Abstract
Purpose
This study aims to proliferate spermatogonial stem cells (SSCs) and compare the in-vitro effects of laminin and growth factors on the proliferation of adult human SSC.
Methods
Isolated testicular cells were cultured in DMEM supplemented with 5 % fetal calf serum (FCS). During the culture, enriched spermatogonial cells were treated with a combination of glial cell line-derived neurotrophic factor (GDNF), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF) and mouse leukemia inhibitory factor (LIF) in the presence or absence of human placental laminin-coated dishes. Cluster assay was performed during culture. Presence of spermatogonia was determined by an ultrastructural study of the cell clusters, reverse transcription polymerase chain reaction (RT-PCR) for spermatogonial markers and xenotransplantation to the testes of busulfan-treated recipient mice. Statistical significance between mean values was determined using statistical ANOVA tests.
Results
The findings indicated that the addition of GDNF, bFGF, EGF and LIF on laminin-coated dishes significantly increased in-vitro spermatogonial cell cluster formation in comparison with the control group (p ≤ 0.001). The expression of spermatogonial markers was maintained throughout the culture period. Furthermore, a transplantation experiment showed the presence of SSC among the cultured cells. In addition, a transmission electron microscopy (TEM) study suggested the presence of spermatogonial cells of typical morphology among the cluster cells.
Conclusions
It can be concluded that human SSCs obtained from non-obstructive azoospermic (NOA) patients had the ability to self-renew in the culture system. This system can be used for the propagation of a small number of these cells from small biopsies.
Keywords: Spermatogonial stem cells, Non- obstructive azoospermia, Testicular biopsy, Culture
Introduction
In 1994, with the successful transplantation of spermatogonial stem cells (SSCs) in the buslfan-treated mouse, a great evolution occurred in the treatment of male infertility [1]. Since then, researchers have proposed the idea that human testicular tissue could be harvested and cryopreserved in children with testicular cancer prior to start of chemotherapy or radiotherapy. Such cells could subsequently be transplanted back into the testis to resume spermatogenesis and sperm production [2, 3]. Until now, autotransplantation has been carried out in a number of animal models such as bovine, goats and monkeys [4–6], but autologous transplantation was only able to successfully resume complete spermatogenesis in bovine. As yet, no evidence has been found in human studies.
SSCs similar to other stem cells are generally rare [7]. It has been demonstrated that the approximate number of SSCs in mice and rats is 0.03% of all germ cells [8]; therefore, we predict that human SSCs may be rare and similar to rodent SSCs. The success rate of transplants depends on the enrichment and concentration of transplanted SSCs in vitro [9–11]. Proliferation of SSCs in vitro enhances SSC numbers [2, 12, 13] and probability of successful transplantation [14]. In addition, it provides large numbers of stem cells for biochemical or molecular analysis [15]. On the other hand, the usual testicular biopsy does not have an adequate number of SSCs for transplantation therapy. Indeed, obtaining a whole testis from a patient is impossible. Therefore, access to sufficient numbers of SSCs is essential for study of their regulations and further biomanipulation [9]. So, in vitro proliferation of a few SSCs to obtain appropriate cell numbers is essential.
Recent studies have shown that soluble growth factors such as glial cell line-derived neurotrophic factor (GDNF), basic fibroblast growth factor (bFGF), epidermal growth factor (EGF) and mouse leukemia inhibitory factor (LIF) along with serial passaging of clusters result in long-term SSC maintenance and stimulate SSC division in animals [16–19] as well as in humans [13]. Also, long term SSC maintenance can be achieved when cultured on laminin coated plates in animals [17, 20] and humans [13, 21].
Controversies present with respect to the use of somatic cells as a feeder layer. Somatic cells are able to differentiate [22] or support mice [23–26] and human SSCs in cultures [13, 27, 28]. Recently, in vitro propagation of human SSCs has been reported with small pieces of normal human testis [13] and small testicular biopsies from azoospermic patients [7, 28] with different culture systems. Mirzapour et al. [28] cultured SSCs from human adult azoospermic testes in co-cultured with Sertoli cells or co-cultured with Sertoli cells with adding LIF and FGF. As a result, SSCs co-cultured with Sertoli cells proliferated with the largest number of colonies [28]. Lim et al. [7] propagated SSCs derived from obstructive azoospermic and non-obstructive azoospermic (NOA) patients for a long-time. The testicular cells were treated with a combination of GDNF, FGF and EGF during culture. They didn’t perform transplantation of obtained germ cell colonies into recipient mice for functional assessment of SSCs [7].
The aim of this study was to compare the in vitro effects of laminin and growth factors on the proliferation of adult human SSC obtained from patients suffered from NOA. To accomplish this objective, isolated testicular cells were treated with a combination of different growth factors in the presence or absence of human placental laminin-coated dishes during culture. Cluster assay was performed during culture. Presence of spermatogonia was determined by ultrastructural study of cell clusters, reverse transcription polymerase chain reaction (RT-PCR) for spermatogonial markers. The presence of functional SSC in culture was confirmed through xenotransplantation into busulfan-treated recipient mouse testes.
Materials and methods
Experimental samples
Testis biopsies (TESE) obtained for the diagnosis of male fertility through the Clinical Urology and Embryology Department of Royan Institute (Tehran, Iran) following informed consent. The use of human testicular biopsies and the experimental protocol were approved by the Ethical and National Research Council guidelines of Royan Institute (Tehran, Iran).
All 20 samples used for this study were obtained from individuals diagnosed with azoospermia due to incomplete or complete maturation arrest (age 32–50 years, during 2008–2009). Each patient in this research had a complete medical history in Royan institute. Semen analysis was performed according to WHO criteria and testicular biopsy was only performed in cases where sperm could not be detected in any of the semen samples collected during 1 year.
Isolation of human spermatogonial stem cells
Testicular cells were isolated using the method described previously, with some modifications [12, 13]. Briefly, after using TESE samples in the andrology and embryology Laboratories, the remainder of the testes tissues (≈50 ± 10 mg) were placed into DMEM medium (DMEM; Gibco, Paisley, UK) supplemented with 13.5 g/L, NaHCO3 (Sigma, St Louis, MO), single-strength non-essential amino acids, 100 IU/ml penicillin, 100 μg/ml streptomycin, 40 μg/ml gentamycin (all from Gibco, Paisley, Uk) and 5 % FBS. Samples were carried to the stem cell laboratory for isolation. Tissues were initially washed two or three times with phosphate buffered saline solution (PBS) supplemented with 1 % pen/strep before being placed in DMEM medium for a second time. Only two or three samples per isolation were pooled and used. Then, tissues were mechanically dissected using two insulin needles and dissociated in PBS. After two washes, tissues were suspended in a DMEM medium containing 1 mg/ml collagenase type I 1 mg/ml hyaluronidase, 1 mg/ml trypsin and 0.05 mg/ml DNase for 30 min with some shaking and pipetting at a temperature of 37 °C. All enzymes were purchased from Sigma Company (Sigma, St Louis, MO). Fragmented tubules, tissues and cells were centrifuged for 2 min at 1100 rpm and washed two–three times in DMEM medium.
