Abstract
Purpose
Natural killer (NK) cells express killer immunoglobulin-like receptors (KIRs) which recognize HLA class I molecules on trophoblasts. KIRs could either activate NK cells or inhibit them to produce soluble factors necessary for the maintenance of pregnancy, thus they are suspected of being involved in the causes of recurrent miscarriage. The aim of this study was to evaluate whether there is any possible association between KIR genes, genotypes and recurrent miscarriage.
Methods
The present study was carried out on 40 women who had unexplained recurrent miscarriage and 90 controls. Sequence-specific oligonucleotide probes analysis were used to investigate 16 KIR genes. All data were statistically analyzed by Fisher Exact Test.
Results
The rate of Bx genotypes that consists elevated number of activating KIR genes was significantly higher (p = 0.014) in women with recurrent miscarriage when compared with the control group. Additionally, the frequency of AA genotype (AA1) of the subjects in the study group was significantly lower than the frequency of the subjects in the control group (p = 0,014). Furthermore, there were no statistically significant differences in the frequencies of the individual KIR genes between women with recurrent miscarriage and the control group.
Conclusions
Inclined balance of KIRs toward an activating state in NK cells may contribute to recurrent miscarriage.
Keywords: Killer cell immunoglobulin-like receptors, KIR, Natural killer cells, Recurrent miscarriage, Trophoblast
Introduction
Recurrent miscarriage is an important clinical entity seen in early periods of pregnant women which has an incidence of 2–5 %. Spontaneous abortions seen 3 times or more until the 20th gestational week have been called as recurrent miscarriage [3]. The cause of a miscarriage may reside in the patients’ genes, anatomy, endocrine, immune and blood-clotting systems, and ⁄or the environment, but in many cases no cause can be found. In fact, the most possible cause can be determined in only about half of the patients [15,24].
Decidual natural killer (NK) cells are known to play an important role in allo-recognition mechanisms in pregnancy [17]. NK cells which are the large granular cells of the immune system are the third major lymphocyte subgroup after T and B lymphocytes, comprising 10–15 % of all lymphocytes in the blood stream [23]. During the first trimester of pregnancy, NK cells generate the CD3-CD16-CD56 phenotypes and dominant decidual cell populations [12,13]. These constitute nearly 70 % of the total leukocyte population on the uterine mucosa and have a different mechanism of action than the NK cells in the blood stream [13]. It is thought that these cells have an important effect on normal pregnancy due to their increase and direct interaction with the invaded trophoblasts [24,25]. While the uterine mucosal NK cells are found during the early pregnancy, they decrease after the 20th week of gestation and totally disappear at the end of pregnancy [13].
The mechanism of how NK cells recognize the target cells and distinguish them from normal cells depends on the balance of the signals produced by the activating and inhibiting receptors [31]. One group of the NK cell receptors is the killer immunoglobulin-like receptors (KIRs). KIRs are glycoproteins classified according to their structure and function. Each KIR molecule consists of two or three extracellular immunoglobulin domains (2D and 3D molecules, respectively), a transmembrane part and a short (S) or a long (L) intracellular tail [25].
KIR gene family is located within the leukocyte receptor cluster on chromosome 19q13.4. According to the order of KIR genes and gene content, haplotypes are divided into A and B. The A haplotype contains six loci coding inhibitory receptors 2DL1, 2DL3, 2DL4, 3DL1, 3DL2, 3DL3, and only one activating KIR 2DS4. The B haplotype is presented by a great variety of subtypes that differ from each other mostly by combinations of stimulatory receptors [18,19,27].
Of the 16 known KIR genes, seven encode the inhibitory receptors KIR2DL1-3, KIR2DL5, and KIR3DL1-3; six encode the activating receptors KIR2DS1-5 and KIR3DS1; one encodes the activating⁄inhibitory receptor KIR2DL4; and two are pseudogenes KIR2DP1 and KIR3DP1 that do not encode any functional KIR receptor [1,4,11]. Seven genes within this KIR repertoire are known to encode receptors for the HLA class I molecules expressed on trophoblasts; KIR2DL1, KIR2DL2, KIR2DL3, KIR2DS1, KIR2DS2, KIR2DS4, and KIR2DL4 [14,30]. KIRs, by their inhibiting and activating isotypes have a substantial role on organizing the NK cell activity [5]. It has been proposed that decrease in inhibiting KIRs or increase in activated KIR-HLA ligand interaction might be related to fatal birth and recurrent miscarriage [16].
