Abstract
Background
Selective declarative memory processes are differentially compromised in chronic alcoholism (ALC) and HIV infection (HIV) and likely reflect neuropathology associated with each condition: frontocerebellar dysfunction in alcoholism and frontostriatal dysfunction in HIV infection. Evidence for disease overlap derives from observed exacerbated impairments in these declarative memory processes in alcoholism-HIV comorbidity. Less is known about nondeclarative memory processes in these disease conditions. Examination of visuomotor learning in chronic alcoholism and HIV infection could provide insight into the differential and combined contribution of selective disease-related injury to visuomotor procedural memory processes.
Methods
We examined component processes of visuomotor learning and retention on the rotary pursuit task in 29 ALC, 23 HIV, 28 ALC+HIV, and 20 control subjects. Participants were given four rotary pursuit learning sessions over two testing days, typically separated by one week, to assess visuomotor learning and retention patterns. Ancillary measures of simple motor, psychomotor, explicit memory, and balance abilities were administered to test which component processes independently predicted visuomotor learning.
Results
All clinical groups showed visuomotor learning across rotary pursuit testing sessions, despite impairment in visuomotor speed in the HIV groups and impairment in explicit memory and psychomotor speed in the alcohol groups. The two alcoholic groups showed retention and consolidation over time (i.e., improved performance without further training), whereas the HIV-infected group showed learning and retention but no consolidation effect. The comorbid group shared impairments associated with the ALC-only group (explicit memory and psychomotor speed) and the HIV-only group (visuomotor speed), although there was no clear compounded effect of alcohol and HIV-infection on visuomotor learning performance.
Conclusions
This study supports the hypothesis that alcoholism and HIV infection exert differential effects on components of visuomotor procedural learning. Further, the results provide behavioral evidence for dissociable influences of frontocerebellar and frontostriatal disruption to visuomotor procedural learning and retention.
Keywords: alcohol, rotary pursuit, visuomotor learning, consolidation, cerebellum
Selective memory processes are differentially compromised in chronic alcoholism (Fein et al., 2006; McGlinchey et al., 2005; Nixon et al., 1995; Oscar-Berman, 1990; Sullivan et al., 2000; Sullivan et al., 1997) and HIV infection (Fama et al., 2009; Gonzalez et al., 2008; Maki et al., 2009; Martin et al., 1993b; Martin et al., 2001; Rothlind et al., 2005). Chronic alcoholism is commonly marked by visuospatially-mediated and working memory impairments, whereas HIV infection is commonly marked by verbally-mediated and episodic memory impairments. These differential patterns of declarative memory impairment in chronic alcoholism and HIV infection likely reflect the neuropathology associated with these conditions: frontocerebellar dysfunction in chronic alcoholism (Sullivan et al., 2003) and frontostriatal dysfunction in HIV infection (Castelo et al., 2006), with limbic system compromise common to both conditions. Persistent cognitive deficits have been documented in both chronic alcoholism with long-term abstinence (e.g., Fein et al., 1990; Fein and Chang, 2006; Rosenbloom et al., 2008) and HIV infection treated with antiretroviral agents (e.g., Cysique and Brew, 2009). In particular, deficits in higher-order executive functions, components of memory, and visuomotor abilities have been documented even after long-term abstinence in alcoholics (Beatty et al., 1996; Brandt et al., 1983; Fein et al., 2006; Goldman et al., 1983), although selective cognitive and motor abilities (e.g., attention, working memory, psychomotor speed) can show some recovery. Further, evidence for disease overlap has been observed with documented compounded impairments in cognitive processes in alcoholism-HIV comorbidity (Fama et al., 2007; Fama et al., 2009; Rothlind et al., 2005).
The majority of memory-related studies in chronic alcoholism and HIV infection has focused on component processes of declarative memory. Less is known about how these conditions and their comorbidity affect nondeclarative memory processes, which could be independently disrupted by neuropathology unique to each condition. Studies of procedural learning, many of which have used rotary pursuit testing to assess visuomotor speed and learning, have generally reported learning deficits in individuals with primary subcortical dysfunction (e.g., Huntington’s disease - (Gabrieli et al., 1997; Heindel et al., 1988); Parkinson’s disease - (Haaland et al., 1997)), whereas these processes are relatively intact in individuals with conditions primarily affecting cortical or medial temporal structures (e.g., Alzheimer’s disease - (Heindel et al., 1989); global amnesia - (Cermak and Butters, 1973; Cohen and Squire, 1980; Corkin, 1968; Tranel et al., 1994)). In addition to subcortical influences on visuomotor learning processes, damage to the cerebellum and associated neural networks is known to impair visuomotor learning, particularly in tasks requiring anticipatory movement (Ivry and Keele, 1989; Schugens et al., 1998).
Martin and colleagues (Martin et al., 1993a) were the first to demonstrate procedural learning deficits in a subgroup of HIV infected subjects. Seven of their 29 HIV-infected subjects (24%) exhibited impaired motor speed and learning on a rotary pursuit task, and this impairment was not associated with CD4 counts. Later, Gonzalez and colleagues (Gonzalez et al., 2008) examined 48 HIV-infected individuals and 48 control subjects on a rotary pursuit task (speed set at 55 rpm for all subjects) and found that the HIV group was impaired compared with the control group on the initial testing session; yet, both groups demonstrated learning across trials. These authors concluded that deficits reported in previous studies on the rotary pursuit task in HIV infection may be due to impairments in complex motor functions and processing speed rather than to a deficit in procedural learning per se.
Doyon and colleagues (Doyon et al., 2003) have hypothesized that learning a visuomotor task comprises three phases: 1) learning, 2) consolidation, and 3) retention. The early, fast learning stage that occurs initially after presentation of a task is hypothesized to be primarily dependent on intact cortico-cerebellar circuitry, whereas the later, retention stage is primarily dependent on intact cortico-striatal circuitry. The consolidation phase, the time between testing sessions, is dependent on limbic structures and surrounding circuitry. Grafton and colleagues (Grafton et al., 1994) using PET provided support for this hypothesis, reporting that initial acquisition of motor skills accompanied recruitment of cortical and cerebellar areas, whereas learning based on continued practice was associated with activity in the striatum. These findings suggest that cortico-cerebellar and cortico-striatal circuits make independent contributions to visuomotor learning tasks. Thus, individuals comorbid for chronic alcoholism and HIV infection may manifest both frontocerebellar and frontostriatal compromise and be dually affected, demonstrating greater impairment in visuoperceptual speed and procedural learning than individuals affected by only one condition.
Visuomotor procedural processes are functionally and structurally multifaceted. Successful learning reflects intact motor, perceptual, memory, and executive functions (cf., Schmidtke et al., 2002). Dysfunction in any one of these component processes contributing to visuomotor learning can contribute to impairment, and processes involved during initial learning may differ from the component processes associated with consolidation and retention. Schmidtke and colleagues (Schmidtke et al., 2002) showed that visuospatial performance in patients with frontostriatal lesions (sum of scaled scores of Block Design and Object Assembly from WAIS-R) predicted initial performance on a rotary pursuit task, whereas verbal fluency scores predicted subsequent learning.
