Abstract
Abnormalities in frontostriatal systems are thought to be central to the pathophysiology of addiction, and may underlie maladaptive processing of the highly generalizable reinforcer, money. Although abnormal frontostriatal structure and function have been observed in individuals addicted to cocaine, it is less clear how individual variability in brain structure is associated with brain function to influence behavior. Our objective was to examine frontostriatal structure and neural processing of money value in chronic cocaine users and closely matched healthy controls. A reward task that manipulated different levels of money was used to isolate neural activity associated with money value. Gray matter volume measures were used to assess frontostriatal structure. Our results indicated that cocaine users had an abnormal money value signal in the sensorimotor striatum (right putamen/globus pallidus) which was negatively associated with accuracy adjustments to money and was more pronounced in individuals with more severe use. In parallel, group differences were also observed in both function and gray matter volume of the ventromedial prefrontal cortex; in the cocaine users, the former was directly associated with response to money in the striatum. These results provide strong evidence for abnormalities in the neural mechanisms of valuation in addiction and link these functional abnormalities with deficits in brain structure. In addition, as value signals represent acquired associations, their abnormal processing in the sensorimotor striatum, a region centrally implicated in habit formation, could signal disadvantageous associative learning in cocaine addiction.
Keywords: sensorimotor striatum, ventromedial prefrontal cortex, reward, addiction, fMRI, VBM
Cocaine addiction is characterized by persistent seeking of cocaine at the expense of other rewarding outcomes and even in the face of negative consequences. Preclinical work suggests that an underlying neurobiological mechanism of addiction may involve adaptations in frontostriatal brain circuits (Kalivas & Volkow, 2005).
The prefrontal cortex, and specifically its ventromedial aspect (VMPFC), participates in evaluating the motivational value of rewards (Grabenhorst & Rolls, 2011), particularly when faced with different reward options (McClure et al., 2004; Kable & Glimcher, 2007; Hare et al., 2009; Smith et al., 2010). In this context of reward processing, the VMPFC has been linked to goal-directed behavior (Hare et al., 2008) and its adaptive adjustment (Hikosaka & Watanabe, 2000). While studies have also supported a role in goal-directed behavior for the ventral striatum and caudate nucleus (O’Doherty et al., 2004; Tricomi et al., 2004; Valentin et al., 2007; Wrase et al., 2007a), the sensorimotor striatum (post-commissural putamen) appears to have a unique, potentially implicit, role in reward processing, as it is engaged as stimulus-response-reward contingencies are learned (Graybiel, 2005; Tricomi et al., 2009). In the context of cocaine addiction, the functions of the VMPFC have been linked to self-control (Baler & Volkow, 2006) and craving (Volkow et al., 2010), the ventral striatum to impulsivity (Bjork et al., 2008) and treatment course (Jia et al., 2011), and the sensorimotor striatum to the habitual aspects of drug-seeking (Everitt & Robbins, 2005; Volkow et al., 2006; Pierce & Vanderschuren, 2010).
Preclinical work suggests that cocaine affects the morphology of dopamine neurons and their target projection structures (like the VMPFC and striatum) to directly contribute to addiction [e.g., (Gerdeman et al., 2003; Robinson & Kolb, 2004; Kauer & Malenka, 2007)]. Studies in humans with poly-substance or cocaine dependence also report changes in gray matter volume, as most notably observed in the VMPFC (Franklin et al., 2002; Matochik et al., 2003; Tanabe et al., 2009; Alia-Klein et al., 2011) and striatum (Chang et al., 2007; Berman et al., 2008), and link these frontostriatal gray matter changes to more compulsive patterns of cocaine use (Ersche et al., 2011).
In the present study, we sought to extend this previous work by directly examining both functioning and gray matter volume of the VMPFC and striatum, and their relevance to behavior, in individuals with chronic cocaine use disorders (CUD) and closely matched healthy controls. We used money, a highly generalizable reinforcer, to target these regions and evaluate their putative dysfunction in addiction. Previous studies examining response to money in cocaine (Jia et al., 2011) or alcohol dependence with comorbid cocaine use (Bjork et al., 2008) found relatively increased activation in the ventral striatum and putamen during the anticipation and receipt of monetary gain, which in the former case predicted poorer treatment outcome. Abnormalities in the VMPFC during notification of success versus failure to win money have also been observed (Bjork et al., 2008). We therefore hypothesized that CUD would show abnormal frontostriatal gray matter and response to money, and that these variables would be differentially associated with behavior.
Materials and Methods
Subjects
Forty-two right-handed native English speakers were recruited using advertisements in local newspapers and by word-of mouth. Exclusion criteria were: (1) history of head trauma or loss of consciousness (> 30 min) or other neurological disease of central origin; (2) abnormal vital signs at time of screening and history of major medical conditions; (3) history of major psychiatric disorder (other than substance abuse or dependence in CUD); note also that subjects in either study group were not excluded for alcohol or nicotine use disorders (except if subjects were intoxicated at the time of the study as determined by clinical observation and study personnel); (4) except for cocaine, positive urine screens for other psychoactive drugs or their metabolites; (5) pregnancy as tested with a urine test in all females; (6) contraindications to the MRI environment; and (7) less than 12 years of education and/or a verbal intelligence score < 70 (as measured with the Wide Range Achievement Test III – Reading Scale). Of a total of 71 subjects who were screened, further exclusions from this study were based on: (1) probable pathological gambling [South Oaks Gambling Screen score > 5] (n=5); (2) psychiatric comorbidity (n=6); (3) failure to successfully complete a structural MR scan (n=16); and (4) age in healthy controls for group matching purposes (n=2). After exclusions, there were 21 CUD and 21 healthy controls included in the analyses, matched on all demographic variables except for cigarette smoking history (see Table 1). Twenty-three (11 CUD and 12 controls) of these 42 subjects were included in our previous report (Goldstein et al., 2009a), which focused on group effects in the functional subdivisions of the anterior cingulate cortex during the most (50¢ drug words) and least (0¢ neutral words) salient conditions of the task; this prior study did not assess neural encoding of money value and did not include gray matter volume measures. All subjects provided written informed consent for their involvement in all study procedures as approved by the local Institutional Review Board at Stony Brook University.
Table 1.
