Abstract
Purpose
The purpose of this study was to determine if the use of antibiotic-impregnated fibrin sealant (AFS) was effective in preventing surgical site infections (SSI) associated with spinal instrumentation.
Methods
In a preliminary study, five pieces of vancomycin-impregnated fibrin sealant, five nuts that were not treated with the sealant, and five nuts that were treated with the sealant were subjected to agar diffusion testing. In a clinical study, the rates of deep SSI were compared between 188 patients who underwent procedures involving spinal instrumentation without AFS (group 1) and 196 patients who underwent procedures involving spinal instrumentation with AFS (group 2).
Results
All five pieces of vancomycin-impregnated fibrin sealant and the five nuts treated with the sealant exhibited antimicrobial efficacy, while the five untreated nuts did not exhibit antimicrobial efficacy in the agar diffusion test. In the clinical study, 11 (5.8 %) of the 188 patients in group 1 acquired a deep SSI, while none (0 %) of the 196 patients in group 2 acquired a deep SSI.
Conclusion
The present study demonstrated that the application of AFS to spinal instrumentation yielded good clinical outcomes in terms of the prevention of postoperative spinal infections. It is hoped that limiting AFS use to patients requiring spinal instrumentation and those with risk factors for SSI will reduce the overall costs while preventing SSIs.
Keywords: Fibrin sealant, Prophylaxis, Spinal instrumentation, Surgical site infection
Introduction
Surgical site infections (SSI), which remain one of the most devastating complications of spinal surgery, result in increased rates of morbidity and mortality, prolonged hospitalizations, long-term antibiotic administration, and reoperations. In addition to the clinical impairments of infected patients, postoperative spinal infections have substantial economic implications for patients, physicians, hospitals, and society as a whole in terms of direct medical costs, resource utilization, and the indirect social costs associated with lost wages and productivity. The costs of care can increase more than fourfold compared with those of uncomplicated cases [1]. Although the development of sterile surgical techniques, the prophylactic intravenous administration of antibiotics, the recognition of predisposing factors, and several advances in anti-infective technology have all contributed to decreasing the infection rates [2, 3], SSIs still exist and remain difficult to treat. Therefore, a more effective means of preventing SSIs is the key to successful spinal surgery.
The prophylactic administration of antibiotics with a drug delivery system that is composed of biodegradable materials is considered an effective means of preventing SSI because a high local concentration of antibiotics can be sustained without systemic complications. Fibrin glue, as well as other biodegradable materials, including high-molecular-weight biodegradable poly(d, l-lactide) cylinders, calcium hydroxyapatite beads, and polyglycolide beads, have been studied as potential local antibiotic delivery systems with successful results [4–7]. Fibrin can exist as a polymer in the body for some time, and it is thereafter absorbed by fibrinolysis. A number of mechanical and biochemical characteristics of fibrin make it an ideal candidate to act as a drug delivery vehicle. First, fibrin is composed of a natural biopolymer and is, therefore, biocompatible, and it has no toxicities or other harmful effects [8]. Second, the local delivery of antibiotics is ensured with fibrin because it is a bioadhesive. Third, because fibrin is a biodegradable material, it does not need to be removed. The aim of this study was to determine if the use of an antibiotic-impregnated fibrin sealant (AFS) could prevent SSIs that are associated with spinal instrumentation.
Materials and methods
Fibrin glue
Fibrin glue was produced from pooled human plasma (Beriplast P Combi-Set; CSL Behring, Tokyo, Japan). This product is composed of Solution A, which contains 80 mg/mL of human fibrinogen, 60 U/mL of human factor 13, and 1,000 KIE/mL of bovine aprotinin, and Solution B, which contains 300 U/mL of human thrombin and 5.88 mg/mL of CaCl2. The human plasma was screened for the hepatitis B virus antigen, the hepatitis C virus antibody, the human immunodeficiency virus-1 antibody, and the human immunodeficiency virus-2 antibody, and it subsequently tested negative for nucleic acids from the human immunodeficiency virus, the hepatitis B virus, the hepatitis C virus, the hepatitis A virus, and parvovirus B19. Virus inactivation by pasteurization was performed at 60 °C for 10 h. Bovine aprotinin was extracted from bovine lung, heated to 70 °C for 1 h, and filtered through a membrane with a pore size of 10 kDa in order to remove prion proteins and viruses.
