Abstract
Purpose
Standard semen parameters are poor predictors of fertility potential. To date, apart from, paternal karyotyping sperm factors are not evaluated in recurrent pregnancy loss (RPL), only recent studies have emphasized the role of sperm factors in early embryonic development as sperm transcribes genes critical for early embryonic development. Sperm DNA integrity is useful diagnostic and prognostic marker and has clinical implications in idiopathic recurrent pregnancy loss (iRPL) following spontaneous conception. The aim of this study was to assess DNA integrity in cases experiencing iRPL following spontaneous conception.
Methods
Semen samples from 45 patients and 20 controls were analyzed as per WHO 1999 guidelines and sperm chromatin structure assay (SCSA) was used to measure DNA fragmentation index (DFI).
Results
By applying receiver operating curve (ROC) analysis, sperm DFI of approximately 26 % was found in male partner of couples experiencing iRPL.
Conclusions
Our data indicate that sperm from men with a history of iRPL have a higher percentage of DNA damage as compared to control group, and this can explain pregnancy loss in these patients. Men with higher DFI are infertile whereas men with lower DFI (26 %) are able to conceive but experience recurrent pregnancy loss. Thus it is important to evaluate sperm DFI in couples experiencing iRPL to understand exact aetiology of RPL and determine prognosis and management.
Keywords: Idiopathic recurrent pregnancy loss, SCSA, DFI, Sperm
Introduction
Recurrent pregnancy loss (RPL) is a devastating reproductive problem affecting approximately 5 % of couples trying to conceive. It is defined as the occurrence of two or more consecutive pregnancy failures. Factors associated with RPL include parental chromosome translocations, uterine malformations, endocrine, haematological and autoimmune factors. In spite of exhaustive investigation, approximately 40 % of RPL cases remain unexplained and are classified as idiopathic RPL (iRPL) [40]. A significant proportions of these cases are believed to be caused by as yet unidentified genetic mechanisms [34]. Several studies have implicated the role of female factor in recurrent miscarriages, but the role of male factor has only recently been realised in couples experiencing sporadic ART failure even after ICSI [3]. Sperm DNA integrity plays a vital role in development of the embryo and fetal well being. DNA damage may result in poorly embryonic development [30]. Sperm DNA damage may occur by at least one of three fundamental mechanisms: defective chromatin condensation or abortive apoptosis during spermatogenesis or transportation of sperm through the male or the female genital tract [38] and oxidative damage to sperm DNA, which results in accumulation of mutagenic bases like 8-hydroxy-2- deoxyguanosine, creates abasic sites, single and double strand breaks. Oxidative stress also damages guanine rich highly conserved hexameric repeats at ends of chromosomes known as telomeres. This results in single strand breaks and shortening of telomeres. Sperm DNA damage is common amongst infertile men with normal and abnormal sperm parameters and may adversely affect the outcome of both natural and assisted conception [44, 47]. Because of its clinical benefit there has been an increase in the use of sperm DNA integrity tests in the evaluation of infertile men. Till date few studies have evaluated the role of sperm DFI in iRPL cases following spontaneous conception [3, 11, 21]. Analysis of DNA integrity in understanding the role of sperm factor in iRPL may reduce the number of cases diagnosed as idiopathic and aid in providing most adapted therapeutics to the couple. This study highlights that men with normal semen parameters may harbour significant DNA damage and the need to evaluate DNA integrity in men with history of RPL.
Materials and methods
Study design
After approval from ethical board of the institute (IESC/T-364), a total of 45 iRPL couples and 20 control men were enrolled for the study. Cases were referred to Laboratory for Molecular Reproduction and Genetics from the department of Obstetrics and Gynecology, All India institute of Medical sciences (AIIMS). Selection criteria included a history of at least three prior pregnancy losses at <20 weeks of gestation, with all pregnancies fathered by the same partner. All the known causes of RPL were ruled out, and only idiopathic cases were recruited. In brief, female partners of couples having normal haemogram (Hb, TLC, DLC, ESR, platelets, PT, CT) with normal ovarian function (USG of ovary for PCOS), a normal uterus confirmed by vaginal ultrasound and/or hysterosalpingography/ hysteroscopy, have normal endocrinological parameters (level of T3, T4,TSH, FSH, LH, PRL), with normal in per speculum and per vaginal examination were enrolled. Other causes of RPL like thrombophilia both inherited (Proglob C) and acquired (anti cardiolipin-IgG and IgM) were also screened and male partner of such females were only included in the study. Both male and female had normal karyotype with no history of autoimmune or endocrine disorders. Male partners of couples who had recently fathered a child, (not older than 2 years) were enrolled as fertile controls and were age matched with cases. All the fertile males had normal karyotype, normal semen profile, and no family history of disease. Other confounding factors (varicocele, antioxidant intake etc.) that may affect DNA damage levels were ruled out in both patient and control. Written informed consent was obtained from both cases and controls.
