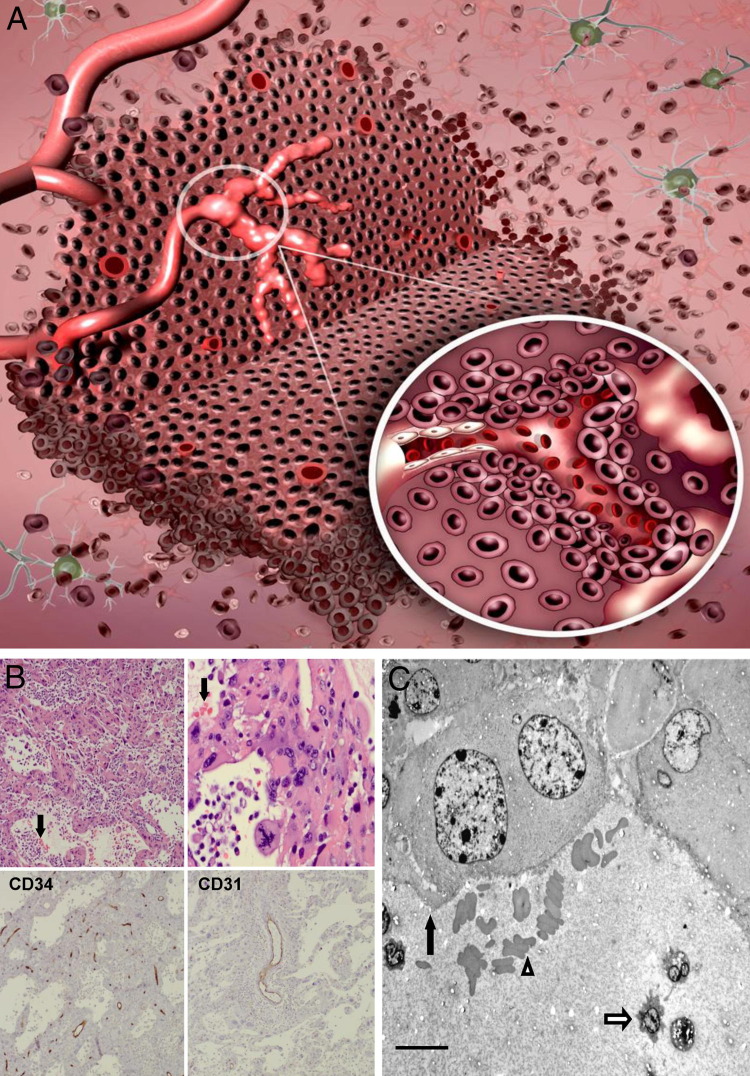
Figure 4.
Vascular mimicry. A: Vascular mimicry is the ability of tumor cells to form functional, perfused, vessel-like networks. These tumor cells lining the vascular channels maintain glioma morphological characteristics and typical glioma markers (inset). B: Photomicrographs demonstrating vascular mimicry. Top row: Low- and high-power views of H&E-stained sections of a human GBM demonstrate perfused vascular networks containing red blood cells (arrows). Bottom row: Immunohistochemical staining of this tumor with CD34 (left, immunoperoxidase) and CD31 (right, immunoperoxidase) reveals immunoreactive vascular channels different from those lined by tumor cells and confirms that these channels are not lined by endothelial cells. C: Electron micrograph shows tumor cells (black arrow) lining a vascular channel containing red blood cells (outlined arrowhead) and polymorphonuclear leukocytes (outlined arrow) in a sectioned human GBM. Scale bar = 10 μm. Original magnification: ×50 (B, top left, bottom left, and bottom right); ×200 (B, top right).
Evidence for vascular mimicry in gliomas has been published. Yue and Chen60 demonstrated the presence of vascular mimicry in 2/45 human astrocytoma samples. Although endothelium-lined vessels dominated the microvasculature, a PAS+CD34− vascular pattern containing red blood cells was identified in two astrocytomas of grade IV. However, the use of PAS as a marker for endothelial cell phenotype is questionable, given that glioma cells also have been described to express PAS.
Shaifer et al61 suggested a link between vascular mimicry in GBMs and vascular radioresistance. Using a three-dimensional organotypic coculture system of GFP-transfected glioma cells and red vital dye-labeled endothelial cells, they showed that endothelial cells form vascular structures first, followed by incorporation of glioma cells within 48 hours, creating a mosaic vascular network. Under angiogenic conditions, glioma cells formed three-dimensional vascular networks, but characterization of these glioma cells consistently revealed the absence of endothelial-specific markers, suggestive of vascular mimicry. Glioma cells did, however, have reduced expression of glial fibrillary acidic protein (GFAP) and increased expression of CD133, indicating a shift to a stem cell phenotype. The presence of glioma cells both stabilized vascular structures and conferred vascular radioresistance in vitro. Shaifer et al61 were also able to demonstrate incorporation of glioma cells into CD31+ perfused vessels in both flank and orthotopic glioma models.
In a study of 101 human glioma samples, Liu et al62 found a correlation between vascular mimicry and World Health Organization tumor grade. Tumors that contained evidence of vascular mimicry, defined immunohistochemically as CD34−PAS+, were more likely to be higher grade and more aggressive, and these patients had shorter overall survival times than those without vascular mimicry. Interestingly enough, tumors exhibiting vascular mimicry had lower microvascular densities than those that did not, indicating that vascular mimicry provides a complementary neovascularization pathway.
Role of Hypoxia in Vascular Mimicry
Although no evidence exists for a direct relationship between hypoxia and vascular mimicry in GBMs, Sun et al63 demonstrated that hypoxia influences vascular mimicry in melanoma models (in which vascular mimicry was first described). They showed in hind limb models that hypoxic tumors (generated by femoral artery ligation) had more vessels containing vascular mimicry than did control tumors that correlated with HIF-1α, VEGF, and MMP levels.
Role of Glioma Stem Cells in Vascular Mimicry
In ubiquitous GFP-transgenic mice with orthotopically injected human GSC-derived tumors, Dong et al64 demonstrated the incorporation of vascular mimicry in the neovascularization process. They identified the formation of patterned tubular networks by tumor cells mimicking endothelial-lined vascular networks. Coexpression of HLA and GFP suggests that cell fusion could be one mechanism by which vascular mimicry occurs.
Chen et al65 studied 48 GBM samples and found non-endothelial-lined blood vessels in viable regions of the tumor in a majority of tumor sections. CD133+ tumor spheres were generated from GBMs that did or did not exhibit vascular mimicry (VM+/VM−). In a three-dimensional Matrigel tube assay, VM+ cells formed a vasculogenic network within 2 days and re-exhibited mimicry when reinjected as xenografts, whereas VM− cells did neither.
Using GBM tissue sections, El Hallani et al66 found both small and large PAS+ tubular structures containing red blood cells lined by CD34− cells on the luminal surface. There were also CD34+ and CD34− portions within the same vessel, further suggesting the role of anastomosis in vascular mimicry. CD34− blood vessels lined by EGFR-amplified cells were identified, implying that the channels were indeed lined by tumor cells. In CD133+ neurosphere cultures of tumors containing tumor-lined vessels (GSC-A) and tumors containing only endothelial-lined vessels (GSC-B), GSC-A tumor cells developed a vascular network in three-dimensional Matrigel culture within 2 days, whereas GSC-B tumor cells did not. Characterization of GSC-A cells showed overexpression of multiple factors involved in vasculogenesis, whereas normal endothelial markers were lacking.
