Abstract
Objective
To compare the dynamics of early development between embryos cultured in single and sequential media.
Design
Randomized, comparative study.
Setting
Private IVF centre.
Patients
A total of 446 metaphase II oocytes from 51 couples who underwent oocyte retrieval procedure for intracytoplasmic sperm injection. Forty-nine resulted in embryo transfer.
Intervention
Oocytes were split between single and sequential media produced by the same manufacturer and cultured in a time-lapse incubator.
Main outcome measures
Morphokinetic parameters until the embryos reached the 5-cell stage (t5), utilization, clinical pregnancy and implantation rates.
Results
Embryos cultured in single media were advanced from the first mitosis cycle and reached 2- to 5-cell stages earlier. There was not any difference between the durations for cell cycle two (cc2 = t3-t2) and s2 (t4-t3). The utilization, clinical pregnancy and implantation rates did not differ between groups. The proportion of cryopreserved day6 embryos to two pronuclei oocytes was significantly higher in sequential than in single media.
Conclusions
Morphokinetics of embryo development vary between single and sequential culture media at least until the 5-cell stage. The overall clinical and embryological parameters remain similar regardless of the culture system.
Keywords: Embryo culture media, Time-lapse recording, Human embryo development
Introduction
Maintaining optimal embryo viability during human in vitro culture is of key importance for the success of assisted reproduction treatments. Although culture media constitutes only one component of in vitro conditions, its interaction with the surrounding environment has a significant impact in determination of the total yield of viable embryos in a cohort [1]. It has been postulated that human embryos are exposed to constant stress under in vitro conditions as none of the culture media fully mimic the in vivo milieu [1]. Adaptation of embryos to stress has consequences such as diminished development due to increased cell death [2] and alterations in expression or imprinting of key genes [3, 4]. Culture media has also been shown to influence development and birth-weight of the fetus [5].
In order to establish an in vitro culture environment with minimal hazardous effect on embryo development, two views compete on the ideal composition of media. According to the ‘back to nature’ approach, culture system should meet demands of the embryos to simulate their passage from the Fallopian tubes to the uterus [6]. During this passage, physiology of the embryo changes before and after compaction, hence ingredients of the media differ in a sequential system. In the ‘let the embryo choose” scheme simultaneous use of all concentration in a mixture (single culture media) is employed with the assumption that the embryo will utilize whatever it requires [7–9].
Among few comparative studies which evaluate potential (dis)advantages of these strategies towards the other, randomization of groups and utilized culture media brands differed significantly. For example, randomization of fertilized oocytes into two commercially available media favored single media because of a higher day5 blastocyst yield despite a similar embryo quality on day3 [10]. The clinical outcome figures remained inconclusive as the majority of transfers included embryos from both groups. Other studies utilizing various accessible media randomized patients [11, 12] or donors [13] into groups. The results supported single media approach due to a higher proportion of compacted day3 embryos to zygotes [13], a higher utilization rate at day3 [12], a higher morula formation at 4 [13], a higher blastocyst yield at day5 [10, 13], and an increased implantation rate as compared to the sequential system [10, 13]. Only in one study [11] there was not any difference in embryo development and clinical outcome.
Predicting the efficiency of culture systems requires comparative studies in which the impact of confounding variables such as patient, cycle and gamete characteristics on embryo development have been minimized. Furthermore, it is recommended to utilize one culture media brand particularly during prolonged culture because under similar conditions, reaction of various media with the in vitro environment differs depending on their diverse ingredients causing homeostatic stress, mainly at the time of fertilization [12] and a subsequent impact on the embryos which may interfere with eventual pregnancy outcome and fetal health [1].
Besides the abovementioned methodological variations, one cause of differences between studies may arise from the conventional static observation technique itself, as it does not allow accurate timing of embryo morphokinetics. Introduction of time-lapse technology into clinical human assisted reproduction treatments [14, 15] enabled a more precise definition of embryo development dynamics and an algorithm has been given to select the embryo with the highest implantation potential [15]. A hierarchical classification of poor to best embryos, beginning from those displaying very poor morphology (i.e., apparently not viable), cleaving from one to three or more cells, showing uneven initial cleavage and multinucleation at the 4-cell stage and eventual selection of the remaining by utilizing parameters t5, s2 and cc2 have been presented. Embryos that met all criteria (cleaving in the pre-defined time zones) have been reported to possess implantation capacities up to 66 %. Yet, this study has been conducted by utilization of a sequential system and whether various culture systems have an impact on pre-defined time points of certain events during development needs to be assessed.
The aim of the present study was to compare morphokinetic parameters of embryo development in single versus sequential media produced by the same manufacturer in a sibling oocyte model and to assess clinical outcome of cycles where transfer embryos have been cultured in respective media. The results will be anticipated to not only contribute to understanding the differences between these strategies but also to whether implantation prediction models based on time-lapse analysis is applicable to various embryo culture systems.
