Abstract
Objective
To investigate a possible association between celiac disease (CD) and systemic lupus erythematosus (SLE). Case series have indicated a possible association, but population-based studies are lacking.
Methods
We compared the risk of SLE in 29,048 individuals with biopsy-verified CD (villous atrophy, Marsh 3) from Sweden’s 28 pathology departments with that in 144,352 matched individuals from the general population identified through the Swedish Total Population Register. SLE was defined as having at least 2 records of SLE in the Swedish Patient Register. We used Cox regression to estimate hazard ratios (HR) for SLE.
Results
During followup, 54 individuals with CD had an incident SLE. This corresponded to an HR of 3.49 (95% CI 2.48–4.90), with an absolute risk of 17/100,000 person-years and an excess risk of 12/100,000. Beyond 5 years of followup, the HR for SLE was 2.54 (95% CI 1.57–4.10). While SLE was predominantly female, we found similar risk estimates in men and women. When we restricted our outcome to individuals who also had a dispensation for a medication used in SLE, the HR was 2.43 (95% CI 1.22–4.87). The HR for having 2 records of SLE diagnoses, out of which at least 1 had occurred in a department of rheumatology, nephrology/dialysis, internal medicine, or pediatrics, was 2.87 (95% CI 1.97–4.17).
Conclusion
Individuals with CD were at a 3-fold increased risk of SLE compared to the general population. Although this excess risk remained more than 5 years after CD diagnosis, absolute risks were low.
Key Indexing Terms: AUTOIMMUNE, CELIAC, GLUTEN, SYSTEMIC LUPUS ERYTHEMATOSUS
Celiac disease (CD) is characterized by small intestinal inflammation and is triggered by gluten exposure in genetically sensitive individuals1. CD occurs in 1%–2% of the Western population2,3 and has been linked to a number of disorders including type 1 diabetes4, sepsis5, lymphoproliferative malignancy6, and excess mortality7.
Systemic lupus erythematosus (SLE) is an immune-mediated disease with a prevalence of about 40/100,000 in Northern Europeans8. It is a multisystem disease with protean manifestations including rash, arthritis, cytopenias, and renal disease. It occurs predominantly in women and is associated with high morbidity and mortality from renal disease or central nervous system lupus9,10.
Several case reports and case series have suggested a possible association between CD and SLE11,12,13,14,15, 16,17,18,19, but earlier research has suffered from low statistical power. At least 2 studies have also reported a high prevalence of antigliadin antibodies in SLE but with substantially lower rates of histologically verified CD17,19, and because of their lack of a control group with non-SLE individuals, these studies were unable to estimate relative risks of SLE in CD. Connective tissue disorders as a group seem to be more frequent among patients with CD, but the small number of cases affected by each specific connective tissue disorder has been a limitation to estimate disease-specific risk20. For example, 19% of 924 patients with CD from France developed an autoimmune disease, but only 2 patients had a subsequent diagnosis of SLE21.
Our aim was to investigate the risk of SLE in a nationwide cohort of patients with biopsy-verified CD compared to individuals matched from the general population.
MATERIALS AND METHODS
We linked nationwide data on biopsy-verified CD from Swedish pathology registers to inpatient and hospital-based outpatient data on SLE obtained from the Swedish Patient Register22 as well as to the Prescribed Drug Register23.
Study participants
Reports on duodenal and jejunal biopsies performed between 1969 and 2008 were collected from all 28 Swedish pathology departments between October 2006 and February 2008 (Table 1). Data were available on date of biopsy, topography (duodenum or jejunum), morphology codes consistent with villous atrophy (VA; Table 2), and personal identity number24. Each individual with CD (as defined below) was matched with up to 5 reference individuals from the Total Population Register on age, sex, calendar year, and county25. After removal of data irregularities, we had information on 29,096 individuals with CD and 144,520 reference individuals.
Table 1.
Characteristics of participants, presented as n (%).
| Characteristics | Reference Individuals | Celiac Disease |
|---|---|---|
| Total | 144,352 | 29,048 |
| Age at study entry, yrs | ||
| 0–19 | 58,846 (40.8) | 11,800 (40.6) |
| 20–39 | 26,356 (18.3) | 5306 (18.3) |
| 40–59 | 32,198 (22.3) | 6455 (22.2) |
| 60+ | 26,952 (18.7) | 5487 (18.9) |
| Sex | ||
| Women | 89,403 (61.9) | 17,965 (61.8) |
| Men | 54,949 (38.1) | 11,083 (38.2) |
| Calendar period | ||
| 1989 | 20,356 (14.1) | 4102 (14.1) |
| 1990–1999 | 59,818 (41.4) | 12,045 (41.5) |
| 2000– | 64,178 (44.5) | 12,901 (44.4) |
Table 2.
