Abstract
Background/Objectives
The effect of a low glycemic load (GL) diet on insulin-like growth factor-1 (IGF-1) concentration is still unknown but may contribute to lower chronic disease risk. We aimed to assess the impact of GL on concentrations of IGF-1 and IGFBP-3.
Subjects/Methods
We conducted a randomized, controlled crossover feeding trial in 84 overweight-obese and normal weight healthy individuals using two 28-day weight-maintaining high- and low-GL diets. Measures were fasting and post-prandial concentrations of insulin, glucose, IGF-1 and IGFBP-3. 20 participants completed post-prandial testing by consuming a test breakfast at the end of each feeding period. We used paired t-tests for diet-component and linear mixed models for biomarker analyses.
Results
The 28-day low-GL diet led to 4% lower fasting concentrations of IGF-1 (10.6 ng/mL, p=0.04) and a 4% lower ratio of IGF-1/IGFBP-3 (0.24, p=0.01) compared to the high-GL diet. The low-GL test breakfast led to 43% and 27% lower mean post-prandial glucose and insulin responses, respectively; mean incremental areas under the curve for glucose and insulin, respectively, were 64.3±21.8 (mmol/L/240min) (p<0.01) and 2253±539 (μU/mL/240min) (p<0.01) lower following the low- compared to the high-GL test meal. There was no effect of GL on mean HOMA-IR or on mean integrated post-prandial concentrations of glucose-adjusted insulin, IGF-1 or IGFBP-3. We did not observe modification of the dietary effect by adiposity.
Conclusions
Low-GL diets resulted in 43% and 27% lower post-prandial responses of glucose and insulin, respectively, and modestly lower fasting IGF-1 concentrations. Further intervention studies are needed to weigh the impact of dietary GL on risk for chronic disease.
MeSH Keywords: Adiposity, Glycemic Index, Insulin Resistance, Insulin-Like Growth Factor I, Insulin-Like Growth Factor Binding Protein 3, Randomized Controlled Trial [Publication Type]
INTRODUCTION
Dietary intervention studies have shown detrimental metabolic effects of high-glycemic load diets, including higher post-prandial glucose and insulin concentrations, less desirable lipid profiles1 and propensity for obesity.2 Glycemic index (or GI) is the numerical classification of a particular food’s blood glucose-raising effect.3 The term “glycemic load” has been used to denote meal- or overall diet-related glycemic effect.4–6 Glycemic load is defined as the product of grams of carbohydrate x GI / 1004 and accounts for both the quantity and quality of dietary carbohydrate. The potentially detrimental metabolic effects are reflected in some of the epidemiologic studies that support an association between high-glycemic load diets and increased risk for type 2 diabetes.5–7
Chronic hyperinsulinemia, as a result of a high glycemic load (or GI) diet, has also been proposed as a risk factor for several types of cancers, though not all studies point toward this risk.8, 9 High insulin-like growth factor-1 (IGF-1) concentration, like hyperinsulinemia, may also contribute to increased risk for some types of cancer;10 however, whether insulin and IGF-1 act through similar mechanisms is unclear. Concentrations of a major binding protein for IGF-1, IGF binding protein 3 (IGFBP-3), affect the bioavailability of IGF-1 (indicated by the molar ratio of IGF-1/IGFBP-3)11 and may also be independently associated with cancer risk.12
Contrary to a proposed cancer protective effect of low IGF-1 concentration, epidemiologic studies suggest a lower ratio of IGF-1/IGFBP-3 is associated with components of the metabolic syndrome.13, 14 The growth hormone-IGF axis is involved in carbohydrate metabolism;15 however, dietary glycemic load has not clearly been associated with IGF-I concentrations in epidemiologic studies.16 Some studies suggest, though evidence is limited, that higher insulin levels may indirectly increase bioavailability of circulating IGF-1.17 Though few intervention studies have examined acute and chronic effects of glycemic load on IGF-1 concentrations,18–20 findings to date suggest a high-glycemic load diet, possibly because of hyperinsulinemia, increases bioavailability of IGF-1.
We hypothesized that a high-glycemic load diet would lead to lower circulating concentrations of IGF-1 and higher concentrations of IGFBP-3 compared to a low-glycemic load diet. To address this hypothesis, we evaluated effects of 28-day, weight-maintaining, high-and low-glycemic load controlled diets on circulating concentrations of fasting and post-prandial glucose, insulin, IGF-1 and IGFBP-3, and to assess modification of dietary effects by adiposity. We conducted a randomized, controlled crossover feeding trial in 84 normal weight (BMI=18.5– 24.9 kg/m2) and overweight-obese (BMI=28.0–40.0 kg/m2) healthy individuals.
SUBJECTS AND METHODS
Study Population
We recruited healthy men and women from the local Seattle area by newspaper, flyer and Fred Hutchinson Cancer Research Center (FHCRC) website announcements. Special efforts were undertaken to recruit African-American and Hispanic individuals at community and cultural events with high African-American or Hispanic attendance.21 Inclusion criteria for the study were that participants 1) had no dietary restrictions, 2) had no physician-diagnosed conditions that might influence metabolic response to diet (e.g., impaired glucose tolerance, diabetes, kidney, thyroid or cardiovascular disease), 3) had a BMI between 18.5 and 25.0 kg/m2 or between 28.0 and 39.9 kg/m2, 4) were not pregnant, lactating or considering pregnancy 5) were not using hormones or over-the-counter medications and 6) did not use tobacco or excessive alcohol (defined as 2 or more cans/bottles of beer, 2 or more glasses of wine or 3 or more ounces of hard liquor daily). We tested fasting blood glucose and excluded those with fasting glucose greater than 5.55 mmol/L. We asked participants to discontinue use of all nutritional supplements prior to the feeding study. Participants completed baseline questionnaires to collect data on sex, race/ethnicity, health history, habitual diet and physical activity. The study protocol was approved by both the Institutional Review Board and Clinic Trials Office of the FHCRC and all participants gave written, informed consent.
