Abstract
Purpose
To assess the efficacy of Rapamycin treatment in chemoprevention and chemotherapy of tumorigenesis in a genetically-defined mouse model of head and neck squamous cell carcinoma (HNSCC).
Experimental design
Knockdown of Tgfbr1 and/or Pten using siRNA-mediated RNA interference was carried out in human HNSCC cell lines to analyze molecular changes in the mTOR pathway. Tgfbr1flox/flox; Ptenflox/flox; K14-CreERtam mice were treated with oral gavage of tamoxifen for the conditional deletion of Tgfbr1 and Pten in oral mucosa, resuting in HNSCC (Bian et al 2011). Tgfbr1 and Pten conditonal deletion (2cKO) mice were treated with Rapamycin before or after the onset of HNSCC, and the efficacy of this treatment was assessed by determining tumor burden, longevity, and molecular analysis of the mTOR pathway. Molecular changes observed in human HNSCC cell lines and 2cKO mice were compared to identify key alterations in the mTOR pathway.
Results
Knockdown of Tgfbr1 and/or Pten in human HNSCC cell lines resulted in activation of mTORC1 and increased levels of survivin. Furthermore, we observed similar changes in HNSCC of the 2cKO mouse. In the human HNSCC tissue array, a loss of Tgfbr1 expression correlated with increased survivin levels. Chemopreventive Rapamycin treatment significantly delayed the onset of the HNSCC tumors and prolonged survival in 2cKO mice. Additionally, we also found that Rapamycin had a therapeutic effect on squamous cell carcinomas in these mice. In 2cKO HNSCC tongue tumors, Rapamycin treatment induced apoptosis, inhibited cell proliferation and phosphorylation of Akt and S6, and decreased survivin expression.
Conclusions
These findings indicate that tumorigenesis in 2cKO HNSCC is associated with activation of the Akt/mTOR/survivin pathway, and inhibition of this pathway by Rapamycin treatment successfully ameliorates the onset and progression of tumorigenesis.
Keywords: TGF-β, Pten, head and neck cancer, mTOR, survivin
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer, with more than 450,000 newly diagnosed cases worldwide every year (1). HNSCC causes significant morbidity and mortality, with a 5-year survival rate of <50%, and it has remained relatively unchanged for the past three decades (2). HNSCC progression involves the sequential acquisition of genetic and epigenetic alterations in tumor suppressor genes and oncogenes (3). Among the multiple molecular mechanisms that are dysregulated in HNSCC, accumulating evidence demonstrates the importance of Akt/mTOR signaling in HNSCC progression (4). Activation of Akt and its downstream target molecule, mTOR, has been observed in more than 80% of HNSCC lesions (5). The activation of mTOR can be the result of enhanced expression and activity of the epithelial growth factor receptor (5), mainly because of the presence of activating mutations in the catalytic subunit of phosphoinositide-3-kinase (PI3K), or from the decreased expression of the PIP3 phosphatase PTEN (Phosphatase and Tensin homolog). Phosphorylation of mTOR activates key eukaryotic translation regulators, including p70-S6 kinase (p70S6K, which directly targets ribosomal S6 protein) and the eukaryotic translation initiation factor 4-E binding protein 1 (4E-BP1). The latter prevents the repressing activity of 4E-BP1 on the eukaryotic initiation factor 4E (eIF4E)(6), ultimately resulting in enhanced translation from a subset of genes that are required for cell growth of HNSCC (4). Activation of mTOR is an early event in HNSCC, as well as one of the most frequent, and it is also related to lymph node metastasis (5, 7). eIF4E-positive surgical margins have more than a 6-fold risk of developing local recurrences (8). Moreover, inhibition of the mTOR activity complex 1 (mTORC1) by specific inhibitors such as Rapamycin (sirolimus) has been shown to induce a rapid regression and prevent recurrence of these tumors in a xenograft mouse model (9). It has also decreased tumor burden and the malignant conversion of potential HNSCC precancerous lesions in genetically defined and chemically induced animal models of HNSCC (10). Survivin, a member of the mammalian inhibitor of apoptosis (IAP), is also regulated by Akt and mTOR (11). Overexpression of survivin often correlates with poor prognosis of HNSCC (12).