For the second digestion step, fragmented tubules was resuspended in DMEM by addition of fresh enzymes and incubated for 30–45 min at 37 °C (with shaking and pipetting). After filtration through a 40 μm nylon filter, collected cells were washed three times with DMEM medium that contained 10 % fetal calf serum (FCS) before using for culturing. After overnight incubation, floating cells were collected and cultured. Prior to culturing, the cell numbers were determined with a hematocytometer. Cell viability was evaluated by the dye exclusion test (0.04 % trypan blue solution).
Spermatogonial stem cell cultures
The obtained cells were incubated at 32 °C and 5 % CO2, in a humidified atmosphere in the presence of 5 % FCS. The culture groups included: (1) control: SSCs cultured on plastic dishes without growth factors and laminin (2) growth factor: SSCs cultured on dishes treated with different growth factors and (3) growth factors plus laminin (Sigma, St Louis, MO): SSCs-cultured on laminin-coated dishes (at a concentration of 20 μg/ml) supplemented with different growth factors. In the treatment group, cells were grown for 2 months in the presence or absence of laminin and different growth factors including recombinant human GDNF 20 ng/ml, recombinant human bFGF 10 ng/ml, mouse EGF 20 ng/ml (all from Sigma, St Louis, MO) and recombinant human LIF 10 ng/ml (Chemicon, Temecula, CA). The cells were cultured in uncoated 4-well culture plates (Cole-Parmer, Vernon Hills, Illinois). Two days post-plating, most testicular cells were attached to the growing surface and the media was changed. Depending on the culture groups, several small clusters were observed on top of the monolayer of testicular cells, approximately after 4 weeks. In order to proliferate these clusters and prevent SSCs from differentiating, every 5–7 days until confluency, cells were passaged with trypsin- EDTA (0.25 %) (Invitrogen) and re-cultured or sub-cultured. Differential plating was performed by considering the ratio of somatic vs. germ cells.
Cluster assay
The cells were cultured for 2 months; the number of clusters which appeared in these cultures as well as the diameter of each cluster was evaluated. An inverted microscope (Zeiss, Jena, Germany) was used to determine the number of the clusters. Furthermore, the diameter of each cluster was measured using Image J software.
Identity confirmation of the spermatogonial cells
RNA extraction and reverse transcription
The presence of spermatogonial cells during culture was determined by the expression of spermatogonial genes based upon previous animal and human studies. Total RNA from the testis samples (positive control), testicular cells obtained before cultivation and cultured testicular cells were extracted using RNX kit standard (Cinnagen, Iran) according to the manufacturer’s instructions. The purity and integrity of RNA was checked by a 260/280 nm ratio measurement. Total RNA was treated with DNase I to remove genomic DNA contamination from samples. First, strand cDNA was performed using oligodT primers and superscript II reverse transcriptase system. All reverse transcription reagents were purchased from Fermentas Corporation (Germany).
Polymerase chain reaction (PCR)
The primers specific for PLZF(promyelocyticleukaemia zinc-finger), DAZL(deleted in azoospermia-like), Oct4(Octamer-binding transcription factor 4), VASA, ITGB1(β1-integrin), ITGA6(α6-integrin), and β -actin genes were designed using previously described human sequences (GenBank) and Gene runner software (version 3.02; Hastings Software) as shown in Table 1. β –actin, a housekeeping gene, was included as an internal control to normalize the PCR reaction. RT-PCR was performed using the prepared cDNA, the primers, and PCR Supermix (Cinnagen) under the following conditions: 35 cycle at 95 ° C for 30 s, specific annealing temperature for each primer (PLZF, 55 °C; DAZL, 62 °C; Oct4, 60 °C; VASA, 62 °C; ITGA6, 52 °C; ITGB1 55 °C; and β-actin, 60 °C) for 45 s, and finally at 72 °C for 45 s. To separate PCR products, 1 μl of each sample was resolved on a 1.7 % agarose gel, then electrophoresis was performed with 1x TAE Loading buffer and a voltage of 95 for 45.
Table 1.
Sequences of the designed primers used for RT-PCR
| Genes | Primer sequences (5′-3′) | Annealing temperature (°C) | Size (bp) |
|---|---|---|---|
| PLZF | F: 5′ GGTCGAGCTTCCTGATAACG 3′ | 55 °C | 396 |
| R: 5′ CCTGTATGTGAGCGCAGGT 3′ | |||
| DAZL | F: 5′ GCC CTT CTTTCAGTGACTTC 3′ | 62 °C | 381 |
| R: 5′ TGCTTCACTCCAACAAAGAC 3′ | |||
| Oct4 | F: 5 ′GTT CTTCATTCACTAAGGAAGG 3′ | 60 °C | 100 |
| R : 5′CAAGAGCATCATTGAACTTCAC 3′ | |||
| VASA | F: 5′ TACTTGCTGGACGAGATTCTG 3′ | 62 °C | 320 |
| R: 5′ ATCCATCAGTCTTCCAGGAG 3′ | |||
| ITGA6 | F: 5′ TCATGGATCTGCAAATGGAA 3′ | 52 °C | 300 |
| R: 5′ GCGGGGTTAGCAGTATATTCA 3′ | |||
| ITGB1 | F: 5′ GTGGGTGGTGCACAAATTC 3′ | 55 °C | 300 |
| R: 5′ GGTCAATGGGATAGTCTTCAGC 3′ | |||
| β-actin | F: 5′ CAAGATCATTGCTCCTCCTG 3′ | 60 °C | 90 |
| R: 5′ ATCCACATCTGCTGGAAGG 3′ |
The bands were visualized by using Gell logic, and images were obtained. The amplified PCR products were sequenced to confirm the identity of the amplified products.
Ultrastructural study of cell clusters
Both clusters of GSCs (small and big) were removed and fixed in 2.5 % glutaraldehyde in PBS (pH 7.4) for 2 h, next post-fixed with 1 % osmium tetroxide in the same buffer for 2 h. After dehydration in an ascending series of ethanol, specimens were placed in propylene oxide and embedded in Epon 812 (TAAB, UK). Semi-thin sections (0.5 mm) were stained with toluidine blue for a light microscopy. Ultrathin sections (60–80 nm) were contrasted with uranyl acetate and lead citrate before being examined by a transmission electron microscopy (TEM; Zeiss EM 900, Germany).