Placental villous trophoblasts do not express HLA-A, HLA-B, or HLA class II molecules which are responsible for T cell dependant graft rejection. On the other hand, trophoblastic cells express combinations of HLA-C, HLA-E, and HLA-G molecules which are specific ligands for NK cell receptors [24]. Of these, only HLA-C is highly polymorphic and interact with KIRs on the NK cell surface [5].
It is proposed that surface receptors encoded by different KIR genes and genotypes, and ligands of these receptors may play a role in autoimmune mechanisms of recurrent miscarriage. Although few, there are studies showing the relationship between decreased inhibiting KIRs or increased activating KIR-HLA ligand relation and recurrent miscarriages [8,10,26,28]. Thus, KIR genotyping in women with a history of recurrent miscarriage might be important in order to understand these mechanisms and in processing a therapeutic approach. So, we aimed to see whether any association between activating and inhibiting KIR genes and recurrent miscarriage exists or not.
Materials and methods
Study subjects and samples
This study was composed of a group of the appropriate female cases from a population study for the determination of KIR genes. The study group was consisted of 40 healthy women with a history of recurrent miscarriage from the Mediterranean coast settled Anatolian Caucasians all of whom were evaluated by an obstetrician. Explained cases with recurrent miscarriage were excluded from the study and only women with unexplained recurrent miscarriage with the same partner were included in the study group. The control group was consisted of 90 healthy multipar women who had at least two healthy children and who had no miscarriage history and matched by ethno-geographic origin. All women in both groups were informed about the study and gave informed consent. Blood samples of all participants were preserved in tubes containing etilendiaminotetraenoic acid in 2–8 °C for genetic evaluation.
KIR genotyping
DNA from venous blood sample of each subject was extracted by DNA isolation kit (Qiagen EZ1, cat no: 951034, QIAGEN Vertriebs GmbH, Vienna, Austria) by using Genovision Geno M-6 Biorobot technology (GenoVision Inc. Philadelphia, USA). Genotyping of the KIR genes was performed using the multiplex KIR-SSO typing kit from Tepnel Lifecodes Corporation (Ref: 545110, Connecticut, USA). This product consists of a mixture of locus-specific oligonucleotide probes coupled to color-coded microspheres (Luminex Corp) and two PCR reactions for the amplification of KIR-exons 4, 5, 7, 8, and 9. To type each sample, PCR was performed and the product was hybridized with the SSO-probe mixture using the manufacturer’s protocol. After hybridization, the sample plate was placed in a Luminex instrument for analysis.
Prediction of group-A and -B KIR haplotypes
Frequencies of group-A and -B KIR haplotypes were deduced from the genotype data [18]. Individuals carrying only KIR3DL3, 2DL3, 2DL1, 2DP1, 3DP1, 2DL4, 3DL1, 2DS4, 3DL2, a fixed gene content characteristic of group-A haplotypes, were considered carrying two copies of group-A KIR haplotypes (AA genotypes). If any of genes KIR2DL2, 2DL5, 3DS1, 2DS1, 2DS2, 2DS3, 2DS5 were present then genotype was taken as having B haplotype (Bx) [18].
Statistics
The percentage of each KIR gene in the study and control groups were determined by direct counting (individuals positive for the gene/individuals tested per population × 100). Data analysis was performed with the statistical soft-ware Minitab Version 15. Differences between two groups in the distribution of each KIR gene and genotype were estimated by two-tailed Fisher Exact test and p < 0.05 was considered to be statistically significant.
