Abstract
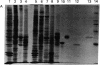
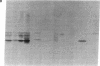
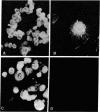
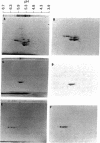
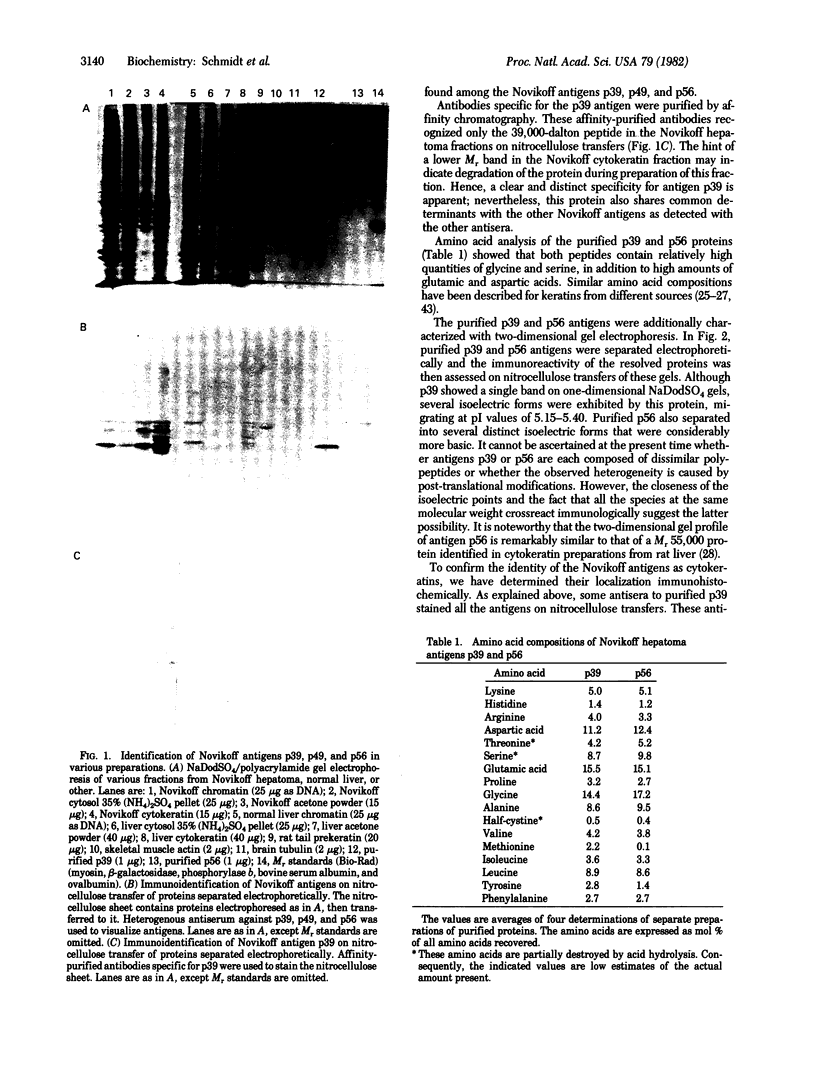
Further characterization of three polypeptide antigens present in Novikoff ascites hepatoma (molecular weights 39,000, 49,000, and 56,000--p39, p49, and p56, respectively), has established the identity of these proteins as cytokeratins. Two-dimensional gel electrophoresis revealed that each of the three proteins exists in several isoelectric forms, all characteristic of the keratins. The antigens p39, and p56 had amino acid compositions compatible with this family of proteins, and immunofluorescence microscopy localized the antigens on elaborate filament arrays indicative of the cytokeratins. Antibodies monospecific for the p39 antigen, purified by using affinity chromatography, were used to demonstrate that protein p39 was specific for Novikoff hepatoma cells. These results suggest that the cytokeratins contain cell-specific that can be useful as markers for differentiation and neoplasia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baden H. P., Bonar L. The alpha-fibrous proteins of epidermis. J Invest Dermatol. 1968 Dec;51(6):478–483. [PubMed] [Google Scholar]
- Baden H. P., Goldsmith L. A., Fleming B. A comparative study of the physicochemical properties of human keratinized tissues. Biochim Biophys Acta. 1973 Oct 18;322(2):269–278. doi: 10.1016/0005-2795(73)90303-6. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Blose S. H., Shelanski M. L., Chacko S. Localization of bovine brain filament antibody on intermediate (100 A) filaments in guinea pig vascular endothelial cells and chick cardiac muscle cells. Proc Natl Acad Sci U S A. 1977 Feb;74(2):662–665. doi: 10.1073/pnas.74.2.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley B. R., Fistel S. H., Marcum J. M., Pardue R. L. Microtubules in cultured cells; indirect immunofluorescent staining with tubulin antibody. Int Rev Cytol. 1980;63:59–95. doi: 10.1016/s0074-7696(08)61757-x. [DOI] [PubMed] [Google Scholar]
- Chytil F., Spelsberg T. C. Tissue differences in antigenic properties of non-histone protein-DNA complexes. Nature. 1971 Oct 13;233(5320):215–218. [PubMed] [Google Scholar]
- Cooke P. A filamentous cytoskeleton in vertebrate smooth muscle fibers. J Cell Biol. 1976 Mar;68(3):539–556. doi: 10.1083/jcb.68.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Appelhans B., Schmid E., Freudenstein C., Osborn M., Weber K. Identification and characterization of epithelial cells in mammalian tissues by immunofluorescence microscopy using antibodies to prekeratin. Differentiation. 1979;15(1):7–25. doi: 10.1111/j.1432-0436.1979.tb01030.x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Denk H., Kalt R., Schmid E. Biochemical and immunological identification of cytokeratin proteins present in hepatocytes of mammalian liver tissue. Exp Cell Res. 1981 Feb;131(2):299–318. doi: 10.1016/0014-4827(81)90234-2. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Grund C., Osborn M., Weber K. The intermediate-sized filaments in rat kangaroo PtK2 cells. I. Morphology in situ. Cytobiologie. 1978 Aug;17(2):365–391. [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Weber K., Osborn M. HeLa cells contain intermediate-sized filaments of the prekeratin type. Exp Cell Res. 1979 Jan;118(1):95–109. doi: 10.1016/0014-4827(79)90587-1. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Weber K., Osborn M., Schmid E., Freudenstein C. Antibody to prekeratin. Decoration of tonofilament like arrays in various cells of epithelial character. Exp Cell Res. 1978 Oct 15;116(2):429–445. doi: 10.1016/0014-4827(78)90466-4. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Multiple keratins of cultured human epidermal cells are translated from different mRNA molecules. Cell. 1979 Jul;17(3):573–582. doi: 10.1016/0092-8674(79)90265-4. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. The expression of keratin genes in epidermis and cultured epidermal cells. Cell. 1978 Nov;15(3):887–897. doi: 10.1016/0092-8674(78)90273-8. [DOI] [PubMed] [Google Scholar]
- Fujitani H., Chiu J. F., Hnilica L. S. Purification of nuclear antigens in Novikoff hepatoma. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1943–1946. doi: 10.1073/pnas.75.4.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson I. K., Anderson R. A. Comparison of 10 nm filaments from three bovine tissues. Exp Cell Res. 1980 Aug;128(2):395–406. doi: 10.1016/0014-4827(80)90075-0. [DOI] [PubMed] [Google Scholar]
- Glass W. F., 2nd, Briggs R. C., Hnilica L. S. Identification of tissue-specific nuclear antigens transferred to nitrocellulose from polyacrylamide gels. Science. 1981 Jan 2;211(4477):70–72. doi: 10.1126/science.7003713. [DOI] [PubMed] [Google Scholar]
- Goldman R. D. The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J Cell Biol. 1971 Dec;51(3):752–762. doi: 10.1083/jcb.51.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Huneeus F. C., Davison P. F. Fibrillar proteins from squid axons. I. Neurofilament protein. J Mol Biol. 1970 Sep 28;52(3):415–428. doi: 10.1016/0022-2836(70)90410-9. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Mitosis and intermediate-sized filaments in developing skeletal muscle. J Cell Biol. 1968 Sep;38(3):538–555. doi: 10.1083/jcb.38.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Hubbard B. D. Immunological characterization of the subunit of the 100 A filaments from muscle cells. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4344–4348. doi: 10.1073/pnas.73.12.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lee L. D., Kubilus J., Baden H. P. Intraspecies heterogeneity of epidermal keratins isolated from bovine hoof and snout. Biochem J. 1979 Jan 1;177(1):187–196. doi: 10.1042/bj1770187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto V. P., Virtanen I., Kurki P. Intermediate filaments anchor the nuclei in nuclear monolayers of cultured human fibroblasts. Nature. 