Abstract
Much research shows early life manipulations have enduring behavioral, neural, and hormonal effects. However, findings of learning and memory performance vary widely across studies. We reviewed studies in which pre-weaning rat pups were exposed to stressors and tested on learning and memory tasks in adulthood. Tasks were classified as aversive conditioning, inhibitory learning, or spatial/relational memory. Variables of duration, type, and timing of neonatal manipulation and sex and strain of animals were examined to determine if any predict enhanced or impaired performance. Brief separations enhanced and prolonged separations impaired performance on spatial/relational tasks. Performance was impaired in aversive conditioning and enhanced in inhibitory learning tasks regardless of manipulation duration. Opposing effects on performance for spatial/relational memory also depended upon timing of manipulation. Enhanced performance was likely if the manipulation occurred during postnatal week 3 but performance was impaired if it was confined to the first two postnatal weeks. Thus, the relationship between early life experiences and adulthood learning and memory performance is multifaceted and decidedly task-dependent.
Keywords: early life stress, neonatal isolation, maternal separation, neonatal handling, aversive conditioning, spatial memory, hippocampus, development
1. Introduction
Mental health disorders can be extremely debilitating to the individual and their family and inflict great costs on society. In the United States, the prevalence of having any disorder in a 12-month period is over 26% with 7% of the population classified as having more than one major disorder (Kessler et al., 2005). Prevention, an effective way to reduce occurrences of the problems, can be enhanced by identifying risk factors. While genetic factors are important, the environmental factor of early life trauma increases susceptibility to depression (Heim and Nemeroff, 2001), post-traumatic stress disorder (PTSD) (Yehuda et al., 2001), schizophrenia (Howes et al., 2004), and addiction (Gordon, 2002). Stress affects neural and hormonal systems that contribute to emotional and cognitive processes associated with mental disorders. Further, acute stress in adulthood can precipitate or exacerbate symptoms of mental disorders. The variability in an individual’s response to stress during adulthood may relate to differences in early life experiences that helped shaped the neural and hormonal responses to stress. A better understanding of the long-term consequences of early life stress could improve prevention strategies for mental disorders, particularly for those that are affected by stress.
Animal models provide an essential tool to understand the mechanisms by which the enduring effects of early life stress become manifest. Research conducted with animals allows control over the environmental manipulation of early life stress. This provides the ability to examine specific parameters of the manipulation, such as the postnatal timing or duration of the stress, in order to determine the critical factors that contribute to the enduring effects of the stress. Animal studies have reduced variability because consistency over factors such as housing or litter size can be maintained and genetic factors controlled by employing rats of a specific strain. Finally, research with animals allows assessing hormonal or neural changes such as neurogenesis or effects on protein levels in specific brain regions. Nonetheless, animal models must be evaluated to ensure their validity because they are only useful if they produce effects comparable to those seen in humans.
The overall purpose of this review is to synthesize results from the literature on early life manipulations in rats in order to determine how it affects learning and memory performance in adulthood. We focus on learning and memory because of the many discrepancies in this literature. There is a need to understand how early life stress alters these processes because it will shed light on early trauma as a risk factor for the many mental disorders that associate with altered learning and memory processing. While other reviews have been written on early life stress, e.g., (Brunson et al., 2003; Catalani et al., 2011; Francis et al., 1999a; Kaufman et al., 2000; Kehoe and Shoemaker, 2001; Lehmann and Feldon, 2000; Levine, 2001; Macri and Wurbel, 2006; Meaney et al., 1996), none have had this focus. Thus, this is a unique perspective to this literature.
Early life manipulations have been reported to enhance, impair, or have no effect on learning or memory performance in the adult. These inconsistencies may reflect procedural variations in early life stress models or differences in assessments of learning and memory. There is a need to find the commonalties and differences within this literature in order to identify parameters that can explain and predict outcomes from studies and enhance the validity of the animal models utilized. Some a priori questions examined in this review include whether brief vs. prolonged manipulations have opposing effects on learning and memory as suggested for effects on stress hormone systems (Francis et al., 1999a). This assumption may not hold for learning and memory tasks (Brunson et al., 2003). In addition to duration, we hypothesize that timing of the manipulation is important in whether learning or memory is altered. Other factors that may play a role in the effects of early life manipulations on performance in learning and memory tasks include sex and strain of the rat. Finally, we deemed that the type of task was important in determining the outcome of the study.
In this paper, we first present our approach to constructing the review. Second, we describe the methods of collecting and synthesizing information from the literature. Third, we briefly describe rat development including maternal effects and changes in stress hormone systems. Next, we explain the various early life manipulations used in publications discussed herein. Then, we summarize results reported in the literature on the effects of early life manipulations on learning and memory tasks presented by task type category and discuss unconditioned effects that may have affected outcomes of these studies. Subsequently, we discuss enduring effects of early life manipulations on the stress hormone and central nervous systems of adult rats. Finally, we present a model to help synthesize results reported upon and provide a heuristic to test predictions in future studies.
2. Approach
We gathered as thorough a collection of published papers as possible and then probed for patterns in the results. A literature search (Medline) of studies published up to January 2012 was performed by combining the key phrases of “early life” or “postnatal stress”, “maternal separation or deprivation”, “neonatal or postnatal handling” or “isolation”. These results were crossed with the keywords of “learning, memory, or conditioning” and then crossed with “rat”. Only studies in which the full article was available in English were included. We also combed these articles for other references that failed to be included in the search results. If these studies met the inclusion criteria (see below), they were included.
The following factors were the inclusion and exclusion criteria for articles. The first premise was that the manipulation was presumed to reflect stress. Second, only studies that utilized postnatal manipulations performed during the preweaning period were included. Studies employing prenatal or post-weaning manipulations were not examined unless combined with such a condition. Note that a prior paper compared effects of postweaning isolation (isolation rearing) to preweaning manipulations (Hall, 1998). We also excluded studies of psychoactive drug or toxin administrations in order to concentrate on general stressors. Thus, most experimental manipulations were considered neonatal handling, maternal separation, or neonatal isolation. We also included studies of foot shock, stress hormone exposure, artificial rearing (complete absence of maternal and littermate contacts), and limited nesting.
Second, with the purpose of having enough results to make reasonable contrasts and comparisons, we focused on aversive classical/instrumental conditioning, spatial/relational memory, and latent inhibition tasks. Studies of appetitive learning were limited and mostly focused on psychoactive drug effects (e.g., drug self-administration). This topic was discussed in other reviews (Gordon, 2002; Kosten and Kehoe, 2007; Moffett et al., 2007). However, appetitive memory tasks, such as the radial arm maze, were included because of its similarity to other spatial tasks, such as Morris water maze. We did not include studies of non-associative tasks (habituation; sensitization) because they are generally forebrain-independent. Finally, we limited our assessment to studies that employed adult (>60-days) rats. Very few mouse studies using early life manipulations exist and we felt it best to not have species as another variable. Finally, too few studies conducted with infant or juvenile rats exist so these were not included although studies with aged rats (>9-mos) were.
3. Methods
We gathered results from the papers and tabulated them in the following way. Significant or trends towards significant findings in specific tasks were categorized as an impairment or an enhancement, or otherwise considered a non-significant effect. Each experiment within a paper was a separate study or case. If rats of both sexes or both adult and aged rats were assessed, these were separate cases. To determine factors that contribute to enduring effects of early life manipulations on learning and memory, we performed discriminant function analysis, a classification method in which values on one or more independent variables or factors are used to predict categorization or group membership in another variable (Cohen and Cohen, 1983)1. Factors examined are defined in Section 4 and include sex, strain, duration (brief vs. prolonged), type (isolation vs. separation), test age (adult vs. aged), and timing (during post-natal week 3 vs. not2). All factors, except strain, were dichotomous and used to predict whether enhancements or impairments in performance in the tasks (e.g., categorization) were seen. Finally, as described below, the task employed was assigned to one of three categories (aversive conditioning, inhibitory learning, or spatial/relational memory) and separate analyses by task category performed.
Results from 231 studies in 77 publications are summarized in Tables 1–3. For ease of presentation, rats of both sexes per paper are combined with a notation made if there were sex-dependent effects. Studies from the same paper that tested adult and aged rats or imposed different early life manipulations are shown on separate lines.
Table 1.
Summary of results of studies on early life stress and aversive conditioning in the adult
| Procedure | Early Life Manipulation | Strain | Sex | Age | Result | Reference |
|---|---|---|---|---|---|---|
| Context-CS freezing (CxtF) | Handling (15-min; PN1-21) | Wistar | male? | adult | Impaired | Meerlo et al. 1999 |
| Handling (3-min; PN1-10) | Wister | male? | adult | impaired (context + cue) | Madruga et al 2006 | |
| Handling (15-min; PN2-22) | Wistar | Both? | adult | impaired memory/enhanced extinction | Guijarro et al 2007 | |
| Handling (15-min; PN2-14) | Lister | female | adult | n.s. | Stevenson et al 2009 | |
| Handling (15-min; PN2-14) | Long-E | male | adult | impaired extinction | Wilber et al 2009 | |
| Neonatal isolation (15-min; PN1-21) | Wistar | both | adult | n.s. | Pryce et al 2003 | |
| Neonatal isolation (15-min; PN1-21) | SD | both | adult | impaired in both sexes (USV) | Kosten et al 2006 | |
| Mat. Sep. (24-hr; PN3) | Wistar | both | adult | enhanced | Oomen et al 2010 | |
| Mat. Sep. (24-hr; PN3) | Wistar | female | adult | n.s. | Oomen et al 2011 | |
| Mat. Sep. (24-hr; PN4) | Wistar | both | adult | n.s. | Lehmann et al 1999 | |
| Mat. Sep. (24-hr; PN9) | Wistar | both | adult | trend impaired | Lehmann et al 1999 | |
| Mat. Sep. (24-hr; PN18) | Wistar | both | adult | trend impaired | Lehmann et al 1999 | |
| Mat. Sep. (3-hr; PN1-10) | Wister | male? | adult | n.s. (context + cue) | Madruga et al 2006 | |
| Mat. Sep. (3-hr; PN2-22) | Wistar | both? | adult | impaired memory or enhanced extinction | Guijarro et al 2007 | |
| Mat. Sep. (6-hr; PN2-14) | Lister | female | adult | n.s. | Stevenson et al 2009 | |
| Neonatal isolation (4-hr; PN1-21) | Wistar | both | adult | n.s. | Pryce et al 2003 | |
| Neonatal isolation (1-hr/day; PN2-9) | SD | both | adult | impair males; enhance female (USV) | Kosten et al 2005 | |
| Neonatal isolation (1-hr/day; PN2-9) | SD | male | adult | Enhance stress-induced fear | Imanaka et al. 2006 | |
| Neonatal isolation (3-hr; PN1-21) | SD | both | adult | impair females (USV) | Kosten et al 2006 | |
|
| ||||||
| Cue-CS freezing (CueF) | Handling (15-min; PN2-22) | Wistar | both? | adult | n.s. | Guijarro et al 2007 |
| Handling (15-min; PN2-14) | Lister | female | adult | enhanced extinction learning | Stevenson et al 2009 | |
| Handling (15-min; PN2-14) | Long-E | male | adult | n.s. | Wilber et al 2009 | |
| Neonatal isolation (15-min; PN1-21) | Wistar | both | adult | impair males (stress hormone) | Pryce et al 2003 | |
| Neonatal isolation (15-min; PN1-21) | SD | both | adult | impaired in both sexes (USV measure) | Kosten et al 2006 | |
| Mat. Sep. (24-hr; PN3) | Wistar | both | adult | enhanced | Oomen et al 2010 | |
| Mat. Sep. (24-hr; PN3) | Wistar | female | adult | enhanced | Oomen et al 2011 | |
| Mat. Sep. (24-hr; PN4) | Wistar | both | adult | n.s. | Lehmann et al 1999 | |
| Mat. Sep. (24-hr; PN9) | Wistar | both | adult | n.s. | Lehmann et al 1999 | |
| Mat. Sep. (24-hr; PN18) | Wistar | both | adult | n.s. | Lehmann et al 1999 | |
| Mat. Sep. (3-hr; PN2-22) | Wistar | both? | adult | n.s. | Guijarro et al 2007 | |
| Mat. Sep. (6-hr; PN2-14) | Lister | female | adult | impaired acquisition and expression | Stevenson et al 2009 | |
| Neonatal isolation (4-hr; PN1-21) | Wistar | both | adult | impair females (stress hormone) | Pryce et al 2003 | |
| Neonatal isolation (1-hr/day; PN2-9) | SD | both | adult | n.s. | Kosten et al 2005 | |
| Neonatal isolation (3-hr; PN1-21) | SD | both | adult | n.s. | Kosten et al 2006 | |
|
| ||||||
| Inhibitory avoidance (IA) | Neonatal isolation (3-min; PN1-21) | SD | both | adult | impaired 24-hr both sexes | Ader 1973 |
| Foot shock (3-min; PN1-21) | SD | both | adult | impaired 24-hr both sexes | Ader 1973 | |
| Neonatal isolation (15-min; PN1-21) | SD | both | adult | impaired 48-hr both sexes; 1-wk females | Kosten et al 2007a | |
| Neonatal isolation (1-hr/day; PN2-9) | SD | both | adult | impaired 48-hr in both sexes | Kosten et al 2007b | |
|
| ||||||
| Fear-potentiated startle (FPS) | Mat Sep (3-hr; PN2-14) | Wistar | both | adult | n.s. | Jongh et al 2005 |
|
| ||||||
| Eyeblink conditioning (EBC) | Handling (15-min; PN2-14) | Long-E | both | adult | impaired males | Wilber et al. 2007 |
| Mat. Sep. (15-min; PN2-14) | Long-E | both | adult | impaired males | Wilber et al. 2007 | |
| Mat. Sep. (1-hr; PN2-14) | Long-E | both | adult | impaired males | Wilber et al. 2007 | |
| Mat. Sep. (1-hr; PN2-14) | Long-E | male | adult | impaired males | Wilber & Wellman 2009 | |
|
| ||||||
| Conditioned taste aversion (CTA) | Neonatal isolation (3-min; PN1-21) | SD | both | adult | n.s. (cyclophosphamide) | Ader 1973 |
| Foot shock (3-min; PN1-21) | SD | both | adult | n.s. (cyclophosphamide) | Ader 1973 | |
| Handling (15-min; PN2-14) | SD | both | adult | n.s. (amphetamine) | Roma et al 2008 | |
| Mat. Sep. (3-hr; PN2-14) | SD | both | adult | n.s. (amphetamine) | Roma et al 2008 | |
Table 3.
