Abstract
The large, elastic arteries, as their name suggests, provide elastic distention and recoil during the cardiac cycle in vertebrate animals. The arteries are distended from the pressure of ejecting blood during active contraction of the left ventricle (LV) during systole, and recoil to their original dimensions during relaxation of the LV during diastole. The cyclic distension occurs with minimal energy loss, due to the elastic properties of one of the major structural extracellular matrix (ECM) components, elastin. The maximum distension is limited to prevent damage to the artery by another major ECM component, collagen. The mix of ECM components in the wall largely determines the passive mechanical behavior of the arteries and the subsequent load on the heart during systole. While much research has focused on initial artery formation, there has been less attention on the continuing development of the artery to produce the mature composite wall complete with endothelial cells (ECs), smooth muscle cells (SMCs), and the necessary mix of ECM components for proper cardiovascular function. This review focuses on the physiology of large artery development, including SMC differentiation and ECM production. The effects of hemodynamic forces and ECM deposition on the evolving arterial structure and function are discussed. Human diseases and mouse models with genetic mutations in ECM proteins that affect large artery development are summarized. A review of constitutive models and growth and remodeling theories is presented, along with future directions to improve understanding of ECM and the mechanics of large artery development.
Keywords: elastin, collagen, biomechanics, aorta
1. Introduction
In vertebrate animals, the large arteries provide an important energy storage and pulse dampening function known as the windkessel effect. The energy from the pressure of ejecting blood from the left ventricle (LV) during systole is stored as strain energy in the distended artery wall. The strain energy is returned during diastole when the LV relaxes and the arteries return to their original dimensions. Because the aortic valve is closed during diastole, the displaced blood moves further down the arterial tree with a dampened pulse wave (Greenwald 2007). The protein elastin, a major component of the large, elastic arteries, provides the elasticity necessary for cyclic deformation of the arterial wall with minimal energy loss. Elastin is only found in vertebrate arteries, indicating an evolutionary requirement for elastic recoil in a closed circulatory system with high blood pressure and pulsatile blood flow (Sage and Gray 1979). In invertebrate animals with an open circulatory system, low blood pressure and constant blood flow, the arterial wall extracellular matrix (ECM) is composed mainly of collagen, which provides strength and limits distension at supraphysiologic pressures. The mix of ECM proteins in the wall of vertebrate arteries is established during development and defines the passive mechanical behavior of the large, elastic arteries. The passive mechanical behavior of these arteries partly determines the load on the heart, as higher systolic pressures are required to generate flow in stiffer arteries (Greenwald 2007), and is therefore a critical determinant of cardiac function.
While much research has focused on vasculogenesis, starting with the formation of endothelial cell (EC) tubes, less attention has been paid to the development of the full arterial wall structure, including ECs, smooth muscle cells (SMCs) and the necessary ECM proteins, including elastin and collagen. This review highlights the physiology of large artery development, including SMC differentiation and ECM production, and reinforces the idea that ECM production is indicative of SMC phenotype. The effects of hemodynamic forces and ECM deposition on evolving arterial structure and function are discussed and it is evident that arterial wall maturation occurs in concert with significant changes in blood pressure and blood flow. There is a focus on large artery development in the mouse, so that developmental timing can be compared for different events in a single animal model. A summary is given of human diseases and genetically modified mice with mutations in ECM proteins that affect large artery development, with a focus on elastin. Genetically modified mice are critical for understanding how the arterial wall adapts and remodels when the necessary wall structure cannot be created. A review of constitutive models and growth and remodeling theories for large arteries is presented, with an emphasis on the limited studies available for embryonic and postnatal development. Several future directions are suggested to improve understanding of ECM and the mechanics of large artery development, including linking models that span from tissue-, to cell- to molecular-level stresses and deformations. This understanding is essential to design beneficial interventions in human cardiovascular disease and intelligent protocols for tissue engineering of large arteries.
2. Large artery development
Vasculogenesis begins with the formation of blood islands in the extra-embryonic mesoderm that contain angioblasts and hematopoetic cells. The blood islands fuse and the angioblasts differentiate into ECs to form the primary capillary plexus (Risau and Flamme 1995). Within the embryo proper, angioblasts from the splanchnic mesoderm migrate toward the midline, fuse and form angioblastic cords that hollow out into tubes and eventually become the major vessels. These two locations of vasculogenesis interconnect, forming a complete vascular loop around embryonic day (E) 8 in the mouse (Jones 2011), just before the heart starts to beat at E8.25. The hematopoetic cells remain in the blood islands until blood flow is detected in the embryo around E8.5 (Ji et al. 2003). Vasculogenesis occurs similarly in other organisms used to study vessel formation, such as birds, but with altered timelines. Once the blood circulation is established, the vascular network is remodeled to change vessel diameter, branching patterns and to recruit other cell types (Jones 2011). The EC tubes remain free of other cells, such as fibroblasts, SMCs and pericytes for up to 24 hours in the quail (Drake 2003). After this time, primordial SMCs are recruited to form layers around the initial EC tubes and are evident at E10.5 in the mouse (Majesky et al. 2011). The SMC layers develop in a ventral to dorsal manner and in a radial pattern beginning with the cells closest to the ECs (Hungerford et al. 1996).
The primordial SMCs in the large arteries come from at least four different embryonic origins: 1) Secondary heart field for the base of the aorta, 2) Neural crest for the ascending aorta and common carotid arteries, 3) Somites for the descending thoracic aorta, and 4) Splanchnic mesoderm for the abdominal aorta (Majesky 2007). Some SMCs may also arise from marrow-derived progenitor cells (Ross et al. 2006), macrophages (Ninomiya et al. 2006), and transdifferentiation of ECs (DeRuiter et al. 1997; Frid et al. 2002). Recent evidence suggests that SMC progenitor cells reside in the adventitial region throughout adult life, providing a source for new SMCs during arterial wall growth, remodeling and repair (Majesky et al. 2011). Presumptive SMCs have a “fibroblast-like” appearance and do not express typical SMC marker proteins until later in development. It is difficult to pinpoint exactly when the presumptive cells become SMCs, as the marker proteins are not necessarily specific for SMCs in general or vascular SMCs (VSMCs) in particular. Additionally, expression varies along the arterial tree and even across the arterial wall thickness. The markers generally accepted for VSMCs are the smooth-muscle-specific isoforms of myosin heavy chains, SM-1 and SM-2, and expression is not detected until the cells have already formed the multilayered vessel wall (Hungerford and Little 1999).
Significant vascular patterning changes occur between E11 and E14 in the mouse. At E11, there are numerous symmetric arches leading off the truncus arteriosis. Over the next three days, they will transform into the aorta, the major branches of the aorta and the pulmonary artery (Effmann et al. 1986). The complete separation of the aorta and pulmonary artery occurs around E13.5 (Vuillemin and Pexieder 1989) and the definitive vascular pattern is established by E14. After this time, the arteries undergo significant changes in diameter, length, wall thickness and wall composition. The composition is altered through the deposition of ECM proteins necessary for the proper mechanical behavior of the wall. The two major structural ECM proteins in the wall of mature, elastic arteries are elastin and collagen. The combination of ECM proteins, specifically the ratio of elastin to collagen, will determine the mechanical properties of a particular artery and will also affect SMC morphology and function. The amount and type of ECM proteins produced may be a better indicator of SMC phenotype than traditional SMC marker proteins (Hungerford and Little 1999). For example, presumptive SMCs surrounding the EC lumen show changes in intracellular architecture suggestive of phenotypic changes at the same time that collagen and basal lamina proteins are deposited between the two cell layers (Murphy and Carlson 1978). In vitro, SMC phenotype is modulated by interactions with the ECM substrate. For example, culturing SMCs on fibronectin or collagen type I promotes a synthetic phenotype, while laminin or elastin promotes a contractile phenotype (Hedin et al. 1988; Qin et al. 2000; Yamamoto et al. 1993).
3. Developmental hemodynamics
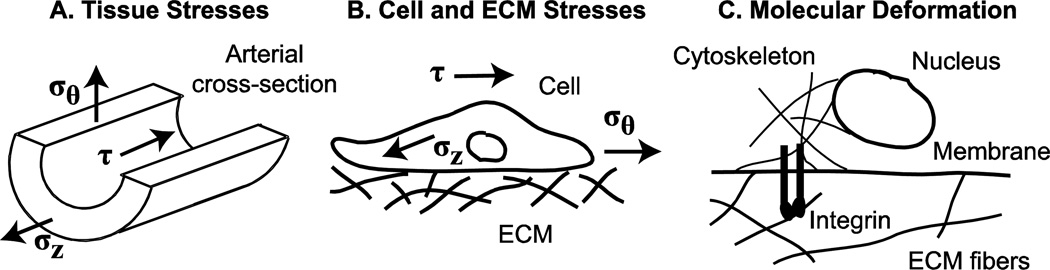
It has been known for over a century that the blood flow, blood pressure and axial force exerted on a growing vessel will influence its form and function. In 1893, Thoma formulated these observations into three postulates 1) vessel lumen size depends on blood flow, 2) vessel wall thickness depends on blood pressure, and 3) vessel length depends on axial force exerted by connective tissues (Clark 1918). These relationships can be better understood by examining the average shear stress (τ) and normal stresses in the circumferential (σθ) and axial (σz) directions on the vessel wall:
| (1) |
where Q = blood flow, µ = blood viscosity, and ri = inner radius;
| (2) |
where P = blood pressure and ro = outer radius, and
| (3) |
where F = total axial force.
Thoma’s observations and the above equations show that changes in blood flow, blood pressure or axial forces, can be counteracted by respective changes in the vessel inner radius, wall thickness or length to maintain constant stresses on the vessel wall. This has been well-studied in adult animals where it is relatively straightforward to perturb the steady state with step changes in blood flow, blood pressure or axial length and observe the resulting changes in vessel wall geometry. The relationships are more difficult to study in growing animals where the hemodynamics and geometry are constantly changing and the stresses are not yet at a steady state. However, understanding the relationships between mechanical stimuli and vessel form and function is necessary for predicting or preventing changes caused by altered hemodynamics in developmental diseases and for recreating the developing hemodynamic environment in arterial tissue engineering protocols.
3.1. Blood flow
Clark (1918) investigated the relationship between blood flow and inner radius by making careful measurements of growing vessels in the tail of frog larvae. Clark found that capillaries with a decrease in blood flow volume would decrease in diameter and eventually disappear, while capillaries with an increase in blood flow volume would increase in diameter and become arterioles or venules. Clark also summarized results of different studies aimed at determining how vessels develop in the absence of blood flow. From these he concluded that extensive capillary development takes place before the heart starts beating and in animals where the heart has been removed or prevented from beating, but this stage “comes to an end relatively early” and further development of the vascular system depends on mechanical factors.
More recent results using genetically-modified mice to disrupt cardiac contractility and pumping show that vascular development is halted at the capillary plexus stage and suggest that remodeling of the capillary plexus to form the mature vascular network depends on blood flow. Vascular defects are apparent around E9 in these mice, soon after circulation is established in normal embryos (Huang et al. 2003; May et al. 2004; Wakimoto et al. 2000). When flow is cut off using a physical approach in bird embryos, no arterial-venous differentiation or patterning is observed, although the capillary plexus continues to grow. Additionally, when normal flow patterns are altered, arteries and veins form in new regions, implying that blood flow determines arteriolar and venular differentiation and patterning (le Noble et al. 2004). While these experiments support a role for blood flow in vascular development, they do not explain why blood flow is necessary for vascular remodeling. It has been hypothesized that the delivery of oxygen or nutrients is necessary for vascular remodeling or that the resultant shear stress is necessary for cell signaling cascades that activate remodeling pathways (Jones et al. 2006). By altering the viscosity of blood and the consequent shear stress (Eqn. 1), but not the velocity and resulting delivery of oxygen or nutrients, Lucitti et al. (2007) showed that shear stress alone is necessary and sufficient to induce vascular remodeling in the mammalian yolk sac. For formation of the large arteries, SMCs must be recruited to surround the remodeled capillary network. In the absence of Kruppel-like factor 2 (KLF2), a transcription factor for several mechanosensitive genes, mouse embryos die at E14.5 from severe hemorrhage. The aorta in KLF2−/− mice does not form a compact medial layer and the SMCs in the vessel wall have altered morphology, reduced expression of SMC marker proteins and reduced deposition of ECM proteins (Kuo et al. 1997), indicating that shear stress may be necessary for SMC recruitment and phenotypic maturation.
It has been shown in vivo and in vitro that fluid flow alters EC morphology, function, and gene expression (Ando and Yamamoto 2009). ECs exposed to unidirectional fluid flow become elongated and align in the direction of flow (Dewey et al. 1981; Langille and Adamson 1981), which is similar to their in vivo arrangement. Increased fluid flow causes increased endothelial nitric oxide synthase (eNOS) expression, which increases NO expression and causes SMC relaxation and vessel dilation (Boo and Jo 2003; Rubanyi et al. 1986). Shear stress alters production of growth factors such as platelet-derived growth factor (PDGF) (Hsieh et al. 1991) and transforming growth factor-β (TGF-β) (Ohno et al. 1995) and affects the production and elimination of reactive oxygen species (ROS) (Laurindo et al. 1994). DNA microarray analysis shows that more than 600 EC genes are up- or down-regulated with different temporal patterns in response to shear stress (Ohura et al. 2003).
ECs are the primary cell type responding to fluid shear stress. Resulting changes in SMC function and arterial wall composition and geometry are generally thought to be the result of EC signals, such as increased NO production and altered levels of ROS. However, in vitro results show that SMCs are also directly responsive to flow induced shear stress. SMCs exposed to unidirectional flow in vitro orient perpendicular to the flow direction (Lee et al. 2002), unlike ECs which orient parallel to the flow direction. In the arterial wall, SMCs are mostly arranged circumferentially, which is perpendicular to the flow direction. Shear stress promotes release of PDGF and basic fibroblast growth factor (bFGF) from SMCs (Sterpetti et al. 1994) and alters expression of numerous other cytokines and signaling molecules (Shi and Tarbell 2011). Fluid flow alters SMC proliferation (Haga et al. 2003; Ekstrand et al. 2010) and contractility (Asada et al. 2005; Civelek et al. 2002), but different studies often show contradictory results. The variations in SMC phenotype, shear stress profiles and differences between two-dimensional in vitro systems and the three-dimensional in vivo environment of the vessel wall, including the ECM proteins, may explain the varying reactions of SMCs to fluid shear stress. In vivo, SMCs are not exposed directly to fluid shear stress because of the EC barrier lining the vessel lumen. However, there is interstitial fluid flow from the inside to the outside of the vessel driven by the pressure difference across the wall. The resulting shear stress is on the order of 1 dyn/cm2 (Wang and Tarbell 1995) and may be up to 100 times higher in the immediate vicinity of pores in the internal elastic lamina (Tada and Tarbell 2000).