Demographic and drug use characteristics.
| Test | Control (n=21) | CUD (n=21) | |
|---|---|---|---|
| Demographics | |||
| Age (years) | t40 = 2.0 | 38.9 ± 1.3 | 43.1 ± 1.6 |
| Gender (male/female) | χ2 = 0.2 | 18/3 | 17/4 |
| Race (African-American/Caucasian/Hispanic/Asian) | χ2 = 2.9 | 12/6/2/1 | 16/4/0/1 |
| Education (years) | t40 = 0.9 | 14.1 ± 0.4 | 13.6 ± 0.4 |
| Verbal IQ: Wide Range Achievement Test III - Reading Scale |
t40 = 1.8 | 101.5 ± 2.5 | 94.9 ± 2.8 |
| Nonverbal IQ: Wechsler Abbreviated Scale of Intelligence – Matrix Reasoning Scale |
t40 = 0.7 | 10.8 ± 0.6 | 10.2 ± 0.8 |
| State Depression: Beck Depression Inventory II | Z = 1.9 | 2.7 ± 0.8 | 5.0 ± 1.0 |
| Socioeconomic Status: Hollingshead Index | t40 = 0.1 | 34.6 ± 2.6 | 35.0 ± 2.9 |
| Drug Use | |||
| Cigarette smokers (current or past/nonsmokers) | χ2 = 11.5† | 5/16 | 16/5 |
| Daily cigarettes (current smokers: N=4/13) | Z = 1.0 | 8.3 ± 2.3 | 12.3 ± 1.8 |
| Time since last cigarette (within 4 hrs./>4 hrs./overnight or more) |
χ2 = 3.8 | 1/3/0 | 7/3/3 |
| Alcohol use lifetime (years) (N=9/20) | t27 = 1.0 | 10.6 ± 3.5 | 15.1 ± 2.8 |
| Cocaine use lifetime (years) b | -- | -- | 17.8 ± 1.5 |
| Duration of current abstinence/time since last cocaine use (days) |
-- | -- | 100.6 ± 86.8 a (median=3) |
| Days/week of cocaine use during the past 12 months c | -- | -- | 3.1 ± 0.5 |
| Withdrawal symptoms: 18-item CSSA (0–126) | -- | -- | 12.7 ± 1.9 |
| Cocaine craving: 5-item Questionnaire (0–45) | -- | -- | 15.2 ± 2.3 |
p<0.005;
When two outliers (days abstinent = 1825 and 210 days, respectively) are excluded, group mean = 4.1 ± 1.0;
Data missing for one subject;
Data missing for two subjects;
CSSA: Cocaine Selective Severity Assessment Scale;
Values are frequencies or means ± standard error of the mean (SEM).
Nineteen of the 21 CUD used crack/cocaine (mostly smoked route) in the past 14 days and all CUD met DSM-IV criteria for current cocaine dependence (n = 15) or abuse (n = 6; five of these subjects met criteria for cocaine dependence in remission); 15 CUD tested positive for cocaine in urine. Current comorbid disorders met by the CUD group were for alcohol and ecstasy abuse and alcohol and marijuana dependence (total of n = 3 CUD). The impact on results of drug urine status and drug use comorbidity was examined as described in Results.
Task Design
Subjects performed a monetary reward paradigm [also described in (Goldstein et al., 2007; Goldstein et al., 2009a; Goldstein et al., 2010)]. This task required successful (fast and accurate) button pressing for the color of drug and neutral words to earn money. There were 4 counterbalanced money conditions (0¢, 1¢, 25¢, or 50¢), presented twice for a total of 8 runs. Each run contained 40 trials (split into two blocks of 20 trials interleaved with three 20 sec fixation periods). Each block began with a 3000 ms window informing subjects of the amount of money they could earn for every trial in that block. In total, there were four blocks (80 trials) for each money condition. The trial structure consisted of fixation (500 ms), the presentation of a word cue (2000 ms), response (500 ms), and a feedback slide indicating the amount gained for a correct response (500 ms); in the case of an error, an “X” rather than money was displayed. During the response window, subjects were instructed to provide fast and accurate responses by pressing 1 of 4 buttons (blue, yellow, green, and red) matching the color of the word they had just read. The total amount of money earned on the task (up to $75) was entirely contingent on performance (mean $65.69 ± 7.21, with no difference between the groups in this amount, p>0.46). We did not observe any significant word × group or money × word × group interaction effects on behavior (p>0.15) or neural activity in the current sample. This was not unexpected as detection of any of these more subtle word effects may have necessitated the use of a more lenient statistical threshold than currently used [e.g., see (Goldstein et al., 2009b)]. Therefore, the analyses described below focused on the effects of money value collapsed across word type. To monitor task engagement, subjects were asked to provide money wanting ratings (“how much do you want money right now” from 0 to 10) at 4 time points during the experiment (before training, before the task, in the middle of the task, and immediately following the task). To assure that the four monetary amounts included in the task did not differ in their subjective value between the groups, subjects also provided subjective value ratings (“how valuable” an amount is to them from 0 to 10) immediately before and after the task but before receiving remuneration.
Image Acquisition
Scanning was performed on a 4T whole-body Varian/Siemens MRI scanner. Blood-oxygen-level-dependent (BOLD) responses were measured as a function of time using a T2*-weighted single-shot gradient-echo EPI sequence (TE/TR = 20/1600 ms, 4 mm slice thickness, 1 mm gap, 33 coronal slices, 20 cm field of view, 64 × 64 matrix size, 90°-flip angle, 200 kHz bandwidth with ramp sampling, 128 time points, and 4 dummy scans, discarded to avoid non-equilibrium effects in the fMRI signal). Anatomical images were collected using a T1-weighted 3D-MDEFT sequence (Lee et al., 1995) (TE/TR = 7/15ms, 0.94 × 0.94 × 0.94 mm spatial resolution, 144 axial slices, 256 readout and 192 × 96 phase-encoding steps, 16 min scan time). A modified T2-weigthed Hyperecho image (TE/TR = 42/10000 ms, echo train length = 16, 256 × 256 matrix size, 30 coronal slices, 0.86 × 0.86 mm in-plane resolution, 5 mm slice thickness, no gap, 2 min scan time) was also acquired and reviewed by a neurologist to rule out gross structural brain abnormalities.
Image Processing and Analysis
Functional Data
Subsequent analyses were performed with the statistical parametric mapping package (SPM8; Wellcome Department of Cognitive Neurology, London UK) running on Matlab version 7.7 (Mathworks Inc., Natick, MA). A six-parameter rigid body transformation (3 rotations, 3 translations) was used for image realignment and to correct for head motion; 2 mm displacement and 2° rotation in any of the axes in any of the task runs were used as criteria for acceptable motion. Spatial normalization to a standard EPI template [Montreal Neurological Institute] was performed using a 12-parameter affine transformation, resulting in a final voxel size of 3 × 3 × 3 mm. An 8 mm3 full-width at half maximum Gaussian kernel was used to smooth the data.
