Abstract
Arousal from sleep is a major defense mechanism in infants against hypoxia and/or hypercapnia. Arousal failure may be an important contributor to SIDS. Areas of the brainstem that have been found to be abnormal in a majority of SIDS infants are involved in the arousal process. Arousal is sleep state dependent, being depressed during AS in most mammals, but depressed during QS in human infants. Repeated exposure to hypoxia causes a progressive blunting of arousal that may involve medullary raphe GABAergic mechanisms. Whereas CB chemoreceptors contribute heavily to arousal in response to hypoxia, serotonergic central chemoreceptors have been implicated in the arousal response to CO2. Pulmonary or chest wall mechanoreceptors also contribute to arousal in proportion to the ventilatory response and decreases in their input may contribute to depressed arousal during AS. Little is known about specific arousal pathways beyond the NTS. Whether CB chemoreceptor stimulation directly stimulates arousal centers or whether this is done indirectly through respiratory networks remains unknown. This review will focus on arousal in response to hypoxia and CO2 in the fetus and newborn and will outline what we know (and don’t know) about the involvement of the carotid body in this process.
Keywords: Arousal, carotid body, newborn, fetus, sleep
1. Introduction
In the newborn and young infant, the development of sleep is coincident with dramatic changes in brain development. Historically, there has been less focus on the development of wakening and arousal processes. In the infant, maturational processes in the brainstem and forebrain underlie the development of wakefulness. Dysfunction in these same systems have been linked to The Sudden Infant Death Syndrome (SIDS), acute life threatening events (ALTE), and sleep apnea (Hayes, 2002). Most agree that the mechanisms of sleep and wakefulness are closely related. Based on ultrasound and fetal heart rate recordings, the fetus appears to be mostly somnolent, but active with a prevalence of active or “REM-like” sleep. There is some speculation that the high level of active sleep during the second half of human pregnancy may serve as a “replacement” for wakefulness, particularly for the development of the visual system (Hopkins, 2002).
Immediately after birth, however, full term infants are mostly awake for the first 6 hours of life (Desmond et al., 1963). This may be related to the “stress” related to the birth process and high levels of adrenaline and noradrenaline (Lagercrantz & Slotkin, 1986). In addition, strong inhibitory influences originating in the placenta, including prostaglandins, are removed at birth resulting in a “release” of inhibition of both breathing and wakefulness present in the fetus (Darnall, 2010). In the full term infant, the amount of time spent awake has been reported to gradually increase from about 24% of the time at 2 weeks of age to 64% by the end of the third month (Hopkins, 2002). Beginning in the neonatal period and continuing through the first year, sub-types of wakening can be distinguished including cry/fuss, quiet alert, active alert, drowsy, etc (Prechtl, 1974). During this same period there is a gradual consolidation of sleep during the night time hours with the establishment of a stable circadian sleep-wake rhythm by 2–3 months (Coons & Guilleminault, 1982).
An often quoted editorial by Phillipson and Sullivan in 1978 entitled “Arousal: The forgotten response to respiratory stimuli” refocused attention on the arousal response to respiratory stimuli. They argued that during sleep the ability to arouse might be the most important response when challenged with hypoxia or hypercapnia (Phillipson & Sullivan, 1978). Arousal from sleep has been considered an essential element for restoration of homeostasis during respiratory and cardiovascular challenges to physiological systems by providing an excitatory drive to vital processes. Cortical activation has been the “gold standard” for the definition of arousal. However, there is a range of “sub-cortical” responses that occur either with or without changes in the EEG. For example, somatosensory and auditory stimuli often result in cardiac, respiratory, or somatic changes without overt cortical activation (Horner, 1996). Arousal can also occur “spontaneously” apparently in response to internal physiological changes. In human infants and developing mammals there is a stereotypical arousal sequence including both subcortical or autonomic and cortical changes that occurs both spontaneously and in response to external stimuli (Lijowska et al., 1997; Dauger et al., 2001; Darnall et al., 2010). This review will touch on spontaneous arousals but will focus on arousal in response to hypoxia and CO2 in the fetus and newborn and will review what we know (and don’t know) about the involvement of the carotid body in this process.
2. Carotid body function in the fetus
Almost all of our information about carotid body (CB) function in the fetus comes from the fetal lamb. Direct recordings of electrical activity in the carotid sinus or aortic nerves in the exteriorized lamb fetus suggest that CB peripheral chemoreceptors are tonically active and respond to decreases in PaO2 and increases in PaCO2 (Blanco et al., 1982, 1984a). In the fetal lamb and human, the resting PaO2 is ~25 mmHg. In the fetal lamb carotid sinus nerve (CSN) electrical activity does not start to increase until PaO2 falls below ~15 mmHg. Details about the postnatal development of carotid body mechanisms and function are addressed in other articles in this series.
3. Hypoxemia in the fetus
Before birth, fetal “breathing” movements (FBMs), characterized by rhythmic contractions of the diaphragm, intercostals and laryngeal muscles can be observed in most mammalian species sometime during the second trimester of pregnancy (Jansen & Chernick, 1983; Jansen & Chernick, 1991; Kobayashi et al., 2001). FBMs characteristically occur during periods of low voltage, high-frequency electrocorticogram (LV-ECoG), rapid eye movements and hypotonia, similar to many features of REM sleep (Dawes et al., 1972). During periods of high voltage low-frequency ECoG (HV-ECoG), the fetus is characteristically apneic. In the fetal lamb FBMs are irregular with an abrupt beginning and ending of diaphragmatic (DIA) EMG electrical activity. There is no evidence for a gradual decrease in DIA EMG activity representing expiratory braking frequently observed in the premature human infant (Harding et al., 1980; Dawes et al., 1982; Rigatto et al., 1986). The incidence of FBMs appears to increase after meals and is associated with increased maternal plasma glucose levels (Patrick et al., 1978).
In the fetus, FBMs do not serve a gas exchange function, but are critical for fetal lung growth (Nijhuis et al., 1986; Savich et al., 1992; Harding et al., 1993). Contrary to what occurs after birth, decreasing PaO2 in the fetus, despite stimulation of CB chemoreceptors, inhibits rather than stimulates fetal breathing (Koos et al., 1987; Jansen & Chernick, 1991). After birth, stimulation of the CB peripheral chemoreceptors by decreasing PaO2 typically results in a rapid increase in respiratory frequency (fR) and tidal volume (VT) followed by a decrease, resulting in a “biphasic ventilatory response” (Rigatto et al., 1975; Darnall, 2010). However, very premature infants with stages of development analogous to the third trimester of pregnancy, there is only depression of breathing, similar to the response in the fetus (Alvaro et al., 1992). In addition, denervation of the CB does not alter fetal breathing or fetal state (Koos et al., 1987; Moore et al., 1989). The inhibition of FBMs during hypoxia likely originates from a region in the upper lateral pons (Johnston & Gluckman, 1989, 1993). Lesions in this region reverse the depression of FBMs caused by hypoxia and also allow CO2 to stimulate FBMs during the HV-ECoG state (Johnston & Gluckman, 1989) suggesting that this region may also provide some level of tonic inhibition, especially during periods of HV-ECoG. Further evidence for the presence of a fetal lateral pontine inhibitory area comes from transection and lesion studies in newborn animals showing that the depressive phase of the “biphasic” hypoxic response can be attenuated by mid-collicular but not pre-collicular transections, and red nucleus lesions in rabbits and rats (Martin-Body & Johnston, 1988; Waites et al., 1996). In newborn animals there is abundant evidence that the central depressive effects of hypoxia including increasing concentrations of GABA and adenosine contribute to the depressive phase of the biphasic response (Darnall, 1985; Xiao et al., 2000; Hehre et al., 2008). It has also been hypothesized that since CB output does not decrease during steady state hypoxia (Blanco et al., 1984b), the late ventilatory decline might also be the result of stimulation of brainstem neurons located near the red nucleus that are inhibitory to respiratory output (Moore et al., 1996).
Whereas hypoxia and presumably stimulation of the CB cause depression of FBMs, the predominant effects of peripheral chemoreceptor stimulation are on the circulation (Hanson, 1988). Changes in heart rate (fH) in response to CO2 and hypoxia are thought to be mediated by CB peripheral chemoreceptors. Both hypoxia and hypercapnia produce substantial decreases in fH. In the case of hypoxia, the change in fH is inversely related to the resting oxyhemoglobin saturation (SaO2) (Boekkooi et al., 1992). The initial cardiovascular responses are reflex in nature and the CB chemoreceptors provide the afferent limb of this reflex. The fall in fH is vagally mediated and the peripheral vasoconstriction is partly α-adrenergic (Giussani et al., 1993). The increase in extracellular adenosine that occurs during hypoxia also plays a significant role in mediating a decrease in fH via adenosine A1 receptors (Rivkees et al., 2001).
4. Does the fetus wake up?
Although controversial, the lamb fetus may spend a very small amount of time in a more active “awake” state characterized by LV-ECoG, fetal breathing, increased tonic and phasic nuchal EMG activity, and increased mean arterial blood pressure (Bissonnette et al., 1995). GABAergic and glutamatergic mechanisms appear to be important in suppressing this rarely observed “awake” state. For example, administration of NMDA antagonists increases the occurence of this state, which is prevented by increasing extracellular GABA by inhibiting reuptake mechanisms (Bissonnette et al., 1995). In the human fetus between 36 and 38 weeks gestation using ultrasound and fetal heart rate recordings with a 3-minute moving time window, both quiet and active wakefulness have been described. These states are difficult to detect and durations are very short but increase as term approaches, when wakefulness is estimated to reach 7% (Nijhuis et al., 1982). Preterm infants matched by gestational age also show an increasing differentiation between sleep and wakefulness as term age is approached (Mirmiran, 1995). Thus, true arousal to wakefulness in response to stimuli such as increasing glucose concentrations probably occurs only rarely, but transitions from HV-ECoG to LV-ECoG active sleep are common. However, little is known about brief spontaneous arousals to wakefulness that might occur in the fetus. Methods used to define state in the fetus may not have been sensitive enough to detect brief arousals as they have been limited to periods of at least 3-minutes. Thus brief 20–30 second spontaneous arousals, commonly observed in infant animals and humans, would not be detected.
5. Definitions of infant arousal
The usual definition of “arousal” or “awakening” from sleep includes a constellation of physiologic responses including increases in fH and blood pressure and muscle tone, a sustained inspiratory effort or breathing pause, and activation of the EEG. In adult human and animal studies the presence of EEG changes consistent with arousal is currently the “gold standard” of arousal. In infants, behavioral, EMG, EOG and EEG criteria have been used to study both spontaneous arousals and those in response to hypoxia or hypercapnia. Comparison between studies has been difficult because of varying definitions of infant “arousal” that include vigorous body movements, eye opening, awakening and/or crying. Thach and colleagues have described in a stereotypical sequence of arousal events that frequently occur associated with both spontaneous and elicited arousals. These events usually begin with an augmented breath, followed by a startle, changes in fH, and then EEG changes and are similar in NREM and REM sleep although the frequency of full cortical arousals appears to be higher in REM (McNamara et al., 1998; McNamara et al., 2002). In potentially asphyxiating environments arousals are often associated with “thrashing” movements that may serve to move the infant out of a potentially dangerous situation (Lijowska et al., 1997). Similar stereotypical changes, either occurring spontaneously or in response to hypoxia, have been observed in piglets and rodent pups (BuSha et al., 2001; Dauger et al., 2001; Darnall et al., 2010).