3.2. Blood pressure
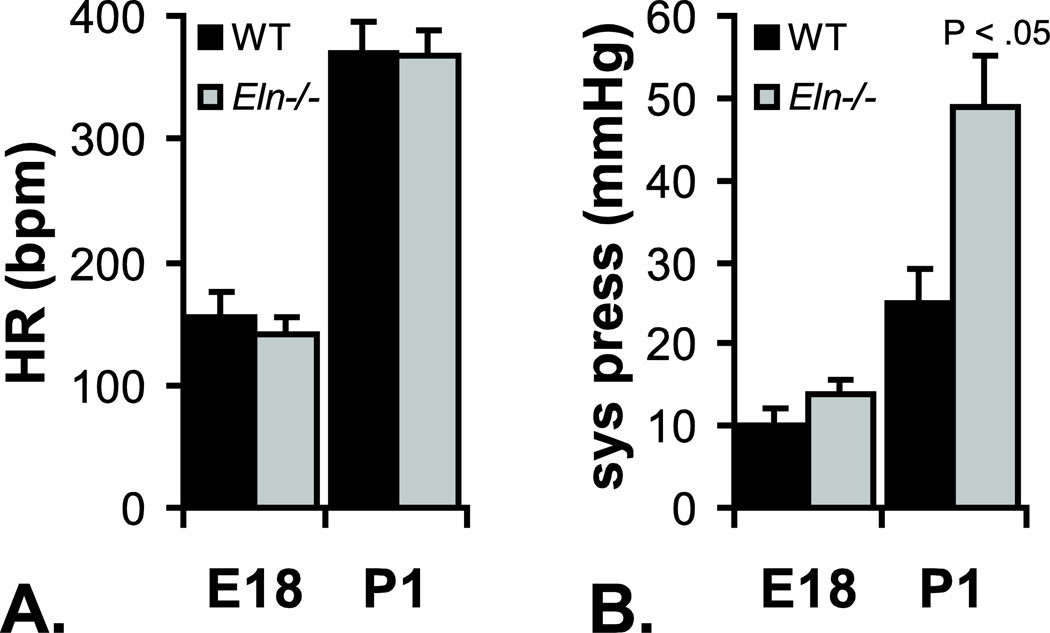
During embryonic development, the pulmonary and systemic circulations are connected by the ductus arteriosis. After birth, the ductus arteriosis closes and the aorta and pulmonary artery carry similar volumetric blood flows, but are exposed to very different blood pressures due to a decrease in peripheral resistance that occurs in the pulmonary circulation at birth. Consistent with Thoma’s postulates and Eqns. 1 and 2, the inner radii of the aorta and pulmonary artery are similar, but the wall thickness increases in the aorta and decreases in the pulmonary artery to adapt to the different pressure in each artery (Leung et al. 1977). In postnatal development, blood pressure and medial wall thickness increase simultaneously in systemic arteries (Gerrity and Cliff 1975). In embryonic development, blood pressure is first measurable soon after blood circulation begins and the heart starts beating. Peak systolic LV pressure increases from 2 to 11 mmHg from E9.5 – E14.5 in the mouse (Ishiwata et al. 2003). Note that these pressure increases occur during the time that SMCs are organizing in layers around the EC lumen, so wall thickness increases through the addition of SMC layers. Around E14, the layers are complete and the SMCs start expressing structural ECM proteins, so wall thickness increases through the addition of elastin and collagen between the cell layers (Figure 1). Systolic LV pressure was measured at 10 mmHg at E18 and 25 mmHg at P1 in mice (Figure 2) (Wagenseil et al. 2009, 2010). Although there are variations between different studies, the overall results show a large pressure increase during the last trimester of mouse development and especially just before birth.
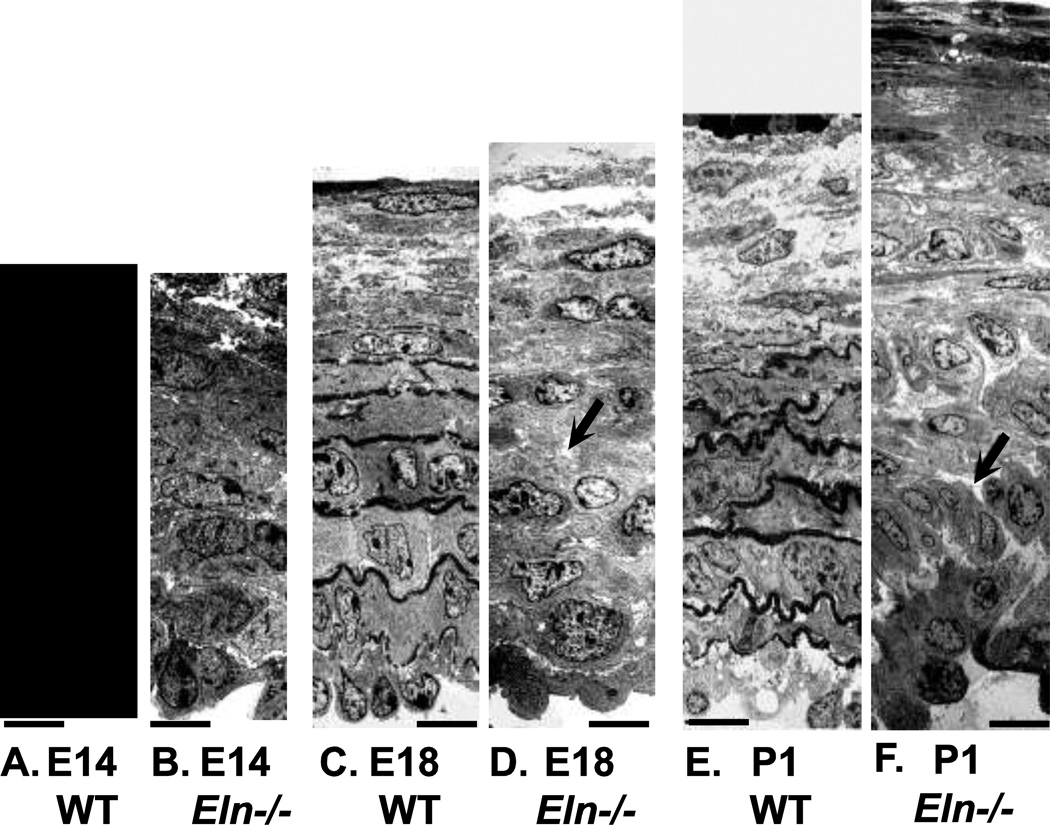
Figure 1.
EM images of WT and Eln−/− mouse aorta at E14, E18 and P1. At E14, WT (A) and Eln−/− (B) aorta look similar, with 6 – 7 layers of circumferentially oriented SMCs. WT E14 aorta has small deposits of elastin (arrow) between cell layers. At E18, WT (C) and Eln−/− (D) aorta still look similar with organized layers of circumferentially oriented SMCs. WT E18 aorta has large deposits of elastin between layers, while Eln−/− E18 aorta has large white spaces (arrow) that are most likely proteoglycans, which do not take up the dark EM stain. At P1, WT (E) aorta has adundant elastin between layers of circumferentially oriented SMCs. At P1, Eln−/− (F) aorta has disorganized SMCs that have lost their circumferential orientation (arrow) and cell-cell contacts and have begun overproliferating. Panels E and F modified from Wagenseil et al. (2009). Scale bars = 11 µm.
Figure 2.
Heart rate (HR) (A) and left ventricular (LV) systolic pressure (sys press) (B) for E18 and P1 WT and Eln−/− mice. Heart rates are similar between genotypes at both ages. LV systolic pressure is similar between genotypes at E18, but Eln−/− pressure is almost double WT by P1. Data from Wagenseil et al. (2009, 2010).
Increased pressure stretches SMCs in the artery wall and increases both the circumferential stress and stretch of the wall. Investigating changes in circumferential stress requires knowledge of the cell mechanical properties, therefore most in vitro studies focus on the effects of changes in stretch. In vitro, SMCs respond to stretch by upregulating production of elastin (Sutcliffe and Davidson 1990), collagen (Durante et al. 2000), and numerous signaling molecules (Katsumi et al. 2002; Li et al. 2003; Mills et al. 1997). In vitro, cyclic stretch has been shown to alter SMC proliferation, apoptosis and expression of SMC marker proteins through the Notch signaling pathway (Morrow et al. 2005a; Morrow et al. 2005b). The Notch pathway is a key regulator of vascular morphogenesis, controlling growth of the blood vessel network, cell proliferation and the differentiation of arteries and veins (Roca and Adams 2007). The involvement of the Notch pathway suggests that cyclic stretch may be an important stimulus for SMC differentiation during early formation of the large arteries. It has been shown that cyclic stretch induces SMC differentiation in embryonic stem cells (Riha et al. 2007; Shimizu et al. 2008). The response of SMCs to stretch is modulated by interactions with the ECM substrate. SMCs on collagen type I or fibronectin coated membranes show increased DNA synthesis in response to stretch, while SMCs on elastin or laminin coated membranes do not (Wilson et al. 1995). SMCs on collagen type I coated membranes also show increased apoptosis in response to stretch, compared to SMCs on elastin or laminin coated membranes (Wernig et al. 2003).
Most in vitro studies apply cyclic stretch to mimic the deformation caused by the pulsatile pressure wave in adult arteries. Although the arterial pulse pressure magnitudes have not been measured in late embryonic development in mice, ultrasound studies show a clear pulsatile deformation of the aortic wall at E18. Interestingly, the percent deformation is approximately the same at P1 (Figure 3), despite large changes in systolic pressure (Figure 2) and presumably pulse magnitude. This implies that the aortic wall is actively growing and remodeling, through modulation of SMC phenotype and deposition of the appropriate mix of ECM proteins, to maintain a constant percent deformation at the physiologic pulse pressure for each age.
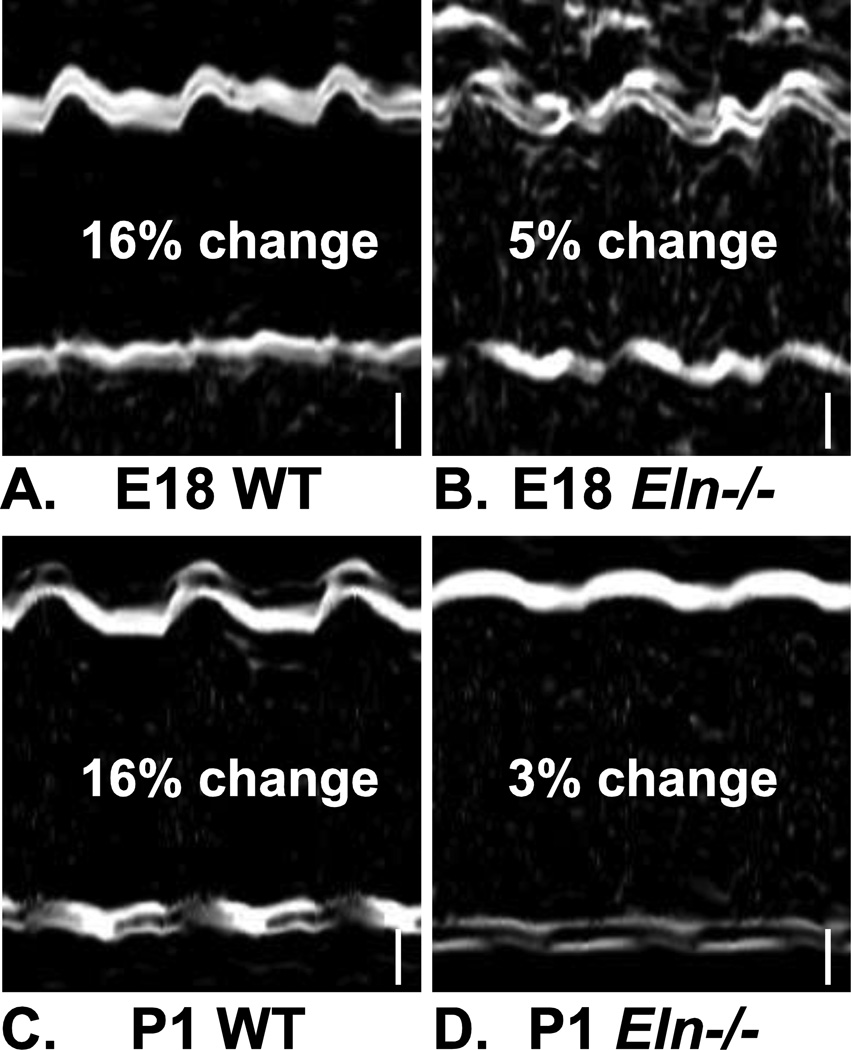
Figure 3.
M-Mode ultrasound images showing in vivo deformation of the aortic wall in E18 and P1 WT and Eln−/− mice. The percent change of the inner diameter between diastole and systole is 16% for WT aorta (A, C) and 3 – 5% for Eln−/− aorta (B, D). Three cardiac cycles are shown. Modified from Wagenseil et al. (2009, 2010). Scale bars = 100 µm.
The tension in the wall is equal to the pressure times the inner radius and is related to the circumferential stress, but is not normalized by the wall thickness. Wolinksy and Glagov (1967) showed that the tension/lamellar unit (one layer of SMCs and elastin) is constant across numerous mammalian species. The lamellar units are formed in late embryonic development and do not change after birth (Cliff 1967; Paule 1963). In the mouse aorta, the number of SMC layers is established before E14 and abundant elastin is deposited between each SMC layer by E18 (Figure 1).
3.3. Axial tension
In his studies on frog larvae, Clark (1918) found that the increase in capillary length in any direction was the same as the growth of the tail in that direction, suggesting that tension exerted by surrounding tissues determines vessel length. In the first 30 days of postnatal development in the mouse, body length and aortic length grow at constant rates, although the rate of body length growth is about twice that of the aorta (Huang et al. 2006). The stretch ratio of the entire aorta (Huang et al. 2006) and of the common carotid artery alone (Le et al. 2011) in the mouse increases about 10% in the first 30 days after birth, which may be a result of the differential growth rate between the arteries and surrounding tissues.
