Abstract
Purpose
Previous studies have demonstrated that the replication checkpoint, which involves the kinases ATR and Chk1, contributes to cytarabine resistance in cell lines. In the present study, we examined whether this checkpoint is activated in clinical AML during cytarabine infusion in vivo and then assessed the impact of combining cytarabine with the recently described Chk1 inhibitor SCH 900776 in vitro.
Experimental design
AML marrow aspirates harvested before and during cytarabine infusion were examined by immunoblotting. Human AML lines treated with cytarabine in the absence or presence of SCH 900776 were assayed for checkpoint activation by immunoblotting, nucleotide incorporation into DNA and flow cytometry. Long-term effects in AML lines, clinical AML isolates, and normal myeloid progenitors were assayed using clonogenic assays.
Results
Immunoblotting demonstrated increased Chk1 phosphorylation, a marker of checkpoint activation, in over half of Chk1-containing AMLs after 48 h of cytarabine infusion. In human AML lines, SCH 900776 not only disrupted cytarabine-induced Chk1 activation and S phase arrest, but also markedly increased cytarabine-induced apoptosis. Clonogenic assays demonstrated that SCH 900776 enhanced the anti-proliferative effects of cytarabine in AML cell lines and clinical AML samples at concentrations that had negligible impact on normal myeloid progenitors.
Conclusions
These results not only provide evidence for cytarabine-induced S phase checkpoint activation in AML in the clinical setting, but also show that a selective Chk1 inhibitor can overcome the S phase checkpoint and enhance the cytotoxicity of cytarabine. Accordingly, further investigation of the cytarabine/SCH 900776 combination in AML appears warranted.
INTRODUCTION
Cytarabine is the single most active agent available for the treatment of acute myelogenous leukemia (AML), particularly in younger patients, and contributes to the high complete remission (CR) rate in this disorder (1, 2). Nonetheless, the majority of AML patients relapse and ultimately die of refractory disease (1–3). As a result, there is considerable interest in identifying mechanisms that contribute to cytarabine resistance and strategies for overcoming this resistance.
A number of mechanisms of cytarabine resistance have been described, including diminished cytarabine import, increased cytarabine degradation, decreased formation or retention of cytarabine triphosphate, or reduced incorporation into DNA if the cells are not cycling. In addition, more recent reports suggest that signaling by the replication checkpoint also contributes to cytarabine resistance (4–7). This checkpoint is activated when replication inhibitors, including cytarabine (4–8), cause DNA polymerases to stall but allow DNA helicases to advance, creating regions of single-stranded DNA that contribute to activation of the ATR (ataxia telangiectasia mutated and Rad3 related) kinase (9–11). Once activated, ATR phosphorylates and activates checkpoint kinase 1 (Chk1, Fig. 1A), which in turn phosphorylates the phosphatase Cdc25A, leading to Cdc25A degradation and cell cycle arrest (12, 13). In addition, Chk1 stabilizes stalled replication forks, activates DNA repair, and suppresses apoptosis (12, 14).
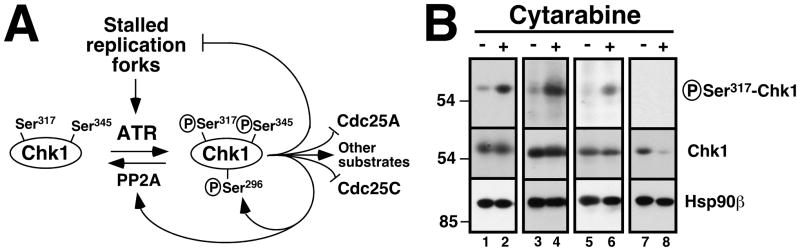
Figure 1. Effect of cytarabine on Chk1 phosphorylation in clinical AML samples in vivo.
A, schematic of the ATR/Chk1 pathway. In response to stalled replication forks, ATR catalyzes the activating phosphorylation of Chk1 on Ser317 and Ser345. Chk1 in turns phosphorylates a number of substrates, including Cdc25A (which contributes to S phase progression but is degraded after phosphorylation), Cdc25C (which contributes to G2/M progression but is inhibited by phosphorylation), substrates that stabilize replication forks, a substrate leading to phosphatase PP2A activation (which leads to Chk1 dephosphorylation), and Chk1 Ser296, an autophosphorylation site. B, marrow mononuclear cells were obtained from patients 6 (lanes 1, 2), 8 (lanes 3, 4), 12 (lanes 5, 6) and 2 (lanes 7, 8) in Cohort 1 before treatment (−) and after 48 h of single-agent cytarabine (+) at 400 mg/m2/day by continuous infusion. All samples shown contained >80% blasts (median 91%). Whole cell lysates were prepared from each fresh sample, subjected to SDS-PAGE, transferred to nitrocellulose, and probed with antibodies that recognize the activating phosphorylation of Chk1 on Ser317 or total Chk1. Hsp90β served as a loading control.
Consistent with these observations, previous studies have shown that Chk1 downregulation enhances the cytotoxicity of cytarabine and other nucleoside analogues (4, 6, 7, 15). These observations provide part of the rationale for the preclinical and early clinical development of Chk1 inhibitors (14, 16, 17). It is important to emphasize, however, that activation of the ATR/Chk1 pathway has not been previously demonstrated in clinical AML during cytarabine therapy.
Two strategies for overcoming replication checkpoint-mediated cytarabine resistance have been previously investigated. Based on the observation that Chk1 requires chaperoning by heat shock protein 90 (Hsp90) to assume an active conformation (18, 19), cytarabine has been combined with the Hsp90 inhibitor tanespimycin. Although tanespimycin enhances the cytotoxicity of cytarabine in AML cell lines and clinical isolates in vitro (6), the combination has proven difficult to administer in vivo because of severe toxicities (20) that likely reflect the effects of tanespimycin on multiple Hsp90 clients in normal tissues. Alternatively, cytarabine has been combined with UCN-01 in vitro and in the clinical setting (21, 22). UCN-01 inhibits Chk1 (23, 24) and a number of other kinases (25), including Chk2 and phosphoinositide-dependent kinase 1 (26, 27), but has also been difficult to develop clinically because of toxicities in normal tissues, possibly reflecting the effect of inhibiting divers kinases. More recently described Chk1 inhibitors also inhibit Chk2 and, in some cases, cyclin-dependent kinases (28). Because cyclin-dependent kinases contribute to the cell cycle progression required for lethal cytarabine incorporation into DNA (29, 30) and Chk2 contributes to apoptosis (31), concern has been raised that these inhibitors might not be as effective at sensitizing cells to replication stress as more selective Chk1 inhibitors (32).