The aims of the present study were (1) to examine visuomotor learning and retention on the rotary pursuit task in individuals with chronic alcoholism, HIV infection, and those comorbid for alcoholism and HIV infection compared with control subjects and (2) to investigate the mnemonic and nonmnemonic component processes underlying learning and retention performance in each clinical group. We tested the following hypotheses: (a) all groups would show visuomotor learning and retention across trials, although the comorbid group would demonstrate the greatest deficits of all the groups in motor and memory related processes; (b) the HIV-infected groups (HIV, ALC+HIV) would demonstrate slowed visuomotor speed compared with the control group; (c) although the single diagnosis groups (ALC, HIV) would not differ from each other on overall visuomotor learning scores, the HIV group would show better early learning than the ALC group (based on ALC-related presumed cerebellar compromise), whereas the ALC group would show better consolidation and retention compared to the HIV group (based on HIV-related presumed striatal compromise); and (d) visuomotor speed and learning in the three clinical groups would be associated with selective cognitive processes (e.g., psychomotor processing speed, memory, executive functions) rather than being associated with simple motor speed ability (e.g., finger movements).
MATERIALS AND METHODS
Based on medical tests and interviews, subjects were categorized as 1) ALC (n=29): men who met DSM-IV criteria for alcohol dependence or abuse within the past 3 years of study entry and who were deemed HIV seronegative by blood test, 2) HIV (n=23): men who tested positive for HIV infection and had never met criteria for alcohol dependence, 3) ALC+HIV (n=28): men who tested positive for HIV infection and also met criteria for alcohol dependence or abuse within the past 3 years of study entry, and 4) NC (n=20): normal control men who were neither HIV-positive nor met alcohol dependence or abuse criteria or any other Axis-I diagnosis in their lifetime. All subjects gave written informed consent to participate in this study, which was approved by the Institutional Review Boards of Stanford University, SRI International, and Santa Clara Valley Medical Center. Demographics of the four subject groups are in Table 1.
Table 1.
Demographic characteristics of subject groups (mean, SD, range)
| Group | Age (yrs) | Eeducation (yrs) | NART IQ† | Lifetime Alcohol Consumption (kg) |
Beck Depression Inventory-II |
CD4 count |
|---|---|---|---|---|---|---|
| Control (NC) | ||||||
| Session 1: n=20 | 43.9 (10.2) |
15.2 (2.0) |
111.3 7.9 |
33.6 (63.4) |
3,0 (3.6) |
NA |
| 22 to 58 | 12 to 18 | 92 to 123 | 0 to 245 | Oto 12 | ||
| Sessions 1-4: n=17 | 42.5 (10.0) |
14.8 (1.9) |
110.1 (7.7) |
38.3 (68.0) |
3 2 (3.8) |
|
| 22 to 58 | 12 to 18 | 92 to 123 | 0 to 264 | Oto 12 | ||
| Alcoholism (ALC) | ||||||
| Session 1; n=29 | 49.3 (9.9) |
13.6 (2.5) |
106.2 (9.4) |
1295 (952.5) |
8.3 (6.5) |
NA |
| 25 to 66 | 10 to 21 | 91 to 124 | 80 to 4376 | Oto 22 | ||
| Sessions 1-4: n=21 | 48.9 (9.2) |
13.8 (2.9) |
107.7 (9.6) |
1352.6 (969.2) |
7.2 (6.3) |
|
| 26 to 66 | 10 to 21 | 91 to 124 | 79 to 4376 | Oto 22 | ||
| HIV infection (HIV) | ||||||
| Session 1: n=23 | 49.6 (8.5) |
13.6 (3.1) |
106.1 (10.3) |
78.9 (63.2) |
11.8 (9.9) |
542.5 (236.3) |
| 28 to 64 | 9 to 19 | 90 to 123 | 0 to 280 | 0 to 28 | 88 to 983 | |
| Sessions 1-4: n=17 | 48.4 (9.4) |
13.2 (3.3) |
106.5 (11.0) |
74.9 (48.4) |
10.9 (10.3) |
544.5 234.4 |
| 28 to 64 | 9 to 19 | 90 to 123 | 8 to 169 | O to 28 | 202 to 983 | |
| Alcoholism with HIV (ALC+HIV) | ||||||
| Session 1: n=28 | 48.9 (9.5) |
13.4 (2.0) |
107.1 (7.6) |
702.6 (519.7) |
10.0 (7.1) |
542.8 (349.4) |
| 22 to 60 | 8 to 18 | 94 to 123 | 31 to 1648 | Oto 24 | 42 to 1259 | |
| Sessions 1-4: n=16 | 47.3 (9.3) |
12.9 (2.4) |
107.5 (8.7) |
729.9 (519.1) |
9.X (6.7) |
488.7 (274.9) |
| 22 to 58 | 8 to 18 | 94 10 123 | 72 to 1648 | Oto 20 | 42 to 1033 | |
|
| ||||||
| Baseline ANOVAs | ns | ns | ns | p=0001 | p=001 | ns |
| Post-hoc t-tcsts | NC<H1V<ALC+HIV<ALC | NC<ALC,HIV,ALC+HIV | ||||
| Longitudinal ANOVAs | ns | ns | ns | p=.0001 | p=012 | ns |
| Post-hoc t-tests | NC<HIV<ALC+H1V<ALC | NC<ALC, HIV, ALC+HIV | ||||
NART- National Adult Reading Test
NA = Not applicable
Participants were part of a larger ongoing longitudinal study on the effects of alcohol and HIV infection on brain and behavior. Although all ALC subjects were initially recruited from local substance abuse treatment programs and ALC+HIV and HIV subjects were recruited from HIV clinics, many participants performed the Rotary Pursuit Test for the first time at a follow-up visit, at which time ALC patients may have been out of treatment for months or even years. Participants in the control group were recruited from the local community. Before initially entering the longitudinal study, all participants (patients and controls) were screened using the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1998) and structured questionnaires on health status. Upon initial assessment subjects were excluded if they had a significant history of a psychiatric or neurological disorder not related to their primary diagnosis, a history of past or present alcohol or drug abuse or dependence for inclusion in the NC group, recent (within the last 3 months) substance dependence other than alcohol in the clinical groups, serious medical condition, or HIV-related opportunistic infection. Participants admitted to the study underwent additional clinical assessments including the Beck Depression Inventory-II (Beck et al., 1996) to assess severity of depression symptoms. and a semi-structured interview (Skinner, 1982; Skinner and Sheu, 1982) to quantify lifetime alcohol consumption.