Cell labeling, recipient mice and transplantation
At passage 7, more than 34 SSC clusters and underlying somatic cells were trypsinized followed by adding 5-Bromo-2-deoxyuridine (BrdU) to the medium for cell labeling and tracing in the recipient mice 72 h before transplantation. Then, spermatogonial cells were transplanted into the seminiferous tubules of recipient mice,age 10 weeks, via the rete testis that was treated with 35 mg/kg busulfan prior to the transplantation. The treated recipient mice were devoid of endogenous spermatogenesis at the time of transplantation (6 weeks after treatment) [29]. Adult recipient mice were anesthetized with 10 % ketamine and 2 % xylazine (Alfasan, Woerden, Netherlands). Approximately, 105 of the cultured cells in 10 μl DMEM were injected into the seminiferous tubules in one testis of each recipient mouse (n = 3). The other testis served as an internal control. Transplantation was performed by retrograde injection through the efferent ducts.
Recipient testes assessment
Transplanted testes of the recipient mice were examined two months after transplantation. The testes were fixed in 4 % paraformaldehyde, dehydrated and embedded in paraffin. Presence of transplanted cells in 5 μm sections was assayed by immunohistochemical detection of BrdU incorporated with a kit according to manufacturer’s instructions (Sigma). For immunohistochemistry, following deparaffination, sections were treated in 25 % formamid in SSC2X for 2h at 60 °C, then washed in SSC2X for 10 min. Antigen retrieval were performed in CaCl2 solution for 20 min at 37 °C and blocked with 10 % goat serum for 1 h at 37 °C (Vector, Burlingame, CA). The slides were incubated for overnight at 4 °C with mouse monoclonal anti BrdU (dilution, 1:300; Sigma). After being extensively washed with PBS, the secondary antibody (goat anti-mouse labeled with fluorescein isothiocyanate (FITC); dilution, 1:100; Sigma) was applied for 45 min. The control slides were under similar conditions except for the removal of the first antibody.
Statistical analysis
Results were expressed as mean ± SD. Data were analyzed using ANOVA and results were assumed significant at p ≤ 0.05.
Results
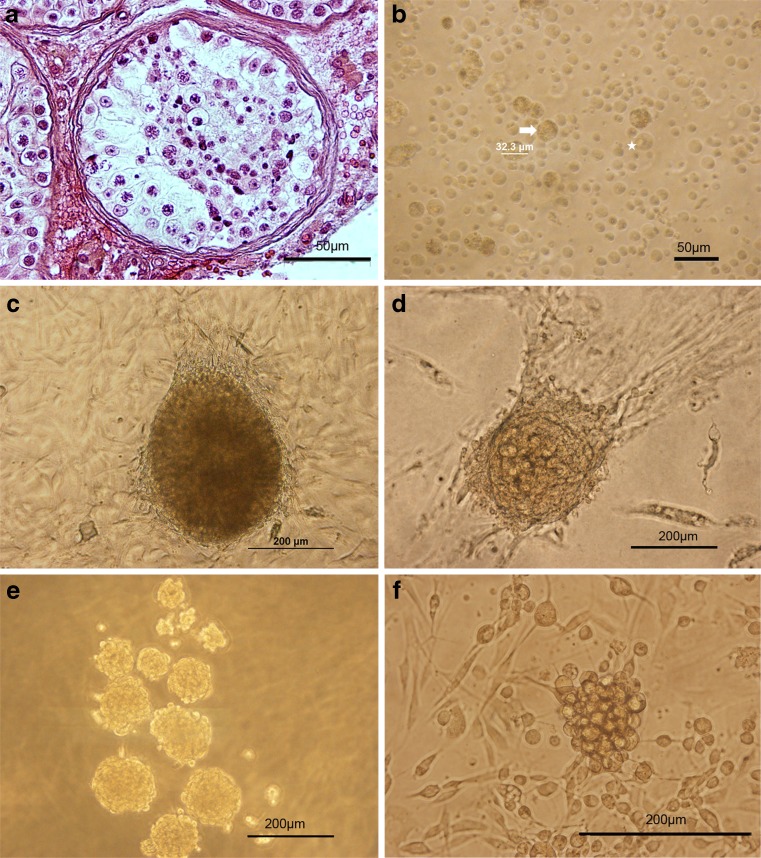
Owing to the presence of a variety of germinal cells in the testes (Fig. 1 a), purification of spermatogonia was difficult after isolation, thus differential plating was used. In addition, the cell population obtained was of different sizes and morphologies. Sertoli cells, as specified by their nuclear morphology, were 32–33 μm in diameter with an irregular outline and a granular appearance (Fig. 1 b). During the first week of culture, they proliferated and created a monolayer of cells. SSC had a diameter of 24–25 μm and a spherical outline with prominent nucleus centrally located (Fig. 1 b). They created embryonic stem cell-like (ES-like) colonies (Fig. 1 c) or big and small germ-line stem cells (GSC) clusters (Fig. 1 d–f) after proliferation over several weeks of culture. Testicular cells could be proliferated for up to 2 months and 7 passages. Of these, only the clusters of GSC were assayed.
Fig. 1.
Isolation and culture of SSCs from testicular tissues of non-obstructive azoospermic (NOA) patients. a Histological appearance of the testes biopsy obtained from azoospermic patient with complete maturation arrest that was stained with H& E and showed spermatogenic cells in tubules. b Cell population obtained from the seminiferous tubules after two steps enzymatic digestion contained different cell types, sizes and morphology. Spermatogonia could be identified as round cells with a large nucleus, one nucleoli and cytoplasmic inclusions (asterisk); whereas, Sertoli cells were large cells with a granular appearance (arrows). After overnight incubation, the non-adhering cells were collected and cultured. c–f The morphology of clusters growing on top of monolayer of somatic cells shows three types of colonies or clusters as observed during 2 months of cultivation. First, ES-like colonies were sharply edged and compact (c); while, big (d) and small (e, f) GSC clusters were smaller and clumpy and their cells were individually recognizable. We assayed the GSC clusters. Scale bars: A, B = 50 μm and C-F = 200 μm
Assessment of spermatogonial stem cell clusters
Cell viability was assessed after isolation of testicular cells by the dye exclusion test (0.04 % trypan blue solution). The results showed that ≥92 % of the cells were viable. Cluster appearance varied between the different experimental groups. The clusters appeared earlier in the growth factors plus laminin group (day 22.3 ± 3.8 of culture) and the growth factor group (day 23.7 ± 6.4 of culture) in comparison with the control group (day ≥55). When these clusters were enzymatically dispersed and replated, their present SSC could start new clusters during 2 months of culture.