SCH 900776 is a recently described inhibitor that is highly selective for Chk1 relative to Chk2 and cyclin dependent kinases (32). Additional studies have shown that SCH 900776 enhances the cytotoxicity of hydroxyurea and gemcitabine in vitro and in vivo without increasing normal tissue toxicities (32). To determine whether there might be a rationale for combining SCH 900776 with cytarabine in AML, the present study first assessed whether the replication checkpoint is activated during cytarabine infusion in the clinical setting and then examined the effect of combining SCH 900776 with cytarabine in human AML cell lines and primary clinical specimens in vitro.
MATERIALS AND METHODS
Materials
SCH 900776 was synthesized as described (32). Additional reagents were purchased as follows: cytarabine and propidium iodide (PI) from Sigma-Aldrich; Q-VD-OPh from SM Biochemicals (Anaheim, CA); mouse monoclonal anti-Chk1 from Santa Cruz Biotechnology; mouse monoclonal anti-phospho-Ser139-histone H2AX from Millipore; rabbit polyclonal anti-phospho-Ser317-Chk1 from R & D Systems; and rabbit polyclonal anti-GAPDH, phospho-Ser296-and phospho-Ser345-Chk1 from Cell Signaling Technology. Murine monoclonal antibodies to heat shock protein 90β (Hsp90β) and poly(ADP-ribose) polymerase 1 (PARP1) were gifts from David Toft (Mayo Clinic, Rochester, MN) and Guy Poirier (Laval University, Ste-Foy, QC, Canada), respectively.
Leukemia samples
After informed consent was obtained under the aegis of Institutional Review Board-approved protocols, bone marrow aspirates were acquired from two cohorts of AML patients. FAB classification and karyotype were determined in both cohorts by standard techniques (20).
Cohort 1 consisted of adult patients with relapsed or refractory acute leukemia who received therapy on a trial of sequential cytarabine and tanespimycin (20). Bone marrow aspirates were harvested from these patients prior to therapy and again after 48 h of treatment with cytarabine, which was administered by continuous infusion at 400 mg/m2/day. Because this second sample was obtained prior to tanespimycin administration, the effects observed reflect the action of cytarabine alone. At the time of harvest, mononuclear cells were isolated on ficoll-Hypaque gradients, washed with ice cold serum-free RPMI 1640 medium, and immediately lysed in buffered guanidine hydrochloride under reducing conditions for SDS-PAGE (33).
Cohort 2 consisted of patients with newly diagnosed or relapsed aggressive myeloid disorders who underwent bone marrow aspiration prior to induction therapy. Immediately after isolation, mononuclear cells from these patients were washed with RPMI 1640 medium, resuspended at 1.5 × 106 cells/ml in Iscove’s modified Dulbecco’s medium containing 20% (vol/vol) heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin G, 100 μg/ml streptomycin, and 2 mM glutamine, and plated at 600,000 cells/plate in MethoCult® methylcellulose (StemCell Technologies, Vancouver, British Columbia) containing the indicated concentrations of cytarabine and SCH 900776. Leukemic colonies were counted after a 14-day incubation (6). Peripheral blood mononuclear cells from normal volunteers were assayed in the same fashion except that normal erythroid and myeloid colonies were counted (34, 35).
Cell culture
HL-60, U937 (American Type Culture Collection, Manassas, VA) and ML-1 cells (a kind gift from Michael Kastan, St. Jude Children’s Hospital, Memphis, TN) were maintained in RPMI 1640 containing 10% FBS, 100 U/ml penicillin G, 100 μg/ml streptomycin, and 2 mM glutamine (medium A) at concentrations below 1 × 106 cells/ml at all times. All lines were passaged less than three months before use. Aliquots were diluted to 3–4 × 105 cells/ml in medium A and treated with cytarabine and SCH 900776 (both added from 1000X stocks in DMSO) or diluent as described for specific assays below.
Replication checkpoint assay
Quadruplicate 100 μl aliquots were treated for 3–4 h with cytarabine, SCH 900776 or the combination, incubated for 20 min with 1 μM 3H-thymidine, and harvested by transferring to glass filters using a Skatron semiautomatic harvester (Skatron As., Lier, Norway). The filter-bound cells were lysed with distilled water. Filter-bound radioactivity was determined by liquid scintillation counting. 3H-thymidine incorporation was calculated as the ratio of drug-treated to diluent (0.2% DMSO)-treated control samples.
Cell cycle analysis
After incubation for 24 h with cytarabine in the absence or presence of SCH 900776 as indicated in the figures, cells were resuspended in 20 mM HEPES, pH 7.4, 120 mM NaCl, 0.025% Triton X-100, 50 μg/mL RNAse A, and 20 μg/mL PI; incubated at 37 °C for 30 min; and analyzed by flow microfluorimetry. Alternatively, cells were lysed in ice cold buffer consisting of 0.1% (wt/vol) sodium citrate, 0.1% (wt/vol) Triton X-100 and 50 μg/ml PI; incubated overnight at 4 °C; and analyzed. No gating was used to generate the histograms.
Immunoblotting
U937 cells were exposed to the indicated concentrations of cytarabine and SCH 900776 for 4 h, washed with PBS, and lysed in 2X SDS-PAGE sample buffer (1 × 107 cells/mL). Lysates (2 × 105 cells/lane) were separated by SDS-PAGE, transferred to Immobilon P, and blotted for the indicated antigens. Alternatively, after treatment with drug or diluent for 24 h, cells were washed once with ice-cold RPMI 1640 medium containing 10 mM HEPES (pH 7.4 at 4°C), and solubilized in buffered 6 M guanidine hydrochloride under reducing conditions (33). Aliquots containing 50 μg protein (determined by the Thermo bicinchoninic acid method) were separated by SDS-PAGE, electrophoretically transferred to nitrocellulose, and probed as described (36).
Additional assays
Assays for apoptotic morphological changes and for the ability of AML cell lines to form colonies in 0.3% agar were performed as described (6).
RESULTS
Replication checkpoint activation in human AML during cytarabine infusion
Although replication checkpoint activation was previously observed in leukemia cell lines treated with nucleoside analogues in vitro, activation of the ATR/Chk1 pathway has not, to our knowledge, been previously evaluated in bone marrow blasts during drug treatment in patients. To address this issue, paired bone marrow aspirates harvested from patients in Cohort 1 prior to therapy and after 48 h of treatment with single-agent cytarabine (Table S1) were immediately upon receipt prepared for immunoblotting, which was performed with antibodies that detect phosphorylation of Chk1 on Ser317, one of two sites phosphorylated by ATR during replication stress (Fig. 1A and ref. 37). If cytarabine were activating the replication checkpoint in vivo, then elevated phosphorylation of Chk1 on Ser317 would be expected during the course of the cytarabine infusion. Among 12 paired samples, three failed to express Chk1 (Table S1). Five of the nine remaining pairs exhibited increased Chk1 phosphorylation after 48 h of single-agent cytarabine infusion (Table S1; Fig. 1B, lanes 2, 4 and 6) and another pair had a marked decrease in total Chk1 on day 3 (Fig. 1B, lane 8), possibly reflecting activation-induced Chk1 degradation (38). Collectively, these results confirm that the Chk1-dependent replication checkpoint is activated during cytarabine administration in over half of AMLs that detectably express Chk1.
