Abstract
Introduction
Previous studies had shown that sagittal spinal and pelvic morphology may be associated with the development and progression of adolescent idiopathic scoliosis, but the predictive value of initial spinal and pelvic morphology on the curve progression during brace treatment is unknown. The objective of this study was to evaluate the relation between initial spinopelvic morphology and the risk of curve progression of adolescent idiopathic scoliosis with the Milwaukee brace.
Materials and methods
From 2002 to 2007, adolescent idiopathic scoliosis (single thoracic curve with apex at or above T8) was treated with the Milwaukee brace in 60 girls. Initial standing, full-length lateral radiographs were made and seven sagittal radiographic parameters of spinal and pelvic alignment were measured. Patients were followed until skeletal maturity or progression of Cobb angle >45°. The progression of curve was defined as an increase of Cobb angle ≥6° at final follow-up or progression to surgery during brace treatment.
Results
The 45 patients (75.0 %) who had successful control of curve progression were initially significantly more skeletally mature (higher mean Risser sign) than the 15 patients (25.0 %) who had curve progression. The initial mean Cobb angle was similar between the stable and progressed groups. The mean pelvic tilt, T1-spinopelvic inclination and T9-spinopelvic inclination angles were significantly greater in the stable group than in the progressed group and these three angles were independent predictors for curve progression during brace treatment. There were no significant differences between the stable and progressed groups in initial mean pelvic incidence, sacral slope, thoracic kyphosis or lumbar lordosis angles. Pre-bracing pelvic tilt ≤−0.5° was strongly predictive and T1-spinopelvic inclination ≤3.5° was moderately predictive of curve progression during the Milwaukee brace treatment.
Conclusions
Initial pelvic tilt and spinopelvic inclination angles may predict the curve progression and treatment outcome of adolescent idiopathic scoliosis with the Milwaukee brace.
Keywords: Adolescent idiopathic scoliosis, Spinopelvic morphology, Brace treatment, Curve progression
Introduction
Adolescent idiopathic scoliosis is a 3-dimensional deformity of the torso consisting of lateral curvature of the spine and vertebral rotation [34]. It affects approximately 1–3 % of adolescents and is more common in girls [18]. The Milwaukee brace is frequently used for non-operative treatment of mild to moderate immature adolescent idiopathic thoracic scoliosis where the apex is at or above T8 [17]. The brace may guide spinal growth and stop deterioration of the deformity. In some patients who use the Milwaukee brace the natural history of scoliosis can be changed and surgery can be avoided [16]. However, the Milwaukee brace may fail to control curve progression in 22–28 % patients [5, 16]. Curve progression and effectiveness of brace treatment are determined by the patient’s age, sex, curve magnitude, curve pattern, and pubertal status [29, 30, 35].
Sagittal spinal alignment and pelvic morphology may contribute to the development and progression of adolescent idiopathic scoliosis [9, 19, 32, 36]. Disproportionate growth of the anterior and posterior spinal columns during the pubertal growth spurt, which contributes to vertebrae malalignment in the sagittal plane may cause spinal buckling and curve formation [9]. Thoracic hypokyphosis from anterior column overgrowth may be associated with faster curve progression in patients with adolescent idiopathic scoliosis [36].
Furthermore, the morphology of the pelvis and the relative position of the pelvis to the spine may influence sagittal spinal alignment and balance [1, 2, 7, 8, 21, 26, 27]. Pelvic incidence (which is the angle measured on a lateral radiograph between the perpendicular to the sacral plate and the line joining the midpoint of the sacral plate and the axis of the femoral heads) is significantly greater in patients who have adolescent idiopathic scoliosis than those who do not have scoliosis [32]. Abnormal sagittal profile may contribute to instability of the spine under self-gravity compression resulting in scoliosis development and progression [10].
Little information is available about the effect of spinopelvic morphology on the control of curve progression during brace treatment in patients with adolescent idiopathic scoliosis. We hypothesized that spinopelvic morphology may influence the outcome of treatment of scoliosis with the Milwaukee brace. The purpose of this radiographic study was to determine the value of spinopelvic morphologic parameters on predicting curve progression during the Milwaukee brace treatment in girls with adolescent idiopathic thoracic scoliosis.
Materials and methods
Subjects
All female patients who had a diagnosis of adolescent idiopathic scoliosis and were treated with a Milwaukee brace between 2002 and 2007 were considered for inclusion in the study. The inclusion criteria were: (1) single thoracic curve with apex at or above T8 (Cobb angle 25°–40°) [17, 25], (2) treatment with Milwaukee brace without any specialized attachments, (3) initial age at bracing 10–15 years, (4) initial Risser sign 0–2, (5) either premenarche or less than 1 year postmenarche, (6) no previous treatment for scoliosis, (7) compliance ratio (defined as the ratio of the actual daily bracing time to the recommended daily time) ≥75 % [24], (8) follow-up until either skeletal maturity or progression of scoliosis (Cobb angle >45° and surgery recommended). Patients with a diagnosis of non-idiopathic scoliosis from congenital, neuromuscular or other connective tissue diseases were excluded from the study. The study was approved by the Clinical Research Ethics Committee of the hospital.
Each patient had been instructed to wear the Milwaukee brace 22 h per day, allowing 2 h for athletic activity and personal hygiene. The brace was checked and adjusted as necessary at the outpatient clinic every 3–6 months. Tapering the brace was started when growth had ceased (less than 1 cm change of height between two consecutive visits with at least 6 months apart) and the iliac crests were ossified (Risser grade 4) [25].