Although there is sufficient evidence to suggest that vascular mimicry does occur in human GBM (Figure 4, B and C), the contribution of this mechanism to the overall neovascularization process on a whole-tumor scale remains unclear. Furthermore, it is very likely that the relative contribution of vascular mimicry varies widely among tumors and tumor types, making it more difficult to confirm its significance. Nonetheless, the implication (by Shaifer et al,61 as discussed above) of vascular mimicry as a complementary neovascularization pathway and its potential role in radiation resistance makes this novel mechanism particularly intriguing in a disease such as GBM, which is characterized by radioresistance.
Glioblastoma-Endothelial Cell Transdifferentiation
The most recently described mechanism of glioma neovascularization involves transdifferentiation of glioma cells into an endothelial phenotype (Figure 5). As with vascular mimicry, the hypothesis of endothelial transdifferentiation of tumor cells originated with human cutaneous melanoma models. The rather recent identification of glioma endothelial cell transdifferentiation has yet to be confirmed, and will need to be further investigated for complete validation.
Figure 5.
Glioblastoma-endothelial cell transdifferentiation. The mechanism of glioma neovascularization involves transdifferentiation of glioma cells into endothelial cells lining vascular channels (inset), including both a phenotypic transformation and the expression of typical endothelial-specific markers.
Role of Hypoxia in Transdifferentiation
Using an orthotopic model of GFP+ GBM in GFAP-Cre recombinase mice, Soda et al67 observed GFP+ endothelial cells in functional vessels, suggesting a tumor-derived endothelial cell population. Using GFP+ tumor cells in DsRed-transgenic nude mice, they further demonstrated that tumor-derived endothelial cells were not a result of fusion between tumor cells and endothelial cells. Similar findings were observed using xenograft tumors of human GBM spheres and in sections of clinical samples of GBM tumors that contained von Willebrand factor (vWF)-positive endothelial cells with EGFR amplifications characteristic of GBM cells. Reduced oxygen concentration enhanced this morphological change. tumor-derived endothelial cells colocalized with hypoxic portions of the tumor, suggesting a role for hypoxia and HIF-1 in the transdifferentiation of GBM cells. In vitro, they showed that growth of GBM cells in endothelial growth medium induced an endothelial-like phenotype. Despite a significant induction of VEGF secretion, this process was independent of VEGF and FGF. Furthermore, treatment with anti-VEGF therapy in an orthotopic GBM mouse model had no effect on tumor growth, and significantly increased the percentage of vessels containing tumor-derived endothelial cells, compared with control-treated mice.
Role of Glioma Stem Cells in Transdifferentiation
The close association between endothelial cells and neural stem cells was demonstrated in a seminal article by Shen et al,68 who established that factors secreted by endothelial cells stimulate self-renewal of neural stem cells and proposed that endothelial cells are an integral component of the stem cell niche. This vascular niche has also been applied to GSCs and is the protective glioma microenvironment, in which GSCs are able to freely proliferate and remain undifferentiated, completely unaffected by any external influences.
Ricci-Vitiani et al69 recently demonstrated the process of endothelial transdifferentiation of GBM stem-like cells. Using 15 archival GBM specimens, they demonstrated that a substantial fraction of CD31+ endothelial cells expressed the same chromosomal aberrations as were present in tumor cells within the specimen, which was verified in freshly dissociated GBM specimens. Further staining revealed a subset of GFAP+ microvascular cells with an aberrant endothelial/glial phenotype, suggesting that at least some endothelial cells originate from the tumor itself. The majority of sorted CD31+/CD144+ GBM cells expressed vWF. Neurospheres and differentiated GBM cells were cultured under endothelial conditions, and only the cells enriched in GSCs generated CD31+ and TIE-2+ microvascular cultures. In GBM neurosphere xenografts, 70% of CD31+ cells lining functional vessels from the inner portion of the tumor were of tumor cell origin. These cells consistently expressed endothelial markers, but not GSC markers. Lastly, in experiments using RFP-GBM neurospheres injected in TIE-2-GFP NOD/SCID mice and confocal microscopy of in vivo angiogenesis, GFP+ mouse vessels were located predominantly outside the tumor.
Another recent study, by Wang et al,70 further demonstrated the role of GBM stem-like cells in the tumor endothelium. In human GBM specimens, the proportion of CD105+ endothelial cells displaying amplification of EGFR and of the chromosome 7 centromeric portion was similar to that of the tumor cells themselves. Dissociated GBM specimens were then fractionated into groups based on CD144 and CD133 expression. The angiogenic endothelial marker CD105 was consistently absent in CD144+/CD133+ double-positive populations; however, when cells were cultured in endothelial medium, CD144 was down-regulated and CD105 and CD31 were up-regulated with coexpression of VEGFR-2 and CD34. Because these investigations relied heavily on CD105 as an exclusive endothelial cell marker, evidence indicating that CD105 can also be expressed by glioma cells71 renders some of the reported observations questionable. In three-dimensional culture, these CD144+/CD133+ double-positive-derived endothelial cells formed abnormal vascular networks characteristic of tumor vasculature, leading the authors to postulate that the CD144+/CD133+ double-positive population represents the neoplastic origin of tumor endothelium.70 When cultured with tumor cells, GFP-labeled CD144+/CD133− cells produced a population of GFP-CD144+/CD133+ cells that, when cultured in collagen, formed vacuoles suggestive of early lumen formation and differentiated into CD105+ and CD31+ cells. This finding suggests that the CD144+/CD133+ double-positive endothelial progenitors within GBM arise from the CD133+ population and can differentiate into an endothelial phenotype. Furthermore, by performing single-cell clonal studies of CD133+/CD144− cells (as well as normal endothelial cells and fibroblasts), they were able to demonstrate both endothelial and neural differentiation potential. The authors suggest that the CD144−/CD133+ population may represent circulating EPCs. Transcriptome analysis was also performed and, although EPCs were found to be highly abnormal, EPCs were near-diploid, making the possibility of nuclear fusion as an explanation for these findings highly unlikely.
More recently, Zhao et al72 also described the characteristic flagstone vascular endothelial cell appearance of glioma stem/progenitor cells (GSPCs) after culture in endothelial differentiation medium, as well as their formation of tubular structures when cultured in Matrigel. Furthermore, when stressed by hypoxia or nutrient deprivation, GSPCs expressed markers of vascular endothelial cells, including CD31, CD34, and vWF.
Dong et al73 also demonstrated the potential of GSPCs to transdifferentiate into endothelial cells. Cultures of GSPCs in transdifferentiation medium resulted in a characteristic flagstone morphology within 10 days, and culture in Matrigel ultimately produced vessel-like structures, with some cells appearing as endotheliocytes. The transdifferentiation process was associated with an increase in transcription and expression levels of markers of vascular endothelial cells. Orthotopic injection of human (h) GSPCs into mice produced tumors that contained functional HLA+ vessels, suggesting that their origin was hGSPCs. Furthermore, examination of clinical GBM specimens showed relatively high levels of nestin expression by tumor vessels, as well as tumor cells coexpressing ABCG2/CD34 or ABCG2/nestin at the intimal layer of tumor vessels. (ABCG2 is a marker of side population cells.) Dong et al73 speculated that this may represent interim cells during the transdifferentiation process from hGSPCs to vascular endothelial cells. This is also supported by the findings of Shaifer et al,61 who found that, under proangiogenic conditions, glioma cells did not acquire endothelial-specific markers, but lost GFAP expression and gained CD133 expression, indicating a shift to a more stem/progenitor phenotype.
