Abstract
Lead (Pb) was one of the first poisons identified, and the developing nervous system is particularly vulnerable to its toxic effects. Relatively low, subclinical doses, of Pb that produce no overt signs of encephalopathy can affect cognitive, emotional, and motor functions. In the present study, the effects of developmental Pb-exposure on behavioral performance and gene expression in BALB/cAnNTac mice were evaluated. Pups were exposed to Pb from gestational-day (gd) 8 to postnatal-day (pnd) 21 and later evaluated in exploratory behavior, rotarod, Morris water maze, and resident-intruder assays as adults. Pb-exposure caused significant alterations in exploratory behavior and water maze performance during the probe trial, but rotarod performance was not affected. Pb-exposed males displayed violent behavior towards their cage mates, but not to a stranger in the resident-intruder assay. Gene expression analysis at pnd21 by microarray and qRT-PCR was performed to provide a molecular link to the behavior changes that were observed. Pb strongly up-regulated gene expression within the signaling pathways of mitogen activated protein kinases (MAPKs), extra-cellular matrix (ECM) receptor, focal adhesion, and vascular endothelial growth-factor (VEGF), but Pb down-regulated gene expression within the pathways for glycan structures-biosynthesis 1, purine metabolism, and N-glycan biosynthesis. Pb increased transcription of genes for major histocompatibility (MHC) proteins, the chemokine Ccl28, chemokine receptors, IL-7, IL7R, and proteases. The qRT-PCR analysis indicated an increase of gene expression in the whole brain for caspase 1 and NOS2. Analysis of IL-1β, caspase 1, NOS2, Trail, IL-18 and IL-33 gene expression of brain regions indicated that Pb perturbed the inter-regional expression pattern of pro-inflammatory genes. Brain region protein concentrations for IL-10, an anti-inflammatory cytokine, showed a significant decrease only within the cortex region. Results indicate that Pb differentially affects the behavior of male and female mice in that females did less exploration and the males were selectively more aggressive. Gene expression data pointed to evidence of neuroinflammation in the brain of both female and male mice. Pb had more of an effect in the males on expression of vomeronasal receptor genes associated with odor detection and social behavior.
Keywords: Behavior, Brain, Cytokines, Gene Expression, Inflammation, Lead, Microarray
1. Introduction
It has been known for millennia that lead (Pb) can be highly toxic, and reports of its effects on the central nervous system go back to the time of the Roman Empire (Needleman, 2009). Yet, despite this knowledge, Pb-exposure continues to be a major public health problem worldwide, including urban centers in the USA (Tong S et al., 2000). Children are particularly sensitive to the effects of Pb (Leggett,1993; Hornung et al., 2009). Asymptomatic Pb-exposures during gestation and early childhood have been found to be neurotoxic in children, since these Pb-exposures (blood Pb concentrations <30 µg/dl) have lasting effects on neuronal function (Bellinger, 2000; Needleman, 2004; Chen et al., 2007; Surkan et al., 2007; Bellinger, 2008; Al-Saleh et al., 2008). The developing nervous system is particularly vulnerable to the toxic effects of Pb (Bressler and Goldstein, 1991; Johnston and Goldstein, 1998), since a greater proportion of systemically circulating Pb gains access to the brain in children, especially those 5 years of age or younger (Faust and Brown, 1987; Brown et al., 1997). Moreover, experimental studies, using rodents and primates, have confirmed a significant interaction between timing of Pb-exposure and the nature and persistence of neurotoxic effects (Cory-Slechta et al., 1983; Cory-Slechta, 1988; Shigeta et al., 1989). In general, the manifestations of Pb neurotoxicity appear to be highly age and dose dependent.
It has become evident over the past decade that the nervous system and the immune system are in continuous communication with each other (Steinman, 2004; Szelenyi and Vizi, 2007). Neurofactors regulate immune activity and cytokine balance. Conversely, factors of the immune system, such as cytokines and major histocompatibility complexes, have a bearing on neural cell differentiation, growth and establishment of neuronal connections. Studies in our laboratory and that of others have demonstrated that Pb exposure will dampen the immune response to bacterial infections (Hemphill et al., 1971; Lawrence 1981; Kowolenko et al., 1991). Moreover, Pb exposure during different windows of embryonic development will modulate the immune system differently in male and female mammals (Snyder et al., 2000; Bunn et al., 2001). Many of the early studies of Pb effects on the immune system were performed using a variety of mouse strains (Lawrence 1981; McCabe and Lawrence, 1990). These studies revealed that the BALB/c mouse strain displayed the greatest effect on the immune system after Pb-exposure. Therefore, the BALB/c strain has been chosen for recent studies on the effects of Pb in our laboratory. The present study was performed in an effort to tie the effects of Pb on the immune system together with those Pb effects observed on the nervous system. We chose an exposure period of gestation and lactation and an environmentally relevant Pb dose that had been demonstrated by our past experiments to have a strong impact on the immune system (Snyder et al., 2000; Dyatlov and Lawrence, 2002). Brain cytokine gene expression in unexposed controls and Pb exposed mice from this study has been reported previously (Kasten-Jolly et al., 2011). These results indicated that there was no difference in brain cytokine gene expression between unexposed male and female mice for each of the cytokines examined. However, when the mice were exposed to Pb gene expression of IL-6 and TGF-β were increased in the brain as a whole primarily in the female mice. Interestingly, these two cytokines play important roles in development of the central nervous system in addition to their immune system functions, as explained in this previous report. Cytokine gene expression and protein concentrations for several pro-inflammatory cytokines in various brain regions suggested that Pb was promoting an inflammatory like response in certain of these regions (Kasten-Jolly et al., 2011). For this reason we decided to investigate the possibility of Pb promoting areas of brain inflammation further by examining the gene expression of more pro-inflammatory cytokines and several factors, such as caspase 1 and NOS2, which are associated with an inflammatory response. Brain region expression of an anti-inflammatory cytokine, IL-10, was also examined in order to find out if expression of this cytokine would be decreased in the presence of increased pro-inflammatory gene expression.
Reports from other investigators have suggested that the presence of Pb or other stressors in the brain triggers responses that resemble those generated by inflammation (Struzynska et al. 2007; Garcia-Bueno et al., 2008). It has been known for many years that Pb accumulates in brain glial cells rather than in neurons and can cause astrocyte activation (Tiffany-Castiglioni et al. 1989). Astrocyte activation promotes increased permeability of the blood-brain-barrier (BBB) through the activity of chemokines (Anthony et al., 1998). Pb is able to potentiate pro-inflammatory cytokine and glutamate-mediated increases in permeability of the BBB in mice, which is probably the result of glutamate-induced oxidative stress (Bradbury and Deane, 1993; Savolainen et al. 1995; Dyatlov et al. 1998). Astrocyte activation was also implicated in a study of Pb-exposed young (15–30 day old) rats. Results showed increased GFAP and S-100b in all brain regions along with increased production of IL-6 in the forebrain and decreased expression of axonal markers synapsin-1 and synaptophysin, suggesting a decrease in axonal growth (Struzynska et al., 2007). The conclusion was that Pb promoted chronic glial cell activation with coexisting inflammatory and neurodegenerative features.
In order to connect the effects of developmental Pb exposure on the immune system to those on the nervous system we evaluated the effects of developmental Pb-exposure using several behavioral assays in adult male and female BALB/cAnNTac mice. Mice were exposed to Pb acetate (0.1 mM) from gestational day 8 (gd8) to post natal day 21 (pnd21) via the dam’s drinking water. We chose gd8 as the starting time to allow for embryo implantation in the absence of Pb, and we chose the termination day of pnd21 so Pb-exposure would end at the time of weaning. After the Pb exposure, mice were either sacrificed at pnd21 for gene expression studies or tested for behavioral changes as adults in terms of exploratory behavior in an automated activity monitor, motor coordination on a rotarod, spatial memory in a Morris water maze, and aggression in the resident-intruder assay. We found that developmental Pb exposure resulted in significant alterations in behavioral performance and that some of these differences were gender specific. Because the female Pb-exposed mice were displaying significant differences in their exploratory behavior from control mice and because previous studies had shown Pb-exposed female mice had more significant differences from control mice in brain cytokine gene expression, it was decided to perform the microarray analysis using the female brain RNA. Furthermore, in behavior studies performed on rats treated with environmentally relevant Pb concentrations combined with prenatal stress, female rats displayed enhanced learning deficits (Cory-Slechta et al., 2010).
The present study extends the work of our previous report by performing behavior studies on the Pb-exposed mice, by doing pathway analysis of the microarray data, by performing qRT-PCR for pro-inflammatory factors in the whole brain and in various brain regions, and by examining the expression of certain vomeronasal receptors associated with social behavior. The behavior studies revealed that the mice were less prone to explore and had some memory impairment after Pb-exposure, while the males displayed selective aggressive behavior after the developmental Pb-exposure. Pathway analysis of the microarray data suggested that Pb was effecting neuronal development and neuronal connectivity by perturbing the focal adhesion and extra-cellular matrix receptor signaling pathways and by changing the expression of major histocompatibility molecules within the developing brain. Increased brain inflammation due to the Pb-exposure was reaffirmed by the increased expression of various pro-inflammatory factors in the whole brain and in select brain regions. Analysis of brain vomeronasal receptor (molecules associated with regulation of social behavior) gene expression in male and female mice indicated that Pb was causing alterations in expression of these receptors, especially in males.
2. Materials and Methods
2.1 Animals
BALB/cAnNTac (BALB/c) breeder pairs were purchased from Taconic Farms (Germantown, NY) and maintained in our colony at Wadsworth Center. Mice were housed in clear Plexiglas cages (29 × 18 × 12.5 cm) with stainless steel wire lids, with food and water freely available. They were kept in a temperature controlled (68–72 °F) room and maintained on a 13:11-hr light–dark cycle with lights on at 0800. All animal procedures had prior approval by the Wadsworth Center‘s IACUC.
2.2 Pb exposure
Adult male and female BALB/c mice were housed individually prior to mating. Animals were mated at 60 – 90 days (two females and one male per cage). On gestational day 0 (gd0) (determined by the presence of a vaginal plug) the dams were returned to their home cages. On gd8, dams were randomly assigned and given either 20ppm (0.1mM Pb acetate) Pb water, or 0 ppm distilled water in plastic bottles. Dams were provided Pb or distilled water until pups were weaned at pnd21. Litters were adjusted to 6 pups per dam immediately after birth.
2.3 Behavioral Procedures
2.3.1 Exploratory Behavior
Exploratory behavior was measured with Digiscan 16-beam automated activity monitors (42 cm × 42 cm × 30 cm; Accuscan, Columbus, OH), which were enclosed in melamine sound-attenuating chambers (65 cm × 55 cm × 55 cm; MED Associates, St. Albans, VT). All testing occurred in dim light within the chambers accompanied by a ceiling fan in the testing room which blunted background noise. A PC computer and Digiscan software recorded all beam breaks. This testing procedure has been previously described in detail (Bolivar et al., 2000; Bolivar 2009) and will only be briefly reviewed here. Approximately 1 hr prior to testing, mice were placed in the testing room to acclimate them to the new environment. At the end of the acclimation period, each mouse was placed in a holding cage for 5 min, and then placed in the center of a clean dark chamber for a 15 min testing period. Following testing, mice were transferred to home cages and returned to the colony room. The variables measured, including total distance traveled, time spent resting, and rotations (clockwise and counterclockwise) were examined directly from the data collected by the Digiscan system. Intrasession habituation, speed, percentage of time spent in the center of the apparatus and distance traveled in the center of the apparatus were calculated from data collected by the system.
2.3.2 Rotarod
For motor ability, rotarod testing utilized a Smartrod Rotating Rod Apparatus (Accuscan Instruments Inc, Columbus, OH). Four individually enclosed boxes (48 cm × 11 cm × 30 cm), with a textured rotating rod that spanned the width of the box positioned 34 cm above a grid floor, were used for testing. Smartrod windows software version 1.7 (Accuscan Instruments Inc.) recorded latency to fall from the rod based on beam breaks. Mice were tested three times each day with a 20 min intertrial interval, on three consecutive days. This procedure has been described in detail previously (McFadyen et al., 2003). Briefly, after a one-hour acclimation period, mice were placed on one of the four rotating rods. Each trial lasted 180s. During the first 60s, the rod accelerated from 0 to 15 rotations per minute (rpm). For the next 110s, the rotation speed was kept constant at 15 rpm. The rod then decelerated from 15 to 0 rpm in the last 10s of the trial. The latency to fall for each trial was recorded, and a mean latency was calculated for each day.
2.3.3 Morris Water Maze (MWM)
Spatial learning and memory were assessed by performance on a hippocampal-dependent visuospatial learning task in the MWM (Schimanski and Nguyen 2004; Moy et al., 2007). Mice were given eight training trials per day, during 4 consecutive days, to find a hidden platform located 1.5cm below the water surface in a pool 1.5m in diameter. The water in the pool was maintained at 24–25°C and made opaque by a nontoxic white paint (Dry Temp Powder Tempera, Palmer Paints, Troy, MI). Within the testing room, only distal visuo-spatial cues were available to the mice for location of the submerged platform. The escape latency, i.e., the time required by the mouse to find and climb onto the platform, was recorded. If the mouse did not successfully reach the platform, a score of 60s was assigned. Each mouse was allowed to remain on the platform for 30s, and was then moved from the maze to its home cage. If the mouse did not find the platform within 60s, it was manually placed on the platform and returned to its home cage after 30s. The inter-trial interval was at least 45min, and the mice were allowed to rest for 60min between trial 4 and 5. On day 4, 1hr following the last training trial, the platform was removed from the pool, and each mouse was tested in a 60s probe trial. Data were recorded by using an Accutrak automated tracking system (Accuscan, Columbus, OH).
2.3.4 Resident-Intruder Test
Aggressive behavior toward a stranger was tested by the resident-intruder assay (Litvin et al., 2007; Patel et al., 2010; Velez et al., 2010; Scattoni et al., 2011). An age-matched, male BALB/c intruder mouse was placed in the home cage of a Pb treated male or a control male mouse. The pairs of mice were filmed for 10 min. As per our previous studies (Bolivar et al., 2002; Bolivar et al., 2007), encounters coded as aggressive included biting, chasing and wrestling. The number of bouts of aggressive behavior and the latency to the first aggressive encounter were recorded.