The patients were organized into two groups according to the final outcome of brace treatment [37]. The stable group included patients for whom the thoracic curve had <6° progression from the time of initial brace prescription to the final follow-up. The progressed group included patients with poor results of treatment evidenced by an increase of the Cobb angle ≥6° from initial brace prescription to the final follow-up or curve progression for which surgical treatment was indicated.
Patient evaluation
The medical records and radiographs of all patients were reviewed. Full-length standing posteroanterior and lateral radiographs were made. The patients stood upright in a relaxed manner with the fingers of both hands placed on the ipsilateral clavicles and the upper arms abducted to approximately 45° from vertical. Forced or unnatural positions were avoided. The Risser sign was noted on each radiograph. All medical records and radiographs were reviewed by the first author who was not involved in the treatment of the patients. In order to evaluate the measuring precision, all measurements were performed twice with an interval of at least 4 weeks using a standard computer program that provided appropriate tools (Surgimap Spine Software, New York, USA). The femoral heads were assumed to be spherical and the centers of the femoral heads were in turn automatically computed.
Radiographic parameters of alignment of the pelvis (three parameters) and spine (four parameters) in the sagittal plane were measured on each lateral radiograph:
Pelvic incidence defined as the angle between the perpendicular to the sacral plate and the line joining the midpoint of the sacral plate and the axis of the femoral heads [23] (Fig. 1).
Sacral slope defined as the angle between the horizontal line and the sacral plate [23] (Fig. 1).
Pelvic tilt defined as the angle between the vertical line and the line joining the midpoint of the sacral plate and the axis of the femoral heads (positive when the hip axis lies in front of the sacral plate midpoint) [23] (Figs. 1, 2).
Thoracic kyphosis defined as the angle between the upper endplate of the T5 vertebra and the lower endplate of the T12 vertebra (negative when the curve is lordotic and positive when the curve is kyphotic) [22].
Lumbar lordosis defined as the angle between the upper endplate of the L1 vertebra and the upper endplate of the S1 vertebra [22].
T1-spinopelvic inclination defined as the angle between the vertical line and the line joining the center of T1 vertebra and the axis of the femoral heads (positive when the hip axis lies in front of the T1 vertebral center) [28] (Figs. 1, 2).
T9-spinopelvic inclination defined as the angle between the vertical line and the line joining the center of T9 vertebra and the axis of the femoral heads (positive when the hip axis lies in front of the T9 vertebral center) [28] (Fig. 1).
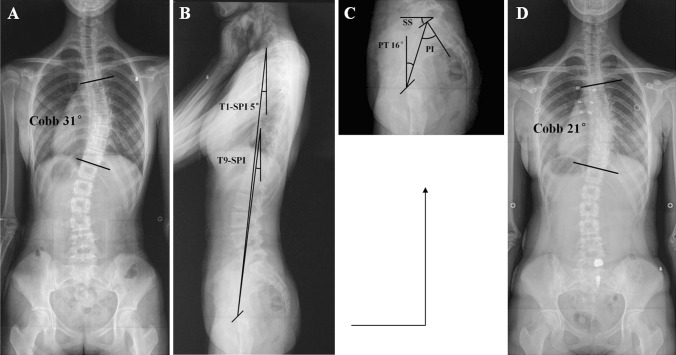
Fig. 1.
a Posteroanterior radiograph of a 13-year-old girl with adolescent idiopathic scoliosis at presentation: Risser grade 0, primary thoracic Cobb angle 31°. b, c Lateral radiographs of the same patient at presentation: T1-spinopelvic inclination (T1-SPI) 5°, pelvic tilt (PT) 16°. d Posteroanterior radiograph of the patient after Milwaukee brace treatment (3 years) shows decreased thoracic Cobb angle (21°)
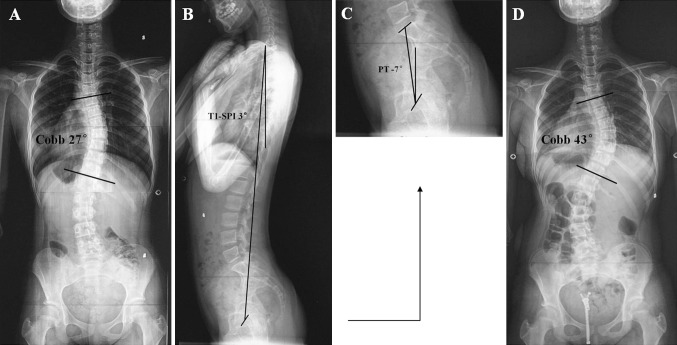
Fig. 2.
a Posteroanterior radiograph of a 12-year-old girl with adolescent idiopathic scoliosis at presentation: Risser grade 0, primary thoracic Cobb angle 27°. b, c Lateral radiographs of the same patient at presentation: T1-spinopelvic inclination (T1-SPI) 3°, pelvic tilt (PT) −7°. d Posteroanterior radiograph after Milwaukee brace treatment (1.5 years) shows increased thoracic Cobb angle (43°) and failure of brace treatment
Data analysis
Mean values of the two measurements were used. The data were analyzed using statistical software (SPSS 13.0, SPSS Inc., Chicago, IL). Average values were reported as mean ± SD. Summary statistics from analyses of variance calculations were used to provide 95 % prediction limits for the error in measurements. Independent samples’ t tests were performed to compare age, Risser sign, Cobb angle, and spinopelvic parameters between the stable and progressed groups. Bivariate correlation tests were done between age, Risser sign, Cobb angle, and spinopelvic parameters. Spearman correlation coefficients were determined to compare the ordinal variables such as Risser sign. Pearson correlation coefficients were determined to compare continuous variables. Multivariate regression analysis was performed to compare spinopelvic parameters between the stable and progressed groups. Statistically significant differences were defined by P < 0.05.
