Abstract
Introduction
Interleukin-6 (IL-6) is thought to play a crucial role in the radicular pain caused by lumbar spinal stenosis. However, efficacy of inhibition of IL-6 for sciatica in patients with lumbar spinal stenosis has not been clarified. The purpose of the current study was to examine the effect of the anti-IL-6 receptor monoclonal antibody, tocilizumab, on radicular pain by its epidural administration onto spinal nerves in patients with lumbar spinal stenosis.
Methods
Sixty patients with low back and radicular leg pain caused by spinal stenosis were investigated. In 30 patients, we infiltrated 2.0 mL of lidocaine and 80 mg of tocilizumab onto the affected spinal nerve, and 2.0 mL of lidocaine and 3.3 mg of dexamethasone were used in 30 patients. Low back pain, leg pain, and leg numbness were evaluated during 1 month after spinal nerve infiltration.
Results
Infiltration of tocilizumab was more effective than dexamethasone for leg pain (3 days, 1, 2, and 4 weeks), low back pain (3 days, 1, 2 and 4 weeks), and leg numbness (3 days, 1 and 2 weeks). No adverse event was observed in either group.
Conclusion
Our results indicate that the epidural administration of an anti-IL-6 receptor monoclonal antibody, tocilizumab, onto the spinal nerve produced reduction of radicular leg pain, numbness, and low back pain without adverse event. IL-6 may be one of the inducers of pain caused by spinal stenosis in humans.
Keywords: Anti-interleukin-6 receptor monoclonal antibody, Sciatica, Lumbar spinal stenosis, Pain, Tocilizumab
Introduction
Radicular pain is a common symptom of lumbar disc herniation and spinal stenosis induced by mechanical compression and inflammation [17, 18]. Cytokines generated at the inflammatory site produce associated pain [12, 19, 20]. Compression of the spinal nerve roots by lumbar spinal stenosis (LSS) is a major clinical problem associated with intermittent claudication, pain, numbness, and lack of normal sensitivity [7, 24]. It has been shown that compression of the spinal nerve roots may induce neurophysiologic dysfunction, degeneration, and reduced blood flow in nerve roots in both animal models and humans [7, 24].
Recently, cytokines such as interleukins 1, 6 (IL-1, IL-6), and tumor necrosis factor-alpha (TNF-α) have been strongly linked to radicular pain [5, 18, 24]. It has also been reported that IL-1beta, IL-6, and TNF-α had neurotoxic to dorsal root axons [21]. It has been reported that IL-6 levels in the cerebrospinal fluid of patients with radicular pain caused by lumbar spinal stenosis was significant correlated with severity of stenosis [15]. On the other hand, concentration of IL-6 was high in joint cartilage and synovium in facet joint in lumbar spinal stenosis patients compared with lumbar disc herniation patients, thus this finding suggested that inflammatory cytokines in degenerated facet joints may have some relation to the cause of pain in degenerative lumbar disorders [4].
Tocilizumab is a humanized anti-IL-6 receptor monoclonal antibody that blocks IL-6 from binding to its receptor [11]. Several clinical trials of tocilizumab in patients with rheumatoid arthritis, both in western countries and in Japan, have shown it to have excellent therapeutic efficacy, as evaluated by the American College of Rheumatology responder rate or the disease activity score [13, 25]. However, the efficacy of inhibition of IL-6 for sciatica in patients with lumbar spinal stenosis has not been clarified.
The purpose of the current study was to examine the effect on radicular pain of epidural administration of the anti-IL-6 receptor monoclonal antibody, tocilizumab, onto spinal nerves in patients with lumbar spinal stenosis. We compared application of the tocilizumab with application of dexamethasone for the treatment of pain.
Methods
The ethics committee of our institution approved the protocol for the human procedures used in this study and informed written consent was obtained from each subject.