Preliminary experimental study
A 2.5-mL portion of Solution A and an equal amount of Solution B were sprayed simultaneously onto a 50-cm2 surface of glass using compressed air, and this fibrin glue layer was then sprinkled with 500 mg of vancomycin (Shionogi & Co., Ltd., Tokyo, Japan). The vancomycin-covered glue layer was subsequently covered with an additional fibrin glue layer that was made from 2.5 mL each of Solution A and Solution B using the same spray method. Five pieces of this vancomycin-impregnated fibrin glue sealant that were obtained as test materials were subjected to agar diffusion testing to determine whether the sealant pieces had antimicrobial activity. The test materials also included five nuts that were not treated with the vancomycin-impregnated fibrin glue sealant and five nuts that were treated with this sealant, and these were included to determine the effects of this treatment. These test materials were placed on Mueller–Hinton agar plates (EIKEN CHEMICAL CO., LTD., Tochigi, Japan) and inoculated with a clinical biofilm-forming strain of methicillin-resistant Staphylococcus aureus (MRSA). The materials were incubated aerobically in an incubation chamber at 35 °C for 24 h. In addition, to determine the duration of the antimicrobial activity, five pieces of vancomycin-impregnated fibrin glue sealant were incubated for 4 weeks. During the incubation period, the antibiotic diffused into the bacteria-seeded agar. The zone of bacterial growth inhibition was evident as a clear region surrounding the test material.
Clinical study
Patients
In total, 384 patients, including 264 males and 120 females, who were aged from 7 to 89 years with a mean age of 55.1 years, underwent procedures involving spinal instrumentation that were performed by the same surgical team at Imakiire General Hospital between June 2003 and August 2010. The first 188 surgeries were performed with spinal instrumentation without AFS between June 2003 and May 2007. The last 196 procedures were performed with spinal instrumentation with AFS between June 2007 and August 2010. This study was approved by the Human Subjects Committee of Imakiire General Hospital, and prior informed consent was obtained from the patients who were included in this study. The following definition of a SSI that was determined by the Centers for Disease Control and Prevention was used in this study: a wound infection that occurred within 1 year of hardware implantation or within 30 days after noninstrumented fusions. A deep SSI was defined as an infection involving the deep soft-tissue muscle and fascia, which was in contrast to a superficial SSI involving only infected skin and subcutaneous tissue. The exclusion criterion was patients with a postoperative follow-up time of less than 1 year. The group of patients who underwent procedures involving spinal instrumentation without AFS and the group of patients who underwent procedures involving spinal instrumentation with AFS comprised group 1 and group 2, respectively. In group 1, there were 188 patients, including 122 males and 62 females, with a mean age of 52.0 years (range 7–89 years). The preoperative diagnoses were spinal trauma in 134, degenerative disease in 43, spinal tumors in 4, and infections in 7 of these patients. In group 2, there were 196 patients, including 142 males and 54 females, with a mean age of 57.9 years (range 12–89 years). The preoperative diagnoses were spinal trauma in 144, degenerative disease in 42, spinal tumors in 2, and infection in 8 of these patients. Patient characteristic data that were collected included patient age, gender, body mass index, serum albumin levels, the presence of diabetes, history of smoking, location of the lesion, diagnosis, surgical approach, number of levels instrumented, estimated blood loss, and duration of the operation. There were no statistically significant differences between group 1 and group 2 except for patient age (Table 1).
Table 1.