1978 Mar 9;272(5649):175–177. doi: 10.1038/272175a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Franke W. W., Weber K. Visualization of a system of filaments 7-10 nm thick in cultured cells of an epithelioid line (Pt K2) by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2490–2494. doi: 10.1073/pnas.74.6.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid E., Tapscott S., Bennett G. S., Croop J., Fellini S. A., Holtzer H., Franke W. W. Differential location of different types of intermediate-sized filaments in various tissues of the chicken embryo. Differentiation. 1979;15(1):27–40. doi: 10.1111/j.1432-0436.1979.tb01031.x. [DOI] [PubMed] [Google Scholar]
- Schmidt W. N., Stryjecka-Zimmer M., Glass W. F., 2nd, Briggs R. C., Hnilica L. S. Tissue specificity and cellular distribution of Novikoff hepatoma antigenic proteins p39, p49, and p56. J Biol Chem. 1981 Aug 10;256(15):8117–8123. [PubMed] [Google Scholar]
- Spelsberg T. C., Hnilica L. S. Proteins of chromatin in template restriction. I. RNA synthesis in vitro. Biochim Biophys Acta. 1971 Jan 1;228(1):202–211. doi: 10.1016/0005-2787(71)90560-0. [DOI] [PubMed] [Google Scholar]
- Steinert P. M. The extraction and characterization of bovine epidermal alpha-keratin. Biochem J. 1975 Jul;149(1):39–48. doi: 10.1042/bj1490039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerhayes I. C., Cheng Y. S., Sun T. T., Chen L. B. Expression of keratin and vimentin intermediate filaments in rabbit bladder epithelial cells at different stages of benzo[a]pyrene-induced neoplastic progression. J Cell Biol. 1981 Jul;90(1):63–69. doi: 10.1083/jcb.90.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. T., Green H. Immunofluorescent staining of keratin fibers in cultured cells. Cell. 1978 Jul;14(3):469–476. doi: 10.1016/0092-8674(78)90233-7. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Green H. Keratin filaments of cultured human epidermal cells. Formation of intermolecular disulfide bonds during terminal differentiation. J Biol Chem. 1978 Mar 25;253(6):2053–2060. [PubMed] [Google Scholar]
- Sun T. T., Shih C., Green H. Keratin cytoskeletons in epithelial cells of internal organs. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2813–2817. doi: 10.1073/pnas.76.6.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. T., Shih C., Green H. Keratin cytoskeletons in epithelial cells of internal organs. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2813–2817. doi: 10.1073/pnas.76.6.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H., Schweizer J. Carcinoma-specific keratin polypeptide patterns in keratinizing epithelia of rodents : independence of species- and tissue-specific variations. Carcinogenesis. 1981;2(7):613–621. doi: 10.1093/carcin/2.7.613. [DOI] [PubMed] [Google Scholar]
- Wu Y. J., Rheinwald J. G. A new small (40 kd) keratin filament protein made by some cultured human squamous cell carcinomas. Cell. 1981 Sep;25(3):627–635. doi: 10.1016/0092-8674(81)90170-7. [DOI] [PubMed] [Google Scholar]