Thus arousals consist of both “subcortical” (autonomic) and “cortical” components. Autonomic components of the arousal response involving changes in fH, blood pressure, and upper airway control may serve to provide cardiovascular support to a full cortical arousal, or maintain airway patency, particularly during obstructive apnea without fully disrupting sleep (Horner, 1996; Horne et al., 2005). Definitions for the subcortical and cortical components of arousal have recently been suggested by an international working group. These scoring definitions rely heavily on visual scoring, have not been universally accepted (International Paediatric Work Group on, 2005) but have been used in many studies in human infants. According to these definitions, subcortical arousals can occur without any change in EEG but have at least two of the following: a gross body movement, an increase in heart rate of at least 10% above baseline, changes in respiratory rate or rhythm while in NREM sleep or an increase in chin EMG tone in REM sleep. Cortical arousals are defined with the above criteria with the addition of an abrupt change in EEG background frequency of at least 1 Hz for a minimum of 3 seconds (Montemitro et al., 2008). Thus using these definitions, a cortical arousal always has a subcortical component.
6. Spontaneous arousals in the neonate
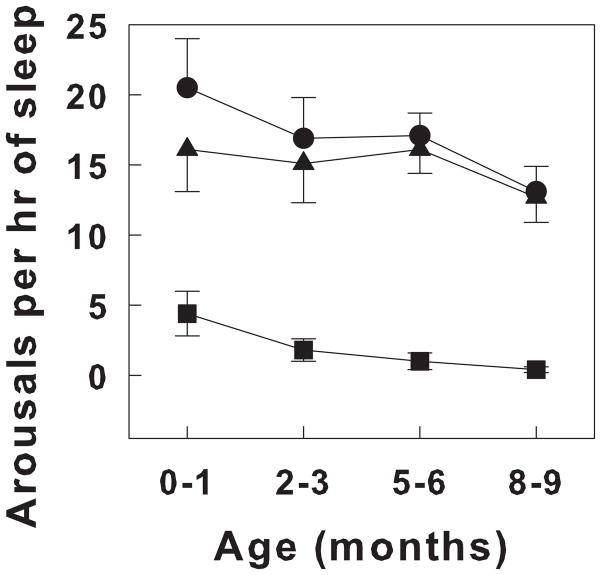
There has been considerable interest in arousals that are not stimulated by any recognized stimulus. It has been argued that the frequency of spontaneous arousals may be a general measure of “arousability”. However, there is somewhat of a blurred line between “mini” or brief arousals that don’t result in a state change and those arousals termed “awakenings” that result in a clear state change to wakefulness. In general, spontaneous arousals have durations of less than a minute. There are no standardized criteria for spontaneous arousals. In infants, many brief spontaneous arousals consist only of subcortical components. When there are EEG changes, they are brief and EEG returns to the pattern associated with the particular state in which the arousal occurred. The number of spontaneous arousals decreases over the first year of development in the human infant. Figure 1 shows the number of spontaneous arousals per hour of sleep in healthy infants over the first 9 months of life (Montemitro et al., 2008).
Figure 1.
The prevalence of cortical and subcortical arousals in the first 9 months of life in healthy full term infants. Sleep was recorded during night polysomnograms. Arousal was scored according to guidelines suggested by the International Paediatric North Group on Arousals (see text). Arousals are quantified as number of arousals per hour of sleep. Cortical arousals are illustrated as filled triangles, subcortical arousals as filled squares and the total number of arousals indicated as closed circles. All values are means ± SEM. Note that the total number of arousals decreases with age, largely related to the decrease in subcortical arousals. Adapted from Montemitro, et al, 2007 (Montemitro et al., 2008).
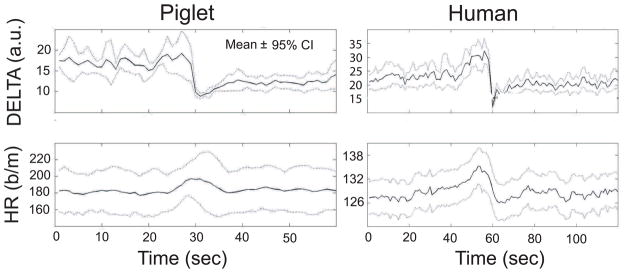
In a study of spontaneous arousals in piglets identified both visually and with a wavelet analysis, we determined that when EEG changes were accompanied by fH and or blood pressure changes, the autonomic changes always preceded the EEG changes. Using an automated analysis, which independently identified changes in BP, fH, and EEG, we found that fH and BP changes often occurred without EEG changes, and less often, EEG changes occurred without fH and BP changes (BuSha et al., 2001). From these data it is tempting to hypothesize that spontaneous arousals originate in the brainstem. Although there is evidence of a temporal relationship between autonomic and EEG changes during arousal, we can only speculate whether there is a cause and effect relationship. Moreover, we found no relationship between the magnitude of the cardiovascular transients and the magnitude of the decrease in delta power suggesting that changes in BP or fH did not “cause” the EEG changes. An alternative explanation would be that EEG and BP or fH transients, when occurring together, arise from a common stimulus that is processed in parallel through the central nervous system with varying temporal profiles. The EEG and fH changes and their temporal relationships in piglets and human infants are strikingly similar. Figure 2 shows changes in EEG delta power and HR in 75 spontaneous arousals in 5 piglets compared to 73 arousals in a single 3 month old infant (Ariagno et al., 2002).
Figure 2.
Delta activity and heart rate activity during spontaneous arousals in the piglet and human infant. Values of delta activity (1–4 Hz) derived from wavelet analyses (upper panels) and heart rate (lower panels) are ensemble averages triggered on the heart rate peak derived from 75 spontaneous arousals in 5 piglets (left panels) and 73 spontaneous arousals in a single 3 month old infant (right panels). Values shown are means ± 95% confidence intervals. Note that the increase in heart rate precedes the fall in delta power. HR = heart rate; DELTA = delta power or activity. Adapted from Ariagno, et al, 2002 (Ariagno et al., 2002).
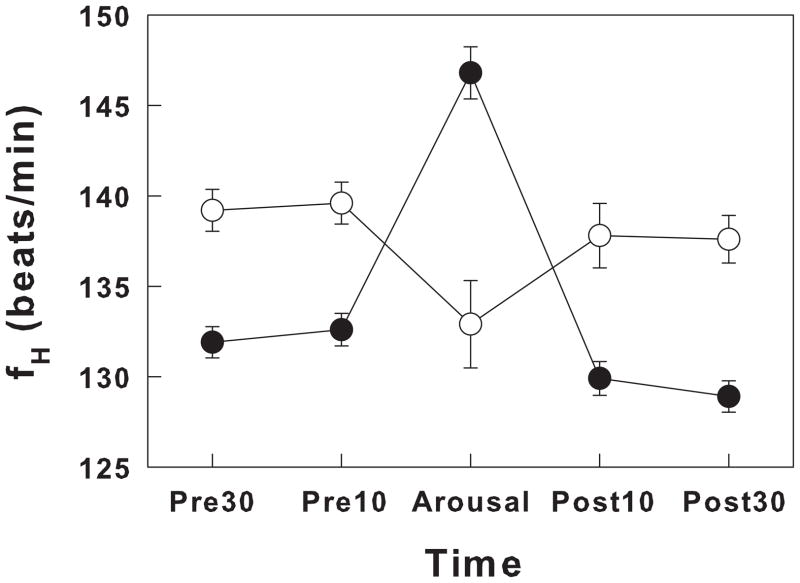
In the human infant, the characteristics and frequency of spontaneous arousals change over the course of development. Spontaneous arousals can be identified in premature infants but become more apparent as term approaches. Although the characteristics of spontaneous arousals in premature and term infants are similar, there are some differences in the fH response. In term infants spontaneous arousals are accompanied or preceded by an increase in fH that averages ~11%; a greater increase occurs with cortical arousals compared to subcortical arousals. In contrast, in preterm infants at ~35 weeks PMA and ~ 20 days of age, fH decreased or remained unchanged with arousal (Hanzer et al., 2007). Figure 3 shows the changes in heart rate during spontaneous arousals in term and premature human infants.
Figure 3.
Heart rate changes during spontaneous arousals in premature (open circles) and term (closed circles) newborns. Heart rate changes occurring before, during, and after arousals in preterm infants (GA: 32 ± 2 wks; PMA at the time of study: 35 ± 1 wk; Chronological age at the time of study: 24 ± 17 days, mean ± SD) and in full term infants (Chronological age at the time of study: 45 ± 12 days, mean ± SD). Values were expressed as heart rate in beats/min during the 30 seconds before arousal (Pre30), the 10 seconds before arousal (Pre10), the arousal period (Arousal), the 10 seconds after the arousal (Post10) and the 30 second period (Post30). All values of heart rate are expressed as means ± SEM. Note that the preterm infants, even when studied at 35 weeks PMA decreased rather than increased heart rate as was observed in the full term infants. Adapted from Hanzer, et al, 2007 (Hanzer et al., 2007).
7. Arousal in response to hypoxia in the human neonate
Studies of arousal in response to hypoxia have generally either evaluated the number of times arousal occurs (the probability of arousal), the time to arousal from the onset of hypoxia (arousal latency), or the PaO2 or SaO2 at which arousal occurs (arousal threshold). Early studies suggested that arousal in response to hypoxia was generally depressed in human newborn infants (Harper & Bandler, 1998). These findings may have reflected that most studies of arousal in response to hypoxia in human infants have been conducted during quiet sleep (QS) (Hunt et al., 1981; McCulloch et al., 1982; Brady & McCann, 1985; van der Hal et al., 1985; Milerad et al., 1989; Davidson-Ward et al., 1992; Dunne et al., 1992; Lewis & Bosque, 1995). Only a few early studies examined arousals in both QS and active sleep (AS) (Ariagno et al., 1980). More recent studies in human infants have shown that sleep state clearly influences the ability to arouse to hypoxia. The probability of arousing to 15% oxygen is very high during AS but is depressed during QS. In addition, arousal latencies are longer during QS compared to AS (Parslow et al., 2003). In both AS and QS arousal latencies in response to 15% oxygen become shorter with age (Parslow et al., 2004) and are shorter than intervals between spontaneous arousals (Horne et al., 2005). Moreover, SaO2 values at arousal during AS and QS are similar suggesting that SaO2 may decrease more rapidly during AS (Horne et al., 2005). When the arousal failure rate is examined, it is also clear that the level of hypoxia is also important. In one study, all infants failed to arouse when exposed to an FiO2 of 0.17 for 10 minutes (Brady & McCann, 1985) whereas in another all infants aroused when exposed to an FiO2 of 0.11 (van der Hal et al., 1985).