SCH 900776 inhibits Chk1, abrogates cytarabine-induced cell cycle arrest and enhances cytarabine-induced killing of human AML cell lines
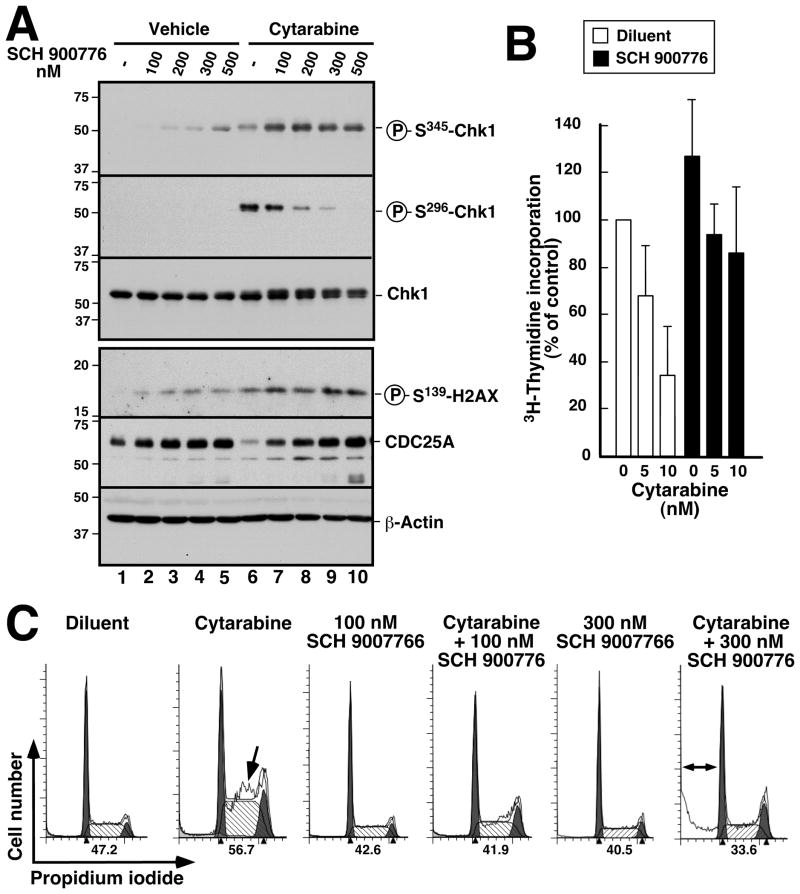
Subsequent experiments examined the effect of combining cytarabine with the Chk1 inhibitor SCH 900776 (32). As was the case with the clinical AML samples, treatment of U937 cells with cytarabine resulted in increased ATR-mediated Chk1 phosphorylation (phospho-Ser345-Chk1, Fig. 2A, lane 6 vs. lane 1). This was accompanied by enhanced phosphorylation of Chk1 at Ser296, an autophosphorylation site, and diminished levels of the cell cycle regulator Cdc25A, which is targeted for proteolysis by Chk1-mediated phosphorylation (Fig. 1A and 2A, lane 6 vs. lane 1). Addition of SCH 900776 decreased the cytarabine-induced Chk1 autophosphorylation at Ser296 and prevented Cdc25A downregulation (Fig. 2A, lanes 7–10 vs. lane 6), suggesting that SCH 900776 was disrupting the replication checkpoint. Consistent with this explanation, SCH 900776 also reversed the cytarabine-induced inhibition of 3H-thymidine incorporation into DNA (Fig. 2B). In addition, the S-phase accumulation of cells induced by a 24-h cytarabine exposure was reduced by co-treatment with 100 nM or 300 nM SCH 900776 even though SCH 900776 had no effect on cell cycle distribution when administered by itself (Fig. 2C). SCH 900776 also increased phosphorylation of Chk1 at Ser345 (Fig. 2A, lanes 7–10 vs. 6), an effect that has been attributed to inhibition of PP2A-mediated dephosphorylation at that site (39) as well as increased DNA damage-induced signaling caused by incomplete replication fork stabilization, aberrant origin firing and/or override of the replication checkpoint (32). Concomitantly, SCH 900776 also induced increased phosphorylation of H2AX, a marker of DNA damage (Fig. 2A, lanes 7–10 vs. 6).
Figure 2. Effect of SCH 900776 on cytarabine-induced replication checkpoint activation.
A, U937 cells were treated for 4 h with the indicated concentrations of SCH 900776 in the presence of diluent (lanes 1–5) or 50 nM cytarabine (lanes 6–10), solubilized in SDS sample buffer, subjected to SDS-PAGE and probed with antibodies to the indicated antigens. β-Actin served as a loading control. B, U937 cells were treated for 4 h with the indicated concentration of cytarabine in the absence or presence of 100 nM SCH 900776, with 3H-thymidine added for the last 20 min. At the completion of the incubation, incorporation of radiolabel into DNA was assayed. Error bars, ± SD of 3 independent experiments. C, U937 cells were treated for 24 h with diluent (0.2% DSMO), 50 nM cytarabine, 100 or 300 nM SCH 900776 or 50 nM cytarabine + SCH 900776 as indicated, stained with PI and subjected to flow microfluorimetry. Arrow, increased S phase population in cells treated with cytarabine alone (see also ref. 6). Double arrow, subdiploid population after cytarabine + SCH 900776. Numbers below each histogram indicate percentage of S phase cells observed in each sample. Additional analysis of DNA fragmentation in U937 cells is contained in Fig. S1.
