Abstract
Surround inhibition is a neural mechanism that assists in the focusing of excitatory drive to muscles responsible for a given movement (agonist muscles) by suppressing unwanted activity in muscles not relevant to the movement (surround muscles). The purpose of the study was to determine the contribution of GABAB receptor mediated intracortical inhibition as assessed by the cortical silent period (CSP) to the generation of surround inhibition in the motor system. Eight healthy adults (5 women and 3 men, 29.8 ± 9 years) performed isometric contractions with the abductor digiti minimi (ADM) muscle in separate conditions with and without an index finger flexion movement. The ADM motor evoked potential (MEP) amplitude and CSP duration elicited by transcranial magnetic stimulation (TMS) were compared between a control condition in which the ADM was activated independently and during conditions involving three phases (premotor, phasic, and tonic) of the index finger flexion movement. The MEP amplitude of the ADM was greater during the control condition compared with the phasic condition. Thus, the presence of surround inhibition was confirmed in the present study. Most critically, the CSP duration of the ADM decreased during the phasic stage of finger flexion compared to the control condition, which indicated a reduction of this type of intracortical inhibition during the phasic condition. These findings indicate that GABAB receptor mediated intracortical inhibition as measured by the duration of the CSP does not contribute to the generation of surround inhibition in hand muscles.
Keywords: facilitation, transcranial magnetic stimulation, inhibition, motor cortex
Introduction
Surround inhibition (lateral inhibition) is a mechanism in sensory system physiology whereby the activation of a neuron is associated with decreased activity of adjacent neurons, a process that sharpens stimulus localization information (Blakemore et al., 1970). This appears to be a fundamental neural organization pattern because it operates in every sensory system (Nabet & Pinter, 1991). In the motor system, evidence for processes analogous to surround inhibition was originally based on the abnormal movements exhibited by patients with basal ganglia disorders (Denny-Brown, 1967; Hallett & Khoshbin, 1980). Subsequently, these observations were refined into a model that proposed that the motor command consists of an excitatory component that executes a desired movement and an inhibitory component that suppresses an unwanted movement (Mink, 1996).
Recent studies have attempted to determine the presence, functional significance, and physiological mechanisms underlying surround inhibition in the motor system using transcranial magnetic stimulation (TMS) (Beck & Hallett, 2011). In these studies, surround inhibition was quantified as the reduction in the motor evoked potential (MEP) obtained from a muscle not involved in a given task. Furthermore, it was shown that surround inhibition was confined to the initiation phase of movement (Beck et al., 2008), modulated by task (Beck et al., 2009b; Shin et al., 2009; Beck & Hallett, 2010), due primarily to supraspinal mechanisms (Sohn & Hallett, 2004a; b; Beck et al., 2008; Beck & Hallett, 2011), and impaired in movement disorders (Sohn & Hallett, 2004a). In addition, paired-pulse TMS techniques were used to establish the cortical circuits involved in the production of surround inhibition (Beck and Hallett, 2011). Although these studies identified the impairment of several cortical circuits in focal hand dystonia (FHD), they were unable to establish the specific intracortical or intercortical pathway responsible for surround inhibition in healthy subjects.
Intracortical inhibition can also be assessed by measurement of the cortical silent period (CSP), which is the interruption of electromyography (EMG) activity following a suprathreshold TMS pulse (Fuhr et al., 1991). The duration of the CSP is a measure of intracortical inhibition due to activation of Gamma-aminobutyric acidB (GABAB) interneurons that synapse on pyramidal neurons (Inghilleri et al., 1996; Chen et al., 1999; McDonnell et al., 2006). There is strong evidence that the mechanisms responsible for the CSP have functional relevance. For example, CSP duration is task-dependent (Tinazzi et al., 2003) in young adults, and prolonged in aging (Sale & Semmler, 2005) as well as in several movement disorders (Priori et al., 1994a; Priori et al., 1994b; Ridding et al., 1995; Hallett, 2011). Based on this apparent importance of GABAB processes in motor function, it therefore seems possible that the mechanisms underlying the CSP could contribute to surround inhibition. However, none of the numerous previous TMS studies on surround inhibition have examined this possible association.
Our purpose was to determine the contribution of GABAB receptor mediated intracortical inhibition as assessed by the duration of the CSP to the generation of surround inhibition in the motor system. This was accomplished by comparing EMG responses to TMS (MEP and CSP) elicited in the ADM (surround muscle) between isolated ADM activation and concurrent ADM activation and index finger flexion. We hypothesized that the ADM MEP amplitude would be reduced and the ADM CSP duration would be increased (greater inhibition) during the initiation of the index finger flexion movement when compared to independent ADM activation. These findings would indicate the contribution of the physiological mechanisms underlying the CSP to the phenomenon of surround inhibition.
Materials and methods
Subjects
Eight right-handed adults (5 women, 29.8 ± 9 years) provided written informed consent before participating in the experiment. A neurological history and physical examination (performed by RWP) indicated that subjects did not have neurological impairments or used medications known to influence neurological function. All experimental procedures were approved by the NIH Institutional Review Board and conducted according to the Declaration of Helsinki.
Experimental arrangement
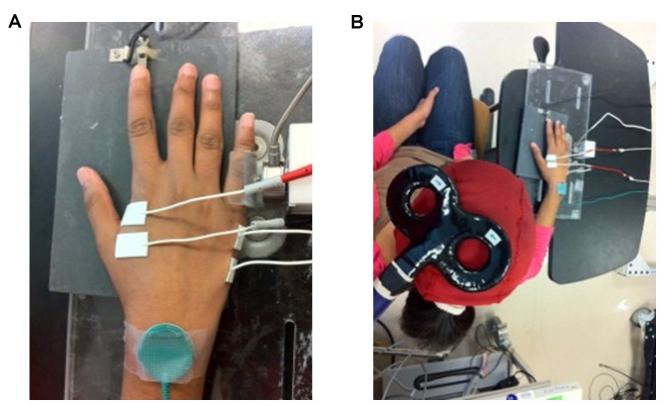
Subjects sat facing a computer monitor with the right arm abducted (~45°), the elbow joint flexed (~90°), and the right hand placed prone on a manipulandum. Index finger flexion force was measured with a force transducer that was placed under the finger pad, and the abduction force exerted by the fifth finger was measured with a force transducer aligned with the proximal interphalangeal joint. This arrangement allowed isometric force production through index finger flexion and fifth finger abduction to be performed simultaneously or with each finger independently when appropriate (Figure 1A).