As the most recently described mechanism of glioma-associated neovascularization, glioblastoma-endothelial cell transdifferentiation represents an exciting area of research. Nonetheless, the relative contribution of this process to the entire neovascularization process and its clinical relevance are as yet undefined (much like the case with vascular mimicry). Although the various reports discussed above have provided evidence for transdifferentiation in archival GBM specimens, as well as in preclinical and in vitro models, the use of questionable markers (such as CD105) and questionable endpoints (such as flagstone morphology as a surrogate for an endothelial phenotype) bring into question the validity of some of these findings.
The demonstration of naturally occurring tumor-specific mutations in vascular cells [such as isocitrate dehydrogenase-1 (IDH-1)74] could prove to be useful in establishing glioblastoma-endothelial cell transdifferentiation as a relevant mechanism in the neovascularization process. Clearly, more work is needed to confirm this process as significant in glioma-associated neovascularization.
Targeting Tumor Neovasculature
For decades, tumor neovasculature has been an attractive target for therapeutic intervention. The rationale for applying antiangiogenic strategies in malignant brain tumors includes i) the high degree of neovascularization in high-grade gliomas, ii) avoidance of problems related to crossing the blood-brain barrier, in contrast to certain chemotherapeutic agents, and iii) normalization of vascular networks leading to synergism with other therapeutic strategies. Antiangiogenic therapy might also represent a way to target GSCs, thought to be responsible for the radioresistant and chemoresistant properties exhibited by GBM. Not only are GSCs a source of proangiogenic factors such as VEGF, but they thrive in the perivascular niche within the tumor microenvironment and could possibly be sensitive to therapy directed at tumor vasculature.75
Several approaches aimed at targeting glioma neovasculature have been proposed. High levels of VEGF have been reported in plasma and tumor fluid in patients with GBM, and VEGF overexpression has been correlated with prognosis in GBM.25 Thus, from among the numerous therapeutic targets identified, the VEGF pathway has been the target of several therapeutic strategies, involving either VEGF [anti-VEGF monoclonal antibody bevacizumab (Avastin)76 or aflibercept (VEGF Trap)77] or its receptors.78 To date, by far the most extensive clinical experience with antiangiogenic therapy in gliomas has been with bevacizumab. Antiangiogenic therapy with bevacizumab has become standard therapy in recurrent high-grade gliomas in adults. Several clinical trials in glioma patients have shown that bevacizumab combined with chemotherapy demonstrates antitumor activity with acceptable toxicity.79,80 Patients at New York University Langone Medical Center (and elsewhere) continue to receive bevacizumab because of i) marked improvement in quality of life, ii) a demonstrable increase in progression-free survival compared with historical controls, and iii) relief from steroid dependence due to diminished tumor edema.80 Beal et al81 reviewed numerous trials using bevacizumab in recurrent GBM and anaplastic astrocytoma, as well as newly diagnosed GBM. As a single agent in prospective trials of recurrent GBM, bevacizumab has demonstrated a radiographical response rate of 28% to 35%, a 6-month progression-free survival of 28% to 35%, and median progression-free survival and overall survival of 3.7 to 4.2 months and 7.2 to 9.2 months, respectively. When combined with chemotherapy (irinotecan, etoposide, temozolomide, or fotemustine) for recurrent GBM, the radiographical response rate was higher (35% to 60%), with 6-month progression-free survival of 37% to 50%. Recent studies have also examined the feasibility of bevacizumab combined with radiation in the recurrent GBM setting, showing promising results with acceptable toxicities. The upfront treatment of newly diagnosed patients with GBM has also been described, showing improvements in progression-free survival. Several ongoing trials have reported interim data on the use of bevacizumab in this setting, with a suggestion of a progression-free survival benefit compared with historic controls. Although these results are not overwhelming, they are exciting, given historical radiographical response rates of 9% and 6-month progression-free survival of 9% to 15% in similar patients without bevacizumab therapy.81
Aflibercept also targets VEGF. This agent acts as a decoy receptor for VEGF and has a high affinity for all isoforms of VEGF-A. In an orthotopic preclinical glioma model, aflibercept was effective in both initial and advanced phases of tumor development in reducing tumor burden and increasing survival. Furthermore, the effect was enhanced in animals treated with prolonged regimens.77
Failure of Antiangiogenic Therapy
Despite promising response rates with antiangiogenic therapy, glioma recurrence is common. Commonly seen after antiangiogenic therapy is a change in vascular phenotype at a cellular level, characterized by decreased microvessel density and normalization in architecture, as well as altered expression of molecules such as CD34 and fascin.82 Additionally, there have been reports of altered clinical relapse patterns. For example, we and others have observed that the pattern of relapse in bevacizumab-treated GBM patients is often characterized by local, as well as distant infiltration of the brain by tumors that demonstrate increased invasiveness.79,80 This problem has become more prevalent as the use of bevacizumab has increased in adult glioma patients. Investigations have been conducted to try to understand why antiangiogenic therapy may alter tumor biology and promote, for example, an invasive phenotype.81 Evidence for similar phenomena after antiangiogenic therapy in other tumor types has been reported.83 Although metastases are a rare occurrence in glioma biology, treatment with antiangiogenic therapy has been shown to increase metastatic potential in both breast and melanoma tumors in preclinical models.84
Experimental evidence suggests that the escape from antiangiogenic therapy such as bevacizumab is at least in part linked to four phenotypic/molecular shifts, many of which are controlled by hypoxia. These include heightened invasion, increased vascular co-option, augmented vasculogenesis, and up-regulation of angiogenic factors other than VEGF. Several experiments have shown that antiangiogenic therapy can increase invasion and, in many cases, vascular co-option.
Rubenstein et al85 showed in an athymic rat model of GBM that anti-VEGF therapy improved outcomes, but resulted in increased perivascular tumor infiltration and vascular co-option evident on histological analysis. Similarly, treatment with DC101 (an anti-VEGFR-2 monoclonal antibody) in GBM mouse models resulted in increased tumor invasion and co-option of existing vasculature.78 Auf et al,86 using a xenograft glioma model, also showed that inhibition of the inositol-requiring protein 1α (IRE1), an important sensor of the unfolded protein response, resulted in both a reduction in the number of blood vessels and an increase of tumor cell invasion and vascular co-option. Gene expression analysis revealed a significant decrease in proangiogenic factors and an increase in proinvasion factors when IRE1 was inhibited, consistent with the observed decrease in tumor vascularity and increase in invasiveness. Using a rat model of GBM, Sakariassen et al87 showed tumor growth and up-regulation of proinvasion genes in the absence of angiogenesis. Of interest, the same observation was made with aflibercept, with resistance leading to increased tumor growth and increased invasiveness. Vascular co-option has been observed (as above) in preclinical models. Zuniga et al88 proposed increased vascular co-option as a mechanism for the observed increase in diffuse relapses seen after antiangiogenic therapy, although they did not demonstrate this mechanism pathologically.
Du et al50 demonstrated that VEGF directly inhibits invasive behavior in vitro, in a Boyden chamber assay. Lucio-Eterovic et al89 found that immunodepletion of VEGF resulted in increased MMP expression and invasion of GBM cells in Matrigel. Pàez-Ribes et al90 reported that decreased VEGF activity resulted in thinner tumor vasculature and improved survival in a genetically transformed astrocyte murine model of GBM, but was also associated with dramatic increase in invasive phenotype of tumor cells. Taken together, these reports confirm that angiogenesis inhibition and particularly the inhibition of VEGF is in many cases associated with an increased invasive phenotype. Thus, clinical strategies of blocking tumor invasion combined with antiangiogenic therapy have been proposed.91
Besides increasing invasion and co-option, antiangiogenic therapy has also been shown to augment vasculogenesis. For example, inhibition of VEGF by AZD2171 (a pan-VEGF tyrosine kinase inhibitor) resulted in an increase in infiltration of CXCR4+ tumor-associated macrophages and recruitment of endothelial and pericyte progenitor cells in response to a transient increase in SDF-1α.92 The importance of monitoring vasculogenesis during antiangiogenic therapy is highlighted by reports that circulating EPCs were elevated in a subset of patients with gliomas93 and increased with tumor progression after interruption of anti-VEGF receptor therapy.94 Because no reliable measure of the biological activity of antiangiogenic agents is yet available, EPCs have been proposed as potential surrogate markers. Other biomarkers under consideration include circulating endothelial cells shed by the tumor vasculature, and plasma levels of SDF-1α and VEGF.