A comparison of different histopathological classification systems.
| Classification Used in This Project | Villous Atrophy | ||
|---|---|---|---|
| Marsh classification26 | Type IIIa | Type IIIb | Type IIIc |
| Marsh description | Flat destructive | ||
| Corazza classification52 | Grade B1 | Grade B2 | |
| SnoMed codes | M58, D6218, M58005 | M58, D6218, M58006 | M58, D6218, M58007 |
| KVAST/Alexander classification | III Partial VA | IV Subtotal VA | IV Total VA |
| Characteristics | |||
| Villous atrophy | + | ++ | ++ |
| IEL | + | + | + |
| Crypt hyperplasia | + | ++ | ++ |
IEL: intraepithelial lymphocytosis.
Celiac disease
CD was defined as VA (Marsh stage 3)26, according to the biopsy report. We did not request a positive CD serology for the diagnosis of CD, but in a subset of individuals with available data, 88% had a positive serology at the time of biopsy. A detailed account of the data collection, including a validation of CD, has been published elsewhere27. Each biopsy report was based on an average of 3 tissue specimens28.
SLE
SLE was defined as having at least 2 records of the following calendar-year-specific international classification of disease (ICD) discharge diagnoses in the National Patient Register: ICD-7: 456.2; ICD-8: 734.1; ICD-9: 710A; ICD-10: M32 (minus drug-induced M32.0).
We then excluded 48 CD individuals and 80 reference individuals with a discharge diagnosis of SLE before CD or study entry. An additional 88 reference individuals were excluded from the study population because their index individual with CD had been excluded and analyses were performed retaining stratum.
Other covariates
Data on the following potential confounding factors were also collected from the government agency Statistics Sweden: country of birth (Nordic vs not Nordic), education level, and socioeconomic status. Education was defined according to 4 a priori categories (≤ 9 years of primary school, 2 years of high school, 3–4 years of high school, college/university) and socioeconomic status according to 6 categories (according to the European Socioeconomic Classification: levels 1, 2, 3+6, 7, 8, and 9; Olén, et al29). Education was missing in 4% (n = 1150), and 31% lacked data on socioeconomic status (n = 8901). These individuals were fitted into separate categories for the statistical analyses. Using the Patient Register, type 1 diabetes and autoimmune thyroid disease (Table 3) were identified because both CD and SLE have been linked to those diseases.
Table 3.
International Classification of Disease (ICD) codes.
| Type 1 diabetes mellitus: Before 1997, the ICD coding for diabetes (ICD-7: 260, ICD-8: 250, ICD-9: 250) did not distinguish between type 1 and type 2 diabetes. We defined individuals with type 1 diabetes as those who were ≤ 30 years of age at their first hospitalization for diabetes (ICD-7-ICD-10). ICD-10: E10. |
| Autoimmune thyroid disease: Defined as follows: ICD-7: 252.00, 252.01, 252.02, 253.10, 253.19, 253.20, 253.29, 254.00; ICD-8: 242.00, 242.09, 244, 245.03; ICD-9: 242A, 242X, 244X, 245C, 245W; ICD-10: E03.5, E03.9, E05.0, E05.5, E05.9, E06.3, E06.5. |
Statistics
Through internally stratified Cox regression we estimated hazard ratios (HR) for SLE. The internal stratification means that the Cox regression resembles a conditional logistic regression in the way that all comparisons are made within the same stratum (defined by the matching), and then a summary HR is calculated. The proportional hazards assumption was examined by visual inspection of log-minus-log curves (Figure 1). The attributable risk percentage (the proportion of all SLE in patients with CD that could be explained by the underlying CD) was estimated by the formula 1 – 1/HR.
Figure 1.
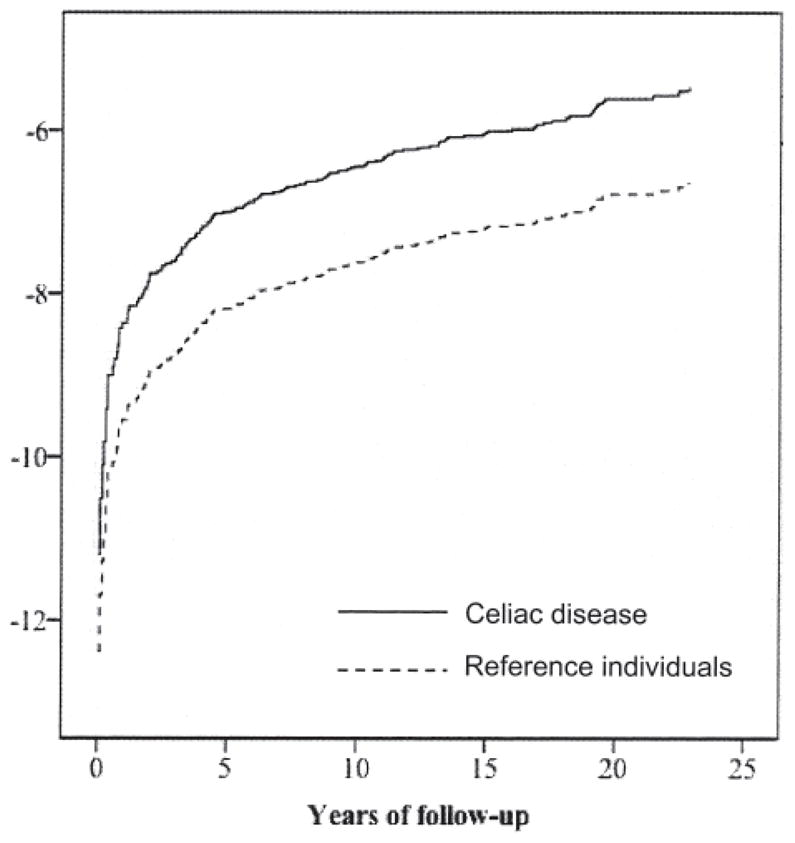
Log-minus-log curve: celiac disease and systemic lupus erythematosus.