Figure 1. Experimental arrangement.
A, the right hand was prone on a manipulandum that was instrumented with two force transducers. The force transducers were arranged so that the index finger flexion and fifth finger abduction force production tasks could be performed concurrently or each independently when desired in the study. B, TMS was applied to the ADM muscle “motor hot spot” of the left hemisphere at appropriate times during the experimental trials. Subjects were seated and facing a monitor with the experimental apparatus positioned on a table in front and to the right of the subject.
TMS was performed using a Magstim 2002 connected to a figure-of-eight coil (inner-loop diameter of 70 mm) that was placed over the “motor hot spot” of the left hemisphere for eliciting MEPs in the right ADM. This position was marked with a pen on a scalp cap to ensure correct coil placement throughout the experiment. The coil was oriented tangential to the scalp with the handle pointing backwards and laterally at 45° from the midline (Figure 1B) (Di Lazzaro et al., 2004). Single TMS pulses were applied at the appropriate times and stimulation intensity during the experimental trial blocks (described below). Surface FDI and ADM EMG was recorded with AgCl electrodes configured in belly-tendon montages. EMG signals were amplified (Nicolet Viking IV, Madison, WI, USA), bandpass filtered (20-1000 Hz), digitized (5000 Hz), and the impedance was below 5 kΩ.
Experimental procedures
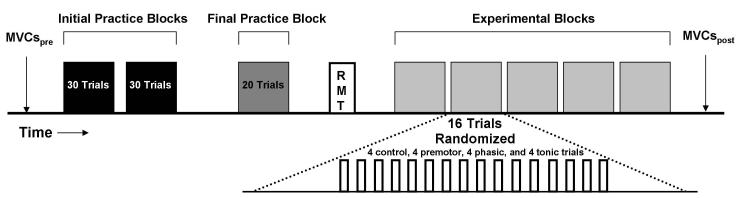
Subjects reported to the laboratory for one experimental session. At the beginning of each session, an investigator gave subjects a visual demonstration of the experimental tasks. Subsequently, the experimental procedures were performed in the order prescribed: (1) maximum voluntary contractions (MVCs) involving index finger flexion (FDI) and fifth finger abduction (ADM); (2) two initial practice trial blocks; (3) a final practice trial block and determination of TMS stimulation times; (4) determination of ADM resting motor threshold (RMT) and TMS stimulation intensity; (5) a series of 5 experimental trial blocks of the motor task with TMS applied during the trials; and (6) MVCs involving index finger flexion and fifth finger abduction. A schematic representation of the experimental protocol is provided in Figure 2.
Figure 2. Experimental protocol.
Schematic representation of the experimental protocol that comprised the MVCpre measurements, initial practice blocks, a final practice block, determination of the RMT, 5 experimental blocks of 16 trials each, and the MVCpost measurements in the order of presentation depicted.
MVCs
Subjects were instructed to independently exert either maximal index finger flexion force or maximal fifth finger abduction force in the shortest time possible and to hold the maximum for 5 seconds (Poston et al., 2008a; b). The average maximal force achieved during the plateau in the force profile was used to determine the target force (5% of MVC for both muscles) for the practice and experimental trials. Three trials were recorded for each muscle at the beginning of the experiment (MVCpre) and one trial for each muscle was conducted at the end of the experiment (MVCpost). The EMG amplitudes during the experimental trials were normalized to the MVC EMG.
Motor task
The motor task involved isometric force production of the ADM muscle of the fifth digit performed with or without an accompanying index finger movement depending on the experimental condition. In the control condition, the ADM was activated independently and matched a target force line (5% of MVC) displayed on the computer monitor for the entire duration of 5 second trials. TMS was delivered randomly between the 1.5 and 3.75 second time points of these control trials in the experimental blocks trial blocks. In the other three experimental conditions, an index finger flexion movement was performed in response to an acoustic tone delivered randomly between the 1.5 and 3.75 second time points of the 5 second trials while the ADM was performing the same isometric force production task throughout the trial as in the control condition. For index finger flexion, subjects were instructed to react as fast as possible to the acoustic tone, rapidly increase the force to the line displayed on the monitor, hold this force throughout the trial, and quickly terminate the force at the end of the trial.
The three experimental conditions involving index finger flexion were distinguished by the time in which TMS was delivered relative to the onset of the FDI EMG and will be referred to as the premotor, phasic, and tonic conditions. These conditions correspond to the following movement phases and TMS delivery times: premotor (20 ms before FDI EMG onset), phasic (the first peak of FDI EMG), and tonic (during contraction at the target force level). In summary, subjects had to accurately maintain a constant target force with the ADM throughout each trial in all conditions, despite sometimes having to concurrently produce a rapid index finger flexion force at random times. This combined with the low target forces and the requirement to use visual feedback to monitor the target forces of both muscles (sometimes simultaneously) made it a difficult motor task. Accordingly, pilot work found that 30-60 practice trials were required for a subject to become proficient.
Initial practice blocks
The goal of the initial practice trial blocks was to provide the subjects with sufficient practice to correctly execute the motor task before progressing to the final practice trial block and the experimental trial block. Accordingly, subjects performed two initial practice blocks of 30 trials. TMS was not applied during these practice blocks. At the end of the initial practice blocks, the investigators and each subject were confident that they could correctly execute the motor task.
