Abstract
Bone graft substitutes have become an essential component in a number of orthopedic applications. Autologous bone has long been the gold standard for bone void fillers. However, the limited supply and morbidity associated with using autologous graft material has led to the development of many different bone graft substitutes. Allogeneic demineralized bone matrix (DBM) has been used extensively to supplement autograft bone because of its inherent osteoconductive and osteoinductive properties. Synthetic and natural bone graft substitutes that do not contain growth factors are considered to be osteoconductive only. Bioactive glass has been shown to facilitate graft containment at the operative site as well as activate cellular osteogenesis. In the present study, we present the results of a comprehensive in vitro and in vivo characterization of a combination of allogeneic human bone and bioactive glass bone void filler, NanoFUSE® DBM. NanoFUSE® DBM is shown to be biocompatible in a number of different assays and has been approved by the FDA for use in bone filling indications. Data are presented showing the ability of the material to support cell attachment and proliferation on the material thereby demonstrating the osteoconductive nature of the material. NanoFUSE® DBM was also shown to be osteoinductive in the mouse thigh muscle model. These data demonstrate that the DBM and bioactive glass combination, NanoFUSE® DBM, could be an effective bone graft substitute.
Keywords: demineralized bone matrix, bioactive glass, osteoconductivity, osteoinductivity
INTRODUCTION
The use of autograft material remains the gold standard for use in orthopedic procedures due to the fact that there is little chance of immune rejection and its innate osteoconductive, osteoinductive and osteogenic potential. Due to the significant levels of pain and morbidity at the donor site, bone graft substitutes are commonly used (Goulet et al. 1997; Heary et al. 2002; Ubhi and Morris 1984; Younger and Chapman 1989). Bone graft substitutes offer a wide range of materials, structures, and delivery systems to be used in bone grafting procedures. Common sources of bone graft materials include allogeneic bone, synthetic calcium phosphate salts, coralline materials and bioactive glass. These materials should possess one or more of the characteristics typical of autograft material including osteoconductivity, osteoinductivity and osteogenicity.
Human derived demineralized bone matrix (DBM) has become a very common bone graft substitute which has shown the ability to aid in new bone formation in many different clinical settings including long bone defects, craniofacial reconstruction and spinal fusion (Glowacki et al. 1981; Mulliken et al. 1981; Rosenthal et al. 1999; Sassard et al. 2000; Tiedeman et al. 1995). DBM in combination with local bone has been shown to perform as well as autograft potentially eliminating the need for autogenous bone harvesting (Sassard et al. 2000). Studies have shown that allogeneic DBM possesses inherent osteoconductive and osteoinductive properties as well as containing numerous bone morphogenic proteins (BMPs) that initiate the cascade of new bone formation (Mulliken et al. 1984; Urist et al. 1983; Urist and Dowell 1968; Urist and Strates 1970). In addition, DBM has been shown to support in vitro proliferation and osteogenic differentiation of human bone marrow stromal cells (Kasten et al. 2003; Mauney et al. 2004a; Mauney et al. 2004b). Studies have shown that the DBM surface roughness plays a significant role in the migration and proliferation of the osteogenic cells (Cornell 1999; Logeart-Avramoglou et al. 2005). The collagen structure of DBM particles provides an osteoconductive effect while the non-collagenous proteins initiate the osteoinductive effect.
During the last couple of decades, the development of new implant technologies have shifted from attempts to create a passive interface between the implant and the native tissue to the design of bioactive materials. Within this category are a wide range of calcium-phosphate ceramics, bioactive glass and bioactive glass-ceramics (Hattar et al. 2002; Kokubo et al. 1990). All these materials possess the common characteristic of generating a carbonated hydroxyapatite layer that is equivalent chemically and structurally to the biological mineral of bone. This is known to be the determining step for biointegration (Hench and Paschall 1973; Ito et al. 1987; Kitsugi et al. 1987). Bioactive glass is the first man-made material to form a direct chemical bond with bone. It has also been shown to have effects on the cell cycle and molecular mechanisms resulting in osteoblastic cell differentiation and proliferation (Xynos et al. 2000b). Unlike traditional soda-lime silica glasses, bioactive glass is extremely biocompatible and, more importantly, is capable of triggering the genetically controlled metabolic mechanisms resulting in bone repair (Xynos et al. 2000a, 2001; Xynos et al. 2000b). During the first few days of cell growth, bioactive glass has been shown to upregulate different families of genes that express osteogenic growth factors and extracellular matrix components required for new bone formation (Xynos et al. 2001). Among the genes upregulated include insulin-like growth factor (IGF-II) and vascular endothelial growth factor (VEGF). IGF-II is known to induce osteoblast proliferation in vitro (Hench et al. 2004) and VEGF promotes angiogenesis required for new bone formation (Xynos et al. 2001). When in contact with surface-reactive bioactive glass, osteoblasts undergo rapid proliferation forming new bone in roughly the same time period as the normal healing process.
Bioactive glass has been proven effective in generating new bone in several different pre-clinical animal studies (Fujishiro et al. 1997; Oonishi et al. 1997; Wheeler et al. 2000; Wheeler et al. 1998), as well as approved products on the market. In addition, only a minimal amount of bioactive glass is required to induce graft bioactivity. Based on these properties of bioactive glass, NanoFUSE® DBM was created to take advantage of osteoconductive and proangiogenic properties of bioactive glass as well as the osteoinductive properties of human-derived DBM. The bioactive glass portion of NanoFUSE® DBM is composed of Hench’s Bioglass® (45S5 composition).