Patients
Patients had low back and leg pain, continuing for at least 1 month. Patients who had previously undergone spinal surgery were excluded from the study. We also excluded patients with spinal tumor, infection, or trauma. Patients were diagnosed with lumbar spinal stenosis on X-ray and magnetic resonance imaging (MRI) and by physical examination. Diagnosis by X-ray was spondylosis and spondylolisthesis (more than 3 mm of anterior slip in a normal position). The degree of spinal stenosis varied from slight to severe. MRI showed central stenosis, stenosis of the lateral recess, and foraminal stenosis. Details are shown in Tables 1 and 2. Patients who showed monoradiculopathy were evaluated. Patients who showed cauda equine syndrome, or polyradiculopathies were excluded from the current study. If a single affected spinal nerve was found using imaging and physical examination, spinal infiltration was performed to confirm the finding. Patients were allowed nonsteroidal anti-inflammatory drugs (NSAIDs) to control low back pain and leg pain. All patients used meloxicam (10 mg meloxicam, 30 min after breakfast) before and for at least 4 weeks after epidural administration.
Table 1.
Demographic characteristics
| Tocilizumab | Dexamethasone | Statistical analysis | |
|---|---|---|---|
| Number of patients | 30 | 30 | NS |
| Sex | Male: 18 | Male: 16 | NS |
| Female: 12 | Female: 14 | ||
| Age mean range (range), years | 67 ± 6.5 (52–80) | 68 ± 5.5 (51–82) | NS |
| Symptom duration, mean (range), months | 3.0 (1–24) | 2.7 (1–24) | NS |
| Use of NSAIDs | 30 | 30 | NS |
| Pain score before infiltration | |||
| Leg pain | |||
| Visual analog scale (VAS) | 6.9 ± 2.1 | 6.5 ± 2.2 | NS |
| Leg numbness | |||
| Visual analog scale (VAS) | 5.9 ± 1.4 | 6.0 ± 1.7 | NS |
| Low back pain | |||
| Visual analog scale (VAS) | 4.0 ± 0.7 | 3.7 ± 0.9 | NS |
| Oswestry Disability Index (ODI) | 34 ± 7.2 | 41 ± 7.8 | NS |
Table 2.
X-ray and MR-imaging evaluation, and affected spinal nerve
| Etanercept | Dexamethasone | Statistical analysis | |
|---|---|---|---|
| Number of patients | 30 | 30 | NS |
| X-ray evaluation | NS | ||
| Spondylosis | 18 | 17 | NS |
| Spondylolisthesis | 12 | 13 | NS |
| MRI evaluation | |||
| Central stenosis | |||
| Stenosis of the lateral recess | 17 | 17 | NS |
| Central stenosis | 11 | 9 | NS |
| Foraminal stenosis | 2 | 4 | NS |
| Affected spinal nerve | L4: 3 | L4: 4 | NS |
| L5: 20 | L5: 21 | ||
| S1: 7 | S1: 5 | ||
Epidural administration
The patients were divided randomly into two groups. The patients were randomized according to the minimization method for injection using etanercept or dexamethasone [22]. We employed sex and age as stratification factors.
Patients received a single spinal nerve block [2.0 mL of lidocaine and 80 mg of tocilizumab (Chugai-Pharm. Co., Japan), tocilizumab group; n = 30] or a spinal nerve block (2.0 mL of lidocaine and 3.3 mg of dexamethasone, dexamethasone group; n = 30). Both groups received 1.5 mL of 1% lidocaine solution into the skin to prevent pain at insertion of needle for block. Then, a 22-gauge spinal nerve-block needle was advanced obliquely to the corresponding spinal nerve under fluoroscopic control and 0.5 mL of the contrast medium Iotorolan (Schering AG, Berlin, Germany) was injected to confirm the position of the spinal nerve. Subsequently unilateral administration of lidocaine and the agent (2.0 mL of lidocaine and 80 mg of tocilizumab, or 2.0 mL of lidocaine and 3.3 mg of dexamethasone) was performed.