Final practice block and determination of TMS stimulation times
After the initial practice blocks, subjects could perform the motor task correctly and displayed consistent reaction times to the acoustic tone. Therefore, the aim of the final practice block was to determine the individual reaction time of each subject in order that TMS could be delivered at the appropriate times relative to the FDI onset in the premotor, phasic, and tonic movement phases in the forthcoming experimental trial blocks (Beck et al., 2008; Beck & Hallett, 2010). Upon completion of the final practice block (20 trials), a custom-written analysis script in Signal 4.07 (Cambridge Electronic Design, Cambridge, UK) was used to determine TMS stimulation times based upon the reaction times and EMG onset times displayed by the subject.
Determination of RMT and TMS stimulation intensity
The RMT of the ADM was determined to the nearest 1% of maximal stimulator output (% MSO) and was defined as the minimal stimulus intensity required to evoke MEPs of at least 50 μV in 5 of 10 consecutive trials (Rossini et al., 1994). The stimulus intensity was set to 130% of RMT and single TMS pulses at this intensity were applied at the appropriate times during the experimental trials.
Experimental trial blocks
Each subject performed 5 blocks of 16 trials of the motor task. The 4 conditions (control, premotor, phasic, and tonic trials) were each presented 4 times in random order within each block of 16 trials. Thus, a total of 20 trials for each condition were collected in the experiment. The presentation order of the conditions within each block was randomized and the times that the acoustic tone was delivered also varied randomly between the 1.5 and 3.75 second time points of the trials. Thus, subjects were unaware at the beginning of each trial of when the acoustic tone would be delivered or when TMS would be applied during the ADM or FDI contractions.
Data analysis
All data were collected using a custom-written data acquisition scripts in Signal and analysed offline with custom-written Matlab programs (Mathworks Inc., Natick, Massachusetts, USA). MEP size was determined by averaging the peak-to-peak amplitudes of the individual MEPs in each experimental condition. The CSP duration was quantified as the time elapsed between the onset of the MEP and the time at which the post-stimulus background EMG returned to the pre-stimulus mean amplitude. These times were determined using a validated algorithm (Garvey et al., 2001) and verified by visual inspection. The average duration of the CSP was obtained for each condition and used for analysis. The average force achieved during the MVCs was denoted as the MVC force for each muscle. Finally, the background EMG activity of the ADM was determined as the average value normalized to MVC over a 100 ms time period before MEP onset.
Statistical analysis
The primary dependent variables were the ADM MEP amplitude and ADM CSP duration. The MVCs (MVCpre, MVCpost) and the ADM background EMG were secondary dependent variables that were used as experimental controls.
Primary dependent variables
Spearman’s rank correlation was used to test for a statistical correlation between the primary dependent variables, ADM MEP amplitude and ADM CSP duration. The Shapiro-Wilk test was used to test the assumption of normality in both primary dependent variables. If the data could be transformed into normal, a one-way repeated measures analysis of variance (ANOVA; parametric test) was applied to the transformed data to examine the effect of Condition (control, premotor, phasic, and tonic). If no transform was effective, a Friedman’s test (non-parametric test) was used to assess the effect of Condition. Post-hoc testing compared the premotor, phasic, and tonic conditions with the control condition in each of the primary dependent variables. To adjust for multiple comparisons, Dunnett’s method was used with parametric testing (ANOVA), and the Bonferroni method was used with non-parametric testing (Friedman’s test). A significance level of 0.025 was used in order to adjust for multiple testing since the study included two primary dependent variables.
Secondary dependent variables
Paired two-tailed t-tests were used to compare the MVCpre and MVCpost for both the FDI and ADM muscles. A one-way repeated measures ANOVA was used to compare the log transformed ADM background EMG means for the four experimental conditions. A significance level of 0.05 was used for the secondary dependent variables.
Results
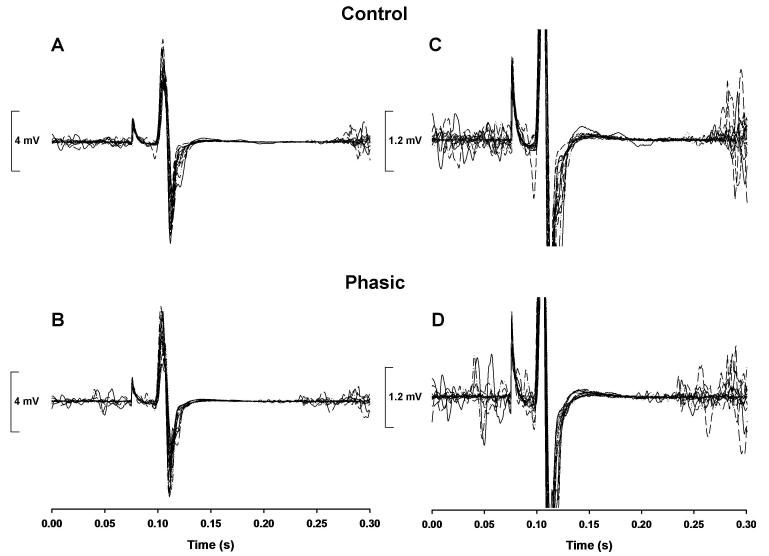
Representative MEP and CSP duration data for the control and phasic conditions are shown in Figure 3.
Figure 3. Representative experimental trials for one subject for the control condition and the phasic condition.
The time-aligned EMG-time profiles of all 20 control condition trials (top panels) and 20 phasic condition trials (bottom panels) performed during the experimental blocks. A-B, the MEP amplitude was greater for the control condition (average MEP amplitude = 9.07 mV) compared with the phasic condition (average MEP amplitude = 8.28 mV) for this subject, which indicated the presence surround inhibition. C-D, the same data are depicted with the scale of the y-axis reduced and the MEPs truncated to make it easier to observe the CSP durations. The CSP duration of the ADM was greater during the control condition (average CSP duration = 183.5 ms) compared with the phasic condition (average CSP duration = 155 ms), which indicated a reduction in the magnitude of this particular type of intracortical inhibition during the phasic condition for this subject.
