Abstract
This short review offers an update on the first and second Multicenter Selective Lymphadenectomy Trials (MSLT-I and MSLT-II) for patients with melanoma, and briefly traces the development of intraoperative lymphatic mapping and sentinel node biopsy.
Keywords: Melanoma, Sentinel node biopsy, Micrometastases, Lymphatic mapping, Phase III multicenter trial
Melanoma is the most rapidly increasing malignancy in the world. It occurs on the surface of the skin, where theoretically it should be easily diagnosed and treated. However, successful management of melanoma is complicated by this cancer’s aggressive nature and its unpredictable metastatic pattern, making it far more lethal than other solid malignancies. For example, a 5-mm primary melanoma is associated with a 50% death rate at 5 years,1 whereas a much larger primary breast cancer (≤20 mm) is associated with a mortality of only 5% at 5 years.
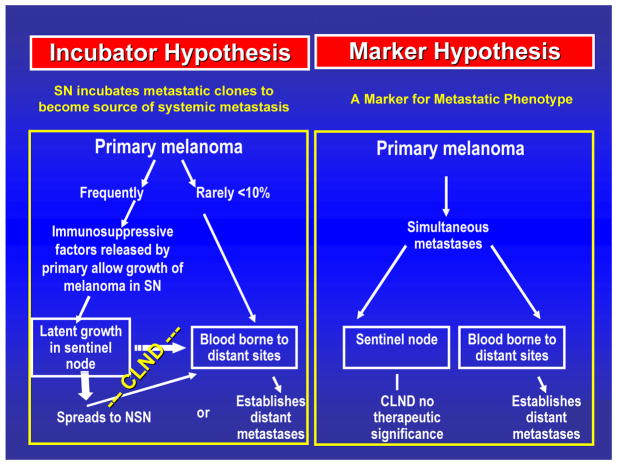
Regional complete lymphadenectomy (CLND) evolved from the observation that most primary cutaneous melanomas spread initially through the intradermal lymphatics to the regional nodes (FIGURE 1) and then move to distant sites.2 According to the incubator hypothesis, the primary melanoma sends immunosuppressive factors to the sentinel node2; these signals foster a nodal microenvironment that favors the growth of tumor cells, which subsequently spread to nonsentinel nodes and then to distant sites. This sequential model of melanoma metastasis has not been universally accepted, and indeed it may not apply in all cases; very thick (>4 mm) primary melanomas may be associated with concurrent nodal and hematogenous metastasis, in which case sentinel node biopsy (SNB) or CLND has no therapeutic relevance.
Fig. 1.
Incubator versus marker hypothesis. Reprinted with permission from Morton et al.2
Routine elective CLND, a practice based on the incubator hypothesis, reveals metastases in only 20% of nodal specimens.3 This means that 80% of patients who undergo nodal surgery will have no clinical benefit yet still be exposed to potential operative morbidity.4 On the other hand, “watch and wait” is not satisfactory because it requires the patient to cope with an uncertain prognosis. Even more important, by the time a nodal deposit becomes clinically evident, the risk of distant disease has substantially increased.
Multiple randomized trials have failed to show any difference in overall survival for patients undergoing wide excision plus elective CLND versus wide excision plus observation, although there may be some survival benefit in certain subgroups.5 However, the numbers in all these trials have been insufficient to detect statistically significant differences. We therefore approached the problem from a different angle. Instead of focusing on the 80% of patients whose nodes are tumor free, we asked how we could identify the 20% of patients who have occult nodal metastases and therefore might benefit from early (therapeutic) CLND. Our answer was intraoperative lymphatic mapping and sentinel node biopsy (SNB).