Summary of results of studies on early life stress and spatial/relational memory in the adult
| Procedure | Early Life Manipulation | Strain | Sex | Age | Result | Reference |
|---|---|---|---|---|---|---|
| Morris Water Maze (MWM) | Handling (15-min; PN1-22) | Long-E | male | aged | decreased aging impairments | Meaney et al. 1988 |
| Handling (10-min * 2/day; PN1-21) | Roman | both? | aged | enhanced RHA (path length) | Escorihuela et al 1995 | |
| Handling (10-min; PN1-22) | SD | male | adult | enhance w/stress | Pham et al. 1997 | |
| Handling (15-min; PN1-21) | SD | male | adult | n.s. | Vallee et al. 1997 | |
| Handling (15-min; PN1-21) | SD | male | adult | n.s. | Vallee et al. 1999 | |
| Handling (15-min; PN1-21) | SD | male | aged | n.s. | Vallee et al. 1999 | |
| Handling + stroking (3-min; PN1-21) | Long-E | both | adult | enhanced (latency; improvement score) | Tang et al 2001 | |
| Handling (10-min; PN1-10) | Wistar | both | adult | Impaired females | Noschang et al 2010 | |
| Handling. (15-min; PN2-14) | Long-E | male | adult | n.s. | Hout et al 2002 | |
| Handling (15-min; PN1-22) | Wistar | male | aged | enhanced (latency) | Lehmann et al. 2002 | |
| Handling (15-min; PN2-9) | SD | male | adult | enhanced (latency) | Fenoglio et al 2005 | |
| Handling (15-min*3/day; PN4-21) | Long-E | both | adult | n.s. | Gibb & Kolb 2005 | |
| Handling (15-min; PN1-21) | Wistar | male | adult | enhanced w/stress | Garoflos et al 2005 | |
| Handling (15-min; PN1-22) | Wistar | both | adult | enhanced w/stress males | Stamatakis et al 2008 | |
| Neonatal isolation (3-min; PN1-PN21) | Roman | both? | aged | enhanced | Fernandez-Teruel et al 1997 | |
| Neonatal isolation (15-min; PN1-21) | Wistar | both | adult | n.s. | Pryce et al 2003 | |
| Novel environment (3-min; PN1-21) | Long-E | male | 7-mon | enhanced | Tang et al 2006 | |
| Mat. Sep. (24-hr; PN3) | Wistar | both | adult | impaired | Oomen et al 2010 | |
| Mat. Sep. (24-hr; PN3) | Wistar | female | adult | n.s. | Oomen et al 2011 | |
| Mat. Sep. (24-hr; PN4) | Wistar | both | adult | enhanced reversal learning | Lehmann et al 1999 | |
| Mat. Sep. (24-hr; PN9) | Wistar | both | adult | enhanced reversal learning | Lehmann et al 1999 | |
| Mat. Sep. (24-hr; PN18) | Wistar | both | adult | enhanced reversal learning | Lehmann et al 1999 | |
| Mat. Sep. (24-hr; PN3) | Brn Norw | male | adult | impaired (latency, distance acq, rev) | Oitzel et al. 2000 | |
| Mat. Sep. (24-hr; PN3) | Brn Norw | male | aged | n.s. | Oitzel et al. 2000 | |
| Mat. Sep. (3-hr; PN2-14) | Long-E | male | adult | impaired (latency; distance) | Hout et al 2002 | |
| Mat. Sep. (3-hr; PN2-21) | Wistar | male | adult | Impaired | Aisa et al 2009 | |
| Mat. Sep. (3-hr; PN2-21) | Wistar | male | adult | impaired | Aisa et al 2009 | |
| Mat. Sep. (3-hr; PN2-21) | Wistar | male | adult | impaired | Solas et al 2010 | |
| Mat. Sep. (3-hr; PN2-21) | Wistar | male | aged | impaired | Solas et al 2010 | |
| Mat. Sep. (6-hr; PN12, 14, 16,&18) | Wistar | male | aged | trend enhance (latency) | Lehmann et al. 2002 | |
| Mat. Sep (12-hr; PN9 and PN11) | Wistar | male | adult | impaired (path length; latency) | Garner et al 2007 | |
| Mat. Sep. (3-hr; PN2-14) | SD | both | adult | Impaired (quadrant time) | Hui et al 2011 | |
| Mat. Sep. (3-hr; PN2-21) | Wistar | both? | adult | impaired retention; n.s. acq | Aisa et al 2007 | |
| Mat. Sep. (24-hr; PN9) | Wistar | male | adult | n.s. | Choy et al 2008 | |
| Neonatal isolation (6-hr/day; PN15-21) | Long-E | both | adult | enhanced | Frisone et al 2002 | |
| Neonatal isolation (1-hr/day; PN2-9) | SD | male | adult | impaired (latency) | Huang et al. 2002 | |
| Neonatal isolation (4-hr; PN1-21) | Wistar | both | adult | enhanced (distance) | Pryce et al 2003 | |
| Neonatal isolation (1-hr/day; PN2-9) | SD | male | adult | n.s. | Lai et al 2006 | |
| Limited nesting (PN2-PN9) | SD | male | adult | trend impairment | Brunson et al 2005 | |
| Limited nesting (PN2-PN9) | SD | male | aged | impaired | Brunson et al 2005 | |
| Limited nesting (PN2-PN9) | SD | male | aged | impaired (quadrant time; latency) | Ivy et al 2010 | |
| CRH (PN10) | SD | male | adult | trend impairment | Brunson et al 2001 | |
| CRH (PN10) | SD | male | adult | impaired | Brunson et al 2001 | |
| CRH (PN10) | SD | male | aged | impaired | Brunson et al 2001 | |
| CRH antagonist (PN10-17) | SD | male | adult | enhanced (latency) | Fenoglio et al 2005 | |
| CRH antagonist (PN10-17) | SD | male | aged | n.s. (quadrant time; latency) | Ivy et al 2010 | |
| Cort (PN1-21) | Wistar | male | adult | enhanced | Catalani et al 1993 | |
| Cort (PN1-21) | Wistar | female | adult | enhanced | Catalani et al 2002 | |
| Artificial rearing | SD | female | adult | some enhancements in retention | Levy et al 2003 | |
|
| ||||||
| Radial arm maze (RAM) | Handling (PN1-21; 15-min) | SD | male | aged | decrease aging impair(<work mem err) | Vallee et al. 1999 |
| Neonatal isolation (6-hr; PN15-21) | Long-E | both | adult | >working errors in males; n.s. females | Sandstrom 2005 | |
| Neonatal isolation (6-hr; PN15-21) | Long-E | males | adult | > working errors; n.s. ref memory | Sandstrom & Hart 2005 | |
| CORT (PN1-PN12) | Wistar | both? | adult | initial better working memory | Roskoden et al 2005a | |
| Artificial rearing | SD | female | adult | decrease latency during initial sessions | Levy et al 2003 | |
|
| ||||||
| Circular maze (CM) | Neonatal isolation (15-min; PN1-21) | SD | both | adult | n.s. | Kosten et al 2007a |
|
| ||||||
| Can test (CT) | Neonatal isolation (15-min; PN2-21) | Wistar | male | aged | enhanced correct responses | Cannizzaro et al 2005 |
| Neonatal isolation (15-min; PN2-21) | Wistar | male | adult | enhanced ref mem & correct responses | Cannizzaro et al 2006 | |
|
| ||||||
| Active avoidance (AA) | Handling (2-min; PN1-21) | Maudsley | female | adult | enhanced | Powell & North-Jones 1974 |
| Handling (15-min; PN1-21) | SD | female | adult | enhanced | Nunez et al 1995 | |
| Neonatal isolation (3-min; PN2-15) | SD & L-E | both | adult | impaired (cort response) | Weinberg & Levine 1977 | |
| Neonatal isolation (15-min; PN1-21) | Wistar | both | adult | enhanced | Pryce et al 2003 | |
| Mat. Sep. (24-hr; PN4) | Wistar | both | adult | impaired males | Lehmann et al 1999 | |
| Mat. Sep. (24-hr; PN9) | Wistar | both | adult | enhanced males | Lehmann et al 1999 | |
| Mat. Sep. (24-hr; PN18) | Wistar | both | adult | n.s | Lehmann et al 1999 | |
| Mat. Sep. (6-hr; PN12, 14, 16,&18) | Wistar | both | adult | enhanced acquisition | Lehmann et al., 2000 | |
| Mat. Sep. (4-hr/day; PN1-21) | SD | both | adult | impaired | Weiss et al., 2001 | |
| Mat. Sep. (3-hr; PN1-16) | Wistar | female | adult | increased avoidance responses Day 1 | Schable et al 2007 | |
| Neonatal isolation (4-hr; PN1-21) | Wistar | both | adult | enhanced | Pryce et al 2003 | |
| Neonatal isolation (4-hr; PN5-11) | SD | male | adult | n.s (2-way shuttle) | Toth et al. 2008 | |
| Cort (PN1-21) | Wistar | male | aged | enhanced | Catalani et al 2000 | |
| Cort (PN1-21) | Wistar | female | aged | enhanced | Catalani et al 2002 | |
|
| ||||||
| T-maze (TM) | Neonatal isolation (PN1-22; 3-min) | Long-E | both | adult | enhanced reversal learning | Wong & Judd 1973 |
| Tact stim/neo iso (<5-min; PN2-9) | Wistar | male | adult | enhanced delayed alternation | Zhang & Cai 2008 | |
| Tact stim/neo iso (<5-min; PN10-17) | Wistar | male | adult | enhanced delayed alternation | Zhang & Cai 2008 | |
| Tact stim/neo iso (1-hr; PN2-9) | Wistar | male | adult | enhanced delayed alternation | Zhang & Cai 2008 | |
| Tact stim/neo iso (1-hr; PN10-17) | Wistar | male | adult | enhanced delayed alternation | Zhang & Cai 2008 | |
| Mat. Sep (12-hr; PN9 and PN11) | Wistar | male | adult | n.s. | Garner et al 2007 | |
| Mat. Sep. (24-hr; PN9) | Wistar | male | adult | no effect on working memory | Choy et al 2008 | |
|
| ||||||
| Y-maze (YM) | Handling (PN1-21; 15-min) | SD | male | adult | n.s. (4,6,8,24-hr ITI) | Vallee et al. 1997 |
| Handling (PN1-21; 15-min) | SD | male | adult | n.s. | Vallee et al. 1999 | |
| Handling (PN1-21; 15-min) | SD | male | aged | decrease aging impairment 24- not 8-hr | Vallee et al. 1999 | |
| Tact stim+novel envt (Pn1-21; 15-min) | Wistar | both | adult | enhance males; n.s. females | Daskalakis et al 2009 | |
| Tact stim (PN1-21; 15-min) | Wistar | both | adult | enhance males; impair females | Daskalakis et al 2009 | |
| Mat. Sep. (24-hr; PN9) | Wistar | male | adult | n.s. | Choy et al 2008 | |
|
| ||||||
| Object recognition (OR) | Handling (15-min; PN2-9) | SD | male | adult | enhanced 24-hr | Fenoglio et al 2005 |
| Neonatal isolation (15-min; PN1-21) | SD | both | adult | enhanced both sexes 3- and 24-hr test | Kosten et al 2007a | |
| Mat. Sep. (3-hr; PN2-21) | Wistar | male | adult | impaired 1-hr | Aisa et al 2007 | |
| Mat. Sep. (3-hr; PN1-10) | Wistar | male | adult | impaired 24-hr | Benetti et al 2009 | |
| Neonatal isolation (1-hr/day; PN2-9) | SD | both | adult | impaired 24-hr but not 3-hr | Kosten et al 2007b | |
| Mat. Sep. (3-hr; PN1-15) | Wistar | male | adult | impaired 24-hr but not 1.5-hr | Martins de Lima et al 2011 | |
| Mat. Sep. (3-hr; PN2-21) | Wistar | male | adult | impaired | Solas et al 2010 | |
| Mat. Sep. (3-hr; PN2-21) | Wistar | male | aged | impaired | Solas et al 2010 | |
| Limited nesting (PN2-PN9) | SD | male | adult | impaired 24-hr | Brunson et al 2005 | |
| Limited nesting (PN2-PN9) | SD | male | aged | impaired 24-hr | Ivy et al 2010 | |
| CRH (PN10) | SD | male | aged | impaired 24-hr | Brunson et al 2001 | |
| CRH antagonist (PN10-17) | SD | male | adult | enhanced 24-hr | Fenoglio et al 2005 | |
| CRH antagonist (PN10-17) | SD | male | aged | n.s. 24-hr | Ivy et al 2010 | |
|
| ||||||
| Social memory (SM) | Handling (1-min; PN1-10) | Wistar | both | adult | n.s. | Todeschin et al 2009 |
| Novel environment (3-min; PN1-21) | Long-E | male | adult | enhanced 24-hr | Reeb-Sutherland & Tang 2011 | |
| Mat. Sep. (3-hr; PN1-10) | Wistar | male | adult | impaired 24-hr | Benetti et al 2009 | |
| Mat. Sep. (3-hr; PN1-14) | Wistar | male | adult | impaired 1-hr | Lukas et al 2011 | |
| Artificial rearing | SD | female | adult | Impaired 24-hr | Levy et al 2003 | |
4. Early life manipulations
Various early life manipulations are believed to be stressful. In most cases, the pup is separated from the dam but may also be isolated from its littermates. Some investigators use the terms “handling” or “maternal separation” regardless of whether the pup was isolated individually or remained huddled with littermates. We distinguish between “separation” and “isolation” such that separation refers to procedures in which pups were allowed to huddle with littermates and isolation refers to procedures in which the pup was isolated individually. We make this distinction because a pup can receive tactile and olfactory stimulation in a huddle and this may ameliorate stress effects of separation from the dam (Cirulli et al., 1992).
A commonly used procedure, neonatal handling, was developed by Levine and colleagues (Levine et al., 1956). In this procedure, pups are removed from the dam and cage for short periods of time. Then, the pups may be placed in another cage or weighed and returned to the dam and home cage. The most common duration of handling is 15-min as shown in Fig. 1. Some studies used less than 15-min and some, 20- or 45-min. For this review, manipulations with durations of less than 60-min are defined as “brief”.
Fig. 1.
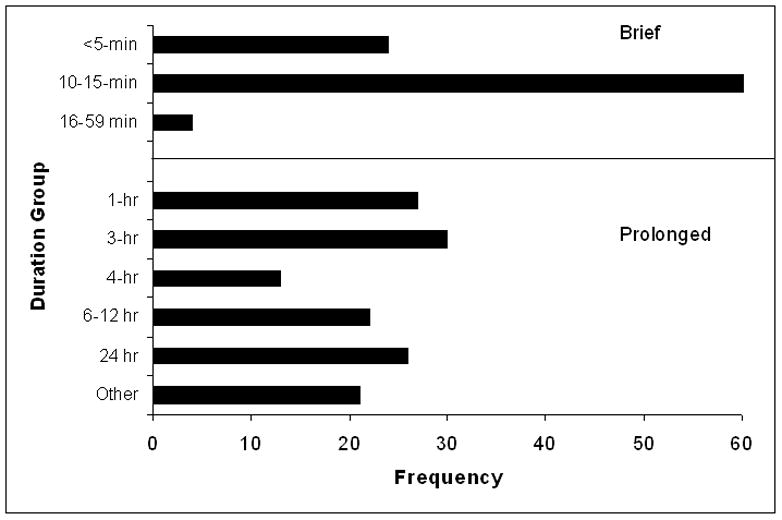
Numbers of studies conducted by duration of early life manipulation are presented by duration type (brief vs. prolonged). Brief is defined as <60-min and prolonged is defined as 1-hr or longer. “Other” includes limited nesting, artificial rearing, and corticosterone or CRH administrations, all of which are considered “prolonged: manipulations.
Maternal separation is another common early life manipulation. The term “maternal separation” typically refers to procedures that employ a prolonged duration of pup removal from the dam and/or littermates. As seen in Fig. 1, there is a range of durations. Note that the longest duration of 24-hr was only imposed on one or two occasions, whereas with shorter durations, the manipulations typically took place on several, consecutive days. All manipulations with durations of 1-hr or more are defined as “prolonged”. In addition to duration, the manner in which maternal separation is conducted across laboratories differs. The number of separations and postnatal days on which they occurred vary widely across studies. These procedural variations may contribute to the discrepancies and inconsistencies in results reported in the literature (Lehmann and Feldon, 2000; Pryce and Feldon, 2003).
Other early life manipulations that likely are stressful to the pups or affect stress hormone levels have been employed. One of the original procedures was foot shock (Ader, 1973; Levine, 1957, 1962). This was done over a short time (e.g., 3-min) so we classified it as a brief and isolated manipulation. Another early life manipulation is corticosterone administration in the drinking water that affects stress hormone levels of the dam (Catalani et al., 2011). Drinking usually occurs in several bouts throughout the day in the home cage and thus we considered it a prolonged duration and a separation type. Other methods of altering stress hormones used include administering corticotrophin-releasing factor (CRF) or an antagonist intracerebrally (Brunson et al., 2001). We considered these manipulations to be prolonged because effects likely lasted for at least 1-hr. Limited nesting material is a stressor used by Brunson and Baram (Brunson et al., 2005). Nesting material is limited around the clock on postnatal days (PN) 2–9 and thus considered a prolonged duration and separation type experience. Tang and colleagues (Tang, 2001; Tang et al., 2006) expose pups to a novel environment for a brief period. We considered this a brief duration and isolation type. Finally, we include a study in which artificial rearing, the complete lack of maternal contact, was employed (Levy et al., 2003). This was defined as prolonged and isolated. All of these aforementioned techniques are classified as “Other” and tabulated in Fig. 1.
There is much variability in the days on which manipulations were performed. Often, separations or isolations took place daily starting on PN 1 or 2 and continued through PN 21, the usual weaning day. Sometimes, manipulations were restricted to the first two postnatal weeks and in other cases, the manipulation was imposed only during the postnatal week 3. Thus, another factor examined was whether the manipulation took place during post-natal week 3 or not. This specific timing dichotomy was suggested by findings in spatial/relational task category and was the clearest division to make based on the various ranges of manipulation timing used. Further, as discussed in Section 12, the first two post-natal weeks represents a “stress hyporesponsive” period and is a time during which much neuronal organization takes place.
5. Stress and the developing rat
For many days after birth, altricial rat pups need a great deal of maternal care in order to survive (Rosenblatt and Snowdon, 1996). Pups actively elicit care from the dam through olfactory, visual, and auditory cues that prompt her to retrieve and groom them (Brewster and Leon, 1980; Smotherman et al., 1974; Stern and Johnson, 1989). Pups are active participants in the licking interaction and nursing that usually follows such grooming (Stern and Johnson, 1989, 1990). Expression of maternal behaviors varies across strains and individual dams (Gomez-Serrano et al., 2002; Liu et al., 1997; Moore et al., 1997; Myers et al., 1989) and these differences can be transmitted across generations (Boccia and Pedersen, 2001; Francis et al., 1999b). Further, maternal behavior is influenced by early life manipulations (Barnett and Urn, 1967; Bell et al., 1971; Hofer, 1983; Kosten and Kehoe, 2010; Lee and Williams, 1974; Levine, 1987; Liu et al., 1997; Marmendal et al., 2004; Pryce et al., 2001). In fact, a hypothesis put forth by Levine (Levine, 1962), is that the immediate and enduring effects of early life manipulations are mediated via the changes such manipulations induce on maternal behavior.
The maternal mediation hypothesis has been discussed in previous reviews (Macri and Wurbel, 2006; Smotherman and Bell, 1980). Briefly, interest in maternally-mediated alterations in mother-pup interactions is due, in large part, to the fact that behavior is linked to functioning of the hypothalamic-pituitary-adrenal (HPA) axis system of the adult offspring (Liu et al., 1997). Both natural variations in maternal care or changes in care due to handling alters HPA axis activity in the adult such that greater arched-back nursing and licking/grooming received as a pup associates with lower stress responsivity (Francis and Meaney, 1999; Meaney, 2001). Further, offspring born to a low-licking dam but raised by a high-licking dam is more similar to pups of high-licking dams than to pups of low-licking dams (Liu et al., 2000). Thus, maternal behavior shapes the responsivity of the adult offspring (see (Meaney, 2001).
Some argue that direct stress effects on the developing pup, in addition to maternally-mediated effects, are necessary to explain the enduring changes in the adult offspring (Macri and Wurbel, 2006; Tang et al., 2006). In the adult rat, stress activates two major systems -- the HPA axis and the sympathetic nervous system (SNS). These functionally-related systems prepare the animal to respond to challenges it faces and then bring the body back to typical conditions when the threat has passed (Chrousos and Gold, 1992). The HPA axis consists of a central component in which CRF is released from the hypothalamus and interacts with receptors in the anterior pituitary. This leads to the synthesis and release of proopiomelanocortin that cleaves into β-endorphin and adrenocorticotrophin hormone (ACTH). ACTH stimulates secretion of glucocorticoids (e.g., corticosterone in rats) from the adrenal cortex that can interact with glucocorticoid (GR) and mineralocorticoid (MR) receptors in the brain, particularly within the hippocampus where these receptors are quite abundant (deKloet and Reul, 1987; McEwen and Sapolsky, 1995). When such receptors are activated, it signals to shut down corticosterone secretion and thus, the hippocampus provides important negative feedback capacity for the animal (Herman and Cullinan, 1997). Stress also activates the SNS resulting in release of adrenaline and norepinephrine (NE), from the adrenal medulla and sympathetic nerves, respectively. A main target of NE is the brainstem structure, the locus coeruleus (LC). LC has widespread efferent connections throughout the brain; it activates the amygdala, hippocampus, and prefrontal cortex (PFC), areas that play pivotal roles in learning and memory. Not surprisingly, stress experience alters learning and memory performance in adult rats. In general, adult rats show impairments in hippocampal dependent memory (e.g., spatial/relational memory) but enhancements in non-hippocampal dependent memory (e.g., aversive Pavlovian conditioning) after stress (Kim and Diamond, 2002) although exceptions to this pattern exist (see Section 12).
6. Early life stress effects on learning and memory: Task categorization
We compiled the studies from over 75 papers and classified them into three task types as seen in Fig. 2. Task categories were initially identified based on known neurobiological and behavioral distinctions as well as by the manner in which early life stress affected performance and this categorization was confirmed by discriminant function analysis. The first category is aversive conditioning. It includes context (CtxF) and cue (CueF) fear conditioning, inhibitory avoidance (IA), eye-blink conditioning (EBC), fear-potentiated startle (FPS), and conditioned taste aversion (CTA). The second category is inhibitory learning and it includes primarily the latent inhibition (LI) tasks. The third category, spatial/relational memory tasks, includes the Morris water maze task (MWM), radial arm maze (RAM), circular maze (CM), can test (CT), active avoidance (AA), T-maze (TM), Y-maze (YM), object recognition (OR), and social memory (SM). AA is in the spatial/relational memory category because it requires the animals to escape the aversive situation like MWM.
Fig. 2.
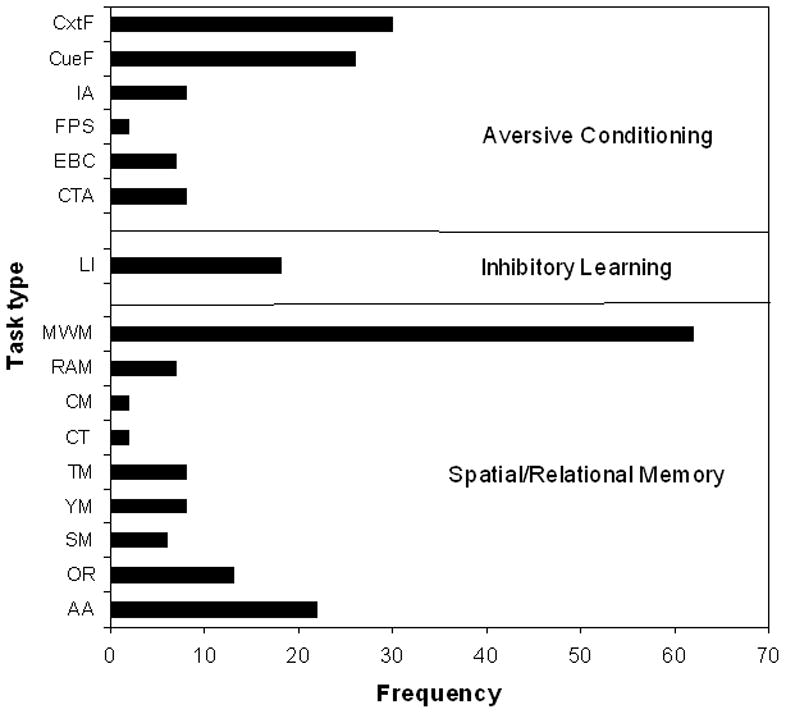
Numbers of studies reported upon in this review are presented by task and task category. Abbreviations: CtxF=context-induced fear; CueF=cue-induced fear; IA=inhibitory avoidance; FPS=fear-potentiated startle; EBC=eyeblink conditioning; CTA=conditioned taste aversion; LI=latent inhibition; MWM=Morris water maze; RAM=radial arm maze; CM=circular maze; CT= can test; TM=T-maze; YM=Y-maze; SM= Social memory; OR=object recognition; AA=active avoidance.
7. Early life stress effects on learning and memory: Aversive conditioning tasks
The first category of tasks includes several aversive conditioning procedures. As seen in Fig 2, much of the research that examined aversive conditioning in adult rats with early life manipulations utilized context or cue fear conditioning procedures (CtxF and CueF). The other aversive conditioning tasks are inhibitory avoidance, fear-potentiated startle, eyeblink conditioning, and conditioned taste aversion (CTA). All of these tasks, except CTA, involve aversive foot shock presentations. The CTA procedures are presumed to involve aversive effects of a drug (or radiation) exposure because the tastant paired with this exposure is subsequently avoided.