Semen collection and analysis
Semen samples were obtained by masturbation in a sterile plastic container after 4 days of sexual abstinence. After liquefaction at 37°C, semen analysis was performed as per the WHO guidelines [46]. An aliquot of 100 μl of the raw semen was stored at −80°C until SCSA analysis.
SCSA
The SCSA was performed as per the protocol described by Evenson et al. [16]. After complete analysis of the sample, the X-mean (red fluorescence) and Y-mean (green fluorescence) values were recorded manually after selecting gate for sperm cells using FlowJo software (Oregon). Extent of DNA denaturation (damage) was expressed as DFI, which is the ratio of red to total (red plus green) fluorescence, i.e., the level of denatured DNA over the total DNA [18]. The percentage of high DNA stainability cells (HDS) were also recorded in each sample manually from the graph plot. HDS represents another distinct population in semen that characterize immature spermatozoa with incomplete chromatin condensation. The DFI of the patients and controls were analysed in duplicate by a single operator, and the mean value was used for comparison.
Statistical analysis
Statistical analyses was performed using MedCalc trial version for Windows (MedCalc Software, Mariakerke, Belgium). Data is represented as mean ± standard deviation. All the comparisons between controls and patients were calculated using Student’s t-test. The two-tailed P value < 0.05 was considered as significant. Receiver operating characteristic (ROC) curve analysis was applied to obtain the cut-off value of DFI to differentiate patient from control.
Results
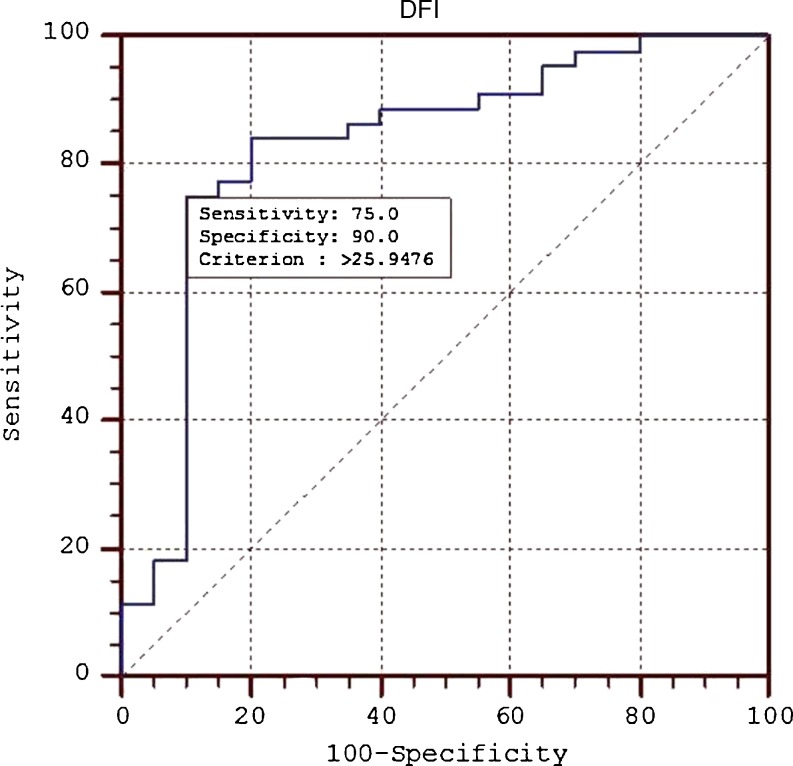
The age of the men was 30–45 years (33.17 ± 5.16) in the RPL group compared to 25–32 years (31.5 ± 5.28) in the proven fertile control. The semen parameters in men, in RPL group were within the reference range and nothing was found significantly different in comparison to controls except sperm morphology (Table 1). The mean DFI of cases was found to be 28.06 ± 4.99 (17.63,44.07), which is 1.2 times higher as compared to controls: 21.75 ± 4.76 (14.84,32.69) (P < 0.05). The median sperm DFI of cases (27.80) was significantly higher in comparison to controls (21.40). Using receiver operating characteristic (ROC) curve analysis, a threshold value of 26 % was obtained to discriminate from the control group. The area under the curve was 0.830 (P < 0.0001; 95 % CI, 0.715 to 0.912), with 75 % sensitivity and 90 % specificity (Fig. 1). From ROC curve analysis, DFI of 26 % was set as the threshold value to differentiate between iRPL cases and controls. The mean value of high DNA stainability cells was 8.8 ± 2.8 and 9.0 ± 3.0 in control and patient group respectively (P > 0.05).