It is also thought that antiangiogenesis may lead to the up-regulation of other proangiogenic factors, such as PDGF, PlGF, bFGF, tumor necrosis factor (TNF-α), interleukins, neuropilins, and angiogenin. In addition, receptors such as the hepatocyte growth factor receptor (also known as proto-oncogene c-Met) and VEGFR-3, are also up-regulated by antiangiogenesis. Up-regulation of both ligands and receptors can lead to resistance to antiangiogenic therapies.89,92 Through this mechanism, inhibition of VEGF can result in a so-called rebound revascularization after discontinuation of anti-VEGF therapy. Similarly, using AZD2171, Batchelor et al94 demonstrated rapid normalization of glioma vascularization, but with reversibility of this process on discontinuation or delay in treatment.
Role of Hypoxia in Antiangiogenic Failure
It has been suggested that antiangiogenic therapy leads to tissue hypoxia95 and may select for hypoxia resistance in cancer cells.96 In fact, one of the initial responses to antiangiogenic therapies is an increase in intratumoral hypoxia.89 Rapisarda et al97 also found that bevacizumab therapy caused a significant increase in intratumoral hypoxia and HIF-1α-dependent gene expression in tumor tissue, and Selvakumaran et al98 concluded that bevacizumab treatment is an effective inducer of a hypoxic environment. Pàez-Ribes et al90 reported similar findings, using both anti-VEGFR-2 and sunitinib treatments. Overexpression of HIF-1α has been correlated with increased tumor invasiveness and resistance to chemotherapy.99 More recently, in an intracranial xenograft model derived from patient tumor neurospheres, Keunen et al100 found that, although anti-VEGF therapy decreased vascular supply to tumors, it also led to a more hypoxic tumor microenvironment. Subsequent up-regulation of HIF-1α resulted in an increase in tumor cell invasion. Thus, it seems that inhibiting blood supply drives the intratumoral accumulation of HIF-1α, the Achilles' heel of antiangiogenic therapy.99
Hypoxia also up-regulates factors involved in recruitment of BMDCs, which in turn promote vasculogenesis. As discussed above, on escape from antiangiogenic therapy tumors can also display increased vasculogenesis. A recent study by Kioi et al58 demonstrated the importance of vasculogenesis in GBM recurrence and resistance to radiation therapy. Irradiation of orthotopic xenografts in mice resulted in a dose-dependent increase in SDF-1α/CXCR4 signaling and in entry of BMDCs (the majority of which were CD11b+ myelomonocytes) into the tumor. Importantly, inhibition of HIF-1 abrogated the increase in CD11b+ monocytes recruited to the tumor and significantly decreased tumor growth. Inhibition of CXCR4 also prevented the return of blood flow in irradiated tumors and completely inhibited the recurrence of tumors after a single high dose or five daily doses of radiation. Both CXCR4 inhibition and VEGF inhibition decreased the number of endothelial cells in the tumor after irradiation, but only CXCR4 blockade caused complete blockage of tumor perfusion. After transplantation of tumors into irradiated normal tissues to eliminate local angiogenesis, HIF-1α inhibition or CXCR4 inhibition completely abrogated tumor growth. In addition, recent work has shown that both HIF-1α and SDF-1α recruit CXCR4/MMP-9+ BMDCs to the tumor, which in turn promotes recruitment of endothelial and pericyte progenitor cells.50 Thus, hypoxia appears to be an important modulating mechanism contributing to the failure of antiangiogenic therapy.
Role of Other Mechanisms in Antiangiogenic Failure
We have learned much about the molecular pathways responsible for the failure of antiangiogenic therapy. Ongoing efforts are providing novel scenarios toward further understanding of the molecular mechanisms associated with acquisition of resistance to antiangiogenesis. For example, Soda et al67 recently described an increase in glioblastoma-endothelial cell transdifferentiation after anti-VEGF therapy in a mouse model, which may represent another strategy of resistance to antiangiogenic therapy.
Interaction of the Glioma-Associated Neovascularization Pathways
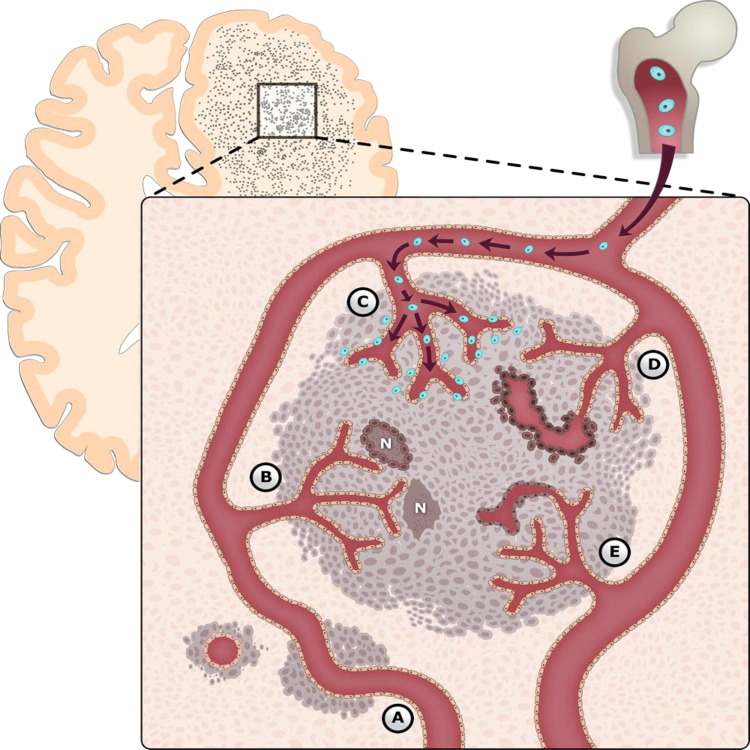
Through continued investigation, it has become increasingly clear that glioma neovascularization is not a simple process, but rather a complex and highly regulated one, dependent on at least five interlinked pathways (Figure 6). For example, Ricci-Vitiani et al69 proposed that vascular mimicry and transdifferentiation may be at the ends of a spectrum, with vascular mimicry representing an incomplete transdifferentiation of cancer stem cells toward endothelial phenotype. Indeed, overlap is evident from recent reports of both vascular mimicry and transdifferentiation. It also appears that these mechanisms are intimately connected, with perturbations in one pathway shifting the balance of the remaining pathways and altering the vascular phenotype. As discussed above, several studies have demonstrated increased vascular co-option, often associated with heightened invasive phenotype, after antiangiogenic therapy.78,85,86 In addition, vasculogenesis became the dominant driver of neovascularization in this setting.58,92
Figure 6.