Research Design
We enrolled 89 participants in the study between June 2006 and July 2009 and block randomized participants by BMI group (18.5–24.9 kg/m2 or 28.0–40.0 kg/m2) and sex to the order of experimental diets. In a cross-over design, each participant consumed a high- or low-glycemic load diet for 28 consecutive days during the first feeding period, followed by a 28-day wash-out period (during which participants consumed their habitual diet) and then consumed the other high- or low-glycemic load diet for 28 days during the second feeding period. Study dietitians and staff prepared all foods and beverages for the dietary intervention periods in the Human Nutrition Laboratory (HNL) of the FHCRC and provided all food for the two feeding periods to the participants.22 A subset of 20 participants completed post-prandial testing by consuming a test breakfast at the end of each feeding period.
We instructed participants to consume only the food and beverages provided by the HNL during the feeding periods with the exception of tea and coffee (whitener and sweetener additives provided by study) which was permitted at stable, continuous levels. During the week, participants ate a daily dinner meal at the HNL under supervision by study staff and ate breakfast, lunch, snack and weekend meals at home. Participants returned unconsumed food (which was weighed and recorded by the HNL) and recorded daily consumption of food provided by the study and any non-study food taken outside of the HNL.
Anthropometry
We measured baseline height, weight and waist and hip circumferences and assessed percentage body fat by whole-body dual-energy X-ray absorptiometry (DXA) scanning, using a GE Lunar DPX- Pro densitometer (GE Healthcare, Milwaukee, WI) prior to the start of the first intervention period. Study staff weighed participants three times per week.
Study Diets
We designed both high- and low- glycemic load diets to be weight-maintaining. We used three-day dietary records and estimates of daily energy needs (calculated from the Mifflin equation23) to predict an individual’s energy needs during the dietary intervention periods. We intended both diets to represent realistic high- and low-glycemic load diets that were similar in daily macronutrient composition (15% energy from protein, 30% energy from fat and 55% energy from total carbohydrate) but differing by glycemic load (glycemic load ≥ 250, GI 78 and glycemic load ≤ 125, GI 34 for high-glycemic load and low-glycemic load diets, respectively). We first created seven-day high- and low-glycemic load reference diets of 2400 kcal/day (see Supplemental Table 1). We next generated variations on the reference diet by adding or subtracting 200-kcal increments in proportional serving sizes of all foods to achieve diets varying in energy content between 1600 and 3600 kcal. This approach met the estimated energy needs of all of our participants. As an example, a diet containing 3600 kcal/day had similar nutrient composition to the diet containing 1600 kcal/day, but differed in portion sizes by proportional increments. In order to maintain a participant’s weight within 3% of baseline weight, we made dietary energy adjustments in 200 kcal increments, if necessary, during the diet intervention periods. While keeping percent energy from carbohydrate similar in both diets, we varied carbohydrate quality across diets to achieve high- or low-glycemic load meals, calculated as previously published24 (glycemic load = GI x total carbohydrate content per serving / 100). We utilized reported glucose-referenced GI values (2002 International Glycemic Index Tables25) and consulted the University of Sydney database (www.glycemicindex.com). Food composition data was obtained from ProNutra®: Metabolic Diet Study Management System (Viocare Technologies, Inc®. Princeton, NJ) and the Nutrition Data System for Research (version 2005, Nutrition Coordinating Center, University of Minnesota), peer reviewed publications of food composition, and food manufacturers. The high- and low-glycemic load diets differed in fiber content (mean fiber content in the 2400 kcal reference diet was 24 g/day and 49 g/day for high-and low-glycemic load diets, respectively) because higher fiber content food is generally found in lower GI foods. Test breakfasts for the postprandial studies were actual meals within the high- and low-glycemic load 28-day intervention diet menus. The high-glycemic load test meal contained high-GI buckwheat pancakes, pancake-syrup, butter, a fruit-flavored drink and milk. The low-glycemic load test meal contained a low-GI oat and buckwheat groats pancake, agave syrup, butter, strawberries, tomato juice and milk.
Sample Collection and Analysis
Before and at the end of each completed 28-day diet period, we obtained blood from fasted participants (12-hour fast). In an ancillary post-prandial study in a subset of participants (n=20), we evaluated post-prandial responses to the test meal breakfasts, corresponding to the high- and low-glycemic load diets of the preceding feeding period. After beginning the meal (time 0), we obtained post-prandial blood draws at time points 15, 30, 45, 60, 90, 120, 180, 240 minutes after the meal. Blood was collected and processed according to a standard protocol and stored at −80°C until analysis.
The assay for serum glucose was performed enzymatically at the Northwest Lipid Research Laboratories (University of Washington), using Roche reagents on a Roche Module P Chemistry autoanalyzer (Roche Diagnostics Inc., Indianapolis, IN). The intra-assay CV was 0.8%. The assay for serum insulin was performed at the Diabetes Endocrinology Research Center Immunoassay Core Laboratory (University of Washington), and quantified by a two site assay using a Tosoh 2000 auto-analyzer (Tosoh Biosciences Inc., South San Francisco, CA). The intra-assay CV was 4.4%. If insulin concentrations were below the detectable range of 2.0 μU/mL, the reported concentration was imputed as 1.0 μU/mL, the midpoint between 0 and 2.0 (μU/mL). The Pollak laboratory conducted the assays for IGF-1 and IGFBP-3 using assays from Diagnostic Systems, Ltd (Webster, TX); they performed ELISA measurements for these analytes using a single production lot of reagents, conducted in duplicate. CVs were < 9% for intra-assay variation.
Statistical Analysis
We aimed to test the effect of a 28-day low- vs. high-glycemic load intervention on fasting serum concentrations and postprandial responses of metabolic biomarkers including glucose, insulin, IGF-1 and IGFBP-3, and to assess modification of dietary effects by adiposity. We used paired t-tests for diet-component analysis and used linear mixed models for biomarker analyses. Biomarker values were transformed by the natural logarithm to improve the normality assumption of the linear models. The linear mixed model allowed us to account for any correlation of paired outcomes from the same participant. In the linear mixed model, diet treatment, diet sequence and diet period were fixed effects and participant was a random effect. We calculated least squared means and 95% confidence intervals for two-sided tests and considered p values < 0.05 to be statistically significant. We adjusted all models for age, sex, baseline biomarker concentrations, diet sequence and feeding period. For the postprandial analyses, we calculated incremental area under the curve (iAUC) by trapezoidal method26 (sum of integrated areas above baseline biomarker values). The iAUC analyses for IGF-1 and IGFBP-3 were performed by additionally subtracting baseline values because post-prandial values were predominantly suppressed below baseline. As an estimate of glucose-adjusted insulin response, we calculated the quotient of post-prandial 240 minute iAUC of insulin divided by that for glucose (iAUC insulin/iAUC glucose).27 Homeostasis model assessment for insulin resistance (HOMA- IR) was calculated by taking the product of the fasting insulin and glucose in mg/dL and dividing this by 405;28 glucose values are presented in SI units (1 mg/dL = 0.0555 mmol/L). We performed a priori subgroup analyses within strata of body fat mass where DXA-measured body fat was classified as high for males with ≥ 25% body fat and females with ≥ 32% body fat.29 We formally assessed for an interaction between diet and body fat by creating cross-product terms and inserting in the linear mixed models. Two participants had missing DXA data and so were assigned to the high body fat mass group based on BMI (30.8 and 40.2 kg/m2). A sample size of 88 participants, calculated a priori, predicted detection of a >30% difference in biomarker concentrations with 80% power. We analyzed the data using SAS (version 9.1.2 SAS Institute, Cary, NC).