All in all, as shown in Table 2, the results indicate that the diameters of the clusters in both of the growth factor groups were varied significantly from that of the control group (p ≤ 0.01). In terms of numbers of clusters, the growth factors plus laminin group was significantly different compared to the control group (p ≤ 0.05). However, the diameters of the clusters in the growth factor group in the first (169.4 ± 84.9) and second months (220.4 ± 54.7) were not significantly different compared with the growth factor plus laminin group in the first (209.9 ± 53.4) and second months (173.8 ± 80.6), respectively. Furthermore, the numbers of the clusters in the growth factor group in the first month (6 ± 3.6) were not significantly different compared with that of the growth factor plus laminin group (8.7 ± 4.7). However, during the second month, the number of clusters in the growth factor group (16.3 ± 4.7) significantly varied from that of the growth factor plus laminin group (28 ± 4) (p ≤ 0.05). Additionally, when the number of clusters and their diameters were analyzed, a higher score was obtained after 2 months of cultivation rather than after one month (p ≤ 0.05).
Table 2.
Comparison of the number (per 105 cells plated) and diameter of the clusters between the control and experimental groups
| Diameters of clusters during culture | Number of clusters/(per 105 cells plated) | ||||
|---|---|---|---|---|---|
| Groups | Day of first cluster formation | After 1 month | After 2 months | After 1 month | After 2 months |
| Control | ≥55 | 0 | 112.3 ± 29.2 | 0 | 3.3 ± 1.5 |
| Growth factors | 23.7 ± 6.4 | 169.4 ± 84.9٭ | 220.4 ± 54.7٭ | 6 ± 3.6٭ | 16.3 ± 4.7٭ |
| Growth factors plus laminin | 22.3 ± 3.8 | 209.9 ± 53.4٭ | 173.8 ± 80.6٭ | 8.7 ± 4.7٭ | 28 ± 4٭, ** |
Results from three separate experiments were used for all groups. Values are the mean cluster numbers and diameters ± SD at different times
*Significant difference vs. control group in the same column (p ≤ 0.05)
**Significant difference vs. growth factors group in the same column (p ≤ 0.05)
Identity confirmation of the spermatogonial cells
RT-PCR
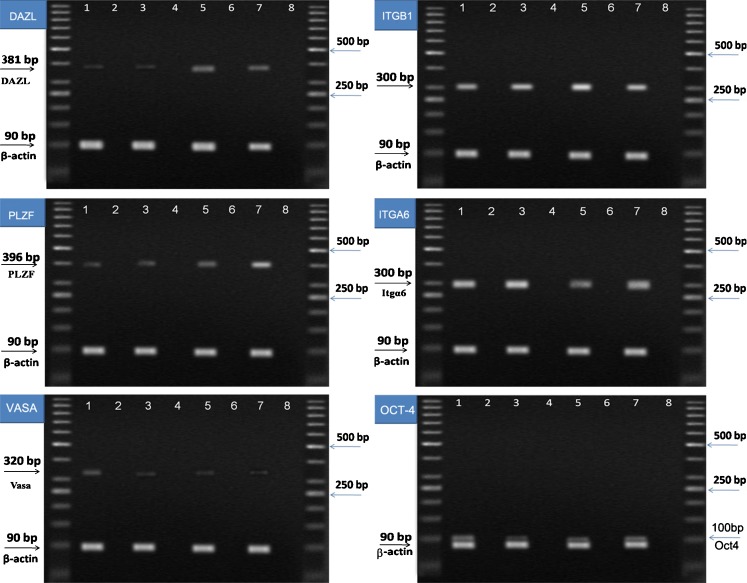
RT- PCR was performed to analyze the expression of specific spermatogonial and germ cell markers in TESE, and the isolated testicular cells and cultured cells after the first and second months. As shown in Fig. 2, all samples expressed specific spermatogonial and germ cell genes: DAZL, PLZF, Oct4, VASA, ITGA6 and ITGB1.
Fig. 2.
Molecular characterization of spermatogonial and germ cells at the RNA level during cell culture. Reverse transcriptase polymerase chain reaction (RT-PCR) was used to determine the expression of specific spermatogonia and germ cell markers. It showed that PLZF (396base pairs); ITGA6 (300base pairs); ITGB1 (300base pairs); VASA (320 base pairs); DAZL (381base pairs); and Oct4 (100 base pairs) genes were expressed in 1) Total testis, 3) isolated testicular cells by two steps of enzymatic digestion before culture, 5) cells one month after cultivation and 7) cells two months after cultivation. 2, 4, 6, 8) Negative control, No cDNA. β-actin was also used as a housekeeping gene (90base pairs). As shown, all samples expressed specific spermatogonial and germ cell genes. PCR products were separated on 1.7 % agarose gel. DAZL (deleted in azoospermia-like), VASA, PLZF (promyelocytic leukemia zinc finger protein), ITGA6 (integrin-α6), and ITGB1 (integrin-β1)
Colonization assay of the transplanted cells
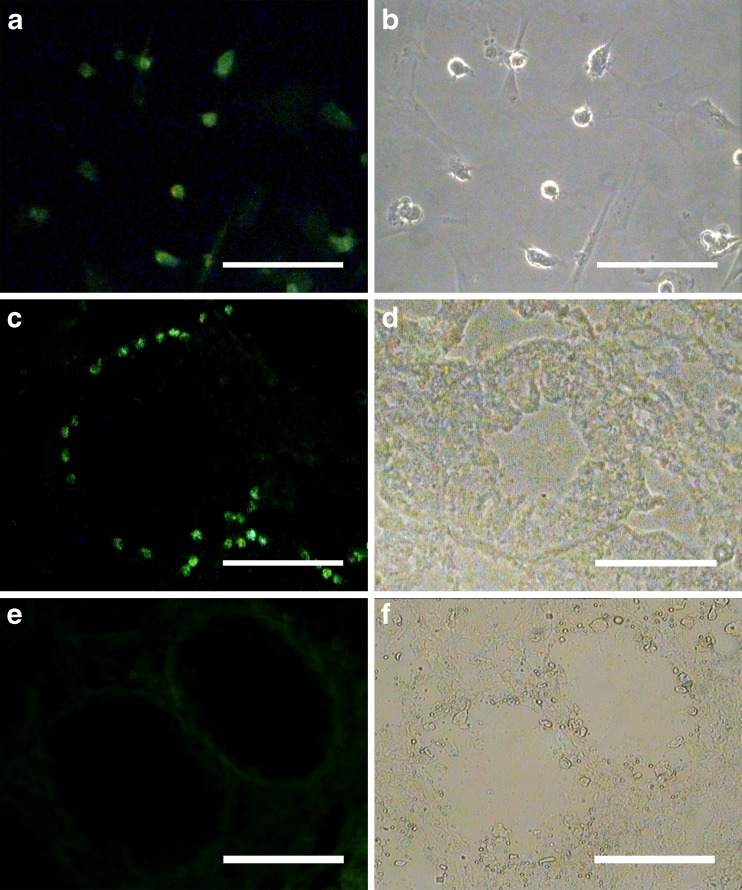
Cell labeling with BrdU was performed before transplantation. Cell staining was examined just before xeno-transplantation. Immunoflourescent cells indicated that a lot of cells (~70 %) were labeled with BrdU before transplantation. After 2 months of cultivation, 105 cells from the growth factor plus laminin group were injected into the seminiferous tubules through the rete testis of the recipient testes in order to confirm the presence of SSCs in clusters and the human SSCs colonization assessment in the testis. Two months after transplantation, the cells whose nuclei stained FITC positive with BrdU were considered as transplanted cells. Two months after xenotransplantation, the transplanted cells were localized in the basal of the seminiferous tubules of the recipient testes as single cells and did not form a cluster. The non-transplanted right testis was considered as the control group.