Mechanisms of glioma-associated neovascularization. Glioma neovascularization is a complex and highly regulated process, dependent on the balance of at least five separate pathways: A, vascular co-option; B, angiogenesis; C, vasculogenesis; D, vascular mimicry; and E, glioblastoma-endothelial cell transdifferentiation. These mechanisms are likely intimately connected, with perturbations in one pathway altering the vascular phenotype. Although there does appear to be a temporal framework by which gliomas attain vasculature, it is likely that several of these processes can and do occur simultaneously in different microdomains of the tumor. BMDCs are shown in blue. N, necrosis.
Hypoxia and GSCs each play a critical role in the molecular events after the modulation of one of the five pathways of vascularization. For example, Du et al50 and Kioi et al58 demonstrated that hypoxia-induced HIF-1 increases levels of SDF-1α, which in turn recruits CXCR4+ BMDCs to tumors, stimulating neovascularization. There are also significant interactions between the vasculature and GSC niches within tumors. Seminal work by Shen et al68 demonstrated a role for endothelial cells in the maintenance of neural stem cells, which led to the concept of the vascular niche. Furthermore, recent reports of transdifferentiation of cancer stem cells toward an endothelial phenotype further demonstrated the complexity of glioma neovascularization.67,69,70,72,73
Although outcomes of antiangiogenic therapy have been generally encouraging, concerns have emerged regarding resistance to therapy, rebound revascularization, and shifts to a more invasive phenotype. Regardless, antiangiogenic therapy is undoubtedly an important step forward in the development of effective targeted therapy. It is clear that neovascularization in GBM is more complex than can be explained by angiogenesis alone. Furthermore, it is also evident that many of these processes occur in tumor types other than glioma. We are confident that a thorough understanding of the contributing molecular processes that control the five modalities we have reviewed should help overcome the treatment resistance that characterizes gliomas and many other types of tumors that escape conventional therapies. Using combinations of therapies (antiangiogenic, antivasculogenic, and anti-invasion) represents a logical approach toward achieving lasting tumor control.
Acknowledgments
We thank Julio Garcia for artwork contributions and Diana Klopsis and Amanda Najjar for editorial assistance.
Footnotes
Supported by NIH grants R01-CA100426-0141 and 1R21-NS074055-01A1 (D.Z.) and the Musella Foundation (D.Z.).
CME Disclosure: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interest to disclose.
References
- 1.Brem S., Cotran R., Folkman J. Tumor angiogenesis: a quantitative method for histologic grading. J Natl Cancer Inst. 1972;48:347–356. [PubMed] [Google Scholar]
- 2.Heddleston J.M., Li Z., Lathia J.D., Bao S., Hjelmeland A.B., Rich J.N. Hypoxia inducible factors in cancer stem cells. Br J Cancer. 2010;102:789–795. doi: 10.1038/sj.bjc.6605551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Semenza G.L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heddleston J.M., Li Z., McLendon R.E., Hjelmeland A.B., Rich J.N. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8:3274–3284. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holash J., Maisonpierre P.C., Compton D., Boland P., Alexander C.R., Zagzag D., Yancopoulos G.D., Wiegand S.J. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 6.Zagzag D., Amirnovin R., Greco M.A., Yee H., Holash J., Wiegand S.J., Zabski S., Yancopoulos G.D., Grumet M. Vascular apoptosis and involution in gliomas precede neovascularization: a novel concept for glioma growth and angiogenesis. Lab Invest. 2000;80:837–849. doi: 10.1038/labinvest.3780088. [DOI] [PubMed] [Google Scholar]
- 7.Reiss Y., Machein M.R., Plate K.H. The role of angiopoietins during angiogenesis in gliomas. Brain Pathol. 2005;15:311–317. doi: 10.1111/j.1750-3639.2005.tb00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rong Y., Durden D.L., Van Meir E.G., Brat D.J. ‘Pseudopalisading’ necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J Neuropathol Exp Neurol. 2006;65:529–539. doi: 10.1097/00005072-200606000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Winkler F., Kienast Y., Fuhrmann M., Von Baumgarten L., Burgold S., Mitteregger G., Kretzschmar H., Herms J. Imaging glioma cell invasion in vivo reveals mechanisms of dissemination and peritumoral angiogenesis. Glia. 2009;57:1306–1315. doi: 10.1002/glia.20850. [DOI] [PubMed] [Google Scholar]
- 10.Simon M.P., Tournaire R., Pouyssegur J. The angiopoietin-2 gene of endothelial cells is up-regulated in hypoxia by a HIF binding site located in its first intron and by the central factors GATA-2 and Ets-1. J Cell Physiol. 2008;217:809–818. doi: 10.1002/jcp.21558. [DOI] [PubMed] [Google Scholar]
- 11.Das B., Yeger H., Tsuchida R., Torkin R., Gee M.F., Thorner P.S., Shibuya M., Malkin D., Baruchel S. A hypoxia-driven vascular endothelial growth factor/Flt1 autocrine loop interacts with hypoxia-inducible factor-1alpha through mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 pathway in neuroblastoma. Cancer Res. 2005;65:7267–7275. doi: 10.1158/0008-5472.CAN-04-4575. [DOI] [PubMed] [Google Scholar]
- 12.Montana V., Sontheimer H. Bradykinin promotes the chemotactic invasion of primary brain tumors. J Neurosci. 2011;31:4858–4867. doi: 10.1523/JNEUROSCI.3825-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brem S. The role of vascular proliferation in the growth of brain tumors. Clin Neurosurg. 1976;23:440–453. doi: 10.1093/neurosurgery/23.cn_suppl_1.440. [DOI] [PubMed] [Google Scholar]
- 14.Zadeh G., Koushan K., Pillo L., Shannon P., Guha A. Role of Ang1 and its interaction with VEGF-A in astrocytomas. J Neuropathol Exp Neurol. 2004;63:978–989. doi: 10.1093/jnen/63.9.978. [DOI] [PubMed] [Google Scholar]
- 15.Hellström M., Phng L.K., Hofmann J.J., Wallgard E., Coultas L., Lindblom P., Alva J., Nilsson A.K., Karlsson L., Gaiano N., Yoon K., Rossant J., Iruela-Arispe M.L., Kalén M., Gerhardt H., Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 16.Sawamiphak S., Seidel S., Essmann C.L., Wilkinson G.A., Pitulescu M.E., Acker T., Acker-Palmer A. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 2010;465:487–491. doi: 10.1038/nature08995. [DOI] [PubMed] [Google Scholar]
- 17.Wang D., Anderson J.C., Gladson C.L. The role of the extracellular matrix in angiogenesis in malignant glioma tumors. Brain Pathol. 2005;15:318–326. doi: 10.1111/j.1750-3639.2005.tb00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindahl P., Johansson B.R., Levéen P., Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 19.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 20.Ridgway J., Zhang G., Wu Y., Stawicki S., Liang W.C., Chanthery Y., Kowalski J., Watts R.J., Callahan C., Kasman I., Singh M., Chien M., Tan C., Hongo J.A., de Sauvage F., Plowman G., Yan M. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 21.Noguera-Troise I., Daly C., Papadopoulos N.J., Coetzee S., Boland P., Gale N.W., Lin H.C., Yancopoulos G.D., Thurston G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 22.Nagy J.A., Dvorak A.M., Dvorak H.F. VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol. 2007;2:251–275. doi: 10.1146/annurev.pathol.2.010506.134925. [DOI] [PubMed] [Google Scholar]