Followup began at first biopsy with CD and on the corresponding index date in the matched reference individuals. Followup ended at the first of the following: first SLE diagnosis, December 31, 2009, emigration, or death.
In predefined analyses, we estimated the risk of SLE according to time since CD diagnosis overall and stratified by sex, age at CD diagnosis, and calendar period. In separate analyses we adjusted for country of birth, education level, socioeconomic status, type 1 diabetes, and autoimmune thyroid disease.
In sensitivity analyses we further restricted the definition of SLE in 4 ways: (1) include that at least 1 SLE discharge diagnosis at some stage occurred in an internal medicine, rheumatology, nephrology/dialysis, or pediatrics department; (2) require at least 12 months between the first and last SLE record, allowing other visits in between; (3) require that the 2 first discharges with SLE-specific ICD codes occurred within 12 months; and (4) require at least 1 dispensing of a medication common in the treatment of SLE from the Prescribed Drug Register (Table 4). The Prescribed Drug Register provides data only on medication dispensations from the pharmacy, therefore any medication administered at an infusion center could not be identified. This analysis was restricted to individuals with followup beyond July 1, 2005, because that was when the Swedish Prescribed Drug Register started (this analysis included both prevalent and incident CD).
Table 4.
Anatomical therapeutic chemical codes for systemic lupus erythematosus medication.
| H02AB06 (prednisolone); H02AB04 (methylprednisolone); P01BA01 (chloroquine); L0AX01 (azathioprine); L04AD01 (cyclosporine); L04AA06 (mycophenolic acid); L04AX03 (methotrexate); L01AA01 (cyclophosphamide); V03AF01 (mesna). |
In a subanalysis we compared the risk of future SLE in women according to whether they were diagnosed with CD before or after assumed menopause (≤ 50 years; ≥ 51 years)30. Several studies have indicated that sex hormones may play a role in SLE etiology31.
A posthoc power analysis using a 0.05 level of significance and 80% power showed that our study could detect an HR of 1.90 for SLE.
We used SPSS 20 to calculate statistics. P values < 0.05 were considered statistically significant.
Ethics
This study was approved by the Regional Ethical Review Board in Stockholm. Because this was a register-based study, no participant was contacted and all data were anonymized prior to data analyses.
RESULTS
The median age at first biopsy with CD was 30 years (range 0–95 yrs). Most patients entered the study after 1990 (Table 1), and the median year of study entry was 1998 (range 1969–2008). The majority of study participants were women (Table 1). The median age at first SLE diagnosis was 52 years in individuals with CD, and 48 years in reference individuals.
Overall risk of future SLE
During followup, there were 54 cases of SLE during 325,770 person-years of observation in individuals with CD [crude incidence rate (IR) = 17 cases/100,000 person-years]. In the general population comparator we observed 81 cases during 1,640,669 person-years (IR = 5 cases/100,000 person-years), yielding an excess risk of 12/100,000 person-years. This corresponded to an HR of 3.49 (95% CI 2.48–4.90). Adjustment for socioeconomic status, education, and country of birth did not appreciably alter the results (HR 3.51; 95% CI 2.49–4.96). The proportion of all SLE in patients with CD that could be explained by the underlying CD (attributable fraction) was 71%. Individuals with CD had an 8-fold increased risk of SLE in the first year of followup, and 5 years after CD diagnosis, the HR decreased to 2.54 (Table 5). Excluding the first year of followup, the HR was 3.05 (95% CI 2.11–4.41), and was generally similar when the first 2 years were excluded (HR 2.88; 95% CI 1.94–4.28).
Table 5.
Risk of SLE in patients with celiac disease, according to followup.
| Followup | HR; 95% CI | p | Person-yrs | No. SLE Events in CD | Incidence Rate* | |
|---|---|---|---|---|---|---|
| CD | Ref | |||||
| All | 3.49; 2.48–4.90 | < 0.001 | 325,770 | 54 | 17 | 5 |
| < 1 yr | 8.85; 3.41–22.98 | < 0.001 | 28,729 | 10 | 35 | 5 |
| 1–5 yrs | 3.87; 2.17–6.89 | < 0.001 | 107,807 | 19 | 18 | 5 |
| > 5 yrs | 2.54; 1.57–4.10 | < 0.001 | 189,233 | 25 | 13 | 5 |
| Beyond 1 yr followup | 3.05; 2.11–4.41 | < 0.001 | 297,041 | 44 | 15 | 5 |
SLE cases per 100,000 person-years of CD. SLE: systemic lupus erythematosus; CD: celiac disease; Ref: reference individuals; HR: hazard ratio.