Carriers are commonly employed in DBM based bone void filler applications. In general, these carriers are natural (collagen, gelatin, alginate, etc) or synthetic (polymer or modified sugars). The NanoFUSE® DBM product employs a novel process to encapsulate the osteoinductive and osteoconductive elements of the product while not interfering with its clinical usefulness. The final product rapidly reconstitutes and is moldable while permitting normal bone healing.
In the present study, we present an in depth characterization of NanoFUSE® DBM. The material was shown to be biocompatible in numerous assays. In addition, data are shown demonstrating the ability of osteogenic cells to attach and proliferate on the particulate structure of the material. Additionally, animal studies demonstrated the material’s osteoinductive properties. This study represents the first comprehensive in vitro and in vivo characterization of a product composed of a combination of bioactive glass and human DBM.
MATERIALS AND METHODS
Human Materials
The DBM used for these studies was harvested from the long bones of generously donated tissue and supplied by Allosource, Inc (Denver, CO). The demineralization process was similar to that described by Urist (Urist and Dowell 1968). The final particle size was a distribution spanning 125 to 710μm.
Bioactive Glass
Bioactive glass, 45S5, was purchased from Mo-Sci Health Care, LLC (Rolla, MO). The composition of the 45S5 (w/w%) was 43 – 47% SiO2; 22.5 – 26.5% CaO; 5 – 7% P2O5; and 22.5 – 26.5% Na2O with a particle size distribution of 90 – 710 μm (≥ 90%).
Scanning Electron Microscopic Analysis
Particle size, morphology, and porosity were examined under vacuum using a scanning electron microscope (SEM) (JEOL 6700 with a cold field emission gun). The microscope is fully controlled through a Windows based interface with image archiving and framestore functions. Dry samples of the bone void filler were prepared by direct adhesion to standard 1″ Aluminum SEM mounts and sputter coated with gold in a precision etching and coating system (PECS) Gatan Model 681. The analysis was performed at an accelerating voltage of 5kV.
Coating Process
Production of NanoFUSE® DBM utilized a patented (USPTO# 7,846,459 and 7,829,105) process to microencapsulate the DBM and bioactive glass particles in porcine gelatin. The NanoFUSE® DBM lots produced were terminally sterilized using ionizing radiation and endotoxin free.
Cell Proliferation
Mouse osteoblasts from calvaria, MC3T3E1 osteoblastic mouse cell line and CMT93 mouse rectum cancer cell line (as a non-osteoblastic cells) were used for these studies. Cells were cultured in α Minimum Essential Medium modified with deoxyribonucleosides and supplemented with 10% fetal bovine serum (FBS) (Tissue Culture Biologicals, Tulare, CA). Cell numbers were determined using the MTT assay following the recommendations of the manufacturer (Invitrogen). NanoFUSE® DBM was aseptically weighted out and reconstituted with growth media (50 mg/1 ml/well of 24 well plate), and one ml of the suspension was placed each well. The cells were added into triplicate wells with or without NanoFUSE® DBM at a concentration of 2 × 104 cells/well. Medium was renewed every second day (the day before MTT assay). On the day of MTT assay, spent medium was replaced with phenol red free medium containing MTT reagent and incubated for 3.5 hrs and developed with MTT solvent solution for 4.0 hrs at 37°C prior to the color measurement at OD 570 nm. Three points from individual culture wells were measured and average values were used to plots the graphs. Substrates alone or empty wells were analyzed similarly and were used as negative controls to adjust for background fluorescence. Cells were visualized using CellTracker reagent following the manufacturer’s procedures (Invitrogen, Carlsbad, CA).
Biocompatibility
All biocompatibility studies were performed by WuXi Apptec (St. Paul, MN)
Cytotoxicity
Cytotoxic effects of NanoFUSE® DBM and of the empty syringe assembly (vented syringe, luer lug, check valve fluid pathway) were evaluated with the test protocol ISO MEM Elution Using L-929 Mouse Fibroblast Cells. The sterilized syringe assembly (fluid path) and sterile sample (4 g) of NanoFUSE® DBM were extracted with Eagle’s Minimal Essential Medium (E-MEM) supplemented with 5% Fetal Bovine Serum (FBS) at 37 ± 1°C for 24–25 hours. The maintenance culture media was removed from the monolayer in the test culture wells and replaced with 1 mL of test media/extract, control media/extract or positive control media spiked with CdCl2 and a negative control (extract from a piece of black rubber). Positive, intermediate, and negative controls were run in parallel with the test articles and all treatments were plated in triplicate. Cell cultures were incubated 72 ± 4 hours at 37 ± 1°C in a humidified atmosphere of 5 ± 1% CO2 in air. Cultures were evaluated for cytotoxic effects by microscopic observation after 24, 48 and 72 ± 4 hour incubation periods. Criteria for evaluating cytotoxicity include morphological changes in cells, such as granulation, crenation, or rounding, and loss of viable cells from the monolayer by lysis or detachment. The degree of toxicity was scored as shown in Table 1.
Table 1.