Morphological characterization of clusters
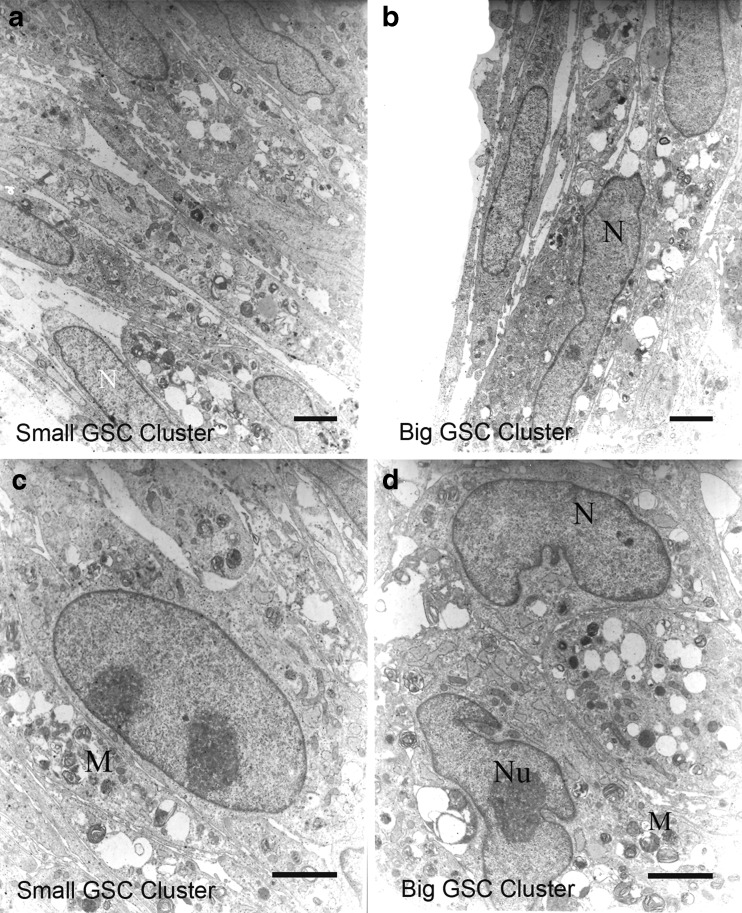
The ultrastructures of the clusters, after 2 months of cultivation, were examined by TEM. The electron micrograph showed that cells from both types of clusters had typical morphology of human spermatogonial cells [30]. As seen in Figs. 3 and 4, both cluster cells had large spherical nuclei that contained one or two prominent nucleoli which were located along the nuclear membrane or in the center of the nucleus. The cell shapes were variable (pear-shaped or round) and contained a long irregular or round nucleus. The cytoplasm was characterized by organelles such as mitochondria, which were mostly located in the perinuclear region. Mitochondria were found singly, in pairs or in groups.
Fig. 3.
Xenotransplantation of human SSCs into recipient mouse testis. In order to determine the functional spermatogonial stem cell activity of cultured cells, xenotransplantation of human SSCs into recipient mouse testis was performed. For these assay, human spermatogonial cells were harvested following culture periods of 60 days and labeled with 5-Bromo-2-deoxyuridine (BrdU). a BrdU was added, and staining was examined in cultured spermatogonial cells before transplantation. Labeled cells were transplanted into the seminiferous tubules of busulfan-treated adult recipient mice through rete testis. Since only spermatogonial stem cells can migrate to seminiferous tubule basement membrane recipient mouse testis. c Transplanted human SSCs were found as single cells or paired at the basal membranes of some of the mouse seminiferous tubules two months after transplantation. These cells were traced in the recipient testes by BrdU staining. e The non-transplanted right testis was considered as the control group. b, d, f Phase contrast. Scale bars = 100 μm
Fig. 4.
Representative transmission electron micrographs from SSCs clusters. To characterize of SSCs, obtained cluster cells from culture were compared using a transmission electron microscopy (TEM). So, both types of GSC clusters were harvested during cultivation and processed for ultrastructure study. The electron micrograph showed cells from small (a, c) and big (b, d). GSC clusters had morphology typical of human spermatogonial cells. The nucleus (N) shown contains a mottled appearance with dark speckles of heterochromatin. In the nuclei, one or two small compact and highly reticulated nucleoli (Nu) were located eccentric position. Also, ratio of their nucleus to cytoplasm was very high and mitochondria (M) were positioned in the cytoplasm in abundance. Scale bar: A, B = 800 nm and C, D = 500 nm
Discussion
In this study, we cultured a small number of adult human testicular cells obtained from NOA patients instead of using abundant normal testicular cells in each culture. We demonstrated that the addition of GDNF, bFGF, EGF and LIF in the presence or absence of laminin-coated dishes significantly increased spermatogonial cell colony formation in SSCs obtained from NOA patients in comparison to the control group (neither growth factor nor laminin). It was also shown that these spermatogonial cell clusters could be successfully cultured and propagated for two months.