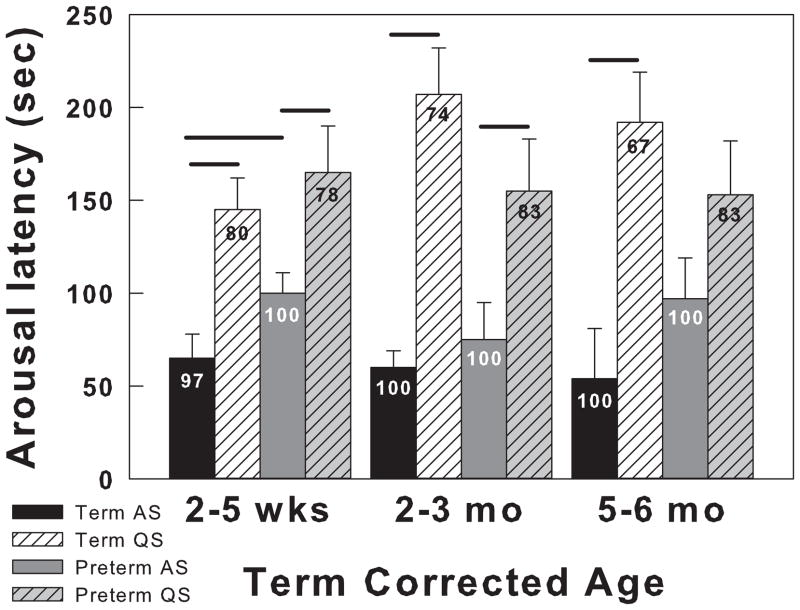
Less is known about arousal to hypoxia during prematurity. Apnea is common in premature infants and when prolonged is accompanied by hypoxia and is frequently terminated with an arousal. Arousal is more common with longer apneas and although hastens recovery, is not essential (Thoppil et al., 1991). In premature infants who were studied at 2–5 weeks, 2–3 and 5–6 months term corrected age, arousal to hypoxia always occurred during AS, but not during QS. During AS, arousal latency was significantly longer in preterm infants compared to term infants. During AS and QS non-arousing tests, preterm infants reached significantly lower SaO2 levels at 2–5 weeks and at 2–3 months during QS. Figure 4 shows arousal latency and probability of arousal in term and premature infants at 2–5 weeks, 2–3 months, and 5–6 months term corrected age exposed to 15% oxygen (Verbeek et al., 2008).
Figure 4.
The effects of sleep state on arousal responses to hypoxia in term and preterm infants at 2–5 weeks, 2–3 months, and 5–6 months of term corrected age. Arousal latencies and the SaO2 at the time of arousal were measured after exposure to 15% oxygen. Preterm infants GA ranged from 29 to 34 weeks (31 ± 0.7; mean ± SEM). Four groups of infants are compared in both QS and AS: Term AS (black bars), Term QS (hatched white bars), Preterm AS (dark gray bars) and Preterm QS (hatched light gray bars). Arousal latency is on the Y-axis and SaO2 are indicated on the bars. Horizontal lines above the bars indicate significant differences (at least P<0.05). In general arousal latencies are shorter and SaO2 values higher during AS. Arousal latencies were longer in term infants only at 2–5 weeks of age. All values are means ± SEM. Adapted from Verbeek et al, 2008 (Verbeek et al., 2008).
8. Arousal in response to hypoxia in the developing mammal
In contrast to findings in human infants, both adult and newborn animals exhibit delayed or impaired arousal in response to hypoxia during AS or REM. Different techniques have been used to produce progressive hypoxia and to detect arousal. Rebreathing 8%–10% oxygen (Rebuck & Campbell, 1974) produces a steady decrease in SaO2 and allows the maintenance of eucapnia. Changes in EEG, neck EMG, ± EOM and behavior are commonly used in adult animals and more precocious newborns to detect arousal. Early studies using these methods in adult dogs showed that the arousal response to hypoxia was markedly delayed during REM sleep even though regression analyses of VI vs SaO2 were similar among REM, NREM and wakefulness (Phillipson et al., 1978). The average arousal latency was ~ 32 seconds during NREM sleep and ~69 seconds during REM; analysis of arousal thresholds showed an SaO2 at arousal of 87.5% and 70.5% for NREM and REM sleep, respectively. Similarly, in newborn calves, arousal occurred at an SaO2 of ~85% during QS and ~59% during AS. In contrast to the findings in adult dogs, during AS, the lower SaO2 thresholds in during REM (AS) in newborn calves were associated with little or no ventilatory response (Jeffery & Read, 1980). Similar results have been reported in newborn lambs; AS was associated with lower SaO2 thresholds and a reduced ventilatory response to hypoxia (Henderson-Smart & Read, 1979). In this same report, however, newborn puppies behaved more like adult dogs with delayed arousal but a normal ventilatory response during AS, suggesting important species and/or maturational differences. More specifically, ribcage collapse was noted during REM in calves and lambs, but not puppies. Thus a species related difference in compliance of the chest wall may have contributed to the differences in the ventilatory response to hypoxia during AS.
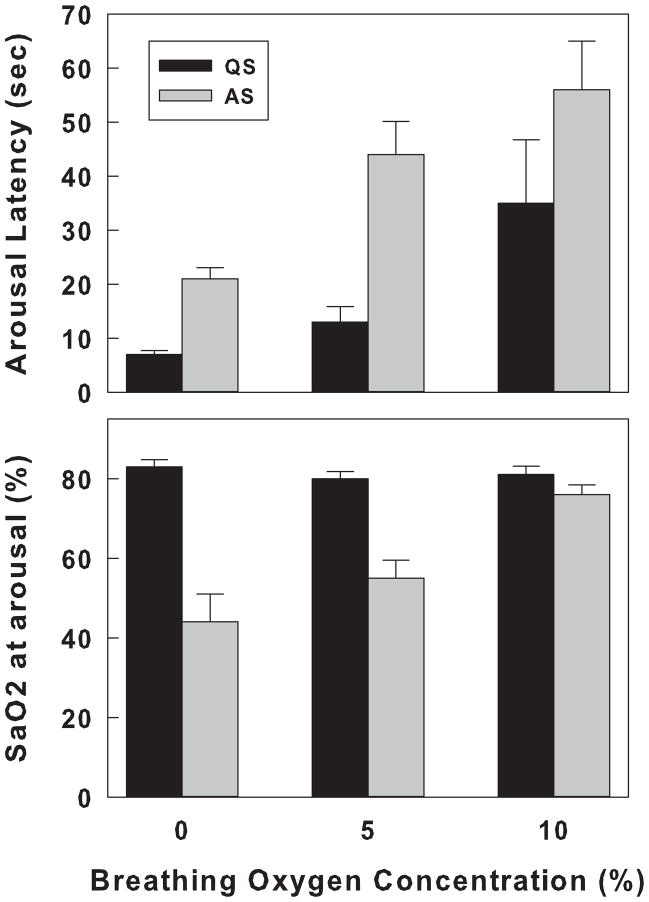
In other studies, decreases in SaO2 were induced by introducing gases of varying oxygen concentrations either directly to the upper airways via a tracheostomy, or into a chamber at different flow rates. In one study in tracheotomized newborn lambs, hypoxia was achieved by switching the inspired gas mixture to 10%, 5%, or 0% oxygen. During both QS and AS, the rate of decrease of SaO2 was inversely proportional to the oxygen concentration. During QS, although SaO2s at the time of arousal were similar (range = 81% to 83%), arousal latencies were shorter when lower oxygen concentrations were used. In contrast, during AS, SaO2 thresholds decreased from 76% during 10% oxygen to 44% during 0%. Similar to what was observed during QS, arousal latencies during AS were longer than those during QS and decreased from 56 sec during 10% oxygen to 21 sec during 0% (Fewell & Baker, 1987). During QS, the ventilatory responses estimated by measurements of the change in fR and in the integrated electrical activity of the diaphragm were inversely proportional to the inspired gas concentration. During AS, however, there was no increase in fR during 10% oxygen whereas changes in ventilation during 5% and 0% oxygen exposure were comparable to those obtained during QS. Figure 5 shows arousal latencies and the SaO2 at arousal for QS and AS when breathing 10%, 5%, and 0% oxygen in new born lambs. One interpretation of these data could be that both chemoreceptor and mechanoreceptor inputs contribute to arousal. During AS chest wall muscle tone decreases and in the lamb causes chest collapse. As the stimulus becomes more intense (in this case 10% → 0% oxygen) and there is no coincident increase in mechanoreceptor input, more input from CB chemoreceptors is necessary for arousal and thus it takes a lower and lower SaO2 to promote arousal.
Figure 5.
Arousal response to rapidly developing hypoxemia in QS and AS in lambs. Arousal latency and SaO2 at the time of arousal are shown in the upper and lower panels, respectively. Three levels of hypoxia were tested: 10%, 5%, and 0% and are shown on the X-axis. Arousal data during QS is shown in the black bars and arousal during AS in the gray bars. All values are expressed as means ± SEM. Note that during QS, the SaO2 at arousal was similar for the different oxygen concentrations, whereas latencies were shortest at 0% (most severe) and longest at 10% oxygen (least severe). During AS both arousal latency and SaO2 at arousal were proportional to the severity of the hypoxia. All values are expressed as means ± SEM. Adapted from Fewell, et al, 1987 (Fewell & Baker, 1987).
In more altricial species including mice and rats, pups transition rapidly between QS, AS and wakefulness and there is no state related electrocortical activity discernible before ~P11 (Frank & Heller, 1997; Seelke & Blumberg, 2008). Thus it has not been possible to study hypoxia related arousal separately during QS and AS. In these models, arousal is detected using behavioral criteria, sometimes with the addition of neck EMG (Blumberg et al., 2005; Balbir et al., 2008). In one study of 3, 12 and 48 hour old mouse pups, using 5% oxygen as a stimulus, the arousal response was present at all ages but arousal latency was longer at 3 vs 12 and 48 hours. The authors suggested that the arousal response became more robust over the period of CB resetting. In addition, arousal always occurred during the secondary decline of the biphasic hypoxic ventilatory response. This was interpreted by the authors as an indication that mechanoreceptor input was less important in promoting arousal (Dauger et al., 2001).
We examined arousal using behavioral criteria in response to hypoxia in P5, P15, and P25 rat pups using 5% oxygen as a stimulus. With this paradigm chamber oxygen concentration fell progressively reaching 6% by 40 seconds at all ages. At 40 seconds, SaO2 approached 90%, 85%, and 76% for the P5, P15, and P25 pups, respectively. Mean arousal latencies ranged from 20 to 26 seconds for the three ages and mean SaO2 at arousal was between 91% to 93%. Neither arousal latencies nor SaO2 at arousal were different among the three ages (Darnall et al., 2010). In all cases, arousal latencies in response to hypoxia were shorter than the interval between spontaneous arousals, which were ~ 42 seconds for P5 pups and ~80 seconds for P15 pups. We also compared arousal latencies and SaO2 thresholds in response to 5% to those using 10% oxygen as stimuli. Similar to what has been reported in lambs, in P15 pups where hypoxia was instituted during putative QS, SaO2s at arousal were similar (93.8 ± 1.4% vs 93.2 ± 1.7 %) for 5% and 10%, respectively, whereas arousal latencies were shorter breathing 5% compared to 10% oxygen (24.7 ± 2 sec vs 27.6 ± 2 sec).
9. Arousal in response to hypercapnia
In tracheotomized lambs the probability of arousal in response to hypercapnia was greater during QS compared to AS (Fewell et al., 1989b; Johnston et al., 2007). Another study in lambs showed that arousal latencies during hyperoxic hypercapnia were significantly longer in AS (58 ± 17 sec) compared to QS (21 ± 10 sec) (Fewell & Baker, 1989). In P15 rat pups, during putative QS, we found that arousal latency in response to 8% CO2 in room air was 34.7 ± 2.3 sec and the CO2 arousal threshold was 4.38 ± 0.28%). There is some evidence that arousal in response to CO2 in adult rodents is dependent on central chemoreceptor activation. In adult mice, arousal to CO2 appears to be dependent on the presence of 5-HT neurons, which have been shown to be chemosensitive after P12 (Hodges & Richerson, 2010b, a). Mice hemizygous for ePet1-Cre and homozygous for floxed Lmx1b (Lmx1bf/f/p), which have no 5-HT neurons fail to arouse to CO2, but seem to arouse normally to hypoxia, sound, and an air puff (Buchanan & Richerson, 2010). These mice were only tested during NREM sleep, however. It is unknown whether 5-HT neurons play such a role during REM sleep, since they are normally silent during this state (Jacobs & Fornal, 1991).