Summary of Biocompatibility Studies
| Test | Result |
|---|---|
| Cytotoxicity | Non-cytotoxic |
| Sensitization | Negative |
| Intracutaneous Reactivity | Non-irritant |
| Genotoxicity/Mutagenicity | Non-mutagenic |
| Acute Systemic Toxicity | Non-toxic |
Sensitization
Sensitization of NanoFUSE® DBM was evaluated using the test protocol Guinea Pig Maximization Sensitization Test Method for Biomaterial Extracts which evaluates if the material stimulates the immune system to produce an allergic response. Eleven guinea pigs were injected in pairs on each side of the dorsal midline with 0.9% saline plus Freund’s complete adjuvant (FCA), extract, and 0.9% saline plus FCA plus extract making it six injection sites per animal. Six negative control guinea pigs were injected in pairs on each side of the dorsal midline with 0.9% saline plus FCA, control vehicle, and 0.9% saline plus FCA plus control vehicle. Two vehicles were used for extraction: normal saline (NS) and cotton seed oil (CSO). On Day 6 of the study, the site was shaved again and topically treated with 0.5 g of 10% (w/w) SLS mixed with mineral oil for a second induction. On Day 7 patches saturated with NanoFUSE® DBM extract or control vehicles were applied and secured for 48 ± 2 hrs. Fourteen days after topical induction, all (test and negative control) animals were challenged by applying and securing a patch saturated with NanoFUSE® DBM extract to a clipped right flank and a patch saturated with control vehicle to a clipped left flank for 24 ± 2 hrs. The extracts for the two induction phases and the challenge were prepared freshly by incubating NanoFUSE® DBM in NS or CSO (1:5 w/v) at 37 ± 2 °C for 72 ± 2 hrs. The dermal patch challenge sites were observed for erythema and response based on dermal scores. An individual score of 1 or greater in test animals indicates sensitization if control animals show a score less than 1 which was the case in this study. The test results are given in percentage of animals showing a sensitization response (score ≥ 1). Dinitrochlorobenzene (DNCB) was used as the positive control.
Intracutaneous Reactivity
Intracutaneous reactivity of NanoFUSE® DBM was evaluated. Two New Zealand white rabbits received five sequential intracutaneous injections along either side of the dorsal midline with NanoFUSE® DBM extracts (2 rows) on the right side and concurrent vehicle control (two rows) on the left side. The vehicles used were 0.9% saline (NS) and cottonseed oil (CSO). NanoFUSE® DBM was extracted for 72 ± 2 hrs at 37 ± 1°C at a 1:5 ratio (w/v). The irritation reaction of the NanoFUSE® DBM extracts were recorded and compared to vehicle controls over a 72-hour period according to the standard ISO Irritation Scoring System. This system scores for erythema and edema on a scale of 0 to 4, 0 being the mildest and 4 the most severe reaction. A difference between the average scores for test article extract (NanoFUSE® DBM) and vehicle control of less than or equal to 1 is considered non-irritating. The final average score is calculated by adding the erythema and edema scores of all five, injection sites for all animals from all observation time points and dividing the sum by 12 (2 animals x 3 observation periods X 2 scoring categories).
Genotoxicity/Mutagenicity
Mutagenicity of NanoFUSE® DBM was evaluated with the test protocol Bacterial Mutagenicity Test – Ames Assay, which evaluates the mutagenicity potential of a test article by measuring its ability to induce back mutations at selected loci of several strains of bacteria in the presence or absence of microsomal enzymes. The Ames Test was used to assess the potential of NanoFUSE® DBM extracts to induce histidine (his) reversions (his− to his+) in five strains S. typhimurium (TA97A, TA98, TA100, TA102, and TA1535) caused by changes or frame shift mutations in the genome of tester organisms. This is an incorporation assay in the presence and absence of an exogenous mammalian activation system (S9). NanoFUSE® DBM was extracted in 0.9% saline (NS) and dimethylsulfoxide (DMSO) by incubating for 72 ± 2 hrs at 50 ± 2 °C according to ISO 10993-12 at a ratio of 1:5 (w/v). Frozen working stocks were used to create working cultures of each bacterial strain. Cultures were grown overnight at 37 ± 2 °C until a density of 0.6–1.6 at 650 nm was reached. NanoFUSE® DBM extract or vehicle control plus bacteria culture plus either PBS or metabolic activation solution (S9) was added to a molten top agar of 0.6% Difco agar in 0.5% NaCl supplemented with an L-histidine/0.5 mM biotin solution. Solution was vortexed and allowed to harden and incubated at for 48–72 hrs at 37 ± 2 °C. All plates were scored using an automatic image analysis system for colony counting. Negatives controls were plated with NS or DMSO extraction blanks, with and without S9. Positive controls consisted of direct acting mutagens i.e. ICR-191 Acridine (IDCR-191), 2-nitrofluorene (2-NF), sodium azide (Na Azide), or cumene and a mutagen requiring metabolic transformation, 2-aminoanthracene (2-AA).
Systemic Toxicity
Systemic toxicity of NanoFUSE® DBM was evaluated with the test protocol ISO Acute Systemic Injection Test. Twenty mice were injected systemically with two extracts of NanoFUSE® DBM (normal saline [NS] or cottonseed oil [CSO]) or the appropriate vehicle. Animals were observed for fatalities/signs of toxicity immediately after injection and at 4, 24, 48, and 72 hours post-injection. Animals were also monitored for weight loss.
Alkaline Phosphatase
Osteoblasts isolated from calvaria of 1-day-old mice were cultured with NanoFUSE® DBM, DBM or bioactive glass as for the cell proliferation assay, except that medium was supplemented with100 μg/ml ascorbic acid and 10 mM β-glycerophosphate to induce osteoblastic differentiation. After 19 days of culture, differentiated cells were fixed with 4% paraformaldehyde/PBS for 30 min. and stained for alkaline phosphatase (AP) activity using a previously published protocol (Narisawa et al. 1992). These experiments were conducted several times and a representative experiment is presented. Microscopic images were taken by inverted TE300 Nikon microscope. Second plate identical to the plate used for AP staining was used for extraction of AP with butanol. The enzymatic activity was measured using para-nitrophosphate as a substrate and recombinant human TNAP as a standard (Hoylaerts et al. 2006).