Results
KIR genes and genotypes in women with recurrent miscarriage history
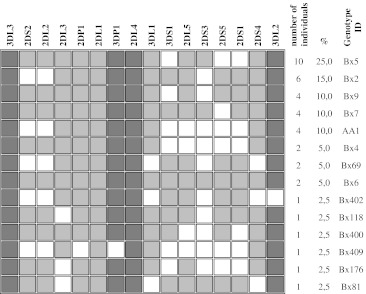
From the four framework genes, KIR2DL4 and 3DL3 were present in all of the samples, but 3DL2 and 3DP1 were present in 97,5 % of women with recurrent miscarriage history. KIR pseudogene KIR2DP1 was present in most samples (in 97,5 % of the study group and 98,9 % of the control group). A tendency was found that inhibitory KIR genes have a higher frequency compared to activating KIR genes in all samples (study and control groups). Among them, inhibitory KIR genes 2DL1, 2DL3 and 3DL1 had higher frequencies in all samples, which were more than 82,2 %. With the exception of KIR2DS4, frequencies of the remaining activating genes were all lower than 65 % (Table 1). Fourteen different genotypes were found in 40 women with recurrent miscarriage history. The most frequent genotypes found in 25 % of participants were consisted from 3DL3, 2DS2, 2DL2, 2DL3, 2DP1, 2DL1, 3DP1, 2DL4, 3DL1, 2DL5, 2DS3, 2DS4, 3DL2 genes which corresponds to Bx genotype (genotype ID: Bx5) (Fig. 1). Only 4 participants (10 %) in the study group demonstrated the presence of AA genotype which consists only one activating gene, 2DS4 (genotype ID: AA1) (Figs. 1 and 2). Other analysed participants in the study group demonstrated the presence of more than one activating gene and thus may be considered as Bx. Only 2 subjects (5 %) from the study group possessed all 16 KIR genes (genotype ID: Bx6). The remaining genotypes contained between 8 to 15 KIR genes. Six genotypes have been seen only once (Fig. 1).
Table 1.
Comparison of the frequencies of each KIR gene of the subjects in the study (n = 40) and control (n = 90) groups
| Gene | Control group number % | Study group number % | P value | |||
|---|---|---|---|---|---|---|
| Inhibitory KIRs | 2DL1 | 89 | 98,9 | 40 | 100 | 1 |
| 2DL2 | 41 | 45,5 | 26 | 65 | 0,057 | |
| 2DL3 | 74 | 82,2 | 37 | 92,5 | 0,179 | |
| 2DL4 | 90 | 100 | 40 | 100 | 1 | |
| 2DL5 | 56 | 56,7 | 32 | 80 | 0,067 | |
| 3DL1 | 81 | 90 | 36 | 90 | 1 | |
| 3DL2 | 90 | 100 | 39 | 97,5 | 0,308 | |
| 3DL3 | 90 | 100 | 40 | 100 | 1 | |
| Activating KIRs | 2DS1 | 31 | 34,4 | 21 | 52,5 | 0,080 |
| 2DS2 | 41 | 45,5 | 26 | 65 | 0,057 | |
| 2DS3 | 29 | 32,2 | 17 | 42,5 | 0,321 | |
| 2DS4 | 82 | 91,1 | 36 | 90 | 1 | |
| 2DS5 | 35 | 38,9 | 22 | 55 | 0,125 | |
| 3DS1 | 37 | 41,1 | 16 | 40 | 1 | |
| pseudogene | 2DP1 | 89 | 98,9 | 39 | 97,5 | 0,522 |
| 3DP1 | 90 | 100 | 39 | 97,5 | 0,308 | |
Fig. 1.
KIR genotype profiles of women with recurrent miscarriage. Fourteen genotypes that differed from each other by the presence (shaded box) and absence (white box) of 16 KIR genes were observed. The figure consisted the percentage frequency and defined the number of individuals carrying the genotype. Genotype ID refers to genotype classification according to www.allelefrequencies.net [18]
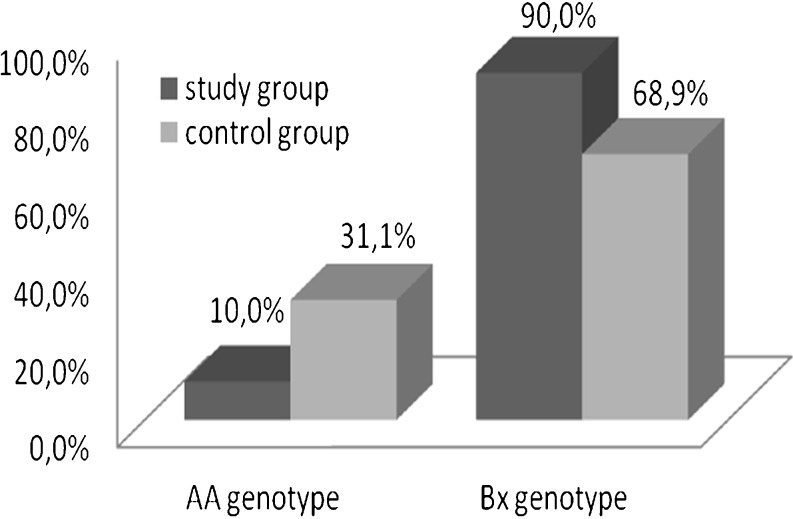
Fig. 2.