Summary of patient characteristics
| Group 1 (N = 188) | Group 2 (N = 196) | |
|---|---|---|
| Age (years) | 52.0 ± 19.4a | 57.9 ± 17.8 |
| Gender | ||
| Male | 122 (64.8) | 142 (72.4) |
| Female | 66 (35.1) | 54 (27.5) |
| Body mass index (kg/m2) | 22.3 ± 3.2 | 22.3 ± 3.5 |
| Serum albumin (g/dL) | 3.6 ± 0.6 | 3.7 ± 0.5 |
| Diabetes mellitus | 26 (13.8) | 24 (12.2) |
| Smoking | 70 (37.2) | 69 (35.2) |
| Location | ||
| Cervical | 55 (29.2) | 75 (38.2) |
| Thoracic | 34 (18.0) | 38 (19.3) |
| Lumbar | 99 (52.6) | 83 (42.3) |
| Diagnosis | ||
| Trauma | 134 (71.2) | 144 (73.4) |
| Degenerative disease | 43 (22.8) | 42 (21.4) |
| Spinal tumor | 4 (2.1) | 2 (1.0) |
| Infection | 7 (3.7) | 8 (4.0) |
| Surgical approach | ||
| Anterior | 47 (25.0) | 47 (23.9) |
| Posterior | 126 (67.0) | 141 (71.9) |
| Combined | 15 (7.9) | 8 (4.0) |
| Number of levels instrumented | ||
| 1–2 | 146 (77.6) | 155 (79.0) |
| 3 | 13 (6.9) | 16 (8.1) |
| 4–6 | 28 (14.8) | 25 (12.7) |
| ≥7 | 1 (0.5) | 0 (0.0) |
| Estimated blood loss (g) | 725.5 ± 719.7 | 682.1 ± 791.6 |
| Duration of operation (min) | 228.1 ± 87.7 | 215.4 ± 76.8 |
Data are presented as mean ± standard deviation or number (%). Patient age, body mass index, serum albumin levels, estimated blood loss, and the duration of the operation were compared between group 1 and group 2 with Student’s t-tests, and gender, the presence of diabetes, history of smoking, the location of the lesion, diagnosis, surgical approach and number of levels instrumented were analyzed using a χ2 test
aP value was < 0.05 for the intergroup comparison
Prophylactic intravenous antibiotics were given 30 min before the skin incisions were made and after the spinal surgery on the day of the surgery, and additional intravenous antibiotics were routinely given to all patients for two more days. Synthetic penicillin was administered unless the patient had a history of a significant allergy to penicillin, including skin eruption, toxic liver dysfunction, and anaphylactic shock. The rates of deep SSI were compared between group 1 and group 2 to assess the efficacy of the AFS in spinal instrumentation surgery.
Clinical application of the antibiotic-impregnated fibrin sealant
After the spinal instrumentation procedure, the surgical field was irrigated with sufficient saline and dried with gauze. Approximately 2.5 mL each of Solution A and Solution B were sprayed simultaneously onto the surface of the surgical field using compressed air, and then 500 mg of vancomycin was sprinkled onto this fibrin layer (Fig. 1a). In addition, an additional 2.5 mL each of Solution A and Solution B were sprayed simultaneously over the fibrin layer that was sprinkled with vancomycin, resulting in a vancomycin-impregnated fibrin sealant (Fig. 1b). The AFS was formed mainly on the spinal instruments, and care was taken so that it did not form on the dura.
Fig. 1.
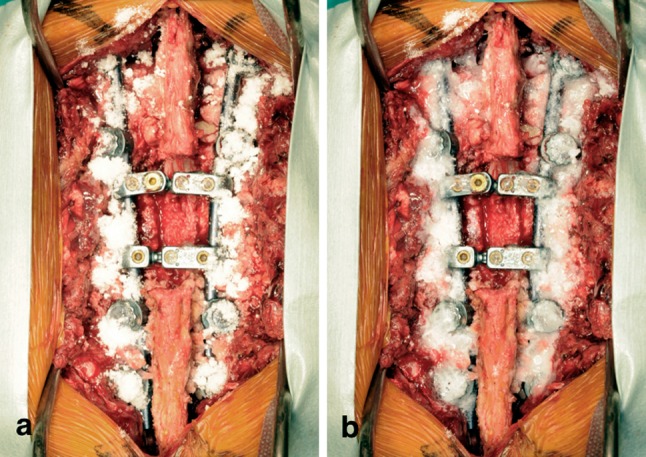
a Photograph showing the surgical field with vancomycin sprinkled onto the fibrin layer. b Photograph showing the surgical field covered with an additional fibrin layer, which resulted in the application of vancomycin-impregnated fibrin sealant
Results
Agar diffusion test
All five pieces of the vancomycin-impregnated fibrin glue sealant and the five nuts that were treated with the sealant exhibited antimicrobial efficacy, which was demonstrated by the presence of an inhibition zone, while the five nuts that were not treated with the sealant did not exhibit antimicrobial efficacy. Furthermore, an inhibition zone was clearly observed at each time point (1, 2, 3, and 4 weeks) after the placement of the five pieces of vancomycin-impregnated fibrin glue sealant onto the Mueller–Hinton agar plates that were inoculated with MRSA, demonstrating the bactericidal activity of AFS through at least 4 weeks. Representative photographs of the findings of this preliminary experimental study are shown in Figs. 2 and 3.