Primary dependent variables: ADM MEP amplitude and CSP Duration
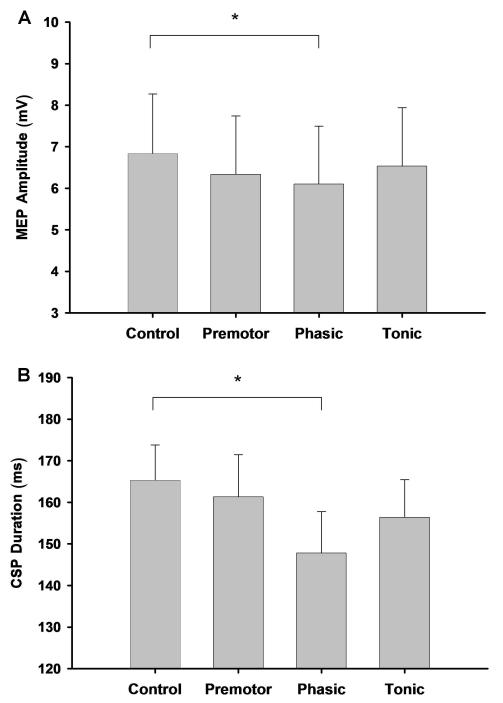
The two primary dependent variables were statistically independent for each of the four experimental conditions (Spearman’s rank correlation, |ρ| < 0.24, P > 0.5 for the control, premotor, phasic, and tonic conditions). The Friedman test on ranks revealed a significant (P = 0.0069) effect for Condition for ADM MEP amplitude. Post hoc analysis with Bonferroni adjustment indicated that the MEP amplitude was greater for the control condition compared with the phasic condition (P = 0.0141; Figure 4A). For the log transformed ADM CSP duration, repeated measures one-way ANOVA showed a significant effect for Condition (P = 0.0012). Post hoc analysis with the Dunnett adjustment revealed that the CSP duration was greater for the control condition compared with the phasic condition (P = 0.0004; Figure 4B).
Figure 4. ADM MEP amplitude and ADM CSP duration for the four conditions.
A, there was a significant effect for Condition and post-hoc analysis revealed that the MEP amplitude of the ADM was greater during the control condition compared with the phasic condition (P = 0.0141), which indicated the presence of surround inhibition during the phasic condition. B, there was a significant effect for Condition and post-hoc analysis revealed that the CSP duration of the ADM was greater during the control condition compared with the phasic condition (P = 0.0004), which indicated a reduction in the magnitude of this particular type of intracortical inhibition during the phasic condition.
Secondary dependent variables: MVCs and ADM background EMG
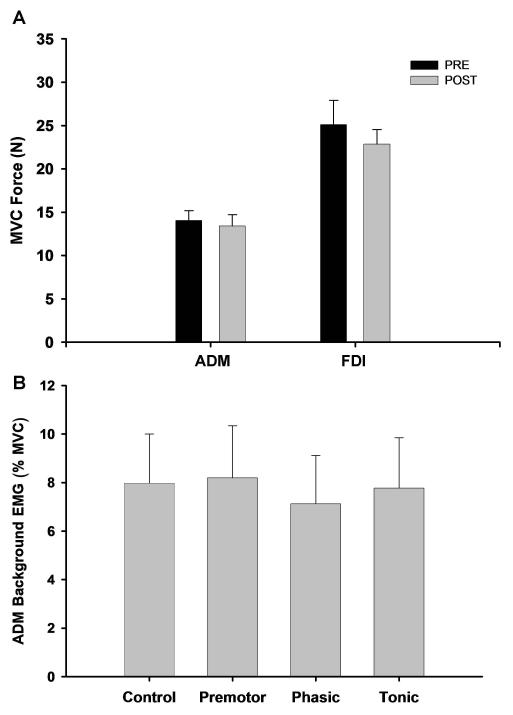
There was no significant difference between the MVCpre and MVCpost for either the ADM muscle (P = 0.385; Figure 5A) or the FDI muscle (P = 0.735; Figure 5A). Furthermore, the ADM background EMG was similar (P = 0.5828) for the four experimental conditions (Figure 5B).
Figure 5. MVCpre and MVCpost for the two muscles and ADM background EMG for the four conditions.
A, MVCpre and MVCpost were similar for both the ADM and the FDI muscles. Thus, muscle fatigue did not influence the experimental results obtained for either muscle. B, the ADM background EMG was similar for the four experimental conditions.
Discussion
The purpose was to determine the contribution of GABAB receptor mediated intracortical inhibition as assessed by the CSP to the generation of surround inhibition. The study produced two main findings. First, ADM MEP amplitude was greater during independent ADM activation (control condition) compared with the phasic movement phase of the index finger flexion. Thus, the presence of surround inhibition was confirmed in the current study. Second, the ADM CSP duration was greater during independent ADM activation compared with the phasic movement phase of the index finger flexion, which indicated that the magnitude of this specific type of intracortical inhibition was reduced during the phasic movement phase. Taken together, these findings indicate that GABAB receptor mediated intracortical inhibition as measured by CSP duration does not contribute to the generation of surround inhibition in hand muscles.
Methodological considerations
A variety of TMS parameters and task details influence MEP magnitude, CSP duration, and the expression of surround inhibition. Therefore, several experimental controls and methodological considerations were employed to minimize confounding influences. For example, muscle fatigue enhances MEP amplitude and CSP duration (Taylor et al., 1996; Taylor et al., 2000). Although the contraction intensities were low and adequate rest periods were given between trial blocks, muscle fatigue was possible due to the number of trials. Nonetheless, the absence of a change between MVCpre and MVCpost for both muscles suggests that muscle fatigue did not influence the results. Another important factor that influences MEP amplitude is the amount of background EMG activity (Capaday, 1997). In the current study, this depended on the ability of the subjects to maintain constant force and ADM EMG levels across conditions, despite having to concurrently produce an index finger flexion movement upon a randomly timed acoustic tone. Accordingly, the similar ADM EMG levels across conditions suggest that motor unit pool excitation was comparable in all cases and not responsible for changes in MEP. Thus, subjects performed the complex task in conformity with the task requirements during the experimental blocks after sufficient practice.
