Abstract
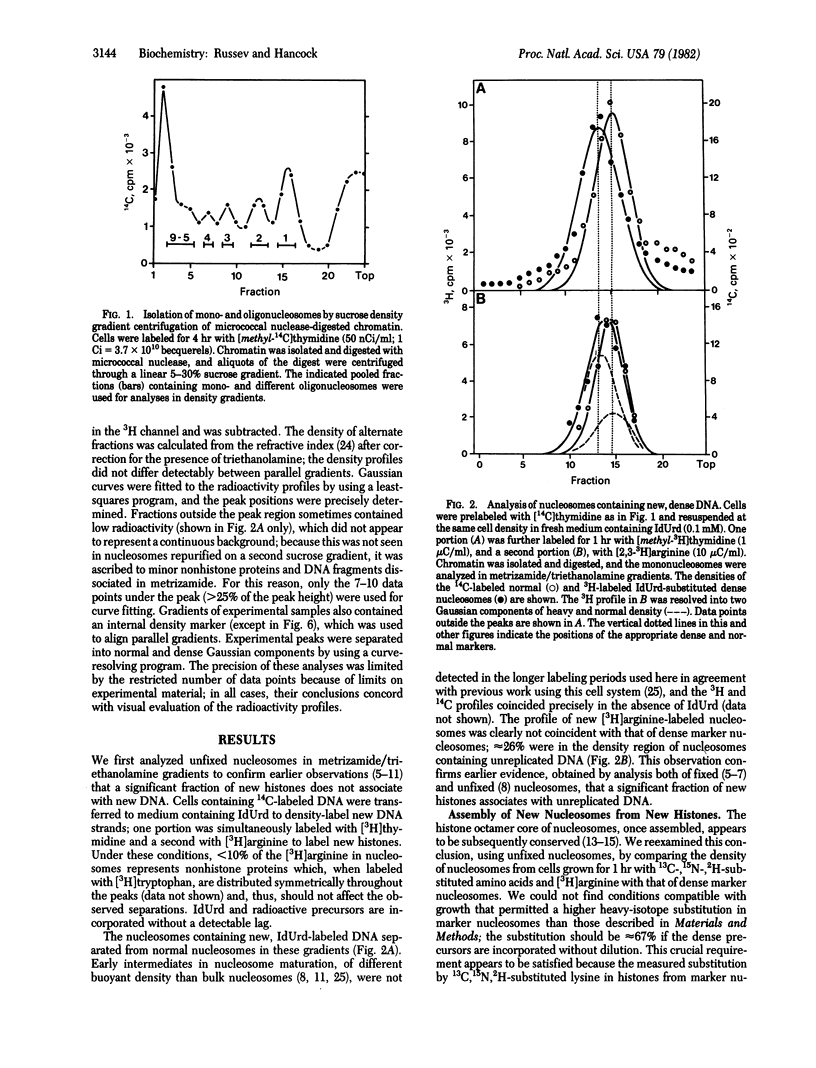
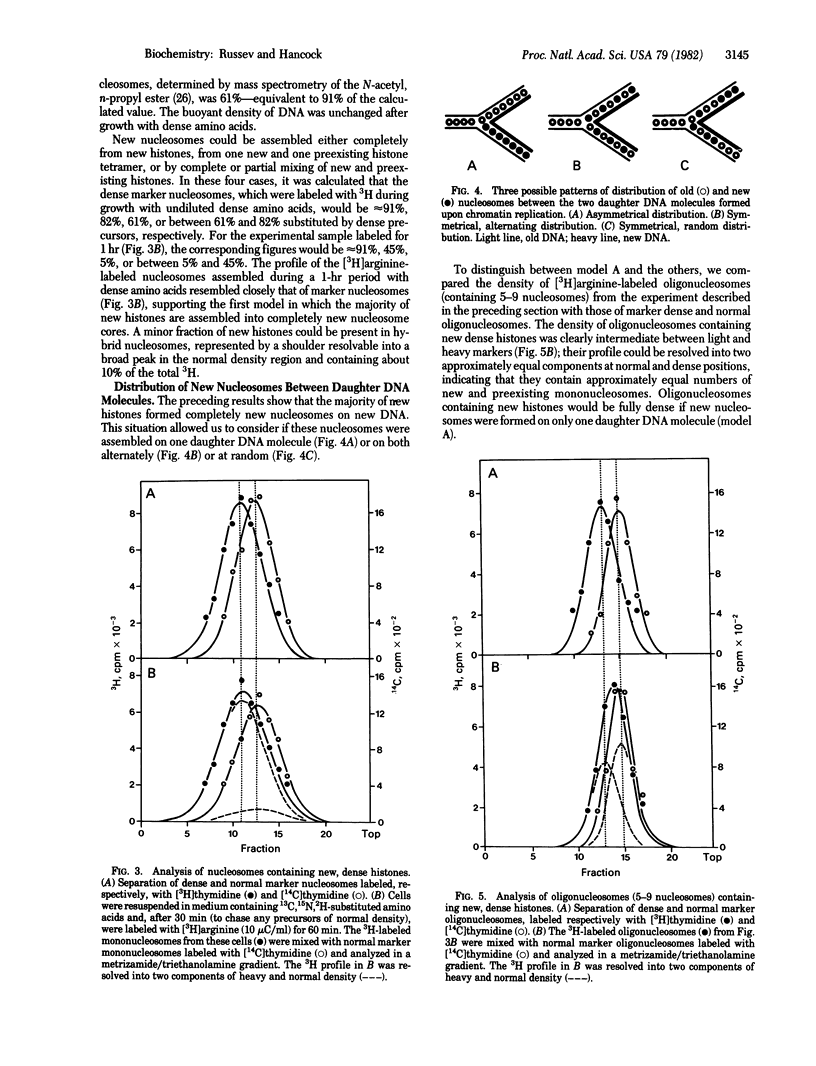
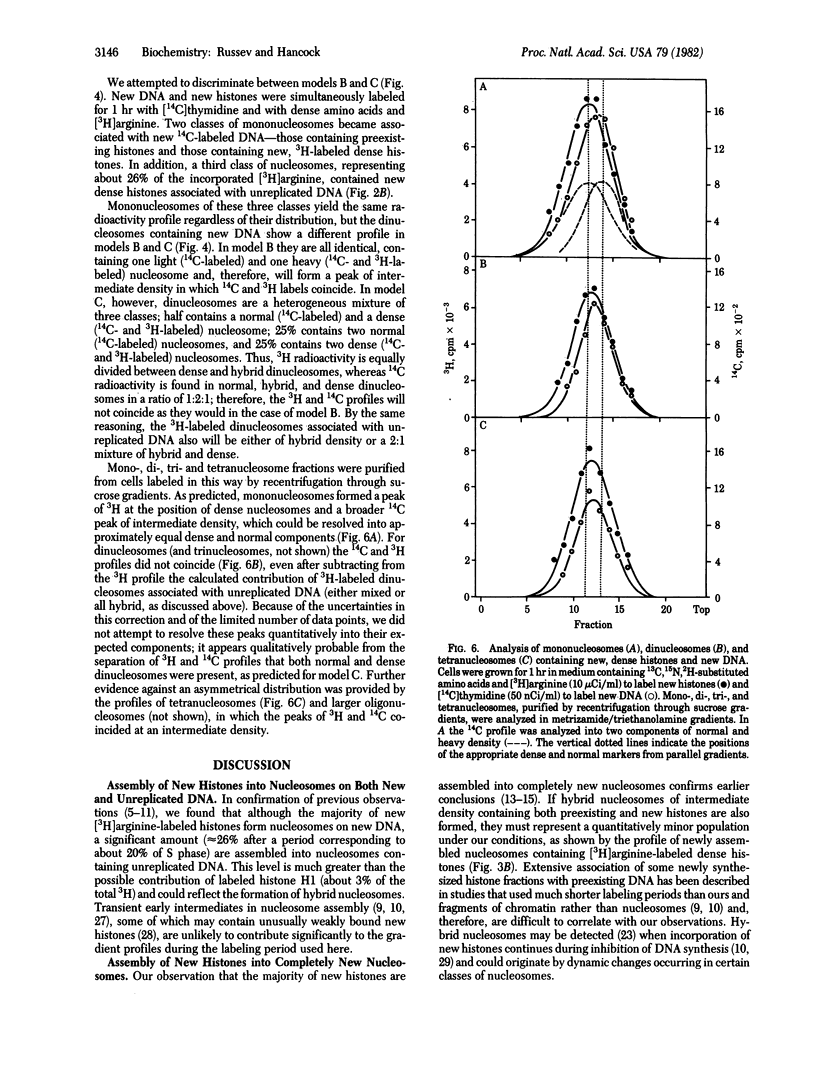
We studied the assembly of new histones into nucleosomes and their distribution in replicating chromatin in growing P815 mouse cells. New histones and new DNA were density-labeled with 13C, 15N, 2H-substituted amino acids together with [3H]arginine or with 5-iododeoxyuridine and [3H]thymidine, respectively, for 1 hr (approximately 20% of S phase). Mono- di-, tri-, tetra- and larger oligonucleosomes were isolated by sucrose gradient centrifugation of micrococcal nuclease-digested chromatin, and their density distribution was analyzed, without fixation, in metrizamide/triethanolamine density gradients [Russev, G. and Tsanev, R. (1976) Nucleic Acids Res. 3, 697-707] in which mono- and oligonucleosomes containing dense amino acids or 5-iododeoxyuridine separate from the corresponding normal nucleosomes. Under these conditions, approximately 74% of the new histones are found in nucleosomes on newly replicated DNA, and the remainder are on unreplicated DNA. The majority of new histones form entirely new nucleosomes; a minor fraction may form hybrid nucleosomes that also contain preexisting histones. New nucleosomes are distributed to both new daughter DNA molecules with approximately equal probability, and our evidence suggests, but does not prove, that they are distributed in a random manner along new DNA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright S. C., Wiseman J. M., Lange R. A., Garrard W. T. Subunit structures of different electrophoretic forms of nucleosomes. J Biol Chem. 1980 Apr 25;255(8):3673–3684. [PubMed] [Google Scholar]