Fig. 2.
a Photograph showing the presence of an inhibition zone around a piece of vancomycin-impregnated fibrin glue sealant. b Photograph showing the absence of an inhibition zone around a nut that was not treated with vancomycin-impregnated fibrin glue sealant. c Photograph showing the presence of a clear inhibition zone around a nut that was treated with vancomycin-impregnated fibrin glue sealant
Fig. 3.
Photographs showing the presence of clear inhibition zones around a representative sample at a 1 week, b 2 weeks, c 3 weeks, and d 4 weeks after the placement of a piece of vancomycin-impregnated fibrin glue sealant onto a Mueller–Hinton agar plate that was inoculated with methicillin-resistant Staphylococcus aureus (MRSA)
Clinical study
Of the 188 patients who underwent procedures with spinal instrumentation without AFS (group 1), 11 (5.8 %) acquired a deep SSI compared with none (0 %) of the 196 patients who underwent procedures with spinal instrumentation with AFS (group 2). This difference was statistically significant (P = 0.0003, Fisher’s exact test). There were seven males and four females in the infected group with a mean age of 55.1 years (range 7–89 years). The average body mass index was 22.7 kg/m2, and the average serum albumin level was 3.7 g/dL. The mean duration of the operation in those that became infected was 284.9 min, and the mean estimated blood loss was 861.3 g. The following pathogens were identified in the 11 patients with deep SSIs in group 1: S. aureus in one patient, methicillin-resistant Staphylococcus epidermidis in one patient, and MRSA in nine patients (81.8 %) (Table 2). We investigated the incidence of deep SSIs in patients with important risk factors for SSI in group 1. Deep SSI was observed in six patients (23.0 %) with diabetes mellitus, eight patients (8.0 %) who underwent lumbar surgeries, six patients (4.4 %) with a traumatized spine, nine patients (7.1 %) who underwent surgeries using a posterior approach, three patients (7.6 %) with an estimated blood loss over 1,000 g, and 6 patients (20.0 %) with operation durations that were greater than 5 h. The incidence of deep SSI was significantly lower in the patients of group 2 with the risk factors of diabetes mellitus, lumbar surgery, traumatized spine, posterior approach, and operation duration of more than 5 h compared with the patients in group 1 with each of the same risk factors (Table 3). No complications associated with the use of the AFS were observed in this study.
Table 2.
Summary of patients who developed deep surgical site infections after spinal instrumentation
| Case | Age/Gender | Diagnosis | Surgical approach | Instrumentation levels | Diabetes | Smoking | Culture |
|---|---|---|---|---|---|---|---|
| 1 | 12/M | Atlantoaxial dislocation | Posterior | O–C2 | − | − | MRSA |
| 2 | 66/M | L1 burst fracture | Posterior | T11–L3 | + | − | SA |
| 3 | 67/M | L3 burst fracture | Anterior | L2–L4 | + | − | MRSA |
| 4 | 57/F | L3 spondylolisthesis | Posterior | L3–L5 | + | + | MRSA |
| 5 | 44/F | L2 fracture dislocation | Posterior | T12–L4 | − | + | MRSA |
| 6 | 26/M | L1 fracture dislocation | Posterior | T11–L4 | − | − | MRSA |
| 7 | 73/F | Tuberculous spondylitis | Posterior | T11–L1 | − | − | MRSA |
| 8 | 80/F | Metastatic spinal tumor | Posterior | T2–T5, T12–L3 | − | − | MRSE |
| 9 | 56/M | L1 fracture dislocation | Posterior | T12–L2 | + | + | MRSA |
| 10 | 66/M | T8 burst fracture | Anterior | T7–T9 | + | − | MRSA |
| 11 | 59/M | L4 spondylolisthesis | Posterior | L4–S1 | + | − | MRSA |
Eight patients (Cases 1, 2, 3, 4, 5, 6, 9, and 11) required removal of spinal instrument as definitive treatment for infection
MRSA Methicillin-resistant Staphylococcus aureus, SAStaphylococcus aureus, MRSE Methicillin-resistant Staphylococcus epidermidis
Table 3.