Table 1.
Semen parameters in RPL male partners and controls
| Parameter | Controls | Patients | P value |
|---|---|---|---|
| Age | 31.5 ± 5.28 | 33.17 ± 5.16 | 0.180 |
| Volume (mL) | 03.10 ± 01.34 | 02.98 ± 01.02 | 0.670 |
| pH | 08.00 ± 00.12 | 07.87 ± 00.29 | 0.045 |
| Concentration | 130 ± 58.96 | 97.90 ± 102.64 | 0.140 |
| Progressive motility (grade a) | 35.21 ± 04.12 | 34.35 ± 05.23 | 0.060 |
| Abnormal forms (%) | 50.33 ± 10.63 | 59.19 ± 11.18 | 0.001* |
| DFI | 21.75 ± 4.76 | 28.06 ± 4.99 | 0.0124* |
P-value <0.05 was considered to be significant
Fig. 1.
Receiver operating curve analysis of DFI in patient and control group
Discussion
Sperm DNA damage leads to increased pre and post implantation losses, increased incidence of miscarriages, high rate of congenital malformations and even childhood cancer [13, 29]. High level of spermatozoal DNA damage may hinder fecundity (fertility potential) of an individual in vivo and outcome of assisted reproduction. Investigating the integrity of sperm DNA has both diagnostic and prognostic implications in couple experiencing repeated pregnancy loss in vivo or vitro. Several studies have proven that semen quality and embryo development are related. Several techniques have also been developed to evaluate paternal DNA integrity independent of other sperm parameters (sperm count, morphology and motility). Abnormal spermatozoa with reduced DNA quality may compromise cleavage rate and thus affect blastocyst formation [25]. Spermatozoa carrying fragmented DNA can fertilize a mature oocyte, but activation of paternal genome further impairs embryonic development, resulting in pre and post implantation losses. DNA strand breaks are usually formed during meiosis (recombination) and chromatin remodelling during maturation, and oxidative stress induces both DNA denaturation and accumulation of mutagenic bases, which causes GC → TA, transversion which lead to single strand and double strand break [1, 33]. In a yet unpublished study from our laboratory, we have found shorter sperm telomere length secondary to oxidative damage and high sperm DFI in cases with iRPL. Zini and colleague [48] reported that percentage of sperm with denatured and fragmented DNA was 25 to 28 % in infertile men as compared to 10 to 13 % in fertile men. They also reported that infertile men have >30 % denatured DNA and is associated with poor embryo quality and miscarriage. A number of repair pathways have been reported that support the maintenance of spermatozoa DNA integrity. Among these, the excision repair pathways remove most of the physical damage to DNA as well as replication errors. However, mature spermatozoa being transcrptionally inert is not at par to repair the damage and the high proportion of embryo DNA damage are thought to be derived from male gamete [2]. Also, genetic polymorphisms of DNA repair genes (XRCC1, XPD6, XPD23) were significantly associated with high DFI [35]. Human germ cell has limited capacity to repair oxidative DNA lesions [33], which may lead to denovo mutation. Thus, transmutation of paternal genome may compromise embryo quality, viability and maintaining pregnancy, ultimately leading to recurrent pregnancy loss. Oocytes are well equipped to handle DNA damage in sperm by different pathways like direct reversal of damage, single-strand damage repair, base excision repair, nucleotide excision repair, mismatch repair that repair sperm DNA damages [26, 31]. Oocytes can repair sperm DNA damage, but there is a threshold beyond which sperm DNA may not be repaired, and accumulation of ethenonucleosides (type of DNA lesion) in sperm may inhibit nucleotide excision repair mechanism. The mutational load thus carried by the embryo after fertilization have a high level of DNA damage is influenced by DNA repair capacity of oocyte. There is little information regarding fidelity and nature of repair mechanism of oocyte, but they are negatively impacted by age. In this study, mean age of female partner was 27.8 ± 4.89, and thus the repair mechanism would be adequate as compared to ageing oocyte in women ≥35 years of age [23]. Because, trans-4-hydroxy-2- nonenal, which is involved in ethenonucleotide synthesis, inhibit NER in human cell [19]. Thus, persistence of sperm DNA damage is associated with poor pregnancy outcome and pre or post implantation loss. With the advent of modern assisted conception techniques, the role of sperm factor in recurrent assisted conception loss is being realized. Although the threshold, for sperm DFI in infertile men is established [5, 8, 15, 44] the role of sperm factor in iRPL following spontaneous conception has not been extensively evaluated [3, 20], and DFI cut -off value has not yet been established. This study has tried to establish a threshold value of sperm DFI in cases with iRPL following spontaneous conception for the first time in India and is amongst the few studies in the world [28, 39]. Studies have suggested that pregnancy is unlikely to occur when sperm nuclear DFI is above a certain threshold value [28, 39]. Using sperm chromatin dispersion test (SCD), Bellver et al [3] observed that sperm DNA fragmentation was higher in the recurrent spontaneous abortion group compared with the sperm donor group. Moreover, Brahem et al [7] also indicated that sperm from men with a history of RPL had a higher incidence of DNA damage and poor motility than sperm from a control group. From large animal and human studies, Evenson and colleagues, who first described SCSA, suggested that threshold of 0–15 %. 