- Crémisi C. Chromatin replication revealed by studies of animal cells and papovaviruses (simian virus 40 and polyoma virus). Microbiol Rev. 1979 Sep;43(3):297–319. doi: 10.1128/mr.43.3.297-319.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakan S., Turner G. N., Pagano J. S., Hancock R. Sites of replication of chromosomal DNA in a eukaryotic cell. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2300–2305. doi: 10.1073/pnas.69.8.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. Assembly of new nucleosomal histones and new DNA into chromatin. Proc Natl Acad Sci U S A. 1978 May;75(5):2130–2134. doi: 10.1073/pnas.75.5.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson V., Chalkley R. A new method for the isolation of replicative chromatin: selective deposition of histone on both new and old DNA. Cell. 1981 Jan;23(1):121–134. doi: 10.1016/0092-8674(81)90277-4. [DOI] [PubMed] [Google Scholar]
- Jackson V., Chalkley R. A reevaluation of new histone deposition on replicating chromatin. J Biol Chem. 1981 May 25;256(10):5095–5103. [PubMed] [Google Scholar]
- Jackson V., Granner D., Chalkley R. Deposition of histone onto the replicating chromosome: newly synthesized histone is not found near the replication fork. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2266–2269. doi: 10.1073/pnas.73.7.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson V., Marshall S., Chalkley R. The sites of deposition of newly synthesized histone. Nucleic Acids Res. 1981 Sep 25;9(18):4563–4581. doi: 10.1093/nar/9.18.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffak I. M., Grainger R., Weintraub H. Conservative assembly and segregation of nucleosomal histones. Cell. 1977 Nov;12(3):837–845. doi: 10.1016/0092-8674(77)90282-3. [DOI] [PubMed] [Google Scholar]
- Mathis D., Oudet P., Chambon P. Structure of transcribing chromatin. Prog Nucleic Acid Res Mol Biol. 1980;24:1–55. doi: 10.1016/s0079-6603(08)60670-4. [DOI] [PubMed] [Google Scholar]