Blood samples were used to determine HIV status, plasma viral load, and CD4 cell count. HIV subjects were also rated on the Karnofsky Scale, which provides a global measure of general abilities in activities of daily living (Karnofsky, 1949). At time of study entry all HIV-positive individuals had CD4>100 cells per mm3 and a Karnofsky score ≥90. The SCID, Beck Depression Inventory-II, alcohol history, Karnofsky Scale, and blood tests were repeated at each follow-up visit.
For ALC and ALC+HIV participants, the SCID interviewer determined whether individuals currently met DSM-IV criteria for a current Alcohol Use Disorder (AUD) and, if not, how many weeks since they had met criteria. The interviewer also noted the date alcohol was last consumed as part of the lifetime alcohol consumption questionnaire. Because some ALC and ALC+HIV subjects reported current light social drinking but had not met DSM-IV criteria for an AUD for many months, we measured their sobriety in terms of weeks since they last met DSM-IV criteria for AUD rather than number of days since they last had an alcoholic drink.
The four groups did not differ significantly in age (F(3,96)=1.56, p=.20). A statistical trend was observed between groups for years of education (F(3,96)=2.42, p=.07) but not on estimated IQ scores, which were based on the NART (National Adult Reading Test; F(3,95)=1.64, p=.19) (Nelson, 1982)(Nelson, 1982). Amount of lifetime alcohol consumption was statistically different between the alcohol groups (ALC: 1350±974 kg; ALC+HIV: 651±491 kg), but both alcohol groups drank significantly more than the HIV or NC groups. The ALC and ALC+HIV did not differ significantly in age of onset of alcoholism (ALC: 22.6±9.4 years of age; ALC+HIV: 21.1±5.9 years of age; Z=.032, p=.975) or duration of alcohol diagnosis (from date first met DSM-IV criteria for AUD to last met criteria) (ALC: 26.7±12.9 years; ALC+HIV: 27.7±11.3 years; Z=.335, p=.738). Weeks in remission tended to be longer in the ALC+HIV group than the ALC group (ALC+HIV: 275.3± 427.7 weeks; ALC: 70.3+92.9 weeks; Z=1.844, p=.065). The HIV and ALC+HIV groups did not differ significantly in CD4 cell count (HIV: 543±236; ALC+HIV: 543±349; t(48)=.004, p=.997) or viral load (HIV: 2.6+1.4; ALC+HIV: 2.2+1.2; t(43)=.496, p=.623) were not different between HIV and ALC+HIV.
Four of the HIV and 4 of the ALC+HIV subjects met CDC criteria for AIDS, having an AIDS defining symptom (e.g., CD4<200), sometime during the course of their illness. At the time of testing, 1 of the 23 HIV subjects and 5 of the 28 ALC+HIV subjects had CD4 cell counts below 200, while 13 HIV and 15 ALC+HIV subjects had CD4 cell counts above 500. Of the 23 HIV subjects, 21 individuals were on highly-active antiretroviral treatment (HAART), and of the 28 ALC+HIV subjects 23 were on HAART. Seven of the HIV and 25 of the ALC+HIV subjects met criteria for a non-alcohol substance abuse diagnosis sometime in their lifetime (but not currently). Six of the HIV, 6 of the ALC, and 11 of the ALC+HIV subjects tested positive for Hepatitis C.
Rotary Pursuit Test
The Rotary Pursuit Test (Corkin, 1968, Buxton and Grant, 1939) is a visuomotor speed and learning task. Subjects were instructed to use a stylus to track a spot of light rotating counterclockwise on a turntable. Subjects held the stylus directly over the light at all times without touching the glass surface and were informed that a clicking sound would indicate when they went on and off the light. Using their preferred hand, subjects were titrated to a turntable speed at which they could keep contact with the target for 5 seconds of a 20 second trial (cf., Heindel et al., 1988). Subjects were started at 10 rpm and speed was increased by 5 or 10 rpm until maximum speed at which a subject could stay on target for at least 5 seconds was reached. This calibrated speed was used for all rotary pursuit sessions for that individual. Subjects received 4 learning sessions spaced over 2 separate test days (2 learning sessions per test day), each session consisted of 8, 20-second trials. A 1-minute rest interval was given between trials 4 and 5 of each learning session. Sessions 1 and 2 and sessions 3 and 4 were separated by at least 1 hour. Sessions 2 and 3 were separated by at least 1 day. Time on target was recorded for each trial. Learning was indicated by increased time on target over session and calculated [Session 4 average time on target score over the 8 trials – Session 1 average time on target score over the 8 trials].
Ancillary Cognitive Tests
Tests of simple motor, psychomotor, memory, and balance were administered to examine the contribution of these motor and cognitive processes to visuomotor speed and learning in chronic alcoholism and HIV infection.
The Fine Finger Movement Test (Corkin et al., 1986) required subjects to turn a knurled rod with their forefinger and thumb, unimanually and then bimanually. The number of turns as indicated by the counter is recorded and averaged across the two trials for each condition. This task required speeded movement and was chosen for its sensitivity to frontostriatal integrity (Cooper et al., 1991; Leonard et al., 1988). The bimanual score was used as the dependent measure from this test.
The Symbol Digit Test (Smith, 1973) required subjects to convert meaningless geometric designs into written number responses using a key which matched nine geometric designs with the numbers from 1 to 9. This test measure primarily assesses visual scanning (Shum, McFarland, and Bain, 1990). The number of correct responses in 90-seconds was used as the dependent measure from this test.
Selective explicit memory abilities were examined with the Wechsler Memory Scale – Revised (WMS-R; (Wechsler, 1987)). Dependent variables included the five computed indices: Verbal Memory (e.g. verbal paired associates and narrative memory), Visual Memory (e.g., visual paired associates and memory for simple designs), Attention (e.g., digit and block span forward and backwards, recitation of numbers and letters), Immediate Memory (e.g., recall of visual and verbal material), and Delayed Memory (e.g., recall after a 30 minute delay of visual and verbal material).
A subtest from the Walk-a-Line Ataxia Battery (Fregly et al., 1972) required subjects to stand on one leg with their arms folded across their chest for 30 seconds. Two trials of each condition (right, then left leg; open eyes, then closed eyes) were administered unless a perfect score was achieved on the initial trial, in which case the subject received full credit for the second trial of that condition. This lower limb motor task assessed balance and was chosen for its demonstrated sensitivity to the integrity of the frontocerebellar motor system, in particular the anterior superior vermis of the cerebellum (Gilman et al., 1990; Sullivan et al., 2006; Sullivan et al., 2000; Victor et al., 1989). The left leg condition was used as the dependent measure from this test, as this condition has been shown to be particularly sensitive to truncal instability, irrespective of handedness (Pitel et al., 2011; Rosenbloom et al., 2007).