10. Role of the carotid body in the arousal response to hypoxia and hypercapnia
CB chemoreceptors are the primary sites for the detection of hypoxia, which in the infant, reflexively increases ventilation. Although active in the fetus, they become more sensitive after birth temporally associated with an abrupt increase in PaO2 and sympathetic activity. From both in vitro and in vivo studies, it appears evident that the strength of the CB reflexes continues to increase with maturation over the first 2–3 weeks of life apparently regardless of the maturity of the species at birth (Kholwadwala & Donnelly, 1992; Carroll et al., 1993; Bamford et al., 1999; Gauda et al., 2009). The time over which CB reflexes mature is less clear in human infants and estimates range from a few days to10 weeks (Calder et al., 1994; Sovik et al., 1999). Several lines of evidence also support the idea that the CB also plays an important role in behavioral arousal in response to hypoxia. In adult dogs, removal of the CB greatly delays arousal coincident with a reduced ventilatory response to hypoxia (Bowes et al., 1981b) or airway occlusion (Bowes et al., 1981a). Similarly, in lambs, denervation of the CB reduces arousal probability in response to both rapidly developing hypoxia and airway obstruction (Fewell et al., 1989a; Fewell et al., 1990). Moreover, in these studies, baseline BP and fH increased after CB denervation in QS whereas during AS only BP was increased. After CB denervation, when arousal did occur, the increase in fR in response to rapidly developing hypoxemia was eliminated and the increase in BP and fH was greatly exaggerated (Fewell & Baker, 1987; Fewell et al., 1989a). Evidence for a role of CB chemoreception in arousal in response to CO2 is less clear. In a study in lambs, CB denervation decreased the arousal response to CO2 in lambs (Fewell et al., 1989b). However, in P15 rat pups, during putative QS, we found that arousal latencies were similar with 8% CO2 in room air and 8% CO2 in 92% oxygen (34.7 ± 2.3 sec vs 35.3 ± 4.1 sec). Similarly CO2 arousal thresholds were similar (4.38 ± 0.28% vs 4.28 ± 0.35%). Also in adult rodents without any 5-HT neurons, there is only a minimal arousal response to CO2 (Buchanan & Richerson, 2010). These data suggest that there may be significant species differences in the mechanisms responsible for arousal during hypercapnia.
11. Arousal habituation in response to acute intermittent hypoxia (AIH) and hypercapnia
Habituation and sensitization are almost universal forms of plasticity and represent the simplest forms of non-associative learning. In simple organisms such as aplysia, there is short term habituation of gill withdrawal in response to a mild tactile stimulation of the siphon (Pinsker et al., 1970; Montarolo et al., 1988). Short term habituation can be produced by activity in neurons containing FMRFamide immunoreactivity, or with a single application of the peptide FMRFamide (Montarolo et al., 1988). In contrast, short term sensitization refers to a progressive increase in the response to a repetitive usually stronger or noxious stimulus, which can be produced by the activity of serotonergic (5-HT) neurons or by the application of serotonin. Long term habituation and sensitization refer to a more longstanding decrease or increase in the strength of the response resulting from more long term presentation of stimuli (Kandel, 2001).
In vertebrates, habituation of the rodent acoustic startle response has been well studied and has provided a model for both short and long term habituation (Koch et al., 1992; Horner et al., 1997). The stimulus is usually provided during wakefulness and cerebellar structures appear to be important, especially for long term habituation (Leaton & Jordan, 1978). Habituation of arousal from sleep has been less well studied. What appears to be habituation of arousal to an auditory stimulus was described in adult cats as early as 1956 (Sharpless & Jasper, 1956). In this model, arousal habituation is specific to the quality, modality or pattern of the stimulus. Moreover, after arousal in response to repeated auditory stimuli was completely extinguished, pairing the auditory stimulus with a novel stimulus such as an air puff temporarily restored the arousal response. Classic dishabituation, however, was not observed; i.e. after pairing with a novel stimulus, the original auditory stimulus failed to produce arousal. Similar results were observed in human infants in response to auditory stimuli (Kisilevsky & Muir, 1993). Habituation of cortical, brainstem, and spinal components of the arousal response to a tactile stimulus has also been studied in human infants up to 5 months of age. In one study it was found that EEG (cortical) arousal habituated first, followed by brainstem, and then spinal responses. At each level, habituation occurred more rapidly during AS (McNamara et al., 1999).
It is thought that the process of habituation serves to allow the elimination of non-essential responses to biologically relevant stimuli (Thompson & Spencer, 1966). Habituation to repeated hypoxia, however, does not seem to be a good strategy, as the lack of an arousal response could be fatal. Nevertheless, a decrement of arousal in response to AIH has been well documented. There are lingering questions about whether this phenomenon is an example of non-associative learning or whether other biochemical processes directly related to the stimulus (i.e. hypoxia) are involved; these will be addressed later. Nevertheless, I will refer to this as “arousal habituation” for convenience. The first description of “arousal habituation” in response to AIH in newborns was provided in 1988 (Fewell et al., 1988). In this study in five tracheostomized lambs instrumented for sleep, arousal latency was longer and SaO2 lower at the time of arousal in response to repeated airway obstructions in AS but not during quiet sleep QS (Fewell et al., 1988). A subsequent report in four additional lambs showed similarly that progressive blunting of arousal occurred in response to repeated exposures to 5% oxygen, although in these experiments, habituation occurred both during QS and AS (Fewell & Konduri, 1989). In contrast, in another study in newborn lambs, Johnston et al found that arousal habituation to AIH readily occurred during AS but was less prominent during QS (Johnston et al., 1998).
Since these initial reports, habituation of the arousal response to repeated exposures to hypoxia has been described in the newborns of several species. In piglets, arousal habituation occurs in response to as few as 4 repeated exposures to hypoxia/hypercapnia. Arousal latencies increased over the 4 trials and when tested again 4 days later, the increase in latency was more pronounced, suggesting the presence of both short and long term habituation (Waters & Tinworth, 2005). Interestingly, the piglets exposed to hypoxia/hypercapnia developed a progressive and relatively severe metabolic acidosis, slept more and had fewer spontaneous arousals during recovery periods. In very young newborn mice (P4), progressive lengthening of arousal latency occurs over the course of 8 trials of hypoxia (5% O2) (Durand et al., 2004). These authors also made simultaneous measurements of fE and determined that arousal occurred during the period of ventilatory decline suggesting that arousal was not secondary to an increase in chest wall movement.
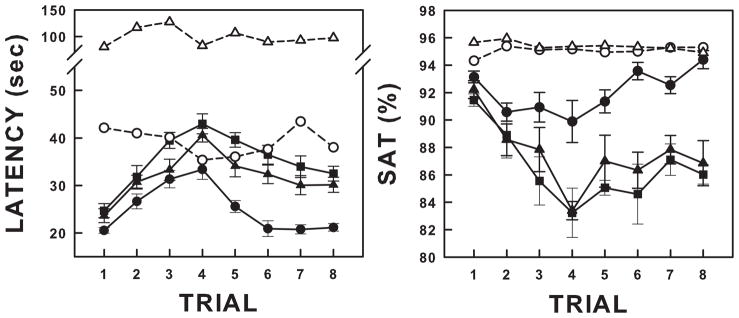
Habituation of arousal in response to AIH depends on age. We recently reported the arousal response to eight 3-minute exposures to 5% O2 in P5, P15 and P25 rat pups (Darnall et al., 2010). Arousal latencies were significantly shorter than the time between spontaneous arousals at all ages. Habituation was more robust in P15 and P25 pups compared to P5 animals measured as the slope of arousal latency across trials. In addition, if O2 was switched back to room air immediately upon arousal after 3–4 trials, the habituation process reversed and arousal latencies approached those observed in trial 1. Figure 6 illustrates the arousal latencies and the SaO2 at the time of arousal, and their reversal, in response to AIH in P5, P15, and P25 pups compared to intervals between spontaneous arousals.
Figure 6.
Arousal latency and SaO2 at arousal in response to repeated exposure to 5% oxygen in P5, P15, and P25 rat pups. Arousal latencies are shown in the left panel and SaO2 (SAT) at arousal in the right panel. For the first 3 trials, each animal was exposed to 3 minutes of 5% oxygen interspersed with 6 minutes of normoxia. Starting with the 4th trial, hypoxia was switched to room air immediately upon arousal. The filled symbols are data from pups exposed to hypoxia. The open symbols represent the times between spontaneous arousals and the SaO2 at spontaneous arousal. P5 hypoxia = closed circles; P5 spontaneous arousal rate = open circles; P15 hypoxia = closed triangles; P15 spontaneous arousals = open triangles; P25 hypoxia = closed squares; P25 spontaneous arousals not shown. Arousal habituation is illustrated by the progressive lengthening of arousal latencies and progressive decrease in SaO2 across trials of hypoxia. Note that arousal latencies progressively increased over the first 4 trials of hypoxia (habituation). When the hypoxia duration was decreased and the recovery time increased by switching to room air at arousal, there was a “reversal” of habituation. All values are expressed as means ± SEM. Adapted from Darnall et al, 2010 (Darnall et al., 2010).
11.1. Possible mechanisms of arousal habituation during AIH
The progressive blunting of arousal that occurs with repeated exposures to hypoxia has many features of “classical” habituation. However, there have been no studies demonstrating “dishabituation” where a novel, usually a more intense non-related stimulus, re-instates the original hypoxic arousal response. It seems unlikely that habituation is due to effector fatigue. In adult rats, there is progressive augmentation of CB afferent activity during acute intermittent hypoxia (Cummings & Wilson, 2005). In newborn mice pups, the increase in fE in response to each hypoxia exposure during AIH did not diminish in the face of increasing arousal latencies (Durand et al., 2004). Thus a progressive reduction in CB output with repeated stimulation does not appear to be responsible for arousal habituation. Indeed, AIH has usually been reported to result in sensitization or facilitation of a behavioral outcome, most often ventilation or phrenic nerve output, sympathetic nerve activity (Xing & Pilowsky, 2010), or blood pressure (Fletcher, 2001). Long term increases in both baseline ventilation (Baker & Mitchell, 2000) and sympathetic discharge (Xing & Pilowsky, 2010) in response to AIH, a form of plasticity termed long term facilitation “LTF”, which is dependent on serotonin and requires protein synthesis (Baker-Herman & Mitchell, 2002). Although similar in some respects to the 5-HT and protein synthesis dependent process of long term synaptic facilitation and behavioral sensitization observed in aplysia, ventilatory LTF has not been shown to involve learning in the classic sense.
In one study in piglets, there was a progressive moderate to severe metabolic acidosis that developed over several bouts of hypoxia (Waters & Tinworth, 2005) that might have contributed to depressed arousal responses. Alternatively, progressive blunting of arousal could be explained by hypoxia induced biochemical processes with a relatively long time constants resulting in arousal inhibition such that a relatively long exposure to hypoxia and a relatively short recovery time result in progressive cumulative inhibition between exposures. We recently showed that if the hypoxia period is shortened and the recovery period lengthened, the inhibition between hypoxia exposures might be attenuated resulting in gradual recovery toward baseline (see Figure 6). Acute hypoxemia is associated with increases in the extracellular concentration (measured with microdialysis) of both excitatory and inhibitory neurotransmitters and/or neuromodulators in the brainstem, including opioids, glutamate, GABA, taurine, adenosine and serotonin in both anesthetized and conscious animals (Hoop et al., 1999; Richter et al., 1999; Tabata et al., 2001; Hehre et al., 2008). Studies in newborn lambs demonstrated that blockade of opioid receptors did not reverse the progressive blunting of arousal with repeated hypoxia exposure (Konduri & Fewell, 1992). Adenosine is a ubiquitous nucleoside that is released from cells into the extracellular space when oxygen supply no longer matches oxygen needs (Darnall & Bruce, 1987). Adenosine could directly inhibit excitatory neurons involved in arousal via A1 receptors, or could indirectly inhibit these neurons by acting on excitatory adenosine A2A receptors located on GABAergic neurons.