Materials and methods
The present is a randomized comparative study in which autologous oocyte retrieval cycles at a single centre between March to November 2011 were assessed. As the standard laboratory procedures and commercially available embryo culture media have been used, Institutional Review Board approval was not required. The maximum number of mature (MII) oocytes was restricted to 12, as this was the capacity of one dish (EmbryoSlide™) in which oocytes/embryos could be cultured and the minimum to 2, because this was the minimum number to be able to make a comparison between groups. Women who were younger than 40 years of age have been included in order to minimize the impact of increased risk of aneuploidy on the results. Cycles in which oocytes/embryos have been subjected to biopsy were also excluded due to the potential risk of delayed embryo development in such cases [14]. Furthermore, cycles where etiology of infertility was endometrial factor were not included due to potential effect on implantation despite good embryo development. There was not any restriction on the number of previous trials and/or sperm source.
All embryo culture media used in the present study were from one manufacturer (Irvine Scientific, Irvine, CA). This manufacturer produces both single (Single Step Media; SSM) and sequential media (Early Cleavage Media; ECM until day3 and MultiBlast Media; MB until day6) containing 25 mM sodium bicarbonate as buffer systems and osmolarity ranging between 280 ± 8 mOsm. These media were supplemented with 10 % synthetic serum substitute (SSS, Irvine Scientific). All oocytes/embryos assessed in the presented study have been cultured in a time-lapse incubator (EmbryoScope™, UniSense Fertilitech, Denmark) which has been set to 37 °C, 6 % CO2 and 5 % O2. The 12-wells of the EmbryoSlides™ dish were filled with SSM and ECM/MB (6 wells each), covered with mineral oil (Irvine Scientific) and were equilibrated overnight before use. The pH of both media has been validated with two independent systems; one daily (pHOnline, MTG, Germany) and other between batches (Beckman-Coulter, CA, USA) to range between 7.28 ± 0.4 under this environment.
Patients underwent ovarian stimulation in an agonist or antagonist protocol combined with a mixed FSH (75–300 IU)/LH (75–150 IU) administration. Oocyte retrieval has been scheduled to 35 h after triggering with 10,000 IU hCG. Oocytes have been rinsed and collected into 50 μl drops of modified human tubal fluid (mHTF, Irvine Scientific) in 10 % SSS which was kept in a humidified, heated and gassed environment through a mini-incubator placed into the laminar flow cabinet. After all oocytes have been retrieved, they were taken into a fresh drop of mHTF and placed into an incubator until denudation, which has been performed approximately after 3 h. Intracytoplasmic sperm injection has been performed 1 h following denudation.
Semen sample has been prepared by discontinuous colloidal silica gel gradient (PureSperm; Nidacon, Sweden) and has been subsequently washed twice in HTF medium. Sperm was selected under high magnification (IMSI) with an appropriate optical system before insemination [16]. Total time for intracytoplasmic sperm injection did not exceed 10 min. Oocytes that extruded a polar body after denudation were not included into the study in order not to introduce bias into groups as the incidence of poor fertilization/poor embryo development has been shown to be higher in late-matured oocytes [17].
Randomization of MII oocytes has been done after intracytoplasmic sperm injection; oocytes were pooled into a 50 μl drop of modified human tubal fluid (mHTF, Irvine Scientific) in 10 % SSS, rinsed and have been distributed in EmbryoSlides™ regardless of injection order. Subsequently they were placed in the EmbryoScope™ and were cultured until day3 without media change. At day3, embryos were transferred into a new EmbryoSlide™ dish in which wells were filled with pre-equilibrated SSM and MB in 10 % SSS. Embryo culture was terminated at day6.
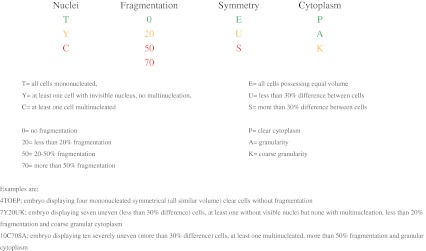
Embryos to be transferred or cryopreserved have been selected according to their morphologic [6, 18, 19] and morphokinetic parameters [15]. Morphological selection criteria included blastomere number, symmetry, nuclear morphology and the proportion of fragmentation to the embryo volume during cleavage stages (Figures 1 and 2) and cell number of the inner cell mass and the trophoectoderm as well as the degree of expansion of the blastocoel cavity in blastocysts (Figure 2). Embryos possessing a good morphology but poor prognosis according to dynamic observation parameters have not been transferred but cryopreserved. These embryos were those which cleaved to more than 2-cells or with severe asymmetry (>30 % difference between blastomere volumes) at the first mitotic cleavage and those with multinucleation at 4-cell stage. According to the legislation, women younger than 35 years of age have been transferred one embryo if they had less than three previous attempts. All others have been transferred two embryos. The embryologist who selected transfer embryos (H.N.C) was blinded to the culture media information. The physicians and the patients have been consulted daily to determine day of transfers which have been scheduled mainly according to the number of good quality embryos to meet the criteria determined by the legislation but sometimes also to patients’ or physicians’ demands. Cryopreservation of surplus embryos has been performed until day6 using a vitrification protocol as described by the manufacturer.
Fig. 1.