16–29 % and >30 % DNA fragmentation index correlate to high, moderate and low fertility potential, respectively [16, 18]. The clinical role of sperm DNA damage assessment by SCSA in assisted reproduction has also been established. Although the different thresholds have applied to assisted reproduction samples, and no IVF or ICSI pregnancies were reported when the DFI of the neat semen sample was >27 % [28]. Similar cut-offs of 28 % for 33 IUI/IVF/ICSI/ cycles [36], 20 % in a group of 104 IVF and ICSI cycles [4] and 27 % for 113 IVF and ICSI cycles have been reported [27]. [10] have also shown the importance of this chromatin assay as an independent predictor in assisted reproduction outcome. The study proved that the outcome parameters like biochemical pregnancy, clinical pregnancy and delivery were significantly decreased in couples with DFI >30 % as compared with couples with DFI ≤30 %. However, ICSI was found to be a better choice than IVF for couples with DFI >30 %. However, some studies did not find a correlation between sperm DNA damage and pregnancy rates at IVF/ICSI [24, 27]. Lack of an association in these studies could be due to selection of morphologically normal sperm and selection of superior quality embryos for transfer at IVF/ICSI and could explain for lack of an association between sperm DNA damage and pregnancy rates in these studies. We found a significant difference between sperm morphology of control and patient group. Alteration in sperm chromatin compaction in infertile men, (protamine deficiency or incomplete sulfhydryl oxidation) may contribute to head morphology defects as described in animal models [14, 41]. Sperm morphology has been correlated with quality of sperm DNA [6, 43], this is in contrast to other studies where no association of sperm morphology and DFI was found [12, 49]. Spermatozoa with compromised genomic integrity may fertilize but may not sustain the pregnancy due to damaged DNA. The variation within DFI in different, independent studies is probably due to inclusion of different patient population and non stringent inclusion criteria. In this study, we included only those cases where both partners were cytogenetically normal, female were normal on clinical and gynaecology examination, and male partner had normal semen parameters and hormonal parameters in both patients were normal and so was the blood profile. As compared to our study in infertile men in which 30 % of sperm DFI was found to be associated with infertility and such couple were unable to conceive, in this study, cases with DFI of approximately 26 % were able to conceive but were unable to sustain pregnancy and experienced recurrent pregnancy loss. As no other cause, of RPL could be identified in these cases the results from this preliminary study show that a high DFI (26 %) did not affect fertilization but adversely affects embryogenesis because post activation of the embryonic genome 3 days post fertilization, DNA damage may lead to increased mutation load in every cell of the embryo if it escapes the oocyte repair mechanism and may result in post-implantation loss. Several other independent studies [37, 42, 45] reported a negative effect on the formation of the blastocyst and implantation rate when there was high DFI, and this was termed as the late paternal effect by Tesarik et al [42] to distinguish from other effects that don’t depend on DFI. The sperm DNA is packaged into two compartments the tightly protamine bound toroid and peripheral histone bound fraction which transcribes genes of developmental importance. It is this outer portion of sperm genome, which is vulnerable to environmental insult and oxidative damage. Oxidative damage may first damage transcriptionally active histone bound peripheral portion of sperm genome and induce oxidative lesions. In a yet unpublished study, we have found shorter telomere length of sperm DNA in infertile and iRPL cases with high ROS levels. Thus, damage to histone bound sperm genome adversely affects genomic integrity and may result in early pregnancy loss. DNA fragmentation in mature male germ cell is usually caused by external factors such as reactive oxygen species (ROS), rather than cell’s own programmed death [24]. Study has proven that DNA fragmentation measured by the SCSA originates (a part) from oxidative products (8-hydroxy-deoxyguanosine) [32]. It is therefore necessary to evaluate sperm DFI and the origin of increased DFI, so that a couple can be provided appropriate therapeutics.