7.1 Fear conditioning
Pavlovian fear conditioning is a commonly used aversive conditioning procedure. Typically, an emotionally neutral light or tone conditioned stimulus (CS) is presented with a mild footshock (unconditioned stimulus or UCS). After one or more pairings, animals show fear responses (e.g., freezing) to the CS in the absence of footshock and to the context in which these pairings occurred. The amygdala is important for fear conditioning and the hippocampus is also involved in fear conditioning to the context (LeDoux, 2000).
The greatest number of studies that assessed the effects of early life manipulations on aversive conditioning in adult rats utilized Pavlovian fear conditioning procedures as seen in Fig. 2 and Table 1. Some papers report results of separate assessments of context- (CtxF) and cue-induced (CueF) fear. However, in some cases, only CtxF was examined and in one study, fear responses were assessed during exposure to both context and cue simultaneously (Madruga et al., 2006). These two procedures are listed separately in Table 1 with the study that assessed both types of fear conditioning at the same time listed with the CtxF studies.
Among 30 cases from 13 published papers, there are 16 reports of significant or trends towards significance of impaired CtxF in rats with early life manipulations as seen in Table 1 and in Fig. 2. In some cases, impairments reflect decreased expression of fear responses (e.g., freezing) upon placement in the context where the foot shocks were delivered (Kosten et al., 2006; Kosten et al., 2005; Lehmann et al., 1999; Madruga et al., 2006; Meerlo et al., 1999) and in other cases, impairments reflect enhanced extinction (Guijarro et al., 2007; Wilber et al., 2009). There are four cases of enhanced CtxF with early life manipulations. In one report, both male and female rats with prolonged maternal separation showed enhanced freezing during the context test (Oomen et al., 2010). In another report, female, but not male rats, with prolonged isolation emitted more ultrasonic vocalizations (USVs) during the context test although freezing was not altered (Kosten et al., 2005). Finally, male rats with the same manipulation showed enhanced CtxF if they were stress-exposed a week prior to training (Imanaka et al., 2006). Isolation experience alone had no effect. Finally, ten studies from five papers report no effect of early life manipulations on CtxF (Lehmann et al., 1999; Madruga et al., 2006; Oomen et al., 2011; Pryce et al., 2003; Stevenson et al., 2009).
There were no obvious differences in training or testing parameters between studies that reported significant impairments vs. those that did not. In most cases, ten shocks were given with a duration range of 0.5 to 4 sec at 0.3 to 1 mA intensity. In fact, negative reports came from papers in which significant effects were seen under the same parameters either with another early life manipulation or in cue tests. Female rats were as likely to show impaired CtxF as male rats and both Wistar and Sprague-Dawley rats were more likely to have impaired CtxF than no effect. All studies were conducted in adult rats. Thus, the factors of sex, strain, and age did not contribute to outcomes in CtxF studies. Type of manipulation did not contribute to results seen. Impaired CtxF occurred in studies using either separation or isolation procedures. However, all four reports of enhanced CtxF were associated with prolonged manipulations that were confined to the first two post-natal weeks. Thus, duration and timing of manipulation may have contributed to effects of early life manipulations on CtxF.
Nine papers with 26 experiments report on effects of early life manipulations on cue-induced fear (CueF) as seen in Table 1 and Fig. 2. Five studies find impaired acquisition, expression, or enhanced extinction (Kosten et al., 2006; Pryce et al., 2003; Stevenson et al., 2009). In some studies, effects on stress hormone or USV levels but not freezing behavior are seen. There are three cases of enhanced CueF in rats with 24-hr separation experienced on PN3 (Oomen et al., 2010; Oomen et al., 2011) although the same manipulation on PN4, 9, or 18 had no effect (Lehmann et al., 1999). No CueF effect is seen in 17 studies (Guijarro et al., 2007; Kosten et al., 2006; Kosten et al., 2005; Lehmann et al., 1999; Wilber et al., 2009).
Similar to CtxF studies, outcomes did not appear to reflect training or testing parameters. There was no pattern suggesting that sex affected the outcome and none of the studies examined aged rats. The factors of strain, or duration, type, and timing of manipulation may have contributed to results. All three studies showing enhanced CtxF used Wistar rats and confined the prolonged separation to the first two post-natal weeks. However, it is difficult to disentangle these variables and all were from the same laboratory (Oomen et al., 2010; Oomen et al., 2011). Moreover, Lehmann and colleagues used same manipulation as in the Oomen studies except it was performed on PN4, 9, or 19 and found no effect on CtxF (Lehmann et al., 1999).
7.2 Inhibitory avoidance
The inhibitory avoidance (IA) task is an aversive conditioning task in which an animal’s response (e.g. entering a dark compartment) determines presentation of an aversive stimulus (e.g. foot shock). The animal’s avoidance of the dark compartment on a subsequent trial is the measure of IA. Both the amygdala and hippocampus play important roles in modulating IA memory (Cahill and McGaugh, 1998). Eight studies from three papers report on the effects of three early life manipulations on IA performance as seen in Table 1 and in Fig 2. IA performance was impaired under all three conditions including a brief exposure to isolation or to foot shocks (Ader, 1973; Kosten et al., 2007b) or after prolonged isolation (Kosten et al., 2007a). Impairments were seen in rats of both sexes. All studies utilized Sprague-Dawley rats, an isolation manipulation, and standard IA procedures. Six of the eight studies timed the manipulation to occur during the third post-natal week and six utilized brief manipulations. Although limited in number, these reports suggest that early life manipulations impaired IA and that duration, type, and timing of the manipulation, as well as sex and strain did not contribute to the outcome.
7.3 Fear-potentiated startle
Fear-potentiated startle (FPS) is an aversive conditioning task in which conditioned fear is measured by elevated startle responses to a loud noise in the presence of a cue previously paired with footshock. The amygdala plays an important role in FPS (Davis, 1992). Two studies from one paper report no effect of prolonged separation on FPS as seen in Table 1 and Fig. 2 (deJongh et al., 2005). The manipulation ended prior to the third post-natal week and used Wistar rats of both sexes. Due to the scarcity of FPS studies, this neither adds to nor detracts from the notion that early life manipulations impair aversive conditioning.
7.4 Eye-blink conditioning
Eye-blink conditioning (EBC) is an aversive conditioning task in which a neutral CS (e.g., light or tone) is paired with an aversive US (e.g., air puff or electric shock) to the eye. While the cerebellum is essential for EBC (Kim and Thompson, 1997), the amygdala plays a modulatory role (Lee and Kim, 2004). Two papers report on seven studies of early life manipulations effects on EBC as seen in Table 1 and Fig. 2. In no case, was enhanced IA reported. Impaired EBC was seen in four studies and these used either brief or prolonged manipulations. Male rats show impaired EBC in all cases whereas no effect is seen in female rats, (Wilber et al., 2007; Wilber and Wellman, 2009). All manipulations were confined to the first two post-natal weeks and employed Long-Evans rats. Results are consistent with the notion that early life manipulations impair aversive conditioning.
7.5 Conditioned taste aversion
Conditioned taste aversion (CTA) is an aversive gustatory conditioning task. If ingestion of a novel tastant (CS) is followed by an illness-inducing situation (US), the animal avoids the tastant (Garcia et al., 1974; Garcia et al., 1955). Eight studies from two papers report on effects of early life manipulations on CTA as seen in Table 1 and Fig. 2. Data suggest that CTA performance was not altered by any manipulation including brief foot-shock exposure or isolation (Ader, 1973), handling (Roma et al., 2008), or prolonged separation (Roma et al., 2008). In all cases, Sprague-Dawley rats of both sexes were employed. Four of the studies utilized a manipulation that took place during post-natal week 3. However, instead of the widely employed LiCl, the US used was atypical; cyclophosphamide was used in the Ader (1973) studies and amphetamine in the Roma et al (2008) studies. These few negative reports with CTA neither add to nor detract from the generalization that early life manipulations impair aversive conditioning.
7.6 Summary for aversive conditioning tasks
Collectively, results from the 81 studies of various aversive conditioning tasks suggest that early life manipulations generally impair acquisition and/or performance of aversive tasks. We evaluated whether duration (brief vs. prolonged) and type (isolation vs. separation) of manipulation, as well as sex and strain (Table 4) affected the outcome. Test age was not included because no aged rats were tested. None of these factors contributed to the outcome (P’s> 0.10). Both brief and prolonged (Fig. 3) or isolation and separation type manipulations (Table 5) associated with impaired performance. However, timing of the manipulation did associate with performance (Fig. 4), F(1,74)=3.99; P<0.05. The only cases of enhanced effects were seen when the manipulation did not occur during post-natal week 3.
Table 4.
Number of studies that show enhanced or impaired performance in the three task categories is presented by rat used. Only the three most commonly used strains are shown. Strain did not alter outcomes in any of the three task categories.
| Strain | Aversive Conditioning (n=81) | Latent Inhibition (n=18) | Spatial/Relational Memory (n=132) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Enhanced | Impaired | Enhanced | Impaired | Enhanced | Impaired | |
| Wistar | 4 | 13 | 13 | 0 | 34 | 15 |
| Sprague-Dawley | 2 | 14 | 3 | 0 | 12 | 19 |
| Long-Evans | 0 | 5 | 0 | 0 | 9 | 5 |
Fig. 3.
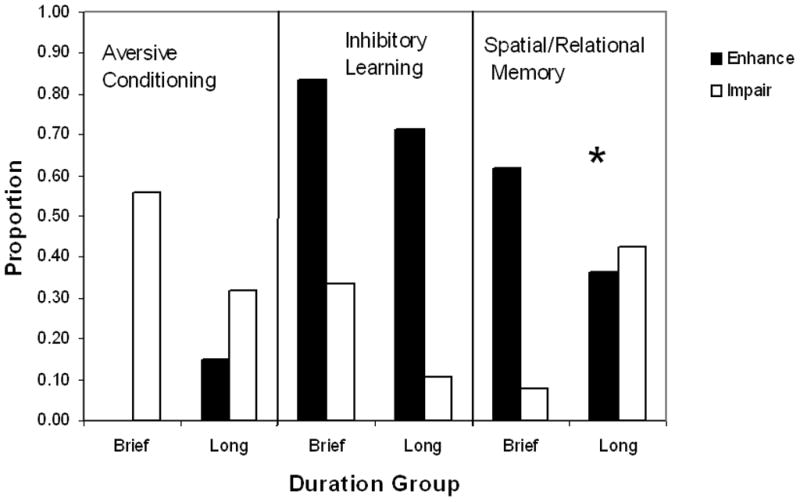
Proportion of studies categorized as aversive conditioning, inhibitory learning, or spatial/relational memory tasks that showed enhanced (solid bars) or impaired (open bars) performance by duration of manipulation and task type. Durations were either brief (<60-min) or prolonged (≥1-hr). Duration has opposing effects on performance in spatial memory tasks but did not affect aversive conditioning or inhibitory learning task performance.
Table 5.
Number of studies that show enhanced or impaired performance in the three task categories is presented by type (separation or isolation) of manipulation. Manipulation type did not alter outcomes in any of the three task categories.
| Manipulation Type | Aversive Conditioning (n=81) | Latent Inhibition (n=18) | Spatial/Relational Memory (n=132) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Enhanced | Impaired | Enhanced | Impaired | Enhanced | Impaired | |
| Separation | 4 | 17 | 18 | 0 | 37 | 30 |
| Isolation | 2 | 16 | 0 | 0 | 23 | 10 |
Fig. 4.
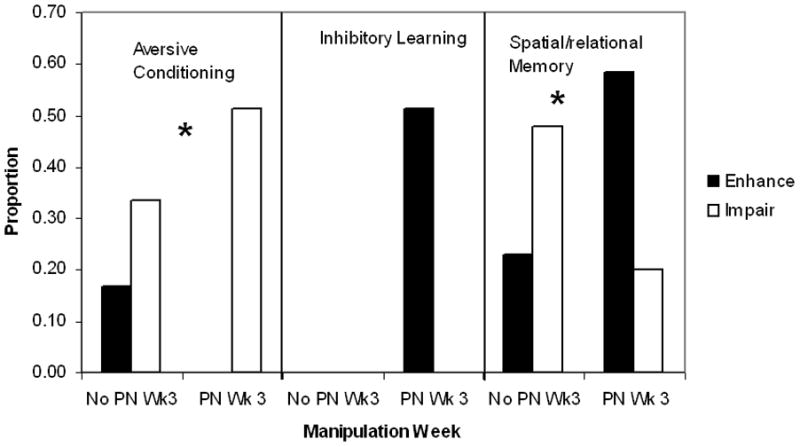
Proportion of studies categorized as aversive conditioning, inhibitory learning, or spatial/relational memory tasks that show enhanced (solid bars) or impaired (open bars) performance by timing of manipulation and task type. Timing refers to manipulations that did not occur during postnatal week 3 (No PN Wk3) or did occur during postnatal week 3 (PN Wk3). Timing has opposing effects on performance in spatial memory tasks but did not affect inhibitory learning task performance. Enhanced aversive conditioning was only seen if the manipulation did not extend into post-natal week 3.
8. Early life stress effects on learning and memory: inhibitory learning
The second category of learning and memory tasks is the inhibitory learning of latent inhibition (LI) tasks. LI refers to impaired or decreased CR to a CS if the animal was exposed to it prior to conditioning (Lubow, 1997). Typically, animals are first given repeated presentations of a light or tone stimulus without US. Then, this stimulus is paired with a US such as food or shock. Overall, results suggest LI performance is enhanced by early life manipulations.
Eighteen experiments in six publications examined effects of an early life manipulation on LI as seen in Table 2 and in Fig 2. Three procedures were used – active avoidance (AA), conditioned emotional responding (CER), and CTA. Enhanced LI was demonstrated in 16 of 18 cases. This was seen with LI of AA learning (Lehmann et al., 2000; Lehmann et al., 1998; Weiner et al., 1985; Weiss et al., 2001), CER (Lehmann et al., 2000; Lehmann et al., 1998; Peters et al., 1991; Weiner et al., 1987), and CTA (Lehmann et al., 1998). None of the studies report impaired LI and there are two cases of non-significant effects (Lehmann et al., 1998; Peters et al., 1991).
Table 2.
Summary of results of studies on early life stress and inhibitory learning in the adult
| Procedure | Early Life Manipulation | Strain | Sex | Age | Result | Reference |
|---|---|---|---|---|---|---|
| Latent inhibition (LI) | Handling (15-min; PN1-22) | Wistar | both | adult | enhanced (active avoidance) | Weiner et al., 1985 |
| Handling (15-min; PN1-22) | Wistar | both | adult | enhanced (CER) | Weiner et al., 1987 | |
| Handling (15-min; PN1-22) | ? | both | adult | enhanced effect in males only (CER) | Peters et al., 1991 | |
| Mat. Sep. (6-hr; PN12, 14, 16,&18) | Wistar | both | adult | enhanced (CER) | Lehmann et al., 1998 | |
| Mat. Sep. (6-hr; PN12, 14, 16,&18) | Wistar | both | adult | enhanced (active avoidance) | Lehmann et al., 1998 | |
| Mat. Sep. (6-hr; PN12, 14, 16,&18) | Wistar | both | adult | enhanced in males only (CTA) | Lehmann et al., 1998 | |
| Mat. Sep. (6-hr; PN12, 14, 16,&18) | Wistar | both | adult | enhanced (CER) | Lehmann et al., 2000 | |
| Mat. Sep. (6-hr; PN12, 14, 16,&18) | Wistar | both | adult | enhanced (active avoidance) | Lehmann et al., 2000 | |
| Mat. Sep. (4-hr/day; PN1-21) | SD | both | adult | enhanced (active avoidance) | Weiss et al., 2001 |
Enhanced LI was seen in rats with brief or prolonged separations and if the manipulation took place throughout the preweaning period or on four days during the end of the second and beginning of the third post-natal week. In some cases, enhanced LI of CTA or CER was found for male but not for female rats. Yet, LI was enhanced in rats of both sexes using CER or AA was also seen. The consistent results may reflect that across studies, there was little variation in many factors. All studies employed a separation procedure imposed during post-natal week 3 in adult rats. Thus, it is not possible to determine whether factors of age, type (Table 5) or timing of the manipulation (Fig. 4) affected LI performance. All but one study used Wistar rats and another study did not report the strain. Thus, a strain effect could not be examined either (Table 4). LI methods across studies were similar within each specific procedure.
9. Early life stress effects on learning and memory: spatial/relational memory tasks
A vast majority of studies reporting on effects of early life manipulations on learning and memory utilize spatial or relational memory tasks and most use Morris water maze as described below and seen in Fig. 2. Other memory tasks include object recognition and other maze tasks such as radial arm, Y- and T-maze tasks. The memory tasks in this category are similar in that they evaluated either working or reference memory or both. Working memory is when the animal acts on information gained during a session such as not re-entering an arm of a maze in which a food reward had been retrieved. Reference memory is when the animal acts on information gained in a prior session such as swimming to the location in which a hidden platform was located during a prior session. Other tasks, such as the social memory, object recognition, and Y-maze tasks, exploit the animal’s natural tendency to explore novel conspecifics, objects, or places and use this behavior to assess memory. Some tasks may use food or water restriction to motivate the animal to move around a maze. Other tasks expose the animal to stressors, such as forced swim in the Morris water maze, or the mild stress of placement in a novel environment. Nonetheless, these tasks are similar in that they assess the spatial or relational memory.
9.1 Morris water maze
Morris water maze (MWM) is a spatial memory task widely used to study hippocampal function (Morris et al., 1982; Silva et al., 1998). Typically, a rat is placed in a pool of opaque water that contains a hidden platform. The location of the platform is fixed in relation to extra maze visual cues so the animal using this information to learn to find the platform. With repeated trials, rats locate the platform faster. To test for long-term memory, the rat is usually returned to the maze 24–48 hours after training and given a probe test. During this test, the platform is removed and the search strategy recorded.
The effects of early life manipulations on MWM performance were assessed in 62 studies from 36 papers as seen in Table 3 and in Fig 2. Across cases, 28 showed enhanced and 19 showed impaired MWM performance. Enhanced performance was seen as decreased latency to reach the platform (Catalani et al., 2002; Catalani et al., 1993; Fenoglio et al., 2005; Frisone et al., 2002; Lehmann et al., 2002a; Stamatakis et al., 2008; Tang et al., 2006), shorter distance to reach the platform (Pryce et al., 2003), or both (Escorihuela et al., 1995; Fernandez-Teruel et al., 1997; Meaney et al., 1988) across trials. In other cases, enhanced memory was demonstrated by greater time spent in the former quadrant location of the platform on a probe test in addition to decreased latency to reach the platform during initial tests (Garoflos et al., 2005; Pham et al., 1997; Stamatakis et al., 2008; Tang, 2001). Finally, enhanced effects of prolonged separation were seen as better reversal learning (Lehmann et al., 1999; Levy et al., 2003). Among studies that report impaired MWM performance, these reflect either increased latency and greater distance to reach the platform (Garner et al., 2007; Huang et al., 2002; Huot et al., 2002; Noschang et al., 2010; Oitzl et al., 2000; Oomen et al., 2010), lower swim distance or time in the former location of the platform during a probe test (Aisa et al., 2009a; Aisa et al., 2009b; Aisa et al., 2007; Brunson et al., 2001; Brunson et al., 2005; Hui et al., 2011; Ivy et al., 2010; Solas et al., 2010), or impaired reversal learning (Ivy et al., 2010; Lehmann et al., 1999; Oitzl et al., 2000). No effect of early life manipulations on MWM was seen in 15 studies (Choy et al., 2008; Gibb and Kolb, 2005; Huot et al., 2002; Ivy et al., 2010; Lai et al., 2006; Oitzl et al., 2000; Oomen et al., 2011; Pryce et al., 2003; Vallee et al., 1999; Vallee et al., 1997).
Most studies utilized standard MWM procedures in which rats were given multiple trials to find the hidden platform over several days. After acquisition, researchers would either run probe tests or reversal tests in which the platform was moved to a new location. A few studies used a procedure in which the platform was moved each session (Tang, 2001; Vallee et al., 1999; Vallee et al., 1997). Yet, none of these differences explain discrepant findings.
Strain and sex of rat did not influence the outcomes. Five different strains were employed and, while most studies utilized only male rats, there were 17 studies that tested female rats or did not specify sex and were assumed to include females. Enhanced or impaired or no effect on MWM performance was just as likely to be seen regardless of sex or strain. Effects of early life manipulations in aged rats were assessed in 15 studies. Eight of these studies showed decreased age-induced impairments, four reported impaired MWM, and two showed no effect.