We previously reported that the deletion of transforming growth factor-β receptor I (Tgfbr1) promotes tumorigenesis of HNSCC mainly through activation of Akt, but does not initiate it (13). In addition, compound deletions of Tgfbr1 and Pten in oral mucosa resulted in spontaneous development of HNSCC with 100% penetrance (14). These observations prompted us to examine whether Tgfbr1-mediated signaling could regulate the activity of mTOR and survivin expression in Tgfbr1/Pten double conditional knock out (2cKO) mice. Additionally, we also examined the effects of an mTOR inhibitor on tumorigenesis of HNSCC in our mouse model. We report here that the activation of mTOR with survivin is a widespread event in spontaneously developed HNSCC of Tgfbr1/Pten 2cKO mice, and that Rapamycin treatment delayed tumorigenesis of HNSCC by inhibiting activation of mTOR and survivin, resulting in prolonged survival.
Materials and Methods
Generation of Tgfbr1/Pten 2cKO mice
The Tgfbr1/Pten 2cKO mice (K14-CreERtam; Tgfbr1flox/flox; Ptenflox/flox) were generated by mating inducible Tgfbr1 conditional knockout mice (Tgfbr1 cKO, K14-CreERtam; Tgfbr1flox/flox) (13) with Ptenflox/flox mice. Pten cKO mice (K14-CreERtam; Ptenflox/flox) were obtained from Jackson Laboratories and maintained as previously described (13, 14). The Tgfbr1/Pten 2cKO mice and the Pten cKO mice and their controls were from the same litter and therefore had the same mixed genetic background of C57BL/6; FVBN; CD1; 129. All animal studies were conducted in accordance with the NIH guidelines for the Care and Use of Laboratory Animals and approved by IACUC, NIDCR. The tamoxifen treatment procedure has been previously described (13, 14). Mice were housed in appropriate sterile filter-capped cages, and fed and watered ad libitum. Only 4- to 8-week-old male and female Tgfbr1/Pten 2cKO mice were included in this study.
Rapamycin treatment
Rapamycin was initially dissolved in 100% ethanol at a concentration of 50 mg/ml, and stored at −20°C. The working solution was further diluted in an aqueous phase of 5.2% Tween 80 and 5.2% polyethylene glycol 400 and prepared immediately before use. For the chemopreventive tumorigenesis studies, 4- weeks after the last oral dose of tamoxifen the mice were randomized into a control group (n = 17 mice) or a group that received 10 mg/kg Rapamycin i.p. every other day (n =17 mice). Mice were treated with this dosing schedule of Rapamycin for 6 weeks, and tumor size was measured weekly. Tumor volume was calculated by multiplying the three dimensions of each tumor using a micrometer caliper. Tumor burden was calculated as the individual tumor volume in each mouse and normalized with relative tumor growth by dividing the final volume by the initial tumor volume. At the end of the tumorigenesis studies, mice were euthanized using CO2 and tumor, lung, and liver tissues were harvested and then fixed in Z-fix overnight and cut into 5um-thick sections. For the chemopreventive survival studies, mice were treated either with vehicle or 10mg/kg Rapamycin every other day for 4 weeks, beginning 2 weeks after the last dose of tamoxifen (n =15 mice from control group and n=14 in Rapamycin group). The mice were euthanized when they reached a humane endpoint (when tumors reached 2 cm in size or when mice were distressed by the tumor burden) that was confirmed independently by a veterinarian. For the chemotherapeutic studies, 4 weeks after the last dose of oral tamoxifen the mice were randomized into a control group (n = 9 mice) or a group that received 10 mg/kg Rapamycin i.p. every other day (n =11 mice) for 1 week, based on our pilot study on the tumorigenesis and survival of 2cKO mice. At the end of the chemotherapeutic studies, mice were euthanized using CO2fixed in Z-fix overnight, and processed for histology.