In vitro, SSCs form three-dimensional aggregations of germ cells on a feeder layer, termed clusters. Disassociation of clusters and serial passaging for extended periods can increase SSCs in number [16]. In our culture system, the addition of GDNF, bFGF, EGF and LIF on laminin-coated dishes increased the numbers of SSCs clusters by self-renewal in vitro. The culture medium used in this study was DMEM supplemented with essential growth factors: GDNF, bFGF, EGF and LIF. These growth factors are secreted by Sertoli cells, their receptors place on SSCs, and increase survival and proliferation in vitro [16, 18, 19, 31]. Majority of studies in humans [7, 13, 28] and animals [9, 12, 17–19, 25] have confirmed the useful effects of the aforementioned growth factors on SSCs. On the other hand, stem cells, in general, need a special microenvironment or niche to establish and maintain their stem cell properties [32, 33]. SSCs niche is provided by Sertoli cells in vivo [34] and probably this microenvironment can be reproduced in vitro [35]. Based on the previous studies, [25, 28, 35, 36]we conclude that in addition to growth factors and existence of somatic cells in culture, likely create a testis-like microenvironment can be more effective in colony formation.
Although laminin-coated dishes could significantly increase SSCs colony formation in vitro, the small clusters GSCs were more abundant in growth factors plus laminin group. This explains why the diameter of clusters decreased after two months of cultivation (Table 2.). Kanatsu-Shinohara et al. has showed that SSCs prefer to attach to laminin [37] and can be enriched 3- to 8-fold after selection on a laminin-coated plate [38, 39]. Also, GS cells on laminin tend to form various types of colonies, ranging from chains to clumps [17]. Previous studies have shown the beneficial effects of laminin on in-vitro normal SSC proliferation [13, 40] and purification [41, 42].
In order to confirm the presence of spermatogonial cells during cultivation, RT-PCR using spermatogonial markers (PLZF, Oct4, DAZL, VASA, ITGA6 and ITGB1) in isolated testicular cells, cluster cells and testes tissue were performed. These markers were predominantly expressed by spermatogonial cells. To date, no SSC-specific marker has been identified for any species but the combination of expression of multiple markers can provide important information about spermatogonial cell types in rodents and other species [43].
PLZF, a marker for spermatogonial stem/progenitor cells, is well-known as a spermatogonial-specific marker in many species including humans [13, 22, 43]. Oct4, a general marker for stem cells, is also expressed in mouse spermatogonial stem/progenitor cells [18, 25, 31, 44, 45] and Human SSCs [7, 27, 46, 47] and may be required for the self-renewal of SSCs [48]. Oct4 expression also reveals the presence of populations of SSCs in the human testes with pluripotent characteristics [47]. ITGA6 and ITGB1, surface markers for spermatogonial stem/progenitor cells, are expressed in rodents [37, 49] and humans [13, 21, 41, 47]. Our findings supported the reports by previous investigators [7, 13, 28, 47]. In our study expression of VASA and DAZL as markers of germ cell identification were also observed in isolated testicular cells, cluster cells and testes tissue. Our finding is in line with Conrad et al. [41] and Mirzapour et al. [28] who mentioned human adult GSCs and SSCs to be positive for stem cell markers such as VASA and Oct4 [28, 41]. Previous studies have revealed that DAZL is presented in nucleus of spermatogonia obtained from rodents [38, 50] and adult rhesus macaque [51].
In addition to confirmation of molecular characteristics, SSCs functional assay and an ultrastructure study of the cluster cells were also performed. As there are no specific biochemical or morphological markers for SSCs in clusters [52, 53]and only the stem cells are able to colonize and repopulate in testes [29, 54], transplantation is performed as a functional assay to determine the presence of SSCs in a cell population. The cultured testicular cells were transplanted into a mouse busulfan azoospermic model. Human SSCs were found as single or paired cells at the basal membranes of some mouse seminiferous tubules. However, because of the large phylogenetic distance between mouse and human, only single or paired cells could be formed at the basal membranes of tubules. Previous reports have also shown similar results8 weeks after transplantation [13, 21, 28, 47]. Although SSCs in the clusters showed pluripotent characteristics, no tumors or teratomas were found in the three recipient mice after transplantation. This demonstrated that human SSCs remained completely committed to the germ line lineage during culture. This finding agreed with the reports by the aforementioned investigators.
Although there have been only a few ultrastructural studies on human spermatogonial cells in colonies or clusters, these studies all confirm the large nucleus to cytoplasm ratio, intensive nucleolus and high heterochromatins in humans and rodents [41, 55–57]. Similarities were found upon the comparison between the ultrastructure of the cluster cells of this study with those of previous studies.
The self-renewal and pluripotency capability of human SSCs from NOA patients in our culture system enables this system to be utilized for proliferation or differentiation of these cells from small biopsies in clinical applications, cell replacement therapy and tissue regeneration.
Acknowledgments
We would like to thank all the patients who donated tissues for research to the lab of Royan institute. We also appreciate the contributions of H. Sadri-ardekani, S.C. Mizrak, R. Aflatounian, and MR. Hadjighassem for comments; M. Moraveji, H. Baharvand, H. Azizi, P. Eftekhari-Yazdi, T. Mirzapour, M. Soleimani, M. Lotfipanah for technical and administrative support; and MR. Akhoond for data analyzing. This work was supported by a grant from Royan Institute, Tehran, Iran (Number: 158-3).
Glossary
- SSCs
spermatogonial stem cells
- GDNF
glial cell line-derived neurotrophic factor
- bFGF
basic fibroblast growth factor
- EGF
epidermal growth factor
- LIF
leukemia inhibitory factor
- NOA
non-obstructive azoospermic
- RT-PCR
reverse transcription polymerase chain reaction
- TESE
Testis biopsies
- PBS
phosphate buffered saline solution
- FCS
fetal calf serum
- PLZF
promyelocytic leukaemia zinc-finger
- DAZL
deleted in azoospermia-like
- Oct4
Octamer-binding transcription factor 4
- ITGB1
β1-integrin
- ITGA6
α6-integrin
- TEM
transmission electron microscopy
- BrdU
5-Bromo-2-deoxyuridine
- ES-like
embryonic stem cell-like
- GSC
germ-line stem cells
Footnotes
Capsule
Proliferation of SSCs obtained from NOA patients are increased by GDNF, bFGF, EGF and LIF in the presence or absence of laminin.
