Abstract
Respiratory disturbances are a common feature of panic disorder and present as breathing irregularity, hyperventilation, and increased sensitivity to carbon dioxide. Common therapeutic interventions, such as tricyclic (TCA) and selective serotonin reuptake inhibitor (SSRI) antidepressants, have been shown to ameliorate not only the psychological components of panic disorder but also the respiratory disturbances. These drugs are also prescribed for generalized anxiety and depressive disorders, neither of which are characterized by respiratory disturbances, and previous studies have demonstrated that TCAs and SSRIs exert effects on basal respiratory activity in animal models without panic disorder symptoms. Whether serotonin-norepinephrine reuptake inhibitors (SNRIs) have similar effects on respiratory activity remains to be determined. Therefore, the current study was designed to investigate the effects of chronic administration of the SNRI antidepressant venlafaxine (VHCL) on basal respiratory output. For these experiments, we recorded phrenic nerve discharge in an in vitro arterially-perfused adult mouse preparation and diaphragm electromyogram (EMG) activity in an in vivo urethane-anesthetized adult mouse preparation. We found that following 28-d VHCL administration, basal respiratory burst frequency was markedly reduced due to an increase in expiratory duration (TE), and the inspiratory duty cycle (TI/Ttot) was significantly shortened. In addition, post-inspiratory and spurious expiratory discharges were seen in vitro. Based on our observations, we suggest that drugs capable of simultaneously blocking both 5-HT and NE reuptake transporters have the potential to influence the respiratory control network in patients using SNRI therapy.
Keywords: serotonin-norepinephrine reuptake inhibitor (SNRI), venlafaxine, panic disorder, respiratory rhythm, breathing
1. Introduction
Respiratory disturbances have emerged as a common feature in individuals suffering from panic disorder and often present as hyperventilation, increased sensations of breathlessness, and/or alterations in the response to carbon dioxide (Nardi et al. 2009; Sardinha et al. 2009). It has generally been accepted that these symptoms are the consequence of a faulty or maladaptive control center within the brainstem (Klein, 1993), and as such neurotransmitter systems within this region have become a potential target for a number of available therapeutics. The most common therapeutic interventions for panic disorder exploit serotonergic (5-HT) and/or noradrenergic (NE) mechanisms, and include tricyclic antidepressants (TCA) (e.g., clomipramine, imipramine, nortriptyline), selective serotonin reuptake inhibitors (SSRI) (e.g., paroxetine, fluoxetine), and serotonin-norepinephrine reuptake inhibitors (SNRI) (e.g., venlafaxine, duloxetine).
To date, both TCAs and SSRIs have been evaluated for their potential to impact the respiratory disturbances associated with panic disorder. While only a limited number of studies have addressed the respiratory phenotype associated with their use, studies examining the efficacy of these therapeutic interventions in panic disorder have demonstrated that these types of drugs can benefit both the psychological (Anderson, 2000; Gorman and Kent, 1999) and physiological (Gorman et al., 1997; Nardi et al., 2009; Pols et al., 1993, 1996) components of the condition. In panic disorder patients, for example, administration of TCAs has been shown to reduce the ventilatory response to carbon dioxide (Gorman et al., 1997; Pols et al., 1993) while administration of SSRI antidepressants has been demonstrated to reduce both the sensitivity to carbon dioxide and breathing frequency (Bocola et al., 1998; Gorman et al., 1997; Pols et al., 1996).
Antidepressants, however, are prescribed for psychiatric disorders other than panic disorder. These include generalized anxiety and depressive conditions, which are generally devoid of respiratory abnormalities. Thus, it is important to understand the influence of these drugs on respiratory activity in individuals that do not exhibit respiratory-related symptoms. Moreover, since both 5-HT and NE have been shown to participate in multiple aspects of central respiratory control (e.g., Al-Zubaidy et al., 1996; Champagnat et al., 1979; Di Pasquale et al., 1992; McCrimmon and Lalley, 1980; Viemari and Hilaire, 2002), it is likely that administration of TCAs, SSRIs, and SNRIs would alter basal respiratory activity. In support of this possibility, animal studies have demonstrated that administration of TCAs and SSRIs influences basal respiratory activity. For example, chronic administration of the SSRI fluoxetine enhances respiratory rate (Annerbrink et al., 2010; Olsson et al., 2004), but elicits negligible effects on resting minute ventilation in rats (Annerbrink et al., 2010; Olsson et al., 2004; Taylor et al., 2004) and goats (Henderson et al., 1999, 2000), observations that are consistent with the proposed excitatory role of 5-HT in central respiratory control (see recent reviews by Hodges and Richerson 2008see recent reviews by Hodges and Richerson 2010). The role of NE in respiratory control is a little less clear and it is complicated by potential interspecies differences. For example, acute administration of exogenous NE results in respiratory excitation in mice (Viemari and Hiliare 2002; Viemari and Ramirez 2006) and respiratory depression in rats (Al-Zubaidy et al. 1996; Champagnat et al. 1979; Errchidi et al. 1990, 1991; Hilaire et al. 1989) while the administration of the noradrenergic TCA desipramine appears to be ineffective in modifying basal tidal volume, respiratory rate, and minute ventilation in wildtype mice (Roux et al., 2007).
While the above studies provide insight into the effects of TCAs and SSRIs on respiratory activity, to date, no studies have evaluated the influence of SNRIs in respiratory control. Furthermore, the above studies using TCAs and SSRIs have provided insight into the effects of NE or 5-HT alone, and not on the influence of 5-HT and NE combined. Therefore, the purpose of the current investigation was to examine the effects of chronic administration of a SNRI antidepressant on basal respiratory timing and patterning in the adult wildtype mouse.
2. METHODS
2.1 General Methods
All experiments were performed under protocols approved by the Institutional Animal Care and Use Committee at the State University of New York at Stony Brook in accordance with Public Health Service Policy on Human Care and Use of Laboratory Animals. Experiments were conducted in 27 male adult C57BL/6 mice weighing 23–30 g.
2.2 Chronic Injection Protocol
Mice received once daily intraperitoneal (i.p.) injections of either venlafaxine hydrochloride (VHCL, 40 mg/kg; dissolved in 0.9% NaCl) or 0.9% NaCl (SAL, vehicle) for 28 days. During the course of the chronic injection protocol, all mice were housed in pairs under standard conditions (12:12 hour light:dark cycle) and provided food and water ad libitum. Mice received injections at the same time each morning, and were monitored daily for body weight, food/water intake, physical condition, and behavior. Following the 28-d injection protocol, respiratory output was evaluated using either an in vitro arterially-perfused adult mouse preparation or an in vivo urethane-anesthetized adult mouse preparation.