Cell culture and RNA interference
HNSCC cell lines CAL27 (ATCC, Manassas, VA) and HSC3 were maintained in Dulbecco’s modified Eagle's medium (DMEM)/F12, 10% fetal bovine serum, at 5% CO2 and 37°C in a humidified incubator as previously described (14) (15). For functional analysis, non-targeting negative control siRNA (Qiagen, Valencia, CA), Pten siRNA, Tgfbr1 siRNA, and combined Pten/Tgfbr1 siRNA were transfected into the appropriate cells using Hiperfect transfection reagent (Qiagen) with a final concentration of 5nM (Supplementary Table 1). For inhibition efficiency and target mRNA transcription studies, RNA was extracted 24h after transfection. For protein extraction, cells were lysed 72h after transfection.
Histology and Immunohistochemistry
Western blot analysis
Human HNSCC tissues array
Tissue arrays of formalin-fixed tissues from human head and neck squamous cell carcinoma (HN803) were retrieved from Biomax US (Rockville, MD, USA). Clinical data, including pathological classification and TNM classification, were provided by Biomax. These tissue microarray slides included 57 confirmed cases of HNSCC, 10 cases of normal tongue mucosa, and 4 cases of lymph node metastasis in HNSCC tissue. Immunohistochemistry for Tgfbr1 (1:100), p-S6 (S235/236, 1:200), and Survivin (1:400) was stained in serial-cut tissue array sections.
Statistical analysis
Data analyses were performed using Graph Pad Prism version 5.00 for Windows (Graph-Pad Software Inc, La Jolla, CA). One-way ANOVA followed by the post-Turkey or Bonferroni multiple comparison tests were used to analyze the differences in immunostaining and protein levels among each group. The Mann–Whitney U test was used to evaluate differences in the total tumor area of the mice treated with Rapamycin and of the untreated control group. Two-way ANOVA with Bonferroni post-test were used for the tumor burden of Rapamycin chemopreventive and chemotherapeutic experiments. One-way ANOVA followed by Dunnett post-test were used to compare the vehicle and Rapamycin-treated chemotherapeutics group (all compared with normalized tumor size of day 0). Log Rank statistics were used to evaluate survival in the Rapamycin chemopreventive and control groups. Two-tailed Pearson statistics were used for correlated expression of Tgfbr1 with p-S6 and survivin after confirmation of the sample with Gaussian distribution. Mean values ± SEM with a difference of P < 0.05 were considered statistically significant.
Results
Knockdown of Tgfbr1 and Pten in human HNSCC cell lines results in activation of PI3K, Akt, p-S6, and survivin
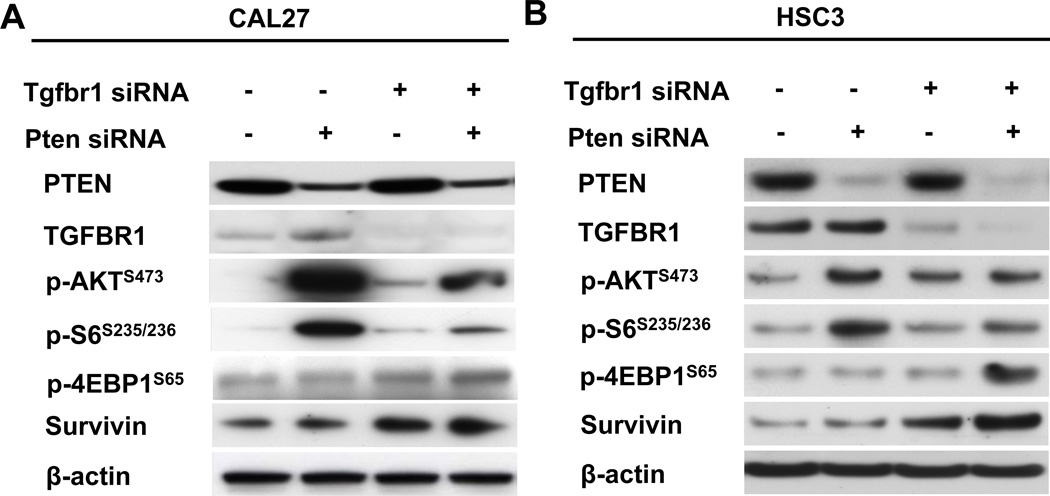