Pain scores
We evaluated the change in low back and leg pain before and after infiltration. To evaluate pain, the visual analog scale (VAS) score (0, no pain; 10, worst pain) was recorded before and 30 min, 3 days, 1, 2, and 4 weeks after infiltration. Oswestry Disability Index (ODI) scores were recorded before and 4 weeks after infiltration.
Subjective outcomes
At 4 weeks after injection, patients were asked to choose one of the following responses regarding their satisfaction with the treatment: (1) treatment met my expectations; (2) I did not improve as much as I had hoped, but I would undergo the same treatment for the same outcome; (3) treatment helped, but I would not undergo the same treatment for the same outcome; or (4) I am the same as or worse than I was before the treatment.
Patients underwent surgery within 6 months after epidural administration
We evaluated the number of patients who underwent surgery within 6 months of epidural administration. Patients were allowed NSAIDs (10 mg meloxicam, 30 min after breakfast) to control low back pain and leg pain after epidural administration.
Complications
Deep or superficial infection including respiratory infection in both groups was evaluated. Spinal nerve injury (motor palsy or sensory disturbance) or other complications in both groups were also evaluated.
Statistical analysis
Data were compared using a Kruskal–Wallis test to compare pain scales between the two groups, a one-way ANOVA with post hoc comparisons for age, symptom duration, and follow-up; and Fisher’s test was used for dichotomous/categorical variables. P < 0.05 was considered statistically significant.
Results
Demographic characteristics of patients in both the tocilizumab and dexamethasone groups are shown in Table 1. There was no significant difference in demographic characteristics of patients between the two groups (P > 0.05). There was no significant difference in scores of leg pain, low back pain, and leg numbness between the two groups (P > 0.05). Affected spinal nerves were mainly in L5 spinal nerves in both groups.
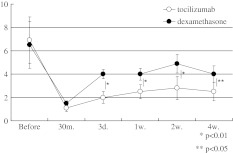
In both groups, treatment was significantly effective in attenuating leg pain, leg numbness, and low back pain 30 min after injection (P < 0.05) (Figs. 1, 2, 3). For leg pain in both groups, treatment was significantly effective in attenuating the pain during the 4 weeks of testing (P < 0.05) (Fig. 1). VAS scores of leg pain in the tocilizumab group were significantly lower than those in the dexamethasone group at 3 days (P < 0.01), 1 (P < 0.01), 2 (P < 0.01), and 4 weeks (P < 0.05) (Fig. 1).
Fig. 1.
Time course of leg pain (VAS). White circles indicate tocilizumab group and black circles indicate dexamethasone group
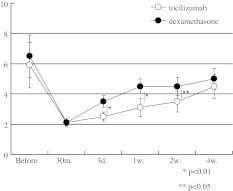
Fig. 2.
Time course of leg numbness (VAS). White circles indicate tocilizumab group and black circles indicate dexamethasone group
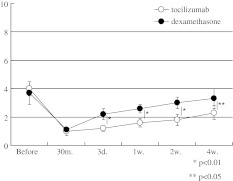
Fig. 3.
Time course of low back pain (VAS). White circles indicate tocilizumab group and black circles indicate dexamethasone group
For leg numbness, treatment was significantly effective in attenuating the numbness during the 4 weeks in both groups (P < 0.05) (Fig. 2). Leg numbness in the tocilizumab group were significantly lower than those in the dexamethasone group at 3 days (P < 0.01), 1 (P < 0.01), and 2 weeks (P < 0.05) (Fig. 2).
Both infiltrations were effective for VAS score of low back pain in both groups during the 4 weeks (P < 0.05) (Fig. 3). VAS scores of low back pain in the tocilizumab group were significantly lower than those in the dexamethasone group at 3 days (P < 0.01), 1 (P < 0.01), 2 (P < 0.01), and 4 weeks (P < 0.05) (Fig. 3).