The frequencies of Bx and AA genotypes of the study and control groups. While the frequency of AA genotype of the study group was significantly lower than the control group, the rate of Bx genotypes was significantly higher in the study group (p = 0,014)
Relationship of KIR genes with recurrent miscarriage
Frequencies of individual KIR genes were compared between two groups (Table 1). All 16 KIR genes and 5 different alleles of 2DS4 were compared by Fisher Exact test. Although activating receptor 2DS2 gene frequency was observed to be higher in the study group, this result was statistically insignificant (p = 0,057) (Table 1). Comparison of the groups in terms of KIR gene frequency showed no statistical significance (p > 0,05) (Table 1). Since the methods used for KIR genotyping permitted to distinguish groups of alleles of activating KIR2DS4, we found that there were no statistically significant differences for 2DS4*001 and 2DS4*003/4/6/7 alleles between the study and control groups (Table 2). On the other hand the rate of Bx genotypes which consists elevated number of activating KIR genes was significantly higher (p = 0.014) in women with recurrent miscarriage (90 %) when compared with the control group (68,9 %). Additionally, the frequency of AA genotype (AA1) of the subjects in the study group (10 %) was significantly lower than the frequency of the subjects in the control group (31,1 %), (p = 0,014), (Figs. 1 and 2).
Table 2.
Comparison of the frequencies of five different alleles of KIR2DS4 gene of the subjects who had this gene in the study (n = 36) and control (n = 82) groups
| Alleles of 2DS4 | Study group (n = 36) n % | Control group (n = 82) n % | p values | ||
|---|---|---|---|---|---|
| 2DS4*001 | 8 | 22,2 | 12 | 14,6 | 0,424 |
| 2DS4*001 and 2DS4*003/4/6/7 | 7 | 19,5 | 20 | 24,4 | 0,639 |
| 2DS4*003/4/6/7 | 21 | 58,3 | 50 | 61,0 | 0,840 |
Discussion
Several factors have been proposed to explain the maternal tolerance to the potentially allogenic fetus, although our understanding of the immunobiology of normal pregnancy and its implications for pregnancy-related pathologies is still limited. Understanding the basis for the observed genetic associations is complicated due to the large repertoire of receptors used by NK cells to interpret their environment. There is extensive polymorphism among KIR haplotypes, which differ not only in nucleotide sequence but also in gene content [22]. This genetic complexity echoes the complications confronted clinically in defining and diagnosing recurrent miscarriage which can be considered not so much a disease or disorder but ‘simply the extreme end of a continuum of characteristics common to all pregnancies’ [5]. Our present study is the first report demonstrating the association of maternal NK cell KIR gene repertoire with recurrent miscarriage among Anatolian women.
There are only a few studies to date where maternal KIR gene repertoires have been investigated for recurrent miscarriages, and these have conflicting conclusions. Varla-Leftherioti et al. [26] proposed that the cause of the miscarriage in women who had experienced an allo-immune abortus is that they have a limited number of inhibiting KIR repertoire and thus trophoblastic HLA class I molecules were not recognized by the decidual NK cells and perceived as foreign. Our study differed from this study as that inhibiting KIR genes did not show significant differences between the study and control groups. In concordant with our study, Witt et al. [29] also did not see a significant relation of KIR gene polymorphisms and recurrent miscarriage. Wang et al. [28] reported that activating KIR genes were higher in women with recurrent abortus according to the fertile control group in a study from a Chinese Han Population. In our study, although the activating receptor gene KIR2DS2 was seen higher in the study group, this was statistically insignificant (p = 0,057).