RESULTS
Baseline characteristics of participants are shown in Table 1. 89 individuals met screening criteria and consented to join the study, 84 participants started the first feeding study and two individuals dropped out of the study during the first feeding period. 80 participants completed both feeding periods and two completed only one feeding period (one completed the high- and the other completed the low-glycemic load diet). We included the 80 participants who completed both diet periods in the analysis.
TABLE 1.
Baseline characteristics of 80 participants by gender and body fat status1
| Low BF %2 | High BF %2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Characteristic | All | Male | Female | All | Male | Female | All | Male | Female |
| Sample (n) | 80 | 40 | 40 | 29 | 16 | 13 | 51 | 24 | 27 |
| Age (years)3 | 29.6 ± 8.2 | 31.0 ± 8.3 | 28.2 ± 7.9 | 28.6 ± 7.1 | 28.0 ± 7.3 | 29.2 ± 6.9 | 30.2 ± 8.8 | 33.0 ± 8.5 | 27.8 ± 8.4 |
| Ethnicity | |||||||||
| Hispanic4 | 20 (25) | 13 | 7 | 9 | 7 | 2 | 11 | 6 | 5 |
| Caucasian4 | 35 (44) | 16 | 19 | 12 | 4 | 8 | 23 | 12 | 11 |
| Black4 | 16 (20) | 6 | 10 | 2 | 1 | 1 | 14 | 5 | 9 |
| Other4 | 9 (11) | 5 | 4 | 6 | 4 | 2 | 3 | 1 | 2 |
| Height (cm)3 | 171.4±10.5 | 178.7 ± 7.4 | 164.1 ± 7.8 | 171.7 ± 10.8 | 179 ± 7.9 | 162.8 ± 6.0 | 171.2 ± 10.5 | 178.5 ± 7.3 | 164.8 ± 8.5 |
| Weight (kg)3 | 81.1 ± 21.7 | 88.4 ± 21.9 | 73.9 ± 19.1 | 77.2 ± 21.5 | 81.1 ± 24.2 | 72.5 ± 17.5 | 83.4 ± 21.7 | 93.3 ± 19.2 | 74.5 ± 20.1 |
| BMI (kg/m2)3 | 27.4 ± 5.9 | 27.5 ± 5.9 | 27.3 ± 6.0 | 26.1 ± 6.1 | 25.1 ± 6.0 | 27.3 ± 6.1 | 28.2 ± 5.7 | 29.2 ± 5.3 | 27.3 ± 6.1 |
| BMI range5 | 19.5 – 40.2 | 19.5 – 40.2 | 20.0 – 38.9 | 19.5 – 40.2 | 19.5 – 40.2 | 20.5 – 38.7 | 20.0 – 39.1 | 20.2 – 39.1 | 20.0 – 38.9 |
| DXA % BF3 | 32.8 ± 11.9 | 31.2 ± 12.1 | 34.4 ± 11.6 | 21.2 ± 6.9 | 20.3 ± 7.5 | 22.3 ± 6.1 | 39.7 ± 8.2 | 38.9 ± 8.0 | 40.5 ± 8.5 |
BF, body fat; DXA Dual-energy X-ray absorptiometry
Low BF <25% for males or <32% for females; High BF 25% for males or 32% for females.
Mean ± SD.
Sample size (%).
Minimum – maximum.
Table 2 shows planned daily mean macronutrient content, dietary GI, dietary glycemic load and distribution of percent energy for both high- and low-glycemic load 2400 kcal reference diets. All diets at each energy level were similar to the reference diet shown. Analyses of self-reported food consumption records and post-meal leftover food weight records revealed the percent energy consumed was not significantly different between diets and adherence to the diets was greater than 98 ± 4%. Weight was maintained throughout both dietary periods and necessary energy adjustments did not differ significantly by diet type or feeding period (data not shown). Table 3 shows planned mean macronutrient content, GI, glycemic load and distribution of percent energy for the high- and low-glycemic load test breakfast from each 2400 kcal reference diet. Test breakfast consumption of protein (g), total carbohydrate (g) and energy (kcal) were statistically different (p < 0.05) by paired t-tests during the high- and low-glycemic load test breakfasts.
Table 2.
Mean energy and macronutrient content of 2400 kcal high and low glycemic load reference diets
| High Glycemic Load Diet | Low Glycemic Load Diet | |
|---|---|---|
| n=7 days | n=7 days | |
| Dietary Descriptor | Mean ± SD | Mean ± SD |
| Energy (kcal2/day) | 2398 ± 8 | 2396 ± 6 |
| Protein (g/day) | 90 ± 1 | 90 ± 0 |
| Energy from protein (% using total carb) | 15 ± 0.2* | 14 ± 0.4* |
| Energy from protein (% using available carb) | 15 ± 0.2 | 16 ± 0.2 |
| Fat (g/day) | 80 ± 0 | 81 ± 1 |
| Energy1 from fat (% using total carb) | 30 ± 0.3* | 29 ± 0.8* |
| % energy from fat (% using available carb) | 31 ± 0.2 | 31 ± 0.6 |
| Total carbohydrate (g/day) | 341 ± 6* | 361 ± 15* |
| Energy from total carbohydrate (%) | 56 ± 0.5* | 57 ± 1.1* |
| Energy from available carbohydrate (%) | 54 ± 0.8 | 53 ± 0.8 |
| Dietary fiber (g/day) | 24 ± 5* | 49 ± 8* |
| Glycemic Index/day | 78 ± 5* | 34 ± 1* |
| Glycemic Load/day | 244 ± 14* | 117 ± 3* |
Asterisk (*) next to p value < 0.05 for paired t-tests comparing high- and low-glycemic load diets.