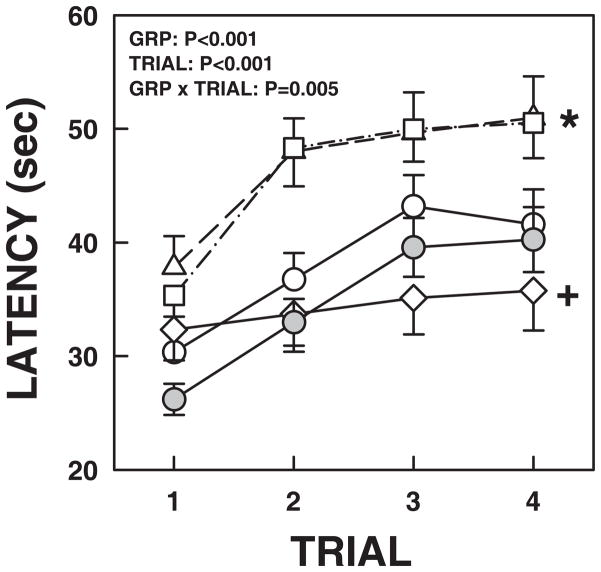
We explored the possible role of GABA in arousal habituation in response to AIH (Darnall et al., 2012). We found in P15 to P25 rat pups that the local application of muscimol, a GABAA receptor agonist, into the medullary raphe, caused a prolongation of arousal latency during 4 repeated trials of hypoxia compared to controls. Similarly blocking GABA reuptake with the GAT antagonist, nipecotic acid, caused a similar prolongation of arousal latency. Finally, local application of bicuculline, a GABAA receptor antagonist, resulted in a dramatic reduction in habituation. In the rodent, there is a broad distribution of GAD containing neurons throughout the medullary raphe with the highest density near the ventral surface and extending laterally (Kihara & Kubo, 1989; Holmes et al., 1994; Lein et al., 2007). Thus, the source of GABA in the medullary raphe most likely is from GABA producing interneurons. However, there may also be substantial projections of remote GABAergic neurons into this region. Finally, some neurons of other phenotypes, including 5-HT neurons, express multiple neurotransmitters, including GABA. It is also clear that GABA producing neurons are present very early in gestation (Lauder et al., 1986), with a distribution pattern at P15–P25 very similar to that of the adult. GABAA receptors are expressed by neurons of many phenotypes. Muscimol would therefore be expected to inhibit the activity of almost all neurons expressing GABAA receptors, and would not help determine the source of GABA. Thus our results after muscimol microinjection could be interpreted as a generalized inhibition of medullary raphe neuronal activity. Nipecotic acid, with a high affinity for the GABA transporters, GAT1 and GAT3, would be expected to block the reuptake of GABA into both neurons (GAT1) and glia (GAT3) (Gether et al., 2006; Kristensen et al., 2011) and may also provide insight as to changing levels of endogenous GABA. The results of our experiments with nipecotic acid suggest that hypoxia results in an increase in ambient GABA and when reuptake is inhibited there is an enhanced progressive increase providing a source of increasing tonic inhibition. In contrast, blocking GABAA receptors with bicuculline did not shorten arousal latency compared to controls, but resulted in an elimination of habituation. These data indicate that the medullary raphe contributes to the phenomenon of arousal habituation and more specifically that activation of medullary raphe GABAA receptors is necessary for arousal habituation. Figure 7 shows the effects of medullary raphe application of muscimol, nipecotic acid, bicuculline, and aCSF on arousal across four trials of hypoxia.
Figure 7.
Arousal latency over the course of 4 exposures to 10% breathing after local application into the medullary raphe of aCSF, muscimol, nipecotic acid, and bicuculline. Under isofluorane anesthesia, neurochemicals were microinjected directly into the medullary raphe in the rostro-caudal dimensions of the facial nucleus in P15–P25 rat pups. After recovery (~30 minutes) pups were exposed to 4 three minute periods of 10% oxygen interspersed with 6 minutes of normoxia. The arousal response after aCSF and no-injection controls (no anesthesia, surgery, or injections are represented by open and gray circles, respectively. Note that the response after aCSF is almost identical to that in non-injected controls. Muscimol (GABAA receptor agonist) and nipecotic acid (GABA reuptake inhibitor) are shown as open triangles and squares, respectively. Note the increase in arousal latency. The results after bicuculline (GABAA receptor antagonist) are shown as open diamonds. Note that in all of the other conditions, there is significant habituation (progressive increase in arousal latency over the 4 hypoxia exposures). After bicuculline application, habituation is abolished. These data suggest that the progressive increase in arousal latency during repeated hypoxia exposures is secondary to increasing levels of ambient GABA and that GABAA receptors activation is necessary for habituation to occur. All values are expressed as means ± SEM. From Darnall, et al, 2012 (Darnall et al., 2012)
There are only a few studies investigating the effects of repeated CO2 exposure on arousal. At least one study in lambs showed that many exposures to CO2 (40 exposures) resulted in little or no habituation of the arousal response measured as the probability of arousal. We have recently examined the effects of intermittent CO2 exposure on arousal in newborn rats. Our preliminary results with four 3-minute exposures to 8% CO2 suggest that 1) arousal latencies in response to repeated exposures to CO2 are shortest when hypercapnia is combined with hypoxia, 2) Arousal latencies in response to hyperoxic hypercapnia are not different from those in response to hypercapnia alone, 3) arousal latencies lengthen progressively over the course of 4 exposures to hypercapnia.
12. Arousal mechanisms
Although it is clear that CB chemoreceptor stimulation contributes to arousal from sleep in response to hypoxia, the specific mechanisms have not been fully elucidated. It remains unclear whether arousal results from a direct stimulation of arousal networks or whether arousal networks are indirectly stimulated either from, or as part of, brainstem respiratory networks proportional to respiratory drive or from mechanoreceptor stimuli arising from the ventilatory apparatus during breathing. Information from the carotid body travels in the carotid sinus nerve which synapses largely in the commissural nucleus of the NTS (com NTS) with some fibers extending directly to the ventral lateral medulla (Finley & Katz, 1992). The com NTS projects to the ventrolateral medulla, retrotrapezoid nucleus, the hypothalamus, amygdala, and the midbrain periaqueductal gray. All of these regions, in turn, project to the medullary raphe. In addition there may be direct projections from the com NTS to the medullary raphe (Takakura et al., 2006). Our findings that arousal in response to repeated hypoxia is influenced by both medullary raphe 5-HT and GABAergic mechanisms suggest that the medullary raphe either lies in an arousal pathway or modulates other ascending arousal pathways activated by hypoxia. There are several lines of evidence suggesting that hypoxia activates medullary raphe neurons. Long term facilitation (LTF) associated with exposure to acute intermittent hypoxia requires the activation of medullary 5-HT neurons that project to the spinal cord (Baker & Mitchell, 2000; Baker-Herman & Mitchell, 2002). Stimulation of the carotid sinus nerves induces FOS-like protein in regions of the medullary raphe (Erickson & Millhorn, 1991). Multi-array extracellular recordings further suggest that midline raphe neurons are critical components of a larger raphe-ponto-medullary network of neurons with respiratory related activity and respond to peripheral chemoreceptor stimulation in concert with the entire network (Nuding et al., 2009).
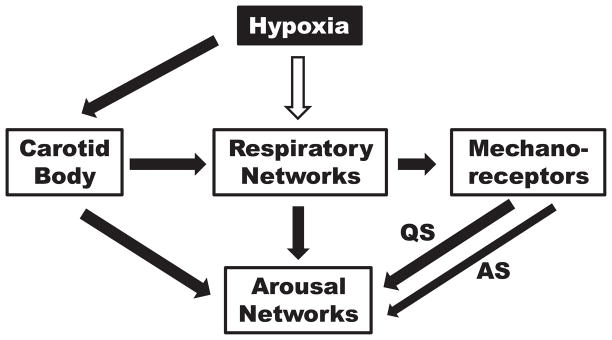
Several lines of evidence suggest that arousal is also influenced by sensory input from the lungs or chest wall movement and that the relative contribution of chemoreceptor and lungs or chest wall may be state related. In adult dogs, the addition of expiratory flow-resistive loads during hypoxic rebreathing significantly decreases the arousal threshold to hypoxia both during NREM and REM sleep (Yasuma et al., 1991). In newborn lambs, however, hyperoxia delayed arousal after airway obstruction during AS but not during QS (Baker & Fewell, 1987). However, during airway occlusion, vagotomy produced a significantly smaller decrement in arousability than CB denervation (Bowes et al., 1981a). Taken together, these data suggest that both CB chemoreceptors and mechanoreceptors contribute to arousal during hypoxia. It is also likely that the medullary raphe contributes to arousal, particularly arousal habituation. During AS, mechanoreceptor activity decreases and does not increase during increasing intensity of the hypoxic stimulus. Figure 8 illustrates possible contributions from the CB, respiratory networks, and pulmonary or chest wall mechanoreceptors on arousal and the reduction of mechanoreceptor input during AS. Although mechanoreceptor output is decreased during REM, the depression of arousal during AS likely depends on central neuronal effects as well.
Figure 8.
Cartoon showing proposed contribution of carotid body chemoreceptors, respiratory networks and pulmonary or chest wall mechanoreceptors to arousal in response to hypoxia. The white arrow indicates inhibition and the black arrows excitation. During AS, there is a decreased contribution from mechanoreceptors indicated by the thinner arrow.
Sleep state plays a major role in arousal, but is highly species dependent. Whereas newborn and adult mammals appear to have depressed arousal during AS, the opposite occurs in human infants. The probability of arousal in response to hypoxia is increased during AS in the human infant and depressed during QS. There are other state related effects on physiological control systems where the human infant appears to be an outlier in the mammalian world. For example, in most small mammals, REM or AS is associated with an attenuation of thermoregulation. Thus brown fat metabolism, shivering and sweating are all suspended during REM (Parmeggiani & Rabini, 1967; Parmeggiani et al., 1977). In the human infant, however, thermoregulation is preserved during REM sleep, and may even be more effective (Stothers & Warner, 1977a, b; Darnall & Ariagno, 1982).
13. Summary
Arousal from sleep is a major defense mechanism in infants against hypoxia and/or hypercapnia during rebreathing, airway obstruction and apnea. The combination of subcortical and cortical arousal allows the infant to move out of a dangerous situation and mount an appropriate physiological response. Decades of research suggests that arousal failure may be an important contributor to SIDS. Areas of the brainstem that have found to be abnormal in a majority of SIDS infants are involved in the arousal process. Arousal is sleep state dependent, being depressed during AS in most mammals, but depressed during QS in human infants. The reasons for the differences are not known. However there are analogous differences in thermoregulatory physiology where human infants mount a metabolic response to a cold stress during AS, unlike the diminished or absent response in most other mammalian species. Thus the ability to arouse and respond to a cold stress during AS may have evolved as an evolutionary advantage in the human. Repeated exposure to hypoxia causes a progressive blunting of arousal that may involve medullary raphe GABAergic mechanisms. Whereas CB chemoreceptors contribute heavily to arousal in response to hypoxia, serotonergic central chemoreceptors have been implicated in the arousal response to CO2. Pulmonary or chest wall mechanoreceptors also contribute to arousal in conjunction with the ventilatory response to hypoxia and decreases in mechanoreceptor input during AS may contribute to depressed arousal during this state. Little is known about specific arousal pathways beyond the comNTS. Whether CB chemoreceptor stimulation directly stimulates arousal networks or whether this is done indirectly through respiratory networks remains unknown. An attractive hypothesis is that medullary raphe, arousal, and respiratory networks are connected and overlapping in that all of these networks are activated in the arousal process. That the medullary raphe contributes to the process of arousal in response to hypoxia has important implications for the Sudden Infant Death Syndrome. Up to 70% of these infants have decreased medullary raphe serotonin and TPH2 levels which may result in a loss or decrease in an important excitatory input to the arousal process, increasing the probability of death when confronted with hypoxia during sleep.