Adult arteries are highly sensitive to changes in axial stress or strain. In response to a mechanical stimulus, growth in the axial direction is much faster (on the order of days) (Davis et al. 2005; Jackson et al. 2002) than in the circumferential direction (on the order of months) (Fridez et al. 2003; Wolinsky 1970). Growth occurs through increased cell proliferation and deposition of ECM proteins. Because of the interrelationship between axial strain and the circumferential stress-strain relationship, both circumferential and axial stresses can be normalized by growth in the axial direction (Humphrey et al. 2009). Unlike shear or circumferential stresses, arteries cannot adapt to reductions in axial stress. When axial stretch is decreased, the artery does not shorten to normalize the changes, but lengthens and becomes tortuous. This axial remodeling depends on breakdown of the ECM proteins, as inhibition of matrix metalloproteinases by doxycycline prevents the lengthening and observed tortuosity (Jackson et al. 2005).
The axial forces in Eqn. 3 are the sum of traction forces in the vessel wall and forces from the blood pressure that tend to lengthen the artery. As pressure increases during the cardiac cycle, the traction forces decrease, while the pressure forces increase, leading to an almost constant total axial force within the physiologic blood pressure range. Experiments with proteases that selectively degrade elastin or collagen show that elastin is largely responsible for the axial traction force (Dobrin 1997).
4. Elastin deposition and large artery mechanics
Elastin provides elasticity and reversible stretch to accommodate pulsatile pressure waves from the heart. The arterial pressure pulse magnitude decreases with distance from the heart, due to the pulse dampening function of the elastic arteries. The amount of elastin also decreases with distance from the heart as less pulse dampening is required in the distal vessels. The amount of collagen, which provides strength and limits stretch at high pressures, remains approximately constant with distance from the heart (Dobrin 1997). The elastic modulus of collagen is 100 – 1000 times higher than elastin, so the decrease in the ratio of elastin to collagen provides a gradient of arterial stiffening down the vascular tree (Greenwald 2007). The large elastic or conducting arteries are those closest to the heart that have relatively high elastin to collagen ratios.
The role of elastin as an arterial pulse dampener, or windkessel (Dobrin 1997), is supported by evolutionary evidence. Elastin is found exclusively in vertebrate animals and its emergence coincides with the appearance of a closed circulatory system (Sage and Gray 1979). In invertebrate animals with an open circulatory system, low pressure and constant flow, the arterial wall ECM is composed mostly of collagen. In invertebrates with highly developed circulatory systems, or lower vertebrates with transitional, partially open circulations, the arterial wall ECM is composed of collagen and microfibrils, but no elastin (Wagenseil and Mecham 2009). Microfibrils consist mostly of the proteins fibrillin-1 and fibrillin-2 and are found in close apposition to developing elastic fibers. The developmental expression pattern of fibrillin-1 is similar to that of elastin, though peak expression occurs earlier (Zhang et al. 1995). After the elastic fibers are fully assembled the microfibrils are no longer visible and have either been removed or completely covered with dense elastin deposits. Fibrillin appears earlier in the evolutionary timeline than elastin and may play a pulse dampening role in transitional circulations. The increase in pressure between E18 and P1 in the mouse, when abundant collagen, microfibrils and elastin deposits are laid down, is similar to the evolutionary pressure increase between invertebrates and jawless vertebrates when the arterial wall ECM changes from mostly collagen to collagen and microfibrils (Wagenseil et al. 2010). In higher vertebrate animals, there is a closed circulatory system, high pressure, pulsatile flow and the arterial wall ECM is composed of collagen, elastin and microfibrils. The lowest systolic blood pressure in adult vertebrates is about 40 mmHg, which occurs around P7 in the mouse (Le et al. 2011), at the same the time that the elastic lamellae in the aorta become complete (Davis 1995). Presumably, the elastic lamellae are not fully functional mechanically before all of the elastin deposits have been connected in continuous layers. It appears that the ECM composition of the arterial wall is directly related to blood pressure throughout evolution and vertebrate development.
Elastin is secreted from cells in its soluble form, tropoelastin. Tropoelastin is crosslinked by lysyl oxidase (LOX) to form elastin globules that are visible by light and electron microscopy (EM) in vitro and in embryonic arterial tissue (Kozel et al. 2005). With the help of additional proteins, such as the fibulins, MAGP and EMILIN, the globules coalesce around microfibrils to form the complete elastic fibers found in the mature arterial wall (Wagenseil and Mecham 2007). Turnover of elastin is slow and assembly of elastic fibers is negligible in adult animals, therefore the elastic fibers created during development must last the lifetime of the animal. Gene array data in developing mouse aorta show that the elastic fiber proteins, and most ECM proteins, follow highly coordinated expression patterns. ECM gene expression in mice begins around E14, rises steadily through P14, then decreases to low, baseline levels around P30 (Kelleher et al. 2004). Similar expression patterns are seen in arteries from sheep, human, and rats (Bendeck and Langille 1991; Gerrity and Cliff 1975; Berry et al. 1972). Note that ECM gene expression in the mouse begins a few days after primordial SMCs are first detected in layers around EC tubes at E10.5 (Majesky et al. 2011) and after the significant patterning changes are complete at E14 (Effmann et al. 1986). ECM gene expression is turned on when the medial cell layers are complete and when the SMC specific proteins SM-1 and SM-2 are first detectable (Hungerford and Little 1999), confirming the observation that ECM gene expression is a suitable indicator of emerging SMC phenotype. ECM gene expression is highest during significant increases in pressure and flow and is essentially turned off as the hemodynamic forces stabilize (Wagenseil and Mecham 2009).
5. Developmental pathology with disrupted ECM
ECM proteins are critical for proper development and function of the large arteries, and of other organ systems, including the lung, bones, skin, and cartilage. There are several human diseases resulting from the loss of function or expression of important ECM proteins. Cardiovascular phenotypes range from narrowing to dilation to rupture of the large arteries, depending on the affected ECM protein. Mouse models have been developed to better understand the effects of absent or reduced levels of ECM proteins. This review will focus on genetic diseases and animal models relating specifically to elastin, elastin-associated proteins and arterial collagens. The major collagens in the large elastic arteries are types I, III, IV, V, and VI (McLean et al. 2005).
5.1. Human diseases
William’s Syndrome (WS) is a neurodevelopmental disorder resulting in a distinct, “elfin” facial appearance along with mild to moderate retardation (Meyer-Lindenberg et al. 2006). While the most salient symptom is the cognitive impairment, many individuals also exhibit a cardiovascular defect known as supravalvular aortic stenosis (SVAS). SVAS is a congenital narrowing of the aortic root just outside the LV outflow tract. If left untreated, it can lead to cardiac hypertrophy and eventual LV failure (Aboulhosn and Child 2006). Isolated SVAS is caused by point mutations in the elastin gene (ELN) that cause functional haploinsufficiency (Li et al. 1997). SVAS arises in WS individuals due to a deletion of a contiguous region of genes that includes ELN (Osborne et al. 1996).
Mutations in the elastin gene have also been associated with the autosomal dominant form of cutis laxa (ADCL). While skin is the major organ affected in ADCL, there are reports of abnormalities in other organs, including the cardiovascular system (Callewaert et al. 2011; Szabo et al. 2006). Autosomal recessive cutis laxa (ARCL) Type 1 arises from mutations in fibulin-4 and -5, which play critical roles in elastic fiber assembly (Yanagisawa and Davis 2010). Mutations in fibulin-5 result in lax skin due to disorganized elastic fibers, with limited involvement of other organ systems (Hu et al. 2006; Loeys et al. 2002; Lotery et al. 2006). Mutations in fibulin-4 result in a much more severe phenotype including lax skin, aortic aneurysm and tortuous arteries at a young age (Dasouki et al. 2007; Hucthagowder et al. 2006).
Marfan syndrome (MFS) is an autosomal dominant disorder affecting connective tissue and causing abnormalities in the cardiovascular, skeletal, and ocular systems (Francke and Furthmayr 1994). Skeletal abnormalities generally manifest as disproportionately long limbs and fingers (arachnodactyly), hypermobility in joints, and a highly arched palate with a narrow jaw, and decreased bone mineral density (osteopenia). Ocular abnormalities include myopia, increased axial length of the eye, corneal flatness, and a displaced lens. In regards to cardiovascular defects, MFS patients typically have aortic and mitral valve regurgitation and aortic root dissection, dilation, and ultimately rupture (Phornphutkul et al. 1973). Histological studies reveal highly disrupted elastic lamellae in MFS aorta (Recchia et al. 1995). MFS pathogenesis is associated with mutations in fibrillin-1 (FBN1) causing improper formation of elastic fibers and dysregulated TGF-β signaling (Dietz et al. 2005).
Ehlers-Danlos syndrome (EDS) is a group of six heritable disorders with variable symptoms in different connective tissue systems. The most common EDS symptoms are thin and loose skin, joint hypermobility, and fragility in blood vessels and other connective tissue (Pyeritz 2000). Classical EDS has been linked to a mutation in the collagen V gene (COL5A1 and COL5A2). Type V collagen is critical for initial fibril formation and functional haploinsufficiency for the gene leads to larger collagen fibrils and a less compact fibril arrangement (Schwarze et al. 2000). Vascular EDS (formerly EDS type IV) carries an increased risk of fatal rupture of the bowels and large arteries with little evidence of dissection or aneurysm (Proske et al. 2006). Vascular EDS is caused by a lack of type III collagen, reducing the burst strength of large arteries (Pope et al. 1975).
5.2. Animal Models
Additional evidence for the role of ECM proteins in large artery development can be obtained from animal models - specifically genetically modified mice. While there are significant differences between the cardiovascular systems of mice and humans, mice allow relatively simple genetic modifications to investigate the absence of, reduced levels of, and mutations in specific ECM proteins. These animal models are essential for providing a better understanding of how alterations in ECM proteins affect cardiovascular function and human health.
5.2.1. Elastin
Mice that do not express the elastin gene (Eln−/−) die within a few days of birth from overproliferating SMCs that occlude the vessel lumen (Li et al. 1998). LV systolic pressure in Eln−/− mice is similar to WT at E18, but almost double WT values by P1, despite similar heart rates (Figure 2). If the large, conduit arteries were rigid tubes instead of compliant windkessels, an infinitely high blood pressure would be required to produce blood flow in systole (Greenwald 2007). Although the Eln−/− aorta is not a rigid tube, it is significantly less compliant than WT at E18 (Wagenseil et al. 2010) and P1 (Wagenseil et al. 2009), which would require higher pressures to generate the necessary blood flow to perfuse distal tissues. There may be a delay between the requirement for increased pressure (at E18) and the manifestation (at P1). The reduced compliance can be seen from in vivo images of the aortic wall deformation (Figure 3). The percent deformation of the Eln−/− aorta from diastole to systole is 3 – 5 times less than WT, significantly reducing the important energy storage and pulse dampening function of the large arteries.
Examination of EM images from Eln−/− and WT aorta during late embryonic development shows the rapid progression from a normal to a pathological wall structure (Figure 1). At E14, when the major arterial patterning changes are complete and ECM expression is just beginning, WT and Eln−/− aorta look similar. Both aortas have 6 – 7 layers of circumferentially aligned SMCs with ECM proteins, including collagen, microfibrils and proteoglycans, deposited between the cell layers. E14 WT aorta has small deposits of elastin between the cell layers that are not present in Eln−/− aorta. At E18, abundant elastin deposits are visible in WT aorta. The WT elastin layers are most complete in the central portion of the wall thickness, with discontinuous layers near the intima and adventitia. In E18 Eln−/− aorta, there are still layers of circumferentially oriented SMCs and there is no evidence of SMC overproliferation. There may be increased production of proteoglycans by the Eln−/− SMCs. At P1, only three days later assuming an average 20 – 21 day gestation period, the SMCs in Eln−/− aorta have lost their circumferential orientation and cell-cell contacts and have begun overproliferating, leading to eventual occlusion of the vessel lumen.
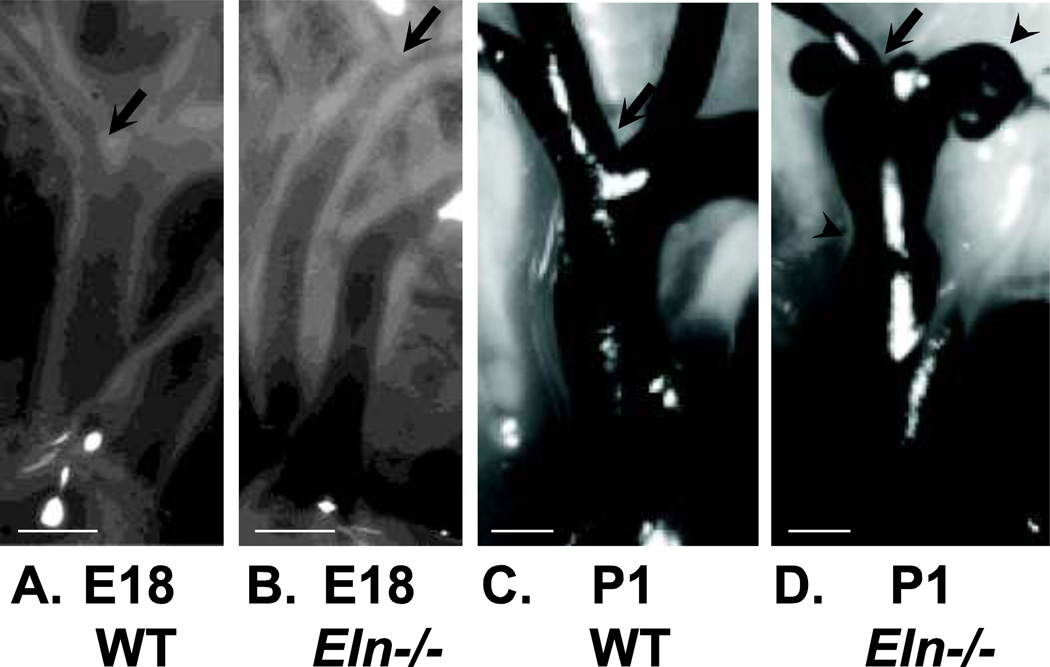
Dramatic changes in arterial structure between E18 and P1 in Eln−/− mice can also be seen from gross morphological examination of the aorta and its branches (Figure 4). At E18, Eln−/− aorta has a smaller diameter and longer length, but is patent, with no severe changes in geometry. At P1, Eln−/− aorta still has a smaller diameter and longer length, but also shows local stenoses and dilations. Tortuosity is present in the P1 Eln−/− carotid arteries (Wagenseil et al. 2010). The increased length and tortuosity of Eln−/− arteries emphasize the role of elastin in the axial mechanical behavior (Dobrin 1997) and the degree to which arteries can remodel in the axial direction in response to altered mechanical stimuli (Jackson et al. 2005; Jackson et al. 2002). Gross morphological and ultrastructural analyses show that the major changes in Eln−/− arteries occur during the last few days of embryonic development. This time period corresponds to a large increase in systolic blood pressure (Figure 2), suggesting that the Eln−/− mouse dies because, without elastin, the arterial wall cannot withstand the stresses caused by the developmental increases in blood pressure.