Cleavage-stage scoring; Traffic lights; green is ‘good for transfer’, yellow is ‘transferable’, and presence of one red is ‘not good for transfer’
Fig. 2.
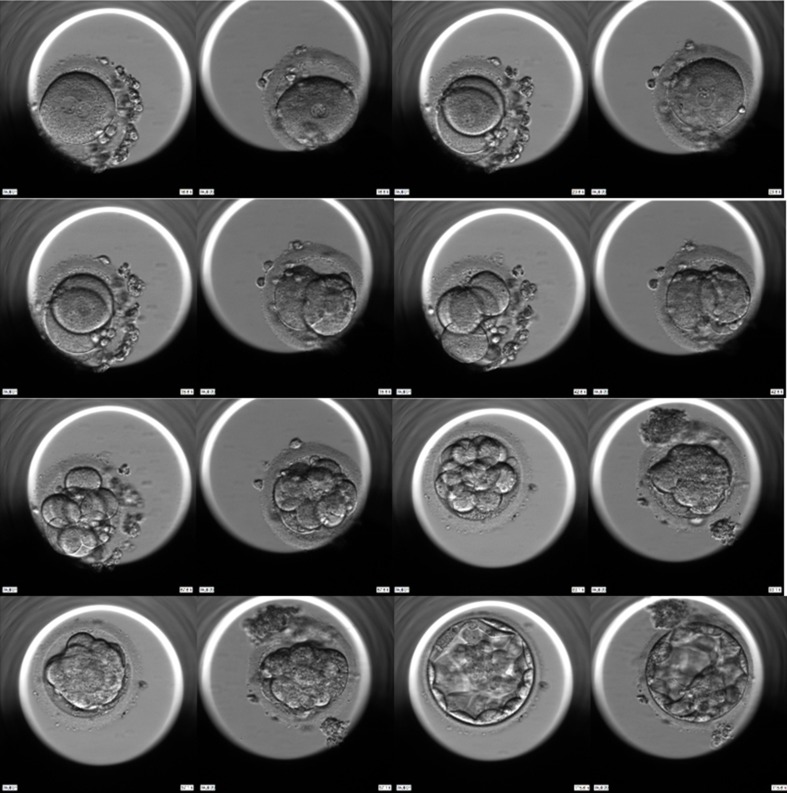
Time-lapse monitoring of two embryos from one patient of which both implanted (from top-left to bottom-right). Please note that the embryo on the left appears to be advanced from the first mitosis cycle and has less fragmentation as compared to the one on the right. However, compaction occurs earlier on the embryo to the right, eventually both forming blastocysts at day 5
The main outcome measures of the study were morphokinetic parameters of good quality (embryos described for calculation of the utilization rate) and implanted (100 % implantation; where the number of transfer embryos matched the number of gestational sacs) embryos to until the 5-cell stage. The utilization rate at day6 which was described as the proportion of mean number of transferred/cryopreserved embryos to fertilized oocytes, the clinical pregnancy (gestational sac detected with ultrasound at 12 weeks) and implantation rates in cycles in which transfer embryos have been cultured in single or sequential media, or from both (mixed group) have also been assessed. Morphokinetic parameters were analyzed as described by Meseguer et al. [15] and these included duration to disappearance of pronuclei, cleavages to 2- to 5- cell stages (t2 to t5), the duration of second cell cycle (cleavage from 2- to 3-cells; cc2) and cell division from 3 to 4 cells (s2). Continuous variables were compared with Mann-Whitney test and categorical variables were compared with Fisher’s exact test using GraphPad InStat version 3.10 for Windows (GraphPad Software, San Diego California USA, www.graphpad.com). A two-sided P-value under 0.05 was considered significant.
Results
A total of 51 oocyte retrieval cycles were recruited into the study. Regardless of the culture media, all embryos displayed severe fragmentation in two cycles, resulting in cancellation without transfer or cryopreservation. In the remaining 49 cycles, the mean age of the patients was 32.8 ± 4.3 and the mean trial number was 2.5 ± 1.7. A total of 446 MII oocytes were randomized into single (n = 231, mean ± sd; 4.7 ± 1.3) and sequential media (215, 4.4 ± 1.4). At day1, 170 oocytes displayed two pronuclei in the former (%; 75.2 ± 22.0) and 149 in the latter groups (%; 71.0 ± 20.8; P = 0.23).
Morphokinetic parameters obtained from all embryos derived from fertilized oocytes (ALL) and those that were transferred and frozen (GOOD) were assessed (Table 1). The duration for disappearance of two pronuclei was significantly shorter when oocytes have been cultured in single as compared to sequential media (P = 0.03 and 0.04 in ALL and GOOD, respectively). Similarly, time for cleavages until 5-cells was shorter when embryos have been cultured in single media (P = 0.001 and 0.009 in t2, 0.01 and 0.02 in t3, 0.02 in both groups in t4, and 0.001 and 0.002 in t5 for ALL and GOOD, respectively). However, the durations for cc2 and s2 were similar between groups.
Table 1.