Other SCSA parameter like percentage of high DNA stainability cells (%HDS) were also analysed. The compactness of sperm nucleus is determined by sperm DNA stainability. An increase in percentage of HDS suggests that the chromatin is less compact as DNA binding dye (acridine orange) has more access to the less condensed sperm DNA. Thus, estimation of sperm %HDS provides information about the sperm chromatin condensation and hence the quality of DNA. This SCSA parameter has statistically been reported to be an independent predictor for the establishment of pregnancy in vivo and vitro, but the result was not consistent in other studies. A threshold value of 15 %HDS was reported in establishing pregnancy in vivo [17] and with lower fertilization rate for in vitro fertilization [45]. However, [9] found that chance of pregnancy and delivery was significantly higher in couple with DFI ≤26 % or HDS ≤10 % as compared to those with DFI ≥27 % or HDS ≥10 %. Further, the same group did not find any upper limit of HDS to predict the outcome of assisted reproduction, neither alone or with DFI. In this study also we did not detect any threshold value of HDS to discriminate between the two groups.
Detecting high DFI and its underlying aetiology is of paramount importance in iRPL as it aids in providing comprehensive counselling and most adapted therapeutics to the couple. Incorporation of DNA fragmentation assay in routine diagnostic workup of couples experiencing idiopathic recurrent pregnancy loss may be helpful [22]. This will certainly reduce the burden of couples experiencing iRPL and increase carry home live birth rate. Thus, sperm is not a mere vector of paternal DNA but also plays a critical role in embryogenesis. Recent studies have documented that paternal DNA damage is associated with increased autosomal dominant disorders, cancer, increased prenatal morbidity, congenital malformations and childhood cancer. This study the first from India established a cut-off value of DFI in cases experiencing recurrent abortions following spontaneous conception. Interestingly, the low threshold value of sperm DFI (26 %) in male partner of iRPL couple in comparison to infertile (30 %) men can be explained. It is postulated, that cases with higher sperm DFI are unable to conceive while men with iRPL couple were successful in conceiving but could not sustain the pregnancy. Thus, knowledge of causative aetiology can help determine strategies to minimise DNA damage and improve pregnancy and live birth rate.
Conclusion
Sperm DNA damage may be the underlying aetiology of poor reproductive outcome including impaired development of the embryo resulting in an increased risk of morbidity in the offspring. Loss of genome integrity may be a risk factor in couples experiencing recurrent iRPL and thus analysis of sperm DNA damage has both diagnostic and prognostic implications in couples experiencing iRPL following spontaneous conception. This study has identified an increased incidence of sperm DNA fragmentation and established a cut-off value of sperm DFI (26 %) in male partner of couples with iRPL following spontaneous conception. Thus, DNA integrity assessment will narrow down the number of RPL cases identified as idiopathic. It also highlights the need to evaluate the cause of raised DFI, so that the appropriate measures can be taken to prevent or minimize DNA damage to achieve a successful pregnancy. Thus, this study highlights the association of increased DFI and iRPL but such finding should be confirmed by large scale studies in different populations.
Acknowledgment
The grant and support from Department of Biotechnology (BT/PR13558/MED/30/282/2010) is highly acknowledged.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
Footnotes
Capsule
Recurrent pregnancy loss affects 3-5% of couples and the aetiology is not known in about 40% of cases. Despite comprehensive analysis of all female factors leading to this condition, the male factor is ignored especially in cases conceived spontaneously. In this article we emphasized role of sperm DNA integrity in etiology of this condition and how it can impact management.
Contributor Information
Kishlay Kumar, Email: kishlaykumar@gmail.com.
Rima Dada, Email: rima_dada@rediffmail.com.