2.4 RNA Isolation
We isolated total RNA on pnd21 using a Midi Lipid Tissue RNA isolation kit and protocol from Qiagen, Inc. (Valencia, CA). Before removal from the cranium, mouse brains were transcardially perfused with 30 ml of sterile, DEPC-treated PBS. For isolation of RNA from whole, perfused brains, two females and two males from each litter were immediately homogenized in Qiazol and RNA was isolated according to manufacturer’s directions. Male and female RNAs were pooled separately from each litter. The litter was considered the unit of measure. Mouse brains to be used for RNA isolation from specific brain regions were snap frozen on dry ice immediately after perfusion and removal from the cranium. Frozen brains were stored at −80°C. The frozen brains were not allowed to thaw during dissection of the brain regions, Frontal cortex (FC), non-frontal cortex [C(nonF)], striatum (S), hypothalamus (HY), hippocampus (HI), cerebellum (CE), and substantia nigra (SN), by performing punches of frozen brain sections. Frozen punches were immediately homogenized in the QIAzol reagent and RNA was isolated according to the manufacturer’s protocol using the lipid tissue mini kit. Concentration and purity of all RNAs was measured using a Beckman DU 640 spectrophotometer (Fullerton, CA). We checked RNA integrity by agarose gel-electrophoresis and bioanalyzer.
2.5 Microarray Gene Expression Analysis
Affymetrix GeneChip hybridization and processing was performed by the Wadsworth Center, Microarray Core Facility (Albany, NY). Quality of the RNA was assessed by means of a bioanalyzer to check the ribosomal RNA S28/S18 ratio. RNA had to have a ratio of 1.8 or higher, if it was to be processed on the GeneChips. Six Affymetrix MG-430A GeneChips were hybridized, three with total RNA from one litter each of female control (distilled water) pups, and three with total RNA from one litter each of female 0.1 mM Pb from gd8 to pnd21 treated experimental pups. Statistical analysis of the microarray data was done by pair-wise comparison using GeneSifter software from VizX Labs (Seattle, WA). Settings included a lower fold change cut-off of 1.2 or 1.5, a quality cut-off of 0.6, log transformation of the raw data, a t-test P value of < 0.05, and a Z score cut off of 2. Control genes were excluded from the analysis and a Benjamini and Hochberg correction was applied to the data analysis. The Z-score was defined as:
where:
R = total number of genes meeting selection criteria
N = total number of genes measured
r = number of genes meeting selection criteria with the specified pathway
n = total number of genes measured with the specific pathway.
Analysis of the microarray data was also performed using the GeneTraffic™UNO software, version 3.0.
2.6 TaqMan Real-Time RT-PCR
A two-step process was employed to quantify the cytokine mRNA transcripts. First, cDNA was synthesized from 2 µg of total RNA by use of the cDNA archive kit from Applied Biosystems, Inc. (Foster City, CA), according to the manufacturer’s directions. After the 2 hr incubation at 37°C, the 100 µl reaction mixtures were diluted with an equal volume of PCR-grade water. Relative quantification was then carried out by use of a TaqMan procedure with primers, probes, and master mixes obtained from Applied Biosystems. Gene expression TaqMan kits from Applied Biosystems were for mouse, IL-7 (Mm00434291_m1), IL-1β (Mm01336189_m1), caspase-1 (Mm00438023_m1), Trail (Tnfsf10) (Mm00437174_m1), IL-18 (Mm00434225_m1), NOS2 (Mm01309902_m1), IL-33 (Mm00505403_m1),), Vomeronasal 1 receptor A5 (Mm00835858_s1), Vomeronasal 1 receptor C7 (Mm00475008_s1), Vomeronasal 2 receptor 12 (Mm00824350_m1), Vomeronasal 2 receptor 16 (Mm00776484_m1), Vomeronasal 2 receptor 42 (Mm02343744_mH), Chymotrypsin C (Mm01348883_m1), Bcl2 (Mm00477631_m1), and GADPH (Mm99999915_g1). The house-keeping gene gapdh (GenBank: NM_008084) was used as the endogenous control for normalization of the cytokine mRNAs. We performed amplification in an Applied Biosystems 7500 real-time PCR instrument under the following conditions: 50°C for 2 min, then 95°C for 10 min followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. Readings were taken during the second step of each cycle. We evaluated relative gene expression two ways: 1.) Using the Applied Biosystems SDS software, with one of the control samples set as a reference, i.e., set at 1.0. 2.) Using the following formula: Gene expression = 2‒(Ct exp – Ct GAPDH) × 1000, where Ct refers to the threshold cycle number. The qRT-PCR results presented in this report are based on the above equation with an average replicate run %CV of 3.31 ± 1.34 SEM.
2.7 Cytokine Protein Detection
Brain regions, Cortex (C), Striatum (S), Hypothalamus (HY), Hippocampus (HI), Cerebellum (CE), and Substania Nigra (SN), were dissected from perfused, brains of 21 day old mouse pups. Dissected tissue was immediately homogenized in an appropriate volume (depending on tissue size) of M-PER protein extraction reagent plus the protease cocktail, HALT, from Pierce (Rockford, IL). Homogenates were spun at 13,000 rpm in a microcentrifuge at 4°C for 20 min to pellet non-solubilized material. Tissues from 2 female mouse pups were pooled from each of 8 litters (3 control and 5 Pb treated litters). Total protein concentrations were determined by means of a kit from Pierce (Rockford, IL). The cytokine IL-10 was detected by using a Fluorokine Map Mouse IL-10 Kit (R & D System, Minneapolis, MN). The assay protocol was provided by the manufacturer. Briefly, a microplate was washed and loaded with diluted microparticle mixture, which contained the beads coated with capture antibody to target cytokine. Then, the serial diluted standards and brain homogenate were added. After incubation for three hours at room temperature on a horizontal microplate shaker, the microplate was washed and biotin antibody cocktail was added. Finally, Streptavidin-PE was used as the fluorescent signal. The assay was run on a Luminex 100 instrument (Luminex Corp. Austin, TX). The results were analyzed by using the software provided by Upstate Biologicals (Lake Placid, NY).
2.8 Statistical Analyses
Exploratory behavior variables were analyzed by 2 × 2 (Treatment × Gender) analyses of variance (ANOVAs) with Treatment (Pb water vs untreated) and Gender (male vs female) as between-subjects variables. To further examine differences, we used Tukey’s honestly significant difference (HSD) post hoc tests, given corresponding significant F values. To evaluate motor coordination on the rotarod, a mixed ANOVA treatment × gender × day, with treatment and gender as between subject variables and day as the repeated measure, was employed. Statistical analysis of the resident-intruder assay and real-time RT-PCR results was performed using the Student’s t-test. Statistical analyses were performed using either StatView (Version 5.0; SAS Institute, Cary, NC) or Statistica (Version 5.0.1). Two way and one way analysis of variance (ANOVA) with a Bonferroni post-test were performed to determine significance in gene expression levels between the various brain regions of control and Pb-exposed mice. Again, significant differences were assumed at a P<0.05.
3. Results
3.1 Exploratory Behaviors
Adult offspring, developmentally exposed to Pb or distilled water (control), were first evaluated in the exploratory behavior assay. A total of six variables were analyzed to completely describe the behavior of mice in this assay (total distance traveled, intra-session habituation, total rest time, average speed, percentage of time spent in the center and percentage of total distance traveled in the center).
3.1.1 Activity Measures
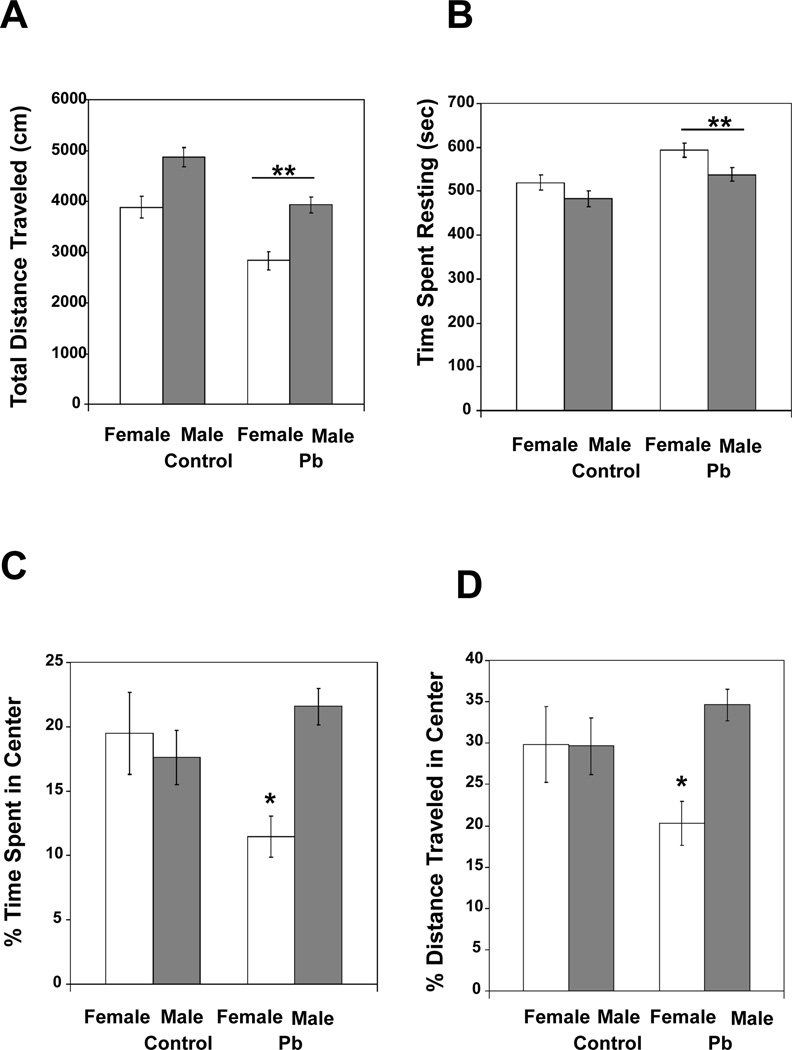
In terms of total distance traveled during the 15 min testing session, there were main effects of Treatment [F (1, 39) = 23.202, p < 0.0001] and Gender [F (1,39) = 25.494, p < 0.0001], but no significant interaction between these two variables. Overall, males traveled more than did females; however, regardless of gender, the Pb-exposed offspring traveled shorter distances than did the controls (Fig. 1A). To measure the change in distance traveled over the full 15 min, the session was broken into three 5 min intervals and intra-session habituation scores were calculated as follows: interval 3 total distance/(interval 1 total distance + interval 3 total distance) (Nadel, 1968; Bolivar, 2009). There was no significant difference in intra-session habituation between the different treatment groups, indicating that the overall difference in distance traveled is consistent across the full 15 min session (data not shown). A similar trend was observed with the amount of time spent resting. There were significant main effects of Treatment [F (1,39) = 13.725, p = 0.0007] and Gender [F (1.39) = 6.974, p = 0.0118], but no interaction between the two variables. Females spent more time resting than did males during the 15 min testing session, and Pb-exposed mice spent more time resting than did controls (Fig. 1B). An approximate speed value could be obtained by dividing total distance traveled by total time the mouse spent moving. Once again, there were main effects of Treatment [F (1,39) = 23.202, p < 0.0001] and Gender (F(1,39) = 25.494, p < 0.0001), but no interaction between the two variables. Males moved faster than females and Pb-exposure reduced speed of movement (Control females, 4.316±0.243 (mean±SEM); Control males, 5.406±0.212; Pb females 3.140±0.210; Pb males, 4.370±0.175). Overall, activity levels were influenced by both Gender and Treatment. Males tended to have increased activity (traveled more, moved more, and moved quicker) compared to females and exposure to Pb reduced the distance traveled, time spent moving and average speed of the mice.
Fig. 1.
Pb exposed mice displayed abnormal exploratory behavior relative to control mice in the activity monitor: (A) Total distance traveled, (B) Time spent resting, (C) percentage of time spent in the center, and (D) percentage of distance traveled in the center. Data are presented as mean ±S.E.M. In Panels A–B, ** indicates a significant difference (p < 0.001) between Pb exposed and control mice (collapsed over sex). In Panels C–D, * indicates a significant difference (p < 0.05) between Pb exposed and control females.
3.1.2 Anxiety Measures
To determine if Pb-exposure had any effect on anxiety-like behavior, activity in the center, relative to the margins, of the apparatus was examined. Although, there were no significant main effects of Treatment or Gender, there was a significant interaction between these two variables [F (1,39) = 6.480, p = 0.0150; Fig. 1C] for the percentage of total time spent in the center of the apparatus. Pb-exposure decreased center time in females, but not males. The same trend was true for percentage of total distance traveled in the center of the apparatus. There was no main effect of Treatment or Gender, but there was a significant Treatment × Gender interaction [F (1,39) = 4.237, p = 0.0463; Fig. 1D].
3.2 Rotarod performance showed no change in motor function
In order to determine whether Pb effects on exploratory behavior were the result of motor ability function, mice were tested for rotarod performance. The motor coordination of adult mice was not affected by Pb-exposure (p>0.05, data not shown). Thus, the marked hypoactivity (travel distance, time spend moving, and speed) in the exploratory behavior assay was not likely due to altered motor systems. Body weight has previously been shown to correlate with rotarod performance (McFadyen et al., 2003); thus, it could have influenced our motor coordination findings. In fact, we found that body weight correlated with performance (−0.477 for trial 1, −0.396 for trial 2, −0.488 for trial 3, all p’s<0.01). However, although females weighed significantly less than males (p<0.05), there was no significant effect of developmental Pb-exposure on body weight (p > 0.05, data not shown).
3.3 Morris Water Maze (MWM) performance suggests Pb impairment of visuo-spatial memory
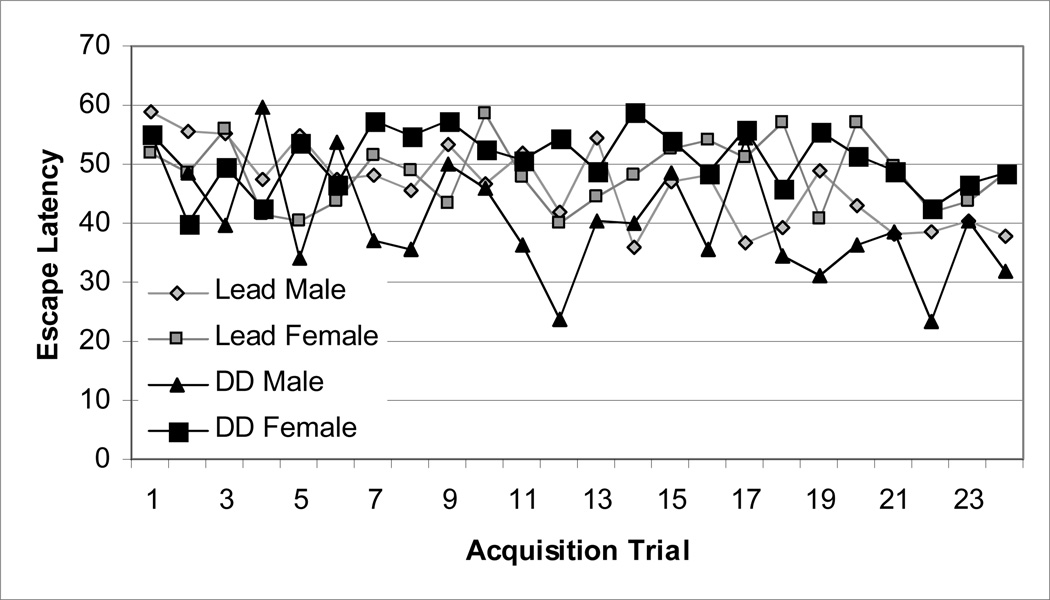
To establish whether learning and memory processes were altered in the adult mice due to the developmental Pb exposure, Pb-exposed BALB/c mice and controls of both genders were examined in the MWM, a hippocampal-dependent visuo-spatial learning memory task. A mixed ANOVA using treatment (Pb-exposed vs. untreated), gender (male vs. female), and trial number as factors was used to evaluate the latencies to reach the platform (escape latency) over the 24 acquisition trials and revealed no significant differences (p> 0.05) (Fig. 2).