Morphokinetic parameters obtained from all (ALL) and transferred/frozen (GOOD) embryos
| dp | t2 | t3 | t4 | t5 | cc2 | s2 | ||
|---|---|---|---|---|---|---|---|---|
| All | Single | 25.12 ± 5.21 (170) | 27.36 ± 4.12 (167) | 37.75 ± 6.64 (164) | 40.07 ± 5.98 (160) | 48.77 ± 9.49 (159) | 10.52 ± 5.99 (164) | 2.54 ± 5.26 (160) |
| Sequential | 25.92 ± 4.99 (149) | 29.09 ± 4.86 (148) | 39.53 ± 6.15 (144) | 41.45 ± 6.07 (138) | 52.22 ± 9.34 (138) | 10.63 ± 4.64 (144) | 1.88 ± 3.52 (138) | |
| Pa | 0.03 | 0.001 | 0.01 | 0.02 | 0.001 | 0.24 | 0.78 | |
| Good | Single | 23.70 ± 3.28 (75) | 26.19 ± 3.36 (75) | 37.25 ± 5.46 (75) | 38.85 ± 4.13 (75) | 49.19 ± 8.40 (75) | 11.06 ± 3.34 (75) | 1.60 ± 2.97 (75) |
| Sequential | 24.75 ± 3.13 (68) | 27.56 ± 3.21 (68) | 39.16 ± 3.93 (68) | 40.33 ± 3.77 (68) | 53.34 ± 6.34 (68) | 11.60 ± 2.64 (68) | 1.17 ± 2.02 (68) | |
| Pa | 0.04 | 0.009 | 0.02 | 0.02 | 0.002 | 0.40 | 0.86 | |
All values (hours) are mean ± SD
The number of embryos is given in parenthesis
aMann Whitney test
dp = disappearance of pronuclei, t(2–5) = cleavage times to (two - five) cells, cc2 = cleavage from 2- to 3-cells (t3-t2), s2 = duration of the period as 3 blastomere embryo (t4-t3)
Data obtained for morphokinetic parameters in implanted embryos (Table 2) showed shorter cleavage times in single as compared to sequential media (P = 0.08 for disappearance of two pronuclei, 0.03 for t2, 0.08 for t3, 0.047 for t4 and 0.08 for t5). The durations for cc2 and s2 remained similar in implanted embryos for both culture systems.
Table 2.
Morphokinetic parameters obtained from embryos with %100 implantation
| dp | t2 | t3 | t4 | t5 | cc2 | s2 | ||
|---|---|---|---|---|---|---|---|---|
| IMP | single | 22.33 ± 3.53 (9) | 24.68 ± 3.56 (9) | 35.74 ± 5.07 (9) | 36.61 ± 4.31 (9) | 47.43 ± 7.93 (9) | 11.07 ± 2.35 (9) | 0.87 ± 1.47 (9) |
| sequential | 25.14 ± 3.61 (9) | 27.92 ± 3.39 (9) | 40.11 ± 4.11 (9) | 41.36 ± 4.52 (9) | 53.87 ± 5.95 (9) | 12.19 ± 1.07 (9) | 1.24 ± 1.29 (9) | |
| Pa | 0.08 | 0.03 | 0.08 | 0.047 | 0.08 | 0.29 | 0.21 | |
All values (hours) are mean ± SD
The number of embryos is given in parenthesis
aMann Whitney test
dp = disappearance of pronuclei, t(2–5) = cleavage times to (two–five) cells, cc2 = cleavage from 2- to 3-cells (t3-t2), s2 = duration of the period as 3 blastomere embryo (t4-t3)
From a total of 49 transfers, in 36 cycles (N embryos = 45) at least one embryo cultured in single and in 32 (N embryos = 35) those cultured in sequential media have been transferred (Table 3). The proportion of mean number of transferred embryos to fertilized oocytes was %29.4 ± 25.3 in single and %27.8 ± 27.4 in sequential media (P = 0.67). A similar proportion for both; %12.4 and %10.7 of fertilized oocytes cultured in single and sequential media, respectively, have cleaved to embryos that have been transferred at days3 and 5. Day4 transfer embryos constituted %1.8 and % 2.0 of fertilized oocytes from respective groups. In all transfer days, there was not any significant difference between the proportions of transfer embryos to fertilized oocytes according to culture media. In 17 and 19 cycles, respectively, at least one supernumerary embryo cultured in single and sequential media were cryopreserved (total number of cryopreserved embryos; 30 in single and 33 in sequential media). Although the proportion of mean number of frozen embryos to fertilized oocytes did not differ between groups (%16.0 ± 27.2 in single and %18.9 ± 29.5 in sequential media, P = 0.63), there were significantly (OR: 0.22 %95 CI: 0.07–0.66) more cryopreserved day6 blastocysts cultured in the sequential system as compared to single media (% cryopreserved embryos to two pronuclei oocytes in single versus sequential media; day3: 3.5 versus 2.0, day5: 11.8 versus 10.1 and day6: 2.4 versus 10.1, respectively).
Table 3.