2.3 In Vitro Arterially-Perfused Adult Mouse Preparation
Mice (VHCL, n=9; SAL, n=7) were deeply anesthetized using 2–5% isoflurane until respiratory movements ceased and no withdrawal reflex or increase in ventilation was elicited in response to a noxious tail pinch. The mice were then transected sub-diaphragmatically and submerged in a bath of chilled artificial cerebrospinal fluid (aCSF) gassed with 95% O2-5% CO2 containing (in mM): 125 NaCl, 24 NaHCO3, 3.0 KCl, 2.5 CaCl2, 1.25 MgSO4, 1.25 KH2PO4, and 10 dextrose. The mice were decerebrated at the pre-collicular level using aspiration, the skin and lungs were removed, and the descending thoracic aorta was separated from the vertebral column. The preparation was transferred to a recording chamber, and the descending thoracic aorta was cannulated and retrogradely perfused with a modified aCSF containing an oncotic agent (2.5% Ficoll 70; Sigma Chemical Co., St. Louis, MO) using a peristaltic pump (Minipuls 3; Gilson, Inc., Middleton, WI). The perfusate was gassed continuously with 95% O2-5% CO2, warmed to ~30 °C (as measured in the cranial vault), passed through a bubble trap to remove gas bubbles and dampen pulsations introduced by the pump, and filtered using a nylon mesh (pore size 40 μM; Millipore, Bedford, MA) prior to entering the aorta. Flow was continuously monitored in the circuit just proximal to the aortic catheter using an in-line microturbine flowmeter (Model G-111 Flo-Meter, McMillan Co., Georgetown, TX). Following aortic cannulation, the rate of the pump was gradually increased until respiratory rhythmic activity resumed after which one or both phrenic nerves were dissected from the surrounding connective tissue and placed over a platinum rod hook electrode for recording. The rate of the pump was further adjusted, if necessary, to obtain stable phrenic nerve discharge that exhibited a eupneic (ramp-like) discharge pattern. Prior to beginning the recording protocol, respiratory-related motor movements were abolished by adding vecuronium bromide (0.5 mg total dose; 2.5 μg/ml) to the perfusate.
2.4 In Vivo Adult Mouse Preparation
Mice (VHCL, n=6; SAL, n=5) were anesthetized with an i.p. injection of urethane (Sigma Aldrich, ~1.6 g/kg) and supplied with a gas mixture of 40% O2 (in a balance of N2) from a nose cone. The adequacy of anesthesia was regularly verified by absence of a withdrawal reflex to a noxious paw pinch, and supplemented as needed (~0.16 g/kg, i.p.). Body temperature was monitored using a rectal probe and maintained at 37.2±0.2°C throughout the experiment using a heating pad and heat lamp as necessary. Diaphragm EMG activity was recorded using a platinum-iridium wire bipolar electrode while the mice breathed spontaneously.
2.5 Data Acquisition and Analyses
Phrenic nerve discharge (in vitro) and diaphragm EMG activity (in vivo) were amplified (10k and 1k, respectively), notch filtered at 60 Hz, analog filtered between 10 Hz and 1 kHz, and a moving average was obtained using a third-order Paynter filter with a 50-ms time constant. Both raw and averaged phrenic (in vitro) or EMG (in vivo) burst activities were recorded on a computer at a sampling rate of 2 kHz (Chart 5.0, PowerLab, ADInstruments, Colorado Springs, CO) and on digital tape (DAT; Model CDAT16 Recorder, Cygnus, Delaware Water Gap, PA) at a sampling rate of 2.5 kHz for off-line analyses (MatLab 7.0.1).
Inspiratory burst frequency, burst duration (TI), the duration between bursts (TE), the duration of the total respiratory cycle (Ttot), inspiratory duty cycle (TI/Ttot), and time-to-peak (Tpeak) were determined under basal conditions. Tpeak was normalized to TI (Tpeak/TI, expressed as a percentage). Average values for these variables were calculated from 10 (in vitro) or 20 (in vivo) consecutive respiratory cycles.
All data are reported as mean±SE. Comparisons were made between the VHCL and SAL (vehicle) treatment groups for variables associated with basal respiratory timing (i.e., burst frequency, TI, TE, Ttot, TI/Ttot) and patterning (i.e., Tpeak/TI) characteristics. For these comparisons, statistical significance was evaluated using an unpaired t-test. For evaluation of the chronic injection protocol on body weight, body weight measurements were compared at injection day 1 versus injection day 28 using a paired t-test. Differences in body weight between the VCHL and SAL treatment groups were also determined for each of these time points using an unpaired t-test. For all comparisons, the criterion level for determination of statistical significance was set to P<0.05.
3. RESULTS
3.1 Effects of chronic VHCL injections
Daily monitoring of the mice revealed that no changes in physical appearance or behavior (i.e., inadequate or excessive grooming, aggression, etc.) were observed in response to the SAL or VHCL injection protocol. Moreover, both SAL- and VHCL-treated mice gained a significant amount of body weight over the course of the 28-d injection protocol. During this time, body weight increased from 22.2±0.5 g to 26.2±0.3 g (P<0.001) in SAL-treated mice and from 22.3±0.4 g to 25.5±0.4 g (P<0.001) in VHCL-treated mice. While the mice in both groups gained weight, no significant differences in weight gain were noted between the groups (P=0.201).
