Abstract
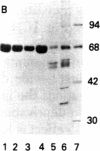
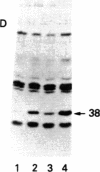
Inhibition of translation in hemin-containing reticulocyte lysates by catalytic subunit (cS) preparations of cAMP-dependent protein kinase from bovine heart, reported earlier by our group, is due to a highly active heat-stable protein contaminant (HS). The specific activity for translational inhibition goes up by a factor of 10 when cS is heated for 10 min at 80 degrees C, which completely destroys histone phosphorylation activity. HS has been purified to homogeneity from bovine heart. It consists of a single polypeptide chain (Mr approximately 68,000). HS inhibits translation with biphasic kinetics similar to those of hemin deficiency and induces pronounced phosphorylation of the alpha subunit of the eukaryotic initiation factor eIF-2. The inhibition is relieved by eIF-2 or GTP but not by high concentrations of double-stranded RNA, thus ruling out involvement of the double-stranded RNA-activated inhibitor. Judged by poly(U) translation, HS has no effect on chain elongation. When added to crude preparations of the proinhibitor form (proHCI) of the heme-controlled translational inhibitor (HCI), HS appears to produce an increase of the HCI-to proHCI ratio. The mode of action of HS is as yet unknown.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benne R., Wong C., Luedi M., Hershey J. W. Purification and characterization of initiation factor IF-E2 from rabbit reticulocytes. J Biol Chem. 1976 Dec 10;251(23):7675–7681. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cohen P. The subunit structure of rabbit-skeletal-muscle phosphorylase kinase, and the molecular basis of its activation reactions. Eur J Biochem. 1973 Apr 2;34(1):1–14. doi: 10.1111/j.1432-1033.1973.tb02721.x. [DOI] [PubMed] [Google Scholar]
- Datta A., de Haro C., Sierra J. M., Ochoa S. Role of 3':5'-cyclic-AMP-dependent protein kinase in regulation of protein synthesis in reticulocyte lysates. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1463–1467. doi: 10.1073/pnas.74.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes J. G., Cohen P. The regulation of glycogen metabolism. Purification and properties of protein phosphatase inhibitor-2 from rabbit skeletal muscle. Eur J Biochem. 1980 Mar;105(1):195–203. doi: 10.1111/j.1432-1033.1980.tb04489.x. [DOI] [PubMed] [Google Scholar]
- Goldstein J., Safer B. Use of heparin-Sepharose for the rapid isolation of initiation and elongation factors. Methods Enzymol. 1979;60:165–181. doi: 10.1016/s0076-6879(79)60014-9. [DOI] [PubMed] [Google Scholar]
- Grankowski N., Kramer G., Hardesty B. No effect of cAMP on protein synthesis in reticulocyte lysates. J Biol Chem. 1979 May 10;254(9):3145–3147. [PubMed] [Google Scholar]
- Gross M., Rabinovitz M. Control of globin synthesis by hemin: factors influencing formation of an inhibitor of globin chain initiation in reticulocyte lysates. Biochim Biophys Acta. 1972 Dec 6;287(2):340–352. doi: 10.1016/0005-2787(72)90383-8. [DOI] [PubMed] [Google Scholar]
- Haas A. L., Rose I. A. Hemin inhibits ATP-dependent ubiquitin-dependent proteolysis: role of hemin in regulating ubiquitin conjugate degradation. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6845–6848. doi: 10.1073/pnas.78.11.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson A. B., Miller A. H., Hardesty B. Multistep regulatory system for activation of a cyclic AMP-independent eukaryotic initiation factor 2 kinase. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2605–2609. doi: 10.1073/pnas.76.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T., Vanderhoff G., London I. M. Control of globin synthesis: the role of heme. J Mol Biol. 1972 May 28;66(3):471–481. doi: 10.1016/0022-2836(72)90427-5. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kishimoto A., Takai Y., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977 Nov 10;252(21):7610–7616. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin D., Ernst V., London I. M. Effects of the catalytic subunit of cAMP-dependent protein kinase (type II) from reticulocytes and bovine heart muscle on protein phosphorylation and protein synthesis in reticulocyte lysates. J Biol Chem. 1979 Aug 25;254(16):7935–7941. [PubMed] [Google Scholar]
- Nimmo G. A., Cohen P. The regulation of glycogen metabolism. Purification and characterisation of protein phosphatase inhibitor-1 from rabbit skeletal muscle. Eur J Biochem. 1978 Jun 15;87(2):341–351. doi: 10.1111/j.1432-1033.1978.tb12383.x. [DOI] [PubMed] [Google Scholar]
- Ochoa S., de Haro C. Regulation of protein synthesis in eukaryotes. Annu Rev Biochem. 1979;48:549–580. doi: 10.1146/annurev.bi.48.070179.003001. [DOI] [PubMed] [Google Scholar]
- Peters K. A., Demaille J. G., Fischer E. H. Adenosine 3':5'-monophosphate dependent protein kinase from bovine heart. Characterization of the catalytic subunit. Biochemistry. 1977 Dec 27;16(26):5691–5697. doi: 10.1021/bi00645a007. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Erlichman J., Rosen O. M. Molecular forms and subunit composition of a cyclic adenosine 3',5'-monophosphate-dependent protein kinase purified from bovine heart muscle. J Biol Chem. 1972 Jan 10;247(1):36–44. [PubMed] [Google Scholar]
- Tahara S. M., Traugh J. A. Cyclic Nucleotide-independent protein kinases from rabbit reticulocytes. Identification and characterization of a protein kinase activated by proteolysis. J Biol Chem. 1981 Nov 25;256(22):11558–11564. [PubMed] [Google Scholar]
- Wilkinson K. D., Urban M. K., Haas A. L. Ubiquitin is the ATP-dependent proteolysis factor I of rabbit reticulocytes. J Biol Chem. 1980 Aug 25;255(16):7529–7532. [PubMed] [Google Scholar]
- de Haro C., Ochoa S. Further studies on the mode of action of the heme-controlled translational inhibitor. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1741–1745. doi: 10.1073/pnas.76.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haro C., Ochoa S. Mode of action of the hemin-controlled inhibitor of protein synthesis: studies with factors from rabbit reticulocytes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2713–2716. doi: 10.1073/pnas.75.6.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]