Rates of deep surgical site infection in patients with risk factors
| Factor | Group 1 | Group 2 | P Value |
|---|---|---|---|
| Total cohort | 11/188 (5.8 %) | 0/196 (0 %) | 0.0003b |
| Diabetes mellitus | 6/26 (23.0 %) | 0/24 (0 %) | 0.023a |
| Lumbar surgery | 8/99 (8.0 %) | 0/83 (0 %) | 0.0082b |
| Trauma | 6/134 (4.4 %) | 0/144 (0 %) | 0.0118a |
| Posterior approach | 9/126 (7.1 %) | 0/141 (0 %) | 0.001b |
| Estimated blood loss over 1,000 g | 3/39 (7.6 %) | 0/39 (0 %) | 0.2403 |
| Duration of operation over 5 h | 6/30 (20.0 %) | 0/23 (0 %) | 0.0303a |
A Fisher’s exact test was used to detect differences in the infection rates between the 2 group
aP < 0.05 and b P < 0.01 for the intergroup comparison
Discussion
The bacteria embedded in biofilms have been shown to develop and colonize on inert surfaces of many medical devices, including orthopedic implants. Large numbers of slime-encased bacteria have been observed by electron microscopy on the surfaces of medical devices that have been the foci of device-related infection [9]. The bacteria within biofilms are protected against host defense mechanisms and antibiotic therapy because activated phagocytes cannot kill bacteria in biofilms, and antibodies that are produced because of stimulation by antigens that are released from sessile bacterial cells and antibiotics fail to penetrate biofilms [10]. Moreover, although phagocytosis cannot be achieved, phagocytic enzymes, which are released by phagocytes, may damage the tissue around biofilms [10]. Therefore, implants must often be removed before the infection can resolve. The use of materials or coatings that release antibiotics in concentrations that kill planktonic bacterial cells around the implant is considered one of the most reasonable strategies for the prevention of biofilm formation.
Besides systemic antibiotic prophylaxis, local antibiotic delivery has also been performed with success [11–13]. The advantage of the use of locally applied antibiotics is that it allows for the treatment of local environments without vascular supplies, such as those involving devitalized tissue, dead space, or pooled hematomas, which intravenous antibiotics cannot reach to sterilize, and high-sustained concentrations of antibiotics are delivered directly to the targeted site without systemic side effects, such as impairments of renal function. Although the use of antibiotic-loaded bone cement has been established with several techniques for the prophylaxis of prosthetic joint infection, no optimal drug delivery system for prophylactic antibiotics for spinal instrumentation has previously been reported. Several drawbacks that exist with the use of polymethylmethacrylate as an antibiotic delivery carrier in the treatment of orthopedic infection, including the requirement of a second surgery for removal, have led to a search for biodegradable carriers. Fibrin glue has been studied as well by several authors as a biodegradable material for locally applied antibiotics in the treatment or prophylaxis of infections [7, 14, 15]. Fibrin, which is a natural biopolymer, has several unique characteristics, including bioadhesiveness, biocompatibility, lack of toxicity, and resorption, making it an ideal candidate to act as a drug delivery carrier.
The release of antibiotics is controlled by the dissolution of the fibrin sealant by fibrinolysis and diffusion through the fibrin matrix. Most experimental studies have revealed a high output of antibiotics within the first several days from the fibrin-antibiotic mixtures, and this relatively short duration of the release of large amounts of antibiotic mainly resulted from rapid diffusion [16–19]. Local hematomas are well known as an excellent culture media for harboring bacteria. In our opinion, we suggest that an antibiotic-impregnated hematoma may be made by the release of antibiotics from the AFS, which has been attributed to the prevention of SSI in this study, even if the hematoma persisted in spite of the drainage.