3.2 Arterially-perfused mouse preparation: effects of VHCL on basal phrenic nerve discharge
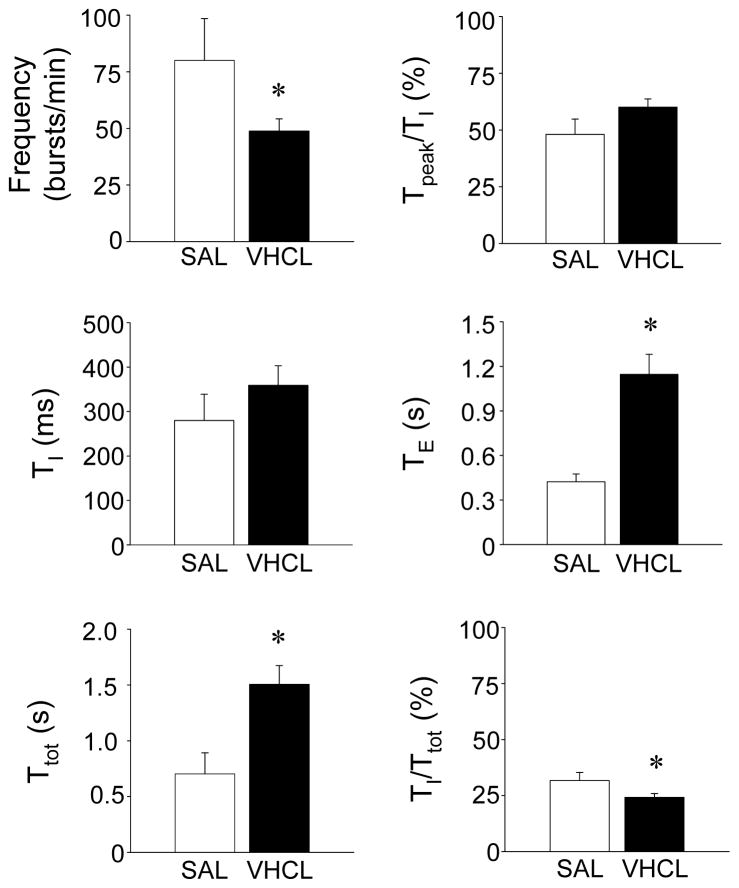
In SAL-treated mice, phrenic nerve discharge was characterized by a regular and fast rhythmic pattern of output (Fig. 1). Phrenic bursts exhibited an augmenting discharge pattern (Fig. 1) albeit the Tpeak/TI corresponded to 48.0±5.0% (Fig. 2). In these experiments, burst frequency averaged 80±18 bursts/min, and TI and TE were 280±59 ms and 423±52 ms, respectively (Fig. 2). TI/Ttot in SAL-treated mice accounted for ~32% of the respiratory cycle. In addition, transient spontaneous apneic periods characteristic of the C57BL/6 mouse strain (as previously reported by Stettner et al. 2007, 2008a,b) were observed throughout the recording protocol (not shown).
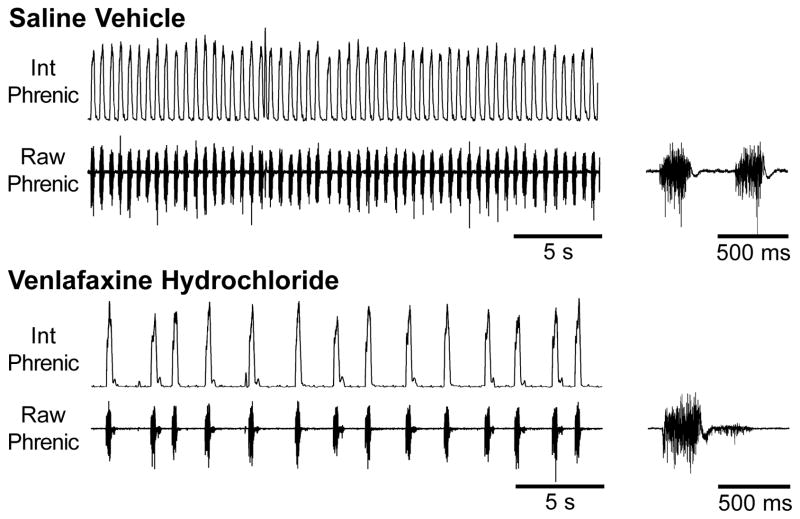
Figure 1. Effects of chronic (28-d) SAL and VHCL treatment on phrenic nerve discharge in the in vitro arterially-perfused adult mouse preparation.
Example traces of integrated (Int) and raw phrenic nerve discharge observed in SAL-treated (upper panel) and VHCL-treated (lower panel) mouse preparations. In VHCL-treated mice, phrenic nerve discharge was regular but slower than that seen in SAL-treated mice. Post-inspiratory discharge was also observed in VHCL-treated mice. Both 30-s duration traces (left) and expanded 1-s traces (right) are shown.
Figure 2. Effects of chronic (28-d) SAL and VHCL treatment on timing and patterning of phrenic nerve discharge.
Summary data showing that VHCL-treated mice exhibited a significant reduction in burst frequency, a substantial prolongation of TE, and a significant decrease in TI/Ttot. No significant differences in TI or Tpeak/TI were noted. Asterisks denote a statistically significant difference (P<0.05) between SAL- and VHCL-treated mice.
In each of the experiments conducted chronic treatment with VHCL altered phrenic nerve discharge. Following chronic administration of VHCL, phrenic nerve discharge was characterized by a regular but slower rhythm than that observed in SAL-treated control mice (Fig. 1). Phrenic burst frequency was significantly reduced to 49.0±5.4 bursts/min (P=0.043), and this reduction was mediated by a significant prolongation of TE (P=0.002; Fig. 2). In VHCL-treated mice, TI was slightly prolonged albeit this increase was not significant (P=0.3; power=0.15). Due to these timing changes, TI/Ttot in VHCL-treated mice accounted for only ~24% of the respiratory cycle, which was a significantly lower than that observed in SAL-treated mice (P=0.004; Fig. 2). In these preparations, phrenic bursts still exhibited an augmenting or ramp-like discharge pattern, with Tpeak/TI being 60.0±3.6% (Fig. 2); however, there was a notable change in phrenic nerve discharge following chronic drug treatment, such that in 7 of the 9 preparations, post-inspiratory activity (Fig. 1) and/or spurious expiratory activity were observed. Spontaneous apneic periods were also observed following VHCL treatment.