A general linear model and a box-car design convolved with a canonical hemodynamic response function and high-pass filter (cut-off frequency: 1/520 sec) were used to calculate individual BOLD-fMRI maps. Contrast maps reflecting % signal change from the fixation baseline were calculated for the 0¢, 1¢, 25¢, and 50¢ money conditions for each subject. These functional contrast maps were then entered into a 4 (Money: 0¢, 1¢, 25¢, 50¢) × 2 (Group: CUD, control) mixed analysis of variance in SPM8.
Structural Data
Voxel-based morphometry (VBM) analysis was conducted with the VBM toolbox (VBM 8) (Gaser, C, University of Jena, Department of Psychiatry, Germany; http://dbm.neuro.uni-jena.de/vbm/), which combines spatial normalization, tissue segmentation, and bias correction into a unified model. The MDEFT scans were first spatially normalized to standard proportional stereotaxic space and segmented into gray matter, white matter, and cerebrospinal fluid tissue classes according to a priori tissue probability maps (Ashburner & Friston, 2000; 2005). The MDEFT sequence is particularly effective for such tissue differentiation, producing the most precise characterization of gray matter tissue compared with other sequences (Tardif et al., 2009). A hidden Markov random field (Cuadra et al., 2005) was applied to maximize the accuracy of the segmentation. Jacobian modulation was used to compensate for the effect of spatial normalization and to restore the original absolute gray matter volume in the gray matter segments. Total brain volume was computed as the sum of the extracted total gray and white matter volumes for each subject. A one-way between-subjects analysis of variance was performed after smoothing the normalized and modulated segments with a 10 mm3 full-width at half maximum Gaussian kernel. Consistent with the VBM literature in addiction (Franklin et al., 2002; Matochik et al., 2003; Makris et al., 2008; Tanabe et al., 2009; Alia-Klein et al., 2011), age and total brain volume were used as covariates of no interest.
Statistical Analyses
Voxels were considered significant if they exceeded a voxel-level threshold of p<0.005 uncorrected and p<0.05 family-wise error (FWE) corrected (within our two a priori regions of interest) and a minimum cluster size of 5 contiguous voxels. The striatum was isolated as an anatomical region of interest, created separately for the left and right side to correspond to the putamen, caudate, and pallidum in PickAtlas (Maldjian et al., 2003). The VMPFC was isolated with an 18-mm radius sphere centered on coordinates reported in Ersche et al. [x=−2, y=32, z=−18; (Ersche et al., 2011)], where individuals with CUD who had more compulsive patterns of cocaine use also had more gray matter loss compared with controls. Regions meeting a cluster-level p<0.05 FWE corrected threshold outside of our a priori regions of interest are also reported but are not the focus of the present study. For follow-up analyses with task behavior across the entire sample and cocaine use variables in CUD, in SPSS 11.5 (SPSS Inc., Chicago, IL), the average percent signal change and gray matter volume in significant coordinates were extracted using the entire cluster around the peak with the EasyROI toolbox (http://www.sbirc.ed.ac.uk/cyril/cp_download.html). Bonferroni correction was used to correct for multiple comparisons in the region of interest analyses with cocaine use variables, which included lifetime years of use, current abstinence, frequency of cocaine use in the past 12 months, withdrawal symptoms, and cocaine craving (0.05/5 = p<0.01; Table 1). Cook’s distance test was used to assess the effect of potential outliers in all regression analyses (cutoff value < 1). The Fisher’s Z-transformation was used to determine differences in correlation coefficients between the groups.
Results
Subjective Ratings and Task Behavior
Motivation to obtain money remained high throughout the task and did not significantly differ between the groups (F<2.03, p>0.14). Similarly, both groups provided higher subjective value ratings for the higher money amounts than the lower ones [main effect of money: 50¢ (mean ± standard error of the mean, 3.89 ± 0.49) > 25¢ (3.18 ± 0.48) > 1¢ (1.92 ± 0.45) > 0¢ (0.87 ± 0.30), F(3,120)=65.78, linear effect, p<0.001] and across subjects and money amounts, these ratings increased following the task (main effect of time: after (2.72 ± 0.45) > before (2.21 ± 0.39), F(1,40)=4.02, p=0.05; all other effects: F<2.35, p>0.09).
These self-reported ratings were reflected in subjects’ behavior on the task. Task accuracy improved with increasing potential gain such that accuracy was higher for the high than the low money conditions [main effect of money: 50¢ = 25¢ > 1¢ = 0¢, F(3,120)=3.82, p=0.01, linear effect, p=0.003; Figure 1]. There were no significant group or money × group interaction effects on accuracy (F<2.43, p>0.069). Possibly because stimulus presentation and response were separated and the former preceded the latter, leaving subjects ample time to prepare a response before the onset of the response window, reaction times for correct (or all) trials did not differ as a function of money amount or group (F<1.11, p>0.35).
Figure 1. Task behavior.
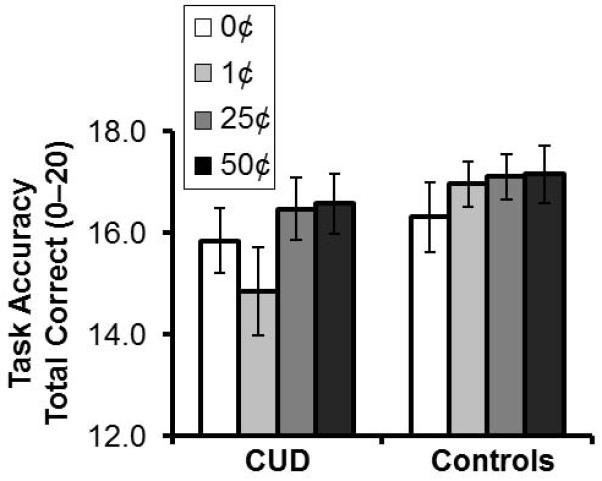
Mean task accuracy for the four monetary reward conditions (linear effect: 50¢>25¢>1¢>0¢, p=0.003). There were no significant group or money × group interaction effects on accuracy (F<2.43, p>0.069). Error bars represent standard error of the mean.