To address the question of why Tgfbr1/Pten 2cKO mice develop spontaneous and rapid tumors with 100% penetrance (10) and Pten cKO mice do not, we carried out siRNA-mediated knockdown of Tgfrb1 or Pten, as well as Tgfbr1 and Pten in human HNSCC cell line CAL27 and metastatic human HNSCC cell line HSC3. Forty-eight hours after siRNA transfection, we found that knockdown of Tgfbr1 or Pten increased p-Akt, p-S6 levels as well as p-4E-BP1 levels (Figs.1A and B), whereas knockdown of both Tgfbr1 and Pten attenuated the increase in p-Akt and p-S6 levels. Therefore, we hypothesized that Tgfbr1 and Pten might regulate downstream targets of mTOR. We further analyzed the knockdown effects of Tgfbr1 and Pten on the protein level of survivin, a downstream molecule in the Akt/mTOR signaling pathway, and found that either knockdown of Pten (P>0.05) or Tgfbr1 (P<0.05 in CAL27 cell line), or knockdown of Tgfbr1 and Pten together (P<0.001 in both cell lines), increased survivin levels in both the HNSCC cell lines (Figs.1A and B, and Supplementary Fig.1A).
Fig. 1. Activation of Akt/mTOR/p-S6/survivin by knockdown of Tgfbr1 and/or Pten in human HNSCC cell lines.
A, B, Western blot analysis for PTEN, Tgfbr1, p-Akt, p-S6, p-4E-BP1, and survivin in the CAL27 and HSC3 HNSCC cell lines 72h after transfection of Tgfbr1 siRNA, Pten siRNA, and combined Tgfbr1/Pten siRNA.
Activation of PI3K, Akt, mTOR and survivin in Tgfbr1/Pten 2cKO mouse HNSCC
We have previously reported the increased activation of p-Akt in HNSCC of the Tgfbr1/Pten 2cKO mice (10). In order to investigate whether these changes cause the activation of mTOR in the tumors, we performed immunostaining of proteins in the mTOR pathway. Our analysis revealed intense staining in the PI3Kα p110 unit, which acts upstream of Akt and mTOR in the Tgfbr1/Pten 2cKO HNSCC, as compared with the Tgfbr1/Pten 2cKO oral mucosa (Figs. 2A and B). We also observed that there was increased staining for survivin in the 2cKO HNSCC sections as compared to the 2cKO tongue (P<0.01) and wild-type tongue (P<0.001, Figs.2A and B). Most importantly, we found increased levels of p-mTORS2448 in 2cKO HNSCC (Fig. 2C). This increase in p-mTORS2448 was also associated with an increase in the levels of PI3Kα p110, p-AktS473, p-S6S235/236, p-4E-BP1S65, and survivin in 2cKO tumors (Fig. 2C, Supplementary Fig. 1B).
Fig. 2. Activation of PI3K/Akt/p-S6/survivin in Tgfbr1/Pten 2cKO mouse HNSCC.
A, Immunohistochemical staining shows increased expression of PI3K p110 subunit, p-Akt, p-S6, and survivin in Tgfbr1/Pten 2cKO mouse HNSCC (high magnification in the right panel), compared with Tgfbr1/Pten 2cKO mouse mucosa and Tgfbr1flox/flox/Ptenflox/flox mucosa (Scale Bars=50um). B, The quantification of immunostaining shows statistics by histoscore (Mean ± SEM,*, P < 0.05; **, P < 0.01;***, P <0.001, One-way ANOVA analysis, Tg, tongue; TSCC, tongue squamous cell carcinoma). C, Western blot shows a significant increase in p-Akt, p-mTOR, p-P70S6K, p-S6, p-4E-BP1, and survivin in Tgfbr1/Pten 2cKO mice HNSCC compared with Tgfbr1flox/flox/Ptenflox/flox tongue.