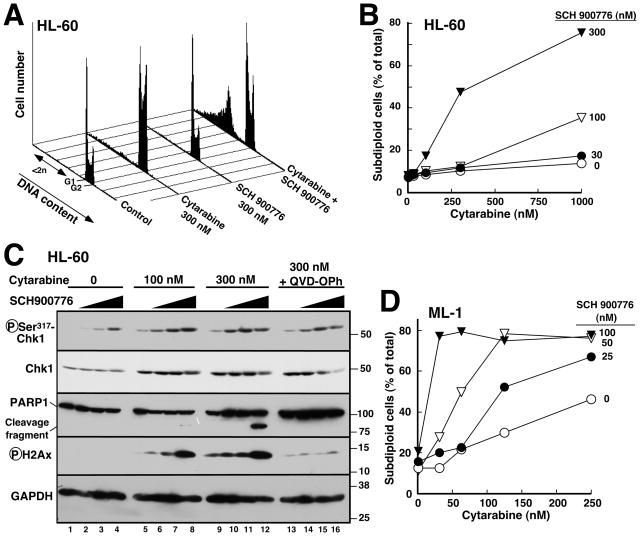
We have previously shown that disruption of the replication checkpoint by gene deletion or RNA interference increases the cytotoxicity of replication stress (5–7, 15). Consistent with these results, treatment with cytarabine and 300 nM SCH 900776 produced an increased number of U937 cells with “subdiploid” DNA content, a feature of apoptosis, compared to either agent alone (Figs. 2C, S1A and S1B). Further experiments demonstrated similar effects in several different AML cell lines, including p53-deficient HL-60 (Fig. 3A, B) and KG1a cells (Fig. S1C) as well as p53 wildtype ML-1 cells (Fig. 3D). Additional assays demonstrated increased numbers of cells with apoptotic morphology (K.S.F. and S.H.K., unpublished observations) and enhanced PARP1 cleavage (Fig. 3C, 3rd panel), a hallmark of caspase activation, confirming increased apoptosis in cells treated with cytarabine + SCH 900776 compared to cytarabine alone. Importantly, however, SCH 900776 was unable to enhance cytarabine-induced apoptosis in K562 cells (Fig. S1D), which are apoptosis-resistant because of Bcr/abl-mediated changes in apoptotic pathways (40) that inhibit processes downstream of DNA damage.
Figure 3. SCH 900776 enhances cytarabine-induced apoptosis in human AML cell lines.
A, HL-60 cells were treated for 24 h with diluent (0.2% DMSO), 300 nM cytarabine, 300 nM SCH 900776 or 300 nM cytarabine + 300 nM SCH 900776. After staining with PI, flow cytometry was performed as indicated in the METHODS. B, percentage of events with <2n DNA content calculated from histograms in panel A and additional samples from the same experiment. C, HL-60 cells were treated for 24 h with the indicated concentrations of cytarabine in the presence of diluent (lanes 1, 5, 9 and 13) or SCH 900776 at 100 (lanes 2, 6, 10 and 14), 300 (lanes 3, 7, 11 and 15) or 1000 nM (lanes 4, 8, 12 and 16). Samples in lanes 13–16 also contained the caspase inhibitor Q-VD-OPh at 5 μM. At the completion of the incubation, cells were lysed in 6 M guanidine hydrochloride under reducing conditions, prepared for SDS-PAGE and subjected to immunoblotting with antibodies to the indicated antigens. D, percentage of subdiploid events observed with ML-1 treated for 24 h with the indicated concentrations of cytarabine and SCH 900776.
At the 24-h time point, the cytarabine-induced increase in ATR-mediated Chk1 Ser317 phosphorylation also remained evident (Fig. 3C, top panel, lanes 1, 5 and 9); and this phosphorylation (like the phosphorylation of Chk1 Ser345 in U937 cells) was increased further by SCH 900776 (Fig. 3C, lanes 6–8 and 10–12). Phosphorylation of H2AX was also increased by combining cytarabine and SCH 900776 (Fig. 3C, 4th panel). Interestingly, the H2AX phosphorylation observed after 24 h of drug treatment, like PARP1 cleavage, was markedly diminished by addition of the caspase inhibitor (41) Q-VD-OPh (Fig. 3C, lanes 13–16 vs. 9–12), suggesting that some of the double strand breaks observed at late time points reflect induction of apoptosis.
SCH 900776 selectively enhances effects of cytarabine on AML cell colony formation
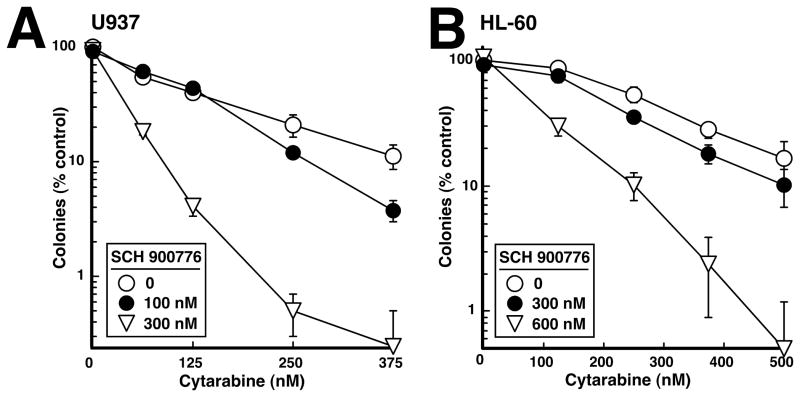
In view of recent results showing that Chk1 inhibition accelerates cisplatin-induced apoptosis without affecting the number of cells ultimately killed (42, 43), we next examined the long-term impact of the cytarabine/SCH 900776 combination using colony forming assays. When U937 (Fig. 4A) or HL-60 cells (Fig. 4B) were exposed to the individual agents or the combination for 24 h, washed, and plated in soft agar, SCH 900776 had no effect on colony formation by itself but nonetheless enhanced the long-term antiproliferative effects of cytarabine in these assays, meeting the definition of synergy (44).
Figure 4. Effect of SCH 900776 on colony formation in human AML cell lines.
U937 (A) or HL-60 (B) were treated for 24 h with diluent or the indicated concentration of SCH 900776 in the absence or presence of the indicated concentration of cytarabine, washed and plated in 0.3% agar. After 10–12 days, colonies containing >50 cells were counted at low magnification. Error bars, ± SD of quadruplicate aliquots. Similar results were obtained in three independent experiments.
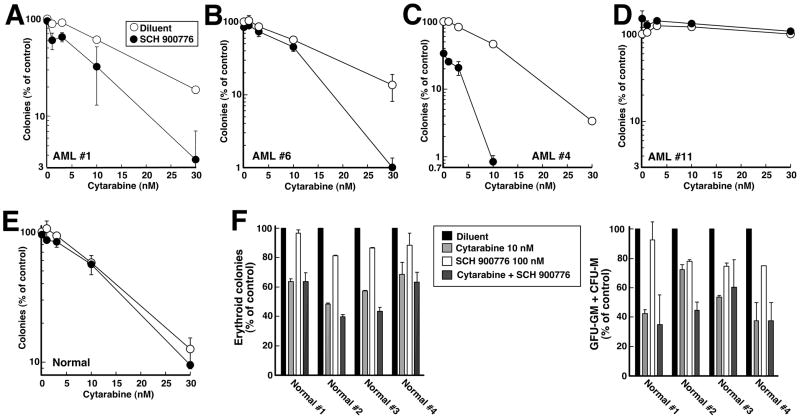
To determine whether similar effects would be observed in primary clinical isolates from patients with myeloid malignancies, leukemic cells from patients in Cohort 2 (Table 1) were exposed to cytarabine ± SCH 900776 or diluent continuously during colony formation in methylcellulose. Four patterns of response were observed (Fig. 5A–D). In some samples, SCH 900776 had no effect by itself but nonetheless enhanced the effects of cytarabine at all concentrations (Fig. 5A) or at the higher concentrations examined (Fig. 5B). In a few samples (Fig. 5C), SCH 900776 not only enhanced the effects of cytarabine, but also inhibited colony formation by itself by 40–70%. In total, sensitization similar to that shown in Figs. 5A–5C was observed in 10 of 14 clinical specimens analyzed (Tables 1 and S2). In contrast, SCH 900776 failed to sensitize 4 of 14 specimens to cytarabine (Fig. 5D, Tables 1 and S2).