Fig. 2.
Average escape latency (time in sec) to find the hidden platform during training acquisition trials in male and female mice exposed to 0.1 mM Pb from gd8 to pnd21 or untreated water. Each point represents the mean across the 24 trials.
However, several mice in all groups exhibited extreme stress/anxiety by “freezing” and remaining motionless in the water maze for the probe trial and for a majority of the training sessions (controls, 2 of 6 males (33%), 2 of 11 females (18%); Pb-exposed, 2 of 13 males (15%), 6 of 13 females (46%).
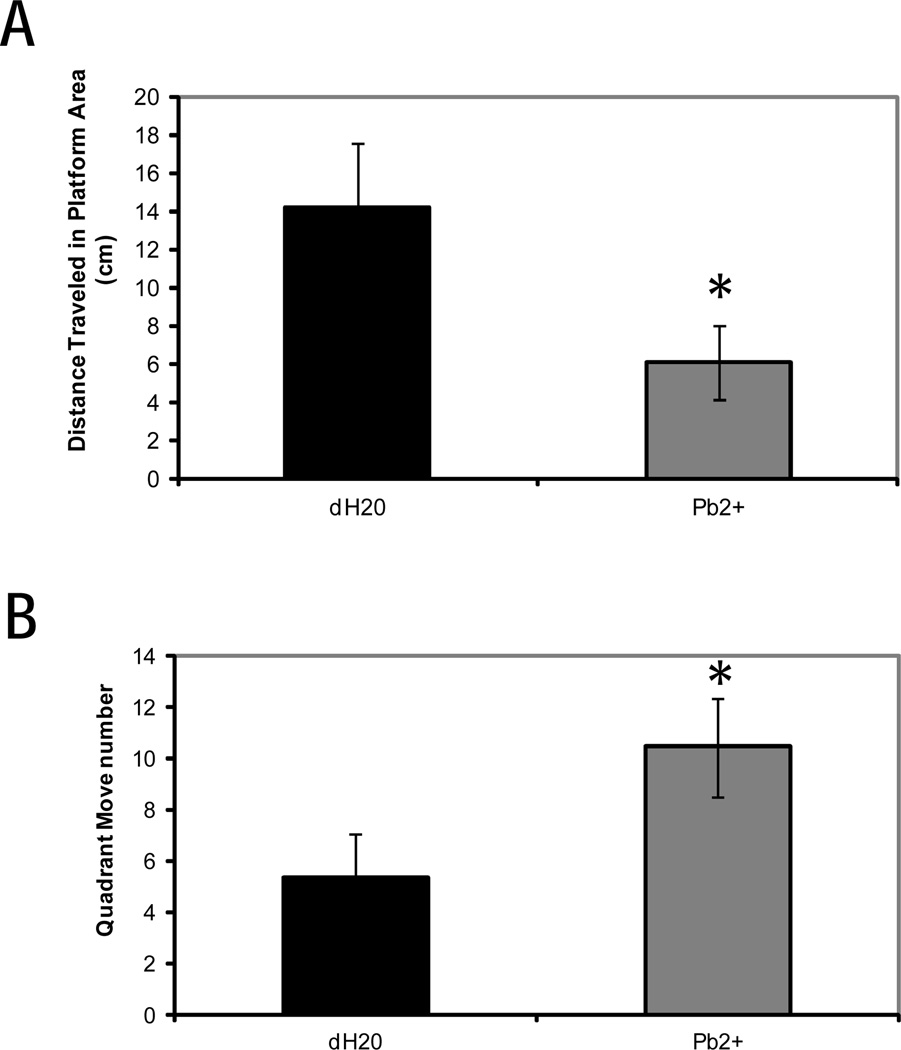
In order to perform a more accurate and detailed analysis of probe data, we excluded mice that swam less than 10s during the probe trial. This analysis revealed a significant main effect of treatment on distance traveled within the platform zone [F (1,21) = 6.44, p < 0.05] (Fig 3). The Pb-exposed mice manifested significant impairment of spatial memory compared with the controls, in that Pb-exposed mice spent less time swimming in the designated platform zone than did the controls. The Pb-exposed mice also spent more time moving between zones than did the controls [F (1,21) = 4.41, p < 0.05].
Fig. 3.
Average distance (mm) traveled in platform area (A) and average number of quadrant move number (B) during probe test trials in male and female mice that completed training successfully by swimming. Mice were exposed to 0.1 mM Pb or untreated water from gd8 to pnd21. Each bar represents the mean ± S.E. An asterisk (*) indicates a significant difference (p< 0.05) between Pb exposed and control mice.
3.4 Selective aggressive behavior by Pb exposed male mice
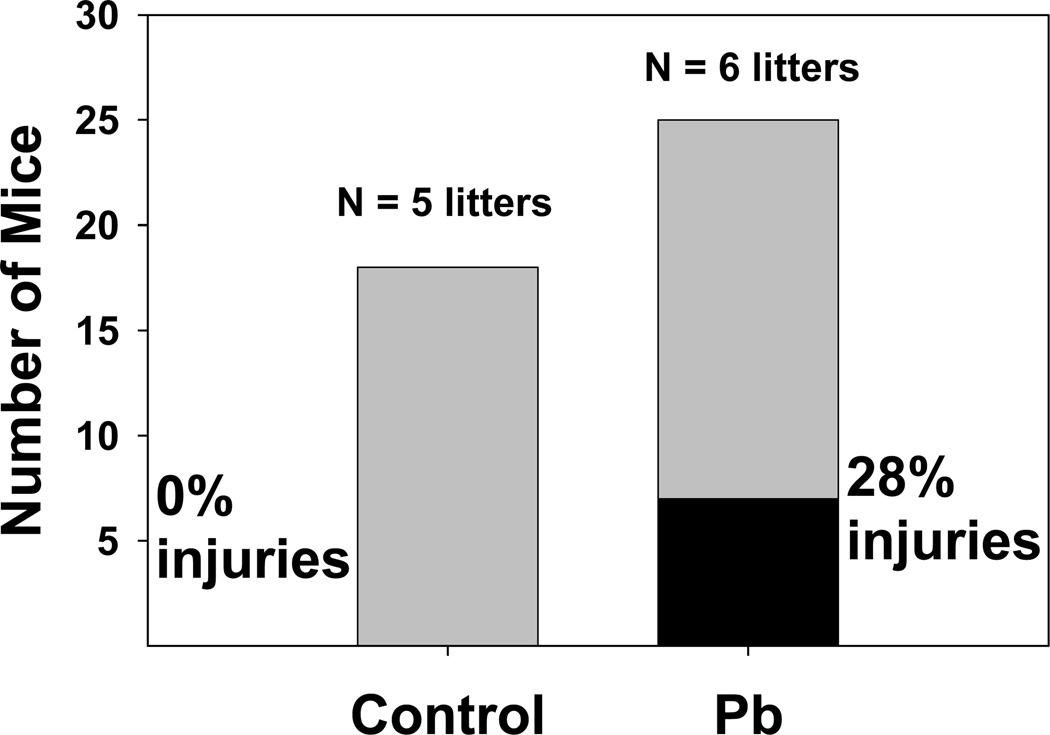
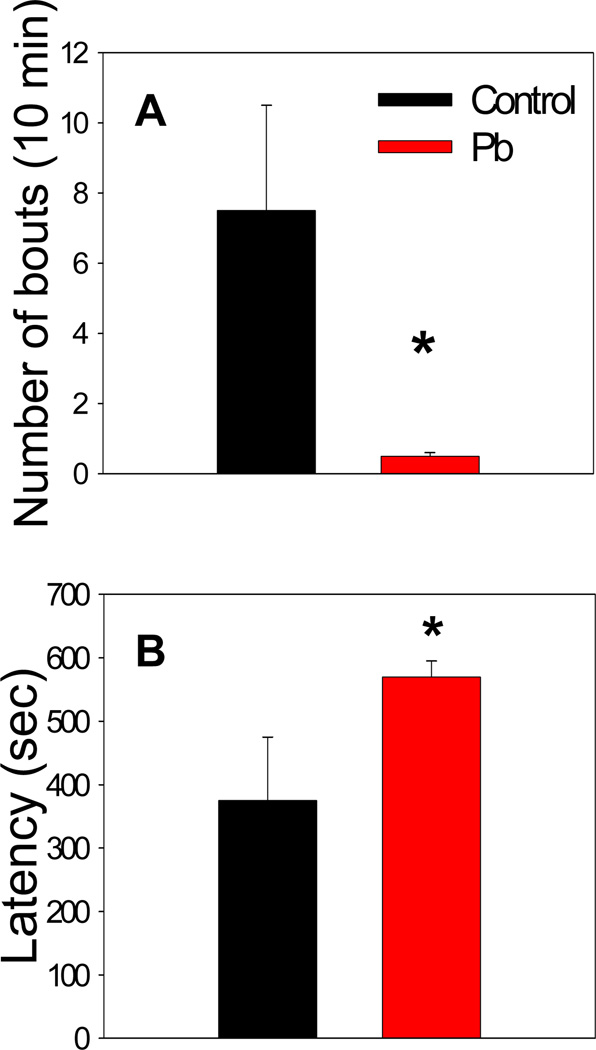
Pb-exposed litter-mates were housed together after weaning with males and females in separate cages. When these mice were approximately 10 months old, it was observed that some of the male mice had been severely bitten, perhaps from a dominant cage mate. Some of the wounds were so severe as to be lethal. These aggression incidents were tabulated (Fig. 4). There were no similar events observed among mice of the same age from the control litters. The aggressive behavior was not likely due to exposure to the behavior assays, as the control mice had also been subjected to the same assays. To study the aggressive behavior in further detail, the resident-intruder assay was performed. It was observed that resident control mice showed relatively high interest in the intruder mouse by sniffing and having short fighting bouts. Resident Pb-treated mice, however, showed very little interest in the intruder mouse and displayed very few fighting bouts within the 10 min observation period (Fig. 5). Thus, the developmental Pb-exposure caused fighting among littermates, but suppressed fighting with a stranger.
Fig. 4.
Pb exposed male mice are aggressive towards litter-mates. Mice were exposed to 0.1 mM Pb from gd8 to pnd21. At the time of these observations mice were > 35 weeks of age. The gray portions of the bars represent total number of mice uninjured, whereas the black portion of the Pb bar represents the number of injured mice.
Fig. 5.
Performance of Pb-exposed and control male mice in the resident-intruder assay: (A) Number of bouts of aggressive behavior, (B) Latency to first bout of aggressive behavior. Data are presented as mean ±S.E.M. An * indicates a significant difference between Pb exposed and control mice (p < 0.05). Developmental Pb exposure attenuates fighting behavior of resident adult male mice. Mice were exposed to 0.1 mM Pb from gd8 to pnd21.
3.5 Affymetrix GeneChip results indicated Pb effected ECM receptor and focal adhesion
Pair-wise comparisons, via the GeneSifter software set at a fold-change (FC) cut-off of 1.5, of whole brain gene expression from pnd21 control and Pb-treated female mice resulted in a list of significantly changed gene expression levels (Table S1). Genes are listed in order of significance of gene expression change, i.e., ascending P-values. A total of 350 genes were affected by Pb-exposure, of these 198 were up-regulated and 152 were down-regulated at a FC of 1.5. Pathway analysis was performed using a FC cut-off of 1.2 and the Kegg pathway analysis showed that the most perturbed by the Pb exposure were pathways for focal adhesion, extracellular matrix (ECM) receptor interaction, Fc epsilon R1 signaling, glycan structures-biosynthesis 1, purine metabolism, N-glycan biosynthesis, and VEGF signaling (Table 1). Genes of the MAPK signaling pathway influenced by developmental Pb-exposure have been listed and discussed in a previous manuscript (Kasten-Jolly et al., 2011). Briefly, MAPK data indicated that Pb up-regulated expression of components of the p38MAPK pathway. Both the focal adhesion and ECM-receptor pathways were relatively strongly up-regulated, based on a Z-score of >4. Genes influenced by Pb-exposure in the pathway for ECM-receptor are presented in Table 2. As indicated, most of the genes affected in this pathway were up-regulated by Pb-exposure, with increases detected in transcripts of several laminin and integrin genes. The most significantly changed gene expression was for integrin beta 1 (fibronectin receptor beta). Genes which are part of the focal adhesion pathway are presented in Table 3. As can be observed, there is some overlap between the members of these pathways, with many laminin and integrin genes being members of both pathways. Additional genes included in this pathway are Rock1 (down), Pdgfd (down), Crk1 (down), Ppp1cb (up), Thbs1 (up), Prkca (up), Akt2 (up), and Pdgfc (up). It is interesting that protein kinase C, alpha (Prkca) expression was slightly increased by Pb, since it has been reported that in vivo PKC activity was not increased in the rat brain after Pb exposure (Cremin and Smith, 2002). However, Pb has been reported to increase PKC activity, in vitro, within a homogenate of the frontal cortex (Cremin and Smith, 2002). Biological process analysis indicated that blood vessel development was up-regulated with a Z-score of 3.49, and that regulation of locomotion was also up with a z-score of 4.06. Genes included in these processes overlap those of the focal adhesion and ECM receptor pathways mentioned above. Pb down-regulated glycan structure biosynthesis as indicated by Z-scores of >3 for glycan structure-biosynthesis 1 and N-glycan biosynthesis pathways (Table 1).
Table 1.