The chosen prophylactic antibiotic should be directed against gram-positive bacteria and at S. aureus and S. epidermidis, which are the pathogens most frequently involved in spinal infections in particular [20–22]. A cephalosporin or synthetic penicillin is commonly recommended in orthopedic surgeries, including spinal surgeries. However, because methicillin-resistant strains of S. aureus and S. epidermidis are increasingly being reported as pathogens in prosthetic surgery, the use of aminoglycosides, such as vancomycin, has been suggested [23], particularly in institutions in which these methicillin-resistant strains are often detected. Vancomycin also has a broad spectrum of protection against gram-positive bacteria, particularly staphylococci, and it was, therefore, selected as the prophylactic antibiotic for impregnation in the fibrin sealant in the present study. Although the gradual development of vancomycin-resistant strains as a result of wide, prolonged, or high-dose administration is a concern, the prudent use of vancomycin may protect against the emergence of vancomycin-resistant strains, such as vancomycin-resistant enterococci. Therefore, prophylaxis using vancomycin should be used only in surgeries with a high risk of infection, such as prosthetic implantations or those involving spinal instrumentation that are performed in institutions with high rates of infection due to methicillin-resistant strains.
In conclusion, to the best of our knowledge, there have been no previous reports of the use of AFS for the prevention of SSI by spinal instrumentation. The present study demonstrated that the use of AFS in spinal instrumentation yielded good clinical outcomes in terms of preventing postoperative spinal infections. It is hoped that limiting AFS use to patients requiring spinal instrumentation and patients having risk factors for SSI will reduce the overall costs, while also preventing a SSI.
Conflict of interest
None.
References
- 1.Calderone RR, Garland DE, Capen DA, Oster H. Cost of medical care for postoperative spinal infections. Orthop Clin North Am. 1996;27:171–182. [PubMed] [Google Scholar]
- 2.Schimmel JJ, Horsting PP, Kleuver M, Wonders G, Limbeek J. Risk factors for deep surgical site infections after spinal fusion. Eur Spine J. 2010;19:1711–1719. doi: 10.1007/s00586-010-1421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Núñez-Pereira S, Pellisé F, Rodríguez-Pardo D, Pigrau C, Sánchez JM, Bagó J, Villanueva C, Cáceres E. Individualized antibiotic prophylaxis reduces surgical site infections by gram-negative bacteria in instrumented spinal surgery. Eur Spine J. 2011;20(Suppl 3):397–402. doi: 10.1007/s00586-011-1906-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garvin KL, Miyano JA, Robinson D, Giger D, Novak J, Radio S. Polylactide/polyglycolide antibiotic implants in the treatment of osteomyelitis. A canine model. J Bone Joint Surg Am. 1994;76:1500–1506. doi: 10.2106/00004623-199410000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Shinto Y, Uchida A, Korkusuz F, Araki N, Ono K. Calcium hydroxyapatite ceramic used as a delivery system for antibiotics. J Bone Joint Surg Br. 1992;74:600–604. doi: 10.1302/0301-620X.74B4.1320622. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Wyss UP, Pichora D, Goosen MF. Biodegradable controlled antibiotic release devices for osteomyelitis: optimization of release properties. J Pharm Pharmacol. 1994;46:718–724. doi: 10.1111/j.2042-7158.1994.tb03890.x. [DOI] [PubMed] [Google Scholar]