References
- 1.Badouard C, Menezo Y, Panteix G, Ravanat JL, Douki T, Cadet J, Favier A. Determination of new types of DNA lesions in human sperm. Zygote. 2008;16(1):9–13. doi: 10.1017/S0967199407004340. [DOI] [PubMed] [Google Scholar]
- 2.Barratt CL, Aitken RJ, Bjorndahl L, Carrell DT, Boer P, Kvist U, Lewis SE, Perreault SD, Perry MJ, Ramos L, Robaire B, Ward S, Zini A. Sperm DNA: organization, protection and vulnerability: from basic science to clinical applications–a position report. Hum Reprod. 2010;25(4):824–838. doi: 10.1093/humrep/dep465. [DOI] [PubMed] [Google Scholar]
- 3.Bellver J, Meseguer M, Muriel L, Garcia-Herrero S, Barreto MA, Garda AL, Remohi J, Pellicer A, Garrido N. Y chromosome microdeletions, sperm DNA fragmentation and sperm oxidative stress as causes of recurrent spontaneous abortion of unknown etiology. Hum Reprod. 2010;25(7):1713–1721. doi: 10.1093/humrep/deq098. [DOI] [PubMed] [Google Scholar]
- 4.Benchaib M, Braun V, Lornage J, Hadj S, Salle B, Lejeune H, Guerin JF. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum Reprod. 2003;18(5):1023–1028. doi: 10.1093/humrep/deg228. [DOI] [PubMed] [Google Scholar]
- 5.Boe-Hansen GB, Fedder J, Ersboll AK, Christensen P. The sperm chromatin structure assay as a diagnostic tool in the human fertility clinic. Hum Reprod. 2006;21(6):1576–1582. doi: 10.1093/humrep/del019. [DOI] [PubMed] [Google Scholar]
- 6.Borini A, Tarozzi N, Bizzaro D, Bonu MA, Fava L, Flamigni C, Coticchio G. Sperm DNA fragmentation: paternal effect on early post-implantation embryo development in ART. Hum Reprod. 2006;21(11):2876–2881. doi: 10.1093/humrep/del251. [DOI] [PubMed] [Google Scholar]
- 7.Brahem S, Mehdi M, Landolsi H, Mougou S, Elghezal H, Saad A. Semen parameters and sperm DNA fragmentation as causes of recurrent pregnancy loss. Urology. 2011. doi:10.1016/j.urology.2011.05.049. [DOI] [PubMed]
- 8.Bungum M, Bungum L, Giwercman A. Sperm chromatin structure assay (SCSA): a tool in diagnosis and treatment of infertility. Asian J Androl. 2011;13(1):69–75. doi: 10.1038/aja.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bungum MP, Humaidan, et al. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod. 2004;19(6):1401–8. doi: 10.1093/humrep/deh280. [DOI] [PubMed] [Google Scholar]
- 10.Bungum MP, Humaidan, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22(1):174–9. doi: 10.1093/humrep/del326. [DOI] [PubMed] [Google Scholar]
- 11.Carrell DT, Wilcox AL, Lowy L, Peterson CM, Jones KP, Erickson L, Campbell B, Branch DW, Hatasaka HH. Elevated sperm chromosome aneuploidy and apoptosis in patients with unexplained recurrent pregnancy loss. Obstet Gynecol. 2003;101(6):1229–1235. doi: 10.1016/S0029-7844(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 12.Cassuto NG, Hazout A, Hammoud I, Balet R, Bouret D, Barak Y, Jellad S, Plouchart JM, Selva J, Yazbeck C. Correlation between DNA defect and sperm-head morphology. Reprod Biomed Online. 2012;24(2):211–218. doi: 10.1016/j.rbmo.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Cho C, Jung-Ha H, Willis WD, Goulding EH, Stein P, Xu Z, Schultz RM, Hecht NB, Eddy EM. Protamine 2 deficiency leads to sperm DNA damage and embryo death in mice. Biol Reprod. 2003;69(1):211–217. doi: 10.1095/biolreprod.102.015115. [DOI] [PubMed] [Google Scholar]
- 14.Cho C, Willis WD, Goulding EH, Jung-Ha H, Choi YC, Hecht NB, Eddy EM. Haploinsufficiency of protamine-1 or −2 causes infertility in mice. Nat Genet. 2001;28(1):82–86. doi: 10.1038/ng0501-82. [DOI] [PubMed] [Google Scholar]
- 15.Erenpreiss J, Elzanaty S, Giwercman A. Sperm DNA damage in men from infertile couples. Asian J Androl. 2008;10(5):786–790. doi: 10.1111/j.1745-7262.2008.00417.x. [DOI] [PubMed] [Google Scholar]
- 16.Evenson D, Jost L. Sperm chromatin structure assay is useful for fertility assessment. Methods Cell Sci. 2000;22(2–3):169–189. doi: 10.1023/A:1009844109023. [DOI] [PubMed] [Google Scholar]