There are also studies indicating that allo-immune abortus has been seen more often in cases where there is a difference in inhibiting KIR receptors (2DL1, 2DL2, 2DL3) between spouses and in women who has a limited number of KIR repertoire [2,20]. Flores et al. [7] conducted similar studies in 30 couples and their findings supported that in recurrent miscarriage subjects, the balance between inhibitory and activating receptors present in natural killer cells is inclined toward an activating state that may contribute to pregnancy loss. They however found an increased prevalence of the inhibitory AA genotypes among the patients’ cohort. Additionally, Hiby et al. [8], in a study on 73 recurrent miscarriage patients, reported an increased prevalence of the maternal AA genotype. On the other hand, in a study of Faridi et al. [5] from a North Indian population noticed that the patients with recurrent misscariage showed a higher prevalence of B haplotypes when compared with A haplotypes. In addition, recently Nowak et al. [21] suggested that KIR AA and HLA-C heterozygous women who have HLA-C C2 homozygous partners showed an increased chance of successful pregnancy. In concordant with these findings, in our study we found a significantly higher frequency of Bx genotype in the study group and higher frequency of AA genotype in the control group. It is important to appreciate the influence of different ethnic groups while conducting such studies on the outcome of KIR frequencies, as KIR genotypes have a wide geographical distribution.
KIR2DL4, by binding to HLA-G synthesized by trophoblasts is one of important receptors in feto-maternal interactions. Yan et al. [30] reported that the surface expression of KIR2DL4 receptors have been significantly higher in women who had recurrent miscarriage than the control group. In our study, we did not assess the surface expression of KIR2DL4, but there was no significant difference between two groups for the KIR2DL4 gene frequency.
The KIR locus is polygenic and polymorphic, giving rise to variable KIR repertoires that are expressed on subsets of NK cells among individuals. It is unclear if and which receptors on uterine NK cells interact with HLA molecules which are expressed on trophoblasts and whether such interactions inhibit NK cell lysis, or lead to production of cytokines that favor normal placental development and maintenance of pregnancy. Some of the studies showed that maternal KIR/trophoblast HLA-C interaction has been important in placentation and recurrent miscarriages [6,9]. Therefore, the necessary difference providing fetal allorejection might be the HLA-C molecules only. Understanding the immune mechanisms on the fetomaternal surface better will help comprehend how the fetus continues her life in the uterus. Findings support that NK cells have an important role during pregnancy. There is a possibility of disarrangement of NK cells and thus end in fetal allograft rejection. Unfortunately there are conflicting results on the immune mechanisms of recurrent miscarriages in the literature. In order to understand the mechanisms better studies should be held in larger groups and in different populations. Examining KIR receptor expression, gene and genotype distribution, and relation between KIR ligands such as HLA-C and HLA-G is also important. Additionally more spesific techniques on the molecular level will help unveil this problematic issue.
Footnotes
Capsule
Bx genotypes that encodes elevated numbers of activating killer cell immunoglobulin-like receptors may be one of the contributing factors of unexplained recurrent miscarriages.