Distribution of transferred (ET) and cryopreserved (CRYO) embryos cultured in single and sequential media
| Single | Sequential | P or OR (%95CI) | ||
|---|---|---|---|---|
| ET | n (cycles; embryos) | 36; 45 | 32; 35 | |
| mean ± SD (%2PN) | 29,4 ± 25,3 | 27,8 ± 27,4 | 0.67 | |
| n Day3 (%2PN) | 21 (12.4) | 16 (10.7) | 1.17 (0.59–2.34) | |
| n Day4 (%2PN) | 3 (1.8) | 3 (2.0) | 0.87 (0.17–4.40) | |
| n Day5 (%2PN) | 21 (12.4) | 16 (10.7) | 1.17 (0.59–2.34) | |
| CRYO | n (cycles; embryos) | 17; 30 | 19; 33 | |
| mean ± SD (%2PN) | 16,0 ± 27,2 | 18,9 ± 29,5 | 0.63 | |
| n Day3 (%2PN) | 6 (3.5) | 3 (2.0) | 1.78 (0.44–7.25) | |
| n Day5 (%2PN) | 20 (11.8) | 15 (10.1) | 1.19 (0.59–2.42) | |
| n Day6 (%2PN) | 4 (2.4) | 15 (10.1) | 0.22 (0.07–0.66) | |
There was not any difference in cumulative clinical pregnancy (%; 52.9 single, 61.5 sequential, 52.6 % mixed) and implantation (%; 46.2 single, 50.0 % sequential, 28.9 % mixed) rates when transfer embryos have been cultured in single and in sequential media or one from each (mixed group, Table 4). However, the implantation rate after day5 transfers in sequential group was significantly higher than the mixed group (OR: 14.00 %95 CI: 1.25–156.70).
Table 4.
Distribution of clinical parameters of embryos cultured in single and sequential media and in mixed transfers
| Single | Sequential | Mixed | Total | |||||
|---|---|---|---|---|---|---|---|---|
| CPR/ET (%) | 9/17 (52.9) | 8/13 (61.5) | 10/19 (52.6) | 27/49 (55.1) | ||||
| IR/ET (%) | 12/26 (46.2) | 8/16 (50.0) | 11/38 (28.9) | 31/80 (38.8) | ||||
| CPR/ET (%) | IR/ET (%) | CPR/ET (%) | IR/ET (%) | CPR/ET (%) | IR/ET (%) | CPR/ET (%) | IR/ET (%) | |
| Day3 | 2/5 (40.0) | 2/7 (28.6) | 0/1 (0) | 0/2 (0) | 8/14 (57.1) | 9/28 (32.1) | 10/20 (50.0) | 11/37 (29.7) |
| Day4 | 1/1 (100) | 2/2 (100) | 0/1 (0) | 0/2 (0) | 1/1 (100) | 1/2 (50.0) | 2/3 (66.7) | 3/6 (50.0) |
| Day5 | 6/11 (54.5) | 8/17 (47.1) | 8/11 (72.2) | 8/12 (66.7)* | 1/4 (25.0) | 1/8 (12.5) | 15/26 (57.7) | 17/37 (45.9) |
CPR/ET = clinical pregnancy rate per embryo transfer, IR/ET = implantation rate per embryo transfer, *OR: 14.00 (%95CI: 1.25–156.70) versus mixed group
Discussion
The results of the present study showed that some early morphokinetics parameters differed between embryos cultured in single and in sequential media however, the overall utilization rate and clinical outcome parameters were similar.
The morphokinetic data obtained from the present study showed that the first cell cycle of embryos cultured in single media was accelerated as compared to those in sequential media although durations between subsequent cleavages (cc2: 2- to 3-cells and s2: 3- to 4-cells) remained similar. Observation of a delayed pronuclear disappearance in sequential culture system is a novel finding. The novelty of this finding can be attributed to the design of the present study as none of the earlier reports randomized sibling oocytes after insemination/microinjection. It is likely that embryos cultured in single media reached the 5-cell stage faster than the sequential system as the initial mitosis cycle was shorter. Two comparative studies reported higher day5 blastocyst yield in single media [10, 13] and another did not find any difference [11] which was in accordance to the results of the present study. However, it is noteworthy to mention that although the overall utilization rate was similar, significantly more day6 blastocysts have been cryopreserved in the sequential culture system than single media. This finding can be interpreted as equalization of embryo utilization to be compensated by developmentally delayed blastocysts in the sequential system, justifying the findings of Reed et al. [10] and Sepulveda et al. [13].