Group Differences in Neural Activity Associated with Money Value
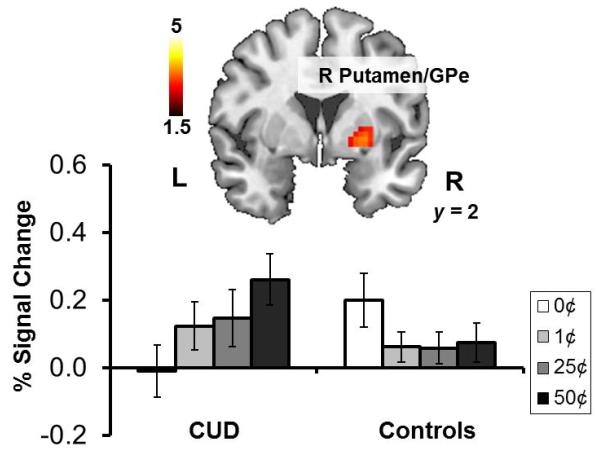
Following the observed linear progression in self-reported subjective value ratings and behavior, we performed region of interest analyses to determine (1) if the VMPFC and striatum similarly tracked money value and (2) if their pattern or magnitude of activation differed between the groups. The 4 (Money: 0¢, 1¢, 25¢, 50¢) × 2 (Group: CUD, control) mixed analysis of variance revealed a significant money main linear effect (50¢ > 25¢ > 1¢ > 0¢) in the left dorsal caudate and left pre/subgenual anterior cingulate and a significant group main effect (Controls > CUD) in the subgenual anterior cingulate extending to the medial orbitofrontal cortex (Figure 3A, Table 2). That is, across the entire sample, the caudate and VMPFC tracked money value. Irrespective of the money amount, CUD additionally deactivated the VMPFC to a greater extent than controls. In addition to these main effects, we also observed a significant money (linear effect) × group interaction in the right putamen extending to the external segment of the globus pallidus (Figure 2, Table 2), which was explained by increased activations in this region to money in CUD but not controls. Follow-up t-contrasts indicated that this interaction was driven by the maximal differential, 50¢>0¢ (CUD>controls); in addition, significant effects in the right putamen were observed within CUD for 50¢>0¢, 25¢>0¢, and any money > 0¢ (all p<0.05 FWE-corrected). Similar pattern of effects was observed within CUD for the left putamen but did not reach significance. Together these differences between the groups in response to money in the putamen parallel previous findings of relatively increased activation in the ventral striatum and putamen during the anticipation and receipt of monetary gain in cocaine (Jia et al., 2011) or alcohol dependence with comorbid cocaine use (Bjork et al., 2008).
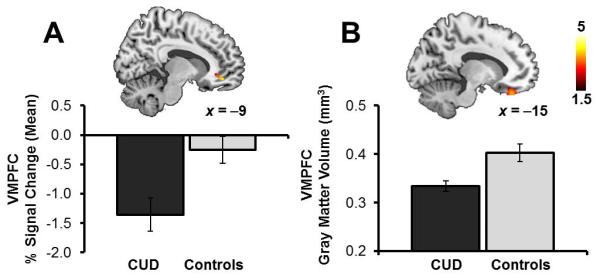
Figure 3. Group differences in activation and gray matter volume of the ventromedial prefrontal cortex.
A, Individuals with cocaine use disorders (CUD) had greater deactivations and B, reduced gray matter volume of the ventromedial prefrontal cortex (VMPFC) as compared with healthy controls. See Tables 2 & 4. Statistical maps are thresholded at p<0.005 voxel-level uncorrected and 5 contiguous voxels for display. Error bars represent standard error of the mean.
Table 2.
VMPFC and striatum region of interest analyses on task activations.
| BA | Side | Voxels | peak Z | p-corrected | x (mm) | y (mm) | z (mm) | |
|---|---|---|---|---|---|---|---|---|
| Money (Linear Contrast: 50¢ > 25¢ > 1¢ > 0¢): All Subjects | ||||||||
| Striatum: Caudate | L | 22 | +3.9 | 0.010 | −18 | −10 | 22 | |
| VMPFC: Pre/Subgenual ACC | 25,11 | L | 43 | −3.7 | 0.016 | −6 0 |
32 26 |
−2 −2 |
| Controls > CUD | ||||||||
| VMPFC: Subgenual ACC/Medial OFC | 25,10,11 | L | 18 | 3.9 | 0.005 | −9 | 44 | −11 |
| CUD > Controls | ||||||||
| None | ||||||||
| Money (Linear Contrast: 50¢ > 25¢ > 1¢ > 0¢): × Group Interaction | ||||||||
| Striatum: Putamen/Globus Pallidus | R | 68 | 3.4 | 0.044 | 27 | 2 | −2 | |
| Money (Linear Contrast: 50¢ > 25¢ > 1¢ > 0¢): CUD | ||||||||
| Striatum: Putamen/Globus Pallidus | R | 85 | +3.8 | 0.015 | 24 | 5 | −2 | |
| Striatum: Putamen/Globus Pallidus | L | 25 | +3.2 | 0.083 | −33 | 2 | 1 | |
| Money (Linear Contrast: 50¢ > 25¢ > 1¢ > 0¢): Controls | ||||||||
| None |
Statistical threshold: p<0.005 uncorrected and p<0.05 family-wise error (FWE) corrected at voxel-level, k >5 voxels;
Striatum was defined anatomically with PickAtlas to correspond to the caudate, putamen, and pallidum;
Ventromedial prefrontal cortex (VMPFC) was defined as an 18-mm radius sphere centered on x=−2, y=32, z=−18 (from Ersche et al., 2011);
ACC: anterior cingulate cortex; OFC: orbitofrontal cortex; R: right; L: left;
+/− Z values indicate direction of significant contrast;
Coordinates in bold font are depicted in the figures.
Figure 2. Money × group interaction on sensorimotor striatum response to money value.
A money (linear contrast) × group interaction was observed in the right putamen, a region extending to the external segment of the globus pallidus, such that only individuals with cocaine use disorders (CUD) but not healthy comparison controls exhibited increased activation in this region to money. See Table 2. Statistical maps are thresholded at p<0.005 voxel-level uncorrected and 5 contiguous voxels for display. Error bars represent standard error of the mean. GPe: globus pallidus externus; R: right; L: left.
Whole-brain significant regional activations are summarized in Table 3. Across subjects, the linear contrast for money revealed increased activation in several brain regions that have been reported in previous reward studies (Liu et al., 2011), including the right cerebellum and left inferior frontal gyrus (a cluster encompassing the left anterior insula). In addition, parametric deactivations with increasing money value were observed in the left pre/subgenual anterior cingulate cortex and bilateral middle/posterior cingulate, consistent with these regions’ roles within the default mode network (Gusnard et al., 2001; Buckner et al., 2008). There were no other significant group or interaction effects that survived whole-brain cluster-level correction for multiple comparisons.
Table 3.