An additional potential confound of the study is the possible dependence of CSP duration on MEP amplitude as some studies have shown a correlation between these variables (Cantello et al., 1992; Taylor et al., 1997; Ho et al., 1998; Orth & Rothwell, 2004). Thus, it could be argued that changes in CSP duration could be exclusively due to concomitant changes in MEP amplitude. However, the evidence for an association between the two variables comes primarily from the aforementioned studies that used a range of stimulus intensities, which would lead to associations since both variables are dependent on stimulus intensity. While one study using a constant stimulus intensity in a single behavioral condition also found an association between CSP duration and MEP amplitude (Orth & Rothwell, 2004), it has been shown conclusively that MEP amplitude and CSP duration can become uncoupled in different behavioral conditions with a constant stimulus intensity and similar background EMG levels (Tinazzi et al., 2003). Therefore, the possible association between CSP duration and MEP amplitude should not have confounded the current study because stimulus intensity was constant, background EMG was similar, and the behavioral state was different between experimental conditions. Accordingly, Spearman’s rank correlation indicated that the two variables were statistically independent for each of the four experimental conditions.
The amount of surround inhibition that can be observed depends on several features of the motor task. Specifically, surround inhibition is greater in the dominant (right) hand (Shin et al., 2009), more pronounced at lower force levels (Beck et al., 2009b), scales with task difficulty (Beck & Hallett, 2010), and is confined to the initiation phase of movement (Sohn & Hallett, 2004b; Beck et al., 2009b; Beck & Hallett, 2011). Therefore, the study included right-handed subjects, a target force of 5% of MVC, a challenging motor task, and measured surround inhibition in the ADM during all of the index finger flexion movement phases as in previous studies (Beck et al., 2008; Beck et al., 2009c). Conversely, the methodological issue of primary importance in interpreting the implications of the CSP duration for a given task is the TMS stimulation intensity, because the CSP does not depend on background EMG activity. In the present study, a stimulation intensity of 130% of RMT was utilized for four reasons. First, preliminary work determined that lower stimulation intensities of 115% of RMT and below, which result in short CSP durations, made it difficult for algorithms or visual inspection to quantify the CSP duration of the ADM at the low activation level of 5% of MVC. Second, short CSP durations (<75 ms) are due to spinal mechanisms, whereas longer silent periods (75 to 300 ms) are due exclusively to cortical mechanisms (Fuhr et al., 1991; Inghilleri et al., 1996; Chen et al., 1999). Because surround inhibition arises primarily from cortical mechanisms (Sohn & Hallett, 2004a; Beck et al., 2008; Beck & Hallett, 2011), the relatively high stimulus intensity assured that the CSP durations elicited by TMS reflected intracortical inhibition. Third, stimulation intensities higher than 130% could have led to ceiling effects in the CSP duration, which could have precluded the ability to observe significant lengthening of the CSP in some experimental conditions. Fourth, stimulation intensities from 130-150% of RMT are the most common in the literature (Orth & Rothwell, 2004). Collectively, these methodological considerations should have optimized the ability to determine the contribution of mechanisms underlying the CSP to surround inhibition.
Surround inhibition and CSP duration
It has been proposed that surround inhibition is an important mechanism that acts to focus excitatory neural drive to muscles responsible for a given movement (agonists) while actively inhibiting activity in muscles not relevant to the movement (surround muscles) (Sohn & Hallett, 2004a; Beck et al., 2008; Beck & Hallett, 2011). Strong support for these contentions comes from observations in movement disorders that are characterized by excessive activation of muscles not required in a given movement (Shin et al., 2010), especially FHD (Hallett, 2011). In contrast to healthy subjects, FHD patients consistently exhibit facilitation as opposed to inhibition of the MEP of the surround muscle during agonist muscle activation, which indicates a loss of surround inhibition (Sohn & Hallett, 2004a; Beck et al., 2008).
Based on these findings, extensive research has focused on the identification of the mechanisms underlying the generation of surround inhibition in healthy subjects and its impairment in motor disorders. Because the MEP reflects net corticospinal excitability and depends on the balance between numerous cortical excitatory and inhibitory interneuronal circuits, a well-accepted strategy involves the application of paired-pulse TMS to establish which pathways contribute to the suppressed MEP indicative of surround inhibition (Beck & Hallett, 2011). Despite the determination of the impairment of some of these pathways in FHD, none of these studies were able to establish the specific cortical pathway underlying the generation of surround inhibition in healthy subjects. For example, intracortical and intercortical circuits including short intracortical inhibition (Sohn & Hallett, 2004a; Beck et al., 2008), long intracortical inhibition (LICI; Sohn & Hallett, 2004b), intracortical facilitation (Sohn & Hallett, 2004b), interhemispheric inhibition (Beck et al., 2009c), dorsal premotor inhibition (Beck et al., 2009a), and ventral premotor inhibition (Houdayer et al., 2012) were not responsible for surround inhibition. Similarly, short afferent inhibition (Richardson et al., 2008), long-latency afferent inhibition (Pirio Richardson et al., 2009), and cerebellar inhibition (Kassavetis et al., 2011) were also not involved. Collectively, these results are surprising given the functional importance and number of the cortical pathways examined in these studies.
The CSP is another index of intracortical inhibition that has been used extensively to study GABAB mediated inhibition processes during voluntary contractions. In the present study, it was hypothesized that the mechanisms underlying the CSP could participate in the generation of surround inhibition. This expectation was based on several interrelated lines of evidence. First, GABAergic neurons are the most numerous and important class of inhibitory interneurons in the motor cortex (Jones, 1993; Keller, 1993). Second, the CSP duration of agonist muscles has been shown to be abnormal in FHD (Ikoma et al., 1996; Chen et al., 1997; Filipovic et al., 1997) and Parkinson’s disease (Priori et al., 1994a; Nakashima et al., 1995), which are the same patient populations that have exhibited impaired surround inhibition (Sohn & Hallett, 2004a; Shin et al., 2007; Beck & Hallett, 2011). Third, the differential modulation of CSP duration in different tasks suggests that this type of intracortical inhibition has functional significance in the execution of fine motor tasks involving hand muscles (Tinazzi et al., 2003; Sale & Semmler, 2005). Fourth, no previous studies had examined the possible role of the CSP in the generation of surround inhibition. In fact, the standard paradigm in these studies did not permit CSP duration quantification because the surround muscle was required to remain at rest during agonist muscle activation. Therefore, a modification of a previously developed experimental methodology (Sohn et al., 2005) was utilized to assess CSP duration in an active surround muscle during remote muscle activation.