- 7.Mader JT, Stevens CM, Stevens JH, Ruble R, Lathrop JT, Calhoun JH. Treatment of experimental osteomyelitis with a fibrin sealant antibiotic implant. Clin Orthop Relat Res. 2002;403:58–72. doi: 10.1097/00003086-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Wood AP, Harner SG. The effect of fibrin tissue adhesive on the middle and inner ears of chinchillas. Otolaryngol Head Neck Surg. 1988;98:104–110. doi: 10.1177/019459988809800202. [DOI] [PubMed] [Google Scholar]
- 9.Khoury AE, Lam K, Ellis B, Costerton JW. Prevention and control of bacterial infections associated with medical devices. ASAIO J. 1992;38:M174–M178. doi: 10.1097/00002480-199207000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 11.Bengtson S, Borgquist L, Lidgren L. Cost analysis of prophylaxis with antibiotics to prevent infected knee arthroplasty. BMJ. 1989;299:719–720. doi: 10.1136/bmj.299.6701.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackburn WD, Jr, Alarcón GS. Prosthetic joint infections. A role for prophylaxis. Arthritis Rheum. 1991;34:110–117. doi: 10.1002/art.1780340118. [DOI] [PubMed] [Google Scholar]
- 13.Trippel SB. Antibiotic-impregnated cement in total joint arthroplasty. J Bone Joint Surg Am. 1986;68:1297–1302. [PubMed] [Google Scholar]
- 14.Woolverton CJ, Fulton JA, Salstrom SJ, Hayslip J, Haller NA, Wildroudt ML, MacPhee M. Tetracycline delivery from fibrin controls peritoneal infection without measurable systemic antibiotic. J Antimicrob Chemother. 2001;48:861–867. doi: 10.1093/jac/48.6.861. [DOI] [PubMed] [Google Scholar]
- 15.Nishimoto K, Yamamura K, Fukase F, Kobayashi M, Nishikimi N, Komori K. Subcutaneous tissue release of amikacin from a fibrin glue/polyurethane graft. J Infect Chemother. 2004;10:101–104. doi: 10.1007/s10156-004-0304-8. [DOI] [PubMed] [Google Scholar]
- 16.Itokazu M, Yamamoto K, Yang WY, Aoki T, Kato N, Watanabe K. The sustained release of antibiotic from freeze-dried fibrin-antibiotic compound and efficacies in a rat model of osteomyelitis. Infection. 1997;25:359–363. doi: 10.1007/BF01740818. [DOI] [PubMed] [Google Scholar]
- 17.Osada T, Yamamura K, Yano K, Fujimoto K, Mizuno K, Sakurai T, Nabeshima T. Distribution and serum concentration of sisomicin released from fibrin glue-sealed dacron graft in the rat and human. J Biomed Mater Res. 2000;52:53–57. doi: 10.1002/1097-4636(200010)52:1<53::AID-JBM7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 18.Tanemoto K, Fujinami H. Experimental study on bacterial colonization of fibrin glue and its prevention. Clin Ther. 1994;16:1016–1027. [PubMed] [Google Scholar]
- 19.Tsourvakas S, Hatzigrigoris P, Tsibinos A, Kanellakopoulou K, Giamarellou H, Dounis E. Pharmacokinetic study of fibrin clot-ciprofloxacin complex: an in vitro and in vivo experimental investigation. Arch Orthop Trauma Surg. 1995;114:295–297. doi: 10.1007/BF00452091. [DOI] [PubMed] [Google Scholar]
- 20.Beiner JM, Grauer J, Kwon BK, Vaccaro AR. Postoperative wound infections of the spine. Neurosurg Focus. 2003;15:E14. doi: 10.3171/foc.2003.15.3.14. [DOI] [PubMed] [Google Scholar]
- 21.Perry JW, Montgomerie JZ, Swank S, Gilmore DS, Maeder K. Wound infections following spinal fusion with posterior segmental spinal instrumentation. Clin Infect Dis. 1997;24:558–561. doi: 10.1093/clind/24.4.558. [DOI] [PubMed] [Google Scholar]
- 22.Roberts FJ, Walsh A, Wing P, Dvorak M, Schweigel J. The influence of surveillance methods on surgical wound infection rates in a tertiary care spinal surgery service. Spine. 1998;23:366–370. doi: 10.1097/00007632-199802010-00016. [DOI] [PubMed] [Google Scholar]
- 23.Gorbach SL, Condon RE, Conte JE, Jr, Kaiser AB, Ledger WJ, Nichols RL. Evaluation of new anti-infective drugs for surgical prophylaxis. Infectious diseases society of America and the food and drug administration. Clin Infect Dis. 1992;15:S313–S338. doi: 10.1093/clind/15.Supplement_1.S313. [DOI] [PubMed] [Google Scholar]