There were several variations in parameters of the early life manipulations such as duration, type, and timing of manipulation as well as the days on which they occurred. In addition to the more commonly used procedures of handling and maternal separation, effects of exposure to limited nesting (Brunson et al., 2005; Ivy et al., 2010) or to novel environments (Tang et al., 2006), stress hormone manipulations (Brunson et al., 2001; Fenoglio et al., 2005; Ivy et al., 2010), or artificial rearing (Levy et al., 2003) manipulations were used. Type of manipulation did not affect MWM performance; enhanced or impaired MWM was just as likely to be seen if the manipulation was of the separation type or of the isolation type. Two factors, duration and timing of the early life manipulation, were important. First, brief manipulations associated with enhanced MWM performance as seen in 13 of 22 such studies. Ten studies, all with aged rats, showed no effect, and one reported impaired effects (see Table 3). In contrast, 18 of 37 studies employing prolonged manipulations reported impairments and 15, enhanced effects. Second, manipulations conducted in post-natal week 3 associated with enhanced MWM as seen in 23 of 38 studies in contrast to only six reports of impaired performance (see Table 3). If the manipulation was confined to the first two post-natal weeks, impaired MWM performance is reported in 13 of 24 studies. Five studies report enhanced effects and six find no effect. In a prior review of early life manipulations, Hall (Hall, 1998) proposed that isolation during the post-weaning period had effects opposite to isolation experienced during the pre-weaning period. Results discussed herein suggest that prolonged manipulations late in the pre-weaning period may be akin to manipulations experienced during the early post-weaning period.
9.2 Radial arm maze
The radial arm maze (RAM) is designed to measure spatial learning and memory in rodents (Olton and Samuelson, 1976). The maze has several arms radiating from a small central platform. Animals use visual and spatial cues to learn to retrieve food rewards located at the end of some arms. The animal’s ability to remember visited arms during a session can measure working memory whereas long-term or reference memory is assessed when the animal returns to arms baited in previous sessions.
RAM performance was assessed in seven studies from five papers as seen in Table 3 and in Fig 2. Four early life manipulations were used. Brief separation reduced impaired working memory in aged male rats (Vallee et al., 1999). Working memory was better during initial trials in adult rats with early corticosterone exposure (Roskoden et al., 2005a). However, impaired working memory was seen in male, but not female, rats with prolonged isolation experienced during post-natal week 3 (Sandstrom, 2005; Sandstrom and Hart, 2005). Finally, artificial rearing led to shorter latencies during initial test days in female rats (Levy et al., 2003).
These studies utilized standard RAM procedures with either an 8-arm or a 12-arm maze and training trials given for 10 to 30 days. In some cases, all arms were baited (Levy et al., 2003; Vallee et al., 1999) so reference memory could not be assessed explicitly. In all but one study (Levy et al., 2003), rats were food-restricted during the training. In one study, decreased latency was seen during initial sessions and in three studies, early life manipulations improved working memory. In contrast, two studies report impaired memory with prolonged isolation although prolonged corticosterone exposure had the opposite effect and the absence of maternal care had no effect on RAM performance.
Three different strains were employed with no consistent pattern seen. The limited number of studies also makes it difficult to ascertain whether the factor of sex affects outcomes. And, only one study examined RAM in aged rats and this was the only study utilizing a brief manipulation. While it is not possible to determine if duration of manipulation is important for RAM performance, the type of manipulation is important. All 3 cases of separation showed enhanced RAM whereas isolation impaired, enhanced, or had no effect on RAM performance. Finally, timing of the manipulation appeared to contribute to results seen. Two of the three studies that confined the manipulation to post-natal week 3 showed impaired RAM, an outcome not seen in the studies in which the manipulation occurred during the first two post-natal weeks. Thus, the results from the RAM studies are consistent with those of MWM and with the notion that duration and timing of manipulations are important (see Figs. 3 and 4).
9.3 Circular maze
In the circular maze (CM) or Barnes maze task, bright illumination in an elevated, dry circular maze motivates rats to escape into one of the dark holes located in the maze periphery (Barnes et al., 1994). Two studies using brief isolation found no effect on CM performance in rats of both sexes (Kosten et al., 2007b). The limited use of CM in early life manipulation studies does not add nor detract from overall findings with spatial/relational tasks.
9.4 Can test
The “can” test (CT) was developed by Cannizzaro and colleagues (Cannizzaro et al., 2005; Cannizzaro et al., 2006) to test place or object memory without the use of aversive stimuli. Instead, cues are paired with the presence of a reward (Popvic et al., 2001). Specifically, aluminum soda cans are inverted so that the well-shaped bottoms can be utilized to hold water. The cans can be positioned within a large space and made distinct (visually or tactilely). Much like RAM, specific cans are baited during training and latency, correct responses, and errors measured to assess working and reference memory during test sessions. Brief isolation throughout the post-weaning period enhanced reference and working memory in adult rats (Cannizzaro et al., 2006) whereas only working memory was enhanced in aged rats (Cannizzaro et al., 2005). CT results are consistent with RAM data in that a brief manipulation decreases working memory errors in aged, male rats. While the data extend the finding to show enhancements in male Wister rats, there are no data on effects of prolonged or separated manipulations or those confined to the first two post-natal weeks. There are also no studies with females or rats of other strains.
9.5 Active avoidance
Active avoidance (AA) is a task in which an animal can avoid an aversive stimulus by making an instrumental response. Typically, an experiment uses a rectangular chamber divided into two compartments with an opening between them to allow the animal to move between sides. A light or tone signals a footshock delivery in one compartment and the animal can avoid shock by moving to the other compartment (Domjan, 2004).
Twenty-two experiments reported upon in 11 papers examined the effects of early life manipulations on AA performance as seen in Table 3 and Fig 2. Twelve experiments showed enhanced AA performance. This was evidenced by greater avoidance responses during testing in most cases (Catalani et al., 2002; Catalani et al., 2000; Lehmann et al., 2000; Nunez et al., 1995; Pryce et al., 2003). In one report, brief manipulation led to shorter escape latencies (Powell and North-Jones, 1974) while another reports enhanced conditioned avoidance responses while escape latencies were unaffected by prolonged separation (Schable et al., 2007). Impaired AA performance was demonstrated in five studies. Two studies (one per sex) report impairments in rats with prolonged separation (Weiss et al., 2001). Another report found impaired performance in male rats that experienced 24-hr of separation on PN4 only (Lehmann et al., 1999). However, if this separation occurred on PN9, male rats showed enhanced AA performance whereas separation on PN18 had no effect. Females were not affected by this manipulation on any of these days. Another study found no effect of prolonged isolation on AA performance in male rats with (Toth et al., 2008). Finally, brief isolation led to lower corticosterone levels when placed in the AA context (Weinberg and Levine, 1977). We considered this to represent two cases of impaired responding (one per sex) assuming that a decreased corticosterone response reflects an impaired response.
There was some variability in the parameters of the AA procedure across studies. Some utilized a light CS and others, a tone, while other studies used a combination of light plus tone. The shock US intensity ranged from 0.2 to 0.8 mA with a range of 2–30 sec duration. Yet, none of these parameters or the number of training trials (range = 75– 250) affected the outcome. Both studies of aged rats report enhanced AA but this was the most common outcome so it is not possible to assess whether age is an important factor. It is unlikely that strain or sex of the rat contributed to the different findings. Most studies employed Wistar rats and only one of these 15 experiments showed impaired AA while four reported non-significant results. Of the four studies that employed Sprague-Dawley rats, two showed impaired AA, one showed enhanced AA performance, and one non-significant effects. Both male and female rats were more likely to show enhanced rather than impaired AA. Thus, it seems unlikely that the ability of early life manipulations to enhance AA is strain- or sex-dependent. The factors of duration and type of manipulation did not affect AA performance. Both brief or prolonged manipulations and separated or isolated procedures were more likely to lead to enhanced vs. impaired AA performance. In contrast, timing of the manipulation contributed to AA performance. If the manipulation took place during post-natal week 3, enhanced rather than impaired AA performance was likely to be seen (Fig. 4). Eleven of 17 studies in which the early life manipulation took place during post-natal week 3 reported enhanced AA. No effect on AA was the most likely outcome in studies in which the manipulation was confined to the first two post-natal weeks.
9.6 T-maze
In the T-maze (TM), one of two short ends is baited with food and the animal is placed in the long end. Studies reported herein utilized a delayed alternation procedure in which the correct response was to enter the end not baited on the previous trial.
None of eight experiments from four papers reported impaired performance as seen in Table 3 and in Fig 2. All brief manipulations enhanced performance (Wong and Judd, 1973; Zhang and Cai, 2008). Prolonged separation did not alter TM performance (Choy et al., 2008; Garner et al., 2007) unless rats experienced tactile stimulation during 1-hr isolation on either PN 2-9 or PN 10-17. In those cases, enhanced performance was found (Zhang and Cai, 2008).
With one exception, all studies used adult male rats and one of two strains, Wistar and Long-Evans. Due to limited variation in these factors, it was not possible to assess effects of strain, sex, or age. Timing of the manipulation could not be assessed either because, in most cases, manipulations were confined to the first two post-natal weeks. However, duration and type of manipulation affected TM performance. All experiments utilizing brief manipulations found enhanced performance, while two studies of prolonged separation showed enhanced effects and two reported negative findings. The two negative findings were seen in the only two studies that used a separation procedure in contrast to findings of enhanced TM in all six isolation type studies.
9.7 Y-maze
The Y-maze (YM) has three arms forming a Y-shape and is surrounded by visual cues. It is akin to TM and measures similar effects. Studies reported here used a procedure that utilized animal’s preference for a novel place by exposing it to two of the arms but not the third. Subsequently, time spent in the novel arm was measured.
Eight studies reported upon in four papers utilized the YM test as seen in Table 3 and Fig 2. Three of the seven studies utilizing brief manipulations reported enhanced YM performance while one reported impaired performance. The one prolonged separation study found no effect (Choy et al., 2008). YM performance was enhanced in male rats (Daskalakis et al., 2009) and aged, male rats had decreased age-induced memory impairments (Vallee et al., 1999) although no effect in non-aged, male rats was reported as well (Vallee et al., 1999; Vallee et al., 1997). Two studies with female rats showed either impairments or no effect (Daskalakis et al., 2009). It was not possible to ascertain whether factors of sex, strain, or duration or type of manipulation affected the results.
9.8 Object recognition
The object recognition (OR) task uses the animal’s natural tendency to prefer novel objects and assesses recognition memory by measuring its preference for a novel object (Ennaceur and Delacour, 1988). When the rat spends more time exploring a new object in the presence of a familiar object, it is inferred that the rat has a memory for the familiar object. Both the hippocampus and the perirhinal cortex contribute to object recognition memory (Baker and Kim, 2002; Broadbent et al., 2009; Winters et al., 2008).
Fifteen experiments reported upon in 11 papers assessed OR as seen in Table 3 and in Fig 2. All three brief manipulation studies reported enhanced OR memory in rats of both sexes when tested either 3- or 24-hr after the initial exposure (Fenoglio et al., 2005; Kosten et al., 2007b). Enhanced OR was seen in one of the 12 studies utilizing prolonged manipulations (Fenoglio et al., 2005) yet the same manipulation, CRH antagonist exposure on PN10-17, had no effect in aged males (Ivy et al., 2010). Ten prolonged manipulation studies showed impaired OR memory (Aisa et al., 2007; Benetti et al., 2009; Brunson et al., 2001; Brunson et al., 2005; deLima et al., 2011; Kosten et al., 2007a; Solas et al., 2010). In some cases, OR memory was not impaired after a short delay but most studies showed impaired OR with a 24-hr delay.
Type of manipulation does not contribute to the results. There is too little variation in sex, strain, or age parameters to determine if any one affected results. Four studies assessed OR performance in aged male rats but all other studies employed non-aged rats. Most studies utilized Sprague-Dawley, male rats only. Only two studies included female rats and five, male Wistar rats. Duration of manipulation appears important for OR performance. All three brief manipulations enhanced OR and all but two prolonged manipulation cases reported impaired OR. Timing of manipulation also affects OR performance. Seven of eight studies in which the manipulation was confined to the first two PN weeks report impaired performance and the eight reports enhanced OR performance. Among seven studies in which the early life manipulation took place during post-natal week 3, three report impaired, three report enhanced, and one found no effect on OR performance.
9.9. Social memory
Like OR and YM tasks, social memory (SM) capitalizes on the animal’s tendency to decrease its investigative behaviors (e.g., sniffing) towards a conspecific after exposure. Thus, it is inferred that a lack of decrease in such behaviors reflects impaired social recognition memory (Thor and Holloway, 1982).
There are six studies of SM from five papers (see Table 3 and Fig. 2). One study shows enhanced SM, three shows impaired SM, and two reports no effect. The one finding of enhanced SM was found in rats that experienced a brief manipulation (Reeb-Sutherland and Tang, 2011) yet, the other two brief manipulation studies show no effect (Todeschin et al., 2009). All three prolonged manipulation studies report impaired SM (Benetti et al., 2009; Levy et al., 2003; Lukas et al., 2011). Only one of the six studies used an isolation type procedure and two performed the manipulation during PN week 3. Two studies were conducted with females and four used Wistar rats. While it appears that prolonged early life manipulations impair SM, it is not possible to tell if any other parameter (strain, sex, timing or type of manipulation) affected the outcome.
9.10 Summary for spatial/relational memory tasks
Results from the 132 studies of spatial/relational memory suggest that whether enhanced or impaired performance occurs depends upon some specific parameters of the early life manipulation. Factors of sex, age, and strain (Table 4) did not contribute to results seen on these tasks (P’s>0.10). Type of manipulation did not affect outcomes either (Table 5; P>0.10). However, duration of manipulation does affect results as seen in Fig 3. While rats exposed to prolonged manipulations are as likely to show either enhanced or impaired performance, only four of the 50 brief manipulation studies report impaired performance on any of these tasks. In fact, almost two-thirds of these studies report enhanced performance. This statement is supported by the significant duration effect, F(1,124) = 7.41; P<0.001. Finally, timing of the manipulation is important as seen in Fig. 4. Rats exposed to manipulations during post-natal week 3 show enhanced performance, whereas rats not exposed to manipulations at that time show impaired performance, F(1,124) = 3.25; P<0.05. Analyzing both duration and timing factors together suggests that timing is the more important factor of the two, P<0.005.
10. Unconditioned effects that may influence learning/memory performance
Learning and memory performance may be altered by early life manipulations due, in part, to primary effects on unconditioned behaviors reflective of fear or anxiety. Although it is beyond the scope of this paper to review this literature, we examined results from the papers discussed in the current review (see Table 6). Two procedures used often are elevated plus maze (EPM) and open field (OF). In EPM, time spent in or entries into open arms reflect decreased unconditioned anxiety. Typical assessments in OF are activity or ambulation and time spent in the center. Increases in these measures reflect decreased unconditioned fear.
Table 6.
Summary of results of select studies on early life stress and unconditioned behaviors
| Procedure | Early Stress | Strain | Sex | Age | Result | Reference |
|---|---|---|---|---|---|---|
| Elevated plus maze (EPM) | Handling (15-min; PN1-21) | Wistar | ? | adult | decreased anxiety | Meerlo et al. 1999 |
| Handling (15-min; PN1-21) | SD | female | adult | decreased anxiety | Nunez et al 1995 | |
| Handling (15-min; PN1-21) | SD | male | adult | decreased anxiety | Vallee et al. 1997 | |
| Neonatal isolation (15-min; PN2-21) | Wistar | male | aged | decreased anxiety | Cannizzaro et al 2005 | |
| Neonatal isolation (15-min; PN2-21) | Wistar | male | adult | n.s. | Cannizzaro et al 2005 | |
| Neonatal isolation (1-hr/day; PN2-9) | SD | male | adult | Enhance stress-induced fear | Imanaka et al. 2006 | |
| Mat. Sep. (24-hr; PN3) | Wistar | female | adult | n.s. | Oomen et al 2011 | |
| Mat. Sep. (24-hr; PN3) | Wistar | both | adult | n.s. | Oomen et al 2010 | |
| Mat. Sep. (3-hr; PN2-21) | Wistar | male | adult | increased anxiety | Aisa et al 2007 | |
| Mat. Sep. (3-hr; PN3-10) | Wistar | both | adult | Increased anxiety | Wigger & Neumann 1999 | |
| Mat. Sep. (24-hr; PN4) | Wistar | both | adult | n.s. | Lehmann et al 1999 | |
| Mat. Sep. (24-hr; PN9) | Wistar | both | adult | n.s. | Lehmann et al 1999 | |
| Mat. Sep. (24-hr; PN18) | Wistar | both | adult | n.s. | Lehmann et al 1999 | |
| Cort (PN1-21) | Wistar | male | aged | decreased anxiety | Catalani et al 2000 | |
| Cort (PN1-21) | Wistar | female | adult | n.s. | Catalani et al 2002 | |
| Limited nesting (PN2-PN9) | SD | male | adult | n.s. | Brunson et al 2005 | |
|
| ||||||
| Open field (OF) | Handling (3-min; PN1-10) | Wister | male? | adult | increased center time; activity | Madruga et al 2006 |
| Handling (2-min; PN1-21) | Maudsley | female | adult | n.s. | Powell & North-Jones 1974 | |
| Handling (10-min; PN1-22) | SD | male | adult | increased activity | Pham et al. 1997 | |
| Handling (15-min; PN2-14) | Lister | female | adult | n.s. | Stevenson et al 2009 | |
| Handling (15-min; PN1-21) | Wistar | ? | adult | n.s. | Meerlo et al. 1999 | |
| Handling (15-min; PN1-21) | SD | female | adult | increased activity | Nunez et al 1995 | |
| Handling (15-min; PN1-22) | SD | males | adult | n.s. | Nunez et al 1996 | |
| Handling (15-min; PN1-21) | SD | males | adult | n.s. | Vallee et al. 1997 | |
| Neonatal isolation (15-min; PN2-21) | Wistar | male | aged | increased activity | Cannizzaro et al 2005 | |
| Neonatal isolation (15-min; PN2-21) | Wistar | male | adult | increased activity | Cannizzaro et al 2005 | |
| Handling (15-min*3/day; PN4-21) | Long-E | both | adult | n.s. | Gibb & Kolb 2005 | |
| Neonatal isolation (1-hr/day; PN2-9) | SD | both | adult | n.s. | Kosten et al 2005 | |
| Neonatal isolation (1-hr/day; PN2-9) | SD | males | adult | n.s. | Imanaka et al. 2006 | |
| Mat. Sep. (3-hr; PN1-10) | Wister | male? | adult | n.s. | Madruga et al 2006 | |
| Mat. Sep. (3-hr; PN2-21) | Wistar | male | adult | n.s. | Aisa et al 2007 | |
| Mat. Sep. (3-hr; PN2-21) | Wistar | male | adult | n.s. | Solas et al 2010 | |
| Mat. Sep. (3-hr; PN2-21) | Wistar | male | aged | n.s. | Solas et al 2010 | |
| Neonatal isolation (4-hr; PN5-11) | SD | male | adult | greater center time | Toth et al. 2008 | |
| Mat. Sep. (6-hr; PN2-14) | Lister | female | adult | n.s. | Stevenson et al 2009 | |
| Mat. Sep (12-hr; PN9 and PN11) | Wistar | male | adult | n.s. | Garner et al 2007 | |
| Mat. Sep. (24-hr; PN4) | Wistar | both | adult | n.s. | Lehmann et al 1999 | |
| Mat. Sep. (24-hr; PN9) | Wistar | both | adult | n.s. | Lehmann et al 1999 | |
| Mat. Sep. (24-hr; PN18) | Wistar | both | adult | n.s. | Lehmann et al 1999 | |
One of our original goals was to determine if brief vs. prolonged manipulations had opposing effects on learning and memory because a commonly held belief is that brief manipulations decrease fear and anxiety whereas prolonged manipulations enhance these effects. There were 21 studies reported upon in the papers in this review that assessed anxiety in EPM (Table 6). Five EPM studies utilized brief manipulations and all but one (Cannizzaro et al., 2005) reports it decreased anxiety (Cannizzaro et al., 2005; Meerlo et al., 1999; Nunez et al., 1995; Vallee et al., 1997). Only one study, using a prolonged manipulation, reports decreased anxiety (Catalani et al., 2000) whereas, three report increased anxiety (Aisa et al., 2007; Imanaka et al., 2006; Wigger and Neumann, 1999). No effect of prolonged manipulations on EPM was seen in 11 studies (Brunson et al., 2005; Catalani et al., 2002; Lehmann et al., 1999; Oomen et al., 2010; Oomen et al., 2011). These results support the notion that brief manipulations decrease anxiety because this was shown in over 3/4 of the studies. However, the converse, that prolonged manipulations increase anxiety, is not supported. Over 2/3 of studies with prolonged manipulations report no significant EPM effects.