The MEP amplitude of the surround ADM muscle was greater during independent activation compared with the phasic movement phase of the accompanying index finger flexion. This finding is consistent with previous studies that found a reduced MEP in the surround muscle during movement initiation, albeit with the surround muscle at rest (Sohn & Hallett, 2004a; b; Beck et al., 2008; Beck et al., 2009b; Beck & Hallett, 2010; Kassavetis et al., 2011). Thus, the presence of surround inhibition was confirmed in the active surround ADM muscle in the current experimental paradigm. Most importantly, this finding coincided with the observation that the CSP duration of the ADM was also greater (more inhibition) during independent activation compared with the phasic movement phase of the index finger flexion. Therefore, the amount of this type of intracortical inhibition was reduced during the phasic movement phase compared with independent activation. Accordingly, these results are contrary to our original hypothesis, which predicted the exact opposite modulation of CSP duration. In summary, the findings indicate that GABAB receptor mediated intracortical inhibition as measured by the duration of the CSP does not contribute to surround inhibition.
CSP duration and LICI
The reduced intracortical inhibition (shortened CSP duration) at first seems counterintuitive. However, the finding is similar to previous results obtained from surround inhibition studies involving other inhibitory pathways. For instance, measures of short afferent inhibition (Richardson et al., 2008), long-latency afferent inhibition (Pirio Richardson et al., 2009), interhemispheric inhibition (Beck et al., 2009c), cerebellar inhibition (Kassavetis et al., 2011), and LICI (Sohn & Hallett, 2004b) all exhibited reductions rather than enhancements in inhibition. The similar modulation of LICI and CSP duration in the two studies is particularly noteworthy because the two measures of intracortical inhibition are thought to reflect similar physiological mechanisms. More specifically, pharmacological studies have determined that both measures involve post-synaptic GABAB mediated inhibition (Chen et al., 1999; Werhahn et al., 1999; McDonnell et al., 2006; Florian et al., 2008). Accordingly, EEG and EMG measures of LICI were significantly associated with CSP duration in the abductor pollicis brevis (Farzan et al., 2010). However, other studies have shown a differential modulation of LICI and CSP duration by drugs (Inghilleri et al., 1996; McDonnell et al., 2006), disease (Berardelli et al., 1996), and fatigue (Benwell et al., 2007). Thus, the balance of the experimental data seems to suggest that the mechanisms underlying LICI and CSP are not identical and display divergent functional responses in various conditions, despite the fact that both measures reflect GABAB mediated inhibition. Furthermore, it has been proposed that CSP may provide a measure of the duration of GABAB receptor mediated inhibition, whereas LICI provides a measure of the depth of this inhibition (Cash et al., 2010). Nonetheless, the reduced LICI observed previously (Sohn & Hallett, 2004b) and the shortened CSP duration found in the present study provides consistent evidence that these types of intracortical inhibition act in the same direction in opposition to surround inhibition processes.
Limitations
Despite the seemingly clear finding that CSP duration and surround inhibition were disassociated and a number of experimental controls were employed, the study had limitations and alternative interpretations of the data are possible. For instance, neither measures of spinal excitability nor the spinal component of the CSP (CSP durations <75 ms) were undertaken and these mechanisms could theoretically contribute to surround inhibition. As cited above, however, a number of the original surround inhibition studies performed control spinal measurements and concluded that surround inhibition was due to supraspinal mechanisms. Therefore, later studies have not seen it to be necessary to perform spinal measurements as it seems highly unlikely that spinal mechanisms could be responsible for surround inhibition in healthy subjects or the loss of surround inhibition in patients. Another alternative explanation for the current findings is that a reduced inhibition of CSP related neurons onto an unknown class of inhibitory interneurons could result in the level of inhibition exerted by these neurons onto surround muscle pyramidal cells to increase, leading to surround inhibition. However, this is highly speculative and unlikely given the known pattern of connections of intracortical neurons mediating the CSP and other forms of intracortical inhibition and facilitation in the motor cortex (Reis et al., 2008). Additionally, this line of reasoning could theoretically apply to almost every other cortical pathway that has been studied and excluded as a possible contributor to surround inhibition. Although such possibilities cannot be ruled out, they also seem highly unlikely given the known connection patterns in the motor cortex and the conclusions of previous studies. The testing of these possibilities would require complicated experimental procedures and could be an avenue of future research.
Conclusions
The presence of surround inhibition in the motor system was confirmed in the current study, but the findings indicated that GABAB receptor mediated intracortical inhibition as measured by the duration of the CSP did not contribute to the generation of surround inhibition. Similar to previous studies (Beck & Hallett, 2011), the results were able to exclude the possible contribution of a specific cortical pathway to surround inhibition, but unable to identify the pathway responsible for the phenomenon. Therefore, future work will examine the remaining candidate cortical inhibitory and excitatory pathways that could be responsible for surround inhibition.
Acknowledgements
This work was supported by the NINDS intramural research program. The authors would like to thank Tianxia Wu for assistance with the statistical analysis.