3.3 In vivo adult mouse preparation: effects of VHCL on basal EMG discharge
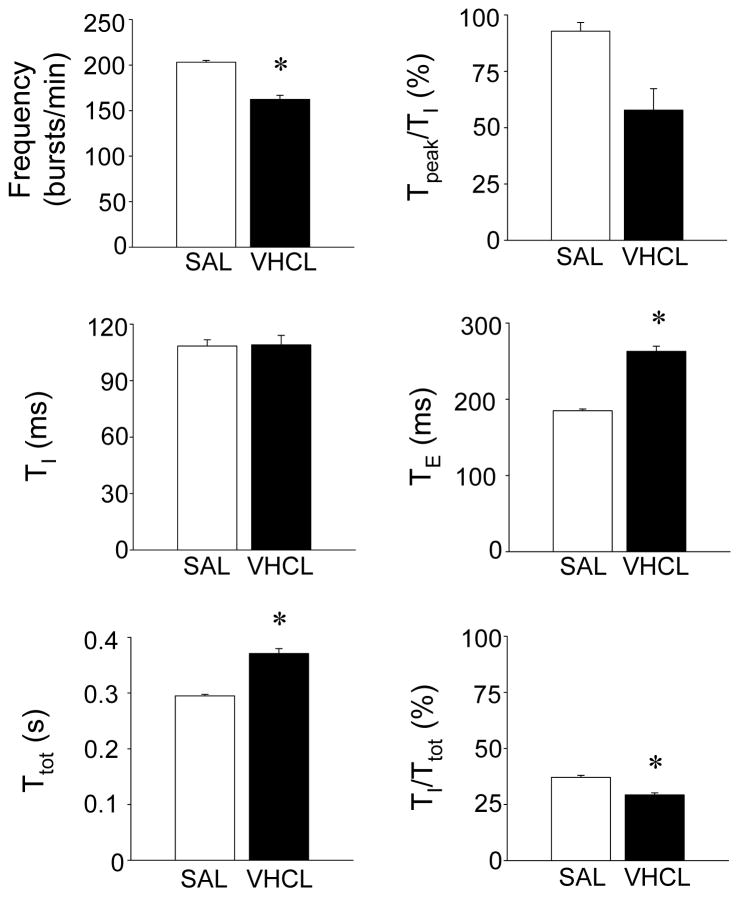
In SAL-treated mice, basal diaphragm EMG activity was characterized by an augmenting discharge pattern (Fig. 3), with Tpeak/TI corresponding to 92.8±3.9%. In these experiments, burst frequency averaged 204.0±1.9 bursts/min, and TI and TE were 109±3 ms and 185±2 ms, respectively (Fig. 4).
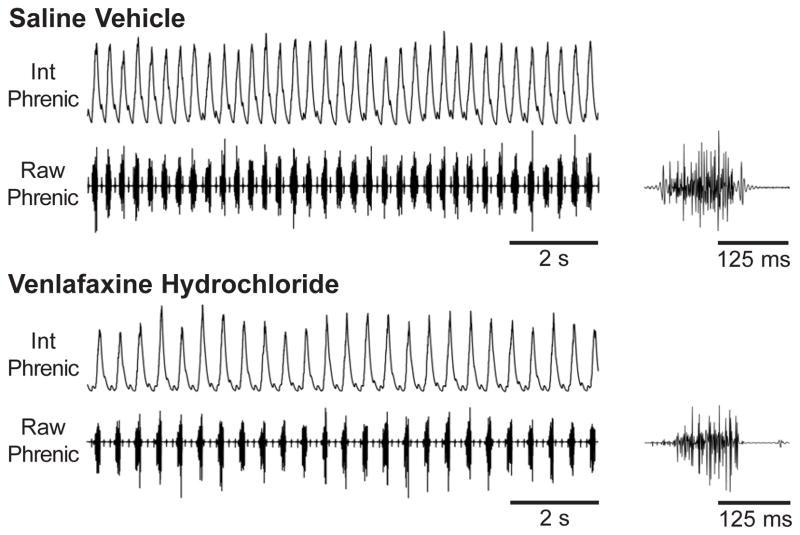
Figure 3. Effects of chronic (28-d) SAL and VHCL treatment on diaphragm EMG activity in the in vivo urethane-anesthetized mouse preparation.
Example traces of integrated (Int) and raw diaphragm EMG activity observed in SAL-treated (upper panel) and VHCL-treated (lower panel) mouse preparations. In VHCL-treated mice, respiratory burst frequency was regular but slower than that seen in SAL-treated mice. Both 10-s duration traces (left) and expanded 250-ms traces (right) are shown.
Figure 4. Effects of chronic (28-d) SAL and VHCL treatment on timing and patterning of diaphragm EMG bursts.
Summary data showing that VHCL-treated mice exhibited a reduced breathing frequency, which resulted from a significant prolongation of TE. A significant decrease in TI/Ttot was also observed. No significant differences in TI or Tpeak/TI were noted. Asterisks denote a statistically significant difference (P<0.05) between SAL- and VHCL-treated mice.
In each of these experiments, chronic treatment with VHCL also modified basal respiratory output. Following chronic administration of VHCL, EMG burst frequency was reduced to 162±4.5 bursts/min (P<0.001), and this reduction was mediated by a prolongation of TE to 263±7 ms (P<0.001; Fig. 4), similar to the effect observed in our in vitro experiments. TI was unaffected by VHCL treatment, but TI/Ttot was reduced to 29.3±0.9% of the respiratory cycle, which was a significantly lower than that observed in SAL-treated mice (37.1±0.9%; P<0.001; Fig. 5). In these preparations, the EMG burst pattern was also slightly modified, though not significantly, with Tpeak/TI shifting to an earlier time point in the burst (57.8±9.4%; P=0.091).
4. Discussion
4.1 Influence of venlafaxine on basal respiratory output
To our knowledge, this is the first study to address the effects of chronic administration of a SNRI on basal respiratory output both in vitro and in vivo. We found that chronic treatment with VHCL reduced respiratory burst frequency by prolonging TE, an effect observed in both the in vitro and in vivo preparations. Drug treatment also reduced the inspiratory duty cycle (TI/Ttot), produced a notable change the pattern of inspiratory motor activity (although it still exhibited an augmenting/ramp-like discharge pattern), and in some cases elicited post-inspiratory and/or spurious expiratory discharges when compared to mice similarly treated with SAL. We interpret these findings to indicate that simultaneous blockade of 5-HT and NE reuptake transporters depresses the central respiratory neural network under basal conditions.