Table 1.
Effect of SCH 900776 on Cytarabine Sensitivity in Samples from Cohort 2
| Patient # | FAB | Age | Karyotype | Prior hemato-logical disorder | FLT3 mutation status | # prior regimens/prior cytarabine | Response to most recent Rx | Sensitive to SCH 900776 alonea,c | Sensitized to cytarabineb,c |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M5 AML | 79 | 46 XY | CLLd | D835S | 0 | − | + | |
| 2 | M2 AML | 53 | 46 XY | D835S | 0 | − | + | ||
| 3 | M1 AML | 56 | 46 XX | ITD | 3/Ye | Refractory | − | − | |
| 4 | M5 AML | 73 | N.D.f | wt | 0 | + | + | ||
| 5 | CMML | 69 | 46 XY | wt | 1/N | - | + | + | |
| 6 | M2 AML | 33 | Complex | wt | 0 | − | + | ||
| 7 | M1 AML | 65 | Complex | wt | 1/Y | Refractory | − | − | |
| 8 | AML, nos | 63 | Complex | MMM | wt | 0 | − | + | |
| 9 | CMML | 66 | 46 XY + 7q | wt | 2/N | Refractory | − | + | |
| 10 | M0 AML | 48 | 46 XX t(12;17) | wt | 1/Y | Refractory | − | + | |
| 11 | M1 AML | 78 | Complex | wt | 0 | − | − | ||
| 12 | AML, nos | 65 | 46 XX | MPN | wt | 1/N | - | − | + |
| 13 | M1 AML | 50 | Complex | wt | 3/Y | Refractory | − | − | |
| 14 | M6 AML | 38 | 46 XY | wt | 0 | − | + |
Samples were defined as sensitive to single-agent SCH 900776 (+) if colony formation was inhibited by ≥40% at 100 nM SCH 900776.
−, not sensitized to cytarabine by SCH 900776.
Quantitative effects of cytarabine, SCH 900776 and the combination are presented in Table S2.
CLL, chronic lymphocytic leukemia; MMM, myelofibrosis with myeloid metaplasia, MPN, myeloproliferative neoplasm, not otherwise specified.
Y indicates treatment of the patient with a cytarabine-containing regimen at some point prior to sample acquisition. N indicates no prior cytarabine exposure.
N.D., not determined. “Complex” indicates the presence of three or more numerical and/or structural abnormalities.
Figure 5. Effect of SCH 900776 and cytarabine on colony formation in primary specimens.
A–D, marrow mononuclear cells from Cohort 2 AML patients 1, 6, 4 and 11 (Table 1) were plated in cytokine-containing Methocult® methylcellulose in the presence of diluent (0.1% DMSO) or 100 nM SCH 900776 and the indicated concentration of cytarabine. Leukemic colonies were counted as previously described (6). E, peripheral blood mononuclear cells from a normal volunteer were assayed for formation of erythroid and myeloid colonies (35), which were combined in this graph. Similar results were observed in samples from three additional normal volunteers. F, effects of 10 nM cytarabine (light grey bars), 100 nM SCH 900776 (white bars) or 10 nM cytarabine + 100 nM SCH 900776 (dark grey bars) on formation of erythroid (left) and myeloid (right) colonies in each of four normal volunteers. Error bars, range of duplicate aliquots at each drug concentration.
Based on the observation that SCH 900776 increases the antineoplastic effects of gemcitabine without enhancing myelosuppression in mice in vivo (32), we also assessed the effect of SCH 900776 on normal myeloid progenitors. In contrast to leukemic samples, progenitors from four normal volunteers exhibited little or no sensitization by SCH 900776 (Fig. 5E and 5F), raising the possibility of a therapeutic window for administering this agent with cytarabine.
In further studies, the relationship between sensitization by SCH 900776 and various features of the malignant myeloid samples was examined in a preliminary fashion. All samples were CD34 positive, reflecting their immature phenotype. As indicated in Table 1, the presence of activating FLT3 mutations did not appear to affect sensitization by SCH 900776, although evaluation of a larger cohort is required to reach a definitive conclusion. The presence of a complex karyotype did not preclude sensitization by SCH 900776, although sensitization was observed in only 2 of 5 samples with a complex karyotype versus 7 of 9 samples without. Importantly, 9 of 10 samples without prior cytarabine exposure were sensitized to cytarabine by addition of SCH 900776. In contrast, only 1 of 4 specimens from patients with prior cytarabine exposure was sensitized (p = 0.041 by Fisher’s exact test); and that sensitization (patient 10) was modest, raising the possibility that prior cytarabine exposure might affect the ability of SCH 900776 to enhance cytarabine sensitivity.
DISCUSSION
Results of the present study demonstrate for the first time that Chk1 undergoes activating phosphorylation in marrow blasts in vivo during cytarabine-containing induction therapy. Building on this result, we also show in human AML cell lines that the selective Chk1 inhibitor SCH 900776 abrogates cytarabine-induced S phase arrest, increases cytarabine-induced apoptosis, and enhances the effects of cytarabine on colony formation. Likewise, SCH 900776 increases the effects of cytarabine in a majority of primary AML isolates, but not normal myeloid progenitors, in vitro. This sensitization was observed at SCH 900776 concentrations far below the ~5 μM SCH 900776 peak levels observed at the maximum tolerated dose in solid tumor patients. These observations have potentially important implications for current efforts to enhance the efficacy of cytarabine-containing AML regimens.
Previous results have shown that cytarabine activates the ATR/Chk1 checkpoint in tissue culture cell lines in vitro (4–7). To determine whether clinically achievable cytarabine concentrations also activate this checkpoint in vivo, whole cell lysates were prepared from marrow aspirates after 48 h of exposure to single-agent cytarabine. This study utilized whole cell lysates to minimize the possibility of inadvertent Chk1 dephosphorylation during cell fractionation; marrow aspirates rather than circulating blasts to maximize the possibility of collecting cells that express Chk1 and incorporate cytarabine into DNA, both of which occur predominantly in S phase; and samples from a unique trial in which cytarabine was administered for 48 h without any anthracycline, thereby eliminating the potential confounding effect of a second agent. Using this approach, we observed that three of 12 paired leukemia samples lacked detectable Chk1 at baseline (Table S1), consistent with our earlier results (45). Because previous studies have shown that Chk1 expression occurs predominantly during S phase (46) and correlates strongly with the proliferation marker PCNA in AML samples (45), it is likely that the lack of detectable Chk1 in these samples reflected a low proliferative fraction. Among the remaining 9 pairs, we observed readily detectable Chk1 phosphorylation at baseline in 7 (Table S1), consistent with observations that the ATR/Chk1 pathway is activated and contributes to survival during normal replication (10). After 48 h of cytarabine infusion, we also observed readily detectable increases in Chk1 phosphorylation in 5 of 9 paired samples that expressed Chk1 as well as decreased Chk1 in an additional sample (Fig. 1B and Table S1), suggesting that the replication checkpoint is activated in at least some leukemias during cytarabine treatment in the clinical setting.