- 17.Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, Purvis K, Angelis P, Claussen OP. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod. 1999;14(4):1039–1049. doi: 10.1093/humrep/14.4.1039. [DOI] [PubMed] [Google Scholar]
- 18.Evenson DP, Larson KL, Jost LK. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl. 2002;23(1):25–43. doi: 10.1002/j.1939-4640.2002.tb02599.x. [DOI] [PubMed] [Google Scholar]
- 19.Feng Z, Hu W, Tang MS. Trans-4-hydroxy-2-nonenal inhibits nucleotide excision repair in human cells: a possible mechanism for lipid peroxidation-induced carcinogenesis. Proc Natl Acad Sci U S A. 2004;101(23):8598–8602. doi: 10.1073/pnas.0402794101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gil-Villa AM, Cardona-Maya W, Agarwal A, Sharma R, Cadavid A. Assessment of sperm factors possibly involved in early recurrent pregnancy loss. Fertil Steril. 2010;94(4):1465–1472. doi: 10.1016/j.fertnstert.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 21.Gil-Villa AM, Cardona-Maya W, Agarwal A, Sharma R, Cadavid Á. Assessment of sperm factors possibly involved in early recurrent pregnancy loss. Fertil Steril. 2010;94(4):1465–1472. doi: 10.1016/j.fertnstert.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 22.Gupta S, Agarwal A, Banerjee J, Alvarez JG (2007) The role of oxidative stress in spontaneous abortion and recurrent pregnancy loss: a systematic review. Obstet Gynecol Surv 62 (5):335–347; quiz 353–334. doi:10.1097/01.ogx.0000261644.89300.df [DOI] [PubMed]
- 23.Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, Dudekula DB, VanBuren V, Ko MS. Age-associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet. 2004;13(19):2263–2278. doi: 10.1093/hmg/ddh241. [DOI] [PubMed] [Google Scholar]
- 24.Henkel R, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, Menkveld R, Gips H, Schill WB, Kruger TF. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil Steril. 2004;81(4):965–972. doi: 10.1016/j.fertnstert.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 25.Janny L, Menezo YJ. Evidence for a strong paternal effect on human preimplantation embryo development and blastocyst formation. Mol Reprod Dev. 1994;38(1):36–42. doi: 10.1002/mrd.1080380107. [DOI] [PubMed] [Google Scholar]
- 26.Jaroudi S, Kakourou G, Cawood S, Doshi A, Ranieri DM, Serhal P, Harper JC, SenGupta SB. Expression profiling of DNA repair genes in human oocytes and blastocysts using microarrays. Hum Reprod. 2009;24(10):2649–2655. doi: 10.1093/humrep/dep224. [DOI] [PubMed] [Google Scholar]
- 27.Larson-Cook KL, Brannian JD, Hansen KA, Kasperson KM, Aamold ET, Evenson DP. Relationship between the outcomes of assisted reproductive techniques and sperm DNA fragmentation as measured by the sperm chromatin structure assay. Fertil Steril. 2003;80(4):895–902. doi: 10.1016/S0015-0282(03)01116-6. [DOI] [PubMed] [Google Scholar]
- 28.Larson KL, DeJonge CJ, Barnes AM, Jost LK, Evenson DP. Sperm chromatin structure assay parameters as predictors of failed pregnancy following assisted reproductive techniques. Hum Reprod. 2000;15(8):1717–1722. doi: 10.1093/humrep/15.8.1717. [DOI] [PubMed] [Google Scholar]
- 29.Lewis SE, Aitken RJ. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res. 2005;322(1):33–41. doi: 10.1007/s00441-005-1097-5. [DOI] [PubMed] [Google Scholar]
- 30.Marchesi DE, Feng HL. Sperm DNA integrity from sperm to egg. J Androl. 2007;28(4):481–489. doi: 10.2164/jandrol.106.002105. [DOI] [PubMed] [Google Scholar]
- 31.Menezo Y, Dale B, Cohen M. DNA damage and repair in human oocytes and embryos: a review. Zygote. 2010;18(4):357–365. doi: 10.1017/S0967199410000286. [DOI] [PubMed] [Google Scholar]
- 32.Oger I, Cruz C, Panteix G, Menezo Y. Evaluating human sperm DNA integrity: relationship between 8-hydroxydeoxyguanosine quantification and the sperm chromatin structure assay. Zygote. 2003;11(4):367–371. doi: 10.1017/S0967199403002442. [DOI] [PubMed] [Google Scholar]