Based on our findings regarding the knockdown of Tgfbr1 in the tissue culture system, which show an increased phosphorylation of Akt and S6, and our in vivo findings of the significant increase in p-Akt, p-S6, and survivin levels, we hypothesized that Tgfbr1 deletion in Tgfbr1/Pten 2cKO mouse HNSCC tumors is responsible for the increase in p-Akt, p-S6, and survivin levels. Using Western blot analysis, we confirmed that there was a dramatic increase in phosphorylation of Akt, S6, and survivin in the 2cKO HNSCC (n=5, Supplementary Fig. 2A), as compared to Pten cKO mice HNSCC (n=4, Supplementary Fig. 2A). This was further confirmed by immunohistochemical staining for p-S6S235/236 and survivin in the Tgfbr1/Pten 2cKO HNSCC and Pten cKO HNSCC (Supplementary Figs. 2B and C). The data suggest that activation of mTOR by deletion of Pten is further compounded by the additional deletion of Tgfbr1, resulting in not only the activation of mTOR but also the activation of survivin and the acceleration of tumorigenesis in Tgfbr1/Pten 2cKO HNSCC.
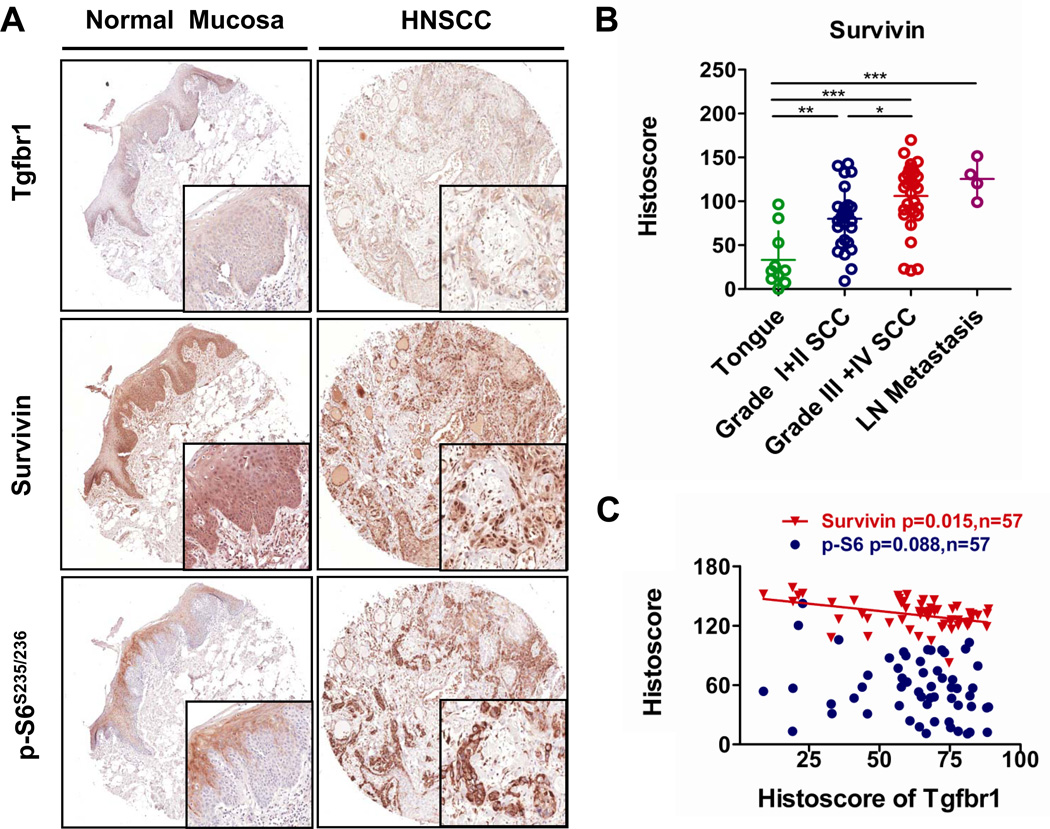
Decreased expression of Tgfbr1 is related to increase of survivin in human HNSCC tissues
In order to assess the correlation between Tgfbr1 expression and the survivin levels in human HNSCCs, we stained the tumor sections from human tissue arrays for HNSCC (n=57) with antibodies for Tgfbr1, p-S6, and survivin and compared them with normal head and neck mucosa samples (n=10). Representative figures of Tgfbr1, survivin, and p-S6 immunostaining are shown in Fig. 3A. Tgfbr1 immunostaining reveals that Tgfbr1 is strongly stained in the cytoplasm and membrane of oral mucosa but that it is significantly decreased in 47.4% of HNSCC cores (Fig. 3A), which is consistent with previous findings (14). We also found that normal mucosa showed mild expression of S6 in the superficial layer but not the basal layer of the epithelium, which is consistent with previous reports (5). Survivin staining was mainly observed in the cytoplasm and membrane of the normal epithelium (n=10), while HNSCC samples showed very strong