4.2 Venlafaxine hydrochloride: binding affinity, selectivity, and potency for 5-HT and NE reuptake transporters
For the current study, we chose to use VHCL for our chronic administration protocol because it has been shown to block both 5-HT and NE reuptake transporters. The binding affinities for these reuptake transporters, however, are not identical, with a number of studies demonstrating that VHCL has a higher affinity for the 5-HT reuptake transporter than for the NE reuptake transporter (Béïque et al., 1998b; Bymaster et al., 2001; Muth et al., 1986; Owens et al., 1997). Furthermore, studies in the adult rat have demonstrated a dose-dependent effect of VHCL, with low doses (i.e., 5 mg/kg) inhibiting only 5-HT reuptake and higher doses (i.e., 40 mg/kg) inhibiting both 5-HT and NE reuptake processes (Béïque et al., 1998a, 1999, 2000a,2000b; Redrobe et al., 1998). A similar dose-dependent effect of venlafaxine treatment on extracellular levels of 5-HT (Béïque et al., 2000b; Dawson et al., 1999; Gur et al., 1999; Hatanaka et al., 2000b) and NE (Béïque et al., 2000b; Dawson et al., 1999; Hatanaka et al., 2000a) has also been noted. For the current study, we selected a high but clinically relevant dose of VHCL (i.e., 40 mg/kg; Béïque et al., 1998a, 1999a, 2000a,b; Redrobe et al., 1998) to ensure that both 5-HT and NE reuptake transporters would be inhibited, and while the exact magnitude of inhibition in the current study is unknown, we are confident that this dose was sufficient to block both reuptake transporters, resulting in an increase in extracellular 5-HT and NE levels.
Administration of VHCL may also affect the sensitivity or density of 5-HT and/or NE receptors. It has been demonstrated that chronic administration of VHCL desensitizes 5-HT1B, but not 5-HT1A, autoreceptors in the hippocampus (Béïque et al., 2000a,b) and reduces 5-HT1A and 5-HT2 receptor expression in cortex in rats (McGrath and Norman, 1998). If VHCL treatment induces a reduction in 5-HT1A and/or 5-HT2 receptors, this could explain, at least in part, the reduced respiratory burst frequency observed in the current study since activation of these receptor subtypes has been shown to increase respiratory burst frequency both in vitro and in vivo (Di Pasquale et al., 1992; Tryba et al., 2006; Valic et al., 2008). Additional studies, however, are needed to identify potential changes in receptor densities that occur within the respiratory brainstem, if any, as a result of chronic VHCL treatment.
4.3 Experimental Considerations
While the objective of numerous studies has been focused on characterizing the behavioral effects of antidepressant treatment in rodents (see File and Tucker, 1986 for review), this was not the primary objective of the current study. We did, however, monitor the mice daily for changes in body weight, food/water intake, general physical condition, and behavior during the 28-d VHCL administration protocol in order to minimize the likelihood of including animals experiencing adverse effects from drug-treatment into our data pool. We found that chronic treatment with this drug did not produce any abnormal changes in body weight (as compared to SAL-treated mice). This finding appears to be in contrast to previous reports from studies utilizing chronic TCA treatment in rats, with most of these studies reporting that treated animals fail to gain (or lose) weight and exhibit a reduction in water consumption (File and Tucker, 1986). Chronic TCA treatment has also been reported to reduce locomotor activity and produce anxiety-like behaviors and aggression in animal models (File and Tucker, 1986; Tucker and File, 1986). We suggest that differences between the previous observations and our current findings may be related to differences in the class (TCA versus SNRI) and/or dosages of the antidepressants used.
In the current study, the respiratory phenotype resulting from chronic VHCL administration was evaluated in both in vitro and in vivo preparations, with both preparations yielding similar observations. The in vitro preparation includes removal of all neural tissue rostral to the superior colliculi (i.e., decerebrate), elimination of vagal input from the lungs, maintenance at a lower working temperature (c.a., 30°C), and perfusion of the vascular system and cranial vault with an aCSF. In this manner, acute elevations of neurotransmitter within the synaptic environment may be “washed out” and the respiratory phenotype observed may reflect changes in receptor expression on cell membranes, and not acute (and/or chronic) neurotransmitter elevations. The removal of neural substrate in this preparation may also impact the degree to which changes in respiratory output are influenced by potential interactions with higher CNS-processing centers. To address some of these limitations, the effect of chronic VHCL administration on respiratory output was also examined in an in vivo mouse preparation, where mice were maintained at normal body temperature (c.a., 37°C), breathed spontaneously, and neural substrate and lung vagal afferent inputs were left intact. We cannot, however, exclude the possibility that the use of anesthesia in vivo may have influenced CNS activity as well as eliminated wakefulness stimuli required to shape respiratory output. The use of anesthesia could also lead to changes in upper airway resistance, which could potentially affect some of the respiratory variables measured in the current study in both the SAL- and VHCL-treated mice. Moreover, since 5-HT neurotransmission participates in the control of hypoglossal motoneuron excitability and upper airway muscle activity (Brandes et al., 2006), the respiratory changes observed in VHCL-treated mice may have been attenuated by the use of anesthesia. Additionally, in the in vivo preparation, vagal afferent input arising from the lungs could reflexively influence respiratory activity and the magnitude of the VHCL-treatment-related effects. We believe that if vagal reflexes contributed to the differences noted, their contribution was negligible since similar changes in respiratory activity were observed in the in vitro preparation, where lung afferents were removed. Regardless of the issues raised above, both preparations employed in the current study demonstrated that following 28-d VHCL administration, basal respiratory rhythm was altered in a similar direction.
4.4 Effects of chronic elevations of endogenous 5-HT and NE on respiratory activity and clinical significance
While the effects of SNRI antidepressant treatment on respiratory activity have not previously been examined, a number of studies have investigated the effects of tricyclic and SSRI antidepressants on various aspects of respiratory control. For example, in unanesthetized rats, systemic administration of SSRI antidepressants has been shown to elicit negligible effects on resting (basal) minute ventilation (Olsson et al., 2004; Taylor et al., 2004) although resting respiratory rate was significantly increased (Annerbrink et al., 2010; Olsson et al., 2004). Systemic administration of SSRI antidepressants has also been shown to be ineffective in altering minute ventilation in unanesthetized goats (Henderson et al., 1999, 2000). With regards to the noradrenergic neurotransmitter system, systemic administration of the noradrenergic TCA desipramine has been shown to be ineffective in modifying basal respiratory activity in wildtype mice (Roux et al., 2007), but in Mecp2-deficient mice, it reduced the number and severity of the apneas observed, thus stabilizing the abnormal breathing pattern seen in these mice (Roux et al., 2007; Zanella et al., 2008). These mice, however, have reduced NE (and 5-HT) content (Viemari et al., 2005); thus, it is not clear whether chronic desipramine treatment normalizes NE or leads to an excess level of NE. Thus, while the above observations from animal studies indicate that treatment with SSRIs and noradrenergic TCAs result in no change to (or enhanced) ventilation, the findings from the current study clearly demonstrate that breathing frequency is markedly reduced by SNRI treatment. While the precise reasons for this difference are not known, the previous studies employing SSRI antidepressants or desipramine were designed to examine the effects of elevated levels of only 5-HT or only NE, respectively, while in the current study, the SNRI VHCL would be expected to simultaneously elevate both 5-HT and NE levels. Additional factors that could explain this difference include differences in the degree of inhibition of the reuptake transporters, the effects of these antidepressants on the expression and sensitivity of various 5-HT and/or NE receptors in the respiratory brainstem, species differences in receptor expression and function, and the contribution of antidepressant-mediated effects from higher brain centers.