Statistical Analyses
Repeated-measures analyses of variance (ANOVAs) were conducted across the four Rotary Pursuit learning sessions to examine group by session interactions and main effects. Paired t-tests within groups assessed learning across sessions. One-way ANOVAs were conducted on all ancillary test measures and demographic variables. Within-group correlational analyses examined the relationships between test scores and the relationships between demographic variables and test scores. Finally, multiple regression analyses examined the unique contribution of select variables to visuomotor speed and learning scores within the clinical groups.
When comparing the 4 participant groups, total amount of learning was calculated as the difference between average time on target across the 8 trials of Session 4 and the average time on target across the 8 trials of Session 1. By contrast, learning scores assessing pattern of performance within and across testing days were calculated as follows: (1) Learning within a testing day: the difference between average time on target on Session 2 (the second session on the first testing day) and average time on target on Session 1 (the first session on the first testing day) and the difference between average time on target on Session 4 (the second session on the second testing day) and the average time on target on Session 3 (the first session on the second testing day); (2) Learning across testing days: the difference between average time on target on Session 3 (the first session on the second testing day) and average time on target on Session 2 (the second session on the first testing day).
RESULTS
Rotary Pursuit Test Baseline Session
Calibrated speed (rpm at which subject could stay on target 5 of 20 seconds) differed significantly between groups (F(3,96)=2.80, p=.04). Follow-up t-tests indicated that the ALC+HIV group calibrated at a significantly slower speed than the control group (t(46)=2.72, p=.009), and the HIV group was modestly slower than the control group (t(41)=1.85, p=.07). Calibrated speed was negatively correlated with time on target in the ALC and NC groups, with faster speed associated with less time on target. No control subject calibrated at less than 40 rpm. Neither age nor years of education was significantly correlated with calibrated speed in any of the four subject groups.
Time on target scores from Session 1 (Baseline trials 1 to 8) examined with repeated measures ANOVA indicated a main effect for trial (F(7,21)=5.62, p=.0001) but no main effect for group (F(3,96)=.625, p= .60) or group × trial interaction (F(21,672)=.732, p=.80) (Figure 1).
Figure 1.
Time on target (mean ± SEM) in seconds for the 8 baseline trials (Session 1) for all subjects (n=100) who were administered the rotary pursuit task.
Performance across Sessions
Subjects were excluded from the across-session analyses if they were not administered Sessions 1 and 2 or Sessions 3 and 4 on the same testing day or if the interval between Testing Day 1 and Testing Day 2 exceeded 3 weeks (Testing Days 1 and 2 were generally within a week of each other). The time between testing days was comparable across groups (F(3,67)=3.2, p=.81). The correlation between time across testing days and change in time on target scores was not significant within any subject group. In addition, only subjects who calibrated at equal to or greater than 40 rpm were included in these analyses to reduce variance in calibrated baseline speed and to be on par with calibration speed of the controls. Of the 100 baseline subjects, 71 subjects met these inclusion criteria across the four testing sessions (HIV n=17; ALC n=21; ALC+HIV n=16; NC n=17; Table 1). Subjects excluded from the across-session analyses were not demographically different from those who were included in these analyses. When a repeated measures ANOVA was conducted for these 71 subjects for baseline scores, similar results to the analysis that included all 100 subjects were observed: a significant main effect for session (F(3,201)=31.4, p=.0001) but not for group (F(3,67)=.08, p=.97) or group × session interaction (F(9,201)=1.47, p=.16).
Group × session interactions, however, were observed for the ALC and HIV groups when examining within-day learning and across-day learning (Day1: Sessions 1 and 2 (F(1,36)=6.81, p=.013) and Day 2: Sessions 3 and 4 (F(1,36)=9.00, p=.005)), with the HIV group showing a larger increase in within-day learning than the ALC group (Figure 2). By contrast, the ALC group showed a trend toward a larger increase in time on target in across test-day learning than the HIV group (F(1,36)=3.34, p=.076). The ALC+HIV group showed a similar pattern to the ALC group, with little to no increase in time on target scores within a testing day (ALC+HIV vs. HIV: Sessions 1 and 2 - group × time on target interaction F(1,31)=7.75, p=.009; Sessions 3 and 4 - group × time on target interaction F(1,31)=8.94, p=.005).
Figure 2.
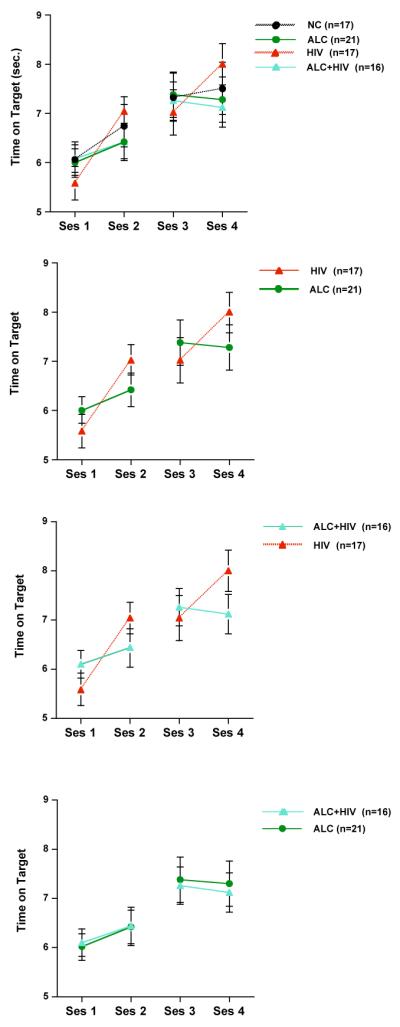
Mean ± SEM of time on target scores at the four rotary pursuit testing sessions. Evident are the different patterns of visuomotor learning between the ALC-only and HIV-only groups. The pattern of learning of the ALC+HIV group was similar to that of the ALC-only group.
Ancillary Cognitive Measures
Raw scores for the ancillary cognitive measures are in Tables 2a and 2b for all groups.
Table 2a.
Descriptive scores for nonmnemonic ancillary tests (mean, SD, range)
| Group | Fine Finger Movement (# of rod turns) |
Symbol Digit Test (# correct in 90 sec) |
Balance (# of seconds) |
|---|---|---|---|
| Control | 88.0 (11.3) |
51.9 (6.3) |
24.9 (19.2) |
| 66 to 108 | 42 to 65 | 0 to 60 | |
| ALC | 79.8 (14.0) |
45.0 (11.5) |
11.0 (9.0) |
| 39 to 104 | 23 to 66 | 0 to 43 | |
| HIV | 80.3 (12.8) |
47.6 (10.1) |
12.6 (15.6) |
| 59 to 112 | 36 to 74 | 0 to 60 | |
| ALC+HIV | 80.2 (13.9) |
38.3 (10.1) |
17.3 (16.6) |
| 52 to 109 | 20 to 62 | 0 to 60 | |
|
| |||
| Baseline ANOVAs | ns | p=.002 | p=.01 |
| Post-hoc t-tests | HIV+ALC<ALC,HIV, NC ALC vs. NC |
ALC,HIV<NC | |
Table 2b.