Figure 4.
Images of the ascending aorta and its major branches in E18 and P1 WT and Eln−/− mice. The arrow in all panels shows the location of the first branch. At E18, the Eln−/− (B) aorta is smaller in diameter and longer in length than WT (A), but shows no other major morphologic differences. At P1, the Eln−/− (D) aorta is still smaller in diameter and longer in length than WT (C), but also shows severe stenoses and dilations (arrowheads). In the P1 images, india ink was injected into the arteries to improve contrast. Modified from Wagenseil et al. (2009, 2010). Scale bars = 300 µm.
In mice that express approximately half the normal amount of elastin (Eln+/-), mean arterial blood pressure is 40% higher than WT and the arteries have smaller diameters, thinner walls and reduced axial stretch ratios (Wagenseil et al. 2005). The typical arterial remodeling response to hypertension is an increase in wall thickness, not a decrease (Berry and Greenwald 1976). The decreased wall thickness in Eln+/- arteries is not caused by an impaired remodeling response, because when blood pressure is increased even further in adult animals, the arterial wall thickness increases, as expected, and to a similar degree as WT animals (Wagenseil et al. 2007). Eln+/- arteries have an increased elastic modulus and an increased number of lamellar units (Faury et al. 2003). Because both the pressure and number of lamellar units are increased, the tension/lamellar unit remains within typical limits seen in mammalian species (Wolinsky and Glagov 1964). Humans with SVAS also have hypertension, smaller arteries and increased arterial lamellar units (Li et al. 1998). It appears that the additional lamellar units are generated in the adventitia around E18 in Eln+/- mice (Wagenseil et al. 2010). These lamellar units may be generated from the population of progenitor cells in the adventitia (Majesky et al. 2011) and changes in hemodynamic forces may be important in signaling the formation of the additional layers. Determining how these layers are formed will be important for recreating functional elastic lamellae in human diseases and arterial tissue engineering. The difference in lamellar count and lack of typical hypertensive remodeling response in Eln+/- arteries, indicates an adaptive remodeling response, as opposed to a pathological one. The arterial geometry, ultrastucture, mechanics and hemodynamics are altered to provide the required cardiovascular function for a normal lifespan in Eln+/- mice, within the constraints caused by reduced elastin levels. Further understanding the adaptive remodeling process may provide strategies for treating SVAS in humans.
Using a bacterial artificial chromosome (BAC) encoding the human elastin gene, elastin can be added back to the Eln−/− or Eln+/- mice. Addition of human elastin rescues the Eln−/− lethal phenotype, despite limited expression of human elastin in the mouse (about 30% of normal). BAC+/+Eln−/− mice have an even more severe phenotype than Eln+/- mice, but most live a normal lifespan. BAC+/+Eln+/- mice have about 80% of normal elastin levels and the cardiovascular phenotypes (high blood pressure, reduced arterial diameter, and increased arterial stiffness) are ameliorated compared to Eln+/- (Hirano et al. 2007). These studies suggest that developmental diseases affecting the elastin gene may be treated by increasing elastin expression levels.
5.2.2. Fibulins and fibrillins
Mice lacking the fibulin-4 or -5 gene recapitulate many of the ARCL phenotypes. Mice lacking fibulin-4 (Fbln4−/−) die soon after birth from severe lung and vascular defects such as emphysema, arterial tortuosity, and aneurysms. Expression of tropoelastin is unaffected in these mice, but histology shows that they do not develop intact elastic fibers (McLaughlin et al. 2006). Mice lacking fibulin-5 (Fbln5−/−) have loose skin and similar lung and vascular irregularities resulting from improper elastic fiber formation, but with less severity than Fbln4−/− mice. Fbln5−/− mice live a normal lifespan (Nakamura et al. 2002; Yanagisawa et al. 2002).
Mice that do not express fibrillin-1 (Fbn1−/−) have thin, fragmented arterial elastic fibers and die soon after birth due to ruptured aortic aneurysm. In contrast, mice lacking fibrillin-2 (Fbn2−/−) have a normal aortic morphology. Compound knockouts for both proteins die in utero and have a more severe vascular phenotype than Fbn1−/−, indicating that fibrillin-2 contributes to the initial microfibril assembly and has partially overlapping functions with fibrillin-1 (Carta et al. 2006). Fbn1+/- mice have larger, stiffer arteries, but live a normal lifespan (Carta et al. 2009). Several other mouse models with mutations in the fibrillin-1 protein have been developed to study MFS. The mgΔ mouse has a fibrillin-1 gene deletion related to a particularly severe form of the human disease. The homozygous mice die suddenly of aortic aneurysms and other cardiovascular complications around three weeks of age (Pereira et al. 1997). Mice that are heterozygous for a known fibrillin-1 missense mutation (C1039G) reproduce many of the human MFS phenotypes including skeletal deformity and progressive deterioration of arterial wall architecture (Judge et al. 2004). The range of phenotypes and lifespans in the different mouse models demonstrates that MFS severity may be modulated by fibrillin-1 amounts.
5.2.3. Collagens
Mouse models with mutations in type I and type III collagens die prematurely from ruptured blood vessels, indicating their necessity for the proper mechanical and structural properties of a mature vessel wall. Mice lacking the ability to synthesize type I collagen (Col1a1−/−) die around E10 from sudden rupture of major blood vessels (Lohler et al. 1984). Mice that cannot express collagen type III (Col3a1−/−) also show perinatal lethality, though about 10% survive to adulthood (Liu et al. 1997). Col3a1−/− mice have a phenotype similar to clinical manifestations of type IV EDS including sudden death due to vessel rupture. Mice lacking type V collagen (ColV−/−) die around E10 with a complete lack of collagen fibrils (Wenstrup et al. 2004). Heterozygous mice (ColV+/-) are viable, but have reduced fibril number and dermal collagen content. Aortic stiffness and tensile strength are reduced compared to WT mice (Wenstrup et al. 2006).
6. Mechanical models
Mechanical models are necessary to predict arterial mechanical behavior for a range of loading conditions and to extrapolate in vivo conditions. Different phenomenological models have been applied to experimental data for arteries [reviewed in (Humphrey 1995; Vito and Dixon 2003)], most of which assume incompressible, pseudoelastic (Fung et al. 1979), nonlinear, transversely isotropic mechanical behavior. The arterial wall has been considered homogeneous, or divided into two homogeneous layers representing the media and adventitia [i.e.(von Maltzahn et al. 1981; von Maltzahn et al. 1984)]. While these models are useful for predicting mechanical behavior for different loading conditions and quantifying differences between groups, they do not provide insight into the mechanical structure-function relationship of the arterial wall components. Additionally, time dependent models are necessary to predict changes in the arterial wall due to mechanically-stimulated remodeling in development. This review focuses on microstructurally-based constitutive models and models of growth and remodeling applied to embryonic and postnatal development.
6.1. Microstructurally-based arterial constitutive models
Microstructurally-based constitutive models account specifically for the amount, organization and mechanical behavior of individual arterial wall components [reviewed in (Holzapfel and Ogden 2010)]. Originally proposed by Holzapfel and Weizsacker (1998) many of these microstructural models assume a non-collagenous substance (usually associated with elastin) with embedded fibers (usually associated with collagen fibers). The non-collagenous substance is often modeled with a neo-Hookean strain energy function, while the embedded fibers are often modeled with a Fung-type strain energy function. Early microstructural models include two families of collagen fibers symmetrically oriented at some angle to the circumferential direction (Holzapfel et al. 2000). Later extensions include viscoelastic effects (Holzapfel et al. 2002), separate material properties for the media and adventitia (Holzapfel et al. 2004), collagen fiber angle dispersion (Holzapfel et al. 2005) and a combination of fiber angle dispersion and collagen fiber waviness (Rodriguez et al. 2008). Other extensions of the fiber-family model include an increase in the number of fiber families to four (angled fibers, plus circumferential and axial fibers), based on multiphoton imaging of arterial collagen organization (Baek et al. 2007a). The four-fiber family model has been used to investigate differences in collagen fiber angles in arteries from hypertensive pigs (Hu et al. 2007), mouse models of muscular dystrophy (Gleason et al. 2008) and mouse models of MFS (Eberth et al. 2009). Additional microstructural models have been developed that use a linear strain energy function for the collagen fibers with a statistical distribution of collagen fiber waviness. The collagen shows nonlinear behavior as more fibers transition from the wavy to the stretched state and begin to contribute to the total tissue stress. Models of this type have been used to describe mechanics of the human aorta (Wuyts et al. 1995; Zulliger and Stergiopulos 2007), rat carotids (Zulliger et al. 2004), and rabbit carotids before and after digestion of the elastin fibers (Fonck et al. 2007). Most constitutive models have focused on explaining the changes in mechanical behavior of adult arteries in health and disease.
A recent model by Cheng et al. (2012) applies a modified version of the two-fiber family model to predict changes in ECM amount and organization with postnatal development and disease in the mouse aorta. The model predicts that the circumferential stress contribution of elastin increases with developmental age up to P21, then remains constant. The predictions are in agreement with measured changes in elastin amount, suggesting that the predicted stress contribution can be directly related to elastin quantity. In support of this assertion, the model also predicts that the circumferential stress contribution of elastin in Eln+/- aorta is reduced compared to WT for all ages. Despite the reduced elastin contribution, the total circumferential stress is similar between genotypes. Protein quantification suggests that the collagen amounts are similar, but the model predicts that the mechanical behavior and organization of the collagen fibers in Eln+/- aorta are altered to provide a higher stress contribution and make up for the reduced elastin contribution. Models of this type help in understanding arterial remodeling in genetic disease and in designing interventions to reverse changes in the arterial wall at the microstructural level.
The contribution of SMCs to arterial mechanics has been included in some microstructural constitutive models. While the passive contribution of SMCs in adult, large elastic arteries is minimal (Faury et al. 1999), SMCs can actively change mechanical behavior by contracting and relaxing in response to chemical and hemodynamic signals. Rachev and Hayashi (1999) proposed a model in which active SMC stress depends on a basal level of SMC tone multiplied by a normalized function of stretch, which recreates the known length-tension relationship of SMCs (Dobrin 1973). Applications of the model assume that the basal level of SMC tone depends on Ca2+ concentration (Baek et al. 2007a; Gleason et al. 2004). Further refinements of the model in the context of cerebral arteries, relate the SMC tone and stress generation to the ratio of circulating constrictors and dilators (Baek et al. 2007b; Valentin et al. 2009). Other mechanical models of SMC contraction are based on original work by A.V. Hill (1938) and expanded by Y.C. Fung (1970). These models include series and parallel elements representing viscous, elastic and contractile components, but do not specifically account for the physiology of cell contraction. Gestrelius and Borgstrom (1986) extended the Hill model to include individual interactions between actin-myosin crossbridges. Hai and Murphy (1988) developed a kinetic model to describe crossbridge cycling based on Ca2+ concentration and rate constants of myosin phosphorylation and stress development. Recent papers have combined mechanical and kinetic models to provide chemomechanical models of SMC activation (Murtada et al. 2010; Stalhand et al. 2008; Yang et al. 2003), but much work remains to couple the chemical and mechanical stimuli for SMC behavior.
6.2. Models of growth and development
It is important to remember that the artery wall is not a static structure, but is in constant flux from protein turnover, changes in protein organization, and cell death, division and migration. This is especially true during development. Observations in the 19th century on trabecular bone growth (Wolff 1986) produced a theory for biological remodeling in response to mechanical factors that has since been generalized to explain the growth of any biological tissue in the presence of mechanical stress (Hsu 1968). Recent developments in constitutive modeling have sought to directly capture this behavior.
Rachev et al. (1996) used a global stress-dependent growth model to investigate the dynamics of wall remodeling in response to changes in blood pressure in adult arteries. This was later adapted for a two-layer model of the arterial wall to investigate remodeling differences in the media and adventitia (Rachev 1997). Fridez et al. (2001) extended Rachev’s model to include SMC contributions to the remodeling process. Tsamis and Stergiopulos (2007) extended Rachev’s model to incorporate microstructurally-based strain energy functions for the arterial wall components. Rodriguez et al. (1994) developed a general stress-dependent growth law for soft tissues based on local growth instead of global growth. The theory was implemented with nonlinear finite elements to investigate growth due to altered flow and pressure in adult arteries (Rodríguez et al. 2007). The above examples include global or local growth of the artery, but do not link the growth to any specific wall component.
Humphrey and Rajagopal (2002) developed a stress-dependent, constrained mixture model in which the growth of individual wall components (elastin, collagen and SMCs) depends on their individual stresses. The model includes stress-dependent turnover and natural configurations for each component. Variations of this model have been applied to remodeling in adult arteries caused by changes in flow (Humphrey and Rajagopal 2003; Gleason et al. 2004), mean pressure (Gleason and Humphrey 2004), pulse pressure (Cardamone et al. 2010), spatial variations in wall components (Alford et al. 2008), and axial extension (Valentin and Humphrey 2009a). A major challenge in these models is determining appropriate values for the required parameters. Parameter sensitivity studies have been performed to examine the model predictions within a range of values (Valentin and Humphrey 2009b), but additional experimental data is needed to further validate the models. As with the SMC contributions discussed above, there is also a need to correlate mechanical stimuli with known changes in chemical signaling. This is necessary to predict the mechanical remodeling effects of pharmaceuticals that directly alter the chemical environment. To this end, recent papers have focused on coupling agent-based models with continuum mechanics models (Hayenga et al. 2011; Thorne et al. 2011). Using parameter refinement and a literature-derived rule-set, the authors show that changes in cellular production of seven key molecules [NO, endothelin-1 (ET-1), TGF-β, PDGF, and various matrix metalloproteinases (MMP-1, -2 and -9] control turnover of cells and ECM proteins to reproduce the expected geometric and mechanical changes in the arterial wall for induced hypertension.