Receiver operator characteristic analysis was done to assess the diagnostic usefulness of different variables for predicting curve progression during brace treatment. For each variable, all possible cut points were selected and the sensitivity and specificity of each cut point were calculated. Receiver operator characteristic curves were then created by plotting the true positive fraction against the false positive fraction for each of the cut points. The area under the receiver operator characteristic curve was a measure of the diagnostic power of the variable, values that were either close to 1.0 (high) or 0.0 (low) suggested strong diagnostic power of the variable, and values close to 0.5 indicated that the variable was no more predictive than random chance [6].
Results
There were 60 girls with adolescent idiopathic scoliosis included in the study and most patients were in the stable group (Table 1). The mean age at presentation was 13.0 ± 1.0 and 12.4 ± 1.1 years for the patients in the stable and progressed group, respectively (P > 0.05). The average follow-up duration was 3.5 years (range 0.9–6.3 years) for all patients.
Table 1.
Radiographic parameters in patients with adolescent idiopathic scoliosis at the initial evaluation
| Radiographic parameter | Stable group | Progressed group | Variability | P† |
|---|---|---|---|---|
| Number (%) patients | 45 (75.0) | 15 (25.0) | – | – |
| Risser sign | 1.2 ± 0.9 | 0.7 ± 0.9 | – | <0.05 |
| Cobb angle (°) | 30.1 ± 5.1 | 28.5 ± 6.4 | 1.3 | NS |
| Pelvic incidence (°) | 43.1 ± 9.7 | 37.4 ± 11.7 | 1.8 | NS |
| Sacral slope (°) | 38.1 ± 9.1 | 42.0 ± 8.6 | 1.3 | NS |
| Pelvic tilt (°) | 5.0 ± 8.8 | -4.6 ± 10.1 | 1.0 | <0.01 |
| Thoracic kyphosis (°) | 11.1 ± 9.5 | 12.4 ± 8.6 | 1.0 | NS |
| Lumbar lordosis (°) | 50.0 ± 11.4 | 52.4 ± 8.0 | 1.8 | NS |
| T1-spinopelvic inclination (°) | 4.9 ± 2.7 | 2.7 ± 2.8 | 0.6 | <0.05 |
| T9-spinopelvic inclination (°) | 5.5 ± 4.3 | 2.7 ± 4.3 | 0.6 | <0.05 |
Reported as mean ± SD
†NS not significant (P > 0.05)
At the initial evaluation (before brace treatment), the patients in the stable group had on average more advanced skeletal maturity (higher average Risser sign) than patients in the progressed group, but both groups had similar initial mean Cobb angle (Table 1). The initial mean pelvic tilt, T1-spinopelvic inclination, and T9-spinopelvic inclination were significantly greater in the stable group than the progressed group (Table 1). There were no differences between the stable and progressed groups in initial mean pelvic incidence, sacral slope, thoracic kyphosis or lumbar lordosis (Table 1).
Bivariate correlation analysis showed that in all patients, age and Risser sign at initial evaluation were inversely associated with sacral slope and lumbar lordosis and age and Risser sign at initial evaluation were positively associated with pelvic tilt (Table 2). In all patients, initial age was also positively correlated with T1- and T9-spinopelvic inclination (Table 2). In the stable group, age and Risser sign at initial evaluation were inversely associated with sacral slope and lumbar lordosis and age and Risser sign at initial evaluation were positively associated with pelvic tilt (Table 2). In the progressed group, Cobb angle at initial evaluation was inversely related to T1- and T9-spinopelvic inclination (Table 2).
Table 2.
Coefficients of correlation between age, Risser sign, Cobb angle and spinopelvic parameters in patients with adolescent idiopathic scoliosis
| Parameters | All patients (n = 60) | Stable group (n = 45) | Progressed group (n = 15) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Risser sign | Cobb angle | Age | Risser sign | Cobb angle | Age | Risser sign | Cobb angle | |
| Pelvic incidence | −0.057 | −0.037 | 0.151 | −0.010 | −0.028 | 0.220 | −0.399 | −0.285 | −0.074 |
| Sacral slope | −0.485* | −0.339 | 0.171 | −0.461** | −0.310* | 0.158 | −0.465 | −0.304 | 0.320 |
| Pelvic tilt | 0.380** | 0.269* | 0.002 | 0.465** | 0.289* | 0.079 | −0.067 | −0.072 | −0.359 |
| Thoracic kyphosis | −0.123 | −0.127 | −0.186 | −0.103 | −0.086 | −0.192 | −0.144 | −0.222 | −0.153 |
| Lumbar lordosis | −0.515** | −0.398** | 0.100 | −0.499** | −0.419** | 0.068 | −0.481 | −0.267 | 0.298 |
| T1-spinopelvic inclination | 0.214* | 0.111 | −0.046 | 0.248 | 0.017 | 0.097 | −0.005 | 0.085 | −0.556* |
| T9-spinopelvic inclination | 0.305* | 0.152 | −0.150 | 0.209 | 0.092 | −0.082 | 0.100 | 0.096 | −0.485* |
* Correlation is significant at the 0.05 level
** Correlation is significant at the 0.01 level
In consideration that the differences of sagittal morphology between the stable and progressed groups may be attributed to the differences of skeletal maturity (Risser sign) between the two groups, we subsequently performed multivariate regression analysis to statistically separate Risser sign from significant spinopelvic parameters (pelvic tilt, T1- and T9-spinopelvic inclination) as independent factors in predicting curve progression. Multivariate regression analysis showed that, after the adjustment for Risser sign and Cobb angle, the stable and progressed groups still differed significantly in initial pelvic tilt, T1-spinopelvic inclination and T9-spinopelvic inclination (Table 3).