- 33.Olsen AK, Duale N, Bjoras M, Larsen CT, Wiger R, Holme JA, Seeberg EC, Brunborg G. Limited repair of 8-hydroxy-7,8-dihydroguanine residues in human testicular cells. Nucleic Acids Res. 2003;31(4):1351–1363. doi: 10.1093/nar/gkg216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;368(9535):601–611. doi: 10.1016/S0140-6736(06)69204-0. [DOI] [PubMed] [Google Scholar]
- 35.Rubes J, Rybar R, Prinosilova P, Veznik Z, Chvatalova I, Solansky I, Sram RJ. Genetic polymorphisms influence the susceptibility of men to sperm DNA damage associated with exposure to air pollution. Mutat Res. 2010;683(1–2):9–15. doi: 10.1016/j.mrfmmm.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Saleh RA, Agarwal A, Nada EA, El-Tonsy MH, Sharma RK, Meyer A, Nelson DR, Thomas AJ. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril. 2003;79(Suppl 3):1597–1605. doi: 10.1016/S0015-0282(03)00337-6. [DOI] [PubMed] [Google Scholar]
- 37.Seli E, Gardner DK, Schoolcraft WB, Moffatt O, Sakkas D. Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil Steril. 2004;82(2):378–383. doi: 10.1016/j.fertnstert.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 38.Shen H, Ong C. Detection of oxidative DNA damage in human sperm and its association with sperm function and male infertility. Free Radic Biol Med. 2000;28(4):529–536. doi: 10.1016/S0891-5849(99)00234-8. [DOI] [PubMed] [Google Scholar]
- 39.Spano M, Bonde JP, Hjollund HI, Kolstad HA, Cordelli E, Leter G. Sperm chromatin damage impairs human fertility. The Danish first pregnancy planner study team. Fertil Steril. 2000;73(1):43–50. doi: 10.1016/S0015-0282(99)00462-8. [DOI] [PubMed] [Google Scholar]
- 40.Stephenson M, Kutteh W. Evaluation and management of recurrent early pregnancy loss. Clin Obstet Gynecol. 2007;50(1):132–145. doi: 10.1097/GRF.0b013e31802f1c28. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka H, Iguchi N, Isotani A, Kitamura K, Toyama Y, Matsuoka Y, Onishi M, Masai K, Maekawa M, Toshimori K, Okabe M, Nishimune Y. HANP1/H1T2, A novel histone H1-like protein involved in nuclear formation and sperm fertility. Mol Cell Biol. 2005;25(16):7107–7119. doi: 10.1128/MCB.25.16.7107-7119.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tesarik J, Greco E, Mendoza C. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum Reprod. 2004;19(3):611–615. doi: 10.1093/humrep/deh127. [DOI] [PubMed] [Google Scholar]
- 43.Trisini AT, Singh NP, Duty SM, Hauser R. Relationship between human semen parameters and deoxyribonucleic acid damage assessed by the neutral comet assay. Fertil Steril. 2004;82(6):1623–1632. doi: 10.1016/j.fertnstert.2004.05.087. [DOI] [PubMed] [Google Scholar]
- 44.Venkatesh S, Singh A, Shamsi MB, Thilagavathi J, Kumar R, Mitra DK, Dada R. Clinical significance of sperm DNA damage threshold value in the assessment of male infertility. Reprod Sci. 2011;18(10):1005–1013. doi: 10.1177/1933719111401662. [DOI] [PubMed] [Google Scholar]
- 45.Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril. 2004;81(5):1289–1295. doi: 10.1016/j.fertnstert.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 46.WHO, editor. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 47.Zini A, Boman JM, Belzile E, Ciampi A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: systematic review and meta-analysis. Hum Reprod. 2008;23(12):2663–2668. doi: 10.1093/humrep/den321. [DOI] [PubMed] [Google Scholar]
- 48.Zini A, Meriano J, Kader K, Jarvi K, Laskin CA, Cadesky K. Potential adverse effect of sperm DNA damage on embryo quality after ICSI. Hum Reprod. 2005;20(12):3476–3480. doi: 10.1093/humrep/dei266. [DOI] [PubMed] [Google Scholar]
- 49.Zini A, Phillips S, Courchesne A, Boman JM, Baazeem A, Bissonnette F, Kadoch IJ, San Gabriel M. Sperm head morphology is related to high deoxyribonucleic acid stainability assessed by sperm chromatin structure assay. Fertil Steril. 2009;91(6):2495–2500. doi: 10.1016/j.fertnstert.2008.03.032. [DOI] [PubMed] [Google Scholar]