Abbreviations
- ADM
Abductor Digiti Minimi
- CSP
Cortical Silent Period
- EMG
Electromyography
- FHD
Focal hand dystonia
- GABAB
Gamma-aminobutyric acidB
- FDI
First Dorsal Interosseus
- LICI
Long Intracortical Inhibition
- MEP
Motor Evoked Potential
- MVC
Maximum Voluntary Contraction
- RMT
Resting Motor Threshold
- TMS
Transcranial Magnetic Stimulation
References
- Beck S, Hallett M. Surround inhibition is modulated by task difficulty. Clin Neurophysiol. 2010;121:98–103. doi: 10.1016/j.clinph.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Hallett M. Surround inhibition in the motor system. Exp Brain Res. 2011;210:165–172. doi: 10.1007/s00221-011-2610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Houdayer E, Richardson SP, Hallett M. The role of inhibition from the left dorsal premotor cortex in right-sided focal hand dystonia. Brain Stimul. 2009a;2:208–214. doi: 10.1016/j.brs.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Richardson SP, Shamim EA, Dang N, Schubert M, Hallett M. Short intracortical and surround inhibition are selectively reduced during movement initiation in focal hand dystonia. J Neurosci. 2008;28:10363–10369. doi: 10.1523/JNEUROSCI.3564-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Schubert M, Richardson SP, Hallett M. Surround inhibition depends on the force exerted and is abnormal in focal hand dystonia. J Appl Physiol. 2009b;107:1513–1518. doi: 10.1152/japplphysiol.91580.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S, Shamim EA, Richardson SP, Schubert M, Hallett M. Inter-hemispheric inhibition is impaired in mirror dystonia. Eur J Neurosci. 2009c;29:1634–1640. doi: 10.1111/j.1460-9568.2009.06710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell NM, Mastaglia FL, Thickbroom GW. Differential changes in long- interval intracortical inhibition and silent period duration during fatiguing hand exercise. Exp Brain Res. 2007;179:255–262. doi: 10.1007/s00221-006-0790-2. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Rona S, Inghilleri M, Manfredi M. Cortical inhibition in Parkinson’s disease. A study with paired magnetic stimulation. Brain. 1996;119(Pt 1):71–77. doi: 10.1093/brain/119.1.71. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Carpenter RH, Georgeson MA. Lateral inhibition between orientation detectors in the human visual system. Nature. 1970;228:37–39. doi: 10.1038/228037a0. [DOI] [PubMed] [Google Scholar]
- Cantello R, Gianelli M, Civardi C, Mutani R. Magnetic brain stimulation: the silent period after the motor evoked potential. Neurology. 1992;42:1951–1959. doi: 10.1212/wnl.42.10.1951. [DOI] [PubMed] [Google Scholar]
- Capaday C. Neurophysiological methods for studies of the motor system in freely moving human subjects. J Neurosci Methods. 1997;74:201–218. doi: 10.1016/s0165-0270(97)02250-4. [DOI] [PubMed] [Google Scholar]
- Cash RFH, Ziemann U, Murray K, Thickbroom GW. Late cortical disinhibition in human motor cortex: A triple-pulse transcranial magnetic stimulation study. J Neurophys. 2010;103:511–518. doi: 10.1152/jn.00782.2009. [DOI] [PubMed] [Google Scholar]
- Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res. 1999;128:539–542. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- Chen R, Wassermann EM, Canos M, Hallett M. Impaired inhibition in writer’s cramp during voluntary muscle activation. Neurology. 1997;49:1054–1059. doi: 10.1212/wnl.49.4.1054. [DOI] [PubMed] [Google Scholar]
- Denny-Brown D. The fundamental organization of motor behavior. In: Yahr M, Purpura D, editors. Neurophysiological basis of normal and abnormal motor activities. Raven; New York: 1967. [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115:255–266. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Farzan F, Barr MS, Levinson AJ, Chen R, Wong W, Fitzgerald PB, Daskalakis ZJ. Reliability of long-interval cortical inhibition in healthy human subjects: a TMS- EEG study. J Neurophysiol. 2010;104:1339–1346. doi: 10.1152/jn.00279.2010. [DOI] [PubMed] [Google Scholar]
- Filipovic SR, Ljubisavljevic M, Svetel M, Milanovic S, Kacar A, Kostic VS. Impairment of cortical inhibition in writer’s cramp as revealed by changes in electromyographic silent period after transcranial magnetic stimulation. Neurosci Lett. 1997;222:167–170. doi: 10.1016/s0304-3940(97)13370-5. [DOI] [PubMed] [Google Scholar]
- Florian J, Muller-Dahlhaus M, Liu Y, Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex a pharmacological TMS study. J Physiol. 2008;586:495–514. doi: 10.1113/jphysiol.2007.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Garvey MA, Ziemann U, Becker DA, Barker CA, Bartko JJ. New graphical method to measure silent periods evoked by transcranial magnetic stimulation. Clin Neurophysiol. 2001;112:1451–1460. doi: 10.1016/s1388-2457(01)00581-8. [DOI] [PubMed] [Google Scholar]
- Hallett M. Neurophysiology of dystonia: The role of inhibition. Neurobiol Dis. 2011;42:177–184. doi: 10.1016/j.nbd.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M, Khoshbin S. A physiological mechanism of bradykinesia. Brain. 1980;103:301–314. doi: 10.1093/brain/103.2.301. [DOI] [PubMed] [Google Scholar]
- Ho KH, Nithi K, Mills KR. Covariation between human intrinsic hand muscles of the silent periods and compound muscle action potentials evoked by magnetic brain stimulation: evidence for common inhibitory connections. Exp Brain Res. 1998;122:433–440. doi: 10.1007/s002210050531. [DOI] [PubMed] [Google Scholar]
- Houdayer E, Beck S, Karabanov A, Poston B, Hallett M. The differential modulation of the ventral premotor-motor interaction during movement initiation is deficient in patients with focal hand dystonia. Eur J Neurosci. 2012;35:478–485. doi: 10.1111/j.1460-9568.2011.07960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikoma K, Samii A, Mercuri B, Wassermann EM, Hallett M. Abnormal cortical motor excitability in dystonia. Neurology. 1996;46:1371–1376. doi: 10.1212/wnl.46.5.1371. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Marchetti P, Manfredi M. Effects of diazepam, baclofen and thiopental on the silent period evoked by transcranial magnetic stimulation in humans. Exp Brain Res. 1996;109:467–472. doi: 10.1007/BF00229631. [DOI] [PubMed] [Google Scholar]