HISTORICAL DEVELOPMENT OF SNB
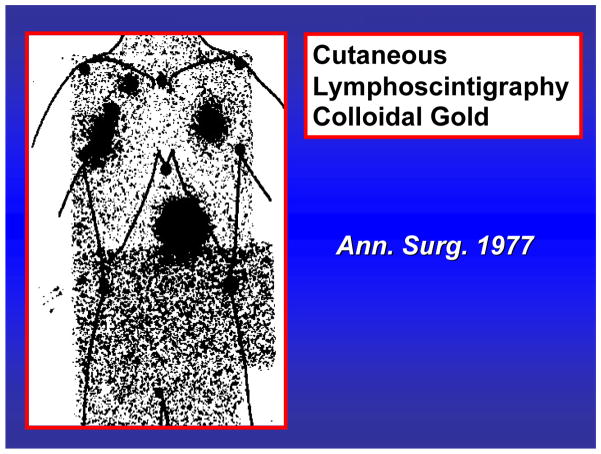
Twenty years ago, elective CLND was common and relatively straightforward when a primary melanoma was on an extremity. However, if the patient had a truncal melanoma, the site of nodal drainage was not clear. Our group therefore introduced cutaneous lymphoscintigraphy to identify the location of tumor-draining nodal basins (FIGURE 2).3, 6 As our technique was refined, we began to see that the drainage tended to localize in one or two nodes. In an effort to determine how we could identify those nodes, we performed mapping studies in cats.7 Results showed that each area of skin is connected to one or two lymph nodes in the regional draining basin.8 When a vital dye was injected at a skin site, it targeted a specific node in the regional drainage basin. This observation was the basis for our hypothesis that lymph draining a primary cutaneous site will target one or two sentinel nodes in the regional lymphatic basin. These sentinel nodes are on the direct drainage pathway from the cutaneous site and therefore the first nodes to receive tumor cells from a primary melanoma at that site. Thus the tumor status of the sentinel node will indicate the tumor status of the regional drainage basin. If the sentinel node is negative then all the nodes should be negative. If the sentinel node is positive then additional nodes might be positive.
Fig. 2.
Cutaneous lymphoscintigraphy performed with colloidal gold shows bilateral drainage from a truncal melanoma. This patient underwent elective bilateral axillary dissections; both nodal specimens were positive. Reprinted with permission from Holmes et al.3
Initially we thought of SNB as a diagnostic or staging procedure to determine who might benefit from CLND. Our proof-of-concept studies were based strictly on vital blue dye, which was an effective but time-consuming mapping agent because of the need to raise flaps and visually follow each lymphatic to the sentinel node. Our seminal study, which was published in 1992,8 reported successful identification of the sentinel node in 194 of 223 mapping procedures. As expected, 20% of the SNB specimens were positive for tumor, however CLND was performed in all cases to check the accuracy of intraoperative dye-directed mapping. Of 3079 total nonsentinel nodes, only 2 (0.06%) contained tumor. This was very convincing proof of the concept.
The next step was to standardize SNB. This was potentially problematic because SNB is a multidisciplinary procedure, a team effort that requires the skill and cooperation of the surgeon, the nuclear medicine physician and the pathologist. The surgeon must be willing to learn the technique, the nuclear medicine physician must be willing to spend time mapping the lymphatic drainage to the sentinel node, and the pathologist must be willing to examine several sections of each sentinel node by immunohistochemistry in order to detect micrometastases.9 At this point, we realized that we needed to validate SNB within the framework of a multicenter trial. We therefore successfully applied for NIH program project funding for the first Multicenter Selective Lymphadenectomy Trial.