Table 3.
Multivariate regression analysis of factors correlating with pelvic tilt, T1-spinopelvic inclination, and T9-spinopelvic inclination in patients with adolescent idiopathic scoliosis
| Factors | Pelvic tilt | T1-spinopelvic inclination | T9-spinopelvic inclination | |||
|---|---|---|---|---|---|---|
| Regression coefficient (r) | P† | Regression coefficient (r) | P† | Regression coefficient (r) | P† | |
| Risser sign | 1.896 | NS | 0.052 | NS | 0.285 | NS |
| Cobb angle | −0.041 | NS | −0.047 | NS | −0.145 | NS |
| Group (stable/progressed) | −8.785 | <0.01 | −2.263 | <0.05 | −2.875 | <0.05 |
†NS not significant (P > 0.05)
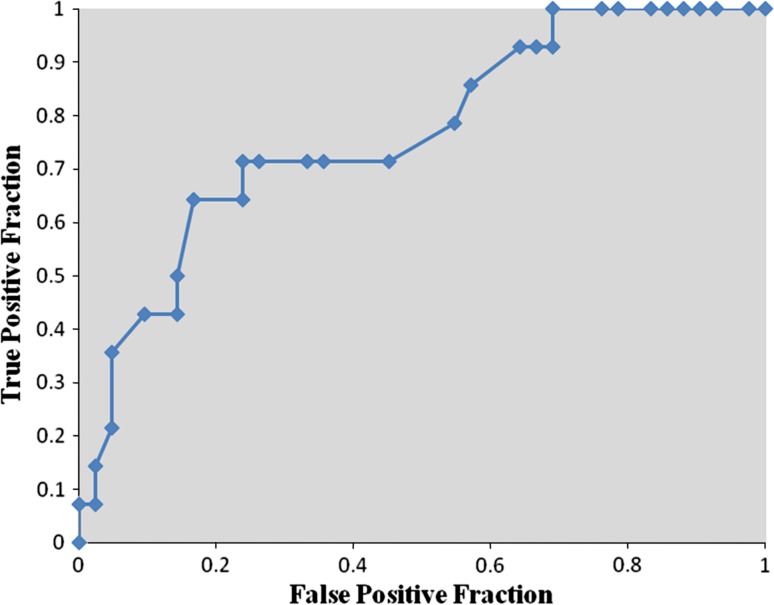
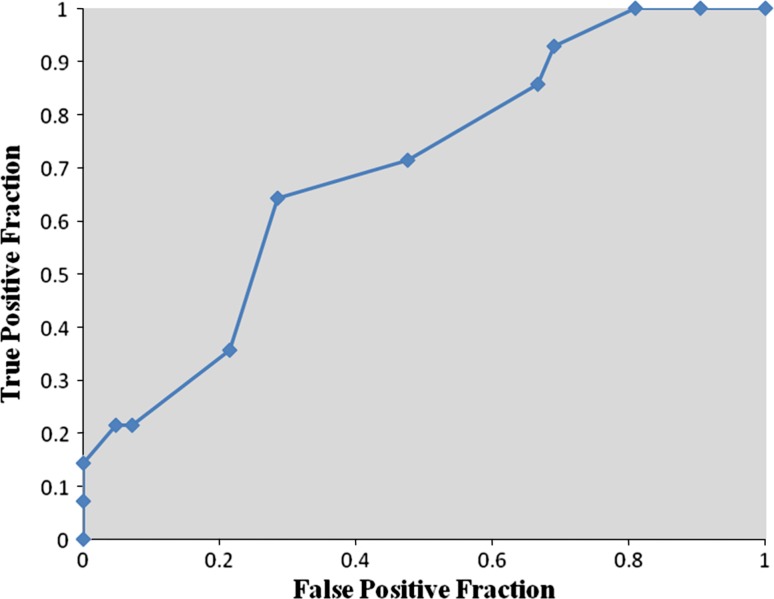
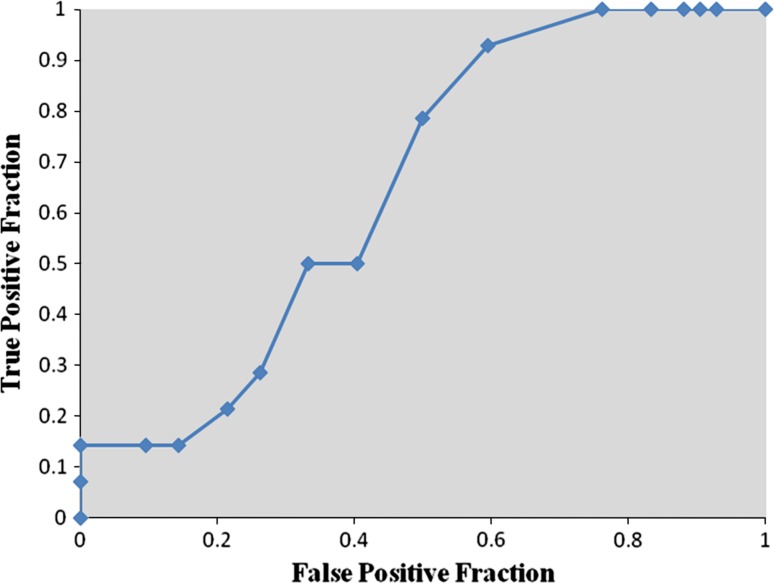
Receiver operator characteristic analysis showed that pre-bracing pelvic tilt ≤−0.5° was strongly predictive of curve progression during the Milwaukee brace treatment (sensitivity 64.3 %, specificity 83.3 %, area under curve 0.766, P < 0.01) (Fig. 3). Pre-bracing T1-spinopelvic inclination ≤3.5° also was predictive of curve progression during the Milwaukee brace treatment (sensitivity 64.3 %, specificity 71.4 %, area under curve 0.696, P < 0.05) (Fig. 4). Although the pre-bracing T9-spinopelvic inclination ≤6.5° was an indicator of increased risk for the progression of scoliosis (sensitivity 92.9 %, specificity 40.5 %), this cut point was only weakly predictive and not significant (area under curve 0.651, P > 0.05) (Fig. 5).
Fig. 3.
Receiver operator characteristic curve for pre-bracing pelvic tilt. Each point is a cut point for pelvic tilt at which the sensitivity and specificity for predicting the failure of bracing is evaluated
Fig. 4.
Receiver operator characteristic curve for pre-bracing T1-spinopelvic inclination. Each point is a cut point for T1-spinopelvic inclination at which the sensitivity and specificity for predicting the failure of bracing is evaluated
Fig. 5.
Receiver operator characteristic curve for pre-bracing T9-spinopelvic inclination. Each point is a cut point for T9-spinopelvic inclination at which the sensitivity and specificity for predicting the failure of bracing is evaluated
Discussion
The significant associations demonstrated between progression of scoliosis and initial pelvic tilt, T1-spinopelvic inclination and T9-spinopelvic inclination (Table 3) are evidence in support of the hypothesis that initial spinopelvic morphology may influence the treatment outcome of scoliosis with the Milwaukee brace. The frequency of stable (75.0 %) and progressed (25.0 %) curves after treatment observed with the Milwaukee brace (Table 1) was consistent with that reported previously [5, 16].