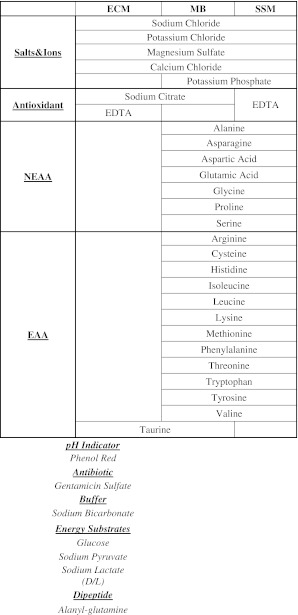
The reason for differences in morphokinetic parameters may be attributed the media ingredients which might affect cell cycle of embryos through metabolic pathways and/or repair mechanisms. According to manufacturer’s declaration, these media basically differ from each other by the presence of essential and non-essential aminoacids during the cleavage stage in the single culture system (Table 5). Aminoacids have several roles in during development of embryos; they are chelators, osmolytes, intracellular pH buffers, antioxidants, biosynthetic precursors, energy metabolism regulators and energy substrates [1]. The impact of presence of aminoacids and other ingredients on morphokinetic parameters of early embryo development remains to be investigated. Furthermore, it should be noted that as composition may vary among various commercially available culture media, data obtained in the present study are subject to change in different studies utilizing other brands.
Table 5.
Components of Early Cleavage Media (ECM), MultiBlast Media (MB) and Single Step Media (SSM) as declared by the manufacturer (concentrations are not known)
Ingredients written in italics at the bottom of the Table are common to all three types. NEAA non-essential aminoacids, EAA essential aminoacids
In the present study, the overall clinical pregnancy and implantation rates remained similar when transfer embryos have been cultured in single and sequential media. However, when cleavage-stage transfers were assessed (Table 4), there were only two cycles (4 embryos) from a total of 13 (16 embryos) in which only embryos cultured in the sequential media were used and these did not result in a pregnancy. In day3 mixed transfers, presence of one twin pregnancy indicated that at least one embryo cultured in sequential media was implanted. Yet, the clinical pregnancy and implantation rates in the remaining eleven day5 transfers were 72.2 % and 66.7 %, respectively. On the other hand, in 6 out of 17 cycles, only cleavage-stage embryos cultured in single media have been transferred (five day 3 and one day 4). Although the clinical data consisted of limited number of cycles in each group, it is tempting to speculate that the observed advanced mitotic pace for embryos cultured in single media caused a better performance in cleavage-stage transfers as compared to the sequential system. Sequential culture system appeared to perform effectively at the blastocyst stage, and the overall clinical success rate of this group was similar, if not slightly better, than the single media. It is also worth to note that the observed lower overall implantation rate in the mixed group (28.9 %) when compared to single (46.2 %) and sequential culture groups (50 %) has been caused by the dominance of cleavage-stage transfers in this group (fourteen at day3, one at day4 and four at day5).
Assessment of impact of culture media on embryo development is rather difficult because several potential confounding variables may interfere with the outcome. In the present study design, such interactions has been minimized as compared to earlier reports: (i) a sibling oocyte model has been used in order to enable the patients to serve as their own controls, (ii) concerns arising from variations between different brands [1, 12] were minimized by utilizing media from the same manufacturer which possessed similar concentrations of buffer systems and osmolarity, (iii) conditions (excluding the media) remained similar during in vitro culture of sibling oocytes/embryos and these include the dish, incubator, environment regarding gas concentration, temperature and humidity, (iv) sperm selection has been performed under high magnification which minimized variations in embryo development through introduction of male genome with different qualities [16]. Furthermore, in the present study, embryos have been cultured in an atmosphere of reduced O2 concentration which has been reported to increase the viability of embryos as compared to culture in 5 % CO2 [20–23] where all previous data obtained from comparison of single and sequential systems were obtained.
In a recent study [12] which randomized patients into groups, a similar early cleavage rate to sequential but higher blastomere numbers at day2 and 3 embryos cultured in single media has been found. This finding indicated that acceleration of mitosis in the single media has been initiated after termination of the first cleavage division. Interestingly, a significantly higher fertilization rate has been reported in oocytes inseminated in single media which contradicted to the results from the present study. However, this finding was accompanied by a higher maturation rate of oocytes cultured in single media which may have caused the difference among groups. Furthermore, as the majority of embryos (135/147) have been transferred at day3, the reported higher implantation rate in the single media group may not have reflected the beneficial effects of sequential media during prolonged culture as observed in the present study.
Another study [10] randomized fertilized oocytes into two media and a similar embryo quality at day3, but higher blastocyst yield at day5 favoring single media has been found. However, as randomization has been done after fertilization of oocytes (insemination was in the sequential medium) the potential effect of single culture media on the first mitosis cycle could have been obscured. In this study, embryo culture with single media has been performed as a continuous system and no refreshment was done at day3. The potential (dis)advantages of continuous culture system on prolonged embryo development has been investigated by Macklon et al. [11] in which patients have been randomized into three groups; sequential, and single media in two arms, with and without day3 refreshment. However, this study did not find any difference in blastulation, ongoing pregnancy and implantation rates between groups.
The sequential culture system used in the present study (ECM/MB) has been compared to a single media from another manufacturer in a study where oocytes have been obtained from donors [13]. The findings favored single media as embryos cleaved faster from the beginning of day 2 with eventual higher blastocyst yield at day5. Although the number of cells in the inner cell mass and the trophectoderm of blastocysts remained similar, the implantation rate was significantly higher in the single media group. The authors concluded that when compared with the single media, sequential systems had a deleterious effect on the potential viability of the embryos that was not apparent by morphologic assessment. However, this study randomized donors and oocytes have been distributed into groups after fertilization.