Microscopic Evaluations
Processing of the slides was performed by Laudier Histology (New York, NY). Freshly prepared samples of NanoFUSE® DBM were fixed in 10% formalin, embedded in methyl methacrylate and then sectioned 5μm thick. The sections were stained with Alizarin red (calcium specific red stain) to visualize the DBM and bioactive glass particles as well as to observe void spaces. Distribution of the particles was also visualized.
Ectopic Bone Formation
The assay was conducted using male athymic nude mice. Each mouse received two intramuscular pouch implants of the test article. A total of ten implants per sample were implanted into the mice. The animals were anesthesized and prepared for surgery. Pockets were formed in the biceps femoris muscle of the mice. The test material (50±5 mg) was placed into each pocket and then the muscle pocket and skin were sutured. The animals recovered from anesthesia and returned to their cages. The animals were sacrificed 28 days later and the bilateral implants were removed. The tissues were fixed in 10% neutral buffered formalin, decalcified, paraffin embedded and cut into 5μm sections. The sections were stained with Hematoxylin-Eosin. Osteoinductivity was analyzed histologically (Edwards et al. 1998). Inflammation was also scored.
RESULTS
Microscopic Evaluation
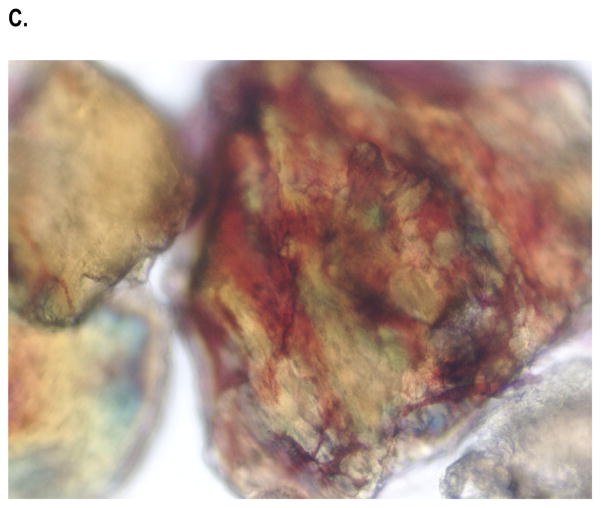
Samples of NanoFUSE® DBM were embedded with methyl methacrylate, sectioned and stained. As shown in Figure 1, the DBM particles as well as the bioactive glass particles were homogeneously dispersed throughout the material. It is interesting to note the red staining surrounding the bioactive glass particles. It is known that bioactive glass will release calcium over time.
Figure 1.
Cross-sectional view of NanoFUSE® DBM embedded with methyl methacrylate. The material was stained with Alizarin red and counterstained with light green. A representative image is shown in A (magnification 2.5x). The material was stained with Alizarin red in image B (magnification 10x). Arrows point out DBM flakes in (A) and DBM flake and bioactive glass in (B). NanoFUSE® DBM from three different batches were examined.
SEM Analysis
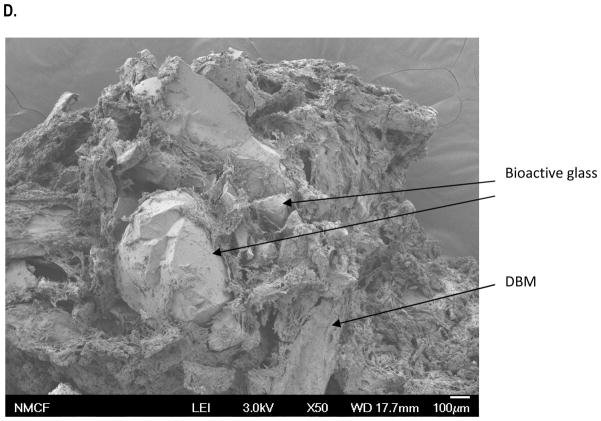
This study was designed to evaluate the shape and size of the bioactive glass and DBM particles in NanoFUSE® DBM. The morphological analyses of the material demonstrated the bioactive glass particles of irregular shape and surface roughness (Figure 2A). The DBM particles were observed to be within the 125–710mm size range (Figure 2B). The morphological analysis of the DBM demonstrated particles of irregular shape and surface roughness. The morphological analysis of NanoFUSE® DBM demonstrated that the gelatin coated particles were larger and often formed clumps as a product of the coating process (Figure 2C). However, the individual bioactive glass and DBM particles are clearly visible. The material was also shown to be highly porous.
Figure 2.

SEM of NanoFUSE® DBM components. Three samples of each were analyzed and representative micrographs are shown. (A) Bioactive glass; (B) Human DBM (50x); (C) Human DBM (250x); (D) NanoFUSE® DBM (50x).
Cell Proliferation and Attachment
The ability of several different types of cells to attach and proliferate on the NanoFUSE® DBM material was investigated over a 9-day period. As shown in Figure 3, the cell numbers increased over time throughout the 9-day incubation period. Figure 3A demonstrates that primary osteoblasts are able to proliferate on the NanoFUSE® DBM in a similar manner as when grown on tissue culture plastic. Similar results were observed when using a mouse osteoblastic cell line (Figure 3B). The cells were observed directly attached to the DBM and bioactive glass particles (Figure 4A and 4B).
Figure 3.

Cell proliferation on NanoFUSE® DBM. Cell numbers were determined at day 3, 5 and 9 days post seeding using alamarBlue reagent. Experiments were run a minimum of three times and a representative experiment is shown. (A) Primary mouse calvarial osteoblasts (closed boxes-cells binding to NanoFUSE® DBM; open boxes- cells binding to tissue culture plastic) (B) Mouse osteoblastic cell line, MC3T3E1 cells (closed boxes) and CMT 93 cells (open boxes).