Of the 14 OF studies in rats with brief manipulations, five studies report increased activity or time in the center (Cannizzaro et al., 2005; Madruga et al., 2006; Nunez et al., 1996; Nunez et al., 1995; Pham et al., 1997) whereas the other nine studies report non-significant findings (Gibb and Kolb, 2005; Meerlo et al., 1999; Nunez et al., 1996; Powell and North-Jones, 1974; Solas et al., 2010; Stevenson et al., 2009; Vallee et al., 1997) as seen in Table 6. Fourteen studies examined OF in rats with prolonged manipulations and most report no effect (Aisa et al., 2007; Garner et al., 2007; Imanaka et al., 2006; Kosten et al., 2005; Lehmann et al., 1999; Madruga et al., 2006; Stevenson et al., 2009). One study of prolonged isolation reports increased time spent in the OF center (Toth et al., 2008) that presumably reflects decreased fear. While brief manipulations appear to decrease fear, prolonged manipulations have little effect on OF.
Results from studies using brief manipulations may have been affected, in part, by decreased unconditioned fear or anxiety. However, such effects would likely differ across task types. Some aversive conditioning tasks (e.g., CtxF, IA) use measures of movement (e.g., freezing or latency to move), whereas other tasks (e.g., EBC, CTA) do not. Yet, impaired performance is seen in most brief manipulation studies of aversive conditioning. Alterations in unconditioned fear or anxiety may have contributed to effects seen in spatial/relational memory tasks but probably does not affect inhibitory learning since LI tasks do not use movement measures to assess learning. Enhanced spatial/relational memory performance is often seen in rats with brief manipulations. A number of these tasks assess latency of the animal to move to a goal (e.g., MWM; RAM). Under some situations decreased fear could appear as improved memory. However, measures of memory in other tasks (e.g., OR, TM) are not based on activity levels and results with these tasks are highly consistent with results from MWM and RAM. Thus, the contributions of alterations in unconditioned fear or anxiety are probably not an important factor for most studies of learning and memory.
11. Enduring hormonal and neural effects of early life manipulations in the adult rat
Because the hippocampus develops and differentiates during the postnatal period in rodents (Altman and Bayer, 1990), early life manipulations may cause enduring effects on cellular or intracellular factors in this region. Indeed, the hippocampus (or amygdala) is well-known for contributing to learning and memory as well as to stress responsivity (Kim and Diamond, 2002; Kim and Jung, 2006; Lupien and McEwen, 1997). In addition to effects on neurochemical and neurohormonal systems, other known effects of chronic stress exposure in adulthood in these areas are decreased long-term potentiation (LTP), and increased long-term depression (LTD), synaptic models of memory, as well as morphological changes such as altered dendritic area and capacity for neurogenesis and increased necrosis. All these effects are consistent with impaired hippocampal-dependent memory. Because results of spatial/relational memory tasks related to duration and timing of the early life manipulations, we concentrate the discussion of this literature on these two factors. Due to limited variations in these factors in studies of other brain regions, much of this discussion focuses on hippocampus, amygdala, and cortex, as well as on HPA axis hormone effects.
11.1 HPA axis
Most early life manipulations discussed herein are considered stressors that activate the HPA axis in adulthood. There are excellent reviews on the effects of early life manipulations on the HPA axis (Catalani et al., 2011; Francis et al., 1999a; Levine, 2001; Macri and Wurbel, 2006; Meaney et al., 1996). A common belief is that brief manipulations reduce stress responsivity and there is much support for this assertion. It is also commonly believed that prolonged manipulations enhance stress responsivity (Francis et al., 1999a; Meaney, 2001). However, these data are quite conflicting.
Brief manipulations during most of the preweaning period either lower basal corticosterone levels or blunt the response to stress (Ader and Grota, 1969; Hess et al., 1969; Lehmann et al., 2002a; Levine, 1962; Meaney et al., 1989; Meerlo et al., 1999; Papaioannou et al., 2002b; Vallee et al., 1999; Vallee et al., 1997; Weinberg and Levine, 1977). Such manipulations prolong the rise in corticosterone or increase negative feedback sensitivity to glucocorticoid administration (Guijarro et al., 2007; Meaney et al., 1989; Viau et al., 1993). In contrast, brief exposure to novelty during the entire preweaning period increase corticosterone response to surprise (Tang et al., 2006). Despite this discrepancy and only two non-significant findings (Durand et al., 1998; Fenoglio et al., 2005), we conclude, as others have (Macri and Wurbel, 2006), that brief early life manipulations lead to HPA axis function more adapted to cope with stressors later in life. Timing of the early life manipulation does not influence HPA axis function. Most reports are based on studies of rats subjected to brief manipulations throughout most of the preweaning period. However, the few studies in which the manipulation did not extend into postnatal week 3 reports decreased HPA axis function as well (Macri et al., 2004; Plotsky and Meaney, 1993).
Prolonged separation throughout most of the preweaning period increased plasma corticosterone and ACTH levels after an acute swim stress (Aisa et al., 2007) or exposure to a shock-associated context (Guijarro et al., 2007) although no effect of restraint was seen in aged rats (Lehmann et al., 2002a). Basal corticosterone levels were increased in adult male rats with prolonged separation although no effect was seen in aged rats (Solas et al., 2010) or in rats with CRH antagonist exposure during PN10-17 (Fenoglio et al., 2005). A single 24-hr separation episode on PN3 also increased basal levels of these hormones (Rots et al., 1996) yet in response to novelty stress, corticosterone response was attenuated in young and aged rats (Workel et al., 2001). This latter finding is consistent with the decreased corticosterone response to stress seen in rats exposed to corticosterone during the entire preweaning period (Catalani et al., 2000; Catalani et al., 1993). Further, restraint stress lowered plasma corticosterone levels and produced a greater inhibition of corticosterone response to dexamethasone in rats with prolonged separations experienced during the entire postweaning period (Muneoka et al., 1994; Ogawa et al., 1994). CRH-induced changes in corticosterone secretion were not altered by prolonged separation (Ogawa et al., 1994). Overall, results do not support the notion that prolonged manipulations affect the HPA axis in a manner opposite to brief manipulations.
Discrepancies among these prolonged manipulation studies cannot be explained by timing of the manipulation. Prolonged isolation experienced during postnatal week 3 only increased plasma corticosterone (Sandstrom and Hart, 2005). Yet, increased HPA axis function was also seen in studies in which the early life manipulation did not occur during postnatal week 3 (Lehmann et al., 2002b; Penke et al., 2001; Rots et al., 1996; Wigger and Neumann, 1999). On the other hand, decreased or no effect on HPA axis function was reported in studies in which the prolonged separation did not continue into postnatal week 3 (Macri et al., 2004; Marais et al., 2008; Workel et al., 2001). Plasma corticosterone levels and adrenal weights were enhanced immediately following termination from prolonged limited nesting on PN 10, but these effects did not endure into adulthood (Brunson et al., 2005). Thus, the only reliable and consistent effect of early life manipulations on HPA axis function is that brief manipulations decrease the responsivity of this system to stress exposure in adulthood.
11.2 Corticosteroids and CRH
Brief manipulations across most of the preweaning period increased GR immunoreactivity or mRNA levels (Garoflos et al., 2005; O’Donnell et al., 1994; Stamatakis et al., 2008) and enhanced GR binding in hippocampus under basal conditions or after an acute stress exposure (Meaney et al., 1985a; Meaney et al., 1985b; Meaney et al., 1989) but not in all cases (Durand et al., 1998). Increased hippocampal GR levels were more likely to be seen if the manipulation occurred earlier rather than later in the preweaning period (Meaney and Aitken, 1985). Levels of MR immunoreactivity were lower in CA1 and CA2 regions under basal conditions but not after acute restraint in rats with brief separations (Garoflos et al., 2005; Stamatakis et al., 2008). Yet, other studies report no effect of brief manipulations on MR levels (Durand et al., 1998; Meaney et al., 1989; O’Donnell et al., 1994). Increased GR or MR likely reflects an enhanced ability to cope with stress and this is consistent with increased HPA axis function often seen in rats with brief early life manipulations (see above). Results of these studies of corticosteroid receptors in rats with brief manipulations are fairly consistent; most studies show increased GR levels in hippocampus.
Results from studies utilizing prolonged manipulation are not consistent. Limited nesting on PN2-9 or CRH administration on PN10 increased hippocampal CRH mRNA levels (Brunson et al., 2001; Ivy et al., 2010). CRH administration during the last 2 postnatal weeks increased GR mRNA expression in the CA1 and dentate gyrus (Fenoglio et al., 2005) whereas prolonged maternal separation throughout the preweaning period decreased hippocampal GR density in adult but not aged rats (Aisa et al., 2009b; Aisa et al., 2007; Solas et al., 2010). Another study showed no change in hippocampal GR mRNA levels in rats with prolonged limited nesting on PN2-9 (Brunson et al., 2005). Hippocampal GR binding levels were unaffected in rats with prolonged separation or corticosterone treatment throughout the preweaning period (Catalani et al., 2002; Catalani et al., 2000; Muneoka et al., 1994). Neither GR nor MR mRNA levels were altered by a single 24-hr separation imposed on PN 3, 5, or 14 (Penke et al., 2001; Rots et al., 1996) although GR binding was reduced in young and aged rats that experienced this separation on PN3 (Workel et al., 2001). However, corticosterone administration throughout the preweaning period increased hippocampal MR binding levels in male but not in female rats (Catalani et al., 2002; Catalani et al., 2000). Timing of manipulation does not explain the discrepancies seen. Although GR concentrations increased in hippocampus of pups during the first postnatal week of brief separation with no effect during the third postnatal week (Meaney and Aitken, 1985), GR and MR levels in adulthood are not altered when the manipulation was confined to the first two postnatal weeks (Brunson et al., 2005; Penke et al., 2001; Rots et al., 1996).
There was no effect of handling during the first two postnatal weeks on GR levels in amygdala or cortex (Meaney and Aitken, 1985). Yet, a similar type manipulation increased GR expression in infralimbic and prelimbic regions of PFC but not in amygdala (Wilber et al., 2009). However, numbers of CRH-immunopositive neurons in the central nucleus of the amygdala (CeA) were decreased in adult rats that had corticosterone administration during the first two postnatal weeks (Roskoden et al., 2005b).
11.3 Neurochemical effects
Prolonged separation during the entire post-weaning period increased NE content in hippocampus in response to a swim stress (Aisa et al., 2007) and elevated basal NE levels in dorsal hippocampus in female, but not male, rats (Matthews et al., 2001). Yet, neither brief nor prolonged isolations extending into postnatal week 3 altered hippocampal beta-adrenoreceptors (Hilakivi-Clarke et al., 1991). A brief manipulation confined to the first postnatal weeks decreased 5-HT2 concentrations with no change in binding in hippocampus (Smythe et al., 1994). If the brief manipulation extended into post-natal week 3, increased 5-HT1A density in hippocampus was seen (Garoflos et al., 2005) although it did not alter 5-HT2 binding or 5-HT levels (Durand et al., 1998; Papaioannou et al., 2002a). Rats with prolonged separation that occurred across the three postnatal weeks showed reduced 5-HT levels in dorsal hippocampus in (Matthews et al., 2001). Results for amygdala and PFC are similar to findings in hippocampus. Prolonged separation throughout the three postnatal weeks increased NE content in PFC in response to a swim stress (Aisa et al., 2007). Prolonged separation across the three postnatal weeks reduced 5-HT levels in PFC of male, but not female rats (Matthews et al., 2001) and 5-HT2 concentrations in PFC were decreased with no change in binding in rats that experienced brief manipulations confined to the first postnatal week (Smythe et al., 1994).
11.4 Cellular changes
Brief manipulations imposed across the entire post-weaning period decreased the rate of aging-induced hippocampal cell loss (Meaney et al., 1988). Both brief and prolonged manipulations during the entire preweaning period reduced CA-field volume but did not alter hippocampal volume (Hui et al., 2011; Lehmann et al., 2002a). Decreased cell numbers and density in dentate gyrus as well as altered dendritic arrangement was seen in rats subjected to a 24-hr maternal separation on postnatal day 3 (Oomen et al., 2011). Limited nesting during the first two post-natal weeks caused dendritic atrophy in CA1 region of adult rats (Ivy et al., 2010) and an expansion and extension of hippocampal mossy fibers in aged rats (Brunson et al., 2005). Hippocampal mossy fiber density was reduced in rats subjected to prolonged separation during the same time frame (Huot et al., 2002). Yet, corticosterone treatment during the same period produced no changes in density or size of the mossy field terminal fields in hippocampus (Roskoden et al., 2005a). A brief manipulation in the first two postnatal weeks increased basal PFC activity (Stevenson et al., 2009). Yet, if it extended into postnatal week 3, numbers of c-fos-positive cell nuclei in hippocampus and CeA of male rats decreased after an acute stress (Abraham and Kovacs, 2000).
11.5 Synaptic and intracellular effects
The presynaptic marker, GAP-43, was not altered in hippocampus by a prolonged manipulation imposed across the entire preweaning period; however, the postsynaptic substrate, neurogranin, increased in various hippocampal areas (McNamara et al., 2002). Spatial learning-induced increases in expression of neural cell adhesion molecule (NCAM) and three of its isoforms in hippocampus were blunted in rats with prolonged separations throughout the preweaning period (Aisa et al., 2009a). Prolonged isolation during the first two postnatal weeks increased levels of breakdown products of spectrin, a cytoskeletal protein, (Kosten et al., 2007a) and decreased pCREB (Huang et al., 2002; Lai et al., 2006) in hippocampus. Yet, neither brief nor prolonged separations during this time frame altered hippocampal or cortical CREB and ΔFosB levels (Lippmann et al., 2007).
Studies of the NMDA/glutamate system showed some consistent results; prolonged manipulations reduced receptors in hippocampus but not in other brain regions. Hippocampal GluR1 and GluR2 levels were decreased by prolonged isolation or separation with no effect in cortex (Kosten et al., 2007a; Pickering et al., 2006). Prolonged separation also lowered hippocampal levels of two NMDA receptor subunits, NR-2A and NR-2B, but did not affect levels of NR-1 or any subunit in cortex (Pickering et al., 2006; Roceri et al., 2002). Brief manipulations increased NR-2B levels in dorsal CA1 and CA3 regions but did not affect NR-1 or NR-2A levels (Stamatakis et al., 2009). Yet, NR-1, NR-2A, and NR-2B expression was decreased in PFC while unaffected in amygdala (Roceri et al., 2002; Wilber et al., 2009). However, levels of NR-2B in amygdala were increased in female, but not male rats (Stamatakis et al., 2009). Further, female, but not male rats, with a similar early life experience showed decreased nitric oxide (NO) production in hippocampus (Noschang et al., 2010).
11.6 LTP
Long term potentation (LTP) is a model of synaptic plasticity often linked to memory (Lynch et al., 1988; Martin and Morris, 2002). We expected that manipulations that impair spatial memory performance to decrease LTP and vice versa. One study reported that brief novelty exposure throughout the preweaning period enhanced LTP of both population spikes and EPSPs in CA1 (Tang and Zou, 2002). The prolonged manipulation of limited nesting on PN2-9 reduced LTP stimulated in stratum radiatum and recorded in CA1 and CA3 in aged, but not in adult rats (Brunson et al., 2005; Ivy et al., 2010). In contrast, prolonged isolation performed during the same time frame as limited nesting enhanced LTP induced in the medial perforant pathway and recorded in dentate gyrus (Kehoe and Bronzino, 1999) and enhanced LTP and LTD in the BLA-dentate gyrus pathway (Blaise et al., 2008). Similarly, separation for 24-hr on PN 3 led to a corticosterone-induced enhancement in LTP recorded from dentate gyrus in male, but not female rats (Oomen et al., 2010; Oomen et al., 2011). Yet, inhibition of corticosterone-induced suppression of LTP stimulated in Schaffer collaterals and recorded in the CA1 region was greater in rats with brief novelty exposure throughout the preweaning period (Zou et al., 2001). Timing of the early life manipulation may be important. The serotonergic agonist, tandospirone, blocked LTP in the CA1 field in rats that received foot shock during the third postnatal week and this inhibition effect was not seen in rats that received the foot shock during the second postnatal week (Matsumoto et al., 2005). Across studies, findings are not consistent but the variability in test parameters makes it difficult to find patterns or to explain discrepancies.
11.7 Neurotrophic factors and neurogenesis
Levels of the neurotrophins, NGF and NT-3, were downregulated in ventral hippocampus but upregulated in dorsal hippocampus in rats with a prolonged manipulation experienced during the first two postnatal weeks (Marais et al., 2008; Reus et al., 2011). A prolonged manipulation that extended into post-natal week 3 also decreased NGF levels in CA1, CA3, and dentate gyrus and reduced NGF and NT3 levels in amygdala with no effect in cortex (Aisa et al., 2009a). Yet, hippocampal NT3 levels were not altered in rats with 24-hr separation on PN9 (Roceri et al., 2002). Finally, brief manipulations throughout the preweaning period did not alter hippocampal NGF levels (Pham et al., 1997)..
Several studies reported on the effects of early life manipulations on levels of brain derived neurotrophic factor (BDNF). Brief manipulations did not alter BDNF levels in hippocampus, amygdala, or cortex (Garoflos et al., 2005; Lippmann et al., 2007). In contrast, many studies of prolonged manipulations showed decreased BDNF mRNA or protein levels in hippocampus (Aisa et al., 2009a; deLima et al., 2011; Lippmann et al., 2007; Roceri et al., 2002; Solas et al., 2010) or found it blocked the stress-induced increases in BDNF (Roceri et al., 2004; Roceri et al., 2002). Yet, there were a few reports of no effects of prolonged manipulations (Reus et al., 2011; Roceri et al., 2004) and one finding of increased BDNF protein levels (Greisen et al., 2005). While no effect of prolonged manipulations on BDNF levels in amygdala were reported (deLima et al., 2011; Lippmann et al., 2007), one study showed decreased protein in amygdala (Reus et al., 2011) and another found increased expression in CeA (Chung et al., 2009). Decreased mRNA or protein levels of BDNF and blunted stress-induced increases (Burton et al., 2007; Roceri et al., 2004) were seen in frontal cortex of rats with prolonged manipulation experience and although other studies reported no effect in this region (deLima et al., 2011; Greisen et al., 2005; Lippmann et al., 2007; Roceri et al., 2002).
Adult neurogenesis in hippocampus was reduced in rats with prolonged manipulations experienced throughout the first two postnatal weeks (Hulshof et al., 2011; Mirescu et al., 2004) and in male rats exposed to a single 24-hr of separation on PN 3 (Oomen et al., 2010) as indicated by lower levels of cell proliferation, cell survival, or neuronal differentiation. Cell proliferation was also reduced in rats subjected to prolonged separation throughout the entire preweaning period (Aisa et al., 2009a). On the other hand, hippocampal neurogenesis increased in females that had 24-hr separation on PN 3 (Oomen et al., 2011). Male rats with brief vs. prolonged separations during the first two post-natal weeks showed no difference in neurogenesis in hippocampus or PFC (Greisen et al., 2005).
11.8 Summary of enduring effects of early life manipulations on HPA axis and CNS
Overall, one effect -- decreased HPA axis reactivity -- appears to be specific to brief manipulations whereas decreased synaptic plasticity appears to be specific to prolonged manipulations. Although data are limited, none of these neural markers seems to relate to the timing of manipulation across the preweaning period.
12. Conclusions
The main findings of this analysis of the literature on the effects of early life manipulations on learning and memory is that the factors of duration and timing of the manipulation contribute to performance in spatial memory tasks in adult rats. Performance in aversive conditioning tasks is impaired in most cases but this effect is less likely to be seen if the manipulations extend into the third post-natal week. Enhanced performance in inhibitory learning is seen regardless of these factors. The convergence of the influences of these factors on spatial memory task performance is depicted in the scheme presented in Fig. 5. As seen in Fig. 5, as duration of the manipulation increases, performance decreases. But, as timing extends to later in the preweaning period, performance increases. Importantly, these relations would not have been seen had the various learning and memory tasks not been classified into the three categories. Indeed, this analysis has demonstrated that the enduring effects of early life manipulations are decidedly task-dependent.