Note that the models discussed above incorporate stress-dependent growth laws, while most in vitro experimental data depend on stretch-dependent SMC reactions, hence there is a disconnect between the modeling assumptions and the available experimental data. While other growth laws are possible, for example stretch-dependent and stretch-rate dependent laws (Cowin 1996), numerous morphologic experiments in embryonic tissues by Beloussov (1998) support the idea of stress-dependent growth. Taber (2008) points out that a stress-based response can produce more varied morphologic behavior because different layers may be stretched the same amount, but experience different stresses due to varying mechanical properties. Humphrey (2001) suggests that cells cannot directly sense stress or stretch and that cellular responses can only correlate with convenient measures of these continuum concepts. He suggests instead that actual mechanisms must depend on more fundamental changes in cellular architecture, such as conformational changes in molecules that result from force changes at the molecular scale. Recent evidence supports a relationship between tissue-level stress or stretch and cellular responses at the molecular level (Figure 5). It is hypothesized that mechanical stimuli applied to the tissue will result in deformation of the ECM, leading to deformation of the cell through integrin connections between the ECM and the cellular cytoskeleton, and subsequent deformation of the cell nucleus which results in altered gene expression [reviewed in (Gieni and Hendzel 2008)]. Hence, the remodeling stimulus, stress at the tissue level and stretch or deformation at the molecular level, may depend on the mechanical scale and it will be important to consider multi-scale, linked genetic-mechanical models.
Figure 5.
Different length scales over which stresses and deformations act to stimulate growth and remodeling in developing arteries. Circumferential (σθ), axial (σz) and shear (τ) stresses depend on the blood pressure, axial tethering forces, and blood flow, respectively, as well as on the arterial wall geometry (Eqns. 1 – 3). These stresses act on the artery as a whole (A), as well as on individual cell and ECM fibers (B). Whole cell and ECM deformations cause local deformations in individual ECM fibers and the cell membrane, which are connected to each other through transmembrane proteins, such as integrins. The local deformations trigger signaling cascades due to conformational changes in the transmembrane proteins directly, and through their connections to cytoskeletal proteins that may link all the way to the cell nucleus (C).
Most growth and remodeling studies focus on changes in stress from the steady-state, homeostatic state in adult arteries. Growth and remodeling in developing arteries, that have constantly changing stresses, is more complicated and has received limited attention in the literature. Taber and Eggers (1996) used local, volumetric growth theory (Rodriguez et al. 1994) to predict growth of the rat aorta due to stresses from pressure increases starting with the first heartbeat in the embryo, through maturity and applied hypertension. They assumed that the composition of the wall changes, from only SMCs in early development to two layers (media and adventitia) with surrounding ECM in postnatal growth. Assuming a constant growth law throughout development, the authors were able to qualitatively reproduce experimental changes in arterial geometry. The model was later extended to include the effects of shear stress from blood flow (Taber 1998) and exhibited more stable growth behavior with less parameter sensitivity. The model has recently been refined (Taber 2008) to include a hyper-restorative response such that the rate of growth depends on the difference between the current and target stress and a stress overshoot is generated by allowing the target stress to change at a rate proportional to the same stress difference. The hyper-restorative model captures experimental behavior of spreading, bending and invagination of a whole embryo, but predicts unbounded growth for embryonic development of a pressure-vessel such as an artery or heart. Taber (2009) found that the unbounded growth of a pressure-vessel could be corrected by assuming that volumetric growth depends on stress rate, instead of stress magnitude. The latest model reproduces geometric changes due to growth in examples from arterial development, wound healing, and sea urchin gastrulation.
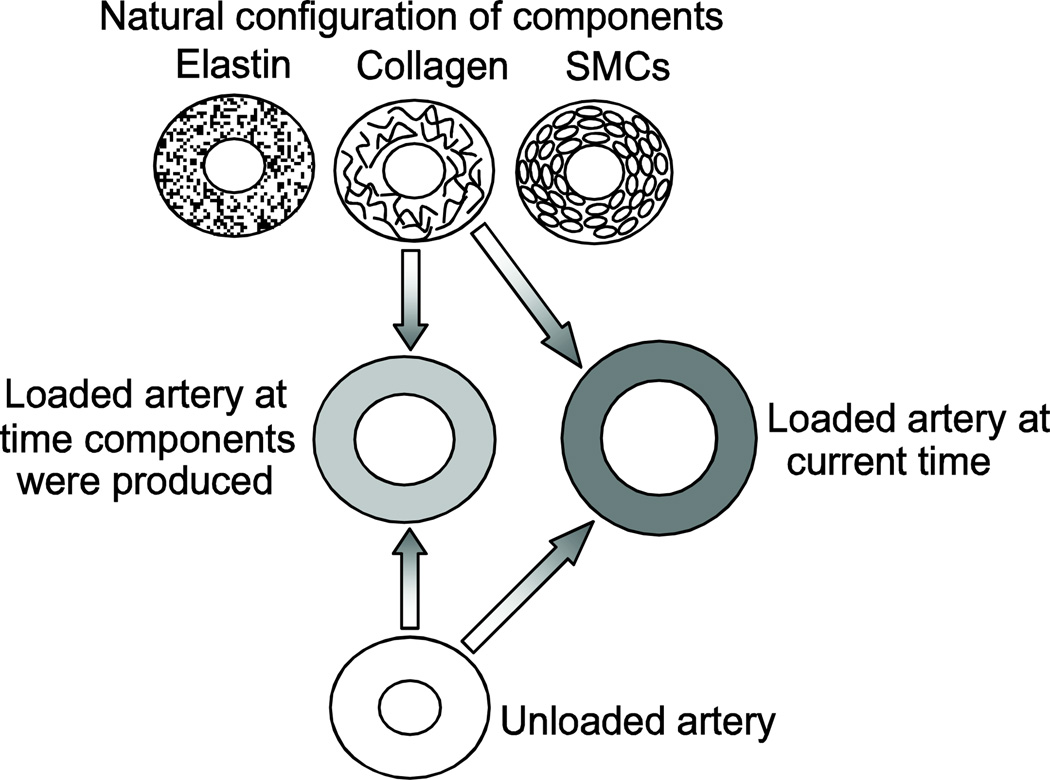
A constrained mixture model with turnover, growth and evolving natural configurations of each wall component (Humphrey and Rajagopal 2002) has been applied to developing arteries (Figure 6) (Wagenseil 2011). It was assumed that geometric changes are stimulated by multiple step changes in pressure, length and flow to normalize the stresses in each direction. The target stresses evolved in multiple steps with postnatal development. The results show that active dilation of SMCs to normalize shear stress in response to changes in blood flow do not fit experimental results for the mouse aorta. Instead, decoupling the geometric changes from the blood flow produce a much better prediction of the experimental data, indicating that developing arteries may have a different shear response than adult arteries or that stress magnitude may not be the appropriate remodeling stimulus for developing arteries. Wagenseil (2011) also found that the material constants and natural configurations for elastin and collagen must evolve to match the experimental changes in total wall mechanical behavior. Alford et al. (2008; 2008) used a similar constrained mixture model, combined with volumetric growth theory (Rodríguez et al. 2007) and including residual strains, to investigate growth and remodeling in the developing aorta. The authors found that the spatial distribution of elastin and collagen through the wall thickness predicted the circumferential residual strain at different locations along the aortic length (Alford et al. 2008). The authors also found that the timing and extent of elastin turnover corresponded with varying residual strains and axial growth. In the absence of elastin, the model predicted unbounded axial growth (Alford and Taber 2008), consistent with the increased length in arteries from Eln−/− mice (Wagenseil et al. 2010). These results emphasize the importance of determining structure-function relationships of the wall components during development, when they are being laid down and the wall is being created, in addition to the adult state.
Figure 6.
Natural configurations of each arterial component (elastin, collagen and SMCs) and the unloaded and loaded configurations of the composite arterial wall in a constrained mixture model applied to postnatal development of the mouse aorta. Results show that the natural configurations and material properties of each component must vary with time to reproduce experimental data. Modified from Wagenseil (2011).
7. Conclusion and future directions
This review has focused on the influence of ECM on the physiology, hemodynamics, and mechanics of large artery development, and on predicting these influences with constitutive models. Correlations between several factors are clear: 1) ECM expression and SMC phenotype, 2) Increasing hemodynamic forces and arterial wall maturation, 3) ECM amounts and arterial mechanics, 4) Tissue-level mechanical stimuli and arterial wall remodeling, and 5) Molecular-level mechanical stimuli and gene expression or chemical signaling; however, causative relationships are still unclear. Much work remains to elucidate mechanistic relationships to connect these factors and predict overall changes in arterial geometry and mechanical properties based on molecular changes in gene expression or chemical signaling caused by mechanical stimuli. This work will depend on linking models with different scales (from tissue- to molecular-level) and different factors (from mechanical, to genetic, to chemical) and will require abundant experimental data where single factors are perturbed and the resulting changes at all levels are catalogued. It will be difficult to isolate single factors and ensure all others remain constant. In fact, it may be necessary to take a systems approach, whereby changes in multiple inputs and outputs must be linked. Creating complex, linked models will allow predictions of how changes in any of these factors affect overall cardiovascular function. The models will allow predictions of the cardiovascular effects of different pharmaceuticals that alter mechanical stimuli, gene expression and/or chemical signaling to inform experimental design and disease treatment. It is important to focus on developing arteries, rather than mature, adult arteries, because this is the state that must be targeted in treating developmental cardiovascular diseases and reproduced in arterial tissue engineering. If individual or combinations of signals can be identified that produce desired remodeling responses, such as the production of functional elastic lamellae, this may be exploited for arterial repair in human disease and arterial reconstruction in tissue engineering.
Acknowledgements
This work was supported, in part, by the National Institutes of Health (Grants R01 HL105314, R00 HL087563, and T32 HL727531). Robert Mecham is acknowledged for providing the Eln−/− and Eln+/- mice, invaluable advice, and editorial assistance. Attila Kovacs is acknowledged for obtaining the ultrasound images in Figure 3. Russel Knusten and Marilyn Levy are acknowledged for assistance with the EM images in Figure 1.
References
- Aboulhosn J, Child JS. Left Ventricular Outflow Obstruction. Circulation. 2006;114(22):2412–2422. doi: 10.1161/CIRCULATIONAHA.105.592089. [DOI] [PubMed] [Google Scholar]
- Alford PW, Humphrey JD, Taber LA. Growth and remodeling in a thick-walled artery model: effects of spatial variations in wall constituents. Biomech Model Mechanobiol. 2008;7(4):245–262. doi: 10.1007/s10237-007-0101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford PW, Taber LA. Computational study of growth and remodelling in the aortic arch. Computer methods in biomechanics and biomedical engineering. 2008;11(5):525–538. doi: 10.1080/10255840801930710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando J, Yamamoto K. Vascular mechanobiology: endothelial cell responses to fluid shear stress. Circulation journal : official journal of the Japanese Circulation Society. 2009;73(11):1983–1992. doi: 10.1253/circj.cj-09-0583. [DOI] [PubMed] [Google Scholar]
- Asada H, Paszkowiak J, Teso D, Alvi K, Thorisson A, Frattini JC, Kudo FA, Sumpio BE, Dardik A. Sustained orbital shear stress stimulates smooth muscle cell proliferation via the extracellular signal-regulated protein kinase 1/2 pathway. J Vasc Surg. 2005;42(4):772–780. doi: 10.1016/j.jvs.2005.05.046. [DOI] [PubMed] [Google Scholar]
- Baek S, Gleason RL, Rajagopal KR, Humphrey JD. Theory of small on large: Potential utility in computations of fluid-solid interactions in arteries. Computer Methods in Applied Mechanics and Engineering. 2007a;196(31–32):3070–3078. [Google Scholar]
- Baek S, Valentin A, Humphrey JD. Biochemomechanics of cerebral vasospasm and its resolution: II. Constitutive relations and model simulations. Ann Biomed Eng. 2007b;35(9):1498–1509. doi: 10.1007/s10439-007-9322-x. [DOI] [PubMed] [Google Scholar]
- Beloussov LV. The dynamic architecture of a developing organism: an interdisciplinary approach to the development of organisms. Kluwer Academic Publishers; 1998. [Google Scholar]
- Bendeck MP, Langille BL. Rapid accumulation of elastin and collagen in the aortas of sheep in the immediate perinatal period. Circulation research. 1991;69(4):1165–1169. doi: 10.1161/01.res.69.4.1165. [DOI] [PubMed] [Google Scholar]
- Berry CL, Greenwald SE. Effects of hypertension on the static mechanical properties and chemical composition of the rat aorta. Cardiovasc Res. 1976;10(4):437–451. doi: 10.1093/cvr/10.4.437. [DOI] [PubMed] [Google Scholar]
- Berry CL, Looker T, Germain J. Nucleic acid and scleroprotein content of the developing human aorta. The Journal of pathology. 1972;108(4):265–274. doi: 10.1002/path.1711080402. [DOI] [PubMed] [Google Scholar]
- Boo YC, Jo H. Flow-dependent regulation of endothelial nitric oxide synthase: role of protein kinases. American journal of physiology Cell physiology. 2003;285(3):C499–C508. doi: 10.1152/ajpcell.00122.2003. [DOI] [PubMed] [Google Scholar]