Total energy calculated as sums of 4 kcal/g protein, 9 kcal/g fat and 4 kcal/g total carbohydrate.
Total energy calculated using available carbohydrate.
Data from ProNutra®: Metabolic Diet Study Management System (Viocare Technologies, Inc®. Princeton, NJ) and/or the Nutrition Data System for Research (version 2005, Nutrition Coordinating Center, University of Minnesota).
Table 3.
Description of test breakfast meal from the 2400 kcal high and low glycemic diets
| High glycemic load test breakfast | ||||||||
|---|---|---|---|---|---|---|---|---|
| Food | Energy (kcal) | Prot (g) | Fat (g) | Total Carb (g) | Fiber (g) | GI food | % Carb x GI | Carb (g) x GI / 100 |
| Reduced fat, 2% milk | 122 | 8 | 5 | 11 | 0 | 30 | 5 | 3 |
| Buckwheat pancake | 160 | 6 | 2 | 31 | 2 | 92 | 38 | 28 |
| Salted butter | 108 | 0 | 12 | 0 | 0 | |||
| Lemon-line Gatorade powder | 58 | 0 | 0 | 15 | 0 | 78 | 16 | 12 |
| Pancake syrup | 66 | 0 | 0 | 17 | 0 | 68 | 16 | 12 |
| Totals | 513 | 14 | 19 | 74 | 2 | 268 | 74 | 55 |
| Percent total energy1 | 10% | 33% | 57% | |||||
| Percent total energy2 | 11% | 33% | 56% | |||||
| Low glycemic load test breakfast | ||||||||
| Reduced fat, 2% milk | 122 | 8 | 5 | 11 | 0 | 30 | 4 | 3 |
| Oat and buckwheat groats pancake | 168 | 9 | 5 | 23 | 3 | 48 | 13 | 11 |
| Salted butter | 72 | 0 | 8 | 0 | 0 | |||
| Frozen, unsweetened strawberries | 49 | 1 | 0 | 13 | 3 | 40 | 6 | 5 |
| Canned tomato juice | 28 | 1 | 0 | 7 | 1 | 33 | 3 | 2 |
| Light agave nectar | 114 | 0 | 0 | 30 | 0 | 32 | 12 | 10 |
| Sugar -free pancake syrup | 14 | 0 | 0 | 4 | 0 | |||
| Totals | 568 | 19 | 18 | 89 | 6 | 183 | 37 | 32 |
| Percent total energy1 | 13% | 27% | 60% | |||||
| Percent total energy2 | 13% | 29% | 58% | |||||
Total energy calculated as sums of 4 kcal/g protein, 9 kcal/ g fat and 4 kcal/g total carbohydrate.
Percent total energy calculated using available carbohydrate. Wt, weight; Prot, protein; Carb, total carbohydrate; GI glycemic index; %, percent of meal carbohydrate; g, grams of total cabohydrate
The overall dietary intervention effects on glucose, insulin HOMA-IR, IGF-1, IGFBP3 and IGF-1/IGFBP3 are shown in Table 4. Fasting serum glucose concentrations were similar between diet treatments in the lean group but not in the overweight/obese group; mean fasting glucose was 0.12 mmol/L higher following the low- compared to the high-glycemic load diet. Fasting insulin concentrations were not different between treatments in the group overall or within body-fat subgroups and similarly, there were no significant differences in HOMA-IR. The low-glycemic load diet led to 4% lower fasting concentrations of IGF-1 (10.6 ng/mL, p = 0.04) and a 4% lower ratio of IGF-1/IGFBP-3 (0.24, p = 0.01) compared to the high-glycemic load diet. The effect of glycemic load on IGF-1 concentration and ratio of IGF-1/IGFBP-3 within body fat subgroups did not reach statistical significance, except for a lower ratio of IGF-1/IGFBP-3 due to the low-glycemic load diet in the high body fat subgroup. Body fat subgroups had similar fasting IGF-1 concentrations (interaction term for diet and adiposity p = 0.8). Diets resulted in no statistically significant difference between fasting concentrations of IGFBP3.
TABLE 4.
Fasting and integrated post-prandial concentrations following 28-day high- or low-glycemic load dietary interventions for all participants and within body fat subgroups1,2,3
| N | High-Glycemic Load | Low-Glycemic Load | Difference | P value | |
|---|---|---|---|---|---|
| Fasting measures4 | |||||
| Glucose (mmol/L) | 80 | 4.92 (4.86–4.98) | 5.00 (4.94–5.06) | −0.08 | 0.04 |
| Low Body Fat | 29 | 4.80 (4.70–4.90) | 4.80 (4.70 – 4.90) | 0.0 | 0.97 |
| High Body Fat | 53 | 5.00 (4.92 – 5.07) | 5.12 (5.04 – 5.19) | −0.12 | 0.02 |
| Insulin (μU/mL) | 80 | 8.3 (7.6–9.1) | 8.2 (7.5–9.0) | 0.2 | 0.73 |
| Low Body Fat | 29 | 5.9 (5.2–6.8) | 5.6 (5.0 – 6.6) | 0.2 | 0.62 |
| High Body Fat | 53 | 10.3 (9.2 – 11.6) | 10.0 (8.9 – 11.2) | 0.4 | 0.63 |
| HOMA-IR | 80 | 1.82 (1.66–2.01) | 1.82 (1.66–2.01) | 0.00 | 0.99 |
| Low Body Fat | 29 | 1.25 (1.08 – 1.46) | 1.23 (1.06 – 1.43) | 0.02 | 0.75 |
| High Body Fat | 53 | 2.29 (2.02 – 2.60) | 2.27 (2.0 – 2.57) | 0.03 | 0.88 |