FIRST MULTICENTER SELECTIVE LYMPHADENECTOMY TRIAL (MSLT-I)
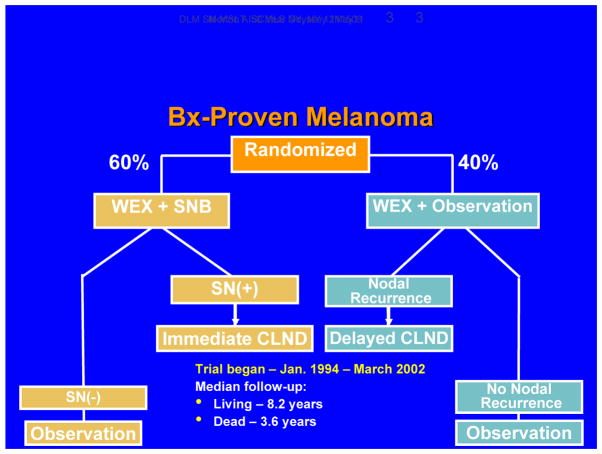
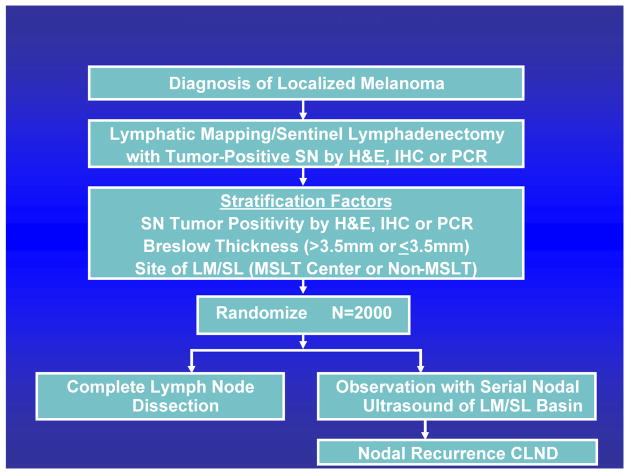
MSLT-I was designed to confirm the accuracy of nodal staging based on SNB and determine whether immediate CLND in patients with tumor-positive sentinel nodes can improve outcome (FIGURE 3). Patients were randomized in a 60:40 ratio to two treatment arms: 1) wide excision plus SNB, with immediate CLND if the SNB specimen was positive; or 2) wide excision plus observation, with delayed CLND for clinical evidence of nodal recurrence during follow-up.
Figure 3.
Design of MSLT-I.
Candidates for MSLT-I had primary melanomas with any Breslow thickness and Clark’s level IV or V. Patients were stratified by the site (extremity, head/neck, trunk) and thickness (1.0–1.19 mm or Clark level ≥ IV, 1.2–1.79 mm, 1.8–3.5 mm, or > 3.5 mm) of the primary melanoma. Our primary cohort comprised the subgroup of patients with intermediate-thickness lesions, i.e., 1.2–3.5 mm, because our large melanoma database showed that these patients would be most likely to have nodal but not distant spread. Thinner lesions were expected to have a low incidence of metastasis, whereas thicker lesions could already have spread to distant sites, so that their removal would confer no benefit.
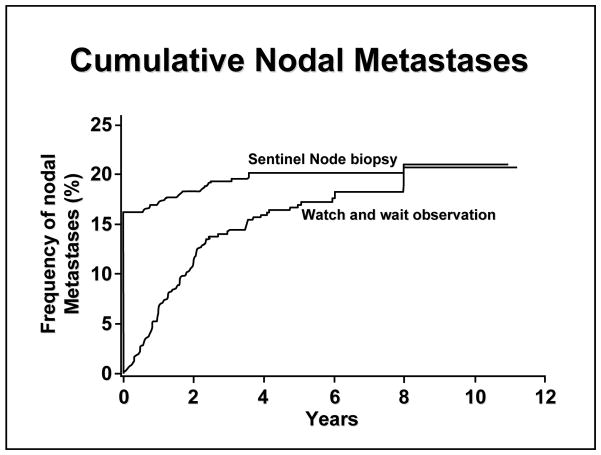
MSLT-I opened in January 1994 and completed accrual of 2001 patients in March 2002, thanks to impressive rates of enrollment at sites in Europe (N=630) and particularly Australia (N=1033). Among patients randomized to SNB, 16% had a tumor-positive SNB specimen. Over the next ten years, an additional 4% of SNB patients developed nodal recurrence. Among patients randomized to observation, the cumulative incidence of nodal recurrence reached 16% by 5 years and 20% by 10 years,10 suggesting that nodal metastases will eventually (albeit slowly) progress to a palpable size if not removed. It is worth noting that we can detect a metastasis as small as 0.05 mm in a sentinel node, whereas clinical detection by palpation usually cannot identify nodal metastases less than 15 to 20 mm in diameter. As shown in FIGURE 4, among patients with intermediate-thickness melanomas, the incidence of nodal metastasis was 19.8% in the SNB group versus 20.5% in the observation group; in the latter group, the median interval before development of palpable disease was 19 months. For lesions thicker than 3.5 mm, the incidence of nodal recurrence increased to 42% in the observation group, with a median interval of 9.6 months before recurrence.