The HR for SLE in individuals with CD diagnosed in childhood or adolescence (< 20 years old at CD diagnosis) was 2.31, and 5.81 in patients diagnosed with CD after age 60 years; this difference was not statistically significant (p = 0.096). In a posthoc analysis restricting followup to < 20 years of age, 6 individuals with CD had an incident diagnosis of SLE, corresponding to an HR of 2.27 (95% CI 0.86–5.97).
Individuals with CD were at a 2.43-fold increased risk of having a later diagnosis of SLE confirmed by medication against SLE (95% CI 1.22–4.87).
Results were similar across the numerous sensitivity analyses considering alternative definitions of SLE. When further requiring an SLE diagnosis from a department of rheumatology, nephrology/dialysis, internal medicine, or pediatrics, 44 individuals with CD had a later diagnosis of SLE (HR 2.87; 95% CI 1.97–4.17). When we restricted our outcome to having at least 2 records of SLE with at least 12 months between the first and the last visit with SLE, the HR was 3.12 (95% CI 2.06–4.71). Examining instead the risk of having SLE with the first and second diagnosis occurring within 12 months, the HR was 3.37 (95% CI 2.34–4.84).
There was no statistically significant difference by sex (p = 0.571; Table 6). We found no difference in HR between women diagnosed with CD before and after assumed menopause (p for interaction = 0.138).
Table 6.
Risk of SLE in patients with celiac disease. Analyses are stratified.
| Subgroup | HR; 95% CI | p | Person-yrs | No. SLE Events in CD | Incidence Rate* | |
|---|---|---|---|---|---|---|
| CD | Ref | |||||
| Age, yrs | ||||||
| < 20 | 2.31; 1.05–5.06 | 0.037 | 147,194 | 9 | 6 | 3 |
| 20–39 | 2.69; 1.35–5.36 | 0.005 | 60,110 | 12 | 20 | 8 |
| 40–59 | 3.91; 2.21–6.91 | < 0.001 | 74,790 | 20 | 27 | 7 |
| ≥ 60 | 5.81; 2.69–12.58 | < 0.001 | 43,676 | 13 | 30 | 4 |
| Sex | ||||||
| Women | 3.47; 2.42–4.98 | < 0.001 | 203,448 | 48 | 24 | 7 |
| Men | 3.55; 1.27–9.91 | 0.016 | 122,323 | 6 | 5 | 1 |
| Calendar period | ||||||
| –1989 | 2.82; 1.35–5.87 | 0.008 | 85,781 | 11 | 13 | 5 |
| 1990–99 | 3.91; 2.40–6.35 | < 0.001 | 161,554 | 28 | 17 | 5 |
| 2000– | 3.35; 1.78–6.28 | < 0.001 | 78,435 | 15 | 19 | 6 |
SLE cases per 100,000 person-years. CD: celiac disease; Ref: reference individuals; SLE: systemic lupus erythematosus.
DISCUSSION
To our knowledge, this is the largest population-based study of CD and risk of future SLE to date. We found a 3-fold increased risk of SLE in CD compared to the general population. However, that still translates into a low absolute risk — we estimated that at most 2 individuals with CD out of 1000 would develop SLE in the 10 years following CD diagnosis.
Several case reports of patients with both CD and SLE were published in the 1980s and 1990s11,12,13,14,15,16. In a report by Rensch, et al, 24 of 103 patients (23.3%) with SLE tested positive for antigliadin antibodies but none were positive for endomysial antibodies nor had small intestinal changes consistent with CD17. Given the lower specificity of gliadin antibodies32, the association with gliadin antibodies but not with endomysial antibodies is less supportive of an association between SLE and CD. In 2008, Freeman described 6 individuals with CD who developed SLE (out of 246 individuals with CD seen at the University of British Columbia Hospital during a period of 25 years)18. Among 24 patients with SLE screened for CD in Tunisia, Ben Abdelghani, et al found a high prevalence of antigliadin antibodies but also 2 patients (8%) with positive tissue transglutaminase antibodies; of them, CD was confirmed by histopathology in 1 patient (4%)19. However, these 3 studies17,18,19 were performed within patient groups with no general population comparator and none included children.
Of 54 CD patients with future SLE, 48 were women (89%), consistent with the well-known predominance of SLE in women33. We observed an increased risk of SLE in both men and women with CD, and the HR were independent of sex. Sex-specific risk factors are otherwise likely to play an important role in the development of immune-mediated diseases, because a female predominance has been shown for a number of diseases (including both CD and SLE)34. We found no evidence that the risk of SLE differed in women diagnosed before or after age 50 years (a proxy for menopause).