| IGF-1 (ng/mL) | 80 | 281.5 (273.3 – 289.9) | 270.9 (263.1 – 279.0) | 10.6 | 0.04 |
| Low Body Fat | 29 | 276.7 (262.5 – 291.7) | 268.7 (254.9 – 283.2) | 8.0 | 0.33 |
| High Body Fat | 53 | 277.9 (267.8 – 288.3) | 267.4 (257.8 – 277.4) | 10.4 | 0.12 |
| IGFBP-3 (ng/mL) | 80 | 4890 (4800 – 4981) | 4908 (4818 – 4999) | −18 | 0.75 |
| Low Body Fat | 29 | 4770(4604 – 4942) | 4669 (4506 – 4838) | 101 | 0.29 |
| High Body Fat | 53 | 4941 (4837 – 5046) | 5028 (4922 – 5135) | −87 | 0.21 |
| IGF1/IGFBP-3 | 80 | 5.76 (5.62–5.90) | 5.52 (5.39–5.66) | 0.24 | 0.01 |
| Low Body Fat | 29 | 5.80 (5.60 – 6.01) | 5.75 (5.55 – 5.96) | 0.05 | 0.69 |
| High Body Fat | 53 | 5.62 (5.44–5.82) | 5.32 (5.14 – 5.50) | 0.31 | 0.02 |
| Postprandial measures5 | |||||
| Glucose iAUC(mmol/L/240 min) | 20 | 148.1 ± 25.4 | 83.9 ± 12.4 | 64.3 ± 21.8 | < 0.01 |
| Low Body Fat | 8 | 183.0 ± 30.2 | 123.7 ± 22.3 | 59.3 ± 34.2 | 0.08 |
| High Body Fat | 12 | 157.5 ± 29.5 | 83.3 ±9.05 | 74.1 ± 24.9 | < 0.01 |
| Insulin iAUC (μU/mL/240 min) | 20 | 8417 ± 808 | 6164 ± 567 | 2253 ± 539 | < 0.01 |
| Low Body Fat | 8 | 9905 ± 1710 | 7622 ± 1402 | 2284 ± 749 | < 0.01 |
| High Body Fat | 12 | 8672 ± 698 | 6240 ± 355 | 2432 ± 685 | < 0.01 |
| Insulin iAUC/Glucose iAUC (pmol/mmol) | 20 | 309.9 ± 131.5 | 169.4 ± 43.2 | 140.5 ± 145.9 | 0.34 |
| Low Body Fat | 8 | 317.1 ± 174.8 | 135.1 ± 73.9 | 183.8 ± 145.9 | 0.21 |
| High Body Fat | 12 | 272.1 ± 156.8 | 203.6 ± 59.5 | 68.5 ± 183.8 | 0.71 |
| IGF-1 iAUC (ng/mL/240 min) A | 20 | −2577 ± 514 | −2359 ± 685 | −218 ± 824 | 0.79 |
| Low Body Fat | 8 | −4271 ± 710 | −4370 ± 979 | 99 ± 1385 | 0.94 |
| High Body Fat | 12 | −2043 ± 695 | −1607 ± 794 | −437 ± 1020 | 0.69 |
| IGFBP-3 iAUC (ng/mL/240 min) A | 20 | −41162 ± 12282 | −19585 ± 13668 | −21577 ± 14980 | 0.15 |
| Low Body Fat | 8 | −90973 ± 16328 | −86212 ± 17185 | −4760 ± 17596 | 0.79 |
| High Body Fat | 12 | −24017 ± 13709 | 6861 ± 10717 | −30877 ± 19649 | 0.11 |
Adjusted for design effects, age, gender and body fat percent.
Low body fat <25% for males or <32% for females; high body fat ≥25% for males or ≥32% for females.
HOMA-IR, Homeostasis model of assessment - insulin resistance iAUC, incremental area under the curve.
Geometric means (CI).
Mean ± SEM.
Negative values for iAUC represent responses for which post-prandial concentrations were predominantly lower than fasting concentrations.
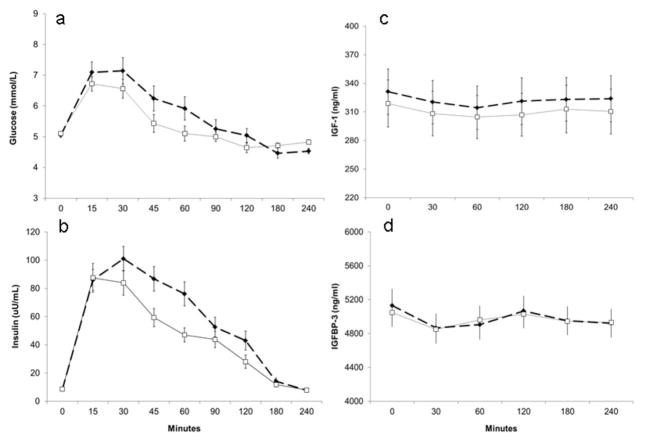
The post-prandial study results for 20 participants are shown in Table 4. Following a high- or low-glycemic load test breakfast, the adjusted mean iAUCs for postprandial glucose and insulin responses were significantly higher (both p < 0.01) following the high-glycemic load breakfast compared to the low-glycemic load breakfast. A difference in diet effect was non-significant for the ratio of iAUC insulin/iAUC glucose over 240 minutes. Concentrations of IGF-1 and IGFBP-3 changed very little between 0 and 240 minutes following the meals; however, there was a statistically significant decline (approximately 4–5% below baseline concentrations) in mean IGF-1 concentrations from fasting during the first 60 minutes after both test meals (paired t-test for difference between mean concentrations at 60 minutes and at baseline p < 0.01). There was no significant decline from baseline at 60 minutes for the ratio of IGF-1/IGFBP-3. Figure 1 shows the mean concentrations of glucose, insulin, IGF-1 and IGFBP-3, beginning at time 0 before the meals and at all time-points following high-and low-glycemic load test meals. The interaction term for diet and body fat category was not significant for fasting and integrated post-prandial concentrations of glucose, insulin, IGF-1, IGFBP-3 and ratio of IGF-1/IGFBP-3.
Figure 1.
Mean (±SEM) concentrations of a) plasma glucose, b) plasma insulin, c) serum insulin-like growth factor-1 (IGF-1) and d) serum insulin-like growth factor-binding protein 3 (IGFBP-3) in 20 healthy lean and overweight-obese participants, who were fasting (time 0) and then consumed a high- (dotted lines and closed squares) or low- (thin line and open squares) glycemic load breakfast. The iAUC are significantly different for glucose and insulin and not significantly different for IGF-1 and IGFBP-3.