There is much more to be learned about the mechanisms involved in arousal and many questions remain. Does the sensitivity of the arousal response change as the carotid bodies mature over the first 2–3 weeks of development? If present, are changes in sensitivity different for hypoxia and hypercapnia? What is the relative contribution of CB and central chemoreceptors to arousal during hypercapnia? Where do sensory inputs converge on ascending arousal networks? Although there appears to be a contribution of medullary raphe 5-HT and GABAergic networks to arousal in response to hypoxia, is the medullary raphe an intrinsic component of the arousal pathway, or does the medullary raphe modulate other arousal networks? Both arousal and thermoregulatory responses are vigorous in the human newborn during REM or AS, this is not the case in other mammalian species where both arousal and thermoregulation are depressed during REM. Is there a relationship between the percent time spent in REM and the strength of arousal and thermoregulatory responses? Given the importance of the arousal response for survival, and the many unanswered questions, there is clearly a need for continued investigation both in animals and in humans.
Acknowledgments
The author is supported by an NIH grant PO1 HD036379 and thanks Don bartlette for reviewing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvaro R, Alvarez J, Kwiatkowski K, Cates D, Rigatto H. Small preterm infants (less than equal to1500 g) have only a sustained decrease in ventilation in response to hypoxia. Pediatr Res. 1992;32:403–406. doi: 10.1203/00006450-199210000-00007. [DOI] [PubMed] [Google Scholar]
- Ariagno R, Mirmiran M, Darnall RA. Arousals in infants during the first year of life: Argument for new definitions and criteria. In: Salzarulo P, Ficca G, editors. Awakening and Sleep-Wake Cycle Across Development. John Benjamins Publishing Company; Philadelphia: 2002. pp. 63–78. [Google Scholar]
- Ariagno RL, Nagel L, Guilleminault C. Waking and ventilatory responses during sleep in infants near-miss for sudden infant death syndrome. Sleep. 1980;3:351–359. doi: 10.1093/sleep/3.3-4.351. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SB, Fewell JE. Effects of hyperoxia on the arousal response to upper airway obstruction in lambs. Pediatr Res. 1987;21:116–120. doi: 10.1203/00006450-198702000-00002. [DOI] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol. 2000;529(Pt 1):215–219. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbir A, Lande B, Fitzgerald RS, Polotsky V, Mitzner W, Shirahata M. Behavioral and respiratory characteristics during sleep in neonatal DBA/2J and A/J mice. Brain Res. 2008;1241:84–91. doi: 10.1016/j.brainres.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford OS, Sterni LM, Wasicko MJ, Montrose MH, Carroll JL. Postnatal maturation of carotid body and type I cell chemoreception in the rat. Am J Physiol. 1999;276:L875–884. doi: 10.1152/ajplung.1999.276.5.L875. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM, Hohimer AR, Knopp SJ. GABAergic and glutamatergic effects on behaviour in fetal sheep. J Physiol. 1995;487.3:677–684. doi: 10.1113/jphysiol.1995.sp020909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco CE, Dawes GS, Hanson MA, Mccooke HB. The Arterial Chemoreceptors in Fetal Sheep and Newborn Lambs. J Physiol-London. 1982;330:P38–P38. doi: 10.1113/jphysiol.1984.sp015229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco CE, Dawes GS, Hanson MA, McCooke HB. The response to hypoxia of arterial chemoreceptors in fetal sheep and new-born lambs. J Physiol. 1984a;351:25–37. doi: 10.1113/jphysiol.1984.sp015229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco CE, Hanson MA, Johnson P, Rigatto H. Breathing pattern of kittens during hypoxia. J Appl Physiol. 1984b;56:12–17. doi: 10.1152/jappl.1984.56.1.12. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Seelke AM, Lowen SB, Karlsson KA. Dynamics of sleep-wake cyclicity in developing rats. Proc Natl Acad Sci U S A. 2005;102:14860–14864. doi: 10.1073/pnas.0506340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekkooi PF, Baan J, Jr, Teitel D, Rudolph AM. Chemoreceptor responsiveness in fetal sheep. Am J Physiol Heart Circ Physiol. 1992;263:H162–H167. doi: 10.1152/ajpheart.1992.263.1.H162. [DOI] [PubMed] [Google Scholar]
- Bowes G, Townsend ER, Bromley SM, Kozar LF, Phillipson EA. Role of the carotid body and of afferent vagal stimuli in the arousal response to airway occlusion in sleeping dogs. Am Rev Respir Dis. 1981a;123:644–647. doi: 10.1164/arrd.1981.123.6.644. [DOI] [PubMed] [Google Scholar]
- Bowes G, Towsend ER, Kozar LF, Bromley SM, Phillipson EA. Effect of carotid body denervation on arousal response to hypoxia in sleeping dogs. J Appl Physiol. 1981b;51:40–45. doi: 10.1152/jappl.1981.51.1.40. [DOI] [PubMed] [Google Scholar]
- Brady JP, McCann EM. Control of ventilation in subsequent siblings of victims of sudden infant death syndrome. J Pediatr. 1985;106:212–217. doi: 10.1016/s0022-3476(85)80289-4. [DOI] [PubMed] [Google Scholar]
- Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci U S A. 2010;107:16354–16359. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BuSha B, Leiter JC, Curran AK, Li A, Nattie EE, Darnall RA. Spontaneous arousals during quiet sleep in piglets: A visual and wavelet-based analysis. Sleep. 2001;24:499–513. doi: 10.1093/sleep/24.5.499. [DOI] [PubMed] [Google Scholar]
- Calder NA, Williams BA, Kumar P, Hanson MA. The respiratory response of healthy term infants to breath-by-breath alternations in inspired oxygen at two postnatal ages. Pediatr Res. 1994;35:321–324. doi: 10.1203/00006450-199403000-00008. [DOI] [PubMed] [Google Scholar]
- Carroll JL, Bamford OS, Fitzgerald RS. Postnatal maturation of carotid chemoreceptor responses to O2 and CO2 in the cat. J Appl Physiol. 1993;75:2383–2391. doi: 10.1152/jappl.1993.75.6.2383. [DOI] [PubMed] [Google Scholar]
- Coons S, Guilleminault C. Development of sleep-wake patterns and non-rapid eye movement sleep stage during the first six months of life in normal infants. Pediatrics. 1982;79:793–798. [PubMed] [Google Scholar]
- Cummings KJ, Wilson RJ. Time-dependent modulation of carotid body afferent activity during and after intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1571–1580. doi: 10.1152/ajpregu.00788.2004. [DOI] [PubMed] [Google Scholar]
- Darnall RA. The role of CO(2) and central chemoreception in the control of breathing in the fetus and the neonate. Respir Physiol Neurobiol. 2010;173:201–212. doi: 10.1016/j.resp.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall RA, Ariagno RL. The effect of sleep state on active thermoregulation in the premature infant. Pediatr Res. 1982;16:512–514. doi: 10.1203/00006450-198207000-00002. [DOI] [PubMed] [Google Scholar]
- Darnall RA, Bruce RD. Effects of adenosine and xanthine derivatives on breathing during acute hypoxia in the anesthetized newborn piglet. Pediatr Pulmonol. 1987;3:110–116. doi: 10.1002/ppul.1950030213. [DOI] [PubMed] [Google Scholar]
- Darnall RA., Jr Aminophylline reduces hypoxic ventilatory depression: possible role of adenosine. Pediatr Res. 1985;19:706–710. doi: 10.1203/00006450-198507000-00014. [DOI] [PubMed] [Google Scholar]
- Darnall RA, McWilliams S, Schneider RW, Tobia CM. Reversible blunting of arousal from sleep in response to intermittent hypoxia in the developing rat. J Appl Physiol. 2010;109:1686–1696. doi: 10.1152/japplphysiol.00076.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall RA, Schneider RW, Tobia CM, Zemel BM. Arousal from sleep in response to intermittent hypoxia in infant rodents is modulated by medullary raphe GABAergic mechanisms. Am J Physiol Regul Integr Comp Physiol. 2012;302:R551–560. doi: 10.1152/ajpregu.00506.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauger S, Aizenfisz S, Renolleau S, Durand E, Vardon G, Gaultier C, Gallego J. Arousal response to hypoxia in newborn mice. Respir Physiol. 2001;128:235–240. doi: 10.1016/s0034-5687(01)00303-6. [DOI] [PubMed] [Google Scholar]
- Davidson-Ward SL, Bautista DB, Keens TG. Hypoxic arousal responses in normal infants. Pediatrics. 1992;89:860–864. [PubMed] [Google Scholar]
- Dawes GS, Fox HE, Leduc BM, Liggins GC, Richards RT. Respiratory movements and rapid eye movement sleep in the foetal lamb. J Physiol. 1972;220:119–143. doi: 10.1113/jphysiol.1972.sp009698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes GS, Gardner WN, Johnston BM, Walker DW. Effects of hypercapnia on tracheal pressure, diaphragm and intercostal electromyograms in unanesthetized fetal lambs. J Physiol. 1982;326:461–474. doi: 10.1113/jphysiol.1982.sp014206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond MM, Franklin RR, Vallvona C, Hill RM, Plumb R, Arnold H, Watts J. The clinical behavior of the newly born. I. The term baby. J Pediatr. 1963;62:307–325. doi: 10.1016/s0022-3476(63)80128-6. [DOI] [PubMed] [Google Scholar]
- Dunne KP, Fox GP, O’Regan M, Matthews TG. Arousal responses in babies at risk of sudden infant death syndrome at different postnatal ages. Ir Med J. 1992;85:19–22. [PubMed] [Google Scholar]
- Durand E, Lofaso F, Dauger S, Vardon G, Gaultier C, Gallego J. Intermittent hypoxia induces transient arousal delay in newborn mice. J Appl Physiol. 2004;96:1216–1222. doi: 10.1152/japplphysiol.00802.2003. discussion 1196. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Millhorn DE. Fos-like protein is induced in neurons of the medulla oblongata after stimulation of the carotid sinus nerve in awake and anesthetized rats. Brain Res. 1991;567:11–24. doi: 10.1016/0006-8993(91)91430-9. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Baker SB. Arousal and Cardiopulmonary Responses to Hyperoxic Hypercapnia in Lambs. J Dev Physiol. 1989;12:21–26. [PubMed] [Google Scholar]
- Fewell JE, Baker SP. Arousal from sleep during rapidly developing hypoxemia in lambs. Pediat Res. 1987;22:471–477. doi: 10.1203/00006450-198710000-00023. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Kondo CS, Dascalu V, Filyk S. Influence of carotid denervation on the arousal and cardiopulmonary response to rapidly developing hypoxemia in lambs. Pediatr Res. 1989a;25:473–477. doi: 10.1203/00006450-198905000-00009. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Kondo CS, Dascalu V, Filyk SC. Influence of carotid-denervation on the arousal and cardiopulmonary responses to alveolar hypercapnia in lambs. J Dev Physiol. 1989b;12:193–199. [PubMed] [Google Scholar]