Fig. 5.
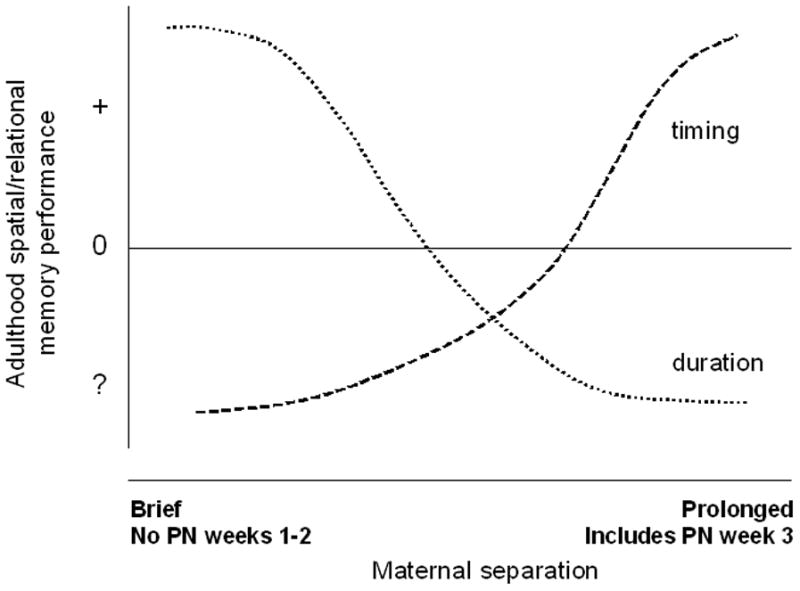
A schematic to explain the competing effects of duration and timing of postnatal manipulation on performance in spatial/relational memory tasks is presented. Performance on these tasks is enhanced (+) when the duration of the manipulation is short (<1-hr). But, as the duration increases (≤1-hr), performance on the task is impaired (−). When the manipulation is not imposed during postnatal week 3, performance is impaired (−). But, performance is enhanced (+) if the timing of the manipulation includes postnatal week 3.
The amygdala, hippocampus, and PFC are important brain regions that contribute to learning and memory processes and they are modulated by stress exposure. The hippocampus is strongly associated with spatial/relational memory tasks and the amygdala with Pavlovian conditioning tasks, especially aversive ones. Yet, the amygdala has important connections to hippocampus that help mediate the effects of stress on the hippocampus. As expected, the effects of stress exposure on the SNS and HPA axis in the rat pup differ from that in the adult because many neural and hormonal systems are still developing during the early postnatal period (Levine, 2001; Rice and Barone, 2000; Sapolsky and Meaney, 1986). From about PN3 to PN15, ending about 1 week prior to typical weaning date, rat pups show attenuated stress hormonal responses to stimuli that normally elicit strong elevations in corticosterone levels in the adult. This period has been characterized as the stress hyporesponsive period (Levine, 2001; Rice and Barone, 2000; Sapolsky and Meaney, 1986). Sapolsky and Meaney (1986) reviewed the literature and suggest several factors that contribute to this hyporesponsive corticosterone response. These include lower hippocampal GR and MR concentrations, lower hypothalamic CRH content, and lower pituitary ACTH content of the rat pup. Levine (2001) points out that early in life, suppression of HPA axis activation is under maternal control. That is, if a pup has contact with its dam, stress evokes minimal ACTH response (Stanton et al., 1988; Suchecki et al., 1995). However, pups do show HPA axis activation after 24-hr of separation (Smith et al., 1997). Some effects are ameliorated by tactile stimulation or feeding, but not by suppressing corticosterone secretion, suggesting that components of maternal behavior suppress HPA axis activation (vanOers et al., 1998). Sapolsky & Meaney (1986) propose that lower level of corticosterone secretion in the pup may help protect structures, such as the hippocampus, that are still developing at this time, from damage. Indeed, the stress hyporesponsive period is a time of rapid brain growth, including neural migration and differentiation, synaptogenesis, gliogenesis and myelination, and maturation of many neurotransmitter systems as well as apoptosis to remove neurons (Dobbing and Sands, 1979; Dumas, 2005; Rice and Barone, 2000). The hippocampus is going through these processes during the first two postnatal weeks whereas the amygdala is more fully developed at this postnatal time. And, the PFC develops later in the post-weaning period.
The general principle, stated by Rice & Barone (2000), is that environmental factors will have the greatest impact if they occur during the cascade of these developmental processes as opposed to occurring before or after the structure has developed to adult conditions. Thus, we expect that early life stress to have enduring effects on hippocampus and consequently modify performance on tasks to which these structures contribute more so than structures like amygdala, that is developed, and PFC, that continues to develop beyond the preweaning period. This is particularly relevant for the first two postnatal weeks. However, early life manipulations may affect the amygdala indirectly by altering the dynamics of its interactions with the developing hippocampus.
A striking observation of this analysis of early life manipulations is that they appear to have the opposite effects to those induced by stress experience in adulthood. In general, stress in adulthood impairs spatial memory (e.g., (deQuervain et al., 1998) but enhances aversive conditioning (e.g., (Cordero et al., 2003). Yet, early life manipulations impair aversive conditioning under most situations whereas spatial memory performance can be impaired or enhanced depending upon the timing and duration of the manipulation (see Fig. 5). Another contrast to adult stress exposure is the failure to find a significant effect of sex on performance in any of the three task types. Indeed, stress experienced in adulthood enhances performance in aversive conditioning tasks and impairs performance in spatial memory tasks in male rats whereas stress has the opposite effects in female rats (Bowman et al., 2003; Conrad et al., 2003; Luine, 2002; Shors et al., 2000; Wood and Shors, 1998). The female pattern of stress effects on learning and memory by task type is similar to effects of brief early life manipulations. This may suggest that brief manipulations alter brain regions that modulate aversive conditioning and spatial memory to become more “female-like”. Alternatively, these brain regions may be “female-like” when the manipulation was imposed and prevent these regions from becoming “masculinized” (Shors and Miesegaes, 2002). Either scenario would result in “female-like” performance in aversive conditioning and spatial memory tasks during adulthood in rats of both sexes that have had brief early life experiences.
Prolonged manipulations impaired performance both on aversive conditioning and on spatial/relational memory tasks. The former effect is “female-like” and may reflect that the amygdala becomes feminized or it is prevented from becoming masculinized possibly by altering its efferent effects on the developing hippocampus. The latter effect is “male-like”. Yet, the probability of showing impaired or enhanced spatial/relational memory with prolonged manipulations is about the same (Fig. 3). Impaired vs enhanced performance in these tasks is more clearly seen with the timing factor (Fig. 4). If the manipulation is confined to postnatal weeks 1–2, then impairment is likely whereas if the manipulation extends into postnatal week 3, enhancement tends to be seen, a female-like effect.
The model shown in Fig. 6 recaps the main findings of this analysis of the effects of early life manipulations on learning and memory. For sake of clarity, we simplified the roles of the three task types so that they reflect mainly amydala for aversive conditioning, hippocampus for spatial memory, and PFC for inhibitory learning. These three brain regions are shown with relative size depicting their stage of development. The amygdala is the most developed of the three and thus, it is depicted larger than the other two structures across the three scenarios. The hippocampus undergoes major development during postnatal weeks 1 and 2 and thus, it is small for the two scenarios in which the manipulation was confined to these two postnatal weeks and larger for the scenario in which the manipulation expanded into postnatal week 3. The PFC is not developed during the entire 3-week preweaning period of the rat and for this reason, it is depicted as small across the three scenarios. The state of development is depicted by the fill of the ovals representing these three brain structures. That is, the amygdala is developed and its ovals are gray-filled. The PFC is yet to develop and its ovals are open (white-filled). Because the hippocampus is in state of development during postnatal weeks 1 and 2 and environmental factors have the greatest impact on a structure when it is undergoing development (Rice and Barone, 2000), we have depicted this structure with wavy-lined fill for the first two scenarios. Finally, the model assumes brief manipulations to not be a “stress” to the pup because the dam modulates its HPA axis responses. Prolonged durations of the manipulations is likely stressful.
Fig. 6.
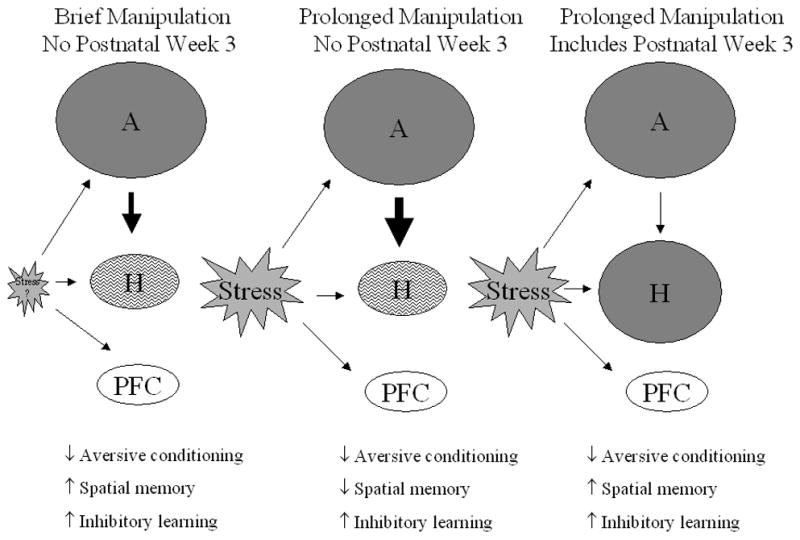
A model of how varying the duration and timing of postnatal manipulations may affect amygdala, hippocampus, and prefrontal cortex to respectively alter performance in aversive conditioning, spatial/relational memory tasks, and inhibitory learning is presented. Ovals represent the brain regions of amygdala (A), hippocampus (H), and prefrontal cortex (PFC) and oval size reflects the degree of development of the structure (small = less developed; large = more developed). Environmental factors have the greatest impact if they occur during the cascade of developmental processes and not before or after (Rice and Barone 2000). Thus, the fill of each brain region oval differs depending upon its state of development. Gray-filled ovals reflect that the structure has developed (e.g., amygdala). Open-filled ovals reflect that the structure is not in a developing state (e.g., PFC). The wavy-lined fill of the hippocampus for the two scenarios in which the manipulation is confined to the first two postnatal weeks depicts that this structure is in a developing state. Note that this structure has a gray-fill (depicting that it is developed) for the scenario in which the manipulation extended into postnatal week 3. Arrow sizes depict the degree of effect. The larger arrows from amygdala to hippocampus for the two scenarios in which the manipulation is confined to the first two postnatal weeks reflects the greater input from amygdala to hippocampus that would likely occur due to the differences in their states of development.
One pattern seen in this figure is that if the early life manipulation occurs at a time when a structure is more developed, impaired performance is seen. For example, the amygdala is the most developed and impairments in aversive conditioning tasks are seen regardless of the timing of the manipulation. In contrast, the PFC is at an early stage of development across the three postnatal weeks and performance on inhibitory learning tasks is enhanced. The hippocampus is developing during the first two postnatal weeks and when the manipulation is brief and possibly, not stressful, enhanced performance is seen in spatial/relational tasks. However, the hippocampus receives amygdalar input the degree of this input likely varies depending on the stage of hippocampal development and whether the manipulation is stressful (prolonged) or not (brief). That is, the presumed relatively greater input from amygdala to hippocampus when the hippocampus is developing and the manipulation is prolonged leads to impaired performance whereas, enhanced performance in spatial/relational memory tasks is seen when the manipulation is brief. Or, these experiences during development may heighten the ability to process spatial information perhaps due to increased GR levels in this region although other processes are likely involved as well. A similar explanation fits the findings of enhanced performance in inhibitory learning regardless of duration or timing of the manipulation as this would reflect that the PFC is developing across the entire preweaning time.
This model is based on a wealth of information (results from over 230 studies) and the synthesis of this information into a manageable schematic that not only describes and explains the data but also provides a method to predict new findings. This heuristic will be valuable for future research on the effects of early life manipulations on learning and memory. It is our hope that the model will be tested and refined further.
*Highlights.
Analyzed over 230 studies from 77 papers and classified into 3 task categories
Duration (brief or prolonged) has opposing effects on spatial/relational memory tasks only.
Timing (during postnatal week 3 or not) led to opposing outcomes on spatial/relational tasks only.
Performance was impaired in aversive conditioning and enhanced in inhibitory learning tasks by early life stress.
Explained discrepancies in literature on early life stress and learning and memory.
Acknowledgments
Funding from the following sources provided support for the preparation of this manuscript: DA 02117 (TAK) and MH64457 (JJK).
Footnotes
Discriminate function analysis (DFA) is the reverse of analysis of variance (ANOVA) and, as such, all assumptions that underlie ANOVA are applicable to DFA. The difference is that in ANOVA, the independent variables are the groups and the dependent variables are the predictors. In DFA, this is reversed; the independent variables are the predictors and dependent variables are the groups.
Other categorization schemas for timing were assessed including during postnatal week 1 only or during postnatal week 3 only. None of these analyses were significant.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham IM, Kovacs KJ. Postnatal handling alters the activation of stress-related neuronal circuitries. European Journal of Neuroscience. 2000;12:3003–3014. doi: 10.1046/j.1460-9568.2000.00176.x. [DOI] [PubMed] [Google Scholar]
- Ader R. Effects of early experiences on shock- and illness-induced passive avoidance behaviors. Developmental Psychobiology. 1973;6:547–555. doi: 10.1002/dev.420060611. [DOI] [PubMed] [Google Scholar]
- Ader R, Grota LJ. Effects of early experience on adrenocortical reactivity. Physiology & Behavior. 1969;4:303–305. [Google Scholar]
- Aisa B, Elizalde N, Tordera R, Lasheras B, DelRio J, Ramirez MJ. Effects of neonatal stress on markers of synaptic plasticity in the hippocampus: implications for spatial memory. Hippocampus. 2009a;19:1222–1231. doi: 10.1002/hipo.20586. [DOI] [PubMed] [Google Scholar]
- Aisa B, Gil-Bea FJ, Marcos B, Tordera R, Lasheras B, DelRio J, Ramirez MJ. Neonatal stress affects vulnerability of cholinergic neurons and cognition in the rat: Involvment of the HPA axis. Psychoneuroendocrinology. 2009b;34:1495–1505. doi: 10.1016/j.psyneuen.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Aisa B, Tordera R, Lasheras B, DelRio J, Ramirez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32:256–266. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. Journal of Comparative Neurology. 1990;301:365–381. doi: 10.1002/cne.903010304. [DOI] [PubMed] [Google Scholar]
- Baker KB, Kim JJ. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learning and Memory. 2002;9:58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, Jung MW, McNaughton BL, Korol DL, Andreasson K, Worley PF. LTP saturation and spatial learning disruption: effects of task variable and saturation levels. Journal of Neuroscience. 1994;14:5793–5806. doi: 10.1523/JNEUROSCI.14-10-05793.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett SA, Urn JB. Early stimulation and maternal behavior. Nature. 1967;213:150–152. doi: 10.1038/213150a0. [DOI] [PubMed] [Google Scholar]
- Bell RW, Nitschke W, Gorry TH, Zachman T. Infantile stimulation and ultrasonic signaling: a possible mediator of early handling phenomena. Developmental Psychobiology. 1971;4:181–191. doi: 10.1002/dev.420040209. [DOI] [PubMed] [Google Scholar]
- Benetti F, Mello PB, Bonini JS, Monteiro S, Cammarota M, Izquierdo I. Early postnatal maternal deprivation in rats induces memory deficits in adult life that can be reversed by donepezil and galantamine. International Journal of Developmental Neuroscience. 2009;27:59–64. doi: 10.1016/j.ijdevneu.2008.09.200. [DOI] [PubMed] [Google Scholar]
- Blaise JH, Koranda JL, Chow U, Haines KE, Dorward EC. Neonatal isolation stress alters bidirectional long-term synaptic plasticity in amygdalo-hippocampal synapses in freely behaving adult rats. Brain Research. 2008;1193:25–33. doi: 10.1016/j.brainres.2007.11.049. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Pedersen CA. Brief vs. long maternal separations in infancy: contrasting relationships with adult maternal behavior and lactation levels of aggression and anxiety. Psychoneuroendocrinology. 2001;26:657–672. doi: 10.1016/s0306-4530(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoamergic activity. Hormones and Behavior. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Brewster JA, Leon M. Relocation of the site of mother young contact: Maternal transport behavior in Norway rats. Journal of Comparative and Physiological Psychology. 1980;94:69–79. [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. learning and Memory. 2009;17 doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Chen Y, Avishai-Eliner S, Baram TZ. Stress and the developing hippocampus. Molecular Neurobiology. 2003;27:121–136. doi: 10.1385/MN:27:2:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proceedings of the National Academy of Science. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Kramar E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G, Baram TZ. Mechanisms of late-onset cognitive decline after early-life stress. Journal of Neuroscience. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton CL, Chatterjee D, Chatterjee-Chakraborty M, Lovic V, Grella SL, Steiner M, Fleming AS. Prenatal restraint stress and motherless rearing disrupts expression of plasticity markers and stres-induced corticosterone release in adult female Sprague-Dawley rats. Brain Research. 2007;1158:28–38. doi: 10.1016/j.brainres.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends in Neurosciences. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Cannizzaro E, Martire M, Mgagliano M, Plescia F, LaBarbera M, Mantia G, Mineo A, Cannizzaro G, Cannizzaro C. Reversal of prenatal diazepam-induced deficit in a spatial-object learning task by brief, periodic maternal separation in adult rats. Behavioural Brain Research. 2005;161:320–330. doi: 10.1016/j.bbr.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Cannizzaro E, Plescia F, Martire M, Gagliano M, Cannizzaro G, Mantia G, Cannizzaro C. Single, intense prenatal stress decreases emotionality and enhances learning performance in the adolescent rat offspring: interaction with a brief, daily maternal separation. Behavioural Brain Research. 2006;169:128–136. doi: 10.1016/j.bbr.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Catalani A, Alema GS, Cinque C, Zuena AR, Casolini P. Maternal corticosterone effects on hypothalamus-pituitary-adrenal axis regulation and behavior of the offspring in rodents. Neuroscience and Biobehavioral Reviews. 2011;35:1502–1517. doi: 10.1016/j.neubiorev.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Catalani A, Casolini P, Cigliana G, Scaccianoce S, Consoli C, Cinque C, Zuena AR, Angelucci L. Maternal corticosterone influences behavior, stress response and corticosteroid receptors in the female rat. Pharmacology Biochemistry and Behavior. 2002;73:105–114. doi: 10.1016/s0091-3057(02)00755-4. [DOI] [PubMed] [Google Scholar]
- Catalani A, Casolini P, Scaccianoce S, Patacchioli FR, Spinozzi P, Angelucci L. Maternal corticosterone during lactation permanently affects brain corticosteriod receptors, stress response and behaviour in rat progeny. Neuroscience. 2000;2:319–325. doi: 10.1016/s0306-4522(00)00277-3. [DOI] [PubMed] [Google Scholar]
- Catalani A, Marinelli M, Scaccianoce S, Nicolai R, Muscolo LAA, Porcu A, Koranyi L, Piazza PV, Angelucci L. Progeny of mothers drinking corticosterone during lactation has lower stress-induced corticosterone secretion and better cognitive performance. Brain Research. 1993;624:209–215. doi: 10.1016/0006-8993(93)90079-3. [DOI] [PubMed] [Google Scholar]
- Choy KHC, deVisser Y, Nichols NR, vandenBuuse M. Combined neonatal stress and young-adult glucocorticoid stimulation in rats reduce BDNF expression in hippocampus: effects on learning and memory. Hippocampus. 2008;18:655–667. doi: 10.1002/hipo.20425. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. Journal of the American Medical Association. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Chung EK, Bian ZX, Xu HX, Sung JJ. Neonatal maternal separation increases brain-derived neurotrophic factor and tyrosine kinase receptor B expression in the descending pain modulatory system. NeuroSignals. 2009;17:231–221. doi: 10.1159/000224631. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Gottlieb SL, Rosenfeld P, Levine S. Maternal factors regulate stress responsiveness in the neonatal rat. Psychobiology. 1992;20:143–152. [Google Scholar]
- Cohen J, Cohen P. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. 2. Lawrence Erlbaum Associates; Hillsdale, NJ: 1983. [Google Scholar]
- Conrad CD, Grote KA, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiology of Learning and Memory. 2003;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Venero C, Kruyt ND, Sandi C. Prior exposure to a single stress session facilitates subsequent contextual fear conditioning in rats: Evidence for a role of corticosterone. Hormones and Behavior. 2003;44:338–345. doi: 10.1016/s0018-506x(03)00160-0. [DOI] [PubMed] [Google Scholar]