Wechsler Memory Scale - Revised (WMS-R) Standard Scores* for all groups (mean, SD, range)
| Group | Verbal | Visual | Attention | Immediate Memory | Delayed Memory |
|---|---|---|---|---|---|
| Control (n=14) | 105.4 (4.6) |
104,2 (4.6) |
106.0 (4.0) |
104,6 (4.6) |
103.8 (4.1) |
| 78 to 138 | 81 to 138 | 77 to 132 | 75 to 135 | 80 to 138 | |
| ALC (n=24) | 96.2 3.6 |
92,4 3.1 |
97.9 3.8 |
93.4 3.9 |
90.5 3.2 |
| 68 to 127 | 60 to 129 | 61 to 127 | 64 to 129 | 66 to 120 | |
| HIV(n=19) | 97.2 (4.6) |
103.2 (3.5) |
96.7 (3.3) |
98.9 (4.5) |
100.1 (4-5) |
| 68 to 138 | 77 to 135 | 74 to 132 | 71 to 135 | 73 to 138 | |
| ALC+HIV(n=21) | 87.5 (3.7) |
92.0 (4.4) |
90.8 (4.0) |
86.0 (4.2) |
87.4 (3.6) |
| 61 to 112 | 50 to 130 | 50 to 118 | 51 to 114 | 60 to 120 | |
|
| |||||
| Baseline ANOVAs | p=0.042 | p-0,040 | p=0.087 | p=0.030 | p-0.014 |
| Post-hoe t-tests | ALC+HIV<NC | ALC<HIV, NC | ALC+HIV<HIV, NC | ALC+H1V-5HIV, NC AI.C<NC |
|
Standard Score = 100±15
Upper Limb Function: Motor Speed
Groups did not differ in fine finger movement speed (F(3,96)=1.94, p=0.13).
Processing Speed: Psychomotor Speed
A significant group difference was observed on Symbol Digit (F(3,74)=5.55, p=.002), with the ALC+HIV group having lower scores than the ALC (t(44)=2.07, p=.04), HIV (t(38)=2.91, p=.006), and NC (t(32)=4.35, p=.0001) groups. The ALC group also performed below the NC group on this measure (t(36)=2.02, p=.05).
Lower Limb Function: Balance
The groups differed on ability to stand on one leg with eyes closed (F(3,95)=3.79, p=.01). Both the ALC and the HIV groups performed significantly worse than the NC group (ALC vs. NC t(47)=3.4, p=.001; HIV vs. NC t(40)=2.3, p=.03).
Episodic Memory and Attention
Of the 100 subjects at baseline, 78 individuals received the Wechsler Memory Scale – Revised (WMS-R). Group differences were observed on several WMS-R indices, including Visual Memory (F(3,74)=3.15, p=.03), Immediate Memory (F(3,74)=2.95, p=.04), and Delayed Memory (F(3,74)=3.78, p=.01), and a modest group difference was noted on Verbal Memory (F(3,74)=2.44, p=.07). No significant group difference was observed on the Attention Index (F(3,74)=1.71, p=.17). Follow-up analyses indicated that the ALC+HIV group scored lower than the HIV and NC groups on Immediate Memory (ALC+HIV vs. HIV: t(38)=2.11, p=.04; ALC+HIV vs. NC: t(32)=2.76, p=.01) and Delayed Memory (ALC+HIV vs. HIV: t(38)=2.21, p=.03; ALC+HIV vs. NC: t(32)=2.86, p=.008). The ALC group scored lower than the HIV and NC groups on Visual Memory (ALC vs. HIV: t(42)=2.40, p=.02; ALC vs. NC: t(36)=2.41, p=.02) and lower than the NC group on Delayed Memory (t(36)=2.56, p=.02). The HIV group did not differ significantly from the NC group on any of WMS-R index scores.
Relations between Rotary Pursuit and Ancillary Cognitive Measures
For within-group correlational analyses the maximum number of subjects in each group was used for analyses (this included subjects that calibrated below 40 rpm) because the primary aim of these analyses was to assess the relationships between motor and cognitive component processes and visuomotor learning scores, regardless of visuomotor speed (calibration speed).
In the ALC group higher calibrated rotary pursuit visuomotor speed at baseline was significantly associated with better Verbal Memory (r=.42, p=.04), Immediate Memory (r=.44, p=.03), and Delayed Memory (r=.47, p=.02) scores. In the ALC+HIV group higher calibrated visuomotor speed was significantly correlated with better Fine Finger Movement (r=.45, p=.02), Symbol Digit (r=.64, p=.002), Visual Memory (r=.47, p=.03), and Delayed Memory (r=.64, p=.002) scores. Calibrated visuomotor speed was not significantly correlated with any of the ancillary test scores in the HIV or NC groups.
Visuomotor learning, as assessed by a positive change in time on target scores over testing sessions (Session 4 mean time on target – Session 1 mean time on target), was positively correlated with Lower Limb Balance (r=.43, p=.022), Verbal Memory (r=.47, p=.033), Immediate Memory (r=.49, p=.023), Delayed Memory (r=.44, p=.045) and Attention (r=.62, p=.003) scores in the ALC+HIV group (Table 3). In the ALC group, higher visuomotor learning scores were not related to higher explicit memory scores on the WMS-R. Greater visuomotor learning in the NC group was related to higher scores on Fine Finger Movement (r=.47, p=.036) and Attention (r=.69, p=.009).
Table 3.
Pearson correlations between ancillary tests and learning on Rotary Pursuit
| Controls | ALC | HIV | ALC+HIV | |
|---|---|---|---|---|
| Fine Finger Movement | .47* | .07 | .02 | .04 |
| Balance | .18 | −.14 | .37 | .43* |
| Symbol Digit | .44 | −.18 | .19 | .05 |
| WMS-R | ||||
| Attention | .69** | −.14 | .26 | .62** |
| Verbal | .04 | −.26 | .23 | .47* |
| Visual | .47 | −.44* | .25 | .36 |
| Immediate Memory | .19 | −.33 | .28 | .49* |
| Delayed Memory | .20 | −.32 | .34 | 44* |
Bivariate correlation significance levels:
p≤.05
p≤.01
p≤.0001
Multiple regression analyses indicated that in the ALC+HIV group visuomotor speed was uniquely predicted from Symbol Digit and Delayed Memory scores after accounting for the variance explained by Fine Finger Movement scores (Table 4). To control for the number of analyses conducted for the ALC+HIV group, Immediate Memory score was chosen as the best possible memory predictor for visuomotor learning because it had the strongest simple correlation of all the memory scores with learning scores on the rotary pursuit task. Visuomotor learning score in the comorbid group was uniquely predicted by Attention score over and above the contribution of Immediate Memory or Balance scores. When tested with multiple regression analyses, none of the memory scores that were significantly correlated with visuomotor speed in the ALC group emerged as a unique predictor.