List of KEGG Pathways Affected by the Pb Exposurea
| KEGG Pathway | Listb | Upc | Downc | Arrayd | z-score (Up)e |
z-score (Down)e |
|---|---|---|---|---|---|---|
| MAPK signaling pathway | 11 | 7 | 4 | 248 | 2.79 | 0.59 |
| Focal adhesion | 9 | 7 | 2 | 164 | 4.08 | 0 |
| Regulation of actin cytoskeleton | 7 | 4 | 3 | 174 | 1.62 | 0.63 |
| Calcium signaling pathway | 6 | 2 | 4 | 150 | 0.32 | 1.66 |
| Wnt signaling pathway | 6 | 3 | 3 | 135 | 1.33 | 1.09 |
| ECM-receptor interaction | 5 | 5 | 0 | 71 | 4.95 | −0.95 |
| Glycan structures - biosynthesis 1 | 5 | 1 | 4 | 81 | 0.15 | 3.09 |
| Leukocyte transendothelial migration | 5 | 3 | 2 | 103 | 1.85 | 0.68 |
| Purine metabolism | 5 | 0 | 5 | 118 | −1.15 | 3.04 |
| Renal cell carcinoma | 5 | 2 | 3 | 60 | 1.72 | 2.7 |
| Cytokine-cytokine receptor interaction | 4 | 2 | 2 | 210 | −0.17 | −0.36 |
| Gap junction | 4 | 1 | 3 | 80 | 0.16 | 2.09 |
| Insulin signaling pathway | 4 | 2 | 2 | 118 | 0.67 | 0.48 |
| Long-term depression | 4 | 2 | 2 | 70 | 1.47 | 1.26 |
| Long-term potentiation | 4 | 2 | 2 | 61 | 1.69 | 1.48 |
| N-Glycan biosynthesis | 4 | 1 | 3 | 33 | 1.1 | 4.14 |
| TGF-beta signaling pathway | 4 | 2 | 2 | 75 | 1.36 | 1.16 |
| Adipocytokine signaling pathway | 3 | 1 | 2 | 64 | 0.39 | 1.4 |
| Apoptosis | 3 | 1 | 2 | 73 | 0.25 | 1.2 |
| Axon guidance | 3 | 1 | 2 | 109 | −0.16 | 0.6 |
| Cell Communication | 3 | 2 | 1 | 93 | 1.03 | −0.13 |
| Cell cycle | 3 | 3 | 0 | 101 | 1.89 | −1.13 |
| ErbB signaling pathway | 3 | 2 | 1 | 71 | 1.45 | 0.15 |
| Fc epsilon RI signaling pathway | 3 | 3 | 0 | 71 | 2.61 | −0.95 |
| GnRH signaling pathway | 3 | 1 | 2 | 87 | 0.07 | 0.93 |
| Melanogenesis | 3 | 1 | 2 | 94 | 0 | 0.82 |
| Melanoma | 3 | 3 | 0 | 66 | 2.77 | −0.91 |
| Pyrimidine metabolism | 3 | 0 | 3 | 76 | −0.92 | 2.19 |
| VEGF signaling pathway | 3 | 3 | 0 | 64 | 2.84 | −0.9 |
Analysis performed by GeneSifter Software on Affymetrix microarray data from 3 control and 3 Pb treated litters.
Number of genes from the KEGG pathway whose expression was significantly changed by Pb, p<0.05.
Number of genes in the pathway whose expression was either increased or decreased by Pb.
Number of genes included in the pathway that were present on the Affymetrix 430A genechip.
Z-score is calculated as described in the methods; dark yellow are down and light yellow are up.
Table 2.
ECM Receptor Pathway Genes Affected by the Pb Treatmenta
| Gene Name | Ratiob | Direction | P-valuec | Gene ID |
|---|---|---|---|---|
| Hyaluronan mediated motility receptor (RHAMM) | 6.69 | Up | 0.04007 | Hmmr |
| integrin alpha V | 2.77 | Up | 0.04776 | - |
| Integrin binding sialoprotein | 2.44 | Down | 0.04873 | Ibsp |
| Laminin, alpha 5 | 2.01 | Up | 0.04195 | Lama5 |
| Laminin, alpha 3 | 1.94 | Down | 0.01957 | Lama3 |
| Laminin, alpha 2 | 1.46 | Up | 0.04436 | Lama2 |
| Laminin, alpha 1 | 1.34 | Up | 0.01835 | Lama1 |
| Thrombospondin 1 | 1.24 | Up | 0.03419 | Thbs1 |
| Fibronectin type III domain containing 3a | 1.22 | Up | 0.02671 | Fndc3a |
| Integrin beta 5 | 1.22 | Up | 0.01022 | Itgb5 |
| Integrin beta 1 (fibronectin receptor beta) | 1.21 | Up | 0.00462 | Itgb1 |
Analysis performed by GeneSifter Software on Affymetrix 430A microarray data on brain RNA from 3 control and 3 Pb treated litters.
Expression fold-change of Pb-treated versus control.
Significant by Student's t-test at p<0.05.
Table 3.
Focal Adhesion Pathway Genes Affected by the Pb Treatmenta
| Gene Name | Ratiob | Direction | P-valuec | Gene ID |
|---|---|---|---|---|
| integrin alpha V | 2.77 | Up | 0.04776 | - |
| Integrin binding sialoprotein | 2.44 | Down | 0.04873 | Ibsp |
| Laminin, alpha 5 | 2.01 | Up | 0.04195 | Lama5 |
| Laminin, alpha 3 | 1.94 | Down | 0.01957 | Lama3 |
| Platelet-derived growth factor, D polypeptide | 1.74 | Down | 0.04081 | Pdgfd |
| Laminin, alpha 2 | 1.46 | Up | 0.04436 | Lama2 |
| Laminin, alpha 1 | 1.34 | Up | 0.01835 | Lama1 |
| Rho-associated coiled-coil containing protein kinase 1 | 1.33 | Down | 0.01335 | Rock1 |
| V-crk sarcoma virus CT10 oncogene homolog (avian)-like | 1.3 | Down | 0.02273 | Crkl |
| Protein phosphatase 1, catalytic subunit, beta isoform | 1.29 | Up | 0.00103 | Ppp1cb |
| Thrombospondin 1 | 1.24 | Up | 0.03419 | Thbs1 |
| Protein kinase C, alpha | 1.24 | Up | 0.03399 | Prkca |
| Thymoma viral proto-oncogene 2 | 1.23 | Up | 0.00759 | Akt2 |
| Platelet-derived growth factor, C polypeptide | 1.22 | Up | 0.02705 | Pdgfc |
| Integrin beta 5 | 1.22 | Up | 0.01022 | Itgb5 |
| Integrin beta 1 (fibronectin receptor beta) | 1.21 | Up | 0.00462 | Itgb1 |
Analysis performed by GeneSifter Software on Affymetrix 430A microarray data on brain RNA from 3 control and 3 Pb treated litters.
Expression fold-change for Pb treated versus control.
Significant by Student's t-test at p<0.05.
3.6 Pb-exposure had high impact on genes associated with the immune-system
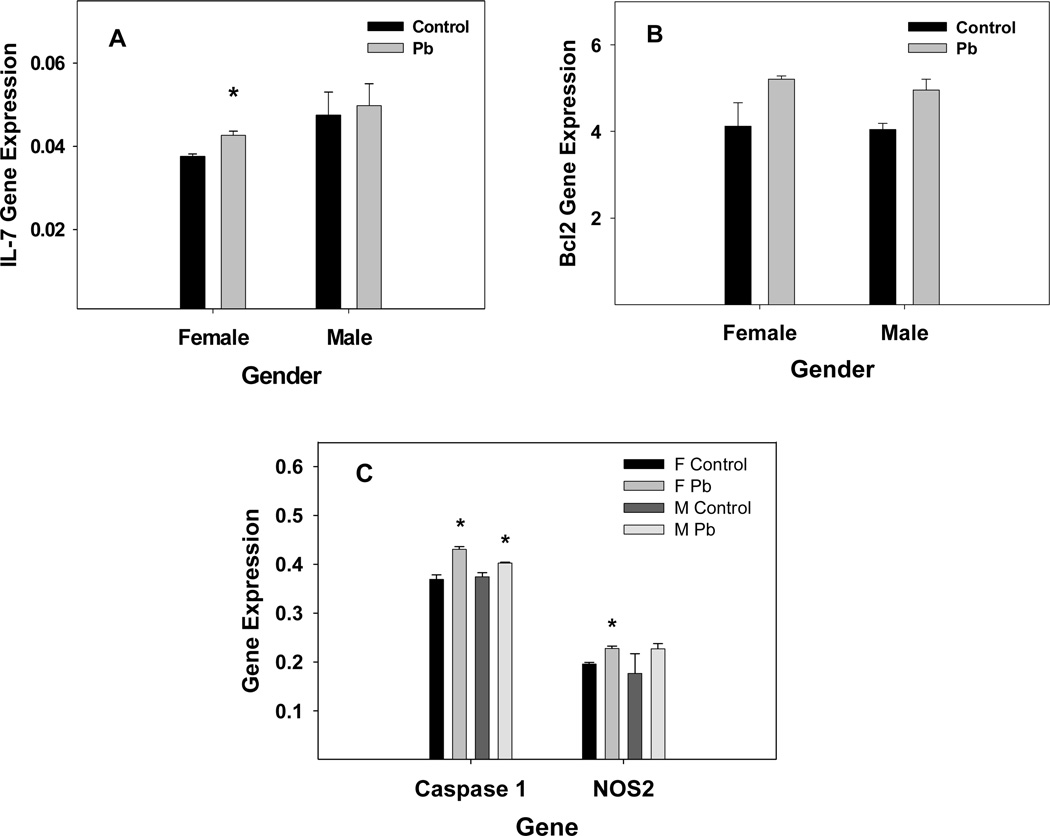
Since genes of the immune system process also have a role in brain development, it is important to note that Pb down-regulated the immune system process with a z-score of 2.20. However, as Table 4 shows, Pb increased expression of certain genes included in the immune system process ontology with a high degree of significance. Most distinctive on this list are several genes, Ccl28 (up), Mb (down), and H2-Aa (up) that are significantly changed (p<0.05) even according to Benjamini and Hockberg restrictions. Pb markedly affected the expression of major histocompatibility molecules (Table 4) and appeared to be down-regulating the complement system and up-regulating chemokine activity by increasing the gene expression of chemokine receptors of the c-c type. The Pb-exposure decreased transcript levels of IL-6 and IFN receptors and increased transcript levels of IL-7 and IL-4 receptors. Pb increased gene expression of IL-7 in the female mouse spleen (Kasten-Jolly et al., 2010) and qRT-PCR measurement of IL-7 gene expression in male and female whole brain indicated that IL-7 gene expression was increased by Pb in females and was not changed by Pb in males (Fig. 6). However, IL-7 expression in whole brain was slightly elevated (P=0.15) in males than in females. The microarray data indicated that Pb-exposure increased gene expression of Bcl2 and decreased gene expression of Traf1, which suggests that Pb in the female whole brain was dampening apoptosis at pnd21. In addition, qRT-PCR results indicated no significant change in gene expression for Trail (data not shown), while gene expression of Bcl2 was slightly increased in both female and male brain (Fig. 6). Conversely, Caspase 1 and NOS2 gene expression measured by qRT-PCR showed increased gene expression in the whole brain (Fig. 6). It might be that the slight increase in Bcl2 expression in males and females is associated with endoplasmic reticulum stress caused by the Pb-exposure (Qian and Tiffany-Castiglioni, 2003). Gene expression of pro-inflammatory cytokines, IL-1β and IL-33, measured by qRT-PCR in the whole brain of males and females did not show any change due to the Pb-exposure (data not shown).
Table 4.
Pb Effects on Immune System Processa
| Gene Name | Ratiob | Direction | p-valuec | Gene ID |
|---|---|---|---|---|
| Histocompatibility 2, class II antigen A, alpha | 1.67 | Up | 0.000037 | H2-Aa |
| Chemokine (C-C motif) ligand 28 | 7.83 | Up | 0.000049 | Ccl28 |
| Myoglobin | 7.2 | Down | 0.000087 | Mb |
| Proteosome (prosome, macropain) subunit, beta type 9 (large multifunctional peptidase 2) | 1.69 | Down | 0.000675 | Psmb9 |
| Histocompatibility 2, class II antigen E alpha | 4.38 | Down | 0.001312 | H2-Ea |
| B-cell leukemia/lymphoma 2 | 2.42 | Up | 0.001448 | Bcl2 |
| T-cell immunoglobulin and mucin domain containing 2 | 2.75 | Up | 0.002678 | Timd2 |
| Retinoic acid receptor, alpha | 2.39 | Down | 0.003058 | Rara |
| Integrin alpha L | 4.14 | Down | 0.003366 | Itgal |
| Tnf receptor-associated factor 1 | 5.14 | Down | 0.003730 | Traf1 |
| Histocompatibility 28 | 4.1 | Down | 0.004093 | H28 |
| Hemoglobin, beta adult major chain | 3.27 | Up | 0.004094 | Hbb-b1 |
| CEA-related cell adhesion molecule 1 | 2.35 | Up | 0.005901 | Ceacam1 |
| Myeloid leukemia factor 1 | 2.49 | Down | 0.006808 | Mlf1 |
| Killer cell lectin-like receptor, subfamily A, member 7 | 4.39 | Down | 0.007948 | Klra7 |
| Recombination activating gene 1 | 10.64 | Down | 0.011999 | Rag1 |
| Interleukin 6 receptor, alpha | 1.61 | Down | 0.013099 | Il6ra |
| Immunoglobulin superfamily, member 6 | 3.19 | Down | 0.015717 | Igsf6 |
| Interleukin 6 receptor, alpha | 1.58 | Down | 0.017767 | Il6ra |
| Immunoglobulin kappa chain variable 1–135 | 2.78 | Down | 0.017858 | Igkv1–135 |
| Complement component 2 (within H-2S) | 4.74 | Up | 0.020775 | C2 |
| Immunoglobulin kappa chain variable 1 (V1) | 5.19 | Up | 0.021944 | Igk-V1 |
| C-type lectin domain family 2, member g | 5.91 | Down | 0.022073 | Clec2g |
| CD79A antigen (immunoglobulin-associated alpha) | 2.59 | Down | 0.022193 | Cd79a |
| Interferon-induced protein with tetratricopeptide repeats 1 | 1.88 | Down | 0.024193 | Ifit1 |
| Macrophage activation 2 like | 2.06 | Up | 0.026871 | Mpa2l |
| Granzyme D | 12.94 | Up | 0.028200 | Gzmd |
| Pre-B-cell leukemia transcription factor 4 | 4.67 | Down | 0.030148 | Pbx4 |
| Interleukin 7 receptor | 2.06 | Up | 0.032943 | Il7r |
| Chemokine (C-C motif) receptor 2 | 6.04 | Up | 0.033524 | Ccr2 |
| mast cell protease 6 | 2.35 | Down | 0.033642 | - |
| Activin A receptor, type II-like 1 | 1.65 | Up | 0.035090 | Acvrl1 |
| Complement component 9 | 3.21 | Down | 0.038159 | C9 |
| Toll-like receptor 5 | 5.91 | Down | 0.039706 | Tlr5 |
| Mannan-binding lectin serine peptidase 1 | 2.37 | Down | 0.039755 | Masp1 |
| Killer cell lectin-like receptor subfamily B member 1F | 1.96 | Up | 0.039827 | Klrb1f |
| Complement factor H-related 1 | 14.51 | Down | 0.039899 | Cfhr1 |
| Chemokine (C-C motif) receptor 8 | 1.7 | Up | 0.040092 | Ccr8 |
| Interleukin 4 receptor, alpha | 3.52 | Up | 0.040116 | Il4ra |
| CEA-related cell adhesion molecule 11 | 6.79 | Up | 0.040410 | Ceacam11 |
| Chemokine (C-C motif) receptor 2 | 4.97 | Up | 0.042378 | Ccr2 |
| Histocompatibility 2, K1, K region | 5.25 | Up | 0.044974 | H2-K1 |
| Signaling lymphocytic activation molecule family member 1 | 3.53 | Down | 0.045683 | Slamf1 |
| Notch gene homolog 4 (Drosophila) | 1.67 | Up | 0.045962 | Notch4 |
| Killer cell lectin-like receptor, subfamily A, member 16 | 8.46 | Down | 0.046071 | Klra16 |
| Interferon (alpha and beta) receptor 2 | 1.77 | Down | 0.047266 | Ifnar2 |
| Inhibin beta E | 4.53 | Up | 0.049989 | Inhbe |
Analysis performed by GeneSifter Software on Affymetrix 430A microarray data on whole brain RNA from 3 control and 3 Pb treated litters.