The prognostic evaluation of brace treatment for patients with adolescent idiopathic scoliosis requires an understanding of risk factors associated with curve progression. Initial age, skeletal maturity and curve magnitude are of prognostic value in predicting the outcome of brace treatment [29, 35]. The current study specifically focused on the role of the initial spinopelvic morphology as a prognostic factor for curve progression during the Milwaukee brace treatment.
In the presence of sagittal malalignment, pelvic tilt may be a compensatory factor to maintain an energy efficient posture and to keep the spine upright [13, 14]. In the present study, initial pelvic tilt was significantly less in the progressed group than in the stable group (Table 1; Figs. 1, 2). Pelvic tilt angle and skeletal maturity (Risser sign) were positively correlated (Table 2) consistent with previously published results [21, 22]. Furthermore, controlling for Risser sign, initial pelvic tilt angle was significantly different between the stable and progressed groups (Table 3) and a pre-treatment pelvic tilt angle of ≤−0.5° was strongly associated with an increased risk of curve progression during brace treatment (Fig. 3), confirming that initial pelvic tilt is an independent factor that may predict success or failure of treatment with the Milwaukee brace. This is consistent with the principle of the compensatory action of pelvic version [3, 12], anteversion of the pelvis (decreased pelvic tilt angle) may cause malalignment of the spine in sagittal plane bringing the apical region of the thoracic curve relatively anterior to the hip axis and this may exert a potentially adverse biomechanical effect on the apical region resulting in increased axial rotational instability and curve progression during brace treatment [10, 15].
The T1- and T9-spinopelvic inclination angles have been used for assessment of the balance and trunk inclination of the sagittal spine [28]. These two angular parameters help avoid error inherent with other parameters that are based on measurement of offset length on non-calibrated radiographs [28]. Initial T1- and T9-spinopelvic inclination angles were significantly smaller in the progressed than the stable group (Table 1), even after adjustment for Risser sign and Cobb angle (Table 3), but the T1-spinopelvic inclination angle (≤3.5°) and not the T9-spinopelvic inclination angle, was significantly (albeit weakly) predictive of curve progression during the treatment with the Milwaukee brace (Figs. 4, 5). The T1-spinopelvic inclination angle is a measure of the position of the T1 vertebra in relation to the pelvis through the hip axis, a smaller T1-spinopelvic inclination angle is noted for patients in whom the femoral heads are closer to a plumb line from the T1 vertebra with a shorter lever arm from the vertical to help maintain balanced forces around the hip joint. Therefore, the smaller mean T1-spinopelvic inclination angle noted in the progressed group (Table 1) may be secondary to pelvic anteversion (lower pelvic tilt angle) and may not be of primary importance.