- Jones EG. GABAergic neurons and their role in cortical plasticity in primates. Cereb Cortex. 1993;3:361–372. doi: 10.1093/cercor/3.5.361-a. [DOI] [PubMed] [Google Scholar]
- Kassavetis P, Hoffland BS, Saifee TA, Bhatia KP, van de Warrenburg BP, Rothwell JC, Edwards MJ. Cerebellar brain inhibition is decreased in active and surround muscles at the onset of voluntary movement. Exp Brain Res. 2011;209:437–442. doi: 10.1007/s00221-011-2575-5. [DOI] [PubMed] [Google Scholar]
- Keller A. Intrinsic synaptic organization of the motor cortex. Cereb Cortex. 1993;3:430–441. doi: 10.1093/cercor/3.5.430. [DOI] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 2006;173:86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Nabet B, Pinter RB. Sensory neural networks: lateral inhibition. Boca Raton; 1991. [Google Scholar]
- Nakashima K, Wang Y, Shimoda M, Sakuma K, Takahashi K. Shortened silent period produced by magnetic cortical stimulation in patients with Parkinson’s disease. J Neurol Sci. 1995;130:209–214. doi: 10.1016/0022-510x(95)00029-2. [DOI] [PubMed] [Google Scholar]
- Orth M, Rothwell JC. The cortical silent period: intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clin Neurophysiol. 2004;115:1076–1082. doi: 10.1016/j.clinph.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Pirio Richardson S, Bliem B, Voller B, Dang N, Hallett M. Long-latency afferent inhibition during phasic finger movement in focal hand dystonia. Exp Brain Res. 2009;193:173–179. doi: 10.1007/s00221-008-1605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston B, Enoka JA, Enoka RM. Endpoint accuracy for a small and a large hand muscle in young and old adults during rapid, goal-directed isometric contractions. Exp Brain Res. 2008a;187:373–385. doi: 10.1007/s00221-008-1309-9. [DOI] [PubMed] [Google Scholar]
- Poston B, Enoka JA, Enoka RM. Practice and endpoint accuracy with the left and right hands of old adults: the right-hemisphere aging model. Muscle Nerve. 2008b;37:376–386. doi: 10.1002/mus.20954. [DOI] [PubMed] [Google Scholar]
- Priori A, Berardelli A, Inghilleri M, Accornero N, Manfredi M. Motor cortical inhibition and the dopaminergic system. Pharmacological changes in the silent period after transcranial brain stimulation in normal subjects, patients with Parkinson’s disease and drug-induced parkinsonism. Brain. 1994a;117(Pt 2):317–323. doi: 10.1093/brain/117.2.317. [DOI] [PubMed] [Google Scholar]
- Priori A, Berardelli A, Inghilleri M, Polidori L, Manfredi M. Electromyographic silent period after transcranial brain stimulation in Huntington’s disease. Mov Disord. 1994b;9:178–182. doi: 10.1002/mds.870090209. [DOI] [PubMed] [Google Scholar]
- Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris-Love M, Perez MA, Ragert P, Rothwell JC, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol. 2008;586:325–351. doi: 10.1113/jphysiol.2007.144824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson SP, Bliem B, Lomarev M, Shamim E, Dang N, Hallett M. Changes in short afferent inhibition during phasic movement in focal dystonia. Muscle Nerve. 2008;37:358–363. doi: 10.1002/mus.20943. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Inzelberg R, Rothwell JC. Changes in excitability of motor cortical circuitry in patients with Parkinson’s disease. Ann Neurol. 1995;37:181–188. doi: 10.1002/ana.410370208. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, et al. Report of an IFCN committee Non- invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Sale MV, Semmler JG. Age-related differences in corticospinal control during functional isometric contractions in left and right hands. J Appl Physiol. 2005;99:1483–1493. doi: 10.1152/japplphysiol.00371.2005. [DOI] [PubMed] [Google Scholar]
- Shin HW, Kang SY, Hallett M, Sohn YH. Extended surround inhibition in idiopathic paroxysmal kinesigenic dyskinesia. Clin Neurophysiol. 2010;121:1138–1141. doi: 10.1016/j.clinph.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Shin HW, Kang SY, Sohn YH. Disturbed surround inhibition in preclinical parkinsonism. Clin Neurophysiol. 2007;118:2176–2179. doi: 10.1016/j.clinph.2007.06.058. [DOI] [PubMed] [Google Scholar]
- Shin HW, Sohn YH, Hallett M. Hemispheric asymmetry of surround inhibition in the human motor system. Clin Neurophysiol. 2009;120:816–819. doi: 10.1016/j.clinph.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Hallett M. Disturbed surround inhibition in focal hand dystonia. Ann Neurol. 2004a;56:595–599. doi: 10.1002/ana.20270. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Hallett M. Surround inhibition in human motor system. Exp Brain Res. 2004b;158:397–404. doi: 10.1007/s00221-004-1909-y. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Kang SY, Hallett M. Corticospinal disinhibition during dual action. Exp Brain Res. 2005;162:95–99. doi: 10.1007/s00221-004-2109-5. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Allen GM, Butler JE, Gandevia SC. Effect of contraction strength on responses in biceps brachii and adductor pollicis to transcranial magnetic stimulation. Exp Brain Res. 1997;117:472–478. doi: 10.1007/s002210050243. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Allen GM, Butler JE, Gandevia SC. Supraspinal fatigue during intermittent maximal voluntary contractions of the human elbow flexors. J Appl Physiol. 2000;89:305–313. doi: 10.1152/jappl.2000.89.1.305. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Allen GM, Gandevia SC. Changes in motor cortical excitability during human muscle fatigue. J Physiol. 1996;490(Pt 2):519–528. doi: 10.1113/jphysiol.1996.sp021163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinazzi M, Farina S, Tamburin S, Facchini S, Fiaschi A, Restivo D, Berardelli A. Task-dependent modulation of excitatory and inhibitory functions within the human primary motor cortex. Exp. Brain Res. 2003;150:222–229. doi: 10.1007/s00221-003-1448-y. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517(Pt 2):591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]