Whole-brain results from the Money × Group analysis of variance on task activations.
| BA | Side | Voxels | peak Z | p-corrected | x (mm) | y (mm) | z (mm) | |
|---|---|---|---|---|---|---|---|---|
| Money (Linear Contrast: 50¢ > 25¢ > 1¢ > 0¢): All Subjects | ||||||||
| Cerebellum: Vermis | R | 220 | +3.9 | 0.046 | 6 21 12 |
−64 −46 −46 |
−17 −50 −17 |
|
| Inf. Frontal G./Insula | 44,45,6 | L | 204 | +3.7 | 0.060 | −45 −33 −30 |
5 11 17 |
13 22 28 |
| VMPFC: Pre/Subgenual ACC | 24,25,11 | L | 459 | −4.3 | 0.001 | −3 −18 |
29 35 |
10 −5 |
| Mid./Posterior Cingulate G. | 23 | R L |
211 | −3.6 | 0.053 | 9 −3 −15 |
−25 −16 −19 |
37 31 52 |
| Controls > CUD | ||||||||
| None | ||||||||
| CUD > Controls | ||||||||
| None | ||||||||
| Money (Linear Contrast: 50¢ > 25¢ > 1¢ > 0¢): × Group Interaction | ||||||||
| None |
Statistical threshold: p<0.005 uncorrected and p<0.05 family-wise error (FWE) corrected at cluster-level, k >5 voxels;
Inf.: inferior; Mid.: middle; VMPFC: ventromedial prefrontal cortex; ACC: anterior cingulate cortex; R: right; L: left;
+/− Z values indicate direction of significant contrast.
Group Differences in Gray Matter Volume
We performed an analysis of variance, statistically controlling for the effects of age and total brain volume, to identify any differences between the groups in gray matter volume within our regions of interest. Compared with controls, CUD had reduced gray matter volume of the medial orbitofrontal cortex extending to the gyrus rectus (Figure 3B, Table 4). No group differences were observed for the striatum. Outside of our regions of interest, gray matter reductions in CUD did not survive whole-brain cluster-level correction for multiple comparisons. Similarly, there were no significant regions of increased gray matter volume in CUD compared with controls.
Table 4.
VMPFC and striatum region of interest analyses on gray matter volume.
| BA | Side | Voxels | peak Z | p-corrected | x (mm) | y (mm) | z (mm) | |
|---|---|---|---|---|---|---|---|---|
| Controls > CUD | ||||||||
| VMPFC: Medial OFC | 11 | L | 347 | 3.5 | 0.029 | −15 −3 |
36 39 |
−25 −28 |
| CUD > Controls | ||||||||
| None |
Statistical threshold: p<0.005 uncorrected and p<0.05 family-wise error (FWE) corrected at voxel-level, k >5 voxels;
Striatum was defined anatomically with PickAtlas to correspond to the caudate, putamen, and pallidum;
Ventromedial prefrontal cortex (VMPFC) was defined as an 18-mm radius sphere centered on x=−2, y=32, z=−18 (from Ersche et al., 2011);
OFC: orbitofrontal cortex; L: left;
Coordinates in bold font are depicted in the figures.
Region of Interest Correlations with Neural Activity and Gray Matter Volume
In follow-up analyses in SPSS we examined the relationship between activity in the VMPFC and putamen using the mean extracted signal in these regions (coordinates from the group effect and the money × group interaction, respectively; Table 2). VMPFC BOLD (50¢>0¢) and putamen BOLD (50¢>0¢) were not significantly correlated across the entire sample (r=−0.21, p=0.18). To address the question of differences in correlations between the groups, we performed the same analysis separately in each group. In CUD, higher activation in the putamen (50¢>0¢) was associated with more VMPFC deactivation (0¢>50¢) (r=−0.47, p=0.03) whereas in controls this effect was not significant (after removing one outlier, Cook’s d > 1; r=−0.11, p=0.64; Figure 4). A comparison of the correlation coefficients using the Fisher’s Z transformation did not indicate that this relationship was significantly stronger in CUD (Z=−1.18, p=0.11).
Figure 4. Correlation between sensorimotor striatum activations and ventromedial prefrontal cortex deactivations.
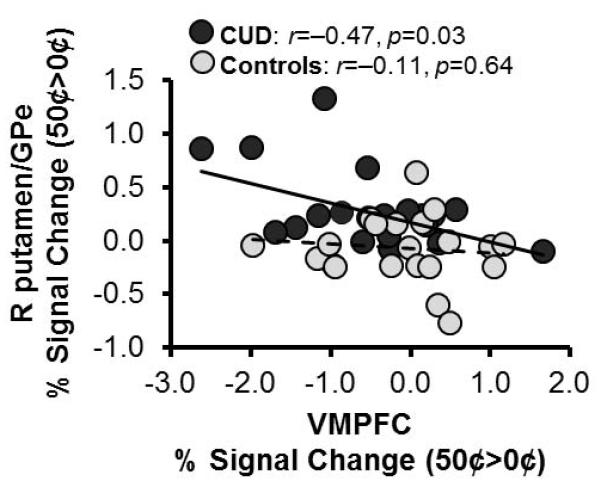
Ventromedial prefrontal cortex (VMPFC) deactivation was associated with increased activation in the sensorimotor striatum to money in individuals with cocaine use disorders (CUD). In controls this relationship was not significant (one outlier was removed, Cook’s d > 1). GPe: globus pallidus externus; R: right.
Because CUD had reduced gray matter volume and greater overall task-related deactivations in the VMPFC, we inspected the relationship between these two measures. However, the relationship between gray matter and overall activity in the VMPFC (average of the four money values) did not reach significance across the entire sample or within each group separately (r<0.28, p>0.068, controlling for age and total brain volume). Similarly, gray matter volume in the VMPFC and response to money in the putamen (BOLD 50¢>0¢) were not significantly correlated across the entire sample or within both subject groups separately (all r<−0.20, p>0.19).
Region of Interest Correlations with Behavior
To establish the specific relationship of money-related brain activations to behavior, we performed correlations with task accuracy and drug use. Behavioral sensitivity to money (differential task-related accuracy, 50¢>0¢) was negatively correlated with activation in the putamen to money (50¢>0¢) when considering the entire sample (r=−0.33, p=0.03), but this effect appeared to be driven by the CUD. We therefore tested this correlation separately in each group. Two outliers (one control, one CUD) were removed (Cook’s d>1 when considering the groups separately). In CUD, accuracy was negatively correlated with putamen BOLD (50¢>0¢) such that higher activity was associated with lower differential accuracy 50¢>0¢ (r=−0.50, p=0.024; Figure 5A). In controls, this relationship was not significant (r=−0.019, p=0.94). This difference in correlations between the groups did not reach nominal significance (Z=−1.55, p=0.060). Because CUD also had greater VMPFC deactivations, which were not specific to a particular money condition, we assessed the relationship between average VMPFC activation (cluster from the group effect, averaged across the four money values) and average accuracy on the task. These two measures were not significantly correlated across the entire sample or within each group separately (r<−0.42, p>0.063).