- 23.Zagzag D., Zhong H., Scalzitti J.M., Laughner E., Simons J.W., Semenza G.L. Expression of hypoxia-inducible factor 1alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer. 2000;88:2606–2618. [PubMed] [Google Scholar]
- 24.Fischer I., Gagner J.P., Law M., Newcomb E.W., Zagzag D. Angiogenesis in gliomas: biology and molecular pathophysiology. Brain Pathol. 2005;15:297–310. doi: 10.1111/j.1750-3639.2005.tb00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamszus K., Ulbricht U., Matschke J., Brockmann M.A., Fillbrandt R., Westphal M. Levels of soluble vascular endothelial growth factor (VEGF) receptor 1 in astrocytic tumors and its relation to malignancy, vascularity, and VEGF-A. Clin Cancer Res. 2003;9:1399–1405. [PubMed] [Google Scholar]
- 26.Plate K.H., Breier G., Weich H.A., Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 27.Zagzag D., Lukyanov Y., Lan L., Ali M.A., Esencay M., Mendez O., Yee H., Voura E.B., Newcomb E.W. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab Invest. 2006;86:1221–1232. doi: 10.1038/labinvest.3700482. [DOI] [PubMed] [Google Scholar]
- 28.Strieter R.M., Belperio J.A., Phillips R.J., Keane M.P. CXC chemokines in angiogenesis of cancer. Semin Cancer Biol. 2004;14:195–200. doi: 10.1016/j.semcancer.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Salmaggi A., Gelati M., Pollo B., Frigerio S., Eoli M., Silvani A., Broggi G., Ciusani E., Croci D., Boiardi A., De Rossi M. CXCL12 in malignant glial tumors: a possible role in angiogenesis and cross-talk between endothelial and tumoral cells. J Neurooncol. 2004;67:305–317. doi: 10.1023/b:neon.0000024241.05346.24. [DOI] [PubMed] [Google Scholar]
- 30.Yang S.X., Chen J.H., Jiang X.F., Wang Q.L., Chen Z.Q., Zhao W., Feng Y.H., Xin R., Shi J.Q., Bian X.W. Activation of chemokine receptor CXCR4 in malignant glioma cells promotes the production of vascular endothelial growth factor. Biochem Biophys Res Commun. 2005;335:523–528. doi: 10.1016/j.bbrc.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 31.Jubb A.M., Pham T.Q., Hanby A.M., Frantz G.D., Peale F.V., Wu T.D., Koeppen H.W., Hillan K.J. Expression of vascular endothelial growth factor, hypoxia inducible factor 1alpha, and carbonic anhydrase IX in human tumours. J Clin Pathol. 2004;57:504–512. doi: 10.1136/jcp.2003.012963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arany Z., Foo S.Y., Ma Y., Ruas J.L., Bommi-Reddy A., Girnun G., Cooper M., Laznik D., Chinsomboon J., Rangwala S.M., Baek K.H., Rosenzweig A., Spiegelman B.M. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 33.Parliament M.B., Allalunis-Turner M.J., Franko A.J., Olive P.L., Mandyam R., Santos C., Wolokoff B. Vascular endothelial growth factor expression is independent of hypoxia in human malignant glioma spheroids and tumours. Br J Cancer. 2000;82:635–641. doi: 10.1054/bjoc.1999.0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaur B., Tan C., Brat D.J., Post D.E., Van Meir E.G. Genetic and hypoxic regulation of angiogenesis in gliomas. J Neurooncol. 2004;70:229–243. doi: 10.1007/s11060-004-2752-5. [DOI] [PubMed] [Google Scholar]
- 35.Ohgaki H., Dessen P., Jourde B., Horstmann S., Nishikawa T., Di Patre P.L., Burkhard C., Schuler D., Probst-Hensch N.M., Maiorka P.C., Baeza N., Pisani P., Yonekawa Y., Yasargil M.G., Lütolf U.M., Kleihues P. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 36.Folkins C., Shaked Y., Man S., Tang T., Lee C.R., Zhu Z., Hoffman R.M., Kerbel R.S. Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1 [Erratum appeared in Cancer Res 2009, 69:8216] Cancer Res. 2009;69:7243–7251. doi: 10.1158/0008-5472.CAN-09-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bao S., Wu Q., Sathornsumetee S., Hao Y., Li Z., Hjelmeland A.B., Shi Q., McLendon R.E., Bigner D.D., Rich J.N. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 38.Lyden D., Hattori K., Dias S., Costa C., Blaikie P., Butros L., Chadburn A., Heissig B., Marks W., Witte L., Wu Y., Hicklin D., Zhu Z., Hackett N.R., Crystal R.G., Moore M.A., Hajjar K.A., Manova K., Benezra R., Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 39.Machein M.R., Renninger S., de Lima-Hahn E., Plate K.H. Minor contribution of bone marrow-derived endothelial progenitors to the vascularization of murine gliomas. Brain Pathol. 2003;13:582–597. doi: 10.1111/j.1750-3639.2003.tb00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruzinova M.B., Schoer R.A., Gerald W., Egan J.E., Pandolfi P.P., Rafii S., Manova K., Mittal V., Benezra R. Effect of angiogenesis inhibition by Id loss and the contribution of bone-marrow-derived endothelial cells in spontaneous murine tumors. Cancer Cell. 2003;4:277–289. doi: 10.1016/s1535-6108(03)00240-x. [DOI] [PubMed] [Google Scholar]
- 41.Spring H., Schüler T., Arnold B., Hämmerling G.J., Ganss R. Chemokines direct endothelial progenitors into tumor neovessels. Proc Natl Acad Sci USA. 2005;102:18111–18116. doi: 10.1073/pnas.0507158102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rafii S., Lyden D. Cancer: A few to flip the angiogenic switch. Science. 2008;319:163–164. doi: 10.1126/science.1153615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furuhata S., Ando K., Oki M., Aoki K., Ohnishi S., Aoyagi K., Sasaki H., Sakamoto H., Yoshida T., Ohnami S. Gene expression profiles of endothelial progenitor cells by oligonucleotide microarray analysis. Mol Cell Biochem. 2007;298:125–138. doi: 10.1007/s11010-006-9359-4. [DOI] [PubMed] [Google Scholar]
- 44.Lewis C.E., De Palma M., Naldini L. Tie2-expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin-2. Cancer Res. 2007;67:8429–8432. doi: 10.1158/0008-5472.CAN-07-1684. [DOI] [PubMed] [Google Scholar]
- 45.Venneri M.A., De Palma M., Ponzoni M., Pucci F., Scielzo C., Zonari E., Mazzieri R., Doglioni C., Naldini L. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007;109:5276–5285. doi: 10.1182/blood-2006-10-053504. [DOI] [PubMed] [Google Scholar]
- 46.Murdoch C., Muthana M., Coffelt S.B., Lewis C.E. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 47.De Palma M., Murdoch C., Venneri M.A., Naldini L., Lewis C.E. Tie2-expressing monocytes: regulation of tumor angiogenesis and therapeutic implications. Trends Immunol. 2007;28:519–524. doi: 10.1016/j.it.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Li B., Sharpe E.E., Maupin A.B., Teleron A.A., Pyle A.L., Carmeliet P., Young P.P. VEGF and PlGF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at the site of tumor neovascularization. FASEB J. 2006;20:1495–1497. doi: 10.1096/fj.05-5137fje. [DOI] [PubMed] [Google Scholar]