- Fewell JE, Konduri GG. Influence of repeated exposure to rapidly developing hypoxaemia on the arousal and cardiopulmonary response to rapidly developing hypoxaemia in lambs. J Dev Physiol. 1989;11:77–82. [PubMed] [Google Scholar]
- Fewell JE, Taylor BJ, Kondo CS, Dascalu V, Filyk SC. Influence of carotid denervation on the arousal and cardiopulmonary responses to upper airway obstruction in lambs. Pediatr Res. 1990;28:374–378. doi: 10.1203/00006450-199010000-00014. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Williams BJ, Szabo JS, Taylor BJ. Influence of repeated upper airway obstruction on the arousal and cardiopulmonary response to upper airway obstruction in lambs. Pediatr Res. 1988;23:191–195. doi: 10.1203/00006450-198802000-00013. [DOI] [PubMed] [Google Scholar]
- Finley JC, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res. 1992;572:108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Fletcher EC. Physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol. 2001;90:1600–1605. doi: 10.1152/jappl.2001.90.4.1600. [DOI] [PubMed] [Google Scholar]
- Frank MG, Heller HC. Development of REM and slow wave sleep in the rat. Am J Physiol. 1997;272:R1792–1799. doi: 10.1152/ajpregu.1997.272.6.R1792. [DOI] [PubMed] [Google Scholar]
- Gauda EB, Carroll JL, Donnelly DF. Developmental maturation of chemosensitivity to hypoxia of peripheral arterial chemoreceptors--invited article. Adv Exp Med Biol. 2009;648:243–255. doi: 10.1007/978-90-481-2259-2_28. [DOI] [PubMed] [Google Scholar]
- Gether U, Andersen PH, Larsson OM, Schousboe A. Neurotransmitter transporters: molecular function of important drug targets. Trends Pharmacol Sci. 2006;27:375–383. doi: 10.1016/j.tips.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Spencer JA, Moore PJ, Bennet L, Hanson MA. Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. J Physiol (Lond) 1993;461:431–449. doi: 10.1113/jphysiol.1993.sp019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MA. The importance of baro- and chemoreflexes in the control of the fetal cardiovascular system. J Dev Physiol. 1988;10:491–511. [PubMed] [Google Scholar]
- Hanzer M, Kerbl R, Urlesberger B, Mueller W, Pichler G, Zotter H. Comparison of heart rate responses during cortical and subcortical arousals in term and preterm infants. Early Human Development. 2007;83:511–515. doi: 10.1016/j.earlhumdev.2006.09.038. [DOI] [PubMed] [Google Scholar]
- Harding R, Hooper SB, Han VK. Abolition of fetal breathing movements by spinal cord transection leads to reductions in fetal lung liquid volume, lung growth, and IGF-II gene expression. Pediatr Res. 1993;34:148–153. doi: 10.1203/00006450-199308000-00008. [DOI] [PubMed] [Google Scholar]
- Harding R, Johnson P, McClelland ME. Respiratory function of the larynx in developing sheep and the influence of sleep state. Respir Physiol. 1980;40:165–179. doi: 10.1016/0034-5687(80)90090-0. [DOI] [PubMed] [Google Scholar]
- Harper RM, Bandler R. Finding the failure mechanism in Sudden Infant Death Syndrome. Nat Med. 1998;4:157–158. doi: 10.1038/nm0298-157. [DOI] [PubMed] [Google Scholar]
- Hayes MJ. Methodological issues in the study of arousals and awakenings during sleep in the human infant. In: Salzarulo P, Ficca G, editors. Awakening and Sleep-Wake Cycle across Development. John Benjamins Publishing Company; Philadelphia: 2002. pp. 23–45. [Google Scholar]
- Hehre DA, Devia CJ, Bancalari E, Suguihara C. Brainstem amino acid neurotransmitters and ventilatory response to hypoxia in piglets. Pediatr Res. 2008;63:46–50. doi: 10.1203/PDR.0b013e31815b4421. [DOI] [PubMed] [Google Scholar]
- Henderson-Smart DJ, Read DJ. Ventilatory responses to hypoxaemia during sleep in the newborn. J Dev Physiol. 1979;1:195–208. [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Medullary serotonin neurons and their roles in central respiratory chemoreception. Respir Physiol Neurobiol. 2010a;173:256–263. doi: 10.1016/j.resp.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. The role of medullary serotonin (5-HT) neurons in respiratory control: contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation. J Appl Physiol. 2010b;108:1425–1432. doi: 10.1152/japplphysiol.01270.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CJ, Mainville LS, Jones BE. Distribution of cholinergic, gabaergic and serotonergic neurons in the medial medullary reticular formation and their projections studied by cytotoxic lesions in the cat. Neuroscience. 1994;62:1155–1178. doi: 10.1016/0306-4522(94)90351-4. [DOI] [PubMed] [Google Scholar]
- Hoop B, Beagle JL, Maher TJ, Kazemi H. Brainstem amino acid neurotransmitters and hypoxic ventilatory response. Resp Physiol. 1999;118:117–129. doi: 10.1016/s0034-5687(99)00072-9. [DOI] [PubMed] [Google Scholar]
- Hopkins B. Development of wakefulness: Re-awakening a neglected topic. In: Salzarulo P, Ficca G, editors. Awakening and Sleep-Wake Cycle across Development. John Benjamins Publishing Company; Philadelphia: 2002. pp. 8–22. [Google Scholar]
- Horne RS, Parslow PM, Harding R. Postnatal development of ventilatory and arousal responses to hypoxia in human infants. Respir Physiol Neurobiol. 2005;149:257–271. doi: 10.1016/j.resp.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Horner RL. Autonomic consequences of arousal from sleep: mechanisms and implications. Sleep. 1996;19:S193–195. doi: 10.1093/sleep/19.suppl_10.s193. [DOI] [PubMed] [Google Scholar]
- Horner RL, Sanford LD, Pack AI, Morrison AR. Activation of a distinct arousal state immediately after spontaneous awakening from sleep. Brain Res. 1997;778:127–134. doi: 10.1016/s0006-8993(97)01045-7. [DOI] [PubMed] [Google Scholar]
- Hunt CE, McCulloch K, Brouillette RT. Diminished hypoxic ventilatory responses in near-miss sudden infant death syndrome. J Appl Physiol. 1981;50:1315–1317. doi: 10.1152/jappl.1981.50.6.1313. [DOI] [PubMed] [Google Scholar]
- International Paediatric Work Group on A. The scoring of arousals in healthy term infants (between the ages of 1 and 6 months) J Sleep Res. 2005;14:37–41. doi: 10.1111/j.1365-2869.2004.00426.x. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Activity of brain serotonergic neurons in the behaving animal. Pharmacol Rev. 1991;43:563–578. [PubMed] [Google Scholar]
- Jansen AH, Chernick V. Development of respiratory control. Physiol Rev. 1983;63:437–483. doi: 10.1152/physrev.1983.63.2.437. [DOI] [PubMed] [Google Scholar]
- Jansen AH, Chernick V. Fetal breathing and development of control of breathing. J Appl Physiol. 1991;70:1431–1446. doi: 10.1152/jappl.1991.70.4.1431. [DOI] [PubMed] [Google Scholar]
- Jeffery HE, Read DJ. Ventilatory responses of newborn calves to progressive hypoxia in quiet and active sleep. J Appl Physiol. 1980;48:892–895. doi: 10.1152/jappl.1980.48.5.892. [DOI] [PubMed] [Google Scholar]
- Johnston BM, Gluckman PD. Lateral pontine lesions affect central chemosensitivity in unanesthetized fetal lambs. J Appl Physiol. 1989;67:1113–1118. doi: 10.1152/jappl.1989.67.3.1113. [DOI] [PubMed] [Google Scholar]
- Johnston BM, Gluckman PD. Peripheral chemoreceptors respond to hypoxia in pontine-lesioned fetal lambs in utero. J Appl Physiol. 1993;75:1027–1034. doi: 10.1152/jappl.1993.75.3.1027. [DOI] [PubMed] [Google Scholar]
- Johnston RV, Grant DA, Wilkinson MH, Walker AM. Repetitive hypoxia rapidly depresses arousal from active sleep in newborn lambs. J Physiol. 1998;2:651–659. doi: 10.1111/j.1469-7793.1998.651bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RV, Grant DA, Wilkinson MH, Walker AM. The effects of repeated exposure to hypercapnia on arousal and cardiorespiratory responses during sleep in lambs. J Physiol. 2007;582:369–378. doi: 10.1113/jphysiol.2007.132415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kholwadwala D, Donnelly DF. Maturation of carotid chemoreceptor sensitivity to hypoxia: In vitro studies in the newborn rat. J Physiol (Lond) 1992;453:461–473. doi: 10.1113/jphysiol.1992.sp019239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara M, Kubo T. Immunocytochemical localization of GABA containing neurons in the ventrolateral medulla oblongata of the rat. Histochemistry. 1989;91:309–314. doi: 10.1007/BF00493006. [DOI] [PubMed] [Google Scholar]
- Kisilevsky BS, Muir DW. Neonatal movement response decrement and recovery to sounds as a function of stimulus intensity. Can J Exp Psychol. 1993;47:639–656. doi: 10.1037/h0078875. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Lemke RP, Greer JJ. Ultrasound measurements of fetal breathing movements in the rat. J Appl Physiol. 2001;91:316–320. doi: 10.1152/jappl.2001.91.1.316. [DOI] [PubMed] [Google Scholar]
- Koch M, Lingenhohl K, Pilz PK. Loss of the acoustic startle response following neurotoxic lesions of the caudal pontine reticular formation: Possible role of giant neurons. Neuroscience. 1992;49:617–625. doi: 10.1016/0306-4522(92)90231-p. [DOI] [PubMed] [Google Scholar]
- Konduri GG, Fewell JE. Naloxone does not alter the arousal response decrement after repeated exposure to hypoxemia during sleep in lambs. PediatrRes. 1992;32:222–225. doi: 10.1203/00006450-199208000-00019. [DOI] [PubMed] [Google Scholar]
- Koos BJ, Sameshima H, Power GG. Fetal breathing, sleep state, and cardiovascular responses to graded hypoxia in sheep. J Appl Physiol. 1987;62:1033–1039. doi: 10.1152/jappl.1987.62.3.1033. [DOI] [PubMed] [Google Scholar]
- Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, Stromgaard K, Gether U. SLC6 Neurotransmitter Transporters: Structure, Function, and Regulation. Pharmacol Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- Lagercrantz H, Slotkin TA. The “stress” of being born. Scientific American. 1986;254:100–107. doi: 10.1038/scientificamerican0486-100. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Han VK, Henderson P, Verdoorn T, Towle AC. Prenatal ontogeny of the GABAergic system in the rat brain: an immunocytochemical study. Neuroscience. 1986;19:465–493. doi: 10.1016/0306-4522(86)90275-7. [DOI] [PubMed] [Google Scholar]