DISCUSSION
In this randomized controlled feeding study, the mean fasting IGF-1 concentration and ratio of IGF-1/IGFBP-3 were 4% lower following 28 days of the low-glycemic load diet compared to the high-glycemic load diet. The effect of glycemic load on fasting ratio of IGF-1/IGBP-3 appeared strongest within the overweight-obese stratum of adiposity, though adiposity was not a statistically significant modifier of dietary effect in these analyses. Higher circulating concentrations of IGF-I may be a risk factor for obesity-linked cancer.30 Mechanistically, some propose diet-induced hyperinsulinemia leads to higher circulating bioactive IGF-1 concentrations by suppression of IGFBP-1,17 and thus may be a causal link between carcinogenesis and these diets.31 In this study, the clinical significance of the low-glycemic load diet effects to moderately lower fasting IGF-1 concentration and ratio IGF-1/IGFBP-3, may be contributing factors in the lower cancer risk ascribed to low-glycemic load diets in epidemiologic studies.8 Our observation, that the effect of a low-glycemic load diet on IGF-1 concentration was especially strong in overweight-obese participants, may be an important finding since these individuals are considered at even higher cancer-risk.10
Aside from regulation by growth hormone, circulating concentrations of IGF-1 and IGFBP-3 are influenced by chronic nutritional status.32–35 Studies of the chronic effects of glycemic load on IGF-1 concentrations during periods of weight stability are limited. Although not testing glycemic load, per se, two previous intervention studies showed no differences in fasting total IGF-1 or IGFBP-3 concentrations following 1 year of a prescribed low-fat, high-fiber diet when compared to 1 year or 4 years of a usual diet.36, 37 In an epidemiologic study, total carbohydrate intake, fiber intake and glycemic load were not associated with IGF-1 or IGFBP-3 concentrations.16 Our finding of a modest effect of glycemic load on IGF-1 concentration after only 28 days is novel and suggests glycemic load may be another important chronic nutritional factor influencing the growth-hormone-IGF-1 axis.
Higher circulating concentrations of IGF-I have been associated with reduced risk of impaired glucose tolerance, type-2 diabetes38 and other components of the metabolic syndrome,11, 13, 14 although these relationships may not be linear.39 In our study, the high-and low-glycemic load diets led to similar estimates of insulin sensitivity (HOMA-IR) between diets after 28 days. This contrasts with some previous intervention study findings that showed a low-glycemic load diet improved insulin sensitivity,40 usually in the context of diabetes or diet-induced weight loss. Our participants were healthy at baseline and weight stable during the intervention periods, thus insulin sensitivity would be less likely to change significantly over 28 days. There was a statistically significant but small (approximately 0.06 mmol/L) higher mean fasting glucose following the low-glycemic load diet; the clinical significance of this may be unimportant. Other studies typically show no change in fasting glucose or a lowering effect due to the low-glycemic load diet.40, 41 One long-term intervention study found that the low-glycemic load diet led to slightly higher mean fasting blood glucose;42 the authors considered the implication of this finding to be minor. Findings for post-prandial effects of glycemic load are more consistent across intervention studies.
High-glycemic load meals, as compared to low-glycemic load meals, reliably lead to higher postprandial glucose and insulin concentrations in both healthy and insulin-resistant individuals.43, 44 Our results are consistent with those previous studies. When post-prandial insulin responses were adjusted for glucose responses (iAUC insulin/iAUC glucose), we did not detect a statistically significant difference between test breakfast types, suggesting β-cell function was not affected by the preceding 28-day glycemic load diets. The effect of glycemic load on β-cell function has varied in previous human and animal intervention studies.45, 46
Our findings do not support an acute post-prandial effect of glycemic load on post-prandial IGF-1 or IGBP-3 concentrations. Our IGF-1 findings are consistent with those of Brand-Miller et al.18 who reported GI had minimal effect on post-prandial free and total IGF-1 concentrations.18 Our findings are consistent with another study in which IGF-I concentration was relatively stable after an oral glucose tolerance test.47 Circulating concentrations of IGF-1 are considered relatively insensitive to acute post-prandial changes47 because of the stabilizing effect of IGFBP-3.48 Perhaps unexpectedly, the high- and low-glycemic load meals led to similar modest post-prandial declines in mean IGF-1 concentration at 60 minutes. On the other hand, neither meal affected concentrations of IGBP-3 in our study, in contrast to findings by Brand-Miller et al.18 who observed divergent GI effects on IGFBP-3. The discrepancy between post-prandial IGFBP-3 responses in both studies might be explained by the large standard error of IGFBP-3 responses.
There are several strengths of this study. The cross-over intervention utilized two controlled diets designed to match distribution of percent energy from macronutrients over a single day and 7 day cycle while maintaining an individual’s weight throughout experimental dietary periods. The two diets were characterized by substantially different glycemic loads and adherence to these diets was very high for both diets, thus ensuring appropriate exposure to high-and low-glycemic carbohydrate loads. We used typically consumed carbohydrate-containing foods with published, validated GI values. The parent study size of 80 participants was relatively large compared to most studies for which all food is provided and adherence is closely monitored. To our knowledge, this is the only study in healthy individuals examining the effects of weight maintaining high- and low-glycemic load diets over several weeks followed by post-prandial testing of IGF-1 and IGFBP-3 on high- and low-glycemic load background diets. There are several important limitations of our study. Mean dietary macronutrient and energy content differed slightly between the high- and low-glycemic load test meals; small differences in mean macronutrient and energy intake between the two breakfasts may obscure an effect of glycemic load. Diets were designed to be realistic and therefore fiber content was naturally higher in the low-glycemic load 28-day diet and the test meal. Consequently, this study cannot distinguish effects of fiber from effects of glycemic load. Post-prandial analyses within body fat subgroup may not have achieved statistical power to detect diet effects due to small sample sizes. Finally, participants for the post-prandial study were sampled by convenience, thereby possibly introducing selection bias. For these reasons, our post-prandial results may not be generalizable to all lean or overweight-obese groups.