Previous studies have shown varied results about the effect of thoracic kyphosis on progression of scoliosis curves. Thoracic hypokyphosis may be a risk factor for scoliosis curve progression based on the “anterior column overgrowth” theory and the results of histomorphometric and magnetic resonance imaging studies that showed disproportionate growth of the anterior and posterior vertebral columns in patients with adolescent idiopathic scoliosis [9, 38]. The progression velocity of scoliosis curves may be greater in patients with thoracic hypokyphosis than those with normal thoracic kyphosis [36], but in another study, thoracic kyphosis was not associated with either the severity of scoliosis at the time of diagnosis or the risk of progression of scoliosis [4]. The present results showed no difference in mean thoracic kyphosis angle in the stable and progressed groups (Table 1). Differences between study results may in part be attributed to different population samples the previous studies [4, 36] included a variety of curve types (thoracic, thoracolumbar, and lumbar curves), but the present study included only single thoracic curves. Although thoracic hypokyphosis may be associated with progression of untreated adolescent idiopathic scoliosis (natural history), it may not be a prognostic factor in predicting curve progression during treatment of single thoracic curves with the Milwaukee brace (Table 1).
Pelvic morphology may influence sagittal spinal alignment and balance [10, 12]. A previous multicenter radiographic study demonstrated that pelvic incidence is greater in patients with adolescent idiopathic scoliosis than normal adolescents [32] possibly as a result of altered spinopelvic morphology. However, no difference in mean pelvic incidence angle was observed between the stable and progressed groups in the present study (Table 1), and values of the pelvic incidence angle in the present study were smaller than those reported previously [19, 32].
It has been well recognized that pelvic tilt (PT) + sacral slope (SS) = pelvic incidence (PI) [13]. With a fixed value of PI, the increase of PT equals to the decrease of SS vice versa. However, in the current study only PT was found to be significantly different between the stable and progressed groups, neither was PI nor SS. In our opinion this could be attributed to the statistical evaluation. Though the independent samples t tests showed that there was no statistical differences of PI and SS between the two groups, the stable group seemed to have a higher PI and a lower SS, whereas the progressed group seemed to have a lower PI and a higher SS (Table 1). These subtle differences of PI and SS between the two groups may not be able to be detected by statistical analysis due to the limited amount of subjects we included, but could potentially cause a cumulative effect on the value of PT via the arithmetical interrelationship of the three parameters (i.e., PT = PI−SS) magnifying the differences of PT between the stable and progressed groups. Moreover previous studies have reported that PI could slightly increase during the pediatric growth, but the trend was very small even in a large group of subjects with a vast range of age from 3 to 18 years [20, 21]. Therefore it was possible to find that PI was mildly greater in the stable group (because the subjects in the stable group were more skeletally mature), but could not be identified as statistically different between the stable and progressed groups, which, in spite of small sample capacity may also be attributed to the limited range of age distribution (10–15 years) in the current study. Based on our results, we regarded that PT (but not PI or SS) possessed preferable clinical relevance and could be used as a predictive factor in curve progression. Further studies with large sample capacities should be conducted in a like manner to verify the logic of our findings.
The present results showed that curve progression during brace treatment was associated with initial lower Risser grade (Table 1) consistent with the findings of previous studies [16, 33]. Brace treatment may not prevent curve progression in the child who is younger (<12 years) and has lower Risser grade (<2) when brace treatment is started [33] and frequency of surgical treatment for scoliosis is higher in patients who are less skeletally mature (Risser sign 0–1) at the time of bracing than patients who are more skeletally mature (Risser sign 2–4) [16]. Previous studies of patients with different scoliosis curve patterns showed that patients who have a larger initial Cobb angle are more likely to have curve progression during brace treatment [11, 31]; however, the initial Cobb angle was not a predictor of curve progression in the present study (Table 1) possibly, because the present patients all had the same initial curve pattern (single thoracic curve) limiting the comparison with previous studies of patients with varied initial curve patterns.
One limitation of the present study is that only one type of bracing was studied; the Boston-type based brace should be studied in a like manner to verify our findings. Another limitation of the present study is the lack of consideration of the immediate effect on pelvic version by bracing. Though each Milwaukee brace was individualized and custom made to make sure that the pelvic module had no extra force on the pelvis, there was still a minute possibility that the pelvic version might be a little bit influenced by wearing the brace. However, in consideration that increased radiation exposure may pose more adverse influences on the immature AIS patients, we did not perform extra lateral radiographs of the patients with the brace on and investigate the effects of Milwaukee brace on the sagittal profile.
Conclusion
Initial pelvic tilt and spinopelvic inclination angles may predict the success or failure of controlling curve progression in adolescent idiopathic scoliosis with the Milwaukee brace. Pre-bracing pelvic tilt ≤−0.5° was strongly predictive and T1-spinopelvic inclination ≤3.5° was moderately predictive of curve progression during the Milwaukee brace treatment. Special attention and particular consultation should be given to the patients who have a lower initial pelvic tilt (≤−0.5°) or T1-spinopelvic inclination (≤3.5°) before and during the Milwaukee brace treatment.