Expression fold-change of Pb-treated versus control.
Significant by Student's t-test at p<0.05.
Fig. 6.
Developmental Pb effects on gene expression of A) IL-7, B) Bcl2, and C) Caspase 1 and NOS2 in the whole brain of females and males as measured by TaqMan RT-PCR. All results were obtained from an N of 3 control and 3 Pb-exposed litters. Data are presented as the mean ± S.E.M. An * indicates a significant increase p<0.05, according to the Student’s t-test.
3.7 Molecular function analysis showed Pb effect on binding activity and proteases
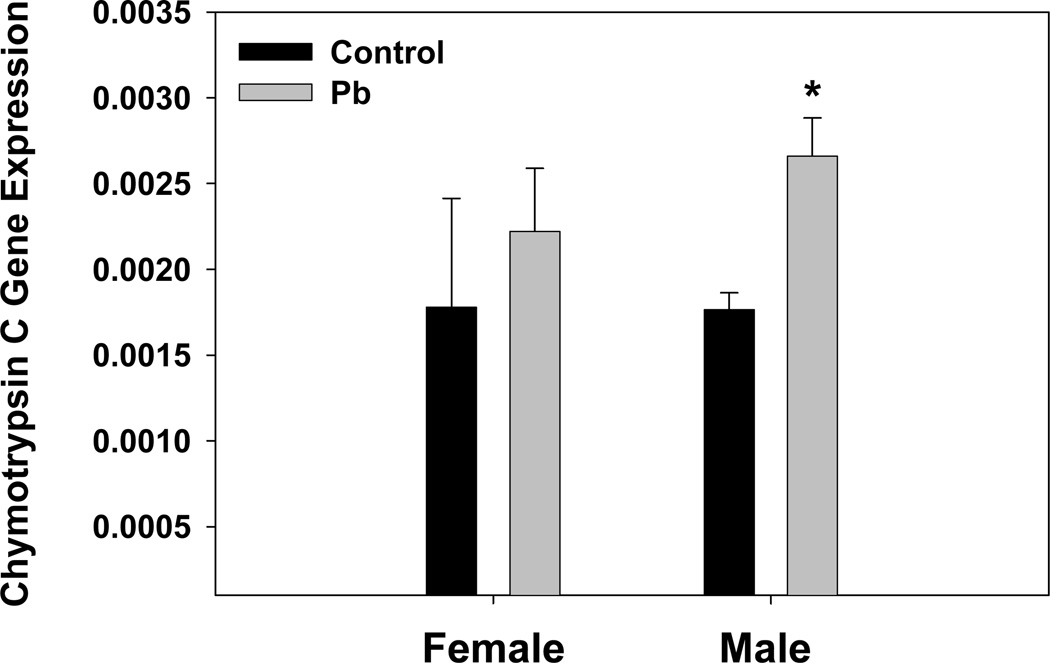
Analysis of molecular functions also indicated strong effects by Pb-exposure. High z-scores were observed for up-regulation of receptor binding, growth factor activity, chymotrypsin activity, glucuronosyltransferase activity, and integrin binding (Table 5). Up-regulation of chymotrypsin activity was re-affirmed by qRT-PCR for mouse chymotyrpsin C gene expression in the brain of developmentally Pb treated mice (Fig. 7). qRT-PCR results showed that chymotrypsin C gene expression was elevated, and males displayed significantly increased gene expression by this method. Although cytokine activity was up, cytokine binding was down in the presence of Pb-exposure (Table 5). Also, highly down-regulated were scavenger receptor activity and galanin receptor activity (Table 5).
Table 5.
Molecular Function Processes Affected by Pb Treatmenta
| Ontology | Listb | Upc | Downc | Arrayd | z-upe | z- downe |
|---|---|---|---|---|---|---|
| ion binding | 125 | 48 | 77 | 2584 | −1.41 | 2.37 |
| metal ion binding | 123 | 47 | 76 | 2522 | −1.37 | 2.46 |
| cation binding | 114 | 41 | 73 | 2326 | −1.67 | 2.78 |
| purine nucleotide binding | 61 | 38 | 23 | 1269 | 2.03 | −1.39 |
| adenyl nucleotide binding | 51 | 34 | 17 | 1025 | 2.54 | −1.57 |
| transcription factor activity | 43 | 25 | 18 | 697 | 2.56 | 0.39 |
| transmembrane receptor activity | 37 | 16 | 21 | 586 | 0.88 | 2 |
| calcium ion binding | 33 | 11 | 22 | 614 | −0.73 | 2.04 |
| receptor binding | 29 | 21 | 8 | 475 | 3.35 | −1 |
| structural molecule activity | 28 | 16 | 12 | 438 | 2.1 | 0.52 |
| iron ion binding | 16 | 5 | 11 | 242 | −0.16 | 2.26 |
| cytokine activity | 12 | 8 | 4 | 183 | 2.01 | −0.16 |
| serine-type endopeptidase activity | 12 | 5 | 7 | 142 | 1.07 | 2.02 |
| growth factor activity | 11 | 9 | 2 | 138 | 3.47 | −0.71 |
| nucleoside-triphosphatase activity | 10 | 7 | 3 | 394 | −0.6 | −2.14 |
| isomerase activity | 9 | 2 | 7 | 123 | −0.44 | 2.44 |
| sugar binding | 9 | 1 | 8 | 131 | −1.13 | 2.83 |
| vitamin binding | 8 | 1 | 7 | 90 | −0.71 | 3.39 |
| cytokine binding | 7 | 3 | 4 | 65 | 1.32 | 2.01 |
| heme binding | 7 | 1 | 6 | 101 | −0.84 | 2.37 |
| tetrapyrrole binding | 7 | 1 | 6 | 101 | −0.84 | 2.37 |
| UDP-glycosyltransferase activity | 7 | 3 | 4 | 65 | 1.32 | 2.01 |
| chymotrypsin activity | 4 | 3 | 1 | 39 | 2.33 | 0.08 |
| pyridoxal phosphate binding | 4 | 1 | 3 | 39 | 0.15 | 2.19 |
| scavenger receptor activity | 4 | 1 | 3 | 24 | 0.65 | 3.27 |
| calcium-dependent phospholipid binding | 3 | 2 | 1 | 19 | 2.47 | 0.83 |
| FAD binding | 3 | 3 | 0 | 43 | 2.13 | −1.02 |
| galanin receptor activity | 3 | 1 | 2 | 3 | 3.67 | 7.33 |
| glucuronosyltransferase activity | 3 | 2 | 1 | 9 | 4.08 | 1.73 |
| integrin binding | 3 | 3 | 0 | 18 | 4.17 | −0.66 |
Analysis performed by GeneSifter Software on Affymetrix 430A microarray data from whole brain RNA from 3 control and 3 Pb treated litters.
Number of genes from the molecular function ontology whose expression was significantly changed by Pb.
Number of genes in the ontology whose expression was either increased or decreased by Pb.
Number of genes included in the ontology that were present on the Affymetrix 430A genechip.
Z-score is calculated as described in the methods; dark yellow designates down and light yellow designates up.
Fig. 7.
Developmental Pb exposure increases Chymotyrpsin C gene expression as measured by TaqMan RT-PCR. All results were obtained from an N of 3 litters. Data are presented as the mean ± S.E.M. An * indicates a significant increase, p<0.05, in chymotrypsin C gene expression between control and Pb treated male members of the litters.
3.8 Pb effect on vomeronasal receptor gene expression
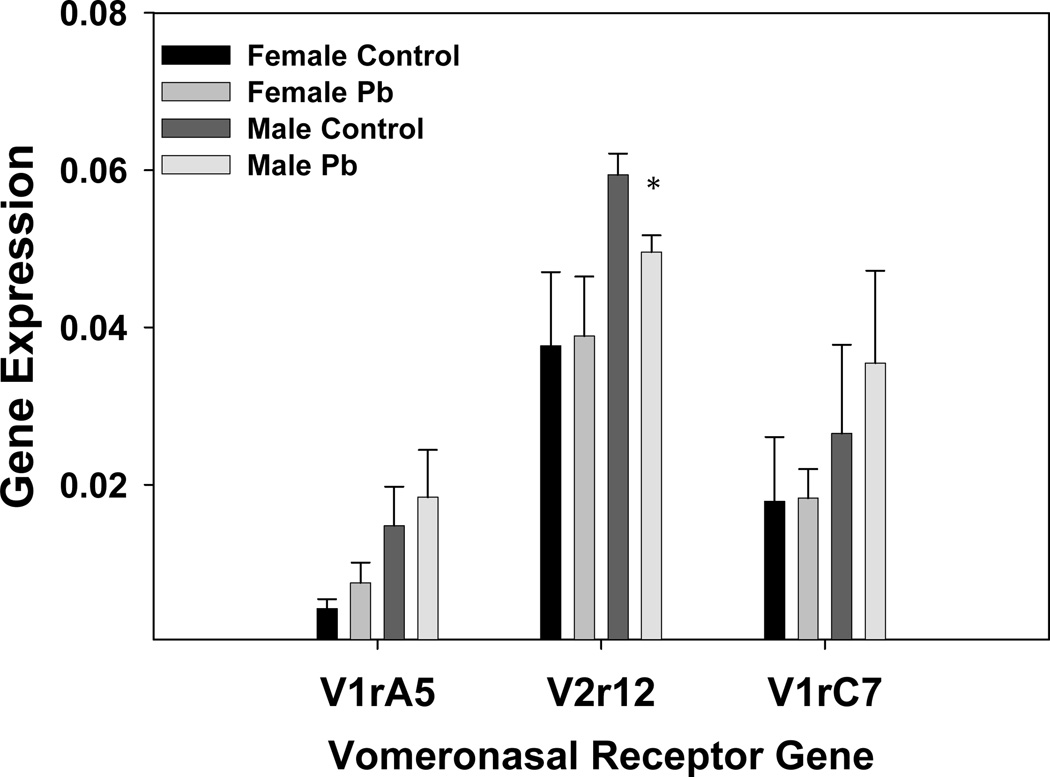
The microarray results suggested that Pb had some effect on expression of the vomeronasal receptor genes. These genes were selected to examine further because of the known vomeronasal receptor connection to various behaviors, including aggression (Insel and Fernald, 2004; He et al., 2008). Expression of these genes is relatively low in the brain (Zhang et al., 2004), which was reflected in our GeneChip data. Although most of the vomeronasal receptor genes represented on the Affymetrix chip had been given a call of “Absent”, there were a few that gave a relatively stronger signal than others. These were selected for quantification by qRT-PCR. Of the five genes mentioned in the methods that were assayed, three genes gave detectable expression (V1rA5, V2r12, and V1rC7). The gene expression results indicated that all of these receptors had slightly increased expression in male mice over those in female mice of the same litter, p<0.01 by paired t-test. The results indicated that Pb significantly decreased vomeronasal receptor expression for V2r12 in male mice (Fig.8), but did not significantly change vomeronasal gene expression in the female mice. V1rA5 expression, via qRT-PCR analysis, was slightly elevated in the female brain by Pb-exposure, and was indicated to be increased according to the GeneChip analysis, as well (Table S1).
Fig. 8.
Developmental Pb exposure modulates Vomeronasal receptor gene expression in male and female mice. Results were generated by TaqMan RT-PCR as described in the Methods section. Data are presented as the mean ± S.E.M. All results are obtained from an N of 3 litters. An * indicates a significant decrease, p<0.05, in V2r12 gene expression in males between control and Pb treated litters.