- Callewaert B, Renard M, Hucthagowder V, Albrecht B, Hausser I, Blair E, Dias C, Albino A, Wachi H, Sato F, Mecham R, Loeys B, Coucke P, De Paepe A, Urban Z. New insights into the pathogenesis of autosomal-dominant cutis laxa with report of five ELN mutations. Hum Mutat. 2011;32(4):445–455. doi: 10.1002/humu.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardamone L, Valentin A, Eberth JF, Humphrey JD. Modelling carotid artery adaptations to dynamic alterations in pressure and flow over the cardiac cycle. Math Med Biol. 2010 doi: 10.1093/imammb/dqq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta L, Pereira L, Arteaga-Solis E, Lee-Arteaga SY, Lenart B, Starcher B, Merkel CA, Sukoyan M, Kerkis A, Hazeki N, Keene DR, Sakai LY, Ramirez F. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J Biol Chem. 2006;281(12):8016–8023. doi: 10.1074/jbc.M511599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta L, Wagenseil JE, Knutsen RH, Mariko B, Faury G, Davis EC, Starcher B, Mecham RP, Ramirez F. Discrete contributions of elastic fiber components to arterial development and mechanical compliance. Arterioscler Thromb Vasc Biol. 2009;29(12):2083–2089. doi: 10.1161/ATVBAHA.109.193227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JK, Mecham RP, Wagenseil JE. A fiber-based constitutive model predicts changes in amount and organization of matrix proteins with development and disease in the mouse aorta. Biomech Model Mechanobiol. 2012 doi: 10.1007/s10237-012-0420-9. In revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelek M, Ainslie K, Garanich JS, Tarbell JM. Smooth muscle cells contract in response to fluid flow via a Ca2+independent signaling mechanism. J Appl Physiol. 2002;93(6):1907–1917. doi: 10.1152/japplphysiol.00988.2001. [DOI] [PubMed] [Google Scholar]
- Clark ER. Studies on the growth of blood-vessels in the tail of the frog larva - by observation and experiment on the living animal. American Journal of Anatomy. 1918;23:37–88. [Google Scholar]
- Cliff WJ. The aortic tunica media in growing rats studied with the electron microscope. Lab Invest. 1967;17(6):599–615. [PubMed] [Google Scholar]
- Cowin SC. Strain or deformation rate dependent finite growth in soft tissues. J Biomech. 1996;29(5):647–649. doi: 10.1016/0021-9290(95)00114-x. [DOI] [PubMed] [Google Scholar]
- Dasouki M, Markova D, Garola R, Sasaki T, Charbonneau N, Sakai L, Chu M. Compound heterozygous mutations in fibulin-4 causing neonatal lethal pulmonary artery occlusion, aortic aneurysm, arachnodactyly, and mild cutis laxa. Am J Med Genet A. 2007;143(22):2635–2641. doi: 10.1002/ajmg.a.31980. [DOI] [PubMed] [Google Scholar]
- Davis EC. Elastic lamina growth in the developing mouse aorta. J Histochem Cytochem. 1995;43(11):1115–1123. doi: 10.1177/43.11.7560894. [DOI] [PubMed] [Google Scholar]
- Davis NP, Han HC, Wayman B, Vito R. Sustained axial loading lengthens arteries in organ culture. Ann Biomed Eng. 2005;33(7):867–877. doi: 10.1007/s10439-005-3488-x. [DOI] [PubMed] [Google Scholar]
- DeRuiter MC, Poelmann RE, VanMunsteren JC, Mironov V, Markwald RR, Gittenberger-de Groot AC. Embryonic endothelial cells transdifferentiate into mesenchymal cells expressing smooth muscle actins in vivo and in vitro. Circ Res. 1997;80(4):444–451. doi: 10.1161/01.res.80.4.444. [DOI] [PubMed] [Google Scholar]
- Dewey CF, Jr, Bussolari SR, Gimbrone MA, Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng. 1981;103(3):177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- Dietz HC, Loeys B, Carta L, Ramirez F. Recent progress towards a molecular understanding of Marfan syndrome. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2005;139C(1):4–9. doi: 10.1002/ajmg.c.30068. [DOI] [PubMed] [Google Scholar]
- Dobrin PB. Influence of initial length on length-tension relationship of vascular smooth muscle. Am J Physiol. 1973;225(3):664–670. doi: 10.1152/ajplegacy.1973.225.3.664. [DOI] [PubMed] [Google Scholar]
- Dobrin PB. Chapter 3: Physiology and Pathophysiology of Blood Vessels. In: Sidawy ANSB, DePalma RG, editors. The Basic Science of Vascular Disease. New York: Futura Publishing; 1997. pp. 69–105. [Google Scholar]
- Drake CJ. Embryonic and adult vasculogenesis. Birth Defects Res C Embryo Today. 2003;69(1):73–82. doi: 10.1002/bdrc.10003. [DOI] [PubMed] [Google Scholar]
- Durante W, Liao L, Reyna SV, Peyton KJ, Schafer AI. Physiological cyclic stretch directs L-arginine transport and metabolism to collagen synthesis in vascular smooth muscle. Faseb J. 2000;14(12):1775–1783. doi: 10.1096/fj.99-0960com. [DOI] [PubMed] [Google Scholar]
- Eastwood M, Porter R, Khan U, McGrouther G, Brown R. Quantitative analysis of collagen gel contractile forces generated by dermal fibroblast and the relationship to cell morphology. Journal of Cellular Physiology. 1996;166:33–42. doi: 10.1002/(SICI)1097-4652(199601)166:1<33::AID-JCP4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Eberth JF, Taucer AI, Wilson E, Humphrey JD. Mechanics of carotid arteries in a mouse model of Marfan Syndrome. Ann Biomed Eng. 2009;37(6):1093–1104. doi: 10.1007/s10439-009-9686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effmann EL, Whitman SA, Smith BR. Aortic arch development. Radiographics. 1986;6(6):1065–1089. doi: 10.1148/radiographics.6.6.3685519. [DOI] [PubMed] [Google Scholar]
- Ekstrand J, Razuvaev A, Folkersen L, Roy J, Hedin U. Tissue factor pathway inhibitor-2 is induced by fluid shear stress in vascular smooth muscle cells and affects cell proliferation and survival. J Vasc Surg. 2010;52(1):167–175. doi: 10.1016/j.jvs.2010.02.282. [DOI] [PubMed] [Google Scholar]
- Faury G, Maher GM, Li DY, Keating MT, Mecham RP, Boyle WA. Relation between outer and luminal diameter in cannulated arteries. Am J Physiol. 1999;277(5 Pt 2):H1745–H1753. doi: 10.1152/ajpheart.1999.277.5.H1745. [DOI] [PubMed] [Google Scholar]
- Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, Minkes RK, Blumer KJ, Kovacs A, Kelly DP, Li DY, Starcher B, Mecham RP. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest. 2003;112(9):1419–1428. doi: 10.1172/JCI19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonck E, Prod'hom G, Roy S, Augsburger L, Rufenacht DA, Stergiopulos N. Effect of elastin degradation on carotid wall mechanics as assessed by a constituent-based biomechanical model. Am J Physiol Heart Circ Physiol. 2007;292(6):H2754–H2763. doi: 10.1152/ajpheart.01108.2006. [DOI] [PubMed] [Google Scholar]
- Francke U, Furthmayr H. Marfan's Syndrome and Other Disorders of Fibrillin. New England Journal of Medicine. 1994;330(19):1384–1385. doi: 10.1056/NEJM199405123301911. [DOI] [PubMed] [Google Scholar]
- Frid MG, Kale VA, Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circ Res. 2002;90(11):1189–1196. doi: 10.1161/01.res.0000021432.70309.28. [DOI] [PubMed] [Google Scholar]
- Fridez P, Rachev A, Meister JJ, Hayashi K, Stergiopulos N. Model of geometrical and smooth muscle tone adaptation of carotid artery subject to step change in pressure. Am J Physiol Heart Circ Physiol. 2001;280(6):H2752–H2760. doi: 10.1152/ajpheart.2001.280.6.H2752. [DOI] [PubMed] [Google Scholar]
- Fridez P, Zulliger M, Bobard F, Montorzi G, Miyazaki H, Hayashi K, Stergiopulos N. Geometrical, functional, and histomorphometric adaptation of rat carotid artery in induced hypertension. J Biomech. 2003;36(5):671–680. doi: 10.1016/s0021-9290(02)00445-1. [DOI] [PubMed] [Google Scholar]
- Fung YC. Mathematical representation of the mechanical properties of the heart muscle. J Biomech. 1970;3(4):381–404. doi: 10.1016/0021-9290(70)90012-6. [DOI] [PubMed] [Google Scholar]
- Fung YC, Fronek K, Patitucci P. Pseudoelasticity of arteries and the choice of its mathematical expression. Am J Physiol. 1979;237(5):H620–H631. doi: 10.1152/ajpheart.1979.237.5.H620. [DOI] [PubMed] [Google Scholar]
- Gerrity RG, Cliff WJ. The aortic tunica media of the developing rat. I. Quantitative stereologic and biochemical analysis. Lab Invest. 1975;32(5):585–600. [PubMed] [Google Scholar]
- Gestrelius S, Borgstrom P. A dynamic model of smooth muscle contraction. Biophys J. 1986;50(1):157–169. doi: 10.1016/S0006-3495(86)83448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieni RS, Hendzel MJ. Mechanotransduction from the ECM to the genome: are the pieces now in place? J Cell Biochem. 2008;104(6):1964–1987. doi: 10.1002/jcb.21364. [DOI] [PubMed] [Google Scholar]
- Gleason RL, Dye WW, Wilson E. Quantification of the mechanical behavior of carotid arteries from wild-type, dystrophin-deficient, and sarcoglycan-δ knockout mice. Journal of Biomechanics. 2008;41(15):3213–3218. doi: 10.1016/j.jbiomech.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason RL, Humphrey JD. A mixture model of arterial growth and remodeling in hypertension: altered muscle tone and tissue turnover. J Vasc Res. 2004;41(4):352–363. doi: 10.1159/000080699. [DOI] [PubMed] [Google Scholar]
- Gleason RL, Taber LA, Humphrey JD. A 2-D Model of Flow-Induced Alterations in the Geometry, Structure and Properties of Carotid Arteries. J Biomech Eng. 2004;126:371–381. doi: 10.1115/1.1762899. [DOI] [PubMed] [Google Scholar]
- Greenwald SE. Ageing of the conduit arteries. The Journal of pathology. 2007;211(2):157–172. doi: 10.1002/path.2101. [DOI] [PubMed] [Google Scholar]
- Haga M, Yamashita A, Paszkowiak J, Sumpio BE, Dardik A. Oscillatory shear stress increases smooth muscle cell proliferation and Akt phosphorylation. J Vasc Surg. 2003;37(6):1277–1284. doi: 10.1016/s0741-5214(03)00329-x. [DOI] [PubMed] [Google Scholar]
- Hai CM, Murphy RA. Cross-bridge phosphorylation and regulation of latch state in smooth muscle. Am J Physiol. 1988;254(1)(Pt 1):C99–C106. doi: 10.1152/ajpcell.1988.254.1.C99. [DOI] [PubMed] [Google Scholar]
- Hayenga HN, Thorne BC, Peirce SM, Humphrey JD. Ensuring congruency in multiscale modeling: towards linking agent based and continuum biomechanical models of arterial adaptation. Ann Biomed Eng. 2011;39(11):2669–2682. doi: 10.1007/s10439-011-0363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedin U, Bottger BA, Forsberg E, Johansson S, Thyberg J. Diverse effects of fibronectin and laminin on phenotypic properties of cultured arterial smooth muscle cells. J Cell Biol. 1988;107(1):307–319. doi: 10.1083/jcb.107.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AV. The heat of shortening and dynamic constants of muscle. Proc R Soc Lond B. 1938;126:136–195. [Google Scholar]
- Hirano E, Knutsen RH, Sugitani H, Ciliberto CH, Mecham RP. Functional rescue of elastin insufficiency in mice by the human elastin gene: implications for mouse models of human disease. Circ Res. 2007;101(5):523–531. doi: 10.1161/CIRCRESAHA.107.153510. [DOI] [PubMed] [Google Scholar]
- Holzapfel GA, Gasser TC, Ogden RW. A new constitutive framework for arterial wall mechanics and a comparative study of material models. Journal of Elasticity. 2000;61(1–3):1–48. [Google Scholar]
- Holzapfel GA, Gasser TC, Ogden RW. Comparison of a multi-layer structural model for arterial walls with a fung-type model, and issues of material stability. Journal of Biomechanical Engineering-Transactions of the Asme. 2004;126(2):264–275. doi: 10.1115/1.1695572. [DOI] [PubMed] [Google Scholar]
- Holzapfel GA, Gasser TC, Stadler M. A structural model for the viscoelastic behavior of arterial walls: Continuum formulation and finite element analysis. European Journal of Mechanics - A/Solids. 2002;21(3):441–463. [Google Scholar]
- Holzapfel GA, Ogden RW. Constitutive modelling of arteries. P Roy Soc a-Math Phy. 2010;466(2118):1551–1596. [Google Scholar]
- Holzapfel GA, Sommer G, Gasser CT, Regitnig P. Determination of layer-specific mechanical properties of human coronary arteries with nonatherosclerotic intimal thickening and related constitutive modeling. Am J Physiol Heart Circ Physiol. 2005;289(5):H2048–H2058. doi: 10.1152/ajpheart.00934.2004. [DOI] [PubMed] [Google Scholar]
- Holzapfel GA, Weizsacker HW. Biomechanical behavior of the arterial wall and its numerical characterization. Comput Biol Med. 1998;28(4):377–392. doi: 10.1016/s0010-4825(98)00022-5. [DOI] [PubMed] [Google Scholar]
- Hsieh HJ, Li NQ, Frangos JA. Shear stress increases endothelial platelet-derived growth factor mRNA levels. Am J Physiol. 1991;260(2 Pt 2):H642–H646. doi: 10.1152/ajpheart.1991.260.2.H642. [DOI] [PubMed] [Google Scholar]
- Hsu FH. The influences of mechanical loads on the form of a growing elastic body. J Biomech. 1968;1(4):303–311. doi: 10.1016/0021-9290(68)90024-9. [DOI] [PubMed] [Google Scholar]
- Hu JJ, Baek S, Humphrey JD. Stress–strain behavior of the passive basilar artery in normotension and hypertension. Journal of Biomechanics. 2007;40(11):2559–2563. doi: 10.1016/j.jbiomech.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Hu Q, Loeys BL, Coucke PJ, De Paepe A, Mecham RP, Choi J, Davis EC, Urban Z. Fibulin-5 mutations: mechanisms of impaired elastic fiber formation in recessive cutis laxa. Hum Mol Genet. 2006;15(23):3379–3386. doi: 10.1093/hmg/ddl414. [DOI] [PubMed] [Google Scholar]
- Huang C, Sheikh F, Hollander M, Cai C, Becker D, Chu PH, Evans S, Chen J. Embryonic atrial function is essential for mouse embryogenesis, cardiac morphogenesis and angiogenesis. Development. 2003;130(24):6111–6119. doi: 10.1242/dev.00831. [DOI] [PubMed] [Google Scholar]
- Huang Y, Guo X, Kassab GS. Axial nonuniformity of geometric and mechanical properties of mouse aorta is increased during postnatal growth. Am J Physiol Heart Circ Physiol. 2006;290(2):H657–H664. doi: 10.1152/ajpheart.00803.2005. [DOI] [PubMed] [Google Scholar]
- Hucthagowder V, Sausgruber N, Kim K, Angle B, Marmorstein L, Urban Z. Fibulin-4: a novel gene for an autosomal recessive cutis laxa syndrome. Am J Hum Genet. 2006;78(6):1075–1080. doi: 10.1086/504304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JD. Mechanics of the arterial wall: review and directions. Crit Rev Biomed Eng. 1995;23(1–2):1–162. [PubMed] [Google Scholar]
- Humphrey JD. Stress, strain, and mechanotransduction in cells. J Biomech Eng. 2001;123(6):638–641. doi: 10.1115/1.1406131. [DOI] [PubMed] [Google Scholar]