Figure 4.
Cumulative incidence of nodal metastases by treatment arm of MSLT-I. Reprinted with permission from Morton et al.10 Copyright 2007 Massachusetts Medical Society. All rights reserved.
Simply comparing the incidence of nodal recurrence after SNB versus nodal observation does not reflect the biology of melanoma. We need to know whether occult nodal disease can spread to other nodes in the same basin. In other words, does “watch and wait” increase the number of tumor-positive nodes in patients who develop clinical evidence of nodal recurrence during observation? MSLT-I data indicate that the answer is yes: in patients with intermediate-thickness melanomas, the mean total number of positive nodes (sentinel plus nonsentinel) was 1.4 ± 0.9 in the SNB group versus 3.2 ± 3.9 in the observation group (P=0.0001). For patients with thicker lesions, the number of positive nodes was 2.0 ± 1.4 in the SNB group versus 3.3 ± 3.5 in the observation group, not statistically significant. The implication is that if microscopic nodal metastases are left alone, they will in time become clinically apparent macroscopic metastases and spread to involve more nodes in the regional lymphatic drainage basin.10 Moreover, as reported by Balch et al,11, 12 patients with multiple tumor-positive nodes have a worse prognosis than patients with only a single positive node.
Is sentinel node status a prognostic factor?
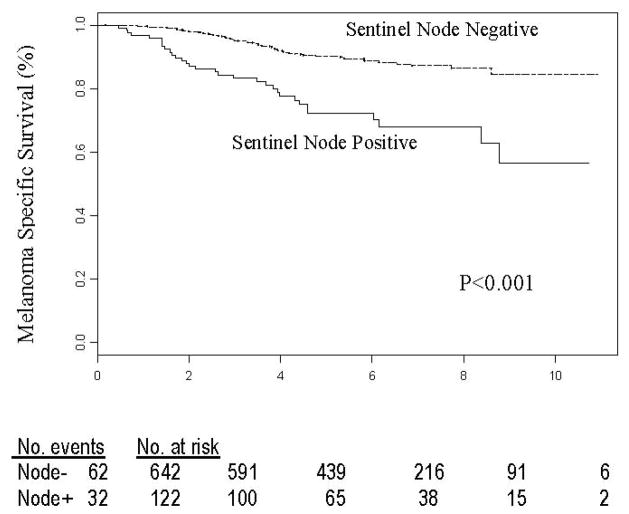
Yes, regardless of the thickness of the primary melanoma. By multivariate analysis the hazard ratio was 3.19 for patients with 1.2–3.5 mm primaries (FIGURE 5), 1.82 for patients with primaries >3.5 mm, and 4.06 for patients with primaries <1.2 mm.
Figure 5.
Melanoma-related survival of 764 patients with tumor-positive versus tumor-negative sentinel node metastases from intermediate-thickness melanomas. Reprinted with permission from Morton et al.13 Copyright 2006 Massachusetts Medical Society. All rights reserved.
Is SNB correlated with disease-free survival?