3.9 Region specific effects of Pb on gene expression of pro-inflammatory factors and protein expression of an anti-inflammatory factor
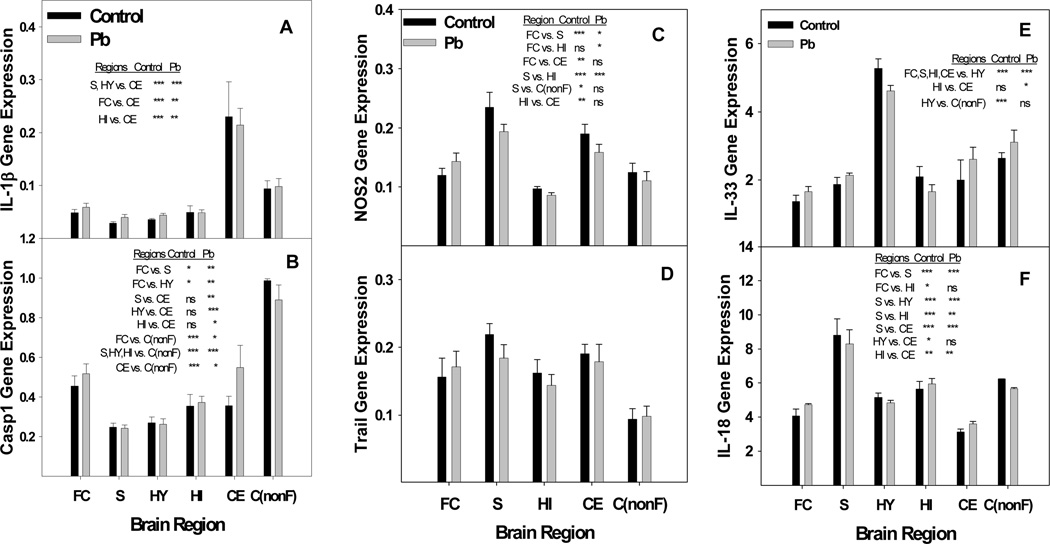
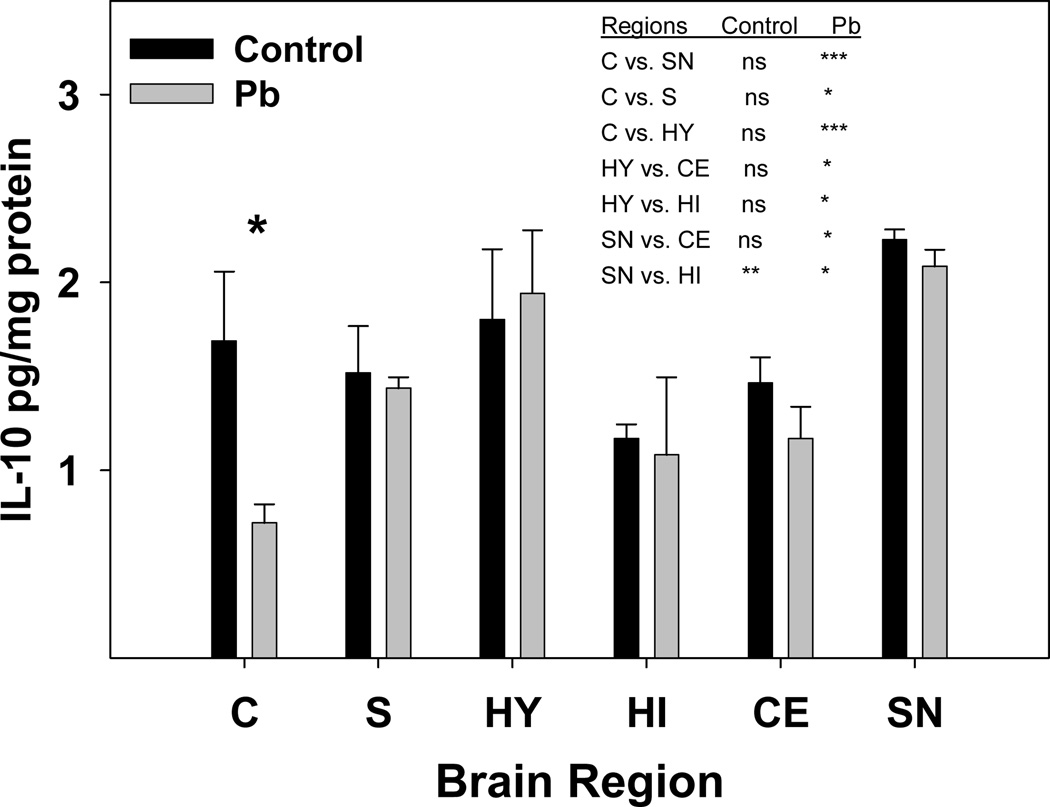
The microarray results suggest that Pb could be generating activity in the brain resembling that which occurs during inflammation. Reports by others have noted similarities between the effects of Pb exposure and neurodegenerative disorders caused by neuroinflammation (Zawia et al., 1998; White et al., 2007). In order to investigate further the possibility that Pb is perturbing expression of factors associated with neuroinflammation, we examined gene expression of certain markers of inflammation in a selection of brain regions. Inflammation associated factors studied were IL-1β, IL-18, caspase-1, NOS2, Trail (TNF-related apoptosis inducing ligand) and IL-33. Expression of each of the genes chosen was slightly elevated by Pb within the frontal cortex region alone (Fig. 9). Further analysis employing ANOVA statistics revealed that Pb-exposure was changing the normal pattern of gene expression for these genes between the designated gene regions (Fig. 9). For example, the difference in gene expression for caspase 1 between the frontal and nonfrontal cortex after Pb-exposure went from a P of <0.001 to a P of <0.05. The general patterns of gene expression between the various regions differ markedly for IL-1β, IL-18, caspase 1, and IL-33. However, the over-all pattern of relative gene expression between the regions presented for NOS2 and Trail appear to be rather similar, even though no significant differences in gene expression were detected between the regions for Trail (Fig. 9). This is perhaps due to the high variation in expression between the litters for Trail gene expression. The results demonstrate a difference in expression level between the frontal cortex and non-frontal cortex and in some cases (as for caspase 1) this difference is quite dramatic (Fig. 9). Gene expression of IL-18 within the frontal cortex and non-frontal cortex according to the Student’s t-test gave P-values of 0.01 and 0.004 for control and Pb-exposed mice, respectively. The influence of developmental Pb exposure on protein concentrations for IL-18 in cortex, striatum, hypothalamus, hippocampus, cerebellum and substantia nigra have been reported previously, and the results showed significantly increased IL-18 protein in the cortex only (Kasten-Jolly et al., 2011). Protein concentration for IL-1β as determined by a Western blot, indicated that the protein was primarily present in its mature form and was slightly increased by Pb in each region except the hippocampus (data not shown). RNA concentrations for IL-10 were very low by qRT-PCR, but we were able to detect the protein in the various brain regions by Luminex analysis. IL-10 concentration was of interest because of its anti-inflammatory properties. Protein concentrations for IL-10 were measured in each brain region, and it was found that the IL-10 concentration was significantly decreased in the cortex (Fig. 10). Here the cortex tissue harvested for the protein determinations was primarily from the frontal region. Inter-region changes in IL-10 protein concentration due to Pb-exposure revealed that Pb dramatically changed the IL-10 protein concentrations between the cortex and the substantia nigra and hypothalamus. Several other regional combinations changed only slightly, such as that for hypothalamus versus hippocampus. Decreased IL-10 protein concentration in the cortex after Pb-exposure correlates with the suggestion that Pb is promoting inflammation like responses within this region.
Fig. 9.
Developmental Pb exposure perturbs regulated gene expression between brain regions of select genes associated with inflammation. A) IL-1β, B) Caspase 1, C) NOS2, D) Trail, E) IL-33, and F) IL-18. Brain region total RNA was isolated as described in the methods from female mice exposed to 0.1 mM Pb from gd8 to pnd21 and distilled water controls. NOS2 data for striatum and hippocampus include an N of 3 litters and all non-frontal cortex data represents an N of 2 litters each for control and Pb-exposed. All other data represent an N of 4 litters for control and Pb-exposed mice. Statistics were performed by ANOVA analysis with Bonferroni post-test restrictions. Asterisks indicate the following P values: * p<0.05, ** p<0.01, *** p<0.001. No significant difference is indicated by ns. Regions are Frontal Cortex (FC), Striatum (S), Hypothalamus (HY), Hippocampus (HI), and Cerebellum (CE), non-frontal Cortex C(nonF). Data are presented as mean ±S.E.M.
Fig. 10.
Pb exposure significantly decreased IL-10 protein concentration in the cortex region. Tissue homogenates were prepared from female brain regions as described in the methods. Two female mice were selected to represent each litter. An N of 3 litters was used to obtain the control values, but the Pb exposed values were obtained with the following number of litters: Cortex (C), N = 5; Hypothalamus (HY), N = 4; Striatum (S), N = 5; Cerebellum (CE), N = 5; Hippocampus (HI), N = 2; and Substantia Nigra (SN), N = 2. Data are presented as mean ±S.E.M. The * represents statistical significance at p<0.05 by the Student’s t-test. Statistical analysis between brain regions was performed by 2-way ANOVA with a Bonferroni post-test. Asterisks indicate the following P values: * p<0.05, ** p<0.01, *** p<0.001.
4. Discussion
The behavioral results presented here indicate that developmental Pb-exposure decreased exploratory activity and spatial memory, but not motor coordination. A decrease in exploratory behavior in the adult mice was not due to impairment of motor function because they performed equally well with the control group during the rotarod test. A decrease in exploratory activity after developmental Pb-exposure was also reported by Moreira et al., (2001) and Reddy et al., (2003). No differences in rotarod performance due to developmental Pb-exposure as measured in adult animals has been reported by other investigators (Moreira et al., 2001; Lim et al., (2005). Our behavior studies showed definite behavioral differences between male and female mice, in that, Pb-exposed females displayed less exploratory activity than males. Differences in behavior between males and females based on blood Pb levels have been reported for several human studies and studies with rodents. A study of 375 children in South Australia indicated that females were more susceptible to the toxic effects of Pb based on IQ scores and blood Pb levels (Tong T et al., 2000). In rats, it was found that prenatal-stress plus Pb-exposure led to prolonged corticosterone levels and elevated frontal cortex and nucleus accumbens dopamine turnover. These effects were more enhanced in the female rats and correlated with an increase in number of attempts to correctly learn response sequences, particularly the sequence LRC (Cory-Slechta et al., 2010). Further human studies measuring IQ of school age children showed a strong negative correlation of blood Pb levels on full scale IQ in addition to performance on reading and mathematics tests (Surkan et al., 2007). This study suggested that Pb may be undermining the children’s working memory, cognitive flexibility, and ability to formulate, test, and adapt hypotheses. These capabilities are considered to be within the realm of executive functions and are under the control of the frontal cortex. These results correlate with the finding in rats that Pb is affecting the frontal cortex, as mentioned earlier. Our results for the MWM are in agreement with these findings in that we found cognitive deficits during the probe trial, but not during acquisition. Absence of a developmental Pb effect on escape latency (acquisition), but presence of a Pb effect on spatial retention (probe trial) during MWM tests as adults have been reported by others (Lim et al., 2005).
Pathway analysis of the microarray data obtained from the female brain RNA indicated that the developmental Pb-exposure perturbed pathways associated with region formation and growth of the developing brain. Pb-exposure caused up-regulation of genes grouped into the focal adhesion (Z score 4.08) and extracellular matrix (ECM) receptor interaction (Z score 4.95) pathways. The majority of these genes coded for laminins or integrins. The ECM components are generally referred to as the basement membrane (BM), whose internal basal lamina (BL) is linked to cellular membranes (Tanzer, 2006; Takagi, 2007). The BL can interact with receptors on the cell membranes that can trigger intracellular signals, which, in turn, control cell migration, proliferation, survival, polarization, shape and differentiation. ECM components, such as laminin, are implicated in myelin formation and play a crucial role in the development of Schwann cells (Court et al., 2006). An effect of childhood low-level Pb exposure on neuronal cell myelination has already been reported (Brubaker et al., 2009). Also, the ECM is a prominent structure of blood vessels and staining for laminin proteins can delineate the blood vessels in the brain which harbor neural stem cells (Shen et al., 2008). Function and differentiation of the neural stem cells is very tightly regulated through their association with the blood vessels and Pb could disturb this process by changing the expression patterns of ECM receptor and focal adhesion pathway proteins. Interestingly, pathways found to be important for neural stem cell proliferation and differentiation during ischemia were the VEGF and TGF-β pathways (Sun et al., 2010). Both of these pathways are perturbed by developmental Pb-exposure (Bouton et al., 2001; Barbeito et al., 2010; Kasten-Jolly et al., 2011).
The microarray data indicated that Pb-exposure was interfering with the formation of neuronal connections within the developing brain. Pb up-regulated expression of MHC class I genes. MHC I has functions in the developing brain distinct from the functions associated with the immune system. In the brain, these MHC I molecules are present postsynaptically on neurons and have been shown to play important roles in remodeling and plasticity of connections in the developing and mature mammalian CNS (Huh et al., 2000; McConnell et al., 2009). Influences of MHC I expression in the hippocampus on neuronal development and function have been demonstrated (Huh et al., 2000; Goddard et al., 2007). Experiments employing retinal ganglion cell (RGC) axons indicated that MHC I expression regulation can influence synapse remodeling and can eventually result in lasting structural changes (Shatz, 2009; Datwani et al., 2009). Therefore, the developmental Pb exposure described here is perturbing pathways and gene expression patterns in the developing brain that would lead to irreversible alterations to the brain. These changes in gene expression during the developmental Pb-exposure would likely have a deleterious effect on cognitive function and memory in the adult animal.
Gene expression changed by Pb within the immune system ontology suggested that Pb was promoting inflammation in the brain. Among the highest up-regulated was Ccl28, a chemokine secreted by epithelial cells and a chemoattractant to T and B lymphocytes bearing the Ccr10 receptor. Ccl28 has also been mechanistically linked to dendritic cells, through a pathway which generates IgE and the high-affinity Fcε receptor, in addition to Ccl28 (Khan et al., 2008). The suggestion here is that increased numbers of T- and B-lymphocytes and dendritic cells might be present in the brains of the developmentally Pb exposed mice. Up-regulation of IL-7 by Pb also hints at increased immune-cell infiltration in the brain. IL-7 is a tissue-derived cytokine whose primary sources are stromal and epithelial cells, but dendritic cells are also capable of producing IL-7 under certain conditions (Fry and Mackall, 2002). Regulation of local availability of IL-7 is the result of its binding to the ECM through attachment to glycosaminoglycan, heparin sulfate, and fibronectin, factors also affected by the Pb-exposure. The function of IL-7 is to increase the growth of immature B-cells and prompt T-cell homing (Fry and Mackall, 2002; Beq et al., 2009). Infiltrating immune cells may be recruited by activated astrocytes through their secretion of chemokines Ccr1 and Ccr2. Correlating with the possibility of increased presence of APCs in the brain is the highly significant increase in MHC class II, antigen A gene expression. However, activated microglia and astrocytes also ezxpress MHC, class II, antigen A (Xiao and Link, 1999). Further, Pb had previously been shown to directly increase MHC, class II on peripheral immune cells of BALB/c mice (McCabe and Lawrence, 1990). Increased infiltration of lymphocytes and monocytes, or activation of microglia and/or astrocyte cells are signs of brain inflammation. Also a sign of inflammation is the increased expression of proteases (Bunnett, 2006). The microarray results indicated that Pb up-regulated gene expression of numerous catabolic enzymes, including carboxypeptidases, chymotrypsin-like proteins, and metalloproteinases (Table S1). In accord with the results reported here the effect of developmental Pb-exposure on protease gene expression has been observed in Drosophila (Ruden et al., 2009) and in mouse spleen (Kasten-Jolly et al., 2010). Brain inflammation is known to generate behavior changes and has been shown to be the root cause of neurodegenerative diseases, such as Alzheimer’s disease.