In a wide variety of clinical disorders, antidepressant treatments have been implemented in an attempt to ameliorate respiratory dysfunction. This includes the use of the 5-HT1A receptor agonist buspirone in eliminating apneustic disturbances in human patients (Wilken et al., 1997) and the noradrenergic TCA desipramine reducing the number and severity of apneas and prolonging the lifespan in a mouse model of Rett Syndrome (Roux et al., 2007; Zanella et al., 2008). With regards to psychiatric conditions which more traditionally utilize these therapies, the utility of 5-HT or NE reuptake inhibitors has been demonstrated by their ability to attenuate respiratory dysfunction in panic disorder patients (Nardi et al., 2003; Yeragani et al., 2004). Of particular importance is the ability of these drugs to influence breathing frequency since panic disorder patients frequently present with periods of hyperventilation during laboratory-induced panic attacks (Gorman et al., 1989; Gorman et al., 1990). Findings from the current study may be interpreted to suggest that administration of the SNRI VHCL may be capable of alleviating the increased breathing frequency experienced by these patients; however, we are cautious in this interpretation because our results demonstrate the efficacy of VHCL to reduce respiratory frequency in a wildtype mouse model, which in this case is not a mouse model of anxiety or panic. Our study does, however, demonstrate that chronic administration of the SNRI VHCL exerts a profound effect on the regulation of basal respiratory rhythm in adult C57BL/6 mice, which we interpret to suggest that chronic treatment with drugs capable of simultaneously blocking the 5-HT and NE reuptake transporters have the ability to modify basal respiratory activity. This is important to recognize, as SNRIs are prescribed to a number of individuals without any observable respiratory dysfunction, including panic disorder patients of the non-respiratory subtype (Briggs et al 1993), patients suffering from generalized anxiety conditions or a wide spectrum of depressive disorders, and patients afflicted with chronic pain (Ormseth et al., 2011) and fibromyalgia (Scholz et al., 2009). Therefore, based on the results of our study, we suggest that changes in respiratory activity may be noted in patients using SNRI therapy.
Highlights.
The current study investigated the effects of 28-d administration of the SNRI antidepressant venlafaxine (VHCL) on basal respiratory output in the adult mouse both in vitro and in vivo.
Following 28-d VHCL administration, basal respiratory burst frequency was markedly reduced both in vitro and in vivo.
Following 28-d VHCL administration, expiratory duration (TE) was increased and the inspiratory duty cycle (TI/Ttot) was significantly shortened both in vitro and in vivo.
Following 28-d VHCL administration, post-inspiratory and spurious expiratory discharges were seen in vitro.
We suggest that drugs capable of simultaneously blocking both 5-HT and NE reuptake transporters have the potential to influence the respiratory control network in patients using SNRI therapy.
Acknowledgments
This work was supported by NIH grants HL63175 and NS045321.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Al-Zubaidy ZA, Erickson RL, Greer JJ. Serotonergic and noradrenergic effects on respiratory neural discharge in the medullary slice preparation of neonatal rats. Pflügers Arch. 1996;431:942–949. doi: 10.1007/s004240050089. [DOI] [PubMed] [Google Scholar]
- Anderson IM. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta-analysis of efficacy and tolerability. J Affect Disord. 2000;58:19–36. doi: 10.1016/s0165-0327(99)00092-0. [DOI] [PubMed] [Google Scholar]
- Annerbrink K, Olsson M, Hedner J, Eriksson E. Acute and chronic treatment with serotonin reuptake inhibitors exert opposite effects on respiration in rats: possible implications for panic disorder. J Psychopharmacol. 2010;24:1793–1801. doi: 10.1177/0269881109106908. [DOI] [PubMed] [Google Scholar]
- Béïque J, de Montigny C, Blier P, Debonnel G. Effects of sustained administration of the serotonin and norepinephrine reuptake inhibitor venlafaxine: I. In vivo electrophysiological studies in the rat. Neuropharmacol. 2000a;39:1800–1812. doi: 10.1016/s0028-3908(00)00017-4. [DOI] [PubMed] [Google Scholar]
- Béïque J, de Montigny C, Blier P, Debonnel G. Effects of sustained administration of the serotonin and norepinephrine reuptake inhibitor venlafaxine: II. In vitro studies in the rat. Neuropharmacol. 2000b;39:1813–1822. doi: 10.1016/s0028-3908(00)00018-6. [DOI] [PubMed] [Google Scholar]
- Béïque JC, de Montigny C, Blier P, Debonnel G. Blockade of 5-hydroxytryptamine and noradrenaline uptake by venlafaxine: a comparative study with paroxetine and desipramine. Br J Pharmacol. 1998a;125:526–532. doi: 10.1038/sj.bjp.0702074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béïque JC, de Montigny C, Blier P, Debonnel G. Venlafaxine: discrepancy between in vivo 5-HT and NE reuptake blockade and affinity for reuptake sites. Synapse. 1999;32:198–211. doi: 10.1002/(SICI)1098-2396(19990601)32:3<198::AID-SYN6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Béïque JC, Lavoie N, de Montigny C, Debonnel G. Affinities of venlafaxine and various reuptake inhibitors for the serotonin and norepinephrine transporters. Eur J Pharmacol. 1998b;349:129–132. doi: 10.1016/s0014-2999(98)00241-6. [DOI] [PubMed] [Google Scholar]
- Bocola V, Trecco MD, Fabbrini G, Paladini C, Sollecito A, Martucci N. Antipanic effect of fluoxetine measured by CO2 challenge test. Biol Psychiatry. 1998;43:612–615. doi: 10.1016/s0006-3223(97)00221-7. [DOI] [PubMed] [Google Scholar]