Yes, in patients with intermediate-thickness (P=0.0074) or thick (P=0.0358) lesions; there were too few events for analysis in patients with thin lesions. In the intermediate-thickness group, rate of 5-year disease-free survival was 78.0 ± 1.6% in the SNB group, as compared with 72.7 ± 2.0% in the observation group. This difference was mostly due to differences in regional nodal metastasis but also reflected differences in distant metastasis. In patients with thick melanomas, rate of 5-year disease-free survival was 56.0 ± 3.0% in the SNB group, as compared with 43.5 ± 4.7% in the observation group.
Within the subgroup of patients with nodal metastases identified at the time of SNB or during nodal observation, the differences were even more marked when the primary melanoma was intermediate in thickness. However, there was no difference in distant disease-free survival among node-positive patients with thicker lesions; this is not surprising because thicker lesions might already have spread beyond the lymph nodes to distant sites at the time of initial therapy.
Is SNB correlated with melanoma-specific survival?
Yes, but only among the subgroup of patients with tumor-positive nodes from intermediate-thickness primaries. These patients had a 5-year melanoma-specific survival of 69.6 ± 4.4% with SNB versus 56.5 ± 5.5% with observation. Among patients with thicker melanomas, 5-year melanoma-specific survival for those with nodal involvement was 60.6 ± 6.6% with SNB, as compared with 53.8 ± 7.6% with observation.
Survival and the dilution effect
The third interim analysis of MSLT-I13 found that although there was a significant treatment-related difference among subgroups of patients with nodal involvement, as described above, there was no significant difference in overall survival for the entire population. This can be explained by the dilution effect,14 the same problem that plagued the early trials of elective versus therapeutic CLND. Remember that only a minority of patients with intermediate-thickness melanomas will have regional node metastasis. Because the rate of sentinel node metastasis was 16% in MSLT-I, the number of patients who could derive benefit from early CLND was diluted by the far larger number of patients whose nodes were tumor-free. A 20% effect on 16% of the population corresponds to an overall 3.2% difference for patients in the trial.
Complications
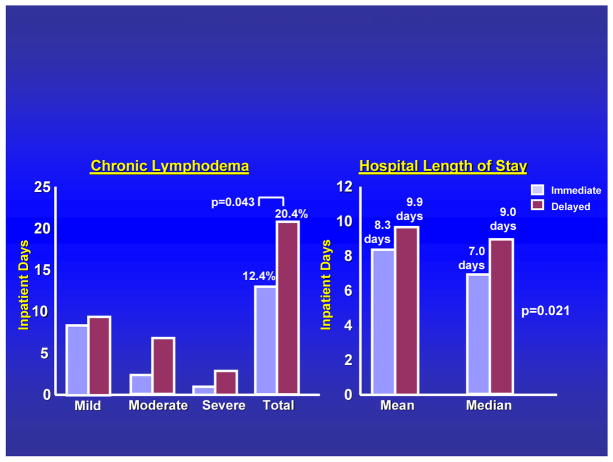
We recently compared the incidence of complications between CLND undertaken immediately after identification of a positive sentinel node versus CLND delayed until clinical appearance of regional node involvement.15 MSLT-I data showed a significant treatment-related difference in the incidence of lymphedema (FIGURE 6). Interestingly, the frequency and severity of lymphedema were greater when CLND was delayed. The reason is unclear but might reflect blockage of lymphatics, or possibly a more extensive resection required for palpable nodal recurrence --- although there was no difference in the number of nodes removed. In addition, the length of hospital stay was shorter with immediate versus delayed CLND.
Figure 6.