There was no significant difference in ODI scores before infiltration between the groups (P > 0.05). The average ODI scores decreased at 4 weeks, and there was significant improvement in both groups compared with that before infiltration (P < 0.05) (Tables 1, 3). There was significant improvement in ODI scores in tocilizumab group compared with dexamethasone group at 4 weeks (P < 0.05) (Table 3).
Table 3.
Pain score 4 weeks after infiltration
| Tocilizumab | Dexamethasone | Statistical analysis | |
|---|---|---|---|
| Leg pain | |||
| Visual analog scale (VAS) | 2.5 ± 0.6 | 4.0 ± 0.9 | P = 0.02 |
| Leg numbness | |||
| Visual analog scale (VAS) | 4.5 ± 0.7 | 5.0 ± 0.9 | NS |
| Low back pain | |||
| Visual analog scale (VAS) | 2.3 ± 0.4 | 3.3 ± 1.0 | P < 0.05 |
| Oswestry Disability Index (ODI) | 20 ± 6.0 | 32 ± 7.0 | P = 0.045 |
Details of subjective outcomes 4 weeks after injection are presented in Table 4. There were more patients showing better outcome in tocilizumab group compared with dexamethasone group, and less patients showing worse outcome in tocilizumab group compared with dexamethasone group.
Table 4.
Subjective outcomes (number of patients)
| Number of patients | ||
|---|---|---|
| Tocilizumab | Dexamethasone | |
| 1. Treatment met my expectations | 20 | 13 |
| 2. I did not improve as much as I had hoped, but I would undergo the same treatment for the same outcome | 9 | 10 |
| 3. Treatment helped, but I would not undergo the same treatment for the same outcome | 1 | 4 |
| 4. I am the same as or worse than I was before the treatment | 0 | 3 |
We evaluated the number of patients who underwent surgery within 6 months of epidural administration. Six patients in the dexamethasone group and three patients in the tocilizumab group underwent surgery within 6 months of epidural administration. The proportion of patients who underwent surgery within 6 months of epidural administration was significantly higher in the dexamethasone group compared with the tocilizumab group (P < 0.05).
Complications
There was no deep or superficial infection in either group. There was no spinal nerve injury or other complications in either group.
Discussion
In the current study, results indicate that single direct application of the anti-IL-6 receptor monoclonal antibody to the spinal nerve produced significantly more pain relief than application of dexamethasone, and produced no adverse event. IL-6 may mediate the radicular pain caused by spinal stenosis in humans.
Of the proinflammatory cytokines, TNF-α has aroused most interest as a potential target for the treatment of sciatica in patients. It has been reported that a single intravenous infusion of infliximab was effective in treating sciatic pain caused by lumbar disc herniation [8]. On the other hand, intravenous infusion of infliximab was compared to a placebo by a Finnish group that conducted the first randomized controlled trial of this inhibitor. The results were disappointing [9, 10]. Cohen et al. [2] have reported a preclinical safety study of transforaminal epidural etanercept for the treatment of sciatica caused by disc herniation in 24 patients. They found effectiveness was dependent on the dose of etanercept (4 groups). In the clinical arm of the study, significant improvements in leg and back pain were collectively noted for the etanercept-treated patients 1 month after treatment, but not for patients in the saline-treated group [2].
IL-6 has also been detected in disc herniation tissue samples, in the cerebrospinal fluid of patients with radicular pain caused by lumbar spinal stenosis, and in joint cartilage and synovium in facet joint in lumbar spinal stenosis patients [3, 4, 15].
IL-6 is an interesting pleiotropic cytokine produced by T-cells, monocytes, macrophages, and synovial fibroblasts. It has several functions in chronic inflammation, acute phase responses, and growth stimulation [6]. It has been reported that IL-6 haplotypes were fivefold overrepresented among Finnish sciatica patients relative to controls (patients 8.4% and controls 1.7%), and the association of IL-6 haplotypes with sciatica was highly significant [14]. Thus, these findings indicated that IL-6 may be a potential target for the treatment of sciatica in patients.