- Briggs AC, Stretch DD, Brandon S. Subtyping of panic disorder by symptom profile. Br J Psychiatry. 1993;163:201–209. doi: 10.1192/bjp.163.2.201. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG, Shaw JL, Thompson L, Nelson DL, Hemrick-Luecke SK, Wong DT. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacol. 2001;25:871–880. doi: 10.1016/S0893-133X(01)00298-6. [DOI] [PubMed] [Google Scholar]
- Champagnat J, Denavit-Saubié M, Henry JL, Leviel V. Catecholaminergic depressant effects on bulbar respiratory mechanisms. Brain Res. 1979;160:57–68. doi: 10.1016/0006-8993(79)90600-0. [DOI] [PubMed] [Google Scholar]
- Dawson LA, Nguyen HQ, Geiger A. Effects of venlafaxine on extracellular concentrations of 5-HT and noradrenaline in the rat frontal cortex: augmentation via 5-HT1A receptor antagonism. Neuropharmacol. 1999;38:1153–1163. doi: 10.1016/s0028-3908(99)00052-0. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Morin D, Monteau R, Hilaire G. Serotonergic modulation of the respiratory rhythm generator at birth: an in vitro study in the rat. Neurosci Lett. 1992;143:91–95. doi: 10.1016/0304-3940(92)90240-8. [DOI] [PubMed] [Google Scholar]
- Errchidi S, Hilaire G, Monteau R. Permanent release of noradrenaline modulates respiratory frequency in the newborn rat: an in vitro study. J Physiol. 1990;429:497–510. doi: 10.1113/jphysiol.1990.sp018269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errchidi S, Monteau R, Hilaire G. Noradrenergic modulation of the medullary respiratory rhythm generator in the newborn rat: an in vitro study. J Physiol. 1991;443:477–498. doi: 10.1113/jphysiol.1991.sp018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Tucker JC. Behavioral consequences of antidepressant treatment in rodents. Neurosci Biobehav Rev. 1986;10:123–134. doi: 10.1016/0149-7634(86)90023-0. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Battista D, Goetz RR, Dillon DJ, Liebowitz MR, Fyer AJ, Kahn JP, Sandberg D, Klein DF. A comparison of sodium bicarbonate and sodium lactate infusion in the induction of panic attacks. Arch Gen Psychiatry. 1989;46:145–150. doi: 10.1001/archpsyc.1989.01810020047008. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Browne ST, Papp LA, Martinez J, Welkowitz L, Coplan JD, Goetz RR, Kent J, Klein DF. Effect of antipanic treatment on response to carbon dioxide. Biol Psychiatry. 1997;42:982–991. doi: 10.1016/s0006-3223(97)00160-1. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Goetz RR, Dillon D, Liebowitz MR, Fyer AJ, Davies S, Klein DF. Sodium D-lactate infusion of panic disorder patients. Neuropsychopharmacol. 1990;3:181–189. [PubMed] [Google Scholar]
- Gorman JM, Kent JM. SSRIs and SNRIs: broad spectrum of efficacy beyond major depression. J Clin Psychiatry. 1999;60(Suppl 4):33–38. [PubMed] [Google Scholar]
- Gur E, Dremencov E, Lerer B, Newman ME. Venlafaxine: acute and chronic effects on 5-hydroxytryptamine levels in rat brain in vivo. Eur J Pharmacol. 1999;372:17–24. doi: 10.1016/s0014-2999(99)00164-8. [DOI] [PubMed] [Google Scholar]
- Hatanaka K, Yatsugi S, Yamaguchi T. Effect of acute treatment with YM992 on extracellular norepinephrine levels in the rat frontal cortex. Eur J Pharmacol. 2000a;395:31–36. doi: 10.1016/s0014-2999(00)00173-4. [DOI] [PubMed] [Google Scholar]
- Hatanaka K, Yatsugi S, Yamaguchi T. Effect of acute treatment with YM992 on extracellular serotonin levels in the rat frontal cortex. Eur J Pharmacol. 2000b;395:23–29. doi: 10.1016/s0014-2999(00)00174-6. [DOI] [PubMed] [Google Scholar]
- Henderson DR, Konkle DM, Mitchell GS. Effects of serotonin re-uptake inhibition on ventilatory control in goats. Respir Physiol. 1999;115:1–10. doi: 10.1016/s0034-5687(98)00103-0. [DOI] [PubMed] [Google Scholar]
- Henderson DR, Konkle DM, Mitchell GS. Serotonin reuptake inhibition does not enhance short term modulation of the exercise ventilatory response. Respir Physiol. 2000;121:45–52. doi: 10.1016/s0034-5687(00)00112-2. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Monteau R, Errchidi S. Possible modulation of the medullary respiratory rhythm generator by the noradrenergic A5 area: an in vitro study in the newborn rat. Brain Res. 1989;485:325–332. doi: 10.1016/0006-8993(89)90577-5. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: neuromodulatory and trophic effects. Respir Physiol Neurobiol. 2008;164:222–232. doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. The role of medullary serotonin (5-HT) neurons in respiratory control: contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation. J Appl Physiol. 2010;108:1425–1432. doi: 10.1152/japplphysiol.01270.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch Gen Psychiatry. 1993;50:306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Lalley PM. Inhibition of respiratory neural discharges by clonidine and 5-hydroxytryptophan. J Pharmacol Exp Ther. 1982;222:771–777. [PubMed] [Google Scholar]
- McGrath C, Norman TR. The effect of venlafaxine treatment on the behavioural and neurochemical changes in the olfactory bulbectomised rat. Psychopharmacology (Berl) 1998;136:394–401. doi: 10.1007/s002130050583. [DOI] [PubMed] [Google Scholar]
- Muth EA, Haskins JT, Moyer JA, Husbands GE, Nielsen ST, Sigg EB. Antidepressant biochemical profile of the novel bicyclic compound Wy-45,030, an ethyl cyclohexanol derivative. Biochem Pharmacol. 1986;35:4493–4497. doi: 10.1016/0006-2952(86)90769-0. [DOI] [PubMed] [Google Scholar]