Incidence of lymphedema and hospital length of stay among patients undergoing immediate lymphadenectomy for a positive sentinel node versus delayed lymphadenectomy for nodal recurrence during observation. Adapted with permission from Faries et al.15
Quality of Life
SNB followed by immediate CLND for sentinel node metastasis improves the patient’s quality of life by eliminating the anxiety of watching and waiting for nodal recurrence. Moreover, our psychometric assessments on MSLT-I patients have shown that there is a definite decrease in quality of life with recurrence as compared to removing the tumor immediately after a positive SNB.16
Summary
Results of MSLT-I have validated SNB as a minimally invasive diagnostic and staging procedure. When performed by an experienced team, SNB can assess the regional nodes in primary melanoma with 95% to 98% accuracy. At most centers, the accuracy of SNB is about 97% with a dual-tracer technique based on a combination of blue dye and radiotracer. SNB identifies the 15% to 20% of patients with nodal metastasis that are candidates for immediate lymphadenectomy, and it identifies the 80% to 85% who do not have metastasis and do not need complete node dissection. Although SNB does not affect the outcome of patients who do not have tumor-involved regional nodes, its low rate of complications and high degree of accuracy mandate its use in all patients with intermediate-thickness melanoma and in selected patients with thinner or thicker lesions. Failure to assess the regional nodes and promptly remove occult nodal metastases can increase the size and number of metastases in patients who develop palpable nodal recurrence, thereby increasing their risk of distant disease and decreasing the likelihood of long-term survival.
SECOND MULTICENTER SELECTIVE LYMPHADENECTOMY TRIAL (MSLT-II)
Although CLND is standard for a patient with a positive SNB specimen, MSLT-I data show that 88% of patients who have a single tumor-containing sentinel node will have no additional nodal metastases when the CLND specimen is examined by hematoxylin-eosin staining (H–E).10, 14 Even when nonsentinel nodes are subjected to the same intense scrutiny used for assessment of sentinel nodes, the rate of additional positive nodes is only 8% to 12%.9, 17
The fact that most sentinel-node positive patients have no nonsentinel node involvement is in accord with the incubator theory and the concept of sequential metastasis. It also suggests that if nodal metastases are limited to one or two sentinel nodes, then SNB might be therapeutic as well as diagnostic. MSLT-II was designed to examine this possibility. Its underlying hypothesis is that CLND can be avoided in most patients with sentinel node metastases.
In brief, MSLT-II is a randomized phase III trial of SNB plus CLND versus SNB plus ultrasound observation of the lymph nodes in patients with sentinel node metastases detected by histopathologic (H–E, immunohistochemistry [IHC]) or molecular (reverse transcriptase-polymerase chain reaction assay [RT-PCR]) techniques (FIGURE 7). The use of ultrasound monitoring in MSLT-II is important because high-definition ultrasonography has the potential to detect metastases as small as 4 millimeters in the regional nodes. MSLT-II’s primary outcome is melanoma-specific survival. Secondary outcomes include overall survival and disease-free survival, prognostic accuracy of histopathology, molecular and immunologic markers, and quality of life.
Figure 7.
Design of MSLT-II.
MSLT-II opened in 2005. It will enroll 1925 subjects with sentinel node metastases; 1960 additional subjects with no evidence of sentinel node metastases by both H&E/IHC and RT-PCR will be followed up for recurrence and survival in an observational study to determine the natural history of melanoma in patients whose SNs are negative by molecular staging. Thus far, enrollment has been excellent. The Data Safety Monitoring Board completed the first interim analysis in January 2011. By April 2011, there were 57 active sites. Of 3992 patients who had been screened, 1219 had been randomized: 993 based on pathology and 226 based on RT-PCR positivity.
OVERVIEW
Although MSLT-I and MSLT-II address separate questions concerning regional metastasis, their careful design and global scope (FIGURE 8) are generating findings that will help determine the clinical significance of sentinel node micrometastases and whether careful nodal observation enhanced by sensitive ultrasound monitoring might be an attractive alternative to complete lymphadenectomy in some patients with sentinel node metastasis. We still do not know exactly when a nodal focus of tumor cells reaches a size that has clinical relevance in terms of metastatic potential. Our ongoing correlative immunologic and molecular studies using blood and tissue specimens from the MSLT cohorts will help us answer this question and further refine the management paradigm for malignant melanoma.
Figure 8.
International sites participating in MSLT-I and MSLT-II.