The increased use of CD serology in the clinical laboratory investigations toward CD diagnosis means that more patients with only minor symptoms of CD are identified and subsequently undergo biopsy to confirm a diagnosis of CD. We found no evidence that the change in diagnostic routines has resulted in a lower risk of SLE, using the last decade of observation as a proxy for changing diagnostic procedures. The HR for SLE in patients diagnosed with CD in the last 10-year period was 3.35 (95% CI 1.78–6.28).
Apart from the nationwide ascertainment of cases, the main strength of our study is the high positive predictive value of our CD diagnosis. We used biopsy report data from all of Sweden’s pathology departments to identify individuals with histopathology Marsh stage 3, equal to VA26. A validation of 114 randomly selected patient charts found that 95% of patients with VA in Sweden have CD27. Some 79% of patients with CD had gastrointestinal symptoms, and when 2 independent researchers manually reviewed > 1500 biopsy reports with either VA or inflammation27, diagnoses other than CD were uncommon (inflammatory bowel disease was mentioned in 0.3% of the biopsy reports, and Helicobacter pylori in 0.2%)27. Biopsy reports also have a high sensitivity for CD because 96%–100% of all Swedish gastroenterologists and pediatricians perform biopsies on their patients before CD diagnosis27.
Although the study population was large, the statistical power was limited by the relatively small number of incident SLE cases observed during followup. We are unaware of any large-scale validation of the SLE diagnosis in Sweden. Investigations within nested case-control studies suggest low positive predictive value when a single inpatient ICD code is used to define SLE35. Therefore, we restricted our outcome (in all analyses) to individuals with at least 2 diagnoses of SLE, and considered both inpatient and outpatient care. Four additional sensitivity analyses restricting the definition of SLE found similar statistically significant associations between CD and SLE. Although only a subanalysis, we included both prevalent and incident cases of CD when we examined the risk of having SLE confirmed by a medication used for SLE. A better approach for this subanalysis would have been to include only individuals with CD and their reference individuals whose biopsy and study entry occurred after July 1, 2005. However, we had insufficient power for that, given the short followup after 2005 and the limited number of SLE events. We lacked data on clinical features in SLE, HLA, and laboratory markers including autoantibodies to further confirm the SLE diagnosis or consider specific phenotypes. Although we lacked data on smoking, this is unlikely to explain the positive association between CD and SLE, because smoking seems to be positively associated with SLE36, but negatively associated37,38 with CD (or not associated at all39). Finally, no information was available on dietary adherence in CD, limiting our ability to evaluate the risk of SLE according to gluten intake. In a subset of individuals with CD, patient chart data showed indications of poor compliance in 17% of individuals with CD27.
Potential mechanisms
The underlying mechanism for the positive association between CD and SLE may be multifactorial, related to shared genetic risk factors, involvement of the innate immune system, especially Toll-like receptors (TLR), as well as several cytokine and chemokine pathways. Thus, the association of CD and SLE could be part of the spectrum of “shared autoimmunity,” a concept also used to explain overlaps between SLE and other autoimmune rheumatic diseases (e.g., “rhupus”)40. Shared patterns of altered gene expression that may lead to immune dysregulation have been observed among unrelated individuals with systemic autoimmune diseases regardless of specific disease diagnosis41,42. Many HLA and non-HLA risk genes are shared between CD and SLE and may act independently or synergistically in disease pathophysiology. Ninety percent of patients with CD carry the DQ2 allele, which is often present with the DR3 haplotype, and two-thirds of patients with SLE carry DR2 or DR3 haplotypes43. The ancestral haplotype B8-DR3-DQ2 is also strongly associated with immunoglobulin A (IgA) deficiency, which is commonly seen in both diseases44. Similarly, single-nucleotide polymorphism in the TNFAIP345,46, UBE2L3, and CLEC16A region may be associated with both CD and SLE, and IgA deficiency. ETS1, a susceptibility gene common to both CD and SLE, codes for a transcription factor that is a negative regulator of Th17 cell differentiation and terminal differentiation of B cells47. Involvement of the innate immune system through activation of dendritic cells through TLR (TLR7 and TLR9 for SLE and TLR7 and TLR8 for CD) and consequent production of type I interferon is another postulated mechanism. In a study of autoantigen profiling, healthy controls with high antinuclear antibody titers showed upregulation of the TGM2 gene, which encodes for the CD autoantigen transglutaminase 248; thus gliadin autoreactivity may be associated with incomplete forms of lupus and some early events in development of lupus may arise in the skin. UV light is a known trigger for lupus flares and induces apoptosis of keratinocytes with expression of self-antigens on the surface of apoptotic blebs that drives the autoimmune response in SLE49,50. It can be speculated that skin lesions of dermatitis herpetiformis, frequently seen in patients with CD, and consequent local inflammatory response may link the immune system to self-antigens and predispose a susceptible individual to lupus autoimmunity.