Table 4.
Multiple regressions predicting visuomotor speed and learning in HIV+ALC group
| ALC+HIV | ||||
|---|---|---|---|---|
| Dependent Measure | Predictors | Beta-Coefficient | R-squared change | |
| Calibrated Speed (rpm) | Symbol Digit | .61 | .30 | ** |
| Fine Finger Movement | .06 | .00 | ||
| Delayed Memory | .58 | .31 | ** | |
| Fine Finger Movement | .14 | .02 | ||
| Symbol Digit | .38 | .07 | ||
| Delayed Memory | .35 | .06 | ||
|
| ||||
| Learning over Sessions (Session 4 -Session 1) |
Attention | 45 | .22 | * |
| Immediate Memory | .32 | .07 | ||
| Attention | .53 | .24 | * | |
| Balance | .21 | .03 | ||
| Immediate Memory | 43 | .20 | * | |
| Balance | .39 | .15 | ||
p<.05
p<.01
p<.001
Relation of Demographic, Depression, and Disease Variables with Rotary Pursuit
Neither age nor years of education was significantly correlated with rotary pursuit learning scores in the ALC, HIV, or ALC+HIV groups. Higher premorbid IQ estimate scores (NART) correlated with higher visuomotor learning scores (r=.59, p=.01) in the HIV group, and a statistical trend in this direction was observed in the ALC+HIV group (r=.48, p=.06). NART score, however, was not a unique predictor over and above the contribution of Attention scores to visuomotor learning score in the comorbid group. NART and learning scores were not significantly correlated in the ALC group (r=−.15, p=.50). Further, learning scores were not related to lifetime alcohol consumption or weeks in remission in the ALC and ALC+HIV groups nor to viral load or CD4 counts in the HIV and ALC+HIV groups.
HIV and ALC+HIV subjects with Hepatitis C did not differ from subjects without Hepatitis C for calibrated speed or time on target scores. ALC subjects who had a Hepatitis C diagnosis achieved modestly lower visuomotor learning scores at Session 1 than ALC subjects without a Hepatitis C diagnosis (Z=1.78, p=.08); however, these subjects were also significantly older (Z=2.48, p=.01) and had drunk more alcohol in their lifetime (Z=2.26, p=.02) than their non-Hepatitis C counterparts. Older age was significantly correlated with less time on target scores in the ALC group (r=−.44, p=.018), but total lifetime alcohol consumption was not significantly related to time on target scores (r=.10, p=.60).
Higher BDI-II scores were correlated with poorer learning scores in the ALC+HIV group (r=−.54, p=.03); however, BDI-II scores were not related to calibrated speed in any group. Multiple regression analyses indicated that the BDI-II score was not a unique predictor of visuomotor learning scores ALC+HIV over and above the contribution of Symbol Digit or Delayed Memory scores.
DISCUSSION
All clinical groups (ALC, HIV, ALC+HIV) showed visuomotor learning and retention on the rotary pursuit task. Learning and retention of this visuomotor procedure occurred in spite of impairment in visuomotor speed in the HIV groups (HIV, ALC+HIV) and impairment in explicit memory processes and psychomotor speed in the alcohol groups (ALC, ALC+HIV) identified with tasks other than rotary pursuit.
As hypothesized, the alcoholic and HIV groups showed different patterns of learning. The HIV group exhibited significant learning across sessions within a day, but a lack of consolidation across testing days. By contrast, both alcohol groups showed little learning across sessions within a day but substantial consolidation and retention across testing days. These patterns of procedural memory performance provide behavioral evidence for the possibility of different brain substrates as supporting different aspects of the visuomotor learning process (cf., Doyon et al., 2003), with frontocerebellar circuitry, which is likely disrupted in alcoholism (Sullivan et al., 2003), supporting early fast learning, and frontostriatal circuitry, which is likely disrupted in HIV infection (Castelo et al., 2006; Heaton et al., 1995), supporting retention and consolidation. The comorbid group showed a learning pattern that was similar to the alcoholic-only group. Alcohol use disorder predated the onset of HIV infection in a majority of our comorbid subjects: of the 27 subjects in the comorbid group, onset data were available for 21, 18 of whom had alcoholism before acquiring HIV infection. There was no clear additive or synergistic effect of chronic alcoholism and HIV infection on visuomotor speed, learning, or retention.
The pattern of spared overall visuomotor learning with concomitant visuomotor speed impairment observed in this study is consistent with previous reports in HIV infection (cf., Gonzalez et al., 2008). Both studies provide behavioral support for the hypothesis that initial reports of visuomotor procedural learning deficits in HIV-infection may have been related to complex motor deficits rather than procedural learning deficits per se. In addition, these complex motor deficits are unlikely due to upper motor peripheral neuropathy because peripheral neuropathy in HIV infection generally involves lower rather than upper limbs (cf., Gonzalez et al., 2008).
Examination of the association and contribution of cognitive component processes and demographic and disease-related variables to visuomotor speed and learning indicated that in the comorbid group psychomotor function predicted visuomotor speed, whereas attention and working memory abilities predicted visuomotor learning. Depressive symptoms were related to visuomotor procedural learning in the comorbid group but did not uniquely predict visuomotor learning over and above psychomotor speed. Neither total amount of alcohol consumed nor time in remission from formal diagnosis was related to visuomotor perceptual learning performance in either alcohol group (ALC or ALC+HIV).
Alcoholics with Hepatitis C showed modestly impaired visuomotor learning initially relative to alcoholics without Hepatitis C, but alcoholics with Hepatitis C were older and had drunk more alcohol in their lifetime than their non Hepatitis C counterparts. However, when the two youngest subjects were excluded from the analyses (both of whom were negative for Hepatitis C), group differences no longer endured. More investigation is needed to determine whether Hepatitis C can negatively influence visuomotor learning in chronic alcoholics.
We examined only men. Before we can confidently generalize these results to both sexes, further studies with women need to be conducted (cf., Maki and Martin-Thormeyer, 2009). Martin and colleagues (Martin et al., 2011) reported a sex difference in overall rotary pursuit performance, with HIV-infected women but not HIV-infected men showing learning impairment compared with their non-infected counterparts. It may be that HIV-infected women and men are differentially affected on visuomotor speed/learning processes or that they may invoke different underlying cognitive processes to complete these types of tasks.