- Leaton RN, Jordan WP. Habituation of the EEG arousal response in rats: short- and long-term effects, frequency specificity, and wake--sleep transfer. J Comp Physiol Psychol. 1978;92:803–814. doi: 10.1037/h0077538. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Lewis KW, Bosque EM. Deficient hypoxia awakening response in infants of smoking mothers: Possible relationship to sudden infant death syndrome. J Pediatr. 1995;127:691–699. doi: 10.1016/s0022-3476(95)70155-9. [DOI] [PubMed] [Google Scholar]
- Lijowska AS, Reed NW, Chiodini BA, Thach BT. Sequential arousal and airway-defensive behavior of infants in asphyxial sleep environments. J Appl Physiol. 1997;83:219–228. doi: 10.1152/jappl.1997.83.1.219. [DOI] [PubMed] [Google Scholar]
- Martin-Body RI, Johnston BM. Central origin of the hypoxic depression of breathing in the newborn. Respir Physiol. 1988;71:25–32. doi: 10.1016/0034-5687(88)90112-0. [DOI] [PubMed] [Google Scholar]
- McCulloch K, Brouillette RT, Guzzetta AJ, Hunt CE. Arousal responses in near-miss sudden infant death syndrome and in normal infants. J Pediatr. 1982;101:911–917. doi: 10.1016/s0022-3476(82)80009-7. [DOI] [PubMed] [Google Scholar]
- McNamara F, Lijowska AS, Thach BT. Spontaneous arousal activity in infants during NREM and REM sleep. J Physiol. 2002;538:263–269. doi: 10.1113/jphysiol.2001.012507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara F, Wulbrand H, Thach BT. Characteristics of the infant arousal response. J Appl Physiol. 1998;85:2314–2321. doi: 10.1152/jappl.1998.85.6.2314. [DOI] [PubMed] [Google Scholar]
- McNamara F, Wulbrand H, Thach BT. Habituation of the infant arousal response. Sleep. 1999;22:320–326. [PubMed] [Google Scholar]
- Milerad J, Hertzberg T, Wennergren G, Lagercrantz H. Respiratory and arousal responses to hypoxia in apnoeic infants reinvestigated. Euro J Pediatr. 1989;148:565–570. doi: 10.1007/BF00441560. [DOI] [PubMed] [Google Scholar]
- Mirmiran M. The function of fetal/neonatal rapid eye movement sleep. Behav Brain Res. 1995;69:13–22. doi: 10.1016/0166-4328(95)00019-p. [DOI] [PubMed] [Google Scholar]
- Montarolo PG, Kandel ER, SS Long-term heterosynaptic inhibition in aplysia. Nature. 1988;333:171–174. doi: 10.1038/333171a0. [DOI] [PubMed] [Google Scholar]
- Montemitro E, Franco P, Scaillet S, Kato I, Groswasser J, Villa MP, Kahn A, Sastre JP, Ecochard R, Thiriez G, Lin JS. Maturation of spontaneous arousals in healthy infants. Sleep. 2008;31:47–54. doi: 10.1093/sleep/31.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PJ, Ackland GL, Hanson MA. Unilateral cooling in the region of locus coeruleus blocks the fall in respiratory output during hypoxia in anaesthetized neonatal sheep. Exp Physiol. 1996;81:983–994. doi: 10.1113/expphysiol.1996.sp003998. [DOI] [PubMed] [Google Scholar]
- Moore PJ, Parkes MJ, Nijhuis JG, Hanson MA. The incidence of breathing movements of fetal sheep in normoxia and hypoxia after peripheral chemodenervation and brainstem transection. J Dev Physiol. 1989;11:147–151. [PubMed] [Google Scholar]
- Nijhuis JG, Jongsma HW, Crijins IJ, de Valk IM, van der Velden JW. Effects of maternal glucose ingestion on human fetal breathing movements at weeks 24 and 28 weeks of gestation. Early Hum Dev. 1986;13:183–188. doi: 10.1016/0378-3782(86)90006-x. [DOI] [PubMed] [Google Scholar]
- Nijhuis JG, Prechtl HF, Martin CB, Jr, Bots RS. Are there behavioural states in the human fetus? Early Hum Dev. 1982;6:177–195. doi: 10.1016/0378-3782(82)90106-2. [DOI] [PubMed] [Google Scholar]
- Nuding SC, Segers LS, Shannon R, O’Connor R, Morris KF, Lindsey BG. Central and peripheral chemoreceptors evoke distinct responses in simultaneously recorded neurons of the raphe-pontomedullary respiratory network. Phil Trans R Soc B. 2009;364:2501–2516. doi: 10.1098/rstb.2009.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmeggiani PL, Rabini C. Shivering and panting during sleep. Brain Res. 1967;6:789–791. doi: 10.1016/0006-8993(67)90139-4. [DOI] [PubMed] [Google Scholar]
- Parmeggiani PL, Zamboni G, Cianci T, Calasso M. Absence of thermoregulatory vasomotor responses during fast wave sleep in cats. Electroencephalogr Clin Neurophysiol. 1977;42:372–380. doi: 10.1016/0013-4694(77)90173-0. [DOI] [PubMed] [Google Scholar]
- Parslow PM, Harding R, Adamson TM, Horne RS. Effects of sleep state and postnatal age on arousal responses induced by mild hypoxia in infants. Sleep. 2004;27:105–109. [PubMed] [Google Scholar]
- Parslow PM, Harding R, Cranage SM, Adamson TM, Horne RS. Arousal responses to somatosensory and mild hypoxic stimuli are depressed during quiet sleep in healthy term infants. Sleep. 2003;26:739–744. doi: 10.1093/sleep/26.6.739. [DOI] [PubMed] [Google Scholar]
- Patrick J, Natale R, Richardson B. Patterns of human fetal breathing activity at 34 to 35 weeks’ gestational age. Am J Obstet Gynecol. 1978;132:507–513. doi: 10.1016/0002-9378(78)90744-5. [DOI] [PubMed] [Google Scholar]
- Phillipson EA, Sullivan CE. Arousal: the forgotten response to respiratory stimuli. Am Rev Respir Dis. 1978;118:807–809. doi: 10.1164/arrd.1978.118.5.807. [DOI] [PubMed] [Google Scholar]
- Phillipson EA, Sullivan CE, Read DJ, Murphy E, Kozar LF. Ventilatory and waking responses to hypoxia in sleeping dogs. J Appl Physiol. 1978;44:512–520. doi: 10.1152/jappl.1978.44.4.512. [DOI] [PubMed] [Google Scholar]
- Pinsker H, Kupfermann I, Castellucci V, Kandel ER. Habituation and dishabituation of the Gill-withdrawal reflex in aplysia. Science. 1970;167:1740–1742. doi: 10.1126/science.167.3926.1740. [DOI] [PubMed] [Google Scholar]
- Prechtl HF. The behavioural states of the newborn infant (a Review) Brain Res. 1974;76:185–212. doi: 10.1016/0006-8993(74)90454-5. [DOI] [PubMed] [Google Scholar]
- Rebuck AS, Campbell EJ. A clinical method for assessing the ventilatory response to hypoxia. Am Rev Respir Dis. 1974;109:345–350. doi: 10.1164/arrd.1974.109.3.345. [DOI] [PubMed] [Google Scholar]
- Richter DW, Schmidt-Garcon P, Pierrefiche O, Bischoff AM, Lalley PM. Neurotransmitters and neuromodulators controlling the hypoxic respiratory response in anaesthetized cats. J Physiol. 1999;514 (Pt 2):567–578. doi: 10.1111/j.1469-7793.1999.567ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigatto H, Brady Jp, Verduzco RT. Chemoreceptor reflexes in preterm infants: I. The effect of gestational and postnatal age on the ventilatory response to inhalation of 100% and 15% oxygen. Pediatrics. 1975;55:604–613. [PubMed] [Google Scholar]
- Rigatto H, Moore M, Cates D. Fetal breathing and behavior measured through a double-wall Plexiglas window in sheep. J Appl Physiol. 1986;61:160–164. doi: 10.1152/jappl.1986.61.1.160. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Zhao Z, Porter G, Turner C. Influences of adenosine on the fetus and newborn. Molecular genetics and metabolism. 2001;74:160–171. doi: 10.1006/mgme.2001.3217. [DOI] [PubMed] [Google Scholar]
- Savich RD, Guerra FA, Lee C-CH, Padbury JF, Kitterman JA. Effects of acute oligohydramnios on respiratory system of fetal sheep. J Appl Physiol. 1992;73:610–617. doi: 10.1152/jappl.1992.73.2.610. [DOI] [PubMed] [Google Scholar]
- Seelke AM, Blumberg MS. The microstructure of active and quiet sleep as cortical delta activity emerges in infant rats. Sleep. 2008;31:691–699. doi: 10.1093/sleep/31.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless S, Jasper H. Habituation of the Arousal Reaction. Brain : a journal of neurology. 1956;79:655. doi: 10.1093/brain/79.4.655. [DOI] [PubMed] [Google Scholar]
- Sovik S, Lossius K, Eriksen M, Grogaard J, Walloe L. Development of oxygen sensitivity in infants of smoking and non-smoking mothers. Early Hum Dev. 1999;56:217–232. doi: 10.1016/s0378-3782(99)00048-1. [DOI] [PubMed] [Google Scholar]
- Stothers JK, Warner RM. Oxygen consumption and sleep state in the newborn. J Physiol. 1977a;269:57. [PubMed] [Google Scholar]
- Stothers JK, Warner RM. Oxygen consumption of the newborn infant in a cool environment, measured within regard to sleep state. J Physiol. 1977b;273:16. [PubMed] [Google Scholar]
- Tabata M, Kurosawa H, Kikuchi Y, Hida W, Ogawa H, Okabe S, Tun Y, Hattori T, Shirato K. Role of GABA within the nucleus tractus solitarii in the hypoxic ventilatory decline of awake rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1411–1419. doi: 10.1152/ajpregu.2001.281.5.R1411. [DOI] [PubMed] [Google Scholar]
- Takakura ACT, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572.2:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychological review. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Thoppil CK, Belan MA, Cowen CP, Mathew OP. Behavioral arousal in newborn infants and its association with termination of apnea. J Appl Physiol. 1991;70:2479–2484. doi: 10.1152/jappl.1991.70.6.2479. [DOI] [PubMed] [Google Scholar]
- van der Hal AL, Rodriguez AM, Sargent CW, Platzker AC, Keens TG. Hypoxic and hypercapneic arousal responses and prediction of subsequent apnea in apnea of infancy. Pediatrics. 1985;75:848–854. [PubMed] [Google Scholar]
- Verbeek MM, Richardson HL, Parslow PM, Walker AM, Harding R, Horne RS. Arousal and ventilatory responses to mild hypoxia in sleeping preterm infants. J Sleep Res. 2008;17:344–353. doi: 10.1111/j.1365-2869.2008.00653.x. [DOI] [PubMed] [Google Scholar]
- Waites BA, Ackland GL, Noble R, Hanson MA. Red nucleus lesions abolish the biphasic respiratory response to isocapnic hypoxia in decerebrate young rabbits. J Physiol. 1996;495:217–225. doi: 10.1113/jphysiol.1996.sp021586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters KA, Tinworth KD. Habituation of arousal responses after intermittent hypercapnic hypoxia in piglets. Am J Respir Crit Care Med. 2005;171:1305–1311. doi: 10.1164/rccm.200405-595OC. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Suguihara C, Hehre D, Devia C, Huang J, Bancalari E. Effects of GABA receptor blockade on the ventilatory response to hypoxia in hypothermic newborn piglets. Pediatr Res. 2000;47:663–668. doi: 10.1203/00006450-200005000-00018. [DOI] [PubMed] [Google Scholar]
- Xing T, Pilowsky PM. Acute intermittent hypoxia in rat in vivo elicits a robust increase in tonic sympathetic nerve activity that is independent of respiratory drive. J Physiol. 2010;588:3075–3088. doi: 10.1113/jphysiol.2010.190454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuma F, Kozar LF, Kimoff RJ, Bradley TD, Phillipson EA. Interaction of chemical and mechanical respiratory stimuli in the arousal response to hypoxia in sleeping dogs. Am Rev Respir Dis. 1991;143:1274–1277. doi: 10.1164/ajrccm/143.6.1274. [DOI] [PubMed] [Google Scholar]