References
- 1.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994;91(24):11298–302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinster RL. Male germline stem cells: from mice to men. Science (New York, NY) 2007;316(5823):404–5. doi: 10.1126/science.1137741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radford J, Shalet S, Lieberman B. Fertility after treatment for cancer. Questions remain over ways of preserving ovarian and testicular tissue. BMJ. 1999;319(7215):935–6. doi: 10.1136/bmj.319.7215.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honaramooz A, Behboodi E, Blash S, Megee SO, Dobrinski I. Germ cell transplantation in goats. Mol Reprod Dev. 2003;64(4):422–8. doi: 10.1002/mrd.10205. [DOI] [PubMed] [Google Scholar]
- 5.Izadyar F, Ouden K, Stout TA, Stout J, Coret J, Lankveld DP, et al. Autologous and homologous transplantation of bovine spermatogonial stem cells. Reproduction. 2003;126(6):765–74. doi: 10.1530/rep.0.1260765. [DOI] [PubMed] [Google Scholar]
- 6.Schlatt S, Kim SS, Gosden R. Spermatogenesisand steroidogenesis in mouse, hamster and monkey testicular tissue after cryopreservation and heterotopic grafting to castrated hosts. Reproduction. 2002;124(3):339–46. doi: 10.1530/rep.0.1240339. [DOI] [PubMed] [Google Scholar]
- 7.Lim JJ, Sung SY, Kim HJ, Song SH, Hong JY, Yoon TK, et al. Long-term proliferation and characterization of human spermatogonial stem cells obtained from obstructive and non-obstructive azoospermia under exogenous feeder-free culture conditions. Cell Prolif. 2010;43(4):405–17. doi: 10.1111/j.1365-2184.2010.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tegelenbosch RA, Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290(2):193–200. doi: 10.1016/0027-5107(93)90159-D. [DOI] [PubMed] [Google Scholar]
- 9.Aponte PM, Soda T, Teerds KJ, Mizrak SC, Kant HJ, Rooij DG. Propagation of bovine spermatogonial stem cells in vitro. Reproduction. 2008;136(5):543–57. doi: 10.1530/REP-07-0419. [DOI] [PubMed] [Google Scholar]
- 10.Brinster RL, Nagano M. Spermatogonial stem cell transplantation, cryopreservation and culture. Semin Cell Dev Biol. 1998;9(4):401–9. doi: 10.1006/scdb.1998.0205. [DOI] [PubMed] [Google Scholar]
- 11.Mirzapour T, Movahedin M, Ibrahim TABT, Haron AW, Makulati Z, Nowroozi M. Effect of donor cells concentration on colonization of human spermatogonial stem cells in recipient mouse testes. J Biol Sci. 2010;10:730–8. doi: 10.3923/jbs.2010.730.738. [DOI] [Google Scholar]
- 12.Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69(2):612–6. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 13.Sadri-Ardekani H, Mizrak SC, Daalen SK, Korver CM, Roepers-Gajadien HL, Koruji M, et al. Propagation of Human Spermatogonial Stem Cells In Vitro. JAMA. 2009;302(19):2127–34. doi: 10.1001/jama.2009.1689. [DOI] [PubMed] [Google Scholar]
- 14.Anjamrooz SH, Movahedin M, Tiraihi T, Mowla SJ. Graft efficiency of co-cultured spermatogonial cells using sperm assay in epididymal lumen of recipient mice. Cell J (Yakhteh) 2006;7:242–9. [Google Scholar]
- 15.Kanatsu-Shinohara M, Inoue K, Lee J, Miki H, Ogonuki N, Toyokuni S, et al. Anchorage-independent growth of mouse male germline stem cells in vitro. Biol Reprod. 2006;74(3):522–9. doi: 10.1095/biolreprod.105.046441. [DOI] [PubMed] [Google Scholar]
- 16.Ebata KT, Yeh JR, Zhang X, Nagano MC. Soluble growth factors stimulate spermatogonial stem cell divisions that maintain a stem cell pool and produce progenitors in vitro. Exp Cell Res. 2011;317(10):1319–29. doi: 10.1016/j.yexcr.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Kanatsu-Shinohara M, Miki H, Inoue K, Ogonuki N, Toyokuni S, Ogura A, et al. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol Reprod. 2005;72(4):985–91. doi: 10.1095/biolreprod.104.036400. [DOI] [PubMed] [Google Scholar]
- 18.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 2004;101(47):16489–94. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng X, Lindahl M, Hyvonen ME, Parvinen M, Rooij DG, Hess MW, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science (New York, NY) 2000;287(5457):1489–93. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 20.Kuijk E, Colenbrander B, Roelen B. The effects of growth factors on in vitro-cultured porcine testicular cells. Reproduction. 2009. doi:10.1530/REP-09-0138. [DOI] [PubMed]
- 21.Sadri-Ardekani H, Akhondi MA, Veen F, Repping S, Pelt AM. In vitro propagation of human prepubertal spermatogonial stem cells. JAMA. 2011;305(23):2416–8. doi: 10.1001/jama.2011.791. [DOI] [PubMed] [Google Scholar]
- 22.Aponte PM, Bragt MP, Rooij DG, Pelt AM. Spermatogonial stem cells: characteristics and experimental possibilities. APMIS. 2005;113(11–12):727–42. doi: 10.1111/j.1600-0463.2005.apm_302.x. [DOI] [PubMed] [Google Scholar]
- 23.Huang YH, Chin CC, Ho HN, Chou CK, Shen CN, Kuo HC, et al. Pluripotency of mouse spermatogonial stem cells maintained by IGF-1- dependent pathway. FASEB J. 2009;23(7):2076–87. doi: 10.1096/fj.08-121939. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Seandel M, Falciatori I, Wen D, Rafii S. CD34+ testicular stromal cells support long-term expansion of embryonic and adult stem and progenitor cells. Stem cells (Dayton, Ohio) 2008;26(10):2516–22. doi: 10.1634/stemcells.2008-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koruji M, Movahedin M, Mowla SJ, Gourabi H, Arfaee AJ. Efficiency of adult mouse spermatogonial stem cell colony formation under several culture conditions. Vitro Cell Dev Biol Anim. 2009;45(5–6):281–9. doi: 10.1007/s11626-008-9169-y. [DOI] [PubMed] [Google Scholar]
- 26.Mohamadi SM, Movahedin M, Koruji SM, Jafarabadi MA, Makoolati Z. Comparison of colony formation in adult mouse spermatogonial stem cells developed in Sertoli and STO coculture systems. Andrologia. 2012;44(1):431–437. doi:10.1111/j.1439-0272.2011.01201.x. [DOI] [PubMed]
- 27.Liu S, Tang Z, Xiong T, Tang W. Isolation and characterization of human spermatogonial stem cells. Reprod Biol Endocrinol. 2011;9:141. doi: 10.1186/1477-7827-9-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirzapour T, Movahedin M, Tengku Ibrahim TA, Koruji M, Haron AW, Nowroozi MR, et al. Effects of basic fibroblast growth factor and leukaemia inhibitory factor on proliferation and short-term culture of human spermatogonial stem cells. Andrologia. 2012. doi:10.1111/j.1439-0272.2010.01135.x. [DOI] [PubMed]