Brain region analysis for expression of factors associated with the presence of inflammation indicated that Pb-exposure changed the expression pattern of these factors between the various regions of the brain. The results indicated up-regulation of caspase 1 and NOS2 after the developmental Pb-exposure. Pro-inflammatory cytokines, IL-1β and IL-18, are both processed to their mature, active forms by caspase-1. Caspase 1, (IL-1 converting enzyme, ICE), showed significantly different inter-region gene expression changes after Pb-exposure. The impact of caspase 1 over-expression on tissue destruction has been demonstrated in a brain ischemia injury model where blocking caspase 1 expression resulted in a decreased infarct size (Sifringer et al., 2007). Caspase 1 regulated over-production of the cleaved, bioactive form of IL-1β results in up-regulation of nitric oxide synthase (iNOS or NOS2) expression and, thus, causes increased production of nitric oxide (NO) (Juttler et al., 2007). The cell primarily responsible for the iNOS-induced release of NO is the activated glial cell. Released NO will create alterations in proteins, DNA, RNA, and lipids culminating in pathological impairment of normal cell functions and ultimately cell death to the cells surrounding these activated glial cells. Increases in NOS2 activity have been reported to occur in rats exposed to sub-chronic levels of Pb (Ramesh and Jadhav, 2001). In both humans and mice, IL-1β and IL-18 are key players in inflammatory processes that increase with aging (Bodles and Barger, 2004; Joseph et al., 2005; Dinarello, 2006; Ojala et al., 2009). IL-33 was expressed in the mouse brain at a relatively high concentration. The protein has been found to be present in the nucleus of astrocyte cells (Hudson et al., 2008). This pro-inflammatory cytokine is released into the cytoplasm and secreted in response to infection or injury. The results presented here show that developmental Pb exposure promotes changes in inter-region gene expression of pro-inflammatory factors IL-1β, IL-18, IL-33, caspase 1, and NOS2 in the brain. Region specific pro-inflammatory cytokine expression has been observed before in studies related to other neural stressors (O’Conner et al. 2009). Consistent with the suggestion of the presence of inflammation in certain brain regions, protein concentration of the anti-inflammatory cytokine, IL-10, was significantly decreased by Pb in the cortex region of the brain. IL-10 has been shown to be capable of directly blocking IL-1 expression (Wong et al., 1997). This cytokine, mainly of the monocytic lineage (including microglia), is considered to be a CD4+ T-helper subset type 2 cytokine and acts as an anti-inflammatory cytokine. In the CNS non-CD4+ T-cell sources of IL-10 are necessary to regulate glial and neuronal processes in order to mediate neuroprotection. This IL-10 mediated protection requires the assistance of CD4+ T-cells (Xin et al., 2011). Therefore, a decrease in IL-10 in the cortex may indicate that neuroprotection is being compromised in this region.
Aggressive behavior due to developmental Pb–exposure has been well documented with respect to studies in humans. Human studies have shown strong direct correlations between early childhood blood Pb levels and increased aggression as adolescents and young adults. Increased blood Pb levels in children at age 7 were found to correlate with externalizing and school problems as well as lowered IQ scores (Chen et al., 2007). A longitudinal study of children from birth to adolescents indicated that prenatal Pb exposure was associated with a covariate-adjusted increase in delinquent and antisocial behaviors as reported by their parents (Dietrich et al., 2001). Prenatal and early childhood blood Pb levels have been linked to arrests in young adults (Wright et al., 2008). Bone Pb levels were found to be higher in adjudicated delinquents than their age-matched control high school students (Needleman et al., 2002). Interestingly, in 1990, homicide rates were higher in US counties that had the highest air Pb concentrations (Wakefield, 2002).
Aggression results in the present report indicated that the extreme aggression due to developmental Pb exposure was directed toward their sibling cage mates. This might be due to a decreased ability of the lead treated males to recognize their siblings. Decreased sibling recognition after Pb-exposure was observed in young herring gulls by measuring their ability to find back to their home nest (Burger, 1998). This lack of sibling recognition might be due to impairment of the sense of smell due to the Pb-exposure, which would make it difficult to pick out the scent of their litter mates. An effect of developmental Pb-exposure on odor discrimination has been reported previously (Lim et al., 2005). For this reason the aggressive behavior observed in the Pb-exposed male-mice might be in part due to aberrant expression and/or function of certain vomeronasal or olfactory receptor genes. Developmental Pb-exposure described here perturbed expression of vomeronasal receptors, V1ra5 (Up) and V2r2 (Up), according to the Affymetrix GeneChip data (females), while qRT-PCR results indicated that Pb significantly decreased expression of V2r12 in the male mice. These receptors are representatives of a huge family of receptors involved in discrimination of odors. Vomeronasal receptors recognize peptide ligands that are presented by MHC class I and class II molecules. For this reason vomeronasal receptors have many features in common with T-cell receptors (Leinders-Zufall et al., 2004; Spehr et al., 2006; He et al., 2008; Leinders-Zufall et al., 2009). In the mouse, the vomeronasal organ has an essential role in detection of chemical cues that regulate social behaviors including male-male territorial aggression, maternal aggression, mating, and recognition between individuals (Insel and Fernald, 2004; Dulac and Wagner, 2006; Muramoto et al., 2007). Since the vomeronasal receptor system is feed-back regulated by Ca2+-Calmodulin (Spehr et al., 2009), Pb may be further interfering with the functioning of this system because Pb disturbs cell Ca2+ homeostasis (Gill et al., 2003). Therefore, malfunctioning of the vomeronasal organ feedback mechanisms and under expression of key vomeronasal receptors could be elements of a mechanistic cause for the extreme aggression incidents that occurred between dominant Pb-treated male mice and their less-dominant male sibling cage mates. Conversely, these same males show little interest in a stranger-intruder placed in their home cage, perhaps because they could not discriminate the scent of the intruder.
5. Conclusions
Results from the present study suggest that exposure to Pb during development can manifest long-term behavioral deficits, some in a gender-dependent manner. Microarray and qRT-PCR results reported here indicate that developmental Pb-exposure affects a number of interrelated pathways associated with brain development and promotes inflammation associated responses in the brain. Gene expression analysis of pro-inflammation factors within brain regions indicated that Pb significantly perturbed the inter-regional expression of these factors and that expression of these factors was slightly higher in the frontal cortex. Pb decreased the protein concentration of an anti-inflammatory cytokine, IL-10, in the cortex, suggesting greater inflammation within this region. Vomeronasal receptor gene expression (measured by qRT-PCR), associated with social behavior, was significantly changed by Pb in the male mice and could be an underlying cause for the aggressive behavior displayed by these mice. Changes in gene expression by Pb of immune system factors reported here suggest mechanisms by which Pb promotes damage in the developing brain.
Supplementary Material
Highlights.
Behavior studies revealed that the mice exposed to Pb during development were less prone to explore and had memory impairment
As adults the Pb exposed males displayed aggressive behavior towards their cage-mates, but ignored a stranger intruder.
Microarray analysis of brain RNA at pnd21 indicated Pb was affecting pathways important for neuronal development.
Brain gene expression analysis at pnd21 indicated inflammation in the brain.
Vomeronasal receptor gene expression, associated with regulation of social behaviors, was altered by Pb in the brain at pnd21.
Acknowledgements
This work was supported by NIH grants ES011135 and ES013857. We thank Michael Ryan and the late Robin Pietropaolo from the Wadsworth Center Microarray Core facility for their assistance in performing the microarray assay. We thank members of Dr. Richard Seegal’s laboratory, Greg Lyng and Richard J. Okoniewski, for their assistance with the dissection of the mouse brain regions used for RNA isolation. We also thank Donghong Gao for her technical assistance with the brain region protein determinations and care of the mice used for these experiments. We acknowledge Mamuka Khvedelidze for his assistance with the ANOVA statistical analysis used in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- Al-Saleh I, Shinwari N, Nester M, Mashhour A, Moncari L, El Din Mohamed G, Rabah A. Longitudinal study of prenatal lead exposure and early cognitive development in Al-Kharj, Saudi Arabia: A preliminary results of cord blood lead levels. J Trop Pediatr. 2008;54:300–307. doi: 10.1093/tropej/fmn019. [DOI] [PubMed] [Google Scholar]
- Anthony D, Dempster R, Fearn S, Clements J, Wells G, Perry H, Walker K. CXC chemokines generate age-related increases in neutrophil-mediated brain inflammation and blood-brain barrier breakdown. Current Biology. 1998;8:923–926. doi: 10.1016/s0960-9822(07)00373-9. [DOI] [PubMed] [Google Scholar]
- Barbeito AG, Martinez-Palma L, Vargas MR, Pehar M, Mañay N, Beckman JS, Barbeito L, Cassina P. Lead exposure stimulates VEGF expression in the spinal cord and extends survival in a mouse model of ALS. Neurobiol Dis. 2010;37:574–580. doi: 10.1016/j.nbd.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC. Effect modification in epidemiologic studies of low-level neurotoxicant exposures and health outcomes. Neurotoxicol Teratol. 2000;22:133–140. doi: 10.1016/s0892-0362(99)00053-7. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. Neurological and behavioral consequences of childhood lead exposure. PLoS Med. 2008;5:0690–0692. doi: 10.1371/journal.pmed.0050115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beq S, Rozlan S, Gautier D, Parker R, Mersseman V, Schilte C, Assouline B, Rance I, Lavedan P, Morre M, Cheynier R. Injection of glycosylated recombinant simian IL-7 provokes rapid and massive T-cell homing in rhesus macaques. Blood. 2009;114:816–825. doi: 10.1182/blood-2008-11-191288. [DOI] [PubMed] [Google Scholar]
- Bodles AM, Barger SW. Cytokines and the aging brain – what we don’t know might help us. TRENDS in Neurosci. 2004;27:621–626. doi: 10.1016/j.tins.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Caldarone BJ, Reilly AA, Flaherty L. Habituation of activity in an open field: A survey of inbred strains and F1 hybrids. Behav Genet. 2000;30:285–293. doi: 10.1023/a:1026545316455. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Ganus JS, Messer A. The development of behavioral abnormalities in the motor neuron degeneration (mnd) mouse. Brain Res. 2002;937:74–82. doi: 10.1016/s0006-8993(02)02470-8. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Walters S, Phoenix JL. Assessing autism-like behavior in mice: Variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar VJ. Intrasession and intersession habituation in mice: From inbred strain variability to linkage analysis. Neurobiol Learn Mem. 2009;92:206–214. doi: 10.1016/j.nlm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton CMLS, Hossain MA, Frelin LP, Laterra J, Pevsner J. Microarray analysis of differential gene expression in lead-exposed astrocytes. Toxicol Appl Pharmacol. 2001;176:34–53. doi: 10.1006/taap.2001.9274. [DOI] [PubMed] [Google Scholar]
- Bradbury MW, Deane R. Permeability of the blood-brain barrier to lead. Neurotoxicology. 1993;14:131–136. [PubMed] [Google Scholar]
- Bressler JP, Goldstein GW. Mechanisms of lead neurotoxicity. Biochem Pharmacol. 1991;41:479–484. doi: 10.1016/0006-2952(91)90617-e. [DOI] [PubMed] [Google Scholar]
- Brown LL, Schneider JS, Lidsky TI. Sensory and cognitive functions of the basal ganglia. Curr Opin Neurobiol. 1997;7:157–163. doi: 10.1016/s0959-4388(97)80003-7. 1997. [DOI] [PubMed] [Google Scholar]
- Brubaker CJ, Schmithorst VJ, Haynes EN, Dietrich KN, Egelhoff JC, Lindquist DM, Lanphear BP, Cecil KM. Altered myelination and axonal integrity in adults with childhood lead exposure: A diffusion tensor imaging study. Neurotoxicology. 2009;30:867–875. doi: 10.1016/j.neuro.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn TL, Parsons PJ, Kao E, Dietert RR. Exposure to lead during critical windows of embryonic development: Differential immunotoxic outcome based on stage of exposure and gender. Toxicol Sci. 2001;64:57–66. doi: 10.1093/toxsci/64.1.57. [DOI] [PubMed] [Google Scholar]
- Bunnett NW. Protease-activated receptors: How proteases signal to cells to cause inflammation and pain. Semin Thromb Hemost. 2006;32(Suppl 1):39–48. doi: 10.1055/s-2006-939553. [DOI] [PubMed] [Google Scholar]
- Burger J. Effects of lead on sibling recognition in young herring gulls. Toxicol Sci. 1998;43:155–160. doi: 10.1006/toxs.1998.2451. [DOI] [PubMed] [Google Scholar]
- Chen A, Cai B, Dietrich KN, Radcliff J, Rogan WJ. Lead exposure, IQ, behavior in urban 5- to 7-year olds: Does lead affect behavior only by lowering IQ? Pediatrics. 2007;119:e650–e658. doi: 10.1542/peds.2006-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Weiss B, Cox C. Delayed behavioral toxicity of lead with increasing exposure concentration. Toxicol Appl Pharmacol. 1983;71:342–352. doi: 10.1016/0041-008x(83)90021-2. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA. Chronic low-level lead exposure: behavioral consequences, biological exposure indices and reversibility. Sci Total Environ. 1988;71:433–440. doi: 10.1016/0048-9697(88)90215-x. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Stern S, Weston D, Allen JL, Liu S. Enhanced learning deficits in female rats following lifetime Pb exposure combined with prenatal stress. Toxicol Sci. 2010;117:427–438. doi: 10.1093/toxsci/kfq221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court FA, Wrabetz L, Feltri ML. Basal Lamina: Schwann cells wrap to the rhythm of space-time. Curr Opin Neurobiol. 2006;16:501–507. doi: 10.1016/j.conb.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Cremin JD, Smith DR. In vitro vs in vivo Pb effects on brain protein kinase C activity. Environ Res. 2002;90:191–199. doi: 10.1016/s0013-9351(02)00007-5. [DOI] [PubMed] [Google Scholar]
- Datwani A, McConnell MJ, Kanold PO, Micheva KD, Busse B, Shamloo M, Smith SJ, Shatz CJ. Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009;64:463–470. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich KN, Ris MD, Succop PA, Berger OG, Bornschein RL. Early exposure to lead and juvenile delinquency. Neurotoxicol Teratol. 2001;23:511–518. doi: 10.1016/s0892-0362(01)00184-2. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr. 2006;83(Suppl):447S–455S. doi: 10.1093/ajcn/83.2.447S. [DOI] [PubMed] [Google Scholar]
- Dulac C, Wagner S. Genetic analysis of brain circuits underlying pheromone signaling. Ann Rev Genet. 2006;40:449–467. doi: 10.1146/annurev.genet.39.073003.093937. [DOI] [PubMed] [Google Scholar]