Figure 5. Correlations between sensorimotor striatum activation and behavior.
A, Higher activations in the right putamen to money were associated with reduced behavioral adjustment to money (differential task-related accuracy, 50¢>0¢) and with B, more frequent cocaine use in the past 12 months in individuals with cocaine use disorders (CUD). One CUD and one control were removed from the analyses with accuracy and one CUD was removed from the analysis with cocaine use (for all excluded subjects, Cook’s d > 1).
Cocaine use days/week in the past year correlated positively with activation in the putamen 50¢>0¢ (after removing one outlier, Cook’s d>1; r=0.62, p=0.006; Figure 5B). There were no other significant correlations with drug use (cocaine use variables in Table 1, p>0.11). For the VMPFC (cluster from the group effect), there was a negative correlation with cocaine craving (p=0.01; all other effects did not survive correction for multiple comparisons, p>0.03), such that subjects who reported more craving deactivated this region to a greater extent overall.
VMPFC gray matter volume was not associated with task accuracy in either group (p>0.58) or with drug use in CUD (p>0.20).
Effects of Subject Exclusions
The potential confounding effects of diagnostic comorbidity were assessed by removing CUD from the main analyses who met DSM-IV criteria for abuse or dependence on any drug other than cocaine or nicotine (n=3 subjects; see Subjects section of Methods). In addition to the main effect of money on accuracy, a money × group interaction was observed in this reduced sample (n=39) which was explained by reduced accuracy for the 1¢ condition in CUD [F(3,111)=2.72, p=0.048]. All other reported main or interaction effects on task activation and gray matter volume, and correlations between task activation with behavior were unchanged (p<0.03). The effect of negative pre-scan drug urine tests (found in n=6 CUD; 3 of whom reported being cocaine abstinent for <7 days, 1 for <14 days, and 2 for >6 months) was similarly inspected. Even after excluding these 6 subjects, all results still mirrored those of the entire sample (p<0.05, except for the correlation between activity in the putamen and task accuracy within CUD which became non-significant, p=0.18). Because of the minimal influence of these factors on our results, CUD with comorbid diagnoses and cocaine urine negative scans were not excluded from the current report to increase generalizability. Because of the almost parallel distribution between cigarette smokers versus non-smokers and group membership, we could not reliably use smoking status as a covariate in our statistical analyses (Miller & Chapman, 2001).
Discussion
In the present study, we evaluated the hypothesis that functioning of the VMPFC and striatum under varying reward contingencies would be altered in cocaine addiction. We further hypothesized that such altered VMPFC-striatum neural activity would be related to alterations in the gray matter volume of these regions and would have negative consequences for behavior in individuals with CUD. Consistent with our first hypothesis, CUD showed abnormal response to money value in the right putamen, a region extending to the external segment of the globus pallidus, and greater overall deactivations in the VMPFC across the money conditions. Consistent with our second hypothesis, CUD also had reduced gray matter volume in the VMPFC. Abnormal responses to money in the putamen and VMPFC, but not gray matter in the VMPFC, were related to task behavior and cocaine use, such that individuals with more severe use and craving, and less behavioral adjustment to money, had the highest activations in the putamen and deactivations in the VMPFC. Thus, our findings, which are consistent with preclinical models of addiction [e.g., (Gerdeman et al., 2003; Robinson & Kolb, 2004; Kauer & Malenka, 2007)] and extend prior work that has separately examined VMPFC and striatum function (Bjork et al., 2008; Jia et al., 2011) or gray matter volume (Franklin et al., 2002; Matochik et al., 2003; Chang et al., 2007; Berman et al., 2008; Tanabe et al., 2009; Alia-Klein et al., 2011; Ersche et al., 2011) in human cocaine addiction, provide strong evidence for frontostriatal abnormalities in the neural mechanisms of valuation in addiction and link these functional abnormalities with deficits in brain structure.
Group Differences in Neural Activity Associated with Money Value
Both CUD and healthy controls reported high levels of motivation to obtain money and did not differ in the subjective value assigned to the different money amounts used in the task. These self-reported ratings were reflected in subjects’ behavior on the task, where subjects were more accurate for the high than the low money amounts. Consistent with previous studies (Liu et al., 2011), across the entire sample, the VMPFC, associative striatum (dorsal caudate), cerebellum, posterior cingulate, and inferior frontal gyrus responded to money, but in CUD there was also an ectopic response in the sensorimotor striatum (right putamen/globus pallidus). The post-commissural putamen (or dorsolateral striatum in rodents) is centrally implicated in habits (Tricomi et al., 2009), stimulus-response associations that render behavior insensitive to outcomes (Yin & Knowlton, 2006; Yin et al., 2006; Balleine & O’Doherty, 2010). With the progression of cocaine addiction (Porrino et al., 2007), this striatal subregion is suggested to underlie the habitual aspects of drug use like cue-induced drug-seeking and craving in both rodents (Vanderschuren et al., 2005; Pierce & Vanderschuren, 2010) and humans (Volkow et al., 2006). The cluster of activation also included the external segment of the globus pallidus, which is a target projection for the indirect D2 dopamine pathway (Hikida et al., 2010) and more recent studies also implicate upregulated dopamine D3 receptor expression (Boileau et al., 2012) and disrupted activity of this pathway (Lobo et al., 2010) in addiction. This increased sensitivity to money in the putamen is in line with previous studies that have revealed hypersensitivity to money in individuals addicted to cocaine (Jia et al., 2011) and marijuana (Nestor et al., 2010). While studies have also reported increased (Bjork et al., 2008) or decreased (Wrase et al., 2007b; Beck et al., 2009) response to money in the ventral striatum in alcohol dependence, likely due to the low level of uncertainty associated with our task, we did not observe activity in this region in either group. Importantly, increased activity in the putamen was associated with reduced adjustments in task accuracy with higher money value and with more frequent cocaine use in CUD. Thus, these differential associations with behavior on the task and drug use outside the lab point to altered neural valuation mechanisms in CUD that may render these individuals less sensitive to potentially positive (e.g., earning more money on the task) or potentially negative (e.g., the physical and emotional impact of their use) behavioral outcomes.