- Humphrey JD, Eberth JF, Dye WW, Gleason RL. Fundamental role of axial stress in compensatory adaptations by arteries. J Biomech. 2009;42(1):1–8. doi: 10.1016/j.jbiomech.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JD, Rajagopal KR. A constrained mixture model for growth and remodeling of soft tissues. Mathematical Models & Methods in Applied Sciences. 2002;12(3):407–430. [Google Scholar]
- Humphrey JD, Rajagopal KR. A constrained mixture model for arterial adaptations to a sustained step change in blood flow. Biomech Model Mechanobiol. 2003;2(2):109–126. doi: 10.1007/s10237-003-0033-4. [DOI] [PubMed] [Google Scholar]
- Hungerford JE, Little CD. Developmental biology of the vascular smooth muscle cell: building a multilayered vessel wall. J Vasc Res. 1999;36(1):2–27. doi: 10.1159/000025622. [DOI] [PubMed] [Google Scholar]
- Hungerford JE, Owens GK, Argraves WS, Little CD. Development of the aortic vessel wall as defined by vascular smooth muscle and extracellular matrix markers. Dev Biol. 1996;178(2):375–392. doi: 10.1006/dbio.1996.0225. [DOI] [PubMed] [Google Scholar]
- Ishiwata T, Nakazawa M, Pu WT, Tevosian SG, Izumo S. Developmental changes in ventricular diastolic function correlate with changes in ventricular myoarchitecture in normal mouse embryos. Circ Res. 2003;93(9):857–865. doi: 10.1161/01.RES.0000100389.57520.1A. [DOI] [PubMed] [Google Scholar]
- Jackson ZS, Dajnowiec D, Gotlieb AI, Langille BL. Partial off-loading of longitudinal tension induces arterial tortuosity. Arterioscler Thromb Vasc Biol. 2005;25(5):957–962. doi: 10.1161/01.ATV.0000161277.46464.11. [DOI] [PubMed] [Google Scholar]
- Jackson ZS, Gotlieb AI, Langille BL. Wall tissue remodeling regulates longitudinal tension in arteries. Circ Res. 2002;90(8):918–925. doi: 10.1161/01.res.0000016481.87703.cc. [DOI] [PubMed] [Google Scholar]
- Ji RP, Phoon CK, Aristizabal O, McGrath KE, Palis J, Turnbull DH. Onset of cardiac function during early mouse embryogenesis coincides with entry of primitive erythroblasts into the embryo proper. Circ Res. 2003;92(2):133–135. doi: 10.1161/01.res.0000056532.18710.c0. [DOI] [PubMed] [Google Scholar]
- Jones EA. Mechanical factors in the development of the vascular bed. Respiratory physiology & neurobiology. 2011;178(1):59–65. doi: 10.1016/j.resp.2011.03.026. [DOI] [PubMed] [Google Scholar]
- Jones EA, le Noble F, Eichmann A. What determines blood vessel structure? Genetic prespecification vs hemodynamics. Physiology (Bethesda) 2006;21:388–395. doi: 10.1152/physiol.00020.2006. [DOI] [PubMed] [Google Scholar]
- Judge DP, Biery NJ, Keene DR, Geubtner J, Myers L, Huso DL, Sakai LY, Dietz HC. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J Clin Invest. 2004;114(2):172–181. doi: 10.1172/JCI20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumi A, Milanini J, Kiosses WB, del Pozo MA, Kaunas R, Chien S, Hahn KM, Schwartz MA. Effects of cell tension on the small GTPase Rac. J Cell Biol. 2002;158(1):153–164. doi: 10.1083/jcb.200201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher CM, McLean SE, Mecham RP. Vascular extracellular matrix and aortic development. Curr Top Dev Biol. 2004;62:153–188. doi: 10.1016/S0070-2153(04)62006-0. [DOI] [PubMed] [Google Scholar]
- Kozel BA, Rongish BJ, Czirok A, Zach J, Little CD, Davis EC, Knutsen RH, Wagenseil JE, Levy MA, Mecham RP. Elastic fiber formation: A dynamic view of extracellular matrix assembly using timer reporters. J Cell Physiol. 2005 doi: 10.1002/jcp.20546. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Veselits ML, Barton KP, Lu MM, Clendenin C, Leiden JM. The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 1997;11(22):2996–3006. doi: 10.1101/gad.11.22.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langille BL, Adamson SL. Relationship between blood flow direction and endothelial cell orientation at arterial branch sites in rabbits and mice. Circ Res. 1981;48(4):481–488. doi: 10.1161/01.res.48.4.481. [DOI] [PubMed] [Google Scholar]
- Laurindo FR, Pedro Mde A, Barbeiro HV, Pileggi F, Carvalho MH, Augusto O, da Luz PL. Vascular free radical release. Ex vivo and in vivo evidence for a flow-dependent endothelial mechanism. Circ Res. 1994;74(4):700–709. doi: 10.1161/01.res.74.4.700. [DOI] [PubMed] [Google Scholar]
- le Noble F, Moyon D, Pardanaud L, Yuan L, Djonov V, Matthijsen R, Breant C, Fleury V, Eichmann A. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development. 2004;131(2):361–375. doi: 10.1242/dev.00929. [DOI] [PubMed] [Google Scholar]
- Le V, Knutsen R, Mecham R, Wagenseil J. Decreased aortic diameter and compliance precedes blood pressure increases in postnatal development of elastin-insufficient mice. Am J Physiol Heart Circ Physiol. 2011;301(1):H221–H229. doi: 10.1152/ajpheart.00119.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AA, Graham DA, Dela Cruz S, Ratcliffe A, Karlon WJ. Fluid shear stress-induced alignment of cultured vascular smooth muscle cells. J Biomech Eng. 2002;124(1):37–43. doi: 10.1115/1.1427697. [DOI] [PubMed] [Google Scholar]
- Leung DY, Glagov S, Mathews MB. Elastin and collagen accumulation in rabbit ascending aorta and pulmonary trunk during postnatal growth. Correlation of cellular synthetic response with medial tension. Circ Res. 1977;41(3):316–323. doi: 10.1161/01.res.41.3.316. [DOI] [PubMed] [Google Scholar]
- Li DY, Faury G, Taylor DG, Davis EC, Boyle WA, Mecham RP, Stenzel P, Boak B, Keating MT. Novel arterial pathology in mice and humans hemizygous for elastin. J Clin Invest. 1998;102(10):1783–1787. doi: 10.1172/JCI4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DY, Toland AE, Boak BB, Atkinson DL, Ensing GJ, Morris CA, Keating MT. Elastin point mutations cause an obstructive vascular disease, supravalvular aortic stenosis. Hum Mol Genet. 1997;6(7):1021–1028. doi: 10.1093/hmg/6.7.1021. [DOI] [PubMed] [Google Scholar]
- Li W, Chen Q, Mills I, Sumpio BE. Involvement of S6 kinase and p38 mitogen activated protein kinase pathways in strain-induced alignment and proliferation of bovine aortic smooth muscle cells. J Cell Physiol. 2003;195(2):202–209. doi: 10.1002/jcp.10230. [DOI] [PubMed] [Google Scholar]
- Liu X, Wu H, Byrne M, Krane S, Jaenisch R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci U S A. 1997;94(5):1852–1856. doi: 10.1073/pnas.94.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeys B, Van Maldergem L, Mortier G, Coucke P, Gerniers S, Naeyaert JM, De Paepe A. Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Hum Mol Genet. 2002;11(18):2113–2118. doi: 10.1093/hmg/11.18.2113. [DOI] [PubMed] [Google Scholar]
- Lohler J, Timpl R, Jaenisch R. Embryonic lethal mutation in mouse collagen I gene causes rupture of blood vessels and is associated with erythropoietic and mesenchymal cell death. Cell. 1984;38(2):597–607. doi: 10.1016/0092-8674(84)90514-2. [DOI] [PubMed] [Google Scholar]
- Lotery A, Baas D, Ridley C, Jones R, Klaver C, Stone E, Nakamura T, Luff A, Griffiths H, Wang T, Bergen A, Trump D. Reduced secretion of fibulin 5 in age-related macular degeneration and cutis laxa. Hum Mutat. 2006;27(6):568–574. doi: 10.1002/humu.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134(18):3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27(6):1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- Majesky MW, Dong XR, Regan JN, Hoglund VJ. Vascular smooth muscle progenitor cells: building and repairing blood vessels. Circ Res. 2011;108(3):365–377. doi: 10.1161/CIRCRESAHA.110.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May SR, Stewart NJ, Chang W, Peterson AS. A Titin mutation defines roles for circulation in endothelial morphogenesis. Dev Biol. 2004;270(1):31–46. doi: 10.1016/j.ydbio.2004.02.006. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Chen Q, Horiguchi M, Starcher BC, Stanton JB, Broekelmann TJ, Marmorstein AD, McKay B, Mecham R, Nakamura T, Marmorstein LY. Targeted disruption of fibulin-4 abolishes elastogenesis and causes perinatal lethality in mice. Molecular and cellular biology. 2006;26(5):1700–1709. doi: 10.1128/MCB.26.5.1700-1709.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean SE, Mecham BH, Kelleher CM, Mariani TJ, Mecham RP. Extracellular matrix gene expression in the developing mouse aorta. Advances in Developmental Biology. 2005;15(05):81–128. [Google Scholar]
- Meyer-Lindenberg A, Mervis CB, Faith Berman K. Neural mechanisms in Williams syndrome: a unique window to genetic influences on cognition and behaviour. Nat Rev Neurosci. 2006;7(5):380–393. doi: 10.1038/nrn1906. doi: http://www.nature.com/nrn/journal/v7/n5/suppinfo/nrn1906_S1.html. [DOI] [PubMed] [Google Scholar]
- Mills I, Cohen CR, Kamal K, Li G, Shin T, Du W, Sumpio BE. Strain activation of bovine aortic smooth muscle cell proliferation and alignment: study of strain dependency and the role of protein kinase A and C signaling pathways. J Cell Physiol. 1997;170(3):228–234. doi: 10.1002/(SICI)1097-4652(199703)170:3<228::AID-JCP2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Morrow D, Scheller A, Birney YA, Sweeney C, Guha S, Cummins PM, Murphy R, Walls D, Redmond EM, Cahill PA. Notch-mediated CBF-1/RBP-J{kappa}-dependent regulation of human vascular smooth muscle cell phenotype in vitro. American journal of physiology Cell physiology. 2005a;289(5):C1188–C1196. doi: 10.1152/ajpcell.00198.2005. [DOI] [PubMed] [Google Scholar]
- Morrow D, Sweeney C, Birney YA, Cummins PM, Walls D, Redmond EM, Cahill PA. Cyclic strain inhibits Notch receptor signaling in vascular smooth muscle cells in vitro. Circ Res. 2005b;96(5):567–575. doi: 10.1161/01.RES.0000159182.98874.43. [DOI] [PubMed] [Google Scholar]
- Murphy ME, Carlson EC. An ultrastructural study of developing extracellular matrix in vitelline blood vessels of the early chick embryo. The American journal of anatomy. 1978;151(3):345–375. doi: 10.1002/aja.1001510304. [DOI] [PubMed] [Google Scholar]
- Murtada SI, Kroon M, Holzapfel GA. A calcium-driven mechanochemical model for prediction of force generation in smooth muscle. Biomech Model Mechanobiol. 2010;9(6):749–762. doi: 10.1007/s10237-010-0211-0. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, Tashiro K, Ross J, Jr, Honjo T, Chien KR. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415(6868):171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- Ninomiya K, Takahashi A, Fujioka Y, Ishikawa Y, Yokoyama M. Transforming growth factor-beta signaling enhances transdifferentiation of macrophages into smooth muscle-like cells. Hypertens Res. 2006;29(4):269–276. doi: 10.1291/hypres.29.269. [DOI] [PubMed] [Google Scholar]
- Ohno M, Cooke JP, Dzau VJ, Gibbons GH. Fluid shear stress induces endothelial transforming growth factor beta-1 transcription and production. Modulation by potassium channel blockade. J Clin Invest. 1995;95(3):1363–1369. doi: 10.1172/JCI117787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohura N, Yamamoto K, Ichioka S, Sokabe T, Nakatsuka H, Baba A, Shibata M, Nakatsuka T, Harii K, Wada Y, Kohro T, Kodama T, Ando J. Global analysis of shear stress-responsive genes in vascular endothelial cells. Journal of atherosclerosis and thrombosis. 2003;10(5):304–313. doi: 10.5551/jat.10.304. [DOI] [PubMed] [Google Scholar]
- Osborne LR, Martindale D, Scherer SW, Shi X-M, Huizenga J, Heng HHQ, Costa T, Pober B, Lew L, Brinkman J, Rommens J, Koop B, Tsui L-C. Identification of Genes from a 500-kb Region at 7q11.23 That Is Commonly Deleted in Williams Syndrome Patients. Genomics. 1996;36(2):328–336. doi: 10.1006/geno.1996.0469. [DOI] [PubMed] [Google Scholar]
- Paule WJ. Electron microscopy of the newborn rat aorta. J Ultrastruct Res. 1963;8:219–235. doi: 10.1016/s0022-5320(63)90004-2. [DOI] [PubMed] [Google Scholar]
- Pereira L, Andrikopoulos K, Tian J, Lee SY, Keene DR, Ono R, Reinhardt DP, Sakai LY, Biery NJ, Bunton T, Dietz HC, Ramirez F. Targetting of the gene encoding fibrillin-1 recapitulates the vascular aspect of Marfan syndrome. Nature genetics. 1997;17(2):218–222. doi: 10.1038/ng1097-218. [DOI] [PubMed] [Google Scholar]
- Phornphutkul C, Rosenthal A, Nadas AS. Cardiac Manifestations of Marfan Syndrome in Infancy and Childhood. Circulation. 1973;47(3):587–596. doi: 10.1161/01.cir.47.3.587. [DOI] [PubMed] [Google Scholar]
- Pope FM, Martin GR, Lichtenstein JR, Penttinen R, Gerson B, Rowe DW, McKusick VA. Patients with Ehlers-Danlos syndrome type IV lack type III collagen. Proc Natl Acad Sci U S A. 1975;72(4):1314–1316. doi: 10.1073/pnas.72.4.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske S, Hartschuh W, Enk A, Hausser I. Ehlers-Danlos syndrome – 20 years experience with diagnosis and classification. JDDG: Journal der Deutschen Dermatologischen Gesellschaft. 2006;4(4):308–318. doi: 10.1111/j.1610-0387.2006.05958.x. [DOI] [PubMed] [Google Scholar]
- Pyeritz RE. Ehlers–Danlos Syndrome. New England Journal of Medicine. 2000;342(10):730–732. doi: 10.1056/NEJM200003093421009. [DOI] [PubMed] [Google Scholar]
- Qin H, Ishiwata T, Wang R, Kudo M, Yokoyama M, Naito Z, Asano G. Effects of extracellular matrix on phenotype modulation and MAPK transduction of rat aortic smooth muscle cells in vitro. Experimental and molecular pathology. 2000;69(2):79–90. doi: 10.1006/exmp.2000.2321. [DOI] [PubMed] [Google Scholar]
- Rachev A. Theoretical study of the effect of stress-dependent remodeling on arterial geometry under hypertensive conditions. J Biomech. 1997;30(8):819–827. doi: 10.1016/s0021-9290(97)00032-8. [DOI] [PubMed] [Google Scholar]