- 49.Tabatabai G., Frank B., Möhle R., Weller M., Wick W. Irradiation and hypoxia promote homing of haematopoietic progenitor cells towards gliomas by TGF-beta-dependent HIF-1alpha-mediated induction of CXCL12. Brain. 2006;129:2426–2435. doi: 10.1093/brain/awl173. [DOI] [PubMed] [Google Scholar]
- 50.Du R., Lu K.V., Petritsch C., Liu P., Ganss R., Passegué E., Song H., Vandenberg S., Johnson R.S., Werb Z., Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubin J.B., Kung A.L., Klein R.S., Chan J.A., Sun Y., Schmidt K., Kieran M.W., Luster A.D., Segal R.A. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci USA. 2003;100:13513–13518. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zagzag D., Esencay M., Mendez O., Yee H., Smirnova I., Huang Y., Chiriboga L., Lukyanov E., Liu M., Newcomb E.W. Hypoxia- and vascular endothelial growth factor-induced stromal cell-derived factor-1alpha/CXCR4 expression in glioblastomas: one plausible explanation of Scherer's structures. Am J Pathol. 2008;173:545–560. doi: 10.2353/ajpath.2008.071197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aghi M., Cohen K.S., Klein R.J., Scadden D.T., Chiocca E.A. Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenotypes. Cancer Res. 2006;66:9054–9064. doi: 10.1158/0008-5472.CAN-05-3759. [DOI] [PubMed] [Google Scholar]
- 54.Smadja D.M., Bièche I., Uzan G., Bompais H., Muller L., Boisson-Vidal C., Vidaud M., Aiach M., Gaussem P. PAR-1 activation on human late endothelial progenitor cells enhances angiogenesis in vitro with upregulation of the SDF-1/CXCR4 system. Arterioscler Thromb Vasc Biol. 2005;25:2321–2327. doi: 10.1161/01.ATV.0000184762.63888.bd. [DOI] [PubMed] [Google Scholar]
- 55.Shaw J.P., Basch R., Shamamian P. Hematopoietic stem cells and endothelial cell precursors express Tie-2, CD31 and CD45. Blood Cells Mol Dis. 2004;32:168–175. doi: 10.1016/j.bcmd.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 56.Udani V., Santarelli J., Yung Y., Cheshier S., Andrews A., Kasad Z., Tse V. Differential expression of angiopoietin-1 and angiopoietin-2 may enhance recruitment of bone-marrow-derived endothelial precursor cells into brain tumors. Neurol Res. 2005;27:801–806. doi: 10.1179/016164105X49319. [DOI] [PubMed] [Google Scholar]
- 57.Murdoch C., Tazzyman S., Webster S., Lewis C.E. Expression of Tie-2 by human monocytes and their responses to angiopoietin-2. J Immunol. 2007;178:7405–7411. doi: 10.4049/jimmunol.178.11.7405. [DOI] [PubMed] [Google Scholar]
- 58.Kioi M., Vogel H., Schultz G., Hoffman R.M., Harsh G.R., Brown J.M. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Demou Z.N., Hendrix M.J. Microgenomics profile the endogenous angiogenic phenotype in subpopulations of aggressive melanoma. J Cell Biochem. 2008;105:562–573. doi: 10.1002/jcb.21855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yue W.Y., Chen Z.P. Does vasculogenic mimicry exist in astrocytoma? J Histochem Cytochem. 2005;53:997–1002. doi: 10.1369/jhc.4A6521.2005. [DOI] [PubMed] [Google Scholar]
- 61.Shaifer C.A., Huang J., Lin P.C. Glioblastoma cells incorporate into tumor vasculature and contribute to vascular radioresistance. Int J Cancer. 2010;127:2063–2075. doi: 10.1002/ijc.25249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu X.M., Zhang Q.P., Mu Y.G., Zhang X.H., Sai K., Pang J.C., Ng H.K., Chen Z.P. Clinical significance of vasculogenic mimicry in human gliomas. J Neurooncol. 2011;105:173–179. doi: 10.1007/s11060-011-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun B., Zhang D., Zhang S., Zhang W., Guo H., Zhao X. Hypoxia influences vasculogenic mimicry channel formation and tumor invasion-related protein expression in melanoma. Cancer Lett. 2007;249:188–197. doi: 10.1016/j.canlet.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 64.Dong J., Zhang Q., Huang Q., Chen H., Shen Y., Fei X., Zhang T., Diao Y., Wu Z., Qin Z., Lan Q., Gu X. Glioma stem cells involved in tumor tissue remodeling in a xenograft model. J Neurosurg. 2010;113:249–260. doi: 10.3171/2010.2.JNS09335. [DOI] [PubMed] [Google Scholar]
- 65.Chen Y., Jing Z., Luo C., Zhuang M., Xia J., Chen Z., Wang Y. Vasculogenic mimicry–potential target for glioblastoma therapy: an in vitro and in vivo study. Med Oncol. 2012;29:324–331. doi: 10.1007/s12032-010-9765-z. [DOI] [PubMed] [Google Scholar]
- 66.El Hallani S., Boisselier B., Peglion F., Rousseau A., Colin C., Idbaih A., Marie Y., Mokhtari K., Thomas J.L., Eichmann A., Delattre J.Y., Maniotis A.J., Sanson M. A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain. 2010;133:973–982. doi: 10.1093/brain/awq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soda Y., Marumoto T., Friedmann-Morvinski D., Soda M., Liu F., Michiue H., Pastorino S., Yang M., Hoffman R.M., Kesari S., Verma I.M. From the Cover: Feature Article: Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc Natl Acad Sci USA. 2011;108:4274–4280. doi: 10.1073/pnas.1016030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen Q., Goderie S.K., Jin L., Karanth N., Sun Y., Abramova N., Vincent P., Pumiglia K., Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 69.Ricci-Vitiani L., Pallini R., Biffoni M., Todaro M., Invernici G., Cenci T., Maira G., Parati E.A., Stassi G., Larocca L.M., De Maria R. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells [Erratum appeared in Nature 2011, 477:238 and in Nature 2011, 469:432] Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 70.Wang R., Chadalavada K., Wilshire J., Kowalik U., Hovinga K.E., Geber A., Fligelman B., Leversha M., Brennan C., Tabar V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 71.Balza E., Castellani P., Zijlstra A., Neri D., Zardi L., Siri A. Lack of specificity of endoglin expression for tumor blood vessels. Int J Cancer. 2001;94:579–585. doi: 10.1002/ijc.1505. [DOI] [PubMed] [Google Scholar]
- 72.Zhao Y., Dong J., Huang Q., Lou M., Wang A., Lan Q. Endothelial cell transdifferentiation of human glioma stem progenitor cells in vitro. Brain Res Bull. 2010;82:308–312. doi: 10.1016/j.brainresbull.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 73.Dong J., Zhao Y., Huang Q., Fei X., Diao Y., Shen Y., Xiao H., Zhang T., Lan Q., Gu X. Glioma stem/progenitor cells contribute to neovascularization via transdifferentiation. Stem Cell Rev. 2011;7:141–152. doi: 10.1007/s12015-010-9169-7. [DOI] [PubMed] [Google Scholar]
- 74.Williams S.C., Karajannis M.A., Chiriboga L., Golfinos J.G., von Deimling A., Zagzag D. R132H-mutation of isocitrate dehydrogenase-1 is not sufficient for HIF-1alpha upregulation in adult glioma. Acta Neuropathol. 2011;121:279–281. doi: 10.1007/s00401-010-0790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Calabrese C., Poppleton H., Kocak M., Hogg T.L., Fuller C., Hamner B., Oh E.Y., Gaber M.W., Finklestein D., Allen M., Frank A., Bayazitov I.T., Zakharenko S.S., Gajjar A., Davidoff A., Gilbertson R.J. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 76.Norden A.D., Drappatz J., Wen P.Y. Antiangiogenic therapies for high-grade glioma. Nat Rev Neurol. 2009;5:610–620. doi: 10.1038/nrneurol.2009.159. [DOI] [PubMed] [Google Scholar]