- Daskalakis NP, Kaperoni M, Koros C, deKloet ER, Kitraki E. Environmental and tactile stimulation modulates the neonatal handling effect on adult rat spatial memory. International Journal of Developmental Neuroscience. 2009;27:747–755. doi: 10.1016/j.ijdevneu.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–373. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- deJongh R, Geyer MA, Olivier B, Groenink L. The effects of sex and neonatal maternal separation on fear-potentiated and light-enhanced startle. Behavioural Brain Research. 2005;161:190–196. doi: 10.1016/j.bbr.2005.02.004. [DOI] [PubMed] [Google Scholar]
- deKloet ER, Reul JM. Feedback action and tonic influence of corticosteroids on brain function: a concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology. 1987;12:83–105. doi: 10.1016/0306-4530(87)90040-0. [DOI] [PubMed] [Google Scholar]
- deLima MNM, Presti-Torres J, Vedana G, Alcalde LA, Stertz L, Fries GR, Roesler R, Andersen ML, Quevedo J, Kapczinski F, Schroder N. Early life stress decreases hippocampal BDNF content and exacerbates recognition memory deficits induced by repeated D-amphetamine exposure. Behavioural Brain research. 2011;224:100–106. doi: 10.1016/j.bbr.2011.05.022. [DOI] [PubMed] [Google Scholar]
- deQuervain D, Roozedaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;304:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Human Development. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Domjan MP. The Essentials of Conditioning and Learning. 3. Wadsworth Publishing; 2004. [Google Scholar]
- Dumas TC. Late postnatal maturation of excitatory synaptic transmission permits adult-like expression of hippocampal behaviors. Hippocampus. 2005;15:562–578. doi: 10.1002/hipo.20077. [DOI] [PubMed] [Google Scholar]
- Durand M, Sarrieau A, Aguerre S, Mormede P, Chaouloff F. Differential effects of neonatal handling on anxiety, corticosterone response to stress, and hippocampal glucocorticoid and serotonin (5-HT)2A receptors in Lewis rats. Psychoneuroendocrinology. 1998;23:323–335. doi: 10.1016/s0306-4530(98)00011-0. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. I. Behavioral data. Behavioural Brain Research. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Escorihuela RM, Tobena A, Fernandez-Teruel A. Environmental enrichment and postnatal handling prevent spatial learning deficits in aged hypoemotional (Roman High-avoidance) and hyperemotional (Roman Low-avoidance) rats. Learning and Memory. 1995;2:40–48. doi: 10.1101/lm.2.1.40. [DOI] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Avishai-Eliner S, Stone BA, Kapadia BJ, Baram TZ. Enduring handling-evoked enhancement of hippocampal memory function and glucocorticoid receptor expression involves activation of the corticotropin-releasing factor Type 1 receptor. Endocrinology. 2005;146:4090–4096. doi: 10.1210/en.2004-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Teruel A, Escorihuela RM, Castellano B, Gonzalez B, Tobena A. Neonatal handling and environmental enrichment effects on emotionality, novelty/reward seeking, and age-related cognitive and hippocampal impairments: focus on the Roman rat lines. Behavior Genetics. 1997;27:513–526. doi: 10.1023/a:1021400830503. [DOI] [PubMed] [Google Scholar]
- Francis DD, Caldji C, Champagne F, Plotsky PM, Meaney MJ. The role of corticotropin-releasing factor-norepinephrine system in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biological Psychiatry. 1999a;46:1153–1166. doi: 10.1016/s0006-3223(99)00237-1. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999b;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Francis DD, Meaney MJ. Maternal care and the development of stress responses. Current Opinion in Neurobiology. 1999;9:128–134. doi: 10.1016/s0959-4388(99)80016-6. [DOI] [PubMed] [Google Scholar]
- Frisone DF, Frye CA, Zimmerberg B. Social isolation stress during the third week of life has age-dependent effects on spatial learning in rats. Behavioural Brain Research. 2002;128:153–160. doi: 10.1016/s0166-4328(01)00315-1. [DOI] [PubMed] [Google Scholar]
- Garcia J, Hankins WG, Rusiniak KW. Behavioral regulation of the milieu interne in man and rat. Science. 1974:824–832. doi: 10.1126/science.185.4154.824. [DOI] [PubMed] [Google Scholar]
- Garcia J, Kimeldorf DJ, Koelling RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–158. [PubMed] [Google Scholar]
- Garner B, Wood SJ, Pantelis C, vandenBuuse M. Early maternal deprivation reduces prepulse inhibition and impairs spatial learning ability in adulthood: no further effect of post-pubertal chronic corticosterone treatment. Behavioural Brain Research. 2007;176:323–332. doi: 10.1016/j.bbr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Garoflos E, Panagiotaropoulos T, Pondiki S, Stamatakis A, Philippidis E, Stylianopoulou F. Cellular mechanisms underlying the effects of an early experience on cognitive abilities and affective states. Annals of General Psychiatry. 2005;4:8. doi: 10.1186/1744-859X-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb R, Kolb B. Neonatal handling alters brain organization but does not influence recovery from perinatal cortical injury. Behavioral Neuroscience. 2005;119:1375–1383. doi: 10.1037/0735-7044.119.5.1375. [DOI] [PubMed] [Google Scholar]
- Gomez-Serrano MA, Sternberg EM, Riley AL. Maternal behavior in F344/N and Lew/N rats: effects on carrageenan-induced inflammatory reactivity and body weight. Physiology & Behavior. 2002;75:493–505. doi: 10.1016/s0031-9384(02)00649-2. [DOI] [PubMed] [Google Scholar]
- Gordon HW. Early environmental stress and biological vulnerability to drug abuse. Psychoneuroendocrinology. 2002;27:115–126. doi: 10.1016/s0306-4530(01)00039-7. [DOI] [PubMed] [Google Scholar]
- Greisen MH, Altar CA, Bolwig TG, Whitehead R, Wortwein G. Increased adult hippocampal brain-derived neurotrophic factor and normal levels of neurogenesis in maternal separation rats. Journal of Neuroscience Research. 2005;79:772–778. doi: 10.1002/jnr.20418. [DOI] [PubMed] [Google Scholar]
- Guijarro JZ, Tiba PA, Ferreira TL, Kawakami SE, Oliveira MGM, Suchecki D. Effects of brief and long maternal separations on the HPA axis activity and the performance of rats on context and tone fear conditioning. Behavioural Brain Research. 2007;184:101–108. doi: 10.1016/j.bbr.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Critical Reviews in Neurobiology. 1998;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamic-pituitary-adrenocortical axis. Trends in Neurosciences. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Hess JL, Denenberg VH, Zarrow MX, Pfeifer WD. Modification of the corticosterone response curve as a function of handling in infancy. Physiology & Behavior. 1969;4:109–112. [Google Scholar]
- Hilakivi-Clarke LA, Turkka J, Lister RG, Linnoila M. Effects of early postnatal handling on brain beta-adrenoceptors and behavior in tests related to stress. Brain Research. 1991;542:286–292. doi: 10.1016/0006-8993(91)91580-t. [DOI] [PubMed] [Google Scholar]
- Hofer MA. The mother-infant interaction as a regulator of infant physiology and behavior. In: Rosenblum L, Moltz H, editors. Symbiosis in parent-offspring interactions. Plenum Press; New York: 1983. pp. 61–75. [Google Scholar]
- Howes OD, McDonald C, Cannon M, Arseneault L, Boydell J, Murray RM. Pathways to schizophrenia: the impact of environmental factors. International Journal of Neuropsychopharmacology. 2004;7:S7–S13. doi: 10.1017/S1461145704004122. [DOI] [PubMed] [Google Scholar]
- Huang LT, Holmes GL, Lai MC, Hung PL, Wang CL, Wang TJ, Yang CH, Liou CW, Yang SN. Maternal deprivation stress exacerbates cognitive deficits in immature rats with recurrent seizures. Epilepsia. 2002;43:1141–1148. doi: 10.1046/j.1528-1157.2002.14602.x. [DOI] [PubMed] [Google Scholar]
- Hui J, Zhang Z, Liu S, Xi G, Zhang X, Teng G, Chan KC, Wu EX, Nie B, Shan B, Li L, Reynolds GP. Hippocampal neurochemistry is involved in the behavioural effects of neonatal maternal separation and their reversal by post-weaning environmental enrichment: a magnetic resonance study. Behavioural Brain Research. 2011;217:122–127. doi: 10.1016/j.bbr.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Hulshof HJ, Novati A, Sgoifo A, Luiten PGM, denBoer JA, Meerlo P. Maternal separation decreases hippocampal cell proliferation and impairs cognitive performance but has little effect on stress sensitivity and anxiety in adult Wistar rats. Behavioural Brain Research. 2011;216:552–560. doi: 10.1016/j.bbr.2010.08.038. [DOI] [PubMed] [Google Scholar]
- Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Research. 2002;950:52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- Imanaka A, Morinobu S, Toki S, Yamawaki S. Importance of early environment in the development of post-traumatic stress disorder-like behavior. Behavioural Brain Research. 2006;173:129–137. doi: 10.1016/j.bbr.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Ivy AS, Rex CS, Chen Y, Dube C, Maras PM, Grigoriadis DE, Gall CM, Lynch G, Baram TS. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. Journal of Neuroscience. 2010;30:13005–13015. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Plotsky PM, Nemeroff CB, Charney DS. Effects of early adverse experiences on brain structure and function: clinical implications. Biological Psychiatry. 2000;48:778–790. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- Kehoe P, Bronzino JD. Neonatal stress alters LTP in freely moving male and female adult rats. Hippocampus. 1999;9:651–658. doi: 10.1002/(SICI)1098-1063(1999)9:6<651::AID-HIPO6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Kehoe P, Shoemaker WJ. Infant stress, neuroplasticity, and behavior. In: Blass E, editor. Developmental Psychobiology. Kluwer Academic/Plenum; New York: 2001. pp. 551–585. [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nature Reviews Neuroscience. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neuroscience and Biobehavioral Reviews. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Thompson RF. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends in Neurosciences. 1997;20:177–181. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Karanian DA, Haile CN, Yeh J, Kim JJ, Kehoe P, Bahr BA. Memory impairments and hippocampal modifications in adult rats with neonatal isolation stress experience. Neurobiology of Learning and Memory. 2007a;88:167–176. doi: 10.1016/j.nlm.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Kehoe P. Early life stress and vulnerability to addiction: translational studies with neonatal isolation of rat pups. In: al’Absi M, editor. Stress and Addiction: Biological and Psychological Mechanisms. Elsevier; San Diego, CA: 2007. pp. 105–126. [Google Scholar]
- Kosten TA, Kehoe P. The immediate and enduring effects of neonatal isolation on maternal behavior in rats. International Journal of Developmental Neuroscience. 2010;28:53–61. doi: 10.1016/j.ijdevneu.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Lee HJ, Kim JJ. Early life stress impairs fear conditioning in adult male and female rats. Brain Research. 2006;1087:142–150. doi: 10.1016/j.brainres.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Lee HJ, Kim JJ. Neonatal handling alters learning in adult male and female rats in a task-specific manner. Brain Research. 2007b;1154:144–153. doi: 10.1016/j.brainres.2007.03.081. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJD, Bombace JC, Lee HJ, Kim JJ. Sex-selective effects of neonatal isolation on fear conditioning and foot shock sensitivity. Behavioural Brain Research. 2005;157:235–244. doi: 10.1016/j.bbr.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Lai MC, Holmes GL, Lee KH, Yang SN, Wang CA, Wu CL, Tiao MM, Hsieh CS, Lee CH, Huang LT. Effect of neonatal isolation on outcome following neonatal seizures in rats -- the role of corticosterone. Epilepsy Research. 2006;68:123–136. doi: 10.1016/j.eplepsyres.2005.10.005. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee M, Williams D. Changes in licking behaviour of rat mother following handling of young. Animal Behavior. 1974;22:679–681. [Google Scholar]
- Lee T, Kim JJ. Differential effects of cerebellar, amygdalar, and hippocampal lesions on classical eyeblink conditioning in rats. Journal of Neuroscience. 2004;24 doi: 10.1523/JNEUROSCI.5382-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Reviews in Neuroscience. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Pryce CR, Bettschen D, Feldon J. The maternal separation paradigm and adult emotionality and cognition in male and female Wistar rats. Pharmacology, Biochemistry and Behavior. 1999;64:705–715. doi: 10.1016/s0091-3057(99)00150-1. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Pryce CR, Jongen-Relo AL, Stohr T, Pothuizen HH, Feldon J. Comparison of maternal separation and early handling in terms of their neurobehavioral effects in aged rats. Neurobiology of Aging. 2002a;23:457–466. doi: 10.1016/s0197-4580(01)00320-7. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Russig H, Feldon J, Pryce CR. Effect of a single maternal separation at different pup ages on the corticosterone stress response in adult and aged rats. Pharmacology Biochemistry and Behavior. 2002b;73:141–145. doi: 10.1016/s0091-3057(02)00788-8. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Stohr T, Feldon J. Long-term effects of prenatal stress experiences and postnatal maternal separation on emotionality and attentional processes. Behavioural Brain Research. 2000;107:133–144. doi: 10.1016/s0166-4328(99)00122-9. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Stohr T, Schuller J, Domeney A, Heidbreder C, Feldon J. Long-term effects of repeated maternal separation on three different latent inhibition paradigms. Pharmacology, Biochemistry and Behavior. 1998;59:873–882. doi: 10.1016/s0091-3057(97)00529-7. [DOI] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405–405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Levine S. Plasma-free corticosteroid response to electric shock in rats stimulated in infancy. Science. 1962;135:795–796. doi: 10.1126/science.135.3506.795-a. [DOI] [PubMed] [Google Scholar]
- Levine S. Psychobiologic consequences of disruption in mother-infant relationships. In: Krasneger N, Blass E, Hofer M, Smotherman W, editors. Perinatal Development: A Psychobiological Perspective. Academic Press; New York: 1987. [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic-pituitary-adrenal axis in the rat. Physiology & Behavior. 2001;73:255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Levine S, Chevalier JA, Korchin SJ. The effects of early shock and handling on later avoidance learning. Journal of Personality. 1956;24:475–493. doi: 10.1111/j.1467-6494.1956.tb01283.x. [DOI] [PubMed] [Google Scholar]
- Levy F, Melo AI, Galef BG, Madden M, Fleming AS. Complete maternal deprivation affects social, but not spatial, learning in adult rats. Developmental Psychobiology. 2003;43:177–191. doi: 10.1002/dev.10131. [DOI] [PubMed] [Google Scholar]
- Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. European Journal of Neuroscience. 2007;25:3091–3098. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nature Neuroscience. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lubow RE. Latent inhibition as a measure of learned inattention: some problems and solutions. Behavioral Brain Research. 1997;88:75–83. doi: 10.1016/s0166-4328(97)02307-3. [DOI] [PubMed] [Google Scholar]
- Luine VN. Sex differences in chronic stress effects on memory in rats. Stress. 2002;5:205–216. doi: 10.1080/1025389021000010549. [DOI] [PubMed] [Google Scholar]
- Lukas M, Bredewold R, Landgraf R, Neumann ID, Veenema AH. Early life stress impairs social recognition due to a blunted response of vasopressin release within the septum of adult male rats. Psychoneuroendocrinology. 2011;36:843–853. doi: 10.1016/j.psyneuen.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Research Reviews. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Lynch G, Muller D, Seubert P, Larson J. Long-term potentiation: persisting problems and recent results. Brain Research Bullletin. 1988;21:363–372. doi: 10.1016/0361-9230(88)90148-7. [DOI] [PubMed] [Google Scholar]
- Macri S, Mason GJ, Wurbel H. Dissociation in the effects of neonatal maternal separations on maternal care and the offspring’s HPA and fear responses in rats. European Journal of Neuroscience. 2004;20:1017–1024. doi: 10.1111/j.1460-9568.2004.03541.x. [DOI] [PubMed] [Google Scholar]
- Macri S, Wurbel H. Developmental plasticity of HPA and fear responses in rats: a critical review of the maternal mediation hypothesis. Hormones and Behavior. 2006;50:667–680. doi: 10.1016/j.yhbeh.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Madruga C, Xavier LL, Achaval M, Sanvitto GL, Lucion AB. Early handling, but not maternal separation, decreases emotional responses in two paradigms of fear without changes in mesolimbic dopamine. Behavioural Brain Research. 2006;166:241–246. doi: 10.1016/j.bbr.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Marais L, vanRensburg SJ, vanZyl JM, Stein DJ, Daniels WMU. Maternal separation of rat pups increases the risk of developing depressive-like behavior after subsequent chronic stress by altering corticosterone and neurotrophin levels in the hippocampus. Neuroscience Research. 2008;61:106–112. doi: 10.1016/j.neures.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Marmendal M, Roman E, Eriksson CJP, Nylander I, Fahlke C. Maternal separation alters maternal care, but has minor effects on behavior and brain opioid peptides in adult offspring. Developmental Psychobiology. 2004;45:140–152. doi: 10.1002/dev.20027. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Morris RG. New life in an old idea: the synaptic plasticity and memory hypothesis revisited. Hippocampus. 2002;12:609–636. doi: 10.1002/hipo.10107. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Higuchi K, Togashi H, Koseki H, Yamaguchi T, Kanno M, Yoshioka M. Early postnatal stress alters the 5-HTergic modulation to emotional stress at postadolescent periods of rats. Hippocampus. 2005;15:775–781. doi: 10.1002/hipo.20100. [DOI] [PubMed] [Google Scholar]
- Matthews K, Dalley JW, Matthews C, Tsai TH, Robbins TW. Periodic maternal separation of neonatal rats produces region- and gender-specific effects on biogenic amine content in postmortem adult brain. Synapse. 2001;40:1–10. doi: 10.1002/1098-2396(200104)40:1<1::AID-SYN1020>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Current Opinion in Neurobiology. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Huot RL, Lenox RH, Plotsky PM. Postnatal maternal separation elevates the expression of the postsynaptic protein kinase C substrate RC3, but not presynaptic GAP-43, in the developing rat hippocampus. Developmental Neuroscience. 2002;24:485–494. doi: 10.1159/000069359. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aiken DH, Bodnoff SR, Iny LJ, Sapolsky RM. The effects of postnatal handling on the development of the glucocorticoid receptor systems and stress recovery in the rat. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1985a;9:731–734. doi: 10.1016/0278-5846(85)90050-8. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH. The effects of early postnatal handling on hippocampal glucocorticoid receptor concentrations: temporal parameters. Brain Research. 1985;354:301–304. doi: 10.1016/0165-3806(85)90183-x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Bodnoff SR, Iny LJ, Tatarewicz JE, Sapolsky RM. Early postnatal handling alters glucocorticoid receptor concentrations in selected brain regions. Behavioral Neuroscience. 1985b;99:765–770. doi: 10.1037//0735-7044.99.4.765. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, vanBerkel C, Bhatnagar S, Sapolski RM. Effects of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239:766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Viau V, Sharma S, Sarrieau A. Neonatal handling alters adrenocortical negative feedback sensitivity and hippocampal type II glucocorticoid receptor binding in the rat. Neuroendocrinology. 1989;50:597–604. doi: 10.1159/000125287. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio R, Francis D, Widdowson J, LaPlante P, Caldji C, Sharm V, Seckl JR, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Developmental Neuroscience. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Horvath KM, Nagy GM, Bohus B, Koohaas JM. The influence of postnatal handling on adult neuroendocrine and behavioural stress reactivity. Journal of Neuroendocrinology. 1999;11:925–933. doi: 10.1046/j.1365-2826.1999.00409.x. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nature Neuroscience. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD, Kuhar MJ. Maternal separation alters drug intake patterns in adulthood in rats. BIochemical Pharmacology. 2007;73:321–330. doi: 10.1016/j.bcp.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CL, Wong L, Daum MC, Leclair OU. Mother-infant interactions in two strains of rats: implications for dissociating mechanism and function of a maternal pattern. Developmental Psychobiology. 1997;30:301–312. [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O’Keefe JO. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Mikuni M, Ogawa T, Kitera K, Takahashi K. Periodic maternal deprivation-induced potentiation of the negative feedback sensitivity to glucocorticoids to inhibit stress-induced adrenocortical response persists throughout the animal’s life-span. Neuroscience Letters. 1994;168:89–92. doi: 10.1016/0304-3940(94)90423-5. [DOI] [PubMed] [Google Scholar]