- Rachev A, Hayashi K. Theoretical study of the effects of vascular smooth muscle contraction on strain and stress distributions in arteries. Ann Biomed Eng. 1999;27(4):459–468. doi: 10.1114/1.191. [DOI] [PubMed] [Google Scholar]
- Rachev A, Stergiopulos N, Meister JJ. Theoretical study of dynamics of arterial wall remodeling in response to changes in blood pressure. J Biomech. 1996;29(5):635–642. doi: 10.1016/0021-9290(95)00108-5. [DOI] [PubMed] [Google Scholar]
- Recchia D, Sharkey AM, Bosner MS, Kouchoukos NT, Wickline SA. Sensitive Detection of Abnormal Aortic Architecture in Marfan Syndrome With High-Frequency Ultrasonic Tissue Characterization. Circulation. 1995;91(4):1036–1043. doi: 10.1161/01.cir.91.4.1036. [DOI] [PubMed] [Google Scholar]
- Riha GM, Wang X, Wang H, Chai H, Mu H, Lin PH, Lumsden AB, Yao Q, Chen C. Cyclic strain induces vascular smooth muscle cell differentiation from murine embryonic mesenchymal progenitor cells. Surgery. 2007;141(3):394–402. doi: 10.1016/j.surg.2006.07.043. [DOI] [PubMed] [Google Scholar]
- Risau W, Flamme I. Vasculogenesis. Annual review of cell and developmental biology. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21(20):2511–2524. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- Rodriguez EK, Hoger A, McCulloch AD. Stress-dependent finite growth in soft elastic tissues. J Biomech. 1994;27(4):455–467. doi: 10.1016/0021-9290(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Rodríguez J, Goicolea JM, Gabaldón F. A volumetric model for growth of arterial walls with arbitrary geometry and loads. Journal of Biomechanics. 2007;40(5):961–971. doi: 10.1016/j.jbiomech.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Rodriguez JF, Ruiz C, Doblare M, Holzapfel GA. Mechanical stresses in abdominal aortic aneurysms: influence of diameter, asymmetry, and material anisotropy. J Biomech Eng. 2008;130(2):021023. doi: 10.1115/1.2898830. [DOI] [PubMed] [Google Scholar]
- Ross JJ, Hong Z, Willenbring B, Zeng L, Isenberg B, Lee EH, Reyes M, Keirstead SA, Weir EK, Tranquillo RT, Verfaillie CM. Cytokine-induced differentiation of multipotent adult progenitor cells into functional smooth muscle cells. J Clin Invest. 2006;116(12):3139–3149. doi: 10.1172/JCI28184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol. 1986;250(6 Pt 2):H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- Sage H, Gray WR. Studies on the evolution of elastin--I. Phylogenetic distribution. Comparative biochemistry and physiology B, Comparative biochemistry. 1979;64(4):313–327. doi: 10.1016/0305-0491(79)90277-3. [DOI] [PubMed] [Google Scholar]
- Schwarze U, Atkinson M, Hoffman GG, Greenspan DS, Byers PH. Null alleles of the COL5A1 gene of type V collagen are a cause of the classical forms of Ehlers-Danlos syndrome (types I and II) American journal of human genetics. 2000;66(6):1757–1765. doi: 10.1086/302933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi ZD, Tarbell JM. Fluid flow mechanotransduction in vascular smooth muscle cells and fibroblasts. Ann Biomed Eng. 2011;39(6):1608–1619. doi: 10.1007/s10439-011-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N, Yamamoto K, Obi S, Kumagaya S, Masumura T, Shimano Y, Naruse K, Yamashita JK, Igarashi T, Ando J. Cyclic strain induces mouse embryonic stem cell differentiation into vascular smooth muscle cells by activating PDGF receptor beta. J Appl Physiol. 2008;104(3):766–772. doi: 10.1152/japplphysiol.00870.2007. [DOI] [PubMed] [Google Scholar]
- Stalhand J, Klarbring A, Holzapfel GA. Smooth muscle contraction: mechanochemical formulation for homogeneous finite strains. Progress in biophysics and molecular biology. 2008;96(1–3):465–481. doi: 10.1016/j.pbiomolbio.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Sterpetti AV, Cucina A, Fragale A, Lepidi S, Cavallaro A, Santoro-D'Angelo L. Shear stress influences the release of platelet derived growth factor and basic fibroblast growth factor by arterial smooth muscle cells. Winner of the ESVS prize for best experimental paper 1993. European journal of vascular surgery. 1994;8(2):138–142. doi: 10.1016/s0950-821x(05)80448-7. [DOI] [PubMed] [Google Scholar]
- Sutcliffe MC, Davidson JM. Effect of static stretching on elastin production by porcine aortic smooth muscle cells. Matrix. 1990;10(3):148–153. doi: 10.1016/s0934-8832(11)80163-0. [DOI] [PubMed] [Google Scholar]
- Szabo Z, Crepeau MW, Mitchell AL, Stephan MJ, Puntel RA, Yin Loke K, Kirk RC, Urban Z. Aortic aneurysmal disease and cutis laxa caused by defects in the elastin gene. J Med Genet. 2006;43:255–258. doi: 10.1136/jmg.2005.034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber LA. A Model for Aortic Growth Based on Fluid Shear and Fiber Stresses. J Biomech Eng. 1998;120:348–467. doi: 10.1115/1.2798001. [DOI] [PubMed] [Google Scholar]
- Taber LA. Theoretical study of Beloussov's hyper-restoration hypothesis for mechanical regulation of morphogenesis. Biomech Model Mechanobiol. 2008;7(6):427–441. doi: 10.1007/s10237-007-0106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber LA. Towards a unified theory for morphomechanics. Philos Transact A Math Phys Eng Sci. 2009;367(1902):3555–3583. doi: 10.1098/rsta.2009.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada S, Tarbell JM. Interstitial flow through the internal elastic lamina affects shear stress on arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 2000;278(5):H1589–H1597. doi: 10.1152/ajpheart.2000.278.5.H1589. [DOI] [PubMed] [Google Scholar]
- Thorne BC, Hayenga HN, Humphrey JD, Peirce SM. Toward a multi-scale computational model of arterial adaptation in hypertension: verification of a multi-cell agent based model. Frontiers in physiology. 2011;2:20. doi: 10.3389/fphys.2011.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsamis A, Stergiopulos N. Arterial remodeling in response to hypertension using a constituent-based model. Am J Physiol Heart Circ Physiol. 2007;293(5):H3130–H3139. doi: 10.1152/ajpheart.00684.2007. [DOI] [PubMed] [Google Scholar]
- Valentin A, Cardamone L, Baek S, Humphrey JD. Complementary vasoactivity and matrix remodelling in arterial adaptations to altered flow and pressure. J R Soc Interface. 2009;6(32):293–306. doi: 10.1098/rsif.2008.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin A, Humphrey JD. Modeling effects of axial extension on arterial growth and remodeling. Med Biol Eng Comput. 2009a;47(9):979–987. doi: 10.1007/s11517-009-0513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin A, Humphrey JD. Parameter sensitivity study of a constrained mixture model of arterial growth and remodeling. J Biomech Eng. 2009b;131(10):101006. doi: 10.1115/1.3192144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vito RP, Dixon SA. Blood vessel constitutive models-1995-2002. Annu Rev Biomed Eng. 2003;5:413–439. doi: 10.1146/annurev.bioeng.5.011303.120719. [DOI] [PubMed] [Google Scholar]
- von Maltzahn WW, Besdo D, Wiemer W. Elastic properties of arteries: a nonlinear two-layer cylindrical model. J Biomech. 1981;14(6):389–397. doi: 10.1016/0021-9290(81)90056-7. [DOI] [PubMed] [Google Scholar]
- von Maltzahn WW, Warriyar RG, Keitzer WF. Experimental measurements of elastic properties of media and adventitia of bovine carotid arteries. J Biomech. 1984;17(11):839–847. doi: 10.1016/0021-9290(84)90142-8. [DOI] [PubMed] [Google Scholar]
- Vuillemin M, Pexieder T. Normal stages of cardiac organogenesis in the mouse: II. Development of the internal relief of the heart. The American journal of anatomy. 1989;184(2):114–128. doi: 10.1002/aja.1001840203. [DOI] [PubMed] [Google Scholar]
- Wagenseil JE. A constrained mixture model for developing mouse aorta. Biomech Model Mechanobiol. 2011;10(5):671–687. doi: 10.1007/s10237-010-0265-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenseil JE, Ciliberto CH, Knutsen RH, Levy MA, Kovacs A, Mecham RP. Reduced vessel elasticity alters cardiovascular structure and function in newborn mice. Circ Res. 2009;104(10):1217–1224. doi: 10.1161/CIRCRESAHA.108.192054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenseil JE, Ciliberto CH, Knutsen RH, Levy MA, Kovacs A, Mecham RP. The importance of elastin to aortic development in mice. Am J Physiol Heart Circ Physiol. 2010;299(2):H257–H264. doi: 10.1152/ajpheart.00194.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenseil JE, Knutsen RH, Li D, Mecham RP. Elastin insufficient mice show normal cardiovascular remodeling in 2K1C hypertension, despite higher baseline pressure and unique cardiovascular architecture. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.00205.2007. [DOI] [PubMed] [Google Scholar]
- Wagenseil JE, Mecham RP. New insights into elastic fiber assembly. Birth Defects Res C Embryo Today. 2007;81(4):229–240. doi: 10.1002/bdrc.20111. [DOI] [PubMed] [Google Scholar]
- Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89(3):957–989. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenseil JE, Nerurkar NL, Knutsen RH, Okamoto RJ, Li DY, Mecham RP. Effects of elastin haploinsufficiency on the mechanical behavior of mouse arteries. Am J Physiol Heart Circ Physiol. 2005;289(3):H1209–H1217. doi: 10.1152/ajpheart.00046.2005. [DOI] [PubMed] [Google Scholar]
- Wakimoto K, Kobayashi K, Kuro OM, Yao A, Iwamoto T, Yanaka N, Kita S, Nishida A, Azuma S, Toyoda Y, Omori K, Imahie H, Oka T, Kudoh S, Kohmoto O, Yazaki Y, Shigekawa M, Imai Y, Nabeshima Y, Komuro I. Targeted disruption of Na? exchanger gene leads to cardiomyocyte apoptosis and defects in heartbeat. J Biol Chem. 2000;275(47):36991–36998. doi: 10.1074/jbc.M004035200. [DOI] [PubMed] [Google Scholar]
- Wang DM, Tarbell JM. Modeling interstitial flow in an artery wall allows estimation of wall shear stress on smooth muscle cells. J Biomech Eng. 1995;117(3):358–363. doi: 10.1115/1.2794192. [DOI] [PubMed] [Google Scholar]
- Wenstrup RJ, Florer JB, Brunskill EW, Bell SM, Chervoneva I, Birk DE. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem. 2004;279(51):53331–53337. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- Wenstrup RJ, Florer JB, Davidson JM, Phillips CL, Pfeiffer BJ, Menezes DW, Chervoneva I, Birk DE. Murine model of the Ehlers-Danlos syndrome. col5a1 haploinsufficiency disrupts collagen fibril assembly at multiple stages. J Biol Chem. 2006;281(18):12888–12895. doi: 10.1074/jbc.M511528200. [DOI] [PubMed] [Google Scholar]
- Wernig F, Mayr M, Xu Q. Mechanical stretch-induced apoptosis in smooth muscle cells is mediated by beta1-integrin signaling pathways. Hypertension. 2003;41(4):903–911. doi: 10.1161/01.HYP.0000062882.42265.88. [DOI] [PubMed] [Google Scholar]
- Wilson E, Sudhir K, Ives HE. Mechanical strain of rat vascular smooth muscle cells is sensed by specific extracellular matrix/integrin interactions. J Clin Invest. 1995;96(5):2364–2372. doi: 10.1172/JCI118293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. In: The law of bone remodeling. Maquet P, Furlong R, translators. Berline: Springer; 1986. [Google Scholar]
- Wolinsky H. Response of the rat aortic media to hypertension. Morphological and chemical studies. Circ Res. 1970;26(4):507–522. doi: 10.1161/01.res.26.4.507. [DOI] [PubMed] [Google Scholar]
- Wolinsky H, Glagov S. Structural Basis for the Static Mechanical Properties of the Aortic Media. Circ Res. 1964;14:400–413. doi: 10.1161/01.res.14.5.400. [DOI] [PubMed] [Google Scholar]
- Wolinsky H, Glagov S. A lamellar unit of aortic medial structure and function in mammals. Circ Res. 1967;20(1):99–111. doi: 10.1161/01.res.20.1.99. [DOI] [PubMed] [Google Scholar]
- Wuyts FL, Vanhuyse VJ, Langewouters GJ, Decraemer WF, Raman ER, Buyle S. Elastic properties of human aortas in relation to age and atherosclerosis: a structural model. Phys Med Biol. 1995;40(10):1577–1597. doi: 10.1088/0031-9155/40/10/002. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Yamamoto K, Noumura T. Type I collagen promotes modulation of cultured rabbit arterial smooth muscle cells from a contractile to a synthetic phenotype. Exp Cell Res. 1993;204(1):121–129. doi: 10.1006/excr.1993.1016. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Davis EC. Unraveling the mechanism of elastic fiber assembly: The roles of short fibulins. Int J Biochem Cell Biol. 2010;42(7):1084–1093. doi: 10.1016/j.biocel.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415(6868):168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- Yang J, Clark JW, Jr, Bryan RM, Robertson C. The myogenic response in isolated rat cerebrovascular arteries: smooth muscle cell model. Medical engineering & physics. 2003;25(8):691–709. doi: 10.1016/s1350-4533(03)00100-0. [DOI] [PubMed] [Google Scholar]
- Zhang H, Hu W, Ramirez F. Developmental expression of fibrillin genes suggests heterogeneity of extracellular microfibrils. J Cell Biol. 1995;129(4):1165–1176. doi: 10.1083/jcb.129.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulliger MA, Fridez P, Hayashi K, Stergiopulos N. A strain energy function for arteries accounting for wall composition and structure. J Biomech. 2004;37(7):989–1000. doi: 10.1016/j.jbiomech.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Zulliger MA, Stergiopulos N. Structural strain energy function applied to the ageing of the human aorta. J Biomech. 2007;40(14):3061–3069. doi: 10.1016/j.jbiomech.2007.03.011. [DOI] [PubMed] [Google Scholar]