nuclear immunostaining for survivin. Expression of survivin was significantly increased in poorly differentiated HNSCC samples (Grade III and Grade IV, n=31): P<0.001 when compared with normal mucosa, P<0.05 when compared with well-differentiated HNSCCs (Grade I and Grad II, n=26, Fig. 3B), and even higher in the lymph node metastasis of HNSCCs (n=4, P<0.001 as compared with normal mucosa, Fig. 3B). This suggests that survivin is a predictor of poor HNSCC differentiation.
Fig. 3. Low expression of Tgfbr1 negatively correlates with increased levels of survivin in human HNSCC tissues.
A, Representative immunohistochemical staining of Tgfbr1, p-S6, and survivin in human head and neck cancer tissue (right panel) compared with oral mucosa (left panel). B, Survivin levels correlate with pathological classification and lymph metastasis of head and neck cancer. C, Negative correlation between the expression of Tgfbr1 and survivin (P <0.05, n=57) in human head and neck cancer tissues. Histoscore based on quantification using Aperio quantification software and statistics with Graph Pad Prism5 (Mean ± SEM, ***, P<0.001, Two tailed Pearson correlation statistics).
Of particular interest is the fact that expression of Tgfbr1 was negatively correlated with a higher expression of survivin (P=0.015, r2=0.1693), but did not correlate significantly with p-S6 expression (P=0.088, r2=0.052, quantification only includes HNSCC tissue, n=57, Fig. 3C). These data suggest that human HNSCCs show decreased expression of Tgfbr1 associated with the increased levels of survivin, and this decreased expression is considered to be an indicator of a poor HNSCC prognosis.
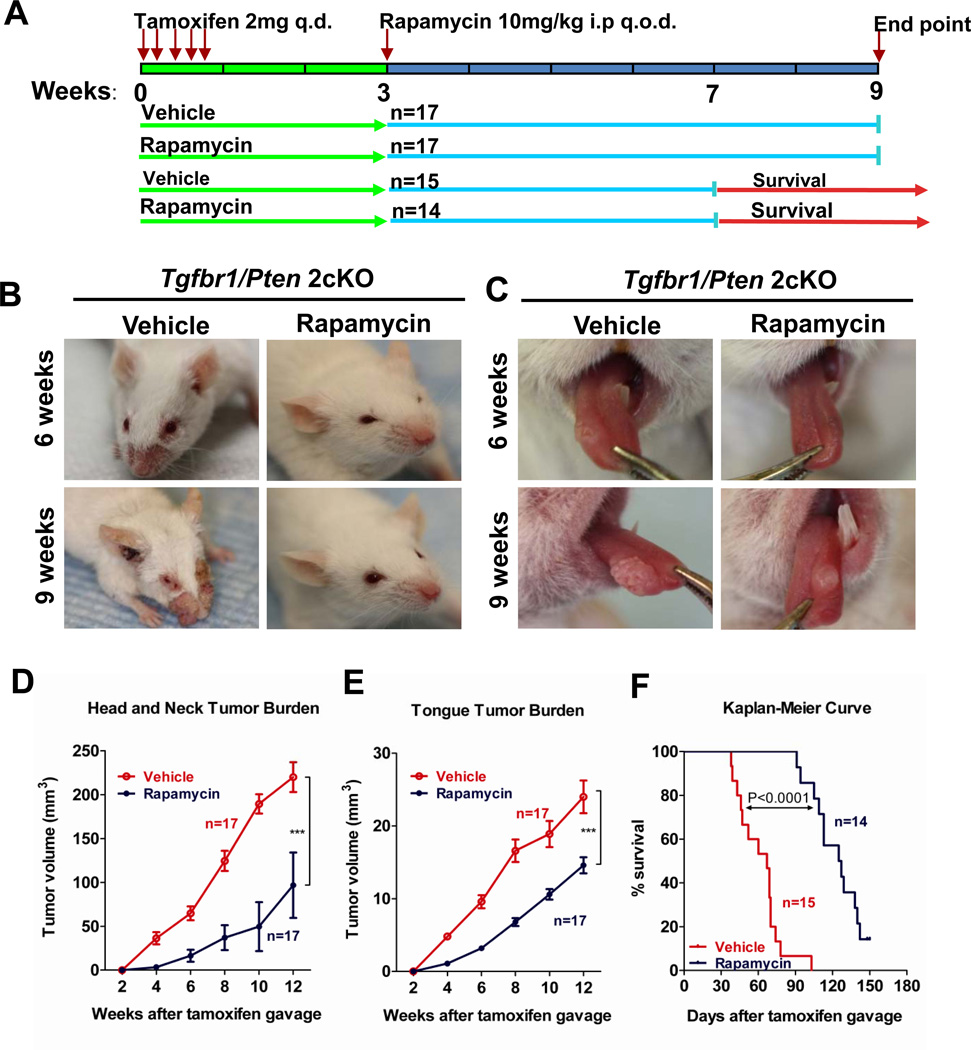
Rapamycin treatment delays the onset and reduces progression of tumorigenesis in Tgfbr /Pten 2cKO mouse HNSCC