- Myers MM, Brunelli SA, Shair HN, Squire JM, Hofer MA. Relationship between maternal behavior of SHR and WKY dams and adult blood pressures of cross-fostered F1 pups. Developmental Psychobiology. 1989;22:55–67. doi: 10.1002/dev.420220105. [DOI] [PubMed] [Google Scholar]
- Noschang CG, Krolow R, Fontella FU, Arcego DM, Diehl LA, Weis SN, Dalmaz NSAC. Neonatal handling impairs spatial memory and leads to altered nitric oxide production and DNA breaks in a sex specific manner. Neurochemical Research. 2010;35:1083–1091. doi: 10.1007/s11064-010-0158-7. [DOI] [PubMed] [Google Scholar]
- Nunez JF, Ferre P, Escorihuela RM, Tobena A, Fernandez-Teruel A. Effects of postnatal handling of rats on emotional, HPA-axis, and prolactin reactivity to novelty and conflict. Physiology & Behavior. 1996;60:1355–1359. doi: 10.1016/s0031-9384(96)00225-9. [DOI] [PubMed] [Google Scholar]
- Nunez JF, Ferre P, Garcia E, Escorihuela RM, Fernandez-Teruel A, Tobena A. Postnatal handling reduces emotionality ratings and accelerates two-way active avoidance in female rats. Physiology & Behavior. 1995;57:831–835. doi: 10.1016/0031-9384(94)00308-r. [DOI] [PubMed] [Google Scholar]
- O’Donnell D, Larocque S, Seckl JR, Meaney MJ. Postnatal handling alters glucocorticoid, but no mineralocorticoid messenger RNA expression in the hippocampus of adult rats. Brain Research Molecular Brain Research. 1994;26:242–248. doi: 10.1016/0169-328x(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Mikuni M, Kuroda Y, Muneoka K, Mori K, Takahashi K. Periodic maternal deprivation alters stress response in adult offspring: potentiates the negative feedback regulation of restraint stress-induced adrenocortical response and reduces the frequencies of open field-induced behaviors. Pharmacology Biochemistry and Behavior. 1994;49:961–967. doi: 10.1016/0091-3057(94)90250-x. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, Workel JO, Fluttert M, Frosch F, Kloet ER. Maternal deprivation affects behaviour from youth to senescence: amplification of individual differences in spatial learning and memory in senescent Brown Norway rats. European Journal of Neuroscience. 2000;12:3771–3780. doi: 10.1046/j.1460-9568.2000.00231.x. [DOI] [PubMed] [Google Scholar]
- Olton DS, Samuelson RJ. Rememberance of places passed: spatial memory in rats. Journal of Experimental Psychology: Animal Behavioral Processes. 1976;2:97–116. [Google Scholar]
- Oomen CA, Soeters H, Audureau N, Vermunt L, Hasselt FNv, Manders EMM, Joels MPJ, Lucassen HK. Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. Journal of Neuroscience. 2010;30:6635–6645. doi: 10.1523/JNEUROSCI.0247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen CA, Soeters H, Audureau N, Vermunt L, vanHasselt FN, Manders EMM, Joels M, Krugers H, Lucassen PJ. Early maternal deprivation affects gentate gyrus structure and emotional learning in adult female rats. Psychopharmacology. 2011;214:249–260. doi: 10.1007/s00213-010-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou A, Dafni U, Alikaridis F, Bolaris S, Stylianopoulou F. Effects of neonatal handling on basal and stress-induced monoamine levels in the male and female rat brain. Neuroscience. 2002a;114:195–206. doi: 10.1016/s0306-4522(02)00129-x. [DOI] [PubMed] [Google Scholar]
- Papaioannou A, Gerozissis K, Prokopiou A, BOlaris S, Stylianopoulou F. Sex differences in the effects of neonatal handling on the animals’s response to stress and the vulnerability for depressive behaviour. Behavioural Brain Research. 2002b;129:131–139. doi: 10.1016/s0166-4328(01)00334-5. [DOI] [PubMed] [Google Scholar]
- Penke Z, Felszeghy K, Fernette B, Sage D, Nyakas C, Burlet A. Postnatal maternal deprivation produces long-lasting modifications of the stress response, feeding and stress-related behaviour in the rat. European Journal of Neuroscience. 2001;14:747–755. doi: 10.1046/j.0953-816x.2001.01691.x. [DOI] [PubMed] [Google Scholar]
- Peters SL, Gray JA, Joseph MH. Pre-weaning non-handling of rats disrupts latent inhibition in males, and results in persisting sex- and area-dependent increases in dopamine and serotonin turnover. Behavioural Pharmacology. 1991;2:215–223. [PubMed] [Google Scholar]
- Pham TM, Soderstrom S, Henriksson BG, Mohammed AH. Effects of neonatal stimulation on later cognitive function and hippocampal nerve growth factor. Behavioural Brain Research. 1997;86:113–120. doi: 10.1016/s0166-4328(96)02252-8. [DOI] [PubMed] [Google Scholar]
- Pickering C, Gustafsson L, Cebere A, Nylander I, Lijequist S. Repeated maternal separation of male Wistar rats alters glutamate receptor expression in the hippocampus but not in the prefrontal cortex. Brain Research. 2006;1099:101–108. doi: 10.1016/j.brainres.2006.04.136. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Molecular Brain Research. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Popvic M, Biessels GJ, Isaacson RL, Gispen WH. Learning and memory in streptozotocin-induced diabetic rats in a novel spatial/object discrimination task. Behavioural Brain Research. 2001;122:201–207. doi: 10.1016/s0166-4328(01)00186-3. [DOI] [PubMed] [Google Scholar]
- Powell BJ, North-Jones M. Effects of early handling on avoidance performance of Maudsley MR and MNR strains. Developmental Psychobiology. 1974;7:145–148. doi: 10.1002/dev.420070207. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Bettschen D, Feldon J. Comparison of the effects of early handling and early deprivation on maternal care in the rat. Developmental Psychobiology. 2001;38:239–251. doi: 10.1002/dev.1018. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Bettschen D, Nanz-Bahr NI, Feldon J. Comparison of the effects of early handling and early deprivation on conditioned stimulus, context, and spatial learning and memory in adult rats. Behavioral Neuroscience. 2003;117:883–893. doi: 10.1037/0735-7044.117.5.883. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Feldon J. Long-term neurobehavioral impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neuroscience and Biobehavioral Reviews. 2003;27:57–71. doi: 10.1016/s0149-7634(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Reeb-Sutherland BC, Tang AC. Dissociation between neonatal novelty-induced preferential maternal care and enhancements in cognitive, social, and emotional functions. Behavioural Brain Research. 2011;224:318–325. doi: 10.1016/j.bbr.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Reus GZ, Stringari RB, Ribeiro KF, Cipriano AL, Panizzutti BS, Stertz L, Lersch C, Kapczinski F, Quevedo J. Maternal deprivation induces depressive-like behaviour and alters neurotrophin levels in the rat brain. Neurochemical Research. 2011;36:460–466. doi: 10.1007/s11064-010-0364-3. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S. Critical periods of vulnerability for the developing nervous system: evidence from human and animal models. Environmental Health Perspectives. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roceri M, Cirulli F, Pessina C, Peretto P, Racagni G, Riva MA. Postnatal repeated maternal deprivation produces age-dependent changes of brain-derived neurotrophic factor expression in selected brain regions. Biological Psychiatry. 2004;55:708–714. doi: 10.1016/j.biopsych.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Roceri M, Hendriks W, Racagni G, Ellenbroek BA, Riva MA. Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Molecular Psychiatry. 2002;7:609–616. doi: 10.1038/sj.mp.4001036. [DOI] [PubMed] [Google Scholar]
- Roma PG, Davis CM, Kohut SJ, Huntsberry ME, Riley AL. Early maternal separation and sex differences in the aversive effects of amphetamine in adult rats. Physiology & Behavior. 2008;93:897–904. doi: 10.1016/j.physbeh.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS, Snowdon CT. Parental care: evolution, mechanisms, and adaptive significance. Academic Press; New York: 1996. [Google Scholar]
- Roskoden T, Linke R, Schwegler H. Transient early postnatal corticosterone treatment of rats leads to accelerated acquisition of a spatial radial maze task and morphological changes in the septohippocampal region. Behavioural Brain Research. 2005a;157:45–53. doi: 10.1016/j.bbr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Roskoden T, Yilmazer-Hanke JHD, Schwegler H. Reduced number of CRF-containing neurons in the central amygdala correlated with enhanced locomotor activity following early postnatal corticosterone treatment in the Wistar rat. Behavioural Brain research. 2005b;165:221–228. doi: 10.1016/j.bbr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Rots N, deJong J, Workel JO, Levine S, Cools AR, DeKloet ER. Neonatal maternally deprived rats have as adults elevated basal pituitary-adrenal activity and enhanced susceptibility to apomorphine. Journal of Neuroendcrinology. 1996;8:501–506. doi: 10.1046/j.1365-2826.1996.04843.x. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ. Sex differences in the long-term effect of preweanling isolation stress on memory retention. Hormones and Behavior. 2005;47:556–582. doi: 10.1016/j.yhbeh.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Hart SR. Isolation stress during the third postnatal week alters radial arm maze performance and corticosterone levels in adulthood. Behavioural Brain Research. 2005;156:289–296. doi: 10.1016/j.bbr.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Research Reviews. 1986;11:65–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Schable S, Poeggel G, Braun K, Gruss M. Long-term consequences of early experience on adult avoidance learning in female rats: role of the dopaminergic system. Neurobiology of Learning and Memory. 2007;87:109–122. doi: 10.1016/j.nlm.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Beylin AV, Wood GE, Gould E. The modulation of Pavlovian memory. Behavioural Brain Research. 2000;110:39–52. doi: 10.1016/s0166-4328(99)00183-7. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G. Testosterone in utero and at birth dictates how stressful experience will affect learning in adulthood. Proceedings of the National Academy of Sciences. 2002;214:131–140. doi: 10.1073/pnas.202199999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Giese KP, Fedorov NB, Frankland PW, Kogan JH. Molecular, cellular, and neuroanatomical substrates of place learning. Neurobiology of Learning and Memory. 1998;70:44–61. doi: 10.1006/nlme.1998.3837. [DOI] [PubMed] [Google Scholar]
- Smith MA, Kim SY, VanOers HJJ, Levine S. Maternal deprivation and stress induce immediated early genes in the infant rat brain. Endocrinology. 1997;138:4622–4628. doi: 10.1210/endo.138.11.5529. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Bell RW. Maternal mediation of early experience. In: Bell RW, Smotherman WP, editors. Maternal Influences and Early Behavior. Spectrum; New York: 1980. pp. 201–210. [Google Scholar]
- Smotherman WP, Bell RW, Starzec J, Elias J. Maternal responses to infant vocalizations and olfactory cues in rats and mice. Behavioral Biology. 1974;12:55–66. doi: 10.1016/s0091-6773(74)91026-8. [DOI] [PubMed] [Google Scholar]
- Smythe JW, Rowe WB, Meaney MJ. Neonatal handling alters serotonin (5-HT) turnover and 5-HT2 receptor binding in selected brain regions: relationship to the handling effect on glucocorticoid receptor expression. Developmental Brain Research. 1994;80:183–189. doi: 10.1016/0165-3806(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Solas M, Aisa B, Mugueta MC, DelRio J, Tordera RM, Rarmirez MJ. Interactions between age, stress and insulin on cognition: implications for Alzheimer’s Disease. Neuropsychopharmacology. 2010;35:1664–1673. doi: 10.1038/npp.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A, Pondiki S, Kitraki E, Diamantopoulou A, Panagiotaropoulos T, Raftogianni A, Stylianopoulou F. Effect of neonatal handling on adult rat spatial learning and memory following acute stress. Stress. 2008;11:148–159. doi: 10.1080/10253890701653039. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Toutountzi E, Fragioudaki K, Kouvelas ED, Stylianopoulou F, Mitsacos A. Selective effects of neonatal handling on rat brain N-methyl-D-aspartate receptors. Neuroscience. 2009;164:1457–1467. doi: 10.1016/j.neuroscience.2009.09.032. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Gutierrez YR, Levine S. Maternal deprivation potentiates pituitary-adrenal stress responses in infant rats. Behavioral Neuroscience. 1988;102:692–700. doi: 10.1037//0735-7044.102.5.692. [DOI] [PubMed] [Google Scholar]
- Stern JM, Johnson SK. Perioral somatosensory determinants of nursing behavior in norway rats (Rattus norvegicus) Journal of Comparative Psychology. 1989;103:269–280. doi: 10.1037/0735-7036.103.3.269. [DOI] [PubMed] [Google Scholar]
- Stern JM, Johnson SK. Ventral somatosensory determinants of nursing behavior in Norway rats. I. Effects of variations in the quality and quantity of pup stimuli. Physiology & Behavior. 1990;47:993–1011. doi: 10.1016/0031-9384(90)90026-z. [DOI] [PubMed] [Google Scholar]
- Stevenson CW, Meredith JP, Spicer CH, Mason R, Marsden CA. Early life programming of innate fear and fear learning in adult female rats. Behavioural Brain Research. 2009;198:51–57. doi: 10.1016/j.bbr.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Nelson DY, VanOers H, Levine S. Activation and inhibition of the hypothalamic-pituitary-adrenal axis of the neonatal rat: effects of maternal deprivation. Psychoneuroendocrinology. 1995;20:169–182. doi: 10.1016/0306-4530(94)00051-b. [DOI] [PubMed] [Google Scholar]
- Tang AC. Neonatal exposure to novel environment enhances hippocampal-dependent memory function during infancy and adulthood. Learning and Memory. 2001;8:257–264. doi: 10.1101/lm.43101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AC, Akers KG, Reeb BC, Romeo RD, McEwen BS. Programming social, cognitive, and neuroendocrine development by early exposure to novelty. Proceedings of the National Academy of Science. 2006;103:15716–15721. doi: 10.1073/pnas.0607374103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AC, Zou B. Neonatal exposure to novelty enhances long-term potentiation in CA1 of the rat hippocampus. Hippocampus. 2002;12:398–404. doi: 10.1002/hipo.10017. [DOI] [PubMed] [Google Scholar]
- Thor D, Holloway W. Social memory of the male laboratory rat. Journal of Comparative and Physiological Psychology. 1982;96:1000–1006. [Google Scholar]
- Todeschin AS, Winkelmann-Duarte EC, Jacob MHV, Aranda BCC, Jacobs S, Fernandes MC, Ribeiro MFM, Sanvitto GL, Lucion AB. Effects of neonatal handling on social memory, social interactions, and number of oxytocin and vasopressin neurons in rats. Hormones and Behavior. 2009;56:93–100. doi: 10.1016/j.yhbeh.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Toth E, Avital A, Leshem M, Richter-Levin G, Braun K. Neonatal and juvenile stress induces change in adult social behavior without affecting cognitive function. Behavioural Brain Research. 2008;190:135–139. doi: 10.1016/j.bbr.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Vallee M, MacCari S, Dellu F, Simon H, LeMoal M, Mayo W. Long-term effects of prenatal stress and postnatal handling on age-related glucocorticoid secretion and cognitive performance: a longitudinal study in the rat. European Journal of Neuroscience. 1999;11:2906–2916. doi: 10.1046/j.1460-9568.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Dellu F, LeMoal M, Simon H, Maccari S. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. Journal of Neuroscience. 1997;17:2626–2636. doi: 10.1523/JNEUROSCI.17-07-02626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vanOers HJJ, deKloet ER, Whelan T, Levine S. Maternal deprivation effect on the infant’s neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. Journal of Neuroscience. 1998;18:10171–10179. doi: 10.1523/JNEUROSCI.18-23-10171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Sharma S, Plotsky PM, Meaney MJ. Increased plasma ACTH responses to stress in nonhandled compared with handled rats require basal levels of corticosterone and are associated with increased levels of ACTH secretagogues in the median eminence. Journal of Neuroscience. 1993;13:1097–1105. doi: 10.1523/JNEUROSCI.13-03-01097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J, Levine S. Early handling influences on behavioral and physiological responses during active avoidance. Developmental Psychobiology. 1977;10:161–169. doi: 10.1002/dev.420100209. [DOI] [PubMed] [Google Scholar]
- Weiner I, Feldon J, Ziv-Harris D. Early handling and latent inhibition in the conditioned suppression paradigm. Developmental Psychobiology. 1987;20:233–240. doi: 10.1002/dev.420200211. [DOI] [PubMed] [Google Scholar]
- Weiner I, Schnabel I, Lubow RE, Feldon J. The effect of early handling on latent inhibition in male and female rats. Developmental Psychobiology. 1985;18:291–297. doi: 10.1002/dev.420180402. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Domeney AM, Moreau JL, Russig H, Feldon J. Dissociation between the effects of pre-weaning and/or post-weaning social isolation on prepulse inhibition and latent inhibition in adult Sprague-Dawley rats. Behavioural Brain Research. 2001;121:207–218. doi: 10.1016/s0166-4328(01)00166-8. [DOI] [PubMed] [Google Scholar]
- Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiology & Behavior. 1999;66:293–302. doi: 10.1016/s0031-9384(98)00300-x. [DOI] [PubMed] [Google Scholar]
- Wilber AA, Southwood CJ, Sokoloff G, Steinmetz JE, Wellman CL. Neonatal maternal separation alters adult eyeblink conditioning and glucocorticoid receptor expression in the interpositus nucleus of the cerebellum. Developmental Neurobiology. 2007;67:1751–1764. doi: 10.1002/dneu.20549. [DOI] [PubMed] [Google Scholar]
- Wilber AA, Southwood CJ, Wellman CL. Brief neonatal maternal separation alters extinction of conditioned fear and corticolimbic glucocorticoid and NMDA receptor expression in adult rats. Developmental Neurobiology. 2009;69:73–87. doi: 10.1002/dneu.20691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber AA, Wellman CL. Neonatal maternal separation-induced changes in glucocorticoid receptor expression in posterior interpositus interneurons but no projection neurons predict deficits in adult eyeblink conditioning. Neuroscience Letters. 2009;460:214–218. doi: 10.1016/j.neulet.2009.05.076. [DOI] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neuroscience Biobehavioral Reviews. 2008;32:1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Wong R, Judd M. Infantile handling and successive spatial reversal learning in rats. Behavioral Biology. 1973;8:391–397. doi: 10.1016/s0091-6773(73)80079-3. [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proceedings of the National Academy of Science. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workel JO, Oitzel MS, Fluttert M, Lesscher H, Karssen A, deKloet ER. Differential and age-dependent effects of maternal deprivation on the hypothalamic-pituitary-adrenal axis of brown norway rats from youth to senescence. Journal of Nueroendocrinology. 2001;13:569–580. doi: 10.1046/j.1365-2826.2001.00668.x. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Grossman R. Childhood trauma and risk for PTSD: relationship to intergenerational effects of trauma, parental PTSD, and cortisol excretion. Development & Psychopathology. 2001;13:733–753. doi: 10.1017/s0954579401003170. [DOI] [PubMed] [Google Scholar]
- Zhang M, Cai JX. Neonatal tactile stimulation enhances spatial working memory, prefrontal long-term potentiation, and D1 receptor activation in adult rats. Neurobiology of Learning and Memory. 2008;89:397–406. doi: 10.1016/j.nlm.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Zou B, Golarai G, Connor JA, Tang AC. Neonatal exposure to a novel environment enhances the effects of corticosterone on neuonal excitability and plasticity in adult hippocampus. Brain Research Developmental Brain Research. 2001;130:1–7. doi: 10.1016/s0165-3806(01)00173-0. [DOI] [PubMed] [Google Scholar]