- Nardi AE, Freire RC, Zin WA. Panic disorder and control of breathing. Respir Physiol Neurobiol. 2009;167:133–143. doi: 10.1016/j.resp.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Nardi AE, Nascimento I, Valença AM, Lopes FL, Mezzasalma MA, Zin WA, Versiani M. Respiratory panic disorder subtype: acute and long-term response to nortriptyline, a noradrenergic tricyclic antidepressant. Psychiatry Res. 2003;120:283–293. doi: 10.1016/s0165-1781(03)00132-x. [DOI] [PubMed] [Google Scholar]
- Olsson M, Annerbrink K, Bengtsson F, Hedner J, Eriksson E. Paroxetine influences respiration in rats: implications for the treatment of panic disorder. Eur Neuropsychopharmacol. 2004;14:29–37. doi: 10.1016/s0924-977x(03)00044-0. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther. 1997;283:1305–1322. [PubMed] [Google Scholar]
- Ormseth MJ, Scholz BA, Boomershine CS. Duloxetine in the management of diabetic peripheral neuropathic pain. Patient Prefer Adherence. 2011;5:343–56. doi: 10.2147/PPA.S16358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pols H, Lousberg H, Zandbergen J, Griez E. Panic disorder patients show decrease in ventilatory response to CO2 after clomipramine treatment. Psychiatry Res. 1993;47:295–296. doi: 10.1016/0165-1781(93)90087-w. [DOI] [PubMed] [Google Scholar]
- Pols HJ, Hauzer RC, Meijer JA, Verburg K, Griez EJ. Fluvoxamine attenuates panic induced by 35% CO2 challenge. J Clin Psychiatry. 1996;57:539–542. doi: 10.4088/jcp.v57n1107. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Bourin M, Colombel MC, Baker GB. Dose-dependent noradrenergic and serotonergic properties of venlafaxine in animal models indicative of antidepressant activity. Psychopharmacology (Berl) 1998;138:1–8. doi: 10.1007/s002130050638. [DOI] [PubMed] [Google Scholar]
- Roux J-C, Dura E, Moncla A, Mancini J, Villard L. Treatment with desipramine improves breathing and survival in a mouse model for Rett syndrome. Eur J Neurosci. 2007;25:1915–1922. doi: 10.1111/j.1460-9568.2007.05466.x. [DOI] [PubMed] [Google Scholar]
- Sardinha A, Freire RCdR, Zin WA, Nardi AE. Respiratory manifestations of panic disorder: causes, consequences and therapeutic implications. J Bras Pneumol. 2009;35:698–708. doi: 10.1590/s1806-37132009000700012. [DOI] [PubMed] [Google Scholar]
- Scholz BA, Hammonds CL, Boomershine CS. Duloxetine for the management of fibromyalgia syndrome. J Pain Res. 2009;2:99–108. [PMC free article] [PubMed] [Google Scholar]
- Stettner GM, Huppke P, Brendel C, Richter DW, Gartner J, Dutschmann M. Breathing dysfunctions associated with impaired control of postinspiratory activity in Mecp2-/y knockout mice. J Physiol. 2007;579:863–876. doi: 10.1113/jphysiol.2006.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettner GM, Zanella S, Hilaire G, Dutschmann M. 8-OH-DPAT suppresses spontaneous central apneas in the C57BL/6J mouse strain. Respir Physiol Neurobiol. 2008a;161:10–15. doi: 10.1016/j.resp.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Stettner GM, Zanella S, Huppke P, Gartner J, Hilaire G, Dutschmann M. Spontaneous central apneas occur in the C57BL/6J mouse strain. Respir Physiol Neurobiol. 2008b;160:21–27. doi: 10.1016/j.resp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Taylor NC, Li A, Green A, Kinney HC, Nattie EE. Chronic fluoxetine microdialysis into the medullary raphe nuclei of the rat, but not systemic administration, increases the ventilatory response to CO2. J Appl Physiol. 2004;97:1763–1773. doi: 10.1152/japplphysiol.00496.2004. [DOI] [PubMed] [Google Scholar]
- Tryba AK, Peña F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JC, File SE. The effects of tricyclic and ‘atypical’ antidpressants on spontaneous locomotor activity in rodents. Neurosci Biobehav Rev. 1986;10:115–121. doi: 10.1016/0149-7634(86)90022-9. [DOI] [PubMed] [Google Scholar]
- Ursini F, Pipicelli G, Grembiale RD. Efficacy and safety of duloxetine in fibromyalgia. Clin Ter. 2010 [PubMed] [Google Scholar]
- Valic M, Pecotic R, Dogas Z. Phrenic nerve activity is enhanced by 5-HT1A receptor agonist 8-OH-DPAT in spontaneously breathing anesthetized rats. J Physiol Pharmacol. 2008;59:17–25. [PubMed] [Google Scholar]
- Viemari JC, Hilaire G. Noradrenergic receptors and in vitro respiratory rhythm: possible interspecies differences between mouse and rat neonates. Neurosci Lett. 2002;324:149–153. doi: 10.1016/s0304-3940(02)00191-x. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Ramirez JM. Norepinephrine differentially modulates different types of respiratory pacemaker and nonpacemaker neurons. J Neurophysiol. 2006;95:2070–2082. doi: 10.1152/jn.01308.2005. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Roux JC, Tryba AK, Saywell V, Burnet H, Pena F, Zanella S, Bevengut M, Barthelemy-Requin M, Herzing LB, Moncla A, Mancini J, Ramirez JM, Villard L, Hilaire G. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J Neurosci. 2005;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilken B, Lalley P, Bischoff AM, Christen HJ, Behnke J, Hanefeld F, Richter DW. Treatment of apneustic respiratory disturbance with a serotonin-receptor agonist. The J Pediatr. 1997;130:89–94. doi: 10.1016/s0022-3476(97)70315-9. [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Rao R, Tancer M, Uhde T. Paroxetine decreases respiratory irregularity of linear and nonlinear measures of respiration in patients with panic disorder. A preliminary report. Neuropsychobiol. 2004;49:53–57. doi: 10.1159/000076410. [DOI] [PubMed] [Google Scholar]
- Zanella S, Mebarek S, Lajard A-M, Picard N, Dutschmann M, Hilaire G. Oral treatment with desipramine improves breathing and life span in Rett syndrome mouse model. Respir Physiol Neurobiol. 2008;160:116–121. doi: 10.1016/j.resp.2007.08.009. [DOI] [PubMed] [Google Scholar]