No effect of cytarabine on Chk1 phosphorylation was observed in the other three leukemias despite the presence of detectable Chk1. Because this failure to detectably activate the ATR/Chk1 pathway in response to cytarabine did not appear to correlate with FAB classification or number of prior cytarabine-containing regimens (Table S1), further study in a larger cohort is required to better define which AMLs activate this checkpoint during treatment and which do not. Likewise, further study is required to determine whether this checkpoint is activated during cytarabine/anthracycline combination therapy, although the observation that anthracyclines activate the ATR/Chk1/Cdc25A pathway (47, 48) certainly makes this likely.
A number of previous publications have shown that deletion or downregulation of components of the replication checkpoint, including Rad9, ATR or Chk1, enhances the cytotoxicity of cytarabine in various cell lines in vitro (5–7). Based on these and additional observations, there have also been several attempts to abrogate this checkpoint in the clinical setting. UCN-01, which inhibits Chk1 (23, 24) and enhances the antiproliferative effects of a number of nucleoside analogs, including cytarabine, in vitro (8, 22, 49), was administered in one such attempt. Unfortunately, UCN-01 had a number of serious drawbacks in the clinic, including a long serum half-life that complicated dosing and severe toxicities when added to other chemotherapeutic agents, possibly reflecting inhibition of a large number of additional kinases (25–27). Likewise, when cytarabine was combined with tanespimycin, which inhibits Hsp90 and thereby prevents folding of catalytically competent Chk1 (19), the combination exhibited severe toxicities in patients with AML (20). Importantly, however, tanespimycin induced little downregulation of Hsp90 client proteins in bone marrow blasts in situ at clinically tolerable concentrations (20), making it impossible to assess the impact of Chk1 downregulation on cytarabine efficacy.
The third-generation Chk1 inhibitor SCH 900776 has several advantages over previous agents used to modulate the replication checkpoint. In contrast to the broad effects of UCN-01 (25), SCH 900776 exhibits selectivity for Chk1 among the ~50 kinases examined (32). Indeed, checkpoint override assays suggest that SCH900776 is selective for Chk1 in cells at concentrations up to 30 μM (32). Moreover, unlike the checkpoint kinase inhibitor AZD7762, SCH 900776 exhibits little effect on the pro-apoptotic kinase Chk2 (32). As is the case with any small molecule inhibitor, however, we cannot rule out the possibility that inhibition of additional kinases contributes to the observed effects of SCH 900776.
In the present study, SCH 900776 diminished cytarabine-induced replication checkpoint activation (Fig. 2) and increased cytarabine-induced apoptosis (Figs. 3 and S1) in AML cell lines. This enhanced killing was observed in cells with wildtype (ML-1), mutant (U937) or absent p53 (HL-60). These effects were accompanied by increased formation of phospho-Ser139-histone H2AX (Figs. 2A and 3C), a commonly used marker of DNA double-strand breaks. While the increased H2AX phosphorylation observed at short time points (e.g., Fig. 2A) likely reflected replication fork collapse that accompanies Chk1 inhibition (32), the increased H2AX phosphorylation seen at later time points paralleled caspase-mediated PARP1 cleavage and was markedly diminished by caspase inhibition (Fig. 3C). The possibility that two distinct processes, replication fork collapse and apoptosis, can contribute to increased H2AX phosphorylation raises an important caution when considering H2AX phosphorylation for inclusion as a pharmacodynamic marker of Chk1 inhibition in future preclinical and clinical studies.
Because the typical AML sample contains only 0.5–5% of cells in S phase, a similar biochemical and cell cycle analysis of the effects of SCH 900776 on cytarabine-induced S phase progression was not feasible using clinical AML samples. Instead, further experiments utilized colony forming assays to examine the effects of the cytarabine/SCH 900776 combination. These studies demonstrated that SCH 900776 enhanced the effects of cytarabine on colony formation by human AML cell lines (Fig. 4) as well as primary AML isolates grown ex vivo (Fig. 5, Tables 1 and S2). Whether this enhancement will be observed under different conditions, e.g., when leukemia cells are adherent to stroma or in xenografts, remains to be determined in future studies. Importantly, normal myeloid progenitors were affected far less by the addition of SCH 900776 (Fig. 5E), suggesting the possibility of a therapeutic window. While these studies were in progress, Guzi et al. reported that SCH 900776 also enhances the antineoplastic effects of gemcitabine in vitro and in human tumor xenografts without appreciably altering the normal tissue toxicities of gemcitabine (32). This selectivity for neoplastic cells, which is also seen with siRNA-mediated knockdown of Chk1, is thought to reflect redundancy in the replication checkpoint in normal cells (7, 50). In view of these results, further studies of the SCH 900776/cytarabine combination appear warranted.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Preclinical studies demonstrated that activation of the replication checkpoint, which involves the sequential action of the kinases ATR and Chk1, contributes to cell cycle arrest and survival of AML cells after cytarabine treatment. Whether this checkpoint is activated during clinical cytarabine treatment and whether checkpoint abrogation will enhance cytarabine cytotoxicity in clinical AML was unknown. To address these questions, sequential marrow aspirates were assayed for ATR-mediated Chk1 phosphorylation. In addition, AML lines, clinical AML isolates and normal myeloid progenitors were examined after treatment with cytarabine ± SCH 900776, a selective Chk1 inhibitor. Results of this analysis not only provide the first evidence for replication checkpoint activation in AML at clinically achievable cytarabine exposures in some patients, but also indicate that Chk1 inhibition can selectively enhance the antiproliferative effects of cytarabine in AML in vitro. These observations suggest that further study of Chk1 inhibitors in combination with cytarabine is warranted.
Acknowledgments
We thank Susan Arbuck and Randi Isaacs for facilitating this project, David Toft and Guy Poirier for reagents, and Deb Strauss for secretarial assistance.
GRANT SUPPORT
This work was supported in part by \U01 CA69912, U01 CA70095, P30 CA06973, R01 CA84321, a Translational Research Grant from the Leukemia & Lymphoma Society of America and educational funds from the Mayo Foundation, including the M.D. Ph.D. Program (ELS) and Clinician Investigator Training Program (BK).