- Murphy R. F., Wallace R. B., Bonner J. Isolation of newly replicated chromatin by using shallow metrizamide gradients. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3336–3340. doi: 10.1073/pnas.77.6.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau P., Oliver D. R., Chalkley R. Effect of inhibition of DNA synthesis on histone synthesis and deposition. Biochemistry. 1978 Nov 14;17(23):4885–4893. doi: 10.1021/bi00616a005. [DOI] [PubMed] [Google Scholar]
- Prior C. P., Cantor C. R., Johnson E. M., Allfrey V. G. Incorporation of exogenous pyrene-labeled histone into Physarum chromatin: a system for studying changes in nucleosomes assembled in vivo. Cell. 1980 Jul;20(3):597–608. doi: 10.1016/0092-8674(80)90306-2. [DOI] [PubMed] [Google Scholar]
- Rickwood D., Birnie G. D. Metrizamide, a new density-gradient medium. FEBS Lett. 1975 Feb 1;50(2):102–110. doi: 10.1016/0014-5793(75)80467-4. [DOI] [PubMed] [Google Scholar]
- Riley D., Weintraub H. Conservative segregation of parental histones during replication in the presence of cycloheximide. Proc Natl Acad Sci U S A. 1979 Jan;76(1):328–332. doi: 10.1073/pnas.76.1.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russev G., Hancock R. Formation of hybrid nucleosomes cantaining new and old histones. Nucleic Acids Res. 1981 Aug 25;9(16):4129–4137. doi: 10.1093/nar/9.16.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russev G., Tsanev R. Distribution of histones in alkali-denatured chromatin studied by isopycnic centrifugation in alkaline metrizamide density gradients. Nucleic Acids Res. 1976 Mar;3(3):697–707. doi: 10.1093/nar/3.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russev G., Vassilev L., Tsanev R. Salt-induced structural changes in nucleosomes. Mol Biol Rep. 1980 Mar 31;6(1):45–49. doi: 10.1007/BF00775754. [DOI] [PubMed] [Google Scholar]