In contrast to differential responses in the striatum, parametric deactivations in the VMPFC were observed with increasing money value in both CUD and controls, in line with this region’s role in processing the motivational value of rewards during goal-directed behavior, including money and drugs of abuse (Grabenhorst & Rolls, 2011). Although the pattern of activation in the VMPFC is consistent with that observed in default mode network regions, where deactivations vary as a function of task engagement or task features (Gusnard et al., 2001; Buckner et al., 2008), the directionality of these results is at odds with previous studies showing positive activations in the VMPFC to reward value. This apparent inconsistency may reflect differences in the tasks used to elicit responses (e.g., blocked designs like ours capture sustained activation while event-related designs capture transient activation). Indeed, because blocked designs may produce initial positive responses in the hemodynamic signal that are followed by sustained negative responses, as has been observed in regions of the default model and particularly the medial prefrontal cortex (Meltzer et al., 2008), the estimate for the block can be negative when the model assumes homogeneity in the signal.
Group Differences in Function and Gray Matter Volume of the VMPFC
Although there was no significant difference between the groups as a function of money value, echoing our prior findings in a partly overlapping sample of CUD and controls (Goldstein et al., 2009a), across the four money conditions, cocaine addicted individuals, and especially those with higher self-reported craving, deactivated the VMPFC to a greater extent than controls. In addition, the VMPFC and putamen were strongly correlated in CUD such that the magnitude of VMPFC deactivation was proportional to the magnitude of striatal activation to money. Thus, enhanced VMPFC deactivation in the cocaine users might represent a compensatory mechanism necessary to maintain comparable levels of task performance, particularly in those individuals with higher activations in the putamen and more severe craving.
Confirming findings from previous studies (Franklin et al., 2002; Matochik et al., 2003; Tanabe et al., 2009; Alia-Klein et al., 2011; Ersche et al., 2011), we further found reduced gray matter volume of the VMPFC in CUD. Although our data cannot answer questions related to causality, functional abnormalities in the VMPFC (and striatum) in CUD could also signal inefficiency of cortical processing due to gray matter volume loss in this region. Interestingly, repeated psychostimulant exposure (Robinson & Kolb, 2004; Everitt & Robbins, 2005; Nelson & Killcross, 2006; Wickens et al., 2007; Briand et al., 2008; Zapata et al., 2010) or lesions of the VMPFC (Corbit & Balleine, 2003; Yin et al., 2005) in experimental animals have been shown to shift control of behavior in response to reinforcers and drug-seeking to the putamen, suggesting that these two regions compete for control of behavior (Balleine & O’Doherty, 2010). Thus, irrespective of the underlying mechanism, frontostriatal abnormalities (i.e., via greater engagement of the putamen, craving, or gray matter loss) may reduce addicted individuals’ ability to inhibit stimulus-driven responses and contribute to disadvantageous behavior (Goldstein & Volkow, 2002; 2011).
Limitations, Future Directions, and Conclusions
Potential limitations of this study include the use of a blocked design, differences in smoking histories between the groups, and the relatively small sample size for investigating possible differences between subgroups of cocaine users (e.g., based on recent cocaine use) or differences in correlations between cocaine users and controls. Because we were interested in identifying which regions represented money value, we used a blocked design. Blocked designs are more sensitive compared with event-related designs in detecting such regional activations because they offer maximal variance in terms of BOLD amplitude changes between conditions (i.e., value in this case) (Huettel, 2008). The cost is in the ability to separate anticipatory from outcome related activity. However, frontostriatal abnormalities in addicted individuals during both anticipation of monetary gain (Wrase et al., 2007b; Beck et al., 2009; Nestor et al., 2010) and at gain outcome (Bjork et al., 2008; Nestor et al., 2010; Jia et al., 2011) suggest that such a separation is not likely to substantially modify our interpretations. Indeed, a recent meta-analysis found vastly overlapping neuroanatomical correlates (including VMPFC and striatum) of anticipation of reward and reward outcome (Liu et al., 2011). This is particularly relevant in the case of money where reward delivery is always delayed and therefore somewhat anticipatory even in event-related studies [e.g., note “real” vs. hypothetical money has similar neural and behavioral correlates (Bickel et al., 2009)]. Because only a few (23%) controls and most (76%) CUD reported a history of cigarette smoking, we were unable to control for differences in smoking status between the groups (Miller & Chapman, 2001). As nicotine use rates in our sample of CUD are comparable to those reported in previous studies (Grant et al., 2004; Kalman et al., 2005; Weinberger & Sofuoglu, 2009; Jia et al., 2011), this may be an inherent feature of this population and future studies would need to recruit more cigarette smoking controls to better address this potential confound. Because most CUD were urine positive for cocaine, our findings may be specific to early abstinence and studies using larger and more heterogeneous samples of CUD are needed to examine the effects of recent use on frontostriatal structure and function. Similarly, although the relationship between response to money in the putamen and task accuracy or between responses in the putamen and VMPFC tended to be stronger in CUD, larger samples may be needed to detect such correlation differences statistically.
In summary, we report that CUD had abnormal value signals in the sensorimotor striatum (right putamen extending to the external globus pallidus), potentially explained by reduced gray matter volume and function of the VMPFC. As value signals represent acquired associations, our results could indicate disadvantageous associative learning in CUD. Indeed, activity in this region was differentially associated with maladaptive task- and drug use-related behaviors. Future studies should directly inspect learning mechanisms in CUD as well as their resistance to extinction procedures to more fully establish the role of habit systems in addiction in humans. In addition, reward processing is only one function of the VMPFC and striatum that may be altered by addiction; future studies could explore the link between gray matter in these regions and the neural correlates of self-control or impulsive choice to determine the functional specificity of these abnormalities. Elucidating the relationship between brain structure and function may not only facilitate better comparison with findings reported in the animal literature, but may also help move beyond reporting of gray matter differences in addiction in humans and attempt to understand the relationship of these differences to behavior and to the underlying function of connected networks of brain regions.
Acknowledgements
This work was supported by grants from the National Institute on Drug Abuse (grant number 1R01DA023579 to R.Z.G.) and General Clinical Research Center (grant number 5-MO1-RR-10710).
Abbreviations
- BOLD
blood oxygen-level-dependent
- CUD
cocaine use disorders
- fMRI
functional magnetic resonance imaging
- FWE
family-wise error
- SPM
statistical parametric mapping
- VBM
voxel-based morphometry
- VMPFC
ventromedial prefrontal cortex
Footnotes
Disclosure/Conflict of Interest
None declared.