In the current study, direct application of the anti-IL-6 receptor monoclonal antibody onto the spinal nerve produced significantly more pain relief than application of dexamethasone. IL-6 has become a common modality for treating rheumatoid disease. Generally speaking, tocilizumab is used by intravenous infusion, and the intravenous infusion of tocilizumab was effective for treating rheumatoid disease. It is not clear whether systemic or direct administration of the antibody is the most effective route for sciatica. Regarding to TNF-α inhibitor, intravenous injection of infliximab was ineffective in treating sciatic pain caused by lumbar disc herniation [9, 10]. However, it has been reported that an efficacy of direct application of TNF-α inhibitor for the treatment of sciatica caused by disc herniation [2]. Cytokines are mainly expressed around or in the disc tissue, where blood supply is generally considered to be insufficient. Therefore, we believe that the supply of cytokine inhibitors is insufficient in case of systemic administration. Furthermore, we selected direct administration without systemic administration, so the current study demonstrated the no adverse event and safety of direct application of the tocilizumab onto spinal nerves.
In the current study, we compared lidocaine + tocilizumab with lidocaine + dexamethasone. The efficacy of steroids for nerve root injection has been reported [1, 23]. Fifty-five patients who were deemed to be surgical candidates were treated and randomized to receive either a selective nerve root injection of betamethasone 6 mg with bupivacaine or a selective nerve root injection of bupivacaine alone. This study showed that 67% of patients in the group receiving both local anesthetic and steroid avoided the need for surgical intervention, compared with 28% in the group receiving local anesthetic alone [23]. A systematic review of therapeutic lumbar transforaminal epidural steroid therapy has shown that the indicated evidence for transforaminal lumbar epidural steroid injections is both short- and long-term pain relief compared with local anesthetic alone [1]. In the current study, we showed that application of tocilizumab produced significantly more pain relief than application of dexamethasone alone. We did not examine a lidocaine only group; however, tocilizumab is probably more effective for pain than application of lidocaine alone by analogy with the previous studies.
Limitations of the current study include its small size and prospective nature, and short follow-up period of only 4 weeks. Moreover, the dose of tocilizumab was only 80 mg. We previously reported that spinal nerve infiltration using 10 mg of etanercept was effective for radicular leg pain without any adverse events. Usually, 10 or 25 mg of etanercept, or 80 mg of tocilizumab is used to treat rheumatoid arthritis patients. Therefore, we selected 80 mg of tocilizumab as the dose in the current study [16]. Furthermore, in the current study, all patients also used NSAIDs. There was a possibility that NSAIDs have the same effect as dexamethasone and that their combinational use may not offer any advantage for pain relief. On the other hand, NSAIDs in combination with tocilizumab may provide an additive effect for pain relief. For ethical reasons, it was not practical to avoid the use of pain medications completely in the current study. Further study is required to clarify these points.
In summary, based on VAS scale and ODI scores, direct application of the anti-IL-6 receptor monoclonal antibody, onto spinal nerves, reduced low back pain, leg pain, and leg numbness caused by spinal stenosis. We did not observe any adverse event in the tocilizumab administration group. Anti-IL-6 receptor monoclonal antibody may therefore be useful tool for the treatment of radicular pain caused by spinal stenosis.
Conflict of interest
The authors did not receive and will not receive any benefits or funding from any commercial party related directly or indirectly to the subject of this article.