- Seale R. L. Studies on the mode of segregation of histone nu bodies during replication in HeLa cells. Cell. 1976 Nov;9(3):423–429. doi: 10.1016/0092-8674(76)90087-8. [DOI] [PubMed] [Google Scholar]
- Seale R. L. Temporal relationships of chromatin protein synthesis, DNA synthesis, and assembly of deoxyribonucleoprotein. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2270–2274. doi: 10.1073/pnas.73.7.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman M. M., Levine A. J., Weintraub H. The asymmetric segregation of parental nucleosomes during chrosome replication. Cell. 1979 Oct;18(2):439–449. doi: 10.1016/0092-8674(79)90063-1. [DOI] [PubMed] [Google Scholar]
- Senshu T., Fukuda M., Ohashi M. Preferential association of newly synthesized H3 and H4 histones with newly replicated DNA. J Biochem. 1978 Oct;84(4):985–988. doi: 10.1093/oxfordjournals.jbchem.a132213. [DOI] [PubMed] [Google Scholar]
- Tsanev R., Sendov B. Possible molecular mechanism for cell differentiation in multicellular organisms. J Theor Biol. 1971 Feb;30(2):337–393. doi: 10.1016/0022-5193(71)90059-2. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Cooperative alignment of nu bodies during chromosome replication in the presence of cycloheximide. Cell. 1976 Nov;9(3):419–422. doi: 10.1016/0092-8674(76)90086-6. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Flint S. J., Leffak I. M., Groudine M., Grainger R. M. The generation and propagation of variegated chromosome structures. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):401–407. doi: 10.1101/sqb.1978.042.01.042. [DOI] [PubMed] [Google Scholar]
- Weintraub H. The assembly of newly replicated DNA into chromatin. Cold Spring Harb Symp Quant Biol. 1974;38:247–256. doi: 10.1101/sqb.1974.038.01.028. [DOI] [PubMed] [Google Scholar]
- Worcel A., Han S., Wong M. L. Assembly of newly replicated chromatin. Cell. 1978 Nov;15(3):969–977. doi: 10.1016/0092-8674(78)90280-5. [DOI] [PubMed] [Google Scholar]