In conclusion, our findings show that consumption of a low-glycemic load diet resulted in lower post-prandial insulin and glucose responses and modestly lower fasting IGF-1 and IGF-1/IGFBP-3 concentrations. There was no observable effect of glycemic load on insulin resistance or glucose-adjusted post-prandial insulin responses in these healthy participants. Low-glycemic load diets induce a metabolic profile that could decrease risk for some cancer types. Further intervention studies are needed to weigh the impact of dietary glycemic load on risk for chronic disease.
Supplementary Material
Acknowledgments
JWL, LMV and MLN designed research protocol; YS and KB conducted dietary interventions; GDC and YS recruited participants; MNP’s laboratory performed IGF-1 and IGBP-3 assays and MNP contributed to data interpretation; SSR, JWL, MLN, CW, CYW analyzed the data; SSR, JWL and MLN wrote the paper and had primary responsibility for the final content. All authors were involved in the preparation of the final manuscript.
Solo® Bars provided by Solo GI Nutrition, Kewlona, BC, Canada
Light Agave Nectar provided by Madhava Natural Sweeteners, Longmont, CO, USA
Supported by: NIH grants U54 CA116847, R03 CA132158, and T32HL007028
List of Abbreviations
- DXA
Dual-energy X-ray absorptiometry
- FHCRC
Fred Hutchinson Cancer Research Center
- GI
Glycemic index
- HNL
Human Nutrition Laboratory
- IGF-1
IGF-1 insulin like growth factor-1
- IGFBP-3
IGF binding protein-3
- iAUC
Incremental area under the curve
- GH
growth hormone
Footnotes
The study was registered at clinicaltrials.gov as NCT00622661.
The authors have no conflicts of interest to disclose.
References
- 1.Opperman AM, Venter CS, Oosthuizen W, Thompson RL, Vorster HH. Meta-analysis of the health effects of using the glycaemic index in meal-planning. Br J Nutr. 2004;92:367–381. doi: 10.1079/bjn20041203. [DOI] [PubMed] [Google Scholar]
- 2.Pawlak DB, Ebbeling CB, Ludwig DS. Should obese patients be counselled to follow a low-glycaemic index diet? Yes. Obes Rev. 2002;3:235–243. doi: 10.1046/j.1467-789x.2002.00079.x. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins DJ, Kendall CW, Augustin LS, Franceschi S, Hamidi M, Marchie A, et al. Glycemic index: overview of implications in health and disease. Am J Clin Nutr. 2002;76:266S–273S. doi: 10.1093/ajcn/76/1.266S. [DOI] [PubMed] [Google Scholar]
- 5.Salmeron J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, et al. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care. 1997;20:545–550. doi: 10.2337/diacare.20.4.545. [DOI] [PubMed] [Google Scholar]
- 6.Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997;277:472–477. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- 7.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 8.Gnagnarella P, Gandini S, La Vecchia C, Maisonneuve P. Glycemic index, glycemic load, and cancer risk: a meta-analysis. Am J Clin Nutr. 2008;87:1793–1801. doi: 10.1093/ajcn/87.6.1793. [DOI] [PubMed] [Google Scholar]
- 9.Mulholland HG, Murray LJ, Cardwell CR, Cantwell MM. Glycemic index, glycemic load, and risk of digestive tract neoplasms: a systematic review and meta-analysis. Am J Clin Nutr. 2009;89:568–576. doi: 10.3945/ajcn.2008.26823. [DOI] [PubMed] [Google Scholar]
- 10.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 11.Juul A, Main K, Blum WF, Lindholm J, Ranke MB, Skakkebaek NE. The ratio between serum levels of insulin-like growth factor (IGF)-I and the IGF binding proteins (IGFBP-1, 2 and 3) decreases with age in healthy adults and is increased in acromegalic patients. Clin Endocrinol (Oxf) 1994;41:85–93. doi: 10.1111/j.1365-2265.1994.tb03788.x. [DOI] [PubMed] [Google Scholar]
- 12.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 13.Sierra-Johnson J, Romero-Corral A, Somers VK, Lopez-Jimenez F, Malarstig A, Brismar K, et al. IGF-I/IGFBP-3 ratio: a mechanistic insight into the metabolic syndrome. Clin Sci (Lond) 2009;116:507–512. doi: 10.1042/CS20080382. [DOI] [PubMed] [Google Scholar]
- 14.Lam CS, Chen MH, Lacey SM, Yang Q, Sullivan LM, Xanthakis V, et al. Circulating insulin-like growth factor-1 and its binding protein-3: metabolic and genetic correlates in the community. Arterioscler Thromb Vasc Biol. 2010;30:1479–1484. doi: 10.1161/ATVBAHA.110.203943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clemmons DR. Role of insulin-like growth factor iin maintaining normal glucose homeostasis. Horm Res. 2004;62 (Suppl 1):77–82. doi: 10.1159/000080763. [DOI] [PubMed] [Google Scholar]
- 16.Holmes MD, Pollak MN, Willett WC, Hankinson SE. Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomarkers Prev. 2002;11:852–861. [PubMed] [Google Scholar]
- 17.Attia N, Tamborlane WV, Heptulla R, Maggs D, Grozman A, Sherwin RS, et al. The metabolic syndrome and insulin-like growth factor I regulation in adolescent obesity. J Clin Endocrinol Metab. 1998;83:1467–1471. doi: 10.1210/jcem.83.5.4827. [DOI] [PubMed] [Google Scholar]
- 18.Brand-Miller JC, Liu V, Petocz P, Baxter RC. The glycemic index of foods influences postprandial insulin-like growth factor-binding protein responses in lean young subjects. Am J Clin Nutr. 2005;82:350–354. doi: 10.1093/ajcn.82.2.350. [DOI] [PubMed] [Google Scholar]
- 19.Smith R, Mann N, Makelainen H, Roper J, Braue A, Varigos G. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res. 2008;52:718–726. doi: 10.1002/mnfr.200700307. [DOI] [PubMed] [Google Scholar]