Acknowledgments
This work was supported by Stryker China Ltd.
Conflict of interest
None.
Footnotes
J. Guo and Z. Liu contributed equally to this work.
References
- 1.Abelin K, Vialle R, Lenoir T, Thevenin-Lemoine C, Damsin JP, Forin V. The sagittal balance of the spine in children and adolescents with osteogenesis imperfecta. Eur Spine J. 2008;17:1697–1704. doi: 10.1007/s00586-008-0793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrey C, Jund J, Noseda O, Roussouly P. Sagittal balance of the pelvis-spine complex and lumbar degenerative diseases. A comparative study about 85 cases. Eur Spine J. 2007;16:1459–1467. doi: 10.1007/s00586-006-0294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrey C, Roussouly P, Perrin G, Huec JC. Sagittal balance disorders in severe degenerative spine. Can we identify the compensatory mechanisms? Eur Spine J. 2011;20(Suppl 5):626–633. doi: 10.1007/s00586-011-1930-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunnell WP. The natural history of idiopathic scoliosis before skeletal maturity. Spine. 1986;11:773–776. doi: 10.1097/00007632-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Carr WA, Moe JH, Winter RB, Lonstein JE. Treatment of idiopathic scoliosis in the Milwaukee brace. J Bone Joint Surg Am. 1980;62:599–612. [PubMed] [Google Scholar]
- 6.Carreon LY, Puno RM, Lenke LG, Richards BS, Sucato DJ, Emans JB, Erickson MA. Non-neurologic complications following surgery for adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2007;89:2427–2432. doi: 10.2106/JBJS.F.00995. [DOI] [PubMed] [Google Scholar]
- 7.Chanplakorn P, Wongsak S, Woratanarat P, Wajanavisit W, Laohacharoensombat W. Lumbopelvic alignment on standing lateral radiograph of adult volunteers and the classification in the sagittal alignment of lumbar spine. Eur Spine J. 2010 doi: 10.1007/s00586-010-1626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endo K, Suzuki H, Tanaka H, Kang Y, Yamamoto K. Sagittal spinal alignment in patients with lumbar disc herniation. Eur Spine J. 2009 doi: 10.1007/s00586-009-1240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo X, Chau WW, Chan YL, Cheng JC. Relative anterior spinal overgrowth in adolescent idiopathic scoliosis. Results of disproportionate endochondral-membranous bone growth. J Bone Joint Surg Br. 2003;85:1026–1031. doi: 10.1302/0301-620X.85B7.14046. [DOI] [PubMed] [Google Scholar]
- 10.Jiang J, Qiu Y, Mao S, Zhao Q, Qian B, Zhu F. The influence of elastic orthotic belt on sagittal profile in adolescent idiopathic thoracic scoliosis: a comparative radiographic study with Milwaukee brace. BMC Musculoskelet Disord. 2010;11:219. doi: 10.1186/1471-2474-11-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz DE, Durrani AA. Factors that influence outcome in bracing large curves in patients with adolescent idiopathic scoliosis. Spine. 2001;26:2354–2361. doi: 10.1097/00007632-200111010-00012. [DOI] [PubMed] [Google Scholar]
- 12.Lazennec JY, Brusson A, Rousseau MA. Hip-spine relations and sagittal balance clinical consequences. Eur Spine J. 2011;20(Suppl 5):686–698. doi: 10.1007/s00586-011-1937-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huec JC, Aunoble S, Philippe L, Nicolas P. Pelvic parameters: origin and significance. Eur Spine J. 2011;20(Suppl 5):564–571. doi: 10.1007/s00586-011-1940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huec JC, Roussouly P. Sagittal spino-pelvic balance is a crucial analysis for normal and degenerative spine. Eur Spine J. 2011;20(Suppl 5):556–557. doi: 10.1007/s00586-011-1943-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huec JC, Saddiki R, Franke J, Rigal J, Aunoble S. Equilibrium of the human body and the gravity line: the basics. Eur Spine J. 2011;20(Suppl 5):558–563. doi: 10.1007/s00586-011-1939-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lonstein JE, Winter RB. The Milwaukee brace for the treatment of adolescent idiopathic scoliosis. A review of one thousand and twenty patients. J Bone Joint Surg Am. 1994;76:1207–1221. doi: 10.2106/00004623-199408000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Lonstein JE (2003) Milwaukee brace treatment of scoliosis. Scoliosis Research Society Bracing Manual (http://www.srs.org/professionals/education_materials/SRS_bracing_manual/index.htm.)