References
- 1.Borrego F, Kabat J, Kim DK, Lieto L, Maasho K, Pena J, Solana R, Coligan JE. Structure and function of major histocompatibility complex (MHC) class I specific receptors expressed on human natural killer (NK) cells. Mol Immunol. 2002;38:637–660. doi: 10.1016/S0161-5890(01)00107-9. [DOI] [PubMed] [Google Scholar]
- 2.Clark DA, Chaouat G, Wong K, Gorczynski RM, Kinsky R. Tolerance mechanisms in pregnancy. A reappraisal of the role of class I paternal MHC antigens. Am J Reprod Immunol. 2010;63:93–103. doi: 10.1111/j.1600-0897.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- 3.Coulam CB, Clark DA, Beer AE, Kutteh WH, Silver R, Kwak J, Stephenson M. Current clinical options for diagnosis and treatment of recurrent spontaneous abortion. Clinical Guidelines Recommendation Committee for Diagnosis and Treatment of Recurrent Spontaneous Abortion. Am J Reprod Immunol. 1997;38:57–74. doi: 10.1111/j.1600-0897.1997.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 4.Du Z, Gjertson DW, Reed EF, Rajalingam R. Receptor-ligand analyses define minimal killer cell Iglike receptor (KIR) in humans. Immunogenetics. 2007;59:1–15. doi: 10.1007/s00251-006-0168-4. [DOI] [PubMed] [Google Scholar]
- 5.Faridi RM, Das V, Tripthi G, Talwar S, Parveen F, Agrawal S. Influence of activating and inhibitory killer immunoglobulin-like receptors on predisposition to recurrent miscarriages. Hum Reprod. 2009;24:1758–1764. doi: 10.1093/humrep/dep047. [DOI] [PubMed] [Google Scholar]
- 6.Faridi RM, Agrawal S. Killer immunoglobulin-like receptors (KIRs) and HLA-C allorecognition patterns implicative of dominant activation of natural killer cells contribute to recurrent miscarriages. Hum Reprod. 2011;26:491–497. doi: 10.1093/humrep/deq341. [DOI] [PubMed] [Google Scholar]
- 7.Flores AC, Marcos CY, Paladino N, Arruvito L, Williams F, Middleton D, Fainboim L. KIR receptors and HLA-C in the maintenance of pregnancy. Tissue Antigens. 2007;69(Suppl 1):112–113. doi: 10.1111/j.1399-0039.2006.762_8.x. [DOI] [PubMed] [Google Scholar]
- 8.Hiby SE, Regan L, Lo W, Farrell L, Carrington M, Moffett A. Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage. Hum Reprod. 2008;23:972–976. doi: 10.1093/humrep/den011. [DOI] [PubMed] [Google Scholar]
- 9.Hiby SE, Apps R, Sharkey AM, Farrell LE, Gardner L, Mulder A, Claas FH, Walker JJ, Redman CC, Morgan L, Tower C, Regan L, Moore GE, Carrington M, Moffett A. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest. 2010;120:4102–4110. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong Y, Wang X, Lu P, Song Y, Lin Q. Killer immunoglobulin-like receptor repertoire on uterine natural killer cell subsets in women with recurrent spontaneous abortions. Euro J Obstet Gynecol Reprod Biol. 2008;140:218–223. doi: 10.1016/j.ejogrb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Hsu KC, Liu XR, Selvakumar A, Mickelson E, O’Reilly RJ, Dupont B. Killer Ig-like receptor haplotype analysis by gene content: evidence for genomic diversity with a minimum of six basic framework haplotypes, each with multiple subsets. J Immunol. 2002;169:5118–5129. doi: 10.4049/jimmunol.169.9.5118. [DOI] [PubMed] [Google Scholar]
- 12.King A, Burrows T, Loke YW. Human uterine natural killer cells. Nat Immunol. 1996;5:41–52. [PubMed] [Google Scholar]
- 13.King A, Burrows T, Verma S, Hiby S, Loke YW. Human uterine lymphocytes. Hum Reprod Update. 1998;4:480–485. doi: 10.1093/humupd/4.5.480. [DOI] [PubMed] [Google Scholar]
- 14.King A, Hiby SE, Gardner L, Joseph S, Bowen JM, Verma S, Burrows TD, Loke YW. Recognition of trophoblast HLA class I molecules by decidual NK cell receptors – a review. Placenta. 2000;21(Suppl. A):S81–S85. doi: 10.1053/plac.1999.0520. [DOI] [PubMed] [Google Scholar]
- 15.Kiwi R. Recurrent pregnancy loss: evaluation and discussion of the causes and their management. Cleve Clin J Med. 2006;73:913–921. doi: 10.3949/ccjm.73.10.913. [DOI] [PubMed] [Google Scholar]