Footnotes
DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
D.P. was an employee of Schering-Plough Biopharma when SCH 900776 was developed. The other authors have no conflict of interest.
AUTHOR CONTRIBUTIONS
Study design: J.E.K. and S.H.K.
Experiments and interpretation of data: E.L.S., B.D.K., K.S.F., K.L.P., L.M.K., S.H.K.
Critical reagents: D.P., A.D.H. and B.D.S.
Writing and editing the manuscript: E.L.S., B.D.K., K.S.F., K.L.P., D.P., A.D.H., B.D.S., J.E.K., L.M.K. and S.H.K.
References
- 1.Tallman MS, Gilliland DG, Rowe JM. Drug therapy of acute myeloid leukemia. Blood. 2005;106:1154–63. doi: 10.1182/blood-2005-01-0178. [DOI] [PubMed] [Google Scholar]
- 2.Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29:487–94. doi: 10.1200/JCO.2010.30.1820. [DOI] [PubMed] [Google Scholar]
- 3.Robak T, Wierzbowska A. Current and emerging therapies for acute myeloid leukemia. Clin Ther. 2009;31(Pt 2):2349–70. doi: 10.1016/j.clinthera.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001;21:4129–39. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loegering D, Arlander SAH, Hackbarth J, Vroman B, Lieberman HB, Karnitz LM, et al. Rad9 protects cells from topoisomerase poison-induced cell death. J Biol Chem. 2004;279:18641–7. doi: 10.1074/jbc.M313536200. [DOI] [PubMed] [Google Scholar]
- 6.Mesa RA, Loegering D, Powell HL, Flatten K, Arlander SAH, Dai NT, et al. Heat shock protein 90 inhibition sensitizes acute myelogenous leukemia cells to cytarabine. Blood. 2005;106:318–27. doi: 10.1182/blood-2004-09-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho SH, Toouli CD, Fujii GH, Crain C, Parry D. Chk1 is essential for tumor cell viability following activation of the replication checkpoint. Cell Cycle. 2005;4:131–9. doi: 10.4161/cc.4.1.1299. [DOI] [PubMed] [Google Scholar]
- 8.Shi Z, Azuma A, Sampath D, Li YX, Huang P, Plunkett W. S-Phase arrest by nucleoside analogues and abrogation of survival without cell cycle progression by 7-hydroxystaurosporine. Cancer Res. 2001;61:1065–72. [PubMed] [Google Scholar]
- 9.Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–8. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 10.Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9:616–27. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zegerman P, Diffley JF. DNA replication as a target of the DNA damage checkpoint. DNA Repair (Amst) 2009;8:1077–88. doi: 10.1016/j.dnarep.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Paulsen RD, Cimprich KA. The ATR pathway: fine-tuning the fork. DNA Repair (Amst) 2007;6:953–66. doi: 10.1016/j.dnarep.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Wagner JM, Kaufmann SH. Prospects for the use of ATR inhibitors to treat cancer. Pharmaceuticals. 2010;3:1311–34. doi: 10.3390/ph3051311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai Y, Grant S. New insights into checkpoint kinase 1 in the DNA damage response signaling network. Clinical Cancer Research. 2010;16:376–83. doi: 10.1158/1078-0432.CCR-09-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karnitz LM, Flatten KS, Wagner JM, Loegering D, Hackbarth JS, Arlander SJ, et al. Gemcitabine-induced activation of checkpoint signaling pathways that affect tumor cell survival. Mol Pharmacol. 2005;68:1636–44. doi: 10.1124/mol.105.012716. [DOI] [PubMed] [Google Scholar]
- 16.Ashwell S, Janetka JW, Zabludoff S. Keeping checkpoint kinases in line: new selective inhibitors in clinical trials. Expert Opin Investig Drugs. 2008;17:1331–40. doi: 10.1517/13543784.17.9.1331. [DOI] [PubMed] [Google Scholar]
- 17.Ma CX, Janetka JW, Piwnica-Worms H. Death by releasing the breaks: CHK1 inhibitors as cancer therapeutics. Trends Mol Med. 2011;17:88–96. doi: 10.1016/j.molmed.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arlander SJH, Eapen AK, Vroman BT, McDonald RJ, Toft DO, Karnitz LM. Hsp90 inhibition depletes Chk1 and sensitizes tumor cells to replication stress. J Biol Chem. 2003;278:52572–7. doi: 10.1074/jbc.M309054200. [DOI] [PubMed] [Google Scholar]
- 19.Arlander SJ, Felts SJ, Wagner JM, Stensgard B, Toft DO, Karnitz LM. Chaperoning checkpoint kinase 1 (Chk1), an Hsp90 client, with purified chaperones. Journal of Biological Chemistry. 2006;281:2989–98. doi: 10.1074/jbc.M508687200. [DOI] [PubMed] [Google Scholar]
- 20.Kaufmann SH, Karp JE, Litzow MR, Mesa RA, Hogan W, Steensma DP, et al. Phase I and pharmacological study of cytarabine and tanespimycin in relapsed and refractory acute leukemia. Haematologica. 2011;96:1619–26. doi: 10.3324/haematol.2011.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S, Wang Z, Grant S. Bryostatin 1 and UCN-01 potentiate 1-beta-D-arabinofuranosylcytosine-induced apoptosis in human myeloid leukemia cells through disparate mechanisms. Mol Pharmacol. 2003;63:232–42. doi: 10.1124/mol.63.1.232. [DOI] [PubMed] [Google Scholar]
- 22.Sampath D, Cortes J, Estrov Z, Du M, Shi Z, Andreeff M, et al. Pharmacodynamics of cytarabine alone and in combination with 7-hydroxystaurosporine (UCN-01) in AML blasts in vitro and during a clinical trial. Blood. 2006;107:2517–24. doi: 10.1182/blood-2005-08-3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Busby EC, Leistritz DF, Abraham RT, Karnitz LM, Sarkaria JN. The radiosensitizing agent 7-hydroxystaurosporine (UCN-01) inhibits the DNA damage checkpoint kinase hChk1. Cancer Research. 2000;60:2108–12. [PubMed] [Google Scholar]
- 24.Graves PR, Yu L, Schwarz JK, Gales J, Sausville EA, O’Connor PM, et al. The Chk1 protein kinase and the Cdc25C regulator pathways are targets of the anticancer agent UCN-01. Journal of Biological Chemistry. 2000;275:5600–5. doi: 10.1074/jbc.275.8.5600. [DOI] [PubMed] [Google Scholar]
- 25.Ruegg UT, Burgess GM. Staurosporine, K-252 and UCN-01: Potent but nonspecific inhibitors of protein kinases. Trends in Pharmacological Sciences. 1989;10:218–20. doi: 10.1016/0165-6147(89)90263-0. [DOI] [PubMed] [Google Scholar]