Finally, nutritional factors may influence the association between CD and SLE. Vitamin D deficiency is common especially in undiagnosed CD and may be a sign of malnutrition, but does also occur in diagnosed CD51. Such deficiency may be a risk factor for SLE52.
Both genetic predisposition and malnutrition in CD could hence contribute to the positive association between CD and SLE in our study. Surveillance bias also may have contributed to the association, especially to the 8-fold increased risk of SLE observed in the first year after CD diagnosis. But when we excluded the first year of followup, individuals with CD were still at a 3-fold increased risk of future SLE.
Individuals with CD seem to be at an increased risk of SLE compared to the general population, but absolute risks are low.
Acknowledgments
Supported by grant K23 AR057815-01A1 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)/US National Institutes of Health (NIH). Dr. Ludvigsson was supported by grants from the Swedish Society of Medicine, the Swedish Research Council, the Swedish Celiac Society, and the Fulbright Commission. Dr. Murray was supported by The National Institutes of Health — DK071003 and DK057892, and by grant support from Alba Therapeutics; he is on the advisory board of Alvine Pharmaceuticals Inc. and Nexpep; he is a consultant for Ironwood Inc., Flamentera, Actogenix, Ferring Research Institute Inc., Bayer Healthcare Pharmaceuticals, Vysera Biomedical, 2G Pharma, Inc., ImmunosanT Inc., and Shire US Inc. Dr. Rubio-Tapia was supported by an American College of Gastroenterology Junior Faculty Development Award. Dr. Chowdhary was supported by a National Institute of Arthritis and Musculoskeletal Diseases — K23 AR057815-01A1, Ronald F. Kinney Executive Dean for Research Career Development Award, Mayo Foundation. Dr. Simard was supported by the Strategic Research Program in Epidemiology at Karolinska Institutet.
References
- 1.Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2012 Feb 16; doi: 10.1136/gutjnl-2011-301346. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dube C, Rostom A, Sy R, Cranney A, Saloojee N, Garritty C, et al. The prevalence of celiac disease in average-risk and at-risk Western European populations: A systematic review. Gastroenterology. 2005;4 (Suppl 1):S57–67. doi: 10.1053/j.gastro.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Walker MM, Murray JA, Ronkainen J, Aro P, Storskrubb T, D’Amato M, et al. Detection of celiac disease and lymphocytic enteropathy by parallel serology and histopathology in a population-based study. Gastroenterology. 2010;139:112–9. doi: 10.1053/j.gastro.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao F, Yu L, Babu S, Wang T, Hoffenberg EJ, Rewers M, et al. One third of HLA DQ2 homozygous patients with type 1 diabetes express celiac disease-associated transglutaminase autoantibodies. J Autoimmun. 1999;13:143–8. doi: 10.1006/jaut.1999.0303. [DOI] [PubMed] [Google Scholar]
- 5.Ludvigsson JF, Olen O, Bell M, Ekbom A, Montgomery SM. Coeliac disease and risk of sepsis. Gut. 2008;57:1074–80. doi: 10.1136/gut.2007.133868. [DOI] [PubMed] [Google Scholar]
- 6.West J, Logan RF, Smith CJ, Hubbard RB, Card TR. Malignancy and mortality in people with coeliac disease: population based cohort study. BMJ. 2004;329:716–9. doi: 10.1136/bmj.38169.486701.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludvigsson JF, Montgomery SM, Ekbom A, Brandt L, Granath F. Small-intestinal histopathology and mortality risk in celiac disease. JAMA. 2009;302:1171–8. doi: 10.1001/jama.2009.1320. [DOI] [PubMed] [Google Scholar]
- 8.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–39. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 9.American College of Rheumatology. 1997 update of the 1982 American College of Rheumatology revised criteria for classification of systemic lupus erythematosus. [Internet. Accessed June 14, 2012]; Available from: http://www.rheumatology.org/practice/clinical/classification/SLE/1997_update_of_the_1982_acr_revised_criteria_for_classification_of_sle.pdf.