- 16.Kulkarni S, Martin MP, Carrington M. The Yin and Yang of HLA and KIR in human disease. Semin Immunol. 2008;20:343–352. doi: 10.1016/j.smim.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Male V, Trundley A, Gardner L, Northfield J, Chang C, Apps R, Moffett A. Natural killer cells in human pregnancy. Methods Mol Biol. 2010;612:447–463. doi: 10.1007/978-1-60761-362-6_30. [DOI] [PubMed] [Google Scholar]
- 18.Middleton D, Menchaca L, Rood H, Komerofsky R (2003) New allele frequency database: http://www.allelefrequencies.net. Tissue Antigens 61:403–407. [DOI] [PubMed]
- 19.Middleton D, Williams F, Halfpenny IA. KIR genes. Transpl Immunol. 2005;14:135–142. doi: 10.1016/j.trim.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Nowak I, Malinowski A, Tchorzewski H, Barcz E, Wilczynski JR, Grybos M, Kurpisz M, Luszczek W, Banasik M, Reszczynska-Slezak D, Majorczyk E, Wisniewski A, Senitzer D, Yao Sun J, Kusnierczyk P. Frequencies of killer immunoglobulin-like receptor genotypes influence susceptibility to spontaneous abortion. J Appl Genet. 2009;50:391–398. doi: 10.1007/BF03195699. [DOI] [PubMed] [Google Scholar]
- 21.Nowak I, Malinowski A, Tchorzewski H, Barcz E, Wilczynski JR, Banasik M, Grybos M, Kurpisz M, Luszczek W, Majorczyk E, Wisniewski A, Senitzer D, Sung JY, Kusnierczyk P. HLA-C C1C2 heterozygosity may protect women bearing the killer immunoglobulin-like receptor AA genotype from spontaneous abortion. J Reprod Immunol. 2011;88:32–37. doi: 10.1016/j.jri.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5:201–214. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- 23.Ravet S, Scott-Algara D, Bonnet E, Tran HK, Tran T, Nguyen N, Truong LX, Theodorou I, Barre-Sinoussi F, Pancino G, Paul P. Distinctive NK-cell receptor repertoires sustain high-level constitutive NK-cell activation in HIV-exposed uninfected individuals. Blood. 2007;109:4296–4305. doi: 10.1182/blood-2006-08-040238. [DOI] [PubMed] [Google Scholar]
- 24.Vargas RG, Bompeixe EP, Franca PP, Moraes MM, Bicalho MG. Activating killer cell ımmunoglobulin-like receptor genes’ association with recurrent miscarriage. Am J Reprod Immunol. 2009;62:34–43. doi: 10.1111/j.1600-0897.2009.00709.x. [DOI] [PubMed] [Google Scholar]
- 25.Varla-Leftherioti M. Role of a KIR/HLA-C allorecognition system in pregnancy. J Reprod Immunol. 2004;62:19–27. doi: 10.1016/j.jri.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Varla-Leftherioti M, Spyropoulou-Vlachou M, Keramitsoglou T, et al. Lack of the appropriate natural killer cell inhibitory receptors in women with spontaneous abortion. Hum Immunol. 2005;66:65–71. doi: 10.1016/j.humimm.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Vilches C, Parham P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu Rev Immunol. 2002;20:217–251. doi: 10.1146/annurev.immunol.20.092501.134942. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, Zhao YR, Jiao YL, et al. Increased activating killer immunoglobulin-like receptor genes and decreased specific HLA-C alleles in couples with recurrent spontaneous abortion. Biochem Biophys Res Commun. 2007;360:696–701. doi: 10.1016/j.bbrc.2007.06.125. [DOI] [PubMed] [Google Scholar]
- 29.Witt CS, Goodridge J, Gerbase-Delima MG, Daher S, Christiansen FT. Maternal KIR repertoire is not associated with recurrent spontaneous abortion. Hum Reprod. 2004;19:2653–2657. doi: 10.1093/humrep/deh483. [DOI] [PubMed] [Google Scholar]
- 30.Yan WH, Lin A, Chen BG, Zhou MY, Dai MZ, Chen XJ, Gan LH, Zhu M, Shi WW, Li BL. Possible roles of KIR2DL4 expression on uNK cells in human pregnancy. Am J Reprod Immunol. 2007;57:233–242. doi: 10.1111/j.1600-0897.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 31.Yokohama WM. Recognition structures on natural killer cells. Curr Opin Immunol. 1993;5:67–73. doi: 10.1016/0952-7915(93)90083-5. [DOI] [PubMed] [Google Scholar]