Because both Tgfbr1 and Pten deletion increased levels of mTOR and its downstream target survivin, we wanted to find out whether the active phosphorylation of mTOR is the main driver for tumorigenesis in Tgfbr1/Pten 2cKO mouse HNSCC. We induced Cre-mediated deletion of Tgfbr1 and Pten deletion by tamoxifen administration, and 2 weeks later we initiated treatment with Rapamycin, a potent inhibitor of mTOR (Fig. 4A). Rapamycin treatment significantly delayed the progression of papilloma and the onset of squamous cell carcinoma in the head and neck region (Fig. 4B) as well as in the oral cavity (Fig. 4C) of these mice. The Rapamycin treatment of Tgfbr1/Pten 2cKO mice prevented the development of head and neck tumors (n=17 for each group, P <0.001; Fig.4D) and oral tumors (n=17 for each group, P <0.001, Fig. 4E) after 6 weeks of Rapamycin treatment. After 6 weeks of treatment, 82.5% (14/17) of mice in the vehicle-treated group had tongue cancer while only 16.6% (3/17) of the Rapamycin-treated group had tongue cancer. Also, Rapamycin treatment significantly increased the life span of these mice (for the vehicle-treated group, median survival=67 days and for the Rapamycin-treated group, median survival=126 days, P<0.0001, Fig. 4F). Following the 4-week Rapamycin treatment, 12/14 mice continued to thrive while 2/14 mice survived without developing any visible tumors during the 5-month observation period.
Fig. 4. Inhibition of mTOR delays onset of Tgfbr1/Pten 2cKO mouse HNSCC.
A, A schematic showing a drug delivery strategy for Rapamycin in the chemopreventive tumorigenesis experiment in Tgfbr1/Pten 2cKO mice. B and C, Head and neck tumorigenesis and tongue tumorigenesis in chemopreventive tumorigenesis experiment. Upper panel shows representative photo of vehicle or Rapamycin treatment 6 weeks after tamoxifen induction, lower panel shows photos with vehicle or Rapamycin treatment 9 weeks after tamoxifen induction on the same mice. Rapamycin chemopreventive tumorigenesis experiment shows that Rapamycin significantly reduces head and neck tumor burden (D) and tongue tumor burden (E) (Mean ± SEM, n=17 for each group P<0.001, Two way-ANOVA analysis with Bonferroni post-test). F, Chemopreventive survival experiment shows that Rapamycin significantly extends the survival of Tgfbr1/Pten 2cKO mice (vehicle group, n=15, Rapamycin group, n=14, Log Rank statistics).