- 29.Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad SciU S A. 1994;91(24):11303–7. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowley MJ, Berlin JD, Heller CG. The ultrastructure of four types of human spermatogonia. Z Zellforsch Mikrosk Anat. 1971;112(2):139–57. doi: 10.1007/BF00331837. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann MC, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; influence of GDNF. Dev Biol. 2005;279(1):114–24. doi: 10.1016/j.ydbio.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116(6):769–78. doi: 10.1016/S0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–31. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 34.Simon L, Ekman GC, Tyagi G, Hess RA, Murphy KM, Cooke PS. Common and distinct factors regulate expression of mRNA for ETV5 and GDNF, Sertoli cell proteins essential for spermatogonial stem cell maintenance. Exp Cell Res. 2007;313(14):3090–9. doi: 10.1016/j.yexcr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Aponte P, Rooij D. Biomanipulation of bovine spermatogonial stem cells. Anim Reprod. 2008;5:16–22. [Google Scholar]
- 36.Dirami G, Ravindranath N, Pursel V, Dym M. Effects of stem cell factor and granulocyte macrophage-colony stimulating factor on survival of porcine type A spermatogonia cultured in KSOM. Biol Reprod. 1999;61(1):225–30. doi: 10.1095/biolreprod61.1.225. [DOI] [PubMed] [Google Scholar]
- 37.Shinohara T, Avarbock MR, Brinster RL. beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 1999;96(10):5504–9. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orwig KE, Shinohara T, Avarbock MR, Brinster RL. Functional analysis of stem cells in the adult rat testis. Biol Reprod. 2002;66(4):944–9. doi: 10.1095/biolreprod66.4.944. [DOI] [PubMed] [Google Scholar]
- 39.Shinohara T, Avarbock MR, Brinster RL. Functional analysis of spermatogonial stem cells in Steel and cryptorchid infertile mouse models. Dev Biol. 2000;220(2):401–11. doi: 10.1006/dbio.2000.9655. [DOI] [PubMed] [Google Scholar]
- 40.Kokil SN, Patil PR, Mahadik KR, Paradkar AR. Effect of molecular weight of hydrolyzed gelatin on its binding properties in tablets: a technical note. AAPS PharmSciTech. 2004;5(3):38–42. doi: 10.1208/pt050341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, et al. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456(7220):344–9. doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- 42.Kanatsu-Shinohara M, Lee J, Inoue K, Ogonuki N, Miki H, Toyokuni S, et al. Pluripotency of a single spermatogonial stem cell in mice. Biol Reprod. 2008;78(4):681–7. doi: 10.1095/biolreprod.107.066068. [DOI] [PubMed] [Google Scholar]
- 43.Hermann B, Sukhwani M, Hansel M, Orwig K. Spermatogonial stem cells in higher primates: are there differences to those in rodents? Reproduction. 2009. doi:10.1530/REP-09-0255. [DOI] [PMC free article] [PubMed]
- 44.Ohbo K, Yoshida S, Ohmura M, Ohneda O, Ogawa T, Tsuchiya H, et al. Identification and characterization of stem cells in prepubertal spermatogenesis in mice small star, filled. Dev Biol. 2003;258(1):209–25. doi: 10.1016/S0012-1606(03)00111-8. [DOI] [PubMed] [Google Scholar]
- 45.Ohmura M, Yoshida S, Ide Y, Nagamatsu G, Suda T, Ohbo K. Spatial analysis of germ stem cell development in Oct-4/EGFP transgenic mice. Arch Histol Cytol. 2004;67(4):285–96. doi: 10.1679/aohc.67.285. [DOI] [PubMed] [Google Scholar]
- 46.Bhartiya D, Kasiviswanathan S, Unni SK, Pethe P, Dhabalia JV, Patwardhan S, et al. Newer insights into premeiotic development of germ cells in adult human testis using Oct-4 as astem cell marker. J Histochem Cytochem. 2010;58(12):1093–106. doi: 10.1369/jhc.2010.956870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Izadyar F, Wong J, Maki C, Pacchiarotti J, Ramos T, Howerton K, et al. Identification and characterization of repopulating spermatogonial stem cells from the adult human testis. Hum Reprod. 2011;26(6):1296–306. doi: 10.1093/humrep/der026. [DOI] [PubMed] [Google Scholar]
- 48.Dann CT, Alvarado AL, Molyneux LA, Denard BS, Garbers DL, Porteus MH. Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem cells (Dayton, Ohio) 2008;26(11):2928–37. doi: 10.1634/stemcells.2008-0134. [DOI] [PubMed] [Google Scholar]
- 49.Kubota H, Avarbock MR, Brinster RL. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci USA. 2003;100(11):6487–92. doi: 10.1073/pnas.0631767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reijo R, Seligman J, Dinulos MB, Jaffe T, Brown LG, Disteche CM, et al. Mouse autosomal homolog of DAZ, a candidate male sterility gene in humans, is expressed in male germ cells before and after puberty. Genomics. 1996;35(2):346–52. doi: 10.1006/geno.1996.0366. [DOI] [PubMed] [Google Scholar]
- 51.Hermann BP, Sukhwani M, Lin CC, Sheng Y, Tomko J, Rodriguez M, et al. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem cells (Dayton, Ohio) 2007;25(9):2330–8. doi: 10.1634/stemcells.2007-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brinster RL. Germline stem cell transplantation and transgenesis. Science (New York, NY. 2002;296(5576):2174–6. doi: 10.1126/science.1071607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McLean DJ. Spermatogonial stem cell transplantation, testicular function, and restoration of male fertility in mice. Methods Mol Biol (Clifton, NJ) 2008;450:149–62. doi: 10.1007/978-1-60327-214-8_11. [DOI] [PubMed] [Google Scholar]
- 54.Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc Natl Acad Sci USA. 2000;97(15):8346–51. doi: 10.1073/pnas.97.15.8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bellve AR, Cavicchia JC, Millette CF, O’Brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol. 1977;74(1):68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiarini-Garcia H, Russell LD. High-resolution light microscopic characterization of mouse spermatogonia. Biol Reprod. 2001;65(4):1170–8. doi: 10.1095/biolreprod65.4.1170. [DOI] [PubMed] [Google Scholar]
- 57.Morena AR, Boitani C, Pesce M, Felici M, Stefanini M. Isolation of highly purified type A spermatogonia from prepubertal rat testis. J Androl. 1996;17(6):708–17. [PubMed] [Google Scholar]