Figure 4.

Cell attachment to NanoFUSE® DBM. Osteoblasts were attached to bioactive glass or NanoFUSE® DBM. Cells were fluorescently labeled using CellTracker. (A) Osteoblasts attached to bioactive glass particles; (B) Osteoblasts attached to NanoFUSE® DBM.
Alkaline Phosphatase
The ability of NanoFUSE® DBM to support osteoblastic phenotypic markers was investigated. Alkaline phosphatase is an early marker for osteoblastic differentiation. Osteoblasts were shown to produce AP in the presence of osteoblastic media when cultured on NanoFUSE® DBM as well as on the subunits of this material (Figure 5A). There appears to be more AP formed when the cells are grown on the NanoFUSE® DBM when compared to either component alone. In addition, the cells were observed to be attached directly to the bioactive glass (Figure 5B) and the DBM particles used to make NanoFUSE® DBM (Figure 5C).
Figure 5.
Alkaline phosphatase induction on NanoFUSE® DBM. Osteoblasts were plated on NanoFUSE® DBM, bioactive glass or DBM alone. After 15 days, the cells were stained for alkaline phosphatase. (A) Total alkaline phosphastase production from osteoblasts (data presented as mean±standard error); (B) Alkaline phosphatase positive staining cells attached directly to bioactive glass beads; (C) Alkaline phosphatase positive staining cells attached directly to NanoFUSE®DBM. Magnification is 50x.
Biocompatibility
The biocompatibility of NanoFUSE® DBM was evaluated using the assays outlined by the ISO 10993 guidelines. A summary of the results is presented in Table 1. As can be seen, NanoFUSE® DBM did not induce a cytotoxic response as measured by the L929 cell assay or a systemic toxic response as measured in mice. The material did not induce a sensitization response as measured in guinea pigs or an irritation response as measured in New Zealand white rabbits. In addition, NanoFUSE® DBM was found to be non-mutagenic. Taken together, NanoFUSE® DBM was found to be biocompatible by all assays that were evaluated.
Ectopic Bone Formation
NanoFUSE® DBM as well as DBM with gelatin and bioactive glass with gelatin were evaluated for their ability to induce new bone formation in the athymic mouse thigh muscle model. The results are presented in Table 2. Comparable results were observed in the NanoFUSE® DBM and the DBM with gelatin formulations with respect to the presence of osteoblasts, new bone formation and the presence of bone marrow. In contrast, no osteoblasts, new bone formation or the presence of bone marrow were observed in any of the bioactive glass with gelatin formulation. Histological evidence of new bone formation is shown in Figure 6. The NanoFUSE® DBM material does induce new bone formation in a similar manner as the DBM mixed with gelatin in this model. Comparable remodeling features associated with new bone formation were observed including visible areas of stromal matrix, pockets of osteoblasts and osteocytes, fatty bone marrow as well as the formation of new blood vessels were observed in the DBM with gelatin and NanoFUSE® DBM samples. Comparable osteoinductivity levels (Edwards et al. 1998) were observed in both sets of samples as well. No adverse inflammatory responses were observed in any of the animals used in the study.
Table 2.
Summary of Osteoinductivity Results in Mice
| NanoFUSE DBM | ||||||
|---|---|---|---|---|---|---|
| Sample Number | Chondrocytes | Osteoblasts | New Bone | Bone Marrow | Original DBM | Osteoinductive |
| 1 | X | X | X | X | Yes | |
| 2 | X | X | X | Yes | ||
| 3 | X | X | X | Yes | ||
| 4 | X | X | X | X | X | Yes |
| 5 | X | X | X | X | X | Yes |
| 6 | X | X | X | X | Yes | |
| 7 | X | X | X | Yes | ||
| 8 | X | X | X | X | Yes | |
| 9 | X | X | X | X | Yes | |
| 10 | X | X | X | X | Yes | |
| DBM/Gelatin | ||||||
|---|---|---|---|---|---|---|
| Sample Number | Chondrocytes | Osteoblasts | New Bone | Bone Marrow | Original DBM | Osteoinductive |
| 1 | X | X | X | X | X | Yes |
| 2 | X | X | X | X | Yes | |
| 3 | X | X | X | X | Yes | |
| 4 | X | X | X | X | Yes | |
| 5 | X | X | X | X | Yes | |
| 6 | X | X | X | X | X | Yes |
| 7 | X | X | X | X | X | Yes |
| 8 | X | X | X | X | Yes | |
| 9 | X | X | X | X | X | Yes |
| 10 | X | X | X | X | X | Yes |
| Bioactive Glass/Gelatin | ||||||
|---|---|---|---|---|---|---|
| Sample Number | Chondrocytes | Osteoblasts | New Bone | Bone Marrow | Original Material | Osteoinductive |
| 1 | X | No | ||||
| 2 | X | No | ||||
| 3 | X | No | ||||
| 4 | X | No | ||||
| 5 | X | No | ||||
| 6 | X | No | ||||
| 7 | X | No | ||||
| 8 | X | No | ||||
| 9 | X | No | ||||
| 10 | X | No | ||||
Figure 6.
Osteoinductivity of NanoFUSE® DBM. The athymic mouse thigh muscle model was used. Explants were removed 28 days post-implantation, fixed in formalin and the sections were stained with H&E.