- 18.Luk KD, Lee CF, Cheung KM, Cheng JC, Ng BK, Lam TP, Mak KH, Yip PS, Fong DY. Clinical effectiveness of school screening for adolescent idiopathic scoliosis: a large population-based retrospective cohort study. Spine. 2010;35:1607–1614. doi: 10.1097/BRS.0b013e3181c7cb8c. [DOI] [PubMed] [Google Scholar]
- 19.Mac-Thiong JM, Labelle H, Charlebois M, Huot MP, Guise JA. Sagittal plane analysis of the spine and pelvis in adolescent idiopathic scoliosis according to the coronal curve type. Spine. 2003;28:1404–1409. doi: 10.1097/01.BRS.0000067118.60199.D1. [DOI] [PubMed] [Google Scholar]
- 20.Mac-Thiong JM, Berthonnaud E, Dimar JR, 2nd, Betz RR, Labelle H. Sagittal alignment of the spine and pelvis during growth. Spine. 2004;29:1642–1647. doi: 10.1097/01.BRS.0000132312.78469.7B. [DOI] [PubMed] [Google Scholar]
- 21.Mac-Thiong JM, Labelle H, Berthonnaud E, Betz RR, Roussouly P. Sagittal spinopelvic balance in normal children and adolescents. Eur Spine J. 2007;16:227–234. doi: 10.1007/s00586-005-0013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mac-Thiong JM, Labelle H, Roussouly P. Pediatric sagittal alignment. Eur Spine J. 2011;20(Suppl 5):586–590. doi: 10.1007/s00586-011-1925-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mac-Thiong JM, Roussouly P, Berthonnaud E, Guigui P. Age- and sex-related variations in sagittal sacropelvic morphology and balance in asymptomatic adults. Eur Spine J. 2011;20(Suppl 5):572–577. doi: 10.1007/s00586-011-1923-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu Y, Sun X, Cheng JC, Zhu F, Li W, Zhu Z, Wang B, Yu Y. Bone mineral accrual in osteopenic and non-osteopenic girls with idiopathic scoliosis during bracing treatment. Spine. 2008;33:1682–1689. doi: 10.1097/BRS.0b013e31817b5b9e. [DOI] [PubMed] [Google Scholar]
- 25.Richards BS, Bernstein RM, D’Amato CR, Thompson GH. Standardization of criteria for adolescent idiopathic scoliosis brace studies: SRS committee on bracing and nonoperative management. Spine. 2005;30:2068–2075. doi: 10.1097/01.brs.0000178819.90239.d0. [DOI] [PubMed] [Google Scholar]
- 26.Roussouly P, Nnadi C. Sagittal plane deformity: an overview of interpretation and management. Eur Spine J. 2010;19:1824–1836. doi: 10.1007/s00586-010-1476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roussouly P, Pinheiro-Franco JL. Biomechanical analysis of the spino-pelvic organization and adaptation in pathology. Eur Spine J. 2011;20(Suppl 5):609–618. doi: 10.1007/s00586-011-1928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwab F, Lafage V, Patel A, Farcy JP. Sagittal plane considerations and the pelvis in the adult patient. Spine. 2009;34:1828–1833. doi: 10.1097/BRS.0b013e3181a13c08. [DOI] [PubMed] [Google Scholar]
- 29.Soucacos PN, Zacharis K, Gelalis J, Soultanis K, Kalos N, Beris A, Xenakis T, Johnson EO. Assessment of curve progression in idiopathic scoliosis. Eur Spine J. 1998;7:270–277. doi: 10.1007/s005860050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan KJ, Moe MM, Vaithinathan R, Wong HK. Curve progression in idiopathic scoliosis: follow-up study to skeletal maturity. Spine. 2009;34:697–700. doi: 10.1097/BRS.0b013e31819c9431. [DOI] [PubMed] [Google Scholar]
- 31.Upadhyay SS, Nelson IW, Ho EK, Hsu LC, Leong JC. New prognostic factors to predict the final outcome of brace treatment in adolescent idiopathic scoliosis. Spine. 1995;20:537–545. doi: 10.1097/00007632-199503010-00006. [DOI] [PubMed] [Google Scholar]
- 32.Upasani VV, Tis J, Bastrom T, Pawelek J, Marks M, Lonner B, Crawford A, Newton PO. Analysis of sagittal alignment in thoracic and thoracolumbar curves in adolescent idiopathic scoliosis: how do these two curve types differ? Spine. 2007;32:1355–1359. doi: 10.1097/BRS.0b013e318059321d. [DOI] [PubMed] [Google Scholar]
- 33.Vijvermans V, Fabry G, Nijs J. Factors determining the final outcome of treatment of idiopathic scoliosis with the Boston brace: a longitudinal study. J Pediatr Orthop B. 2004;13:143–149. doi: 10.1097/00009957-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Weinstein SL, Dolan LA, Cheng JCY, Danielsson A, Morcuende JA. Adolescent idiopathic scoliosis. Lancet. 2008;371:1527–1537. doi: 10.1016/S0140-6736(08)60658-3. [DOI] [PubMed] [Google Scholar]
- 35.Wu H, Ronsky JL, Cheriet F, Harder J, Kupper JC, Zernicke RF. Time series spinal radiographs as prognostic factors for scoliosis and progression of spinal deformities. Eur Spine J. 2011;20:112–117. doi: 10.1007/s00586-010-1512-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ylikoski M. Growth and progression of adolescent idiopathic scoliosis in girls. J Pediatr Orthop B. 2005;14:320–324. doi: 10.1097/01202412-200509000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Yrjonen T, Ylikoski M, Schlenzka D, Poussa M. Results of brace treatment of adolescent idiopathic scoliosis in boys compared with girls: a retrospective study of 102 patients treated with the Boston brace. Eur Spine J. 2007;16:393–397. doi: 10.1007/s00586-006-0167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu F, Qiu Y, Yeung HY, Lee KM, Cheng JC. Histomorphometric study of the spinal growth plates in idiopathic scoliosis and congenital scoliosis. Pediatr Int. 2006;48:591–598. doi: 10.1111/j.1442-200X.2006.02277.x. [DOI] [PubMed] [Google Scholar]