- 20.Smith RN, Mann NJ, Braue A, Makelainen H, Varigos GA. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57:247–256. doi: 10.1016/j.jaad.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 21.Coronado GD, Ondelacy S, Schwarz Y, Duggan C, Lampe JW, Neuhouser ML. Recruiting underrepresented groups into the carbohydrates and related biomarkers (CARB) cancer prevention feeding study. Contemp Clin Trials. 2012;33:641–646. doi: 10.1016/j.cct.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neuhouser ML, Schwarz Y, Wang C, Breymeyer K, Coronado G, Wang CY, et al. A low-glycemic load diet reduces serum C-reactive protein and modestly increases adiponectin in overweight and obese adults. J Nutr. 2012;142:369–374. doi: 10.3945/jn.111.149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 24.Brand-Miller JC, Thomas M, Swan V, Ahmad ZI, Petocz P, Colagiuri S. Physiological validation of the concept of glycemic load in lean young adults. J Nutr. 2003;133:2728–2732. doi: 10.1093/jn/133.9.2728. [DOI] [PubMed] [Google Scholar]
- 25.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 26.Wolever TM, Jenkins DJ. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr. 1986;43:167–172. doi: 10.1093/ajcn/43.1.167. [DOI] [PubMed] [Google Scholar]
- 27.Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care. 2009;32:335–341. doi: 10.2337/dc08-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudenski AS, Matthews DR, Levy JC, Turner RC. Understanding “insulin resistance”: both glucose resistance and insulin resistance are required to model human diabetes. Metabolism. 1991;40:908–917. doi: 10.1016/0026-0495(91)90065-5. [DOI] [PubMed] [Google Scholar]
- 29.Flegal KM, Shepherd JA, Looker AC, Graubard BI, Borrud LG, Ogden CL, et al. Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr. 2009;89:500–508. doi: 10.3945/ajcn.2008.26847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 31.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131:3109S–3120S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 32.Giovannucci E, Pollak M, Liu Y, Platz EA, Majeed N, Rimm EB, et al. Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiol Biomarkers Prev. 2003;12:84–89. [PubMed] [Google Scholar]
- 33.Isley WL, Underwood LE, Clemmons DR. Dietary components that regulate serum somatomedin-C concentrations in humans. J Clin Invest. 1983;71:175–182. doi: 10.1172/JCI110757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thissen JP, Ketelslegers JM, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr Rev. 1994;15:80–101. doi: 10.1210/edrv-15-1-80. [DOI] [PubMed] [Google Scholar]
- 35.McGreevy KM, Hoel BD, Lipsitz SR, Hoel DG. Impact of nutrients on insulin-like growth factor-I, insulin-like growth factor binding protein-3 and their ratio in African American and white males. Public Health Nutr. 2007;10:97–105. doi: 10.1017/S1368980007217999. [DOI] [PubMed] [Google Scholar]
- 36.Gann PH, Kazer R, Chatterton R, Gapstur S, Thedford K, Helenowski I, et al. Sequential, randomized trial of a low-fat, high-fiber diet and soy supplementation: effects on circulating IGF-I and its binding proteins in premenopausal women. Int J Cancer. 2005;116:297–303. doi: 10.1002/ijc.21042. [DOI] [PubMed] [Google Scholar]
- 37.Flood A, Mai V, Pfeiffer R, Kahle L, Remaley AT, Rosen CJ, et al. The effects of a high-fruit and -vegetable, high-fiber, low-fat dietary intervention on serum concentrations of insulin, glucose, IGF-I and IGFBP-3. Eur J Clin Nutr. 2008;62:186–196. doi: 10.1038/sj.ejcn.1602726. [DOI] [PubMed] [Google Scholar]
- 38.Sandhu MS, Heald AH, Gibson JM, Cruickshank JK, Dunger DB, Wareham NJ. Circulating concentrations of insulin-like growth factor-I and development of glucose intolerance: a prospective observational study. Lancet. 2002;359:1740–1745. doi: 10.1016/S0140-6736(02)08655-5. [DOI] [PubMed] [Google Scholar]
- 39.Brugts MP, van Duijn CM, Hofland LJ, Witteman JC, Lamberts SW, Janssen JA. Igf-I bioactivity in an elderly population: relation to insulin sensitivity, insulin levels, and the metabolic syndrome. Diabetes. 2010;59:505–508. doi: 10.2337/db09-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livesey G, Taylor R, Hulshof T, Howlett J. Glycemic response and health--a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am J Clin Nutr. 2008;87:258S–268S. doi: 10.1093/ajcn/87.1.258S. [DOI] [PubMed] [Google Scholar]
- 41.Vrolix R, Mensink RP. Effects of glycemic load on metabolic risk markers in subjects at increased risk of developing metabolic syndrome. Am J Clin Nutr. 2010;92:366–374. doi: 10.3945/ajcn.2009.28339. [DOI] [PubMed] [Google Scholar]
- 42.Wolever TM, Gibbs AL, Mehling C, Chiasson JL, Connelly PW, Josse RG, et al. The Canadian Trial of Carbohydrates in Diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: no effect on glycated hemoglobin but reduction in C-reactive protein. Am J Clin Nutr. 2008;87:114–125. doi: 10.1093/ajcn/87.1.114. [DOI] [PubMed] [Google Scholar]
- 43.Lan-Pidhainy X, Wolever TM. Are the glycemic and insulinemic index values of carbohydrate foods similar in healthy control, hyperinsulinemic and type 2 diabetic patients? Eur J Clin Nutr. 2011;65:727–734. doi: 10.1038/ejcn.2011.28. [DOI] [PubMed] [Google Scholar]
- 44.Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2003;26:2261–2267. doi: 10.2337/diacare.26.8.2261. [DOI] [PubMed] [Google Scholar]
- 45.Andersson U, Rosen L, Wierup N, Ostman E, Bjorck I, Holm C. A low glycaemic diet improves oral glucose tolerance but has no effect on beta-cell function in C57BL/6J mice. Diabetes Obes Metab. 2010;12:976–982. doi: 10.1111/j.1463-1326.2010.01288.x. [DOI] [PubMed] [Google Scholar]
- 46.Solomon TP, Haus JM, Kelly KR, Cook MD, Filion J, Rocco M, et al. A low-glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am J Clin Nutr. 2010;92:1359–1368. doi: 10.3945/ajcn.2010.29771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frystyk J, Grofte T, Skjaerbaek C, Orskov H. The effect of oral glucose on serum free insulin-like growth factor-I and -II in health adults. J Clin Endocrinol Metab. 1997;82:3124–3127. doi: 10.1210/jcem.82.9.4259. [DOI] [PubMed] [Google Scholar]
- 48.Murphy LJ. The role of the insulin-like growth factors and their binding proteins in glucose homeostasis. Exp Diabesity Res. 2003;4:213–224. doi: 10.1155/EDR.2003.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.