DISCUSSION
The need for bone graft materials is an ongoing challenge in orthopedics. The use of commercially available DBM as a supplement to autogenous bone is becoming increasingly common (Berven et al. 2001; Morone and Boden 1998; Rosenthal et al. 1999; Sassard et al. 2000). However, autogenous bone remains the gold standard for use in orthopedic procedures due to its osteoinductve, osteoconductive, and osteogenic potential. Due to postoperative morbidity and in revision cases where the autogenous iliac crest bone graft is limited, the search continues for effective alternatives. The development of novel bone graft substitutes with novel properties can expand the use of these materials in orthopedic treatments. Bone graft substitutes should possess one or more of the characteristics typical of autograft. These materials should be biocompatible, possess osteoconductive as well as osteoinductive properties, and should degrade in concert with bony replacement. The NanoFUSE® DBM material described herein was shown to be biocompatible as well as possess osteoconductive and osteoinductive properties.
It is very important that any implanted material does not induce any adverse biological responses. The data provided herein clearly demonstrate that NanoFUSE® DBM is biocompatible. The material did not induce a cytotoxic response as measured in the mouse L929 cell assay and was not genotoxic or mutagenic. This material did not elicit a sensitization response as measured by erythema on the dermis of guinea pigs when extracted in normal saline or cotton-seed oil. In addition, there was no irritation response as measured in rabbits. When an extract of the material was given systemically to mice, there were no adverse clinical signs observed throughout the study period.
The integration of bone void fillers is dependent on the activity of the surrounding bone cell and their precursors. Migration and proliferation of these osteogenic cells is influenced by the interaction of the surface of the bone graft with cells. The attachment and proliferation of these osteogenic cells is dependent on the surface porosity and roughness. The surface, of the coated DBM particles, was shown to be porous as well as possessing a very rough surface. Similar features were also noted for the bioactive glass particles that are found in this bone graft substitute. Data is presented to demonstrate that NanoFUSE® DBM supports the attachment and proliferation of osteogenic cells and their precursors. It is important for the NanoFUSE® DBM material to support the growth of these cells because these are the cells responsible for the initial healing and the new bone formation.
The results presented herein also demonstrate the ability of this material to support the in vitro induction of osteoblastic phenotypic markers, as shown by the expression of alkaline phosphatase. These properties of the novel bone graft substitute are important for new bone formation and osteoinduction. As described above, an ideal bone graft substitute should possess osteoconductive properties similar to the ones demonstrated in this study.
NanoFUSE® DBM was also shown to be osteoinductive in the athymic mouse thigh muscle pouch model. The new bone formation was characterized by regions of osteoid pockets of osteoblasts and osteocytes as well as the formation of new blood vessels. Previous studies have shown that materials that are osteoinductive in a muscle pouch model were also shown to be positive in a rat spinal fusion model (Biswas et al. 2010). Indeed, NanoFUSE® DBM was shown to induce spinal fusion in athymic rats (unpublished data). Clearly this material has the ability to support new bone formation.
Bioactive glass is the first man-made material to form a direct chemical bond with bone. It is also the first man-made material to exert a positive effect on osteoblastic differentiation and osteoblast proliferation (Xynos et al. 2000b). The bioactive glass portion of NanoFUSE® DBM is composed of Hench’s Bioglass. Years of testing, preclinical and clinical use have demonstrated the safety and efficacy of this material (Wilson et al. 1981). Bioactive glass has traditionally been employed for its osteoconductive and osteostimulative properties (Xynos et al. 2000a, 2001; Xynos et al. 2000b). Recently, data has been presented demonstrating the proangiogenic potential of bioactive glass in vitro and in vivo (Xynos et al. 2001). In addition, these studies have shown that the soluble dissolution products of bioactive glass can stimulate the production of proangiogenic factors thereby providing a potentially promising strategy to enhance neovascularization and resultant bone formation. Data presented herein demonstrates the ability of 45S5 bioactive glass to not only support cell attachment and proliferation, but was also able to support alkaline phosphatase production. In addition, it appears that the combination of bioactive glass and DBM resulted in higher levels of alkaline phosphatase than either one alone. It is clear from the data presented herein that the combination of DBM and bioactive glass present in the NanoFUSE® DBM product resulted in new bone formation and increased neovascularization.
The results of these studies demonstrate the biocompatibility of the NanoFUSE® DBM material. It also demonstrates that the NanoFUSE® DBM material meets the criteria for an ideal bone graft substitute. NanoFUSE® DBM combines the osteoconductive and proangiogenic properties of bioactive glass with the osteoinductive properties of human DBM. While each of these is important, it is the osteoinductive nature of the DBM that enables bone generation to occur throughout a defect rather than simply at the edges (Mulliken et al. 1981). NanoFUSE® DBM represents the first FDA-approved product to combine osteoinductive DBM with the osteoconductive and osteostimulatory bioactive glass. The fact that is a combination of bioactive glass and human DBM means that it is unique when compared with the many other products currently on the market.
NanoFUSE® DBM is a registered trademark of Nanotherapeutics, Inc.
Acknowledgments
The authors would like to thank the donors and their families for their selfless gift of tissue donation, without which this research would not have been possible. The authors would also like to thank Nanotherapeutics, Inc. for their continued support of this research.