The present study differed from the earlier by utilization of dynamic embryo development monitoring technique. As compared to the static observation method, this technique enabled a more precise definition of events occurring during growth of embryos. Morphokinetic data included parameters from all embryos that have cleaved from fertilized oocytes cultured in respective systems. Yet, the majority of these embryos were not viable and abnormal cleavage patterns were frequent in this entire cohort. Data obtained from good quality embryos (those which have been transferred and frozen) were more informative, yet the viability and implantation capacities of cryopreserved embryos remained uncertain. The morphokinetic data shown for implanted embryos indicated that viable embryos differed in some morphokinetic parameters according to the culture media. To our knowledge, this is a novel finding as none of the earlier studies comparing these culture systems have reported data on the cleavage rates of embryos with known implantation.
Some morphokinetics parameters of embryo development have been reported as a tool to predict implantation outcome [15]. However, the algorithm was defined when embryos have been cultured in a sequential media system under 5 % CO2 in an ambient atmosphere. According to the data obtained from the present study, application of the described algorithm to various culture conditions may require revision on certain parameters. For example, from the pre-determined parameters, duration of cleavage to the 5-cell stage (t5) may not be as predictive as s2 and cc2 as the latter two time zones remained similar under different culture conditions. It is tempting to speculate that morphokinetic parameters based on durations between cleavages, especially those signifying synchronicity (3- to 4-cells and 5- to 8-cells) will gain more importance in the future when compared to those based on time periods to reach to a certain cell number.
The long-term consequences of human embryo culture under in vitro conditions are well-recognized [24, 25]. Studies which evaluate the impact of treatments and manipulations on human embryos should consider fetal development and health for assessment of feasibility. The results presented in this study need to be assessed in prospective randomized studies with larger datasets which will enable a better prediction of the impact of culture media systems in clinical outcomes. Furthermore, results should be extended to include data on delivery rates, health of the fetus and the newborn and subsequently, the incidence of epigenetic disorders from both groups.
In conclusion, in vitro culture according to ‘let the embryo choose’ strategy appears to enable a faster development than ‘back to nature’ and the difference between the morphokinetics of embryos begins at the first cell cycle. In extended culture, although utilization rate of both strategies are similar, the beneficial effect of the latter emerges at a later stage of embryo development than the former. The clinical relevance of these findings can be adaptation of the former strategy to cycles where cleavage-stage transfers have been planned and the latter exclusively to blastocysts.
Footnotes
Capsule
Sibling oocytes were cultured until day 6 in single and sequential media in a time-lapse incubator. Morphokinetics of initial embryo development varied however overall clinical and embryological parameters remained similar.
References
- 1.Lane M, Garner DK. Embryo culture medium: which is the best? Best Pract Res Clin Obstetr Gynecol. 2007;21:83–100. doi: 10.1016/j.bpobgyn.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Brison DR, Schultz RM. Apoptosis during mouse blastocyst formation: evidence for a role for survival factors including transforming growth factor alpha. Biol Reprod. 1997;56:1088–1096. doi: 10.1095/biolreprod56.5.1088. [DOI] [PubMed] [Google Scholar]
- 3.Doherty AS, Schultz RM. Culture of preimplantation mouse embryos. Methods Mol Biol. 2000;135:47–52. doi: 10.1385/1-59259-685-1:47. [DOI] [PubMed] [Google Scholar]
- 4.Fauque P, Jouannet P, Lesaffre C, Ripoche MA, Dandolo L, Vaiman D, Jammes H. Assisted Reproductive Technology affects developmental kinetics, H19 Imprinting Control Region methylation and H19 gene expression in individual mouse embryos. BMC Dev Biol. 2007;18:116. doi: 10.1186/1471-213X-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dumoulin JC, Land JA, Montfoort AP, Nelissen EC, Coonen E, Derhaag JG, Schreurs IL, Dunselman GA, Kester AD, Geraedts JP, Evers JL. Effect of in vitro culture of human embryos on birthweight of newborns. Hum Reprod. 2010;25:605–612. doi: 10.1093/humrep/dep456. [DOI] [PubMed] [Google Scholar]
- 6.Gardner DK, Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum Reprod Update. 1997;3:367–382. doi: 10.1093/humupd/3.4.367. [DOI] [PubMed] [Google Scholar]