- 26.Yu Q, La Rose J, Zhang H, Takemura H, Kohn KW, Pommier Y. UCN-01 inhibits p53 up-regulation and abrogates gamma-radiation-induced G(2)-M checkpoint independently of p53 by targeting both of the checkpoint kinases, Chk2 and Chk1. Cancer Res. 2002;62:5743–8. [PubMed] [Google Scholar]
- 27.Sato S, Fujita N, Tsuruo T. Interference with PDK1-Akt survival signaling pathway by UCN-01 (7-hydroxystaurosporine) Oncogene. 2002;21:1727–38. doi: 10.1038/sj.onc.1205225. [DOI] [PubMed] [Google Scholar]
- 28.Janetka JW, Almeida L, Ashwell S, Brassil PJ, Daly K, Deng C, et al. Discovery of a novel class of 2-ureido thiophene carboxamide checkpoint kinase inhibitors. Bioorg Med Chem Lett. 2008;18:4242–8. doi: 10.1016/j.bmcl.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 29.Graham FL, Whitmore GF. Studies in mouse L-cells on the incorporation of 1-beta-D-arabinofuranosylcytosine 5′-triphosphatase. Cancer Res. 1970;30:2636–44. [PubMed] [Google Scholar]
- 30.Major PP, Egan EM, Beardsley GP, Minden MD, Kufe DW. Lethality of human myeloblasts correlates with the incorporation of arabinofuranosylcytosine into DNA. Proc Natl Acad Sci U S A. 1981;78:3235–9. doi: 10.1073/pnas.78.5.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H, et al. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287:1824–7. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- 32.Guzi TJ, Paruch K, Dwyer MP, Labroli M, Shanahan F, Davis N, et al. Targeting the replication checkpoint using SCH 900776, a potent and functionally selective CHK1 inhibitor identified via high content screening. Mol Cancer Ther. 2011;10:591–602. doi: 10.1158/1535-7163.MCT-10-0928. [DOI] [PubMed] [Google Scholar]
- 33.Kaufmann SH, Svingen PA, Gore SD, Armstrong DK, Cheng Y-C, Rowinsky EK. Altered formation of topotecan-stabilized topoisomerase I-DNA adducts in human leukemia cells. Blood. 1997;89:2098–104. [PubMed] [Google Scholar]
- 34.Eaves C, Lambie K. Atlas of human hematopoietic colonies. Vancouver: StemCell Technologies Inc; 1995. [Google Scholar]
- 35.Mesa RA, Tefferi A, Gray LA, Reeder T, Schroeder G, Kaufmann SH. In vitro antiproliferative activity of the farnesyltransferase inhibitor R115777 in hematopoietic progenitors from patients with myelofibrosis with myeloid metaplasia. Leukemia. 2003;17:849–55. doi: 10.1038/sj.leu.2402901. [DOI] [PubMed] [Google Scholar]
- 36.Kaufmann SH. Reutilization of immunoblots after chemiluminescent detection. Analytical Biochemistry. 2001;296:283–6. doi: 10.1006/abio.2001.5313. [DOI] [PubMed] [Google Scholar]
- 37.Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–59. [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y-W, Otterness DM, Chiang GG, Xie W, Liu Y-C, Mercurio F, et al. Genotoxic stress targets human Chk1 for degradation by the ubiquitin-proteasome pathway. Mol Cell. 2005;19:607–18. doi: 10.1016/j.molcel.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 39.Leung-Pineda V, Ryan CE, Piwnica-Worms H. Phosphorylation of Chk1 by ATR is antagonized by a Chk1-regulated protein phosphatase 2A circuit. Mol Cell Biol. 2006;26:7529–38. doi: 10.1128/MCB.00447-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amarante-Mendes GP, Kim CN, Liu L, Huang Y, Perkins C, Green DR, et al. Bcr-Abl exerts its antiapoptotic effect against diverse apoptotic stimuli through blockage of mitochondrial release of cytochrome c and activation of caspase-3. Blood. 1998;91:1700–5. [PubMed] [Google Scholar]
- 41.Caserta TM, Smith AN, Gultice AD, Reedy MA, Brown TL. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis. 2003;8:345–52. doi: 10.1023/a:1024116916932. [DOI] [PubMed] [Google Scholar]
- 42.Wagner JM, Karnitz LM. Cisplatin-induced DNA damage activates replication checkpoint signaling components that differentially affect tumor cell survival. Mol Pharmacol. 2009;76:208–14. doi: 10.1124/mol.109.055178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montano R, Chung I, Garner KM, Parry D, Eastman A. Preclinical development of the novel Chk1 inhibitor SCH900776 in combination with DNA-damaging agents and antimetabolites. Mol Cancer Ther. 2012;11:427–38. doi: 10.1158/1535-7163.MCT-11-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berenbaum MC. What is Synergy? Pharmacological Reviews. 1989;41:93–141. [PubMed] [Google Scholar]
- 45.Kaufmann SH, Karp JE, Letendre L, Kottke TJ, Safgren S, Greer J, et al. Phase I and pharmacological study of infusional topotecan and carboplatin in relapsed and refractory leukemia. Clin Cancer Res. 2005;11:6641–9. doi: 10.1158/1078-0432.CCR-05-0817. [DOI] [PubMed] [Google Scholar]
- 46.Kaneko YS, Watanabe N, Morisaki H, Akita H, Fujimoto A, Tominaga K, et al. Cell-cycle-dependent and ATM-independent expression of human Chk1 kinase. Oncogene. 1999;18:3673–81. doi: 10.1038/sj.onc.1202706. [DOI] [PubMed] [Google Scholar]
- 47.Agner J, Falck J, Lukas J, Bartek J. Differential impact of diverse anticancer chemotherapeutics on the Cdc25A-degradation checkpoint pathway. Exp Cell Res. 2005;302:162–9. doi: 10.1016/j.yexcr.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 48.Ho CC, Siu WY, Chow JP, Lau A, Arooz T, Tong HY, et al. The relative contribution of CHK1 and CHK2 to Adriamycin-induced checkpoint. Exp Cell Res. 2005;304:1–15. doi: 10.1016/j.yexcr.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 49.Sampath D, Shi Z, Plunkett W. Inhibition of cyclin dependent kinase 2 by the Chk1-Cdc25A pathway during the S-phase checkpoint activated by fludarabine: dysregulation by 7-hydroxystaurosporine. Mol Pharmacol. 2002;62:680–8. doi: 10.1124/mol.62.3.680. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez-Bravo V, Guaita-Esteruelas S, Salvador N, Bachs O, Agell N. Different S/M checkpoint responses of tumor and non tumor cell lines to DNA replication inhibition. Cancer Res. 2007;67:11648–56. doi: 10.1158/0008-5472.CAN-07-3100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.