REFERENECES CITED
- Berven S, Tay BK, Kleinstueck FS, Bradford DS. Clinical applications of bone graft substitutes in spine surgery: consideration of mineralized and demineralized preparations and growth factor supplementation. Eur Spine J. 2001;10(Suppl 2):S169–177. doi: 10.1007/s005860100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas D, Bible JE, Whang PG, Miller CP, Jaw R, Miller S, Grauer JN. Augmented demineralized bone matrix: a potential alternative for posterolateral lumbar spinal fusion. American journal of orthopedics. 2010;39 (11):531–538. [PubMed] [Google Scholar]
- Cornell CN. Osteoconductive materials and their role as substitutes for autogenous bone grafts. Orthop Clin North Am. 1999;30 (4):591–598. doi: 10.1016/s0030-5898(05)70112-7. [DOI] [PubMed] [Google Scholar]
- Edwards JT, Diegmann MH, Scarborough NL. Osteoinduction of human demineralized bone: characterization in a rat model. Clin Orthop Relat Res. 1998;(357):219–228. doi: 10.1097/00003086-199812000-00028. [DOI] [PubMed] [Google Scholar]
- Fujishiro Y, Hench LL, Oonishi H. Quantitative rates of in vivo bone generation for Bioglass and hydroxyapatite particles as bone graft substitute. J Mater Sci Mater Med. 1997;8(11):649–652. doi: 10.1023/a:1018527621356. 173658 [pii] [DOI] [PubMed] [Google Scholar]
- Glowacki J, Kaban LB, Murray JE, Folkman J, Mulliken JB. Application of the biological principle of induced osteogenesis for craniofacial defects. Lancet. 1981;1 (8227):959–962. doi: 10.1016/s0140-6736(81)91730-x. [DOI] [PubMed] [Google Scholar]
- Goulet JA, Senunas LE, DeSilva GL, Greenfield ML. Autogenous iliac crest bone graft. Complications and functional assessment. Clin Orthop Relat Res. 1997;(339):76–81. doi: 10.1097/00003086-199706000-00011. [DOI] [PubMed] [Google Scholar]
- Hattar S, Berdal A, Asselin A, Loty S, Greenspan DC, Sautier JM. Behaviour of moderately differentiated osteoblast-like cells cultured in contact with bioactive glasses. European cells & materials. 2002;4:61–69. doi: 10.22203/ecm.v004a05. [DOI] [PubMed] [Google Scholar]
- Heary RF, Schlenk RP, Sacchieri TA, Barone D, Brotea C. Persistent iliac crest donor site pain: independent outcome assessment. Neurosurgery. 2002;50 (3):510–516. doi: 10.1097/00006123-200203000-00015. discussion 516–517. [DOI] [PubMed] [Google Scholar]
- Hench LL, Paschall HA. Direct chemical bond of bioactive glass-ceramic materials to bone and muscle. J Biomed Mater Res. 1973;7 (3):25–42. doi: 10.1002/jbm.820070304. [DOI] [PubMed] [Google Scholar]
- Hench LL, Xynos ID, Polak JM. Bioactive glasses for in situ tissue regeneration. J Biomater Sci Polym Ed. 2004;15 (4):543–562. doi: 10.1163/156856204323005352. [DOI] [PubMed] [Google Scholar]
- Hoylaerts MF, Ding L, Narisawa S, Van Kerckhoven S, Millan JL. Mammalian alkaline phosphatase catalysis requires active site structure stabilization via the N-terminal amino acid microenvironment. Biochemistry. 2006;45 (32):9756–9766. doi: 10.1021/bi052471+. [DOI] [PubMed] [Google Scholar]
- Ito G, Matsuda T, Inoue N, Kamegai T. A histological comparison of the tissue interface of bioglass and silica glass. J Biomed Mater Res. 1987;21 (4):485–497. doi: 10.1002/jbm.820210408. [DOI] [PubMed] [Google Scholar]
- Kasten P, Luginbuhl R, van Griensven M, Barkhausen T, Krettek C, Bohner M, Bosch U. Comparison of human bone marrow stromal cells seeded on calcium-deficient hydroxyapatite, beta-tricalcium phosphate and demineralized bone matrix. Biomaterials. 2003;24 (15):2593–2603. doi: 10.1016/s0142-9612(03)00062-0. [DOI] [PubMed] [Google Scholar]
- Kitsugi T, Nakamura T, Yamamura T, Kokubu T, Shibuya T, Takagi M. SEM-EPMA observation of three types of apatite-containing glass-ceramics implanted in bone: the variance of a Ca-P-rich layer. J Biomed Mater Res. 1987;21 (10):1255–1271. doi: 10.1002/jbm.820211008. [DOI] [PubMed] [Google Scholar]
- Kokubo T, Ito S, Huang ZT, Hayashi T, Sakka S, Kitsugi T, Yamamuro T. Ca, P-rich layer formed on high-strength bioactive glass-ceramic A-W. J Biomed Mater Res. 1990;24 (3):331–343. doi: 10.1002/jbm.820240306. [DOI] [PubMed] [Google Scholar]
- Logeart-Avramoglou D, Anagnostou F, Bizios R, Petite H. Engineering bone: challenges and obstacles. Journal of cellular and molecular medicine. 2005;9 (1):72–84. doi: 10.1111/j.1582-4934.2005.tb00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauney JR, Blumberg J, Pirun M, Volloch V, Vunjak-Novakovic G, Kaplan DL. Osteogenic differentiation of human bone marrow stromal cells on partially demineralized bone scaffolds in vitro. Tissue engineering. 2004a;10 (1–2):81–92. doi: 10.1089/107632704322791727. [DOI] [PubMed] [Google Scholar]