- 10.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–21. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 11.Pena AS. Systemic lupus erythematosus, Sjogren’s syndrome, and purpura in a patient with coeliac disease. Neth J Med. 1987;31:305–7. [PubMed] [Google Scholar]
- 12.Mukamel M, Rosenbach Y, Zahavi I, Mimouni M, Dinari G. Celiac disease associated with systemic lupus erythematosus. Isr J Med Sci. 1994;30:656–8. [PubMed] [Google Scholar]
- 13.Komatireddy GR, Marshall JB, Aqel R, Spollen LE, Sharp GC. Association of systemic lupus erythematosus and gluten enteropathy. South Med J. 1995;88:673–6. doi: 10.1097/00007611-199506000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Romano C, Bartolone S, Sferlazzas C, Larosa D, Magazzu G. Systemic lupus erythematosus and coeliac disease. Clin Exp Rheumatol. 1997;15:582–3. [PubMed] [Google Scholar]
- 15.Calvani M, Jr, Parisi P, Guaitolini C, Parisi G, Paolone G. Latent coeliac disease in a child with epilepsy, cerebral calcifications, drug-induced systemic lupus erythematosus and intestinal folic acid malabsorption associated with impairment of folic acid transport across the blood-brain barrier. Eur J Pediatr. 2001;160:288–92. doi: 10.1007/s004310100728. [DOI] [PubMed] [Google Scholar]
- 16.Mirza N, Bonilla E, Phillips PE. Celiac disease in a patient with systemic lupus erythematosus: A case report and review of literature. Clin Rheumatol. 2007;26:827–8. doi: 10.1007/s10067-006-0344-9. [DOI] [PubMed] [Google Scholar]
- 17.Rensch MJ, Szyjkowski R, Shaffer RT, Fink S, Kopecky C, Grissmer L, et al. The prevalence of celiac disease autoantibodies in patients with systemic lupus erythematosus. Am J Gastroenterol. 2001;96:1113–5. doi: 10.1111/j.1572-0241.2001.03753.x. [DOI] [PubMed] [Google Scholar]
- 18.Freeman HJ. Adult celiac disease followed by onset of systemic lupus erythematosus. J Clin Gastroenterol. 2008;42:252–5. doi: 10.1097/MCG.0b013e31802e70a1. [DOI] [PubMed] [Google Scholar]
- 19.Ben Abdelghani K, Mouelhi L, Hriz A, Hajri S, Najjar T, Mahfoudhi M, et al. Systemic lupus erythematosus and celiac disease. Joint Bone Spine. 2012;79:202–3. doi: 10.1016/j.jbspin.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Ventura A, Magazzu G, Greco L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. SIGEP Study Group for Autoimmune Disorders in Celiac Disease. Gastroenterology. 1999;117:297–303. doi: 10.1053/gast.1999.0029900297. [DOI] [PubMed] [Google Scholar]
- 21.Cosnes J, Cellier C, Viola S, Colombel JF, Michaud L, Sarles J, et al. Incidence of autoimmune diseases in celiac disease: protective effect of the gluten-free diet. Clin Gastroenterol Hepatol. 2008;6:753–8. doi: 10.1016/j.cgh.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 22.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad Olausson P, Bergman U, et al. The new Swedish Prescribed Drug Register — Opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16:726–35. doi: 10.1002/pds.1294. [DOI] [PubMed] [Google Scholar]
- 24.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: Possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24:659–67. doi: 10.1007/s10654-009-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johannesson I. New possibilities and better quality. Örebro: Statistics Sweden; 2002. The total population register of Statistics Sweden. [Google Scholar]
- 26.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’) Gastroenterology. 1992;102:330–54. [PubMed] [Google Scholar]
- 27.Ludvigsson JF, Brandt L, Montgomery SM, Granath F, Ekbom A. Validation study of villous atrophy and small intestinal inflammation in Swedish biopsy registers. BMC Gastroenterol. 2009;9:19. doi: 10.1186/1471-230X-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ludvigsson JF, Brandt L, Montgomery SM. Symptoms and signs in individuals with serology positive for celiac disease but normal mucosa. BMC Gastroenterol. 2009;9:57. doi: 10.1186/1471-230X-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olen O, Bihagen E, Rasmussen F, Ludvigsson JF. Socioeconomic position and education in patients with coeliac disease. Dig Liver Dis. 2012;44:471–6. doi: 10.1016/j.dld.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Rodstrom K, Bengtsson C, Milsom I, Lissner L, Sundh V, Bjourkelund C. Evidence for a secular trend in menopausal age: A population study of women in Gothenburg. Menopause. 2003;10:538–43. doi: 10.1097/01.GME.0000094395.59028.0F. [DOI] [PubMed] [Google Scholar]