Acknowledgments
Supported by a grant (CA 29605) from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Cancer Institute or the National Institutes of Health.
Thanks to all members of the MSLT Study Group and to all patients in the United States, Europe and Australia whose participation in these important trials has ensured their timely completion and successful outcome.
Footnotes
Presented at the Fourth International Symposium on Cancer Metastasis and the Lymphovascular System, May 12, 2011, New York, NY.
References
- 1.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–34. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 2.Morton DL, Hoon DS, Cochran AJ, et al. Lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: therapeutic utility and implications of nodal microanatomy and molecular staging for improving the accuracy of detection of nodal micrometastases. Ann Surg. 2003;238:538–49. doi: 10.1097/01.sla.0000086543.45557.cb. discussion 49–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmes EC, Moseley HS, Morton DL, Clark W, Robinson D, Urist MM. A rational approach to the surgical management of melanoma. Ann Surg. 1977;186:481–90. doi: 10.1097/00000658-197710000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morton DL, Cochran AJ, Thompson JF, et al. Sentinel node biopsy for early-stage melanoma: accuracy and morbidity in MSLT-I, an international multicenter trial. Ann Surg. 2005;242:302–11. doi: 10.1097/01.sla.0000181092.50141.fa. discussion 11–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balch CM, Soong S, Ross MI, et al. Long-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0 to 4.0 mm). Intergroup Melanoma Surgical Trial. Ann Surg Oncol. 2000;7:87–97. doi: 10.1007/s10434-000-0087-9. [DOI] [PubMed] [Google Scholar]
- 6.Robinson DS, Sample WF, Fee HJ, Holmes C, Morton DL. Regional lymphatic drainage in primary malignant melanoma of the trunk determined by colloidal gold scanning. Surg Forum. 1977;28:147–8. [PubMed] [Google Scholar]
- 7.Wong JH, Cagle LA, Morton DL. Lymphatic drainage of skin to a sentinel lymph node in a feline model. Ann Surg. 1991;214:637–41. doi: 10.1097/00000658-199111000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–9. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 9.Morton DL, Thompson JF, Essner R, et al. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: a multicenter trial. Multicenter Selective Lymphadenectomy Trial Group. Ann Surg. 1999;230:453–63. doi: 10.1097/00000658-199910000-00001. discussion 63–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morton DL, Cochran AJ, Thompson JF. Sentinel node biopsy in melanoma (letter) N Engl J Med. 2007;356:419–20. [Google Scholar]
- 11.Balch CM, Soong SJ, Murad TM, Ingalls AL, Maddox WA. A multifactorial analysis of melanoma: III. Prognostic factors in melanoma patients with lymph node metastases (stage II) Ann Surg. 1981;193:377–88. doi: 10.1097/00000658-198103000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–48. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 13.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–17. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 14.Morton DL, Cochran AJ, Thompson JF. Authors’ response to a letter to the editor re: Sentinel node biopsy for early-stage melanoma. Ann Surg. 2007;245:828–9. doi: 10.1097/01.sla.0000261157.79250.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faries MB, Thompson JF, Cochran A, et al. The impact on morbidity and length of stay of early versus delayed complete lymphadenectomy in melanoma: results of the Multicenter Selective Lymphadenectomy Trial (I) Ann Surg Oncol. 2010;17:3324–9. doi: 10.1245/s10434-010-1203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garreau JR, Faries M, Ye X. Mood state and melanoma outcome in the Multicenter Selective Lymphadenectomy Trial (Abstract 9603) J Clin Oncol. 2009:27. [Google Scholar]
- 17.Cochran AJ, Wen DR, Huang RR, Wang HJ, Elashoff R, Morton DL. Prediction of metastatic melanoma in nonsentinel nodes and clinical outcome based on the primary melanoma and the sentinel node. Mod Pathol. 2004;17:747–55. doi: 10.1038/modpathol.3800117. [DOI] [PubMed] [Google Scholar]