Notice
This manuscript has been authored by Brookhaven Science Associates, LLC under Contract No. DE-AC02-98CHI-886 with the U.S. Department of Energy. The United States Government retains, and the publisher, by accepting the article for publication, acknowledges, a world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for the United States Government purposes.
References
- Alia-Klein N, Parvaz MA, Woicik PA, Konova AB, Maloney T, Shumay E, Wang R, Telang F, Biegon A, Wang GJ, Fowler JS, Tomasi D, Volkow ND, Goldstein RZ. Gene x disease interaction on orbitofrontal gray matter in cocaine addiction. Arch Gen Psychiatry. 2011;68:283–294. doi: 10.1001/archgenpsychiatry.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hagele C, Knutson B, Heinz A, Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Berman S, O’Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Ann N Y Acad Sci. 2008;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Pitcock JA, Yi R, Angtuaco EJ. Congruence of BOLD response across intertemporal choice conditions: fictive and real money gains and losses. J Neurosci. 2009;29:8839–8846. doi: 10.1523/JNEUROSCI.5319-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Hommer DW. Striatal sensitivity to reward deliveries and omissions in substance dependent patients. Neuroimage. 2008;42:1609–1621. doi: 10.1016/j.neuroimage.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J, Wilkins D, Selby P, George TP, Zack M, Furukawa Y, McCluskey T, Wilson AA, Kish SJ. Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. J Neurosci. 2012;32:1353–1359. doi: 10.1523/JNEUROSCI.4371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Flagel SB, Garcia-Fuster MJ, Watson SJ, Akil H, Sarter M, Robinson TE. Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology. 2008;33:2969–2980. doi: 10.1038/npp.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behav Brain Res. 2003;146:145–157. doi: 10.1016/j.bbr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Cuadra MB, Cammoun L, Butz T, Cuisenaire O, Thiran JP. Comparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain images. IEEE Trans Med Imaging. 2005;24:1548–1565. doi: 10.1109/TMI.2005.857652. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2011;134:2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Carrillo JH, Maloney T, Woicik PA, Wang R, Telang F, Volkow ND. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc Natl Acad Sci U S A. 2009a;106:9453–9458. doi: 10.1073/pnas.0900491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Honorio Carrillo J, Maloney T, Woicik PA, Wang R, Telang F, Volkow ND. Dopaminergic response to drug words in cocaine addiction. J Neurosci. 2009b;29:6001–6006. doi: 10.1523/JNEUROSCI.4247-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, Telang F, Alia-Klein N, Volkow ND. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144:1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Woicik PA, Maloney T, Tomasi D, Alia-Klein N, Shan J, Honorio J, Samaras D, Wang R, Telang F, Wang GJ, Volkow ND. Oral methylphenidate normalizes cingulate activity in cocaine addiction during a salient cognitive task. Proc Natl Acad Sci U S A. 2010;107:16667–16672. doi: 10.1073/pnas.1011455107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci. 2011;15:56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol. 2005;15:638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Watanabe M. Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cereb Cortex. 2000;10:263–271. doi: 10.1093/cercor/10.3.263. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G. Functional Magnetic Resonance Imaging. Sinauer Associates, Inc.; Sunderland, MA: 2008. [Google Scholar]
- Jia Z, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC, Pearlson GD, Potenza MN. An Initial Study of Neural Responses to Monetary Incentives as Related to Treatment Outcome in Cocaine Dependence. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict. 2005;14:106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Lee JH, Garwood M, Menon R, Adriany G, Andersen P, Truwit CL, Ugurbil K. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magn Reson Med. 1995;34:308–312. doi: 10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biol Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Meltzer JA, Negishi M, Constable RT. Biphasic hemodynamic responses influence deactivation and may mask activation in block-design fMRI paradigms. Hum Brain Mapp. 2008;29:385–399. doi: 10.1002/hbm.20391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of abnormal psychology. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Nelson A, Killcross S. Amphetamine exposure enhances habit formation. J Neurosci. 2006;26:3805–3812. doi: 10.1523/JNEUROSCI.4305-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor L, Hester R, Garavan H. Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. Neuroimage. 2010;49:1133–1143. doi: 10.1016/j.neuroimage.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Vanderschuren LJ. Kicking the habit: the neural basis of ingrained behaviors in cocaine addiction. Neurosci Biobehav Rev. 2010;35:212–219. doi: 10.1016/j.neubiorev.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Smith DV, Hayden BY, Truong TK, Song AW, Platt ML, Huettel SA. Distinct value signals in anterior and posterior ventromedial prefrontal cortex. J Neurosci. 2010;30:2490–2495. doi: 10.1523/JNEUROSCI.3319-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, Banich M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry. 2009;65:160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif CL, Collins DL, Pike GB. Sensitivity of voxel-based morphometry analysis to choice of imaging protocol at 3 T. Neuroimage. 2009;44:827–838. doi: 10.1016/j.neuroimage.2008.09.053. [DOI] [PubMed] [Google Scholar]
- Tricomi E, Balleine BW, O’Doherty JP. A specific role for posterior dorsolateral striatum in human habit learning. Eur J Neurosci. 2009;29:2225–2232. doi: 10.1111/j.1460-9568.2009.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41:281–292. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- Valentin VV, Dickinson A, O’Doherty JP. Determining the neural substrates of goal-directed learning in the human brain. J Neurosci. 2007;27:4019–4026. doi: 10.1523/JNEUROSCI.0564-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, Ma Y, Pradhan K, Wong C, Swanson JM. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. NeuroImage. 2010;49:2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Sofuoglu M. The impact of cigarette smoking on stimulant addiction. Am J Drug Alcohol Abuse. 2009;35:12–17. doi: 10.1080/00952990802326280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens JR, Horvitz JC, Costa RM, Killcross S. Dopaminergic mechanisms in actions and habits. J Neurosci. 2007;27:8181–8183. doi: 10.1523/JNEUROSCI.1671-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Kahnt T, Schlagenhauf F, Beck A, Cohen MX, Knutson B, Heinz A. Different neural systems adjust motor behavior in response to reward and punishment. Neuroimage. 2007a;36:1253–1262. doi: 10.1016/j.neuroimage.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Beck A, Strohle A, Juckel G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007b;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Inactivation of dorsolateral striatum enhances sensitivity to changes in the action-outcome contingency in instrumental conditioning. Behav Brain Res. 2006;166:189–196. doi: 10.1016/j.bbr.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- Zapata A, Minney VL, Shippenberg TS. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. J Neurosci. 2010;30:15457–15463. doi: 10.1523/JNEUROSCI.4072-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]