- 31.Namjou B, Scofield RH, Kelly JA, Goodmon E, Aberle T, Bruner GR, et al. The effects of previous hysterectomy on lupus. Lupus. 2009;18:1000–5. doi: 10.1177/0961203309104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill ID. What are the sensitivity and specificity of serologic tests for celiac disease? Do sensitivity and specificity vary in different populations? Gastroenterology. 2005;4 (Suppl 1):S25–32. doi: 10.1053/j.gastro.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Simard JF, Costenbader KH. What can epidemiology tell us about systemic lupus erythematosus? Int J Clin Pract. 2007;61:1170–80. doi: 10.1111/j.1742-1241.2007.01434.x. [DOI] [PubMed] [Google Scholar]
- 34.Pennell LM, Galligan CL, Fish EN. Sex affects immunity. J Autoimmun. 2012;38:J282–91. doi: 10.1016/j.jaut.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Lofstrom B, Backlin C, Sundstrom C, Hellstrom-Lindberg E, Ekbom A, Lundberg IE. Myeloid leukaemia in systemic lupus erythematosus — A nested case-control study based on Swedish registers. Rheumatology. 2009;48:1222–6. doi: 10.1093/rheumatology/kep204. [DOI] [PubMed] [Google Scholar]
- 36.Costenbader KH, Kim DJ, Peerzada J, Lockman S, Nobles-Knight D, Petri M, et al. Cigarette smoking and the risk of systemic lupus erythematosus: A meta-analysis. Arthritis Rheum. 2004;50:849–57. doi: 10.1002/art.20049. [DOI] [PubMed] [Google Scholar]
- 37.West J, Logan RF, Hill PG, Lloyd A, Lewis S, Hubbard R, et al. Seroprevalence, correlates, and characteristics of undetected coeliac disease in England. Gut. 2003;52:960–5. doi: 10.1136/gut.52.7.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Austin AS, Logan RF, Thomason K, Holmes GK. Cigarette smoking and adult coeliac disease. Scand J Gastroenterol. 2002;37:978–82. doi: 10.1080/003655202760230973. [DOI] [PubMed] [Google Scholar]
- 39.Ludvigsson JF, Montgomery SM, Ekbom A. Smoking and celiac disease: A population-based cohort study. Clin Gastroenterol Hepatol. 2005;3:869–74. doi: 10.1016/s1542-3565(05)00414-3. [DOI] [PubMed] [Google Scholar]
- 40.Alarcon-Segovia D. Shared autoimmunity: The time has come. Curr Rheumatol Rep. 2004;6:171–4. doi: 10.1007/s11926-004-0063-7. [DOI] [PubMed] [Google Scholar]
- 41.Aune TM, Maas K, Parker J, Moore JH, Olsen NJ. Profiles of gene expression in human autoimmune disease. Cell Biochem Biophys. 2004;40:81–96. doi: 10.1385/CBB:40:2:081. [DOI] [PubMed] [Google Scholar]
- 42.O’Hanlon TP, Rider LG, Gan L, Fannin R, Paules RS, Umbach DM, et al. Gene expression profiles from discordant monozygotic twins suggest that molecular pathways are shared among multiple systemic autoimmune diseases. Arthritis Res Ther. 2011;13:R69. doi: 10.1186/ar3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graham RR, Ortmann W, Rodine P, Espe K, Langefeld C, Lange E, et al. Specific combinations of HLA-DR2 and DR3 class II haplotypes contribute graded risk for disease susceptibility and autoantibodies in human SLE. Eur J Hum Genet. 2007;15:823–30. doi: 10.1038/sj.ejhg.5201827. [DOI] [PubMed] [Google Scholar]
- 44.Wang N, Shen N, Vyse TJ, Anand V, Gunnarson I, Sturfelt G, et al. Selective IgA deficiency in autoimmune diseases. Mol Med. 2011;17:1383–96. doi: 10.2119/molmed.2011.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trynka G, Zhernakova A, Romanos J, Franke L, Hunt KA, Turner G, et al. Coeliac disease-associated risk variants in TNFAIP3 and REL implicate altered NF-kappa-B signalling. Gut. 2009;58:1078–83. doi: 10.1136/gut.2008.169052. [DOI] [PubMed] [Google Scholar]
- 46.Ramos PS, Criswell LA, Moser KL, Comeau ME, Williams AH, Pajewski NM, et al. A comprehensive analysis of shared loci between systemic lupus erythematosus (SLE) and sixteen autoimmune diseases reveals limited genetic overlap. PLoS Genet. 2011;7:e1002406. doi: 10.1371/journal.pgen.1002406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trynka G, Wijmenga C, van Heel DA. A genetic perspective on coeliac disease. Trends Mol Med. 2010;16:537–50. doi: 10.1016/j.molmed.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Li QZ, Karp DR, Quan J, Branch VK, Zhou J, Lian Y, et al. Risk factors for ANA positivity in healthy persons. Arthritis Res Ther. 2011;13:R38. doi: 10.1186/ar3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casciola-Rosen L, Rosen A. Ultraviolet light-induced keratinocyte apoptosis: A potential mechanism for the induction of skin lesions and autoantibody production in LE. Lupus. 1997;6:175–80. doi: 10.1177/096120339700600213. [DOI] [PubMed] [Google Scholar]
- 51.Stenson WF, Newberry R, Lorenz R, Baldus C, Civitelli R. Increased prevalence of celiac disease and need for routine screening among patients with osteoporosis. Arch Intern Med. 2005;165:393–9. doi: 10.1001/archinte.165.4.393. [DOI] [PubMed] [Google Scholar]
- 52.Ritterhouse LL, Crowe SR, Niewold TB, Kamen DL, Macwana SR, Roberts VC, et al. Vitamin D deficiency is associated with an increased autoimmune response in healthy individuals and in patients with systemic lupus erythematosus. Ann Rheum Dis. 2011;70:1569–74. doi: 10.1136/ard.2010.148494. [DOI] [PMC free article] [PubMed] [Google Scholar]