- Dyatlov V, Platoshin AV, Lawrence DA, Carpenter DO. Lead potentiates cytokine- and glutamate-mediated increases in permeability of the blood-brain barrier. Neurotoxicology. 1998;19:283–292. [PubMed] [Google Scholar]
- Dyatlov VA, Lawrence DA. Neonatal lead exposure potentiates sickness behavior induced by Listeria monocytogenes infection of mice. Brain Behav Immun. 2002;16:477–492. doi: 10.1006/brbi.2001.0641. [DOI] [PubMed] [Google Scholar]
- Faust D, Brown J. Moderately elevated blood lead levels: Effects on neuropsychologic functioning in children. Pediatrics. 1987;80:623–629. [PubMed] [Google Scholar]
- Fry TJ, Mackall CL. Interleukin-7: From bench to clinic. Blood. 2002;99:3892–3904. doi: 10.1182/blood.v99.11.3892. [DOI] [PubMed] [Google Scholar]
- Garcia-Bueno B, Caso JR, Leza JC. Stress as a neuroinflammatory condition in brain: Damaging and protective mechanisms. Neuro Biobehav Rev. 2008;32:1136–1151. doi: 10.1016/j.neubiorev.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Gill KD, Gupta V, Sandhir R. Ca2+/calmodulin-mediated neurotransmitter release and neurobehavioral deficits following lead exposure. Cell Biochem Funct. 2003;21:345–353. doi: 10.1002/cbf.1030. [DOI] [PubMed] [Google Scholar]
- Goddard CA, Butts DA, Shatz CJ. Regulation of CNS synapses by neuronal MHC class I. Proc Natl Acad Sci USA. 2007;104:6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Ma L, Kim S, Nakai J, Yu CR. Encoding gender and individual information in the mouse vomeronasal organ. Science. 2008;320:535–538. doi: 10.1126/science.1154476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemphill FE, Kaeberle ML, Buck WB. Lead suppression of mouse resistance to Salmonella typimurium. Science. 1971;172:1031–1032. doi: 10.1126/science.172.3987.1031. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Lanphear BP, Dietrich KN. Age of greatest susceptibility to childhood lead exposure: A new statistical approach. Environ Health Perspect. 2009;117:1309–1312. doi: 10.1289/ehp.0800426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson CA, Christophi GP, Gruber RC, Wilmore JR, Lawrence DA, Massa PT. Induction of IL-33 expression and activity in central nervous system glia. J Leukoc Biol. 2008;84:631–643. doi: 10.1189/jlb.1207830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class 1 MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: Searching for the social brain. Annu Rev Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Johnston MV, Goldstein GW. Selective vulnerability of the developing brain to lead. Curr Opin Neurol. 1998;11:689–693. doi: 10.1097/00019052-199812000-00013. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Casadesus G, Fisher D. Oxidative stress and inflammation in brain aging: Nutritional considerations. Neurochem Res. 2005;30:927–935. doi: 10.1007/s11064-005-6967-4. [DOI] [PubMed] [Google Scholar]
- Juttler E, Bonmann E, Spranger M, Kolb-Bachofen V, Suschek CV. A novel role of interleukin-1-converting enzyme in cytokine-mediated inducible nitric oxide synthase gene expression: Implications for neuroinflammatory diseases. Mol Cell Neurosci. 2007;34:612–620. doi: 10.1016/j.mcn.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Kasten-Jolly J, Heo Y, Lawrence DA. Impact of developmental lead exposure on splenic factors. Toxicol Appl Pharmacol. 2010;247:105–115. doi: 10.1016/j.taap.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten-Jolly J, Heo Y, Lawrence DA. Central nervous system cytokine gene expression: Modulation by lead. J Biochem Mol Toxicol. 2011;25:41–54. doi: 10.1002/jbt.20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SH, Park SS, Sirajuddin IA, Grayson MH. Respiratory vitus and asthma: The role of immunoglobulin E. Clin Ther. 2008;30:1017–1024. doi: 10.1016/j.clinthera.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Kowolenko M, Tracy L, Lawrence DA. Early effects of lead on bone marrow cell responsiveness in mice challenged with Listeria monocytogenes. Fund Appl Toxicol. 1991;17:75–82. doi: 10.1016/0272-0590(91)90240-5. [DOI] [PubMed] [Google Scholar]
- Lawrence DA. In vivo and in vitro effects of lead on humoral and cell-mediated immunity. Infect Immun. 1981;31:136–143. doi: 10.1128/iai.31.1.136-143.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett RW. An age-specific kinetic model of lead metabolism in humans. Environ Health Perspect. 1993;101:598–616. doi: 10.1289/ehp.93101598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T, Brennan P, Widmeyer P, Chandramani P, Maul-Pavici A, Jager M, Li X-H, Zufall F, Boehm T. MHC class 1 peptides as chemosensory signals in the vomeronasal organ. Science. 2004;306:1033–1037. doi: 10.1126/science.1102818. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Ishii T, Mombaerts P, Zufall F, Eoehm T. Structural requirements for the activation of vomeronasal sensory neurons by MHC peptides. Nature Neurosci. 2009;12:1551–1558. doi: 10.1038/nn.2452. [DOI] [PubMed] [Google Scholar]
- Lim S-Y, Doherty JD, McBride K, Miller-Ihli NJ, Carmona GN, Stark KD, Salem N., Jr Lead exposure and (N-3) fatty acid deficiency during rat neonatal development affect subsequent spatial task performance and olfactory discrimination. J Nutr. 2005;135:1019–1026. doi: 10.1093/jn/135.5.1019. [DOI] [PubMed] [Google Scholar]
- Litvin Y, Blanchard DC, Pentkowski NS, Blanchard RJ. A pinch of lesion: A reconceptualization of biting consequences in mice. Aggress Behav. 2007;33:545–551. doi: 10.1002/ab.20222. [DOI] [PubMed] [Google Scholar]
- McCabe MJ, Lawrence DA. The heavy metal lead exhibits B cell-stimulatory factor activity by enhancing B cell Ia expression and differentiation. J Immunol. 1990;145:671–677. [PubMed] [Google Scholar]
- McConnell MJ, Husng YH, Datwani A, Shatz CJ. H2-Kb and H2-Db regulate cerebellar long-term depression and limit motor learning. Proc Natl Acad Sci USA. 2009;106:6784–6789. doi: 10.1073/pnas.0902018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadyen MP, Kusek G, Bolivar VJ, Flaherty L. Differences among eight inbred strains of mice in motor ability and motor learning on a rotorod. Genes Brain Behav. 2003;2:214–219. doi: 10.1034/j.1601-183x.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- Moreira EG, Vassilieff I, Vassilieff VS. Developmental lead exposure: Behavioral alterations in the short and long term. Neurotoxicol Teratol. 2001;23:489–495. doi: 10.1016/s0892-0362(01)00159-3. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto K, Hashimoto M, Kaba H. Target regulation of V2R expression and functional maturation in vomeronasal sensory neurons in vitro. European J Neurosci. 2007;26:3382–3394. doi: 10.1111/j.1460-9568.2007.05954.x. [DOI] [PubMed] [Google Scholar]
- Nadel L. Dorsal and ventral hippocampal lesions and behavior. Physiol Behav. 1968;3:891–900. [Google Scholar]
- Needleman H. Lead Poisoning. Annu Rev Med. 2004;55:209–222. doi: 10.1146/annurev.med.55.091902.103653. [DOI] [PubMed] [Google Scholar]
- Needleman H. Low level lead exposure: History and discovery. Ann Epidemiol. 2009;19:235–238. doi: 10.1016/j.annepidem.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Needleman HL, McFarland C, Ness RB, Fienberg SE, Tobin MJ. Bone lead levels in adjudicated delinquents: A case control study. Neurotoxicol Teratol. 2002;24:711–717. doi: 10.1016/s0892-0362(02)00269-6. [DOI] [PubMed] [Google Scholar]
- O’Conner M-F, Irwin MR, Wellisch DK. When grief heats up: Pro-inflammatory cytokines predict regional brain activation. Neuroimage. 2009;47:891–896. doi: 10.1016/j.neuroimage.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojala J, Alafuzoff I, Herukka S-K, van Groen T, Tanila H, Pirttila T. Expression of interleukin-18 is increased in the brains of Alzheimer’s disease patients. Neurobiol Aging. 2009;30:198–209. doi: 10.1016/j.neurobiolaging.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Patel A, Siegel A, Zalcman SS. Lack of aggression and anxiolytic-like behavior in TNF receptor (TNF-R1 and TNF-R2) deficient mice. Brain Behav Immun. 2010;24:1276–1280. doi: 10.1016/j.bbi.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Tiffany-Castiglioni E. Lead-induced endoplasmic reticulum (ER) stress responses in the nervous system. Neurochem Res. 2003;28:153–162. doi: 10.1023/a:1021664632393. [DOI] [PubMed] [Google Scholar]
- Ramesh GT, Jadhav AL. Levels of protein kinase C and nitric oxide synthase activity in rats exposed to sub chronic low level lead. Molec Cell Biochem. 2001;223:27–33. doi: 10.1023/a:1017549003114. [DOI] [PubMed] [Google Scholar]
- Reddy GR, Basha MR, Devi BC, Suresh A, Baker JL, Shafeck A, Heinz J, Chetty CS. Lead induced effects on acetylcholinesterase activity in cerebellum and hippocampus of developing rat. Int J Dev Neurosci. 2003;21:347–352. doi: 10.1016/s0736-5748(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Ruden DM, Chen L, Possidente D, Possidente B, Rasouli P, Wang L, Lu X, Garfinkel MD, Hirsch HVB, Page GP. Genetical toxicogenomics in Drosophila identifies master-modulatory loci that are regulated by developmental exposure to lead. Neurotoxicology. 2009;30:898–914. doi: 10.1016/j.neuro.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen KM, Loikkanen J, Naarala J. Amplification of glutamate-induced oxidative stress. Toxicol Letters. 1995;82/83:399–405. doi: 10.1016/0378-4274(95)03490-0. [DOI] [PubMed] [Google Scholar]
- Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2011;10:44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimanski LA, Nguyen PV. Multidisciplinary approaches for investigating the mechanisms of hippocampus-delendent memory: A focus on inbred mouse strains. Neurosci Behav Rev. 2004;28:463–483. doi: 10.1016/j.neubiorev.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Shatz CJ. MHC class I: An unexpected role in neuronal plasticity. Neuron. 2009;64:40–45. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang S-M, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: A quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeta S, Miyake K, Misawa T. Critical period of brain development in learning caused by lead exposure in rats. J Exp Clin Med. 1989;14:147–152. [PubMed] [Google Scholar]
- Sifringer M, Stefovska V, Endesfelder S, Stahel PF, Genz K, Dzietko M, Ikonomidou C, Felderhoff-Mueser U. Activation of caspase-1 dependent interactions in developmental brain trauma. Neurobiol Dis. 2007;25:614–622. doi: 10.1016/j.nbd.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Snyder JE, Filipov NM, Parsons PJ, Lawrence DA. The efficiency of maternal transfer of lead and its influence on plasma IgE and splenic cellularity of mice. Toxicol Sci. 2000;57:87–94. doi: 10.1093/toxsci/57.1.87. [DOI] [PubMed] [Google Scholar]
- Spehr M, Kelliher KR, Li X-H, Boehm T, Leinders-Zufall T, Zufall F. Essential role of the main olfactory system in social recognition of major histocompatibility complex peptide ligands. J Neurosci. 2006;26:1961–1970. doi: 10.1523/JNEUROSCI.4939-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. Elaborate interactions between the immune and nervous systems. Nature Immunology. 2004;5:575–581. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- Struzynska L, Dabrowska-Bouta B, Koza K, Sulkowski G. Inflammation-like glial response in lead-exposed immature rat brain. Toxicol Sci. 2007;95:156–162. doi: 10.1093/toxsci/kfl134. [DOI] [PubMed] [Google Scholar]
- Sun J, Zhou W, Sha B, Yang Y. Ischemia induced neural stem cell proliferation and differentiation in neonatal rat involved vascular endothelial growth factor and transforming growth factor-beta pathways. Brain Devel. 2010;32:191–200. doi: 10.1016/j.braindev.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Surkan PJ, Zhang A, Trachtenberg F, Daniel DB, McKinlay S, Bellinger DC. Neuropsychological function in children with blood lead levels <10 µg/dL. Neurotoxicology. 2007;28:1170–1177. doi: 10.1016/j.neuro.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szelenyi J, Vizi ES. The catecholamines-cytokine balance: Interaction between the brain and the immune system. Ann NY Acad Sci. 2007;1113:311–324. doi: 10.1196/annals.1391.026. [DOI] [PubMed] [Google Scholar]
- Takagi J. Structural basis for ligand recognition by integrins. Curr Opin Cell Biol. 2007;19:557–564. doi: 10.1016/j.ceb.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Tanzer ML. Current concepts of extracellular matrix. J Orthop Sci. 2006;11:326–331. doi: 10.1007/s00776-006-1012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany-Castiglioni E, Sierra EM, Wu JN, Rowles TK. Lead toxicity in neuroglia. Neurotoxicology. 1989;10:417–443. [PubMed] [Google Scholar]
- Tong T, McMicheal AJ, Baghurst PA. Interactions between environmental lead exposure and sociodemographic factors on cognitive development. Arch Environ Health. 2000;55:330–335. doi: 10.1080/00039890009604025. [DOI] [PubMed] [Google Scholar]
- Tong S, Von Schirnding YE, Prapamontol T. Environmental lead exposure: A public health problem of global dimensions. Bull World Health Organ. 2000;78:1068–1077. [PMC free article] [PubMed] [Google Scholar]
- Velez L, Sokoloff G, Miczek KA, Palmer AA, Dulawa SC. Differences in aggressive behavior and DNA copy number variants between BALB/cJ and BALB/cByJ substrains. Behav Genet. 2010;40:201–2010. doi: 10.1007/s10519-009-9325-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield J. The lead effect? Environ Health Perspect. 2002;110:A574–A580. doi: 10.1289/ehp.110-a574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LD, Cory-Slechta DA, Gilbert ME, Tiffany-Castiglioni E, Zawia NH, Virgolini M, Rossi-George A, Lasley SM, Qian YC, Riyaz Basha MD. New and evolving concepts in the neurotoxicology of lead. Toxicol Appl Pharmacol. 2007;225:1–27. doi: 10.1016/j.taap.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Wong M-L, Bongiorno PB, Rettori V, McCann SM, Lici J. Interleukin (IL) 1β, IL-1 receptor antagonist, IL-10, and IL-13 gene expression in central nervous system and anterior pituitary during systemic inflammation with pathophysiological implications. Proc Natl Acad Sci USA. 1997;94:227–232. doi: 10.1073/pnas.94.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JP, Dietrich KN, Ris MD, Hornung RW, Wessel SD, Lanphear BP, Ho M, Rea MN. Association of prenatal and childhood blood lead concentrations with criminal arrests in early adulthood. PLoS Med. 2008;5:0732–0740. doi: 10.1371/journal.pmed.0050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B-G, Link H. Is there a balance between microglia and astrocytes in regulating Th1/Th2-cell responses and neuropathologies? Immunol Today. 1999;10:477–479. doi: 10.1016/s0167-5699(99)01501-7. [DOI] [PubMed] [Google Scholar]
- Xin J, Wainwright DA, Mesnard NA, Serpe CJ, Sanders VM, Jones KJ. IL-10 within the CNS is necessary for CD4+ T cells to mediate neuroprotection. Brain Behav Immun. 2011;25:820–829. doi: 10.1016/j.bbi.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawia NH, Sharan R, Brydie M, Oyama T, Crumpton T. Sp1 as a target site for metal-induced perturbation of transcriptional regulation of developmental brain gene expression. Devel Brain Res. 1998;107:291–298. doi: 10.1016/s0165-3806(98)00023-6. [DOI] [PubMed] [Google Scholar]
- Zhang Xin, Rogers M, Tian H, Zhang Xia, Zou D-J, Liu J, Ma M, Shepherd GM, Firestein SJ. High-throughput microarray detection of olfactory receptor gene expression in the mouse. Proc Natl Acad Sci USA. 2004;101:14168–14173. doi: 10.1073/pnas.0405350101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.