References
- 1.Buenaventura RM, Datta S, Abdi S, Smith HS (2009) Systematic review of therapeutic lumbar transforaminal epidural steroid injections. Pain Physician 12:233–251 (review) [PubMed]
- 2.Cohen SP, Bogduk N, Dragovich A, Buckenmaier CC, 3rd, Griffith S, Kurihara C, Raymond J, Richter PJ, Williams N, Yaksh TL. Randomized, double-blind, placebo-controlled, dose-response, and preclinical safety study of transforaminal epidural etanercept for the treatment of sciatica. Anesthesiology. 2009;110:1116–1126. doi: 10.1097/ALN.0b013e3181a05aa0. [DOI] [PubMed] [Google Scholar]
- 3.Hurri H, Karppinen J. Discogenic pain. Pain. 2004;112:225–228. doi: 10.1016/j.pain.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Igarashi A, Kikuchi S, Konno S, Olmarker K. Inflammatory cytokines released from the facet joint tissue in degenerative lumbar spinal disorders. Spine. 2004;29:2091–2095. doi: 10.1097/01.brs.0000141265.55411.30. [DOI] [PubMed] [Google Scholar]
- 5.Ito T, Ohtori S, Inoue G, Koshi T, Doya H, Ozawa T, Saito T, Moriya H, Takahashi K. Glial phosphorylated p38 MAP kinase mediates pain in a rat model of lumbar disc herniation and induces motor dysfunction in a rat model of lumbar spinal canal stenosis. Spine. 2007;32:159–167. doi: 10.1097/01.brs.0000251437.10545.e9. [DOI] [PubMed] [Google Scholar]
- 6.Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther. 2006;8(Suppl 2):S2. doi: 10.1186/ar1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konno S, Yabuki S, Sato K, Olmarker K, Kikuchi S (1995) A model for acute, chronic, and delayed graded compression of the dog cauda equina: presentation of the gross, microscopic, and vascular anatomy of the dog cauda equina and accuracy in pressure transmission of the compression model. Spine 20:2758–2764 [DOI] [PubMed]
- 8.Korhonen T, Karppinen J, Malmivaara A, Autio R, Niinimäki J, Paimela L, Kyllönen E, Lindgren KA, Tervonen O, Seitsalo S, Hurri H. Efficacy of infliximab for disc herniation-induced sciatica: one-year follow-up. Spine. 2004;29:2115–2119. doi: 10.1097/01.brs.0000141179.58778.6c. [DOI] [PubMed] [Google Scholar]
- 9.Korhonen T, Karppinen J, Paimela L, Malmivaara A, Lindgren KA, Järvinen S, Niinimäki J, Veeger N, Seitsalo S, Hurri H. The treatment of disc herniation-induced sciatica with infliximab: results of a randomized, controlled, 3-month follow-up study. Spine. 2005;30:2724–2728. doi: 10.1097/01.brs.0000190815.13764.64. [DOI] [PubMed] [Google Scholar]
- 10.Korhonen T, Karppinen J, Paimela L, Malmivaara A, Lindgren KA, Bowman C, Hammond A, Kirkham B, Järvinen S, Niinimäki J, Veeger N, Haapea M, Torkki M, Tervonen O, Seitsalo S, Hurri H. The treatment of disc-herniation-induced sciatica with infliximab: one-year follow-up results of FIRST II, a randomized controlled trial. Spine. 2006;31:2759–2766. doi: 10.1097/01.brs.0000245873.23876.1e. [DOI] [PubMed] [Google Scholar]
- 11.Mihara M, Kasutani K, Okazaki M, Nakamura A, Kawai S, Sugimoto M, Matsumoto Y, Ohsugi Y. Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family. Int Immunopharmacol. 2005;5:1731–1740. doi: 10.1016/j.intimp.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Myers RR, Wagner R, Sorkin LS. Hyperalgesic actions of cytokines on peripheral nerves. In: Watkins LR, Maier SF, editors. Cytokines and pain. Basel: Birkhauser; 1999. pp. 133–157. [Google Scholar]