- 7.Biggers JD. Thoughts on embryo culture conditions. Reprod Biomed Online. 2001;4:30–38. doi: 10.1016/S1472-6483(12)60009-1. [DOI] [PubMed] [Google Scholar]
- 8.Summers MC, Biggers JD. Chemically defined media and the culture of mammalian preimplantation embryos: historical perspective and current issues. Hum Reprod Update. 2003;9:557–582. doi: 10.1093/humupd/dmg039. [DOI] [PubMed] [Google Scholar]
- 9.Biggers JD, McGinnis LK, Lawitts JA. One-step versus two-step culture of mouse preimplantation embryos: is there a difference? Hum Reprod. 2005;20:3376–3384. doi: 10.1093/humrep/dei228. [DOI] [PubMed] [Google Scholar]
- 10.Reed ML, Hamic A, Thompson DJ, Caperton CL. Continuous uninterrupted single medium culture without medium renewal versus sequential media culture: a sibling oocyte study. Fertil Steril. 2009;92:1783–1786. doi: 10.1016/j.fertnstert.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Macklon NS, Pieters MHEC, Hassan MA, Jeucken PHM, Eijkemans MJC, Fauser BCJM. A prospective randomized comparison of sequential versus monoculture systems for in-vitro blastocyst development. Hum Reprod. 2002;17:2700–2705. doi: 10.1093/humrep/17.10.2700. [DOI] [PubMed] [Google Scholar]
- 12.Paternot G, Debrock S, D’Hooghe TM, Spiessens C. Early embryo development in a sequential versus single medium: a randomized study. Reprod Biol Endocrinol. 2010;8:83–90. doi: 10.1186/1477-7827-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sepulveda S, Garcia J, Arriaga E, Diaz J, Noriega-Portella L, Noriega-Hoces L. In vitro development and pregnancy outcomes for human embryos cultured in either a single medium or in a sequential media system. Fertil Steril. 2009;91:1765–1770. doi: 10.1016/j.fertnstert.2008.02.169. [DOI] [PubMed] [Google Scholar]
- 14.Kirkegaard K, Hindkjaer JJ, Ingerslev HJ. Human embryonic development after blastomere removal: a time-lapse analysis. Hum Reprod. 2012;27:97–105. doi: 10.1093/humrep/der382. [DOI] [PubMed] [Google Scholar]
- 15.Meseguer M, Herrero J, Tejera A, Hilligsoe KM, Ramsing NB, Remohi J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26:2658–2671. doi: 10.1093/humrep/der256. [DOI] [PubMed] [Google Scholar]
- 16.Berkovitz A, Eltes F, Yaari S, Katz N, Barr I, Fishman A, Bartoov B. The morphological normalcy of sperm nucleus and pregnancy rate of intracytoplasmic injection with morphologically selected sperm. Hum Reprod. 2004;20:185–190. doi: 10.1093/humrep/deh545. [DOI] [PubMed] [Google Scholar]
- 17.Moon JH, Hyun CS, Lee SW, Son WY, Yoon SH, Lim HJ. Visualization of metaphase II meiotic spindle in living human oocytes using the Polscope enables the prediction of embryonic developmental competence after ICSI. Hum Reprod. 2003;18:817–820. doi: 10.1093/humrep/deg165. [DOI] [PubMed] [Google Scholar]
- 18.Ciray HN, Karagenc L, Ulug U, Bener F, Bahceci M. Use of both early cleavage and mononucleation to predict embryos with high implantation potential in intracytoplasmic sperm injection cycles. Fertil Steril. 2005;84:1411–1416. doi: 10.1016/j.fertnstert.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Mesut N, Ciray HN, Mesut A, Aksoy T, Bahceci M. Cryopreservation of blastocysts is the most feasible strategy in good responder patients. Fertil Steril. 2011;96:1121–1125. doi: 10.1016/j.fertnstert.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Kovacic B, Vlaisavljevic V. Influence of atmospheric versus reduced oxygen concentration on development of human blastocysts in vitro: a prospective study on sibling oocytes. Reprod Biomed Online. 2008;17:229–236. doi: 10.1016/S1472-6483(10)60199-X. [DOI] [PubMed] [Google Scholar]
- 21.Ciray HN, Aksoy T, Yaramanci K, Karayaka I, Bahceci M. In vitro culture under physiologic oxygen concentration improves blastocyst yield and quality: a prospective randomized survey on sibling oocytes. Fertil Steril. 2009;91:1459–1461. doi: 10.1016/j.fertnstert.2008.07.1707. [DOI] [PubMed] [Google Scholar]
- 22.Gomes Sobrinho DB, Oliveira JB, Petersen CG, Mauri AL, Silva LF, Massaro FC, Baruffi RL, Cavagna M, Franco JG., Jr IVF/ICSI outcomes after culture of human embryos at low oxygen tension: a meta-analysis. Reprod Biol Endocrinol. 2011;9:143. doi: 10.1186/1477-7827-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nanassy L, Peterson CA, Wilcox AL, Peterson CM, Hammoud A, Carrell DT. Comparison of 5% and ambient oxygen during days 3–5 of in vitro culture of human embryos. Fertil Steril. 2010;93:579–585. doi: 10.1016/j.fertnstert.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 24.Huntriss J, Picton HM. Epigenetic consequences of assisted reproduction and infertility on the human preimplantation embryo. Hum Fertil (Camb) 2008;11:85–94. doi: 10.1080/14647270802116250. [DOI] [PubMed] [Google Scholar]
- 25.Montfoort AP, Hanssen LL, Sutter P, Viville S, Geraedts JP, Boer P. Assisted reproduction treatment and epigenetic inheritance. Hum Reprod Update. 2012;18:171–197. doi: 10.1093/humupd/dmr047. [DOI] [PMC free article] [PubMed] [Google Scholar]