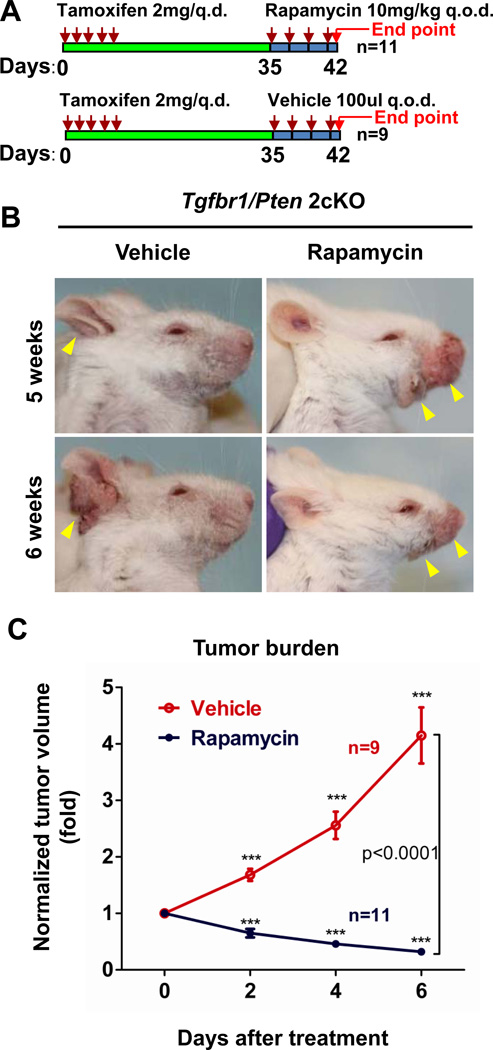
Rapamycin treatment results in regression of established HNSCC tumors in Tgfbr1/Pten 2cKO mice
The success of Rapamycin treatment in delaying the initiation and reducing the progression of HNSCCs in these mice prompted us to explore the consequences of treating the mice that were already harboring HNSCC tumors with Rapamycin. 5 weeks after the tamoxifen-induced deletion of Tgfbr1 and Pten60% of the mice developed head and neck papilloma or squamous cell carcinoma, and at this time-point we initiated Rapamycin treatment (Fig. 5A). As shown in Figs. 5B and C, the untreated mice displayed rapid tumor growth with a 4.15±1.49-fold (n=11) increase in 1 week; however, the tumors began to regress in the treated group just after 1 dose of Rapamycin. The normalized tumor size was found to regress by 0.32±0.19-fold compared to the original tumor volume (P<0.0001 n=11, Fig.5C).
Fig. 5. Experimental therapeutic treatment of Rapamycin in Tgfbr1/Pten 2cKO mice HNSCC.
A, A schematic for drug delivery strategy of Rapamycin in the chemotherapeutic experiment of Tgfbr1/Pten 2cKO mice. B, Shows typical photo of vehicle or Rapamycin treatment 5 weeks (upper panel) after tamoxifen induction, lower panel shows head and neck tumorigenesis photos after one week of vehicle or Rapamycin treatment (6 weeks) on the same mice. D, Shows that Rapamycin significantly reduces head and neck tumor burden (n=9 for vehicle group and n=11 for Rapamycin group, P<0.0001, a two-way ANOVA test between vehicle and Rapamycin treated groups. ***, P<0.001, at day 2, day 4, and day 6 of the vehicle-treated as well as the Rapamycin-treated group compared with normalized tumor size on day 0, using one-way ANOVA followed by Dunnett post-test).
Rapamycin treatment decreases cell proliferation and increases apoptosis in Tgfbr1/Pten 2cKO mice HNSCC