- Mauney JR, Sjostorm S, Blumberg J, Horan R, O’Leary JP, Vunjak-Novakovic G, Volloch V, Kaplan DL. Mechanical stimulation promotes osteogenic differentiation of human bone marrow stromal cells on 3-D partially demineralized bone scaffolds in vitro. Calcif Tissue Int. 2004b;74 (5):458–468. doi: 10.1007/s00223-003-0104-7. [DOI] [PubMed] [Google Scholar]
- Morone MA, Boden SD. Experimental posterolateral lumbar spinal fusion with a demineralized bone matrix gel. Spine (Phila Pa 1976) 1998;23 (2):159–167. doi: 10.1097/00007632-199801150-00003. [DOI] [PubMed] [Google Scholar]
- Mulliken JB, Glowacki J, Kaban LB, Folkman J, Murray JE. Use of demineralized allogeneic bone implants for the correction of maxillocraniofacial deformities. Ann Surg. 1981;194 (3):366–372. doi: 10.1097/00000658-198109000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulliken JB, Kaban LB, Glowacki J. Induced osteogenesis--the biological principle and clinical applications. J Surg Res. 1984;37 (6):487–496. doi: 10.1016/0022-4804(84)90218-x. [DOI] [PubMed] [Google Scholar]
- Narisawa S, Hofmann MC, Ziomek CA, Millan JL. Embryonic alkaline phosphatase is expressed at M-phase in the spermatogenic lineage of the mouse. Development. 1992;116 (1):159–165. doi: 10.1242/dev.116.1.159. [DOI] [PubMed] [Google Scholar]
- Oonishi H, Kushitani S, Yasukawa E, Iwaki H, Hench LL, Wilson J, Tsuji E, Sugihara T. Particulate bioglass compared with hydroxyapatite as a bone graft substitute. Clin Orthop Relat Res. 1997;(334):316–325. [PubMed] [Google Scholar]
- Rosenthal RK, Folkman J, Glowacki J. Demineralized bone implants for nonunion fractures, bone cysts, and fibrous lesions. Clin Orthop Relat Res. 1999;(364):61–69. doi: 10.1097/00003086-199907000-00009. [DOI] [PubMed] [Google Scholar]
- Sassard WR, Eidman DK, Gray PM, Block JE, Russo R, Russell JL, Taboada EM. Augmenting local bone with Grafton demineralized bone matrix for posterolateral lumbar spine fusion: avoiding second site autologous bone harvest. Orthopedics. 2000;23 (10):1059–1064. doi: 10.3928/0147-7447-20001001-17. discussion 1064-1055. [DOI] [PubMed] [Google Scholar]
- Tiedeman JJ, Garvin KL, Kile TA, Connolly JF. The role of a composite, demineralized bone matrix and bone marrow in the treatment of osseous defects. Orthopedics. 1995;18 (12):1153–1158. doi: 10.3928/0147-7447-19951201-05. [DOI] [PubMed] [Google Scholar]
- Ubhi CS, Morris DL. Fracture and herniation of bowel at bone graft donor site in the iliac crest. Injury. 1984;16 (3):202–203. doi: 10.1016/0020-1383(84)90162-1. [DOI] [PubMed] [Google Scholar]
- Urist MR, DeLange RJ, Finerman GA. Bone cell differentiation and growth factors. Science. 1983;220 (4598):680–686. doi: 10.1126/science.6403986. [DOI] [PubMed] [Google Scholar]
- Urist MR, Dowell TA. Inductive substratum for osteogenesis in pellets of particulate bone matrix. Clin Orthop Relat Res. 1968;61:61–78. [PubMed] [Google Scholar]
- Urist MR, Strates BS. Bone formation in implants of partially and wholly demineralized bone matrix. Including observations on acetone-fixed intra and extracellular proteins. Clin Orthop Relat Res. 1970;71:271–278. [PubMed] [Google Scholar]
- Wheeler DL, Eschbach EJ, Hoellrich RG, Montfort MJ, Chamberland DL. Assessment of resorbable bioactive material for grafting of critical-size cancellous defects. J Orthop Res. 2000;18 (1):140–148. doi: 10.1002/jor.1100180120. [DOI] [PubMed] [Google Scholar]
- Wheeler DL, Stokes KE, Hoellrich RG, Chamberland DL, McLoughlin SW. Effect of bioactive glass particle size on osseous regeneration of cancellous defects. J Biomed Mater Res. 1998;41 (4):527–533. doi: 10.1002/(SICI)1097-4636(19980915)41:4<527::AID-JBM3>3.0.CO;2-E. [pii] [DOI] [PubMed] [Google Scholar]
- Wilson J, Pigott GH, Schoen FJ, Hench LL. Toxicology and biocompatibility of bioglasses. J Biomed Mater Res. 1981;15 (6):805–817. doi: 10.1002/jbm.820150605. [DOI] [PubMed] [Google Scholar]
- Xynos ID, Edgar AJ, Buttery LD, Hench LL, Polak JM. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem Biophys Res Commun. 2000a;276(2):461–465. doi: 10.1006/bbrc.2000.3503 S0006-291X(00)93503-4. [pii] [DOI] [PubMed] [Google Scholar]
- Xynos ID, Edgar AJ, Buttery LD, Hench LL, Polak JM. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass 45S5 dissolution. J Biomed Mater Res. 2001;55(2):151–157. doi: 10.1002/1097-4636(200105)55:2<151::AID-JBM1001>3.0.CO;2-D. [pii] [DOI] [PubMed] [Google Scholar]
- Xynos ID, Hukkanen MV, Batten JJ, Buttery LD, Hench LL, Polak JM. Bioglass 45S5 stimulates osteoblast turnover and enhances bone formation In vitro: implications and applications for bone tissue engineering. Calcif Tissue Int. 2000b;67(4):321–329. doi: 10.1007/s002230001134. [pii] [DOI] [PubMed] [Google Scholar]
- Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3 (3):192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]