- 77.Gomez-Manzano C., Holash J., Fueyo J., Xu J., Conrad C.A., Aldape K.D., de Groot J.F., Bekele B.N., Yung W.K. VEGF Trap induces antiglioma effect at different stages of disease. Neuro Oncol. 2008;10:940–945. doi: 10.1215/15228517-2008-061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kunkel P., Ulbricht U., Bohlen P., Brockmann M.A., Fillbrandt R., Stavrou D., Westphal M., Lamszus K. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001;61:6624–6628. [PubMed] [Google Scholar]
- 79.Norden A.D., Young G.S., Setayesh K., Muzikansky A., Klufas R., Ross G.L., Ciampa A.S., Ebbeling L.G., Levy B., Drappatz J., Kesari S., Wen P.Y. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 80.Narayana A., Kelly P., Golfinos J., Parker E., Johnson G., Knopp E., Zagzag D., Fischer I., Raza S., Medabalmi P., Eagan P., Gruber M.L. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival. J Neurosurg. 2009;110:173–180. doi: 10.3171/2008.4.17492. [DOI] [PubMed] [Google Scholar]
- 81.Beal K., Abrey L.E., Gutin P.H. Antiangiogenic agents in the treatment of recurrent or newly diagnosed glioblastoma: analysis of single-agent and combined modality approaches. Radiat Oncol. 2011;6:2–15. doi: 10.1186/1748-717X-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fischer I., Cunliffe C.H., Bollo R.J., Raza S., Monoky D., Chiriboga L., Parker E.C., Golfinos J.G., Kelly P.J., Knopp E.A., Gruber M.L., Zagzag D., Narayana A. High-grade glioma before and after treatment with radiation and Avastin: initial observations. Neuro Oncol. 2008;10:700–708. doi: 10.1215/15228517-2008-042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ebos J.M., Kerbel R.S. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis [Erratum appeared in Nat Rev Clin Oncol 2011, 8:316 and in Nat Rev Clin Oncol 2011, 8:221] Nat Rev Clin Oncol. 2011;8:210–221. doi: 10.1038/nrclinonc.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ebos J.M., Lee C.R., Cruz-Munoz W., Bjarnason G.A., Christensen J.G., Kerbel R.S. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rubenstein J.L., Kim J., Ozawa T., Zhang M., Westphal M., Deen D.F., Shuman M.A. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2:306–314. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Auf G., Jabouille A., Guérit S., Pineau R., Delugin M., Bouchecareilh M., Magnin N., Favereaux A., Maitre M., Gaiser T., von Deimling A., Czabanka M., Vajkoczy P., Chevet E., Bikfalvi A., Moenner M. Inositol-requiring enzyme 1alpha is a key regulator of angiogenesis and invasion in malignant glioma. Proc Natl Acad Sci USA. 2010;107:15553–15558. doi: 10.1073/pnas.0914072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sakariassen P.Ø., Prestegarden L., Wang J., Skaftnesmo K.O., Mahesparan R., Molthoff C., Sminia P., Sundlisaeter E., Misra A., Tysnes B.B., Chekenya M., Peters H., Lende G., Kalland K.H., Øyan A.M., Petersen K., Jonassen I., van der Kogel A., Feuerstein B.G., Terzis A.J., Bjerkvig R., Enger P.Ø. Angiogenesis-independent tumor growth mediated by stem-like cancer cells. Proc Natl Acad Sci USA. 2006;103:16466–16471. doi: 10.1073/pnas.0607668103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zuniga R.M., Torcuator R., Jain R., Anderson J., Doyle T., Ellika S., Schultz L., Mikkelsen T. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol. 2009;91:329–336. doi: 10.1007/s11060-008-9718-y. [DOI] [PubMed] [Google Scholar]
- 89.Lucio-Eterovic A.K., Piao Y., de Groot J.F. Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res. 2009;15:4589–4599. doi: 10.1158/1078-0432.CCR-09-0575. [DOI] [PubMed] [Google Scholar]
- 90.Pàez-Ribes M., Allen E., Hudock J., Takeda T., Okuyama H., Viñals F., Inoue M., Bergers G., Hanahan D., Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barcellos-Hoff M.H., Newcomb E.W., Zagzag D., Narayana A. Therapeutic targets in malignant glioblastoma microenvironment. Semin Radiat Oncol. 2009;19:163–170. doi: 10.1016/j.semradonc.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.di Tomaso E., Snuderl M., Kamoun W.S., Duda D.G., Auluck P.K., Fazlollahi L., Andronesi O.C., Frosch M.P., Wen P.Y., Plotkin S.R., Hedley-Whyte E.T., Sorensen A.G., Batchelor T.T., Jain R.K. Glioblastoma recurrence after cediranib therapy in patients: lack of “rebound” revascularization as mode of escape. Cancer Res. 2011;71:19–28. doi: 10.1158/0008-5472.CAN-10-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zheng P.P., Hop W.C., Luider T.M., Sillevis Smitt P.A., Kros J.M. Increased levels of circulating endothelial progenitor cells and circulating endothelial nitric oxide synthase in patients with gliomas. Ann Neurol. 2007;62:40–48. doi: 10.1002/ana.21151. [DOI] [PubMed] [Google Scholar]
- 94.Batchelor T.T., Sorensen A.G., di Tomaso E., Zhang W.T., Duda D.G., Cohen K.S., Kozak K.R., Cahill D.P., Chen P.J., Zhu M., Ancukiewicz M., Mrugala M.M., Plotkin S., Drappatz J., Louis D.N., Ivy P., Scadden D.T., Benner T., Loeffler J.S., Wen P.Y., Jain R.K. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gerstner E.R., Frosch M.P., Batchelor T.T. Diffusion magnetic resonance imaging detects pathologically confirmed, nonenhancing tumor progression in a patient with recurrent glioblastoma receiving bevacizumab. J Clin Oncol. 2010;28:e91–e93. doi: 10.1200/JCO.2009.25.0233. [DOI] [PubMed] [Google Scholar]
- 96.Graeber T.G., Osmanian C., Jacks T., Housman D.E., Koch C.J., Lowe S.W., Giaccia A.J. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 97.Rapisarda A., Hollingshead M., Uranchimeg B., Bonomi C.A., Borgel S.D., Carter J.P., Gehrs B., Raffeld M., Kinders R.J., Parchment R., Anver M.R., Shoemaker R.H., Melillo G. Increased antitumor activity of bevacizumab in combination with hypoxia inducible factor-1 inhibition. Mol Cancer Ther. 2009;8:1867–1877. doi: 10.1158/1535-7163.MCT-09-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Selvakumaran M., Yao K.S., Feldman M.D., O'Dwyer P.J. Antitumor effect of the angiogenesis inhibitor bevacizumab is dependent on susceptibility of tumors to hypoxia-induced apoptosis. Biochem Pharmacol. 2008;75:627–638. doi: 10.1016/j.bcp.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blagosklonny M.V. Hypoxia-inducible factor: Achilles' heel of antiangiogenic cancer therapy (review) Int J Oncol. 2001;19:257–262. doi: 10.3892/ijo.19.2.257. [DOI] [PubMed] [Google Scholar]
- 100.Keunen O., Johansson M., Oudin A., Sanzey M., Rahim S.A., Fack F., Thorsen F., Taxt T., Bartos M., Jirik R., Miletic H., Wang J., Stieber D., Stuhr L., Moen I., Rygh C.B., Bjerkvig R., Niclou S.P. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci USA. 2011;108:3749–3754. doi: 10.1073/pnas.1014480108. [DOI] [PMC free article] [PubMed] [Google Scholar]