In summary, this study demonstrates that visuomotor procedural learning and retention remains relatively intact in chronic alcoholism, HIV infection, and their comorbidity. Complex motor abilities, as assessed by speed on the rotary pursuit task, are impaired in HIV infection but can be dissociable from visuomotor procedural learning processes. Despite overall performance comparability to controls, patterns of visuomotor learning processes are distinctly different in chronic alcoholism and HIV infection. Further, these results provide behavioral support for our hypothesis proposing differential contributions of frontocerebellar and frontostriatal systems to visuomotor procedural learning and retention.
Acknowledgment
This research was supported by grants from the National Institute on Alcohol Abuse and Alcoholism AA017347, AA005965, AA010723, and AA017168.
REFERENCES
- Beatty WW, Hames KA, Blanco CR, Nixon SJ, Tivis LJ. Visuospatial perception, construction and memory in alcoholism. J. Stud. Alcohol. 1996;57:136–143. doi: 10.15288/jsa.1996.57.136. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Brandt J, Butters N, Ryan C, Bayog R. Cognitive loss and recovery in long-term alcohol abusers. Arch Gen Psychiatry. 1983;40:435–442. doi: 10.1001/archpsyc.1983.01790040089012. [DOI] [PubMed] [Google Scholar]
- Buxton CE, Grant DA. Retroaction and gains in motor learning: II. Sex differences, and a further analysis of gains. J Experimental Psych. 1939;25(2):198–208. [Google Scholar]
- Castelo JM, Sherman SJ, Courtney MG, Melrose RJ, Stern CE. Altered hippocampal-prefrontal activation in HIV patients during episodic memory encoding. Neurology. 2006;66(11):1688–95. doi: 10.1212/01.wnl.0000218305.09183.70. [DOI] [PubMed] [Google Scholar]
- Cermak LS, Butters N. Information processing deficits of alcoholic Korsakoff patients. Q J Stud Alcohol. 1973;34(4):1110–32. [PubMed] [Google Scholar]
- Cohen NJ, Squire LR. Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing how and knowing that. Science. 1980;210(4466):207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Sagar HJ, Jordan N, Harvey N, Sullivan EV. Cognitive impairment in early, untreated Parkinson’s disease and its relationship to motor disability. Brain. 1991;114:2095–2122. doi: 10.1093/brain/114.5.2095. [DOI] [PubMed] [Google Scholar]
- Corkin S. Acquisition of motor skills after bilateral medial temporal lobe excision. Neuropsychologia. 1968;6:255–256. [Google Scholar]
- Corkin S, Growdon JH, Sullivan EV, Nissen MJ, Huff FJ. Assessing treatment effects from a neuropsychological perspective. In: Poon L, editor. Handbook of Clinical Memory Assessment in Older Adults. American Psychological Association; Washington DC: 1986. pp. 156–167. [Google Scholar]
- Cysique LA, Brew BJ. Neuropsychological Functioning and Antiretroviral Treatment in HIV/AIDS: A Review. Neuropsychol. Rev. 2009;19(2):169–185. doi: 10.1007/s11065-009-9092-3. [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41(3):252–62. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Fama R, Eisen JC, Rosenbloom MJ, Sassoon SA, Kemper CA, Deresinski S, Pfefferbaum A, Sullivan EV. Upper and lower limb motor impairments in alcoholism, HIV infection, and their comorbidity. Alcohol. Clin. Exp. Res. 2007;31(6):1038–44. doi: 10.1111/j.1530-0277.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- Fama R, Rosenbloom MJ, Nichols BN, Pfefferbaum A, Sullivan EV. Working and episodic memory in HIV infection, alcoholism, and their comorbidity: Baseline and 1-year follow-up examinations. Alcohol. Clin. Exp. Res. 2009;33(10):1815–1824. doi: 10.1111/j.1530-0277.2009.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Bachman L, Fisher S, Davenport L. Cognitive impairments in abstinent alcoholics. West J Med. 1990;152:531–537. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Chang M. Visual P300s in long-term abstinent chronic alcoholics. Alcohol. Clin. Exp. Res. 2006;30(12):2000–7. doi: 10.1111/j.1530-0277.2006.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Torres J, Price LJ, Di Sclafani V. Cognitive performance in long-term abstinent alcoholic individuals. Alcoholism: Clinical and Experimental Research. 2006;30(9):1538–44. doi: 10.1111/j.1530-0277.2006.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Version 2.0. Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1998. [Google Scholar]
- Fregly AR, Graybiel A, Smith MS. Walk on floor eyes closed (WOFEC): A new addition to an ataxia test battery. Aerosp Med. 1972;43(4):395–399. [PubMed] [Google Scholar]
- Gabrieli JD, Stebbins GT, Singh J, Willingham DB, Goetz CG. Intact mirror-tracing and impaired rotary-pursuit skill learning in patients with Huntington’s disease: evidence for dissociable memory systems in skill learning. Neuropsychology. 1997;11(2):272–81. doi: 10.1037//0894-4105.11.2.272. [DOI] [PubMed] [Google Scholar]
- Gilman S, Adams K, Koeppe RA, Berent S, Kluin KJ, Modell JG, Kroll P, Brunberg JA. Cerebellar and frontal hypometabolism in alcoholic cerebellar degeneration studied with Positron Emission Tomography. Ann Neurol. 1990;28(6):775–785. doi: 10.1002/ana.410280608. [DOI] [PubMed] [Google Scholar]
- Goldman MS, Williams DL, Klisz DK. Recoverability of psychological functioning following alcohol abuse: prolonged visuo-spatial dysfuntion in older alcoholics. J Consult Clin Psychol. 1983;51:370–378. doi: 10.1037//0022-006x.51.3.370. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Jacobus J, Amatya AK, Quartana PJ, Vassileva J, Martin EM. Deficits in complex motor functions, despite no evidence of procedural learning deficits, among HIV+ individuals with history of substance dependence. Neuropsychology. 2008;22(6):776–86. doi: 10.1037/a0013404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Woods RP, Tyszka M. Functional imaging of procedural motor learning; relating cerebral blood flow with individual subject performance. Hum. Brain Mapp. 1994;1:221–234. doi: 10.1002/hbm.460010307. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL, O’Brien S, Hermanowicz N. Cognitive-motor learning in Parkinson’s disease. Neuropsychology. 1997;11(2):180–6. doi: 10.1037//0894-4105.11.2.180. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, McCutchan JA, Taylor MJ, Kelly MD, Ellis RJ, Wolfson T, Velin R, Marcotte TD, Hesselink JR, Jernigan TL, Chandler J, Wallace M, Abramson I, HIV Neurobehavioral Research Center The HNRC 500--neuropsychology of HIV infection at different disease stages. J Int Neuropsychol Soc. 1995;1(3):231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Heindel WC, Butters M, Salmon DP. Impaired learning of a motor skill in patients with Huntington’s disease. Behav Neurosci. 1988;102(1):141–147. doi: 10.1037//0735-7044.102.1.141. [DOI] [PubMed] [Google Scholar]
- Heindel WC, Salmon DP, Shults CW, Walicke PA, Butters N. Neuropsychological evidence for multiple implicit memory systems: A comparison of Alzheimer’s, Huntington’s, and Parkinson’s disease patients. The Journal of Neuroscience. 1989;9:582–587. doi: 10.1523/JNEUROSCI.09-02-00582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivry RB, Keele SW. Timing functions of the cerebellum. J Cogn Neurosci. 1989;1(2):136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- Karnofsky DA. In: The clinical evaluation of chemotherapeutic agents in cancer, in Evaluation of Chemotherapeutic Agents. MacLeod CM, editor. Columbia University Press; New York: 1949. pp. 191–205. [Google Scholar]
- Leonard G, Jones L, Milner B. Residual impairment in handgrip strength after unilateral frontal-lobe lesions. Neuropsychologia. 1988;26:555–564. doi: 10.1016/0028-3932(88)90112-1. [DOI] [PubMed] [Google Scholar]
- Maki PM, Cohen MH, Weber K, Little DM, Fornelli D, Rubin LH, Perschler P, Gould F, Martin E. Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: a preliminary study. Neurology. 2009;72(19):1661–8. doi: 10.1212/WNL.0b013e3181a55f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Martin-Thormeyer E. HIV, Cognition and Women. Neuropsychol. Rev. 2009 doi: 10.1007/s11065-009-9093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Heyes MP, Salazar AM, Law W, Williams J. Impaired motor-skill learning, slowed reaction time, and elevated cerebrospinal-fluid quinolinic acid in a subgroup of HIV-infected individuals. Neuropsychology. 1993a;7(2):149–157. [Google Scholar]
- Martin E, Gonzalez R, Vassileva J, Maki P. HIV+ men and women show different performance patterns on procedural learning tasks. J Clin Exp Neuropsychol. 2011:1–9. doi: 10.1080/13803395.2010.493150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EM, Robertson LC, Sorensen DJ, Jagust WJ, Mallon KF, Chirurgi VA. Speed of memory scanning is not affected in early HIV-1 infection. J Clin Exp Neuropsychol. 1993b;15(2):311–320. doi: 10.1080/01688639308402565. [DOI] [PubMed] [Google Scholar]
- Martin EM, Sullivan TS, Reed RA, Fletcher TA, Pitrak DL, Weddington W, Harrow M. Auditory working memory in HIV-1 infection. J Int Neuropsychol Soc. 2001;7(1):20–26. doi: 10.1017/s1355617701711022. [DOI] [PubMed] [Google Scholar]
- McGlinchey RE, Fortier CB, Capozzi SM, Disterhoft JF. Trace eyeblink conditioning in abstinent alcoholic individuals: effects of complex task demands and prior conditioning. Neuropsychology. 2005;19(2):159–70. doi: 10.1037/0894-4105.19.2.159. [DOI] [PubMed] [Google Scholar]
- Nelson HE. The National Adult Reading Test (NART) Nelson Publishing Company; Windsor, Canada: 1982. [Google Scholar]
- Nixon SJ, Tivis R, Parsons OA. Behavioral dysfunction and cognitive efficiency in male and female alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19(3):577–581. doi: 10.1111/j.1530-0277.1995.tb01551.x. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M. Learning and memory deficits in detoxified alcoholics. NIDA Res Monogr. 1990;101:136–155. [PubMed] [Google Scholar]
- Pitel A-L, Zahr NM, Jackson K, Sassoon S, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Signs of preclinical Wernicke’s Encephalopathy and thimine levels as predictors of neuropsychological deficits in acoholism without Korsakoff’s syndrome. Neuropsychopharmacology. 2011;36(3):580–588. doi: 10.1038/npp.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom MJ, Rohlfing T, O’Reilly A, Sassoon SA, Pfefferbaum A, Sullivan EV. Improvement in memory and static balance with abstinence in alcoholic men and women: Selective relations with changes in regional ventricular volumes. Psychiatry Research: Neuroimaging. 2007;155:91–102. doi: 10.1016/j.pscychresns.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom MJ, Sullivan EV, Pfefferbaum A. Consequences of excessive chronic alcohol consumption on the brain. In: Brick J, editor. Handbook of Medical Consequences of Alcohol and Drug Abuse. Second Edition The Haworth Press; New York: 2008. pp. 97–122. [Google Scholar]
- Rothlind JC, Greenfield TM, Bruce AV, Meyerhoff DJ, Flenniken DL, Lindgren JA, Weiner MW. Heavy alcohol consumption in individuals with HIV infection: effects on neuropsychological performance. J Int Neuropsychol Soc. 2005;11(1):70–83. doi: 10.1017/S1355617705050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtke K, Manner H, Kaufmann R, Schmolck H. Cognitive procedural learning in patients with fronto-striatal lesions. Learn Mem. 2002;9(6):419–29. doi: 10.1101/lm.47202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schugens MM, Breitenstein C, Ackermann H, Daum I. Role of the striatum and the cerebellum in motor skill acquisition. Behav Neurol. 1998;11(3):149–157. [PubMed] [Google Scholar]
- Skinner HA. Development and Validation of a Lifetime Alcohol Consumption Assessment Procedure. Addiction Research Foundation; Toronto, Canada: 1982. [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices: The lifetime drinking history and the MAST. J. Stud. Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Smith A. The Symbol Digit Modalities Test Manual. Western Psychological Services; Los Angeles: 1973. [Google Scholar]
- Sullivan EV, Harding AJ, Pentney R, Dlugos C, Martin PR, Parks MH, Desmond JE, Chen SHA, Pryor MR, De Rosa E, Pfefferbaum A. Disruption of frontocerebellar circuitry and function in alcoholism. Alcoholism: Clinical and Experimental Research. 2003;27:301–309. doi: 10.1097/01.ALC.0000052584.05305.98. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rose J, Pfefferbaum A. Effect of vision, touch, and stance on cerebellar vermian-related sway and tremor: A quantitative MRI and physiological study. Cereb Cortex. 2006;16:1077–1086. doi: 10.1093/cercor/bhj048. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcoholism: Clinical and Experimental Research. 2000;24(5):611–621. [PubMed] [Google Scholar]
- Sullivan EV, Shear PK, Zipursky RB, Sagar HJ, Pfefferbaum A. Patterns of content, contextual, and working memory impairment in schizophrenia and nonamnesic alcoholism. Neuropsychology. 1997;11(2):195–206. doi: 10.1037//0894-4105.11.2.195. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio AR, Damasio H, Brandt JP. Sensorimotor skill learning in amnesia: additional evidence for the neural basis of nondeclarative memory. Learn Mem. 1994;1(3):165–79. [PubMed] [Google Scholar]
- Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders Due to Alcoholism and Malnutrition. 2nd edition F.A. Davis Co; Philadelphia: 1989. [Google Scholar]
- Wechsler D. Wechsler Memory Scale - Revised. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]