- 13.Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J, Kishimoto T. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol. 2009;19:12–19. doi: 10.1007/s10165-008-0125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noponen-Hietala N, Virtanen I, Karttunen R, Schwenke S, Jakkula E, Li H, Merikivi R, Barral S, Ott J, Karppinen J, Ala-Kokko L. Genetic variations in IL6 associate with intervertebral disc disease characterized by sciatica. Pain. 2005;114:186–194. doi: 10.1016/j.pain.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Ohtori S, Suzuki M, Koshi T, Takaso M, Yamashita M, Inoue G, Yamauchi K, Orita S, Eguchi Y, Kuniyoshi K, Ochiai N, Kishida S, Nakamura J, Aoki Y, Ishikawa T, Arai G, Miyagi M, Kamoda H, Suzuki M, Toyone T, Takahashi K (2011) Proinflammatory cytokines in the cerebrospinal fluid of patients with lumbar radiculopathy. Eur Spine J 20:942–946 [DOI] [PMC free article] [PubMed]
- 16.Ohtori S, Miyagi M, Eguchi Y, Inoue G, Orita S, Ochiai N, Kishida S, Kuniyoshi K, Nakamura J, Aoki Y, Ishikawa T, Arai G, Kamoda H, Suzuki M, Takaso M, Furuya T, Toyone T, Takahashi K (2011) Epidural administration of spinal nerves with the tumor necrosis factor-alpha inhibitor, etanercept, compared with dexamethasone for treatment of sciatica in patients with lumbar spinal stenosis: a prospective randomized study. Spine (Epub ahead of print) [DOI] [PubMed]
- 17.Olmarker K, Rydevik B, Nordborg C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine. 1993;18:1425–1432. [PubMed] [Google Scholar]
- 18.Olmarker K, Larsson K. Tumor necrosis factor alpha and nucleus-pulposus-induced nerve root injury. Spine. 1998;23:2538–2544. doi: 10.1097/00007632-199812010-00008. [DOI] [PubMed] [Google Scholar]
- 19.Olmarker K, Nutu M, Størkson R. Changes in spontaneous behavior in rats exposed to experimental disc herniation are blocked by selective TNF-alpha inhibition. Spine. 2003;28:1635–1642. doi: 10.1097/01.BRS.0000083162.35476.FF. [DOI] [PubMed] [Google Scholar]
- 20.Onda A, Murata Y, Rydevik B, Larsson K, Kikuchi S, Olmarker K. Infliximab attenuates immunoreactivity of brain-derived neurotrophic factor in a rat model of herniated nucleus pulposus. Spine. 2004;29:1857–1861. doi: 10.1097/01.brs.0000137054.08788.b2. [DOI] [PubMed] [Google Scholar]
- 21.Ozaktay AC, Kallakuri S, Takebayashi T, Cavanaugh JM, Asik I, DeLeo JA, Weinstein JN. Effects of interleukin-1 beta, interleukin-6, and tumor necrosis factor on sensitivity of dorsal root ganglion and peripheral receptive fields in rats. Eur Spine J. 2006;15:1529–1537. doi: 10.1007/s00586-005-0058-8. [DOI] [PubMed] [Google Scholar]
- 22.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. doi: 10.2307/2529712. [DOI] [PubMed] [Google Scholar]
- 23.Riew KD, Yin Y, Gilula L, Bridwell KH, Lenke LG, Lauryssen C, Goette K. The effect of nerve root injections on the need for operative treatment of lumbar radicular pain. J Bone Jt Surg Am. 2000;82A:1589–1593. doi: 10.2106/00004623-200011000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Sekiguchi M, Kikuchi S, Myers RR. Experimental spinal stenosis: relationship between degree of cauda equina compression, neuropathology, and pain. Spine. 2004;29:1105–1111. doi: 10.1097/00007632-200405150-00011. [DOI] [PubMed] [Google Scholar]
- 25.Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, OPTION Investigators Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebocontrolled, randomised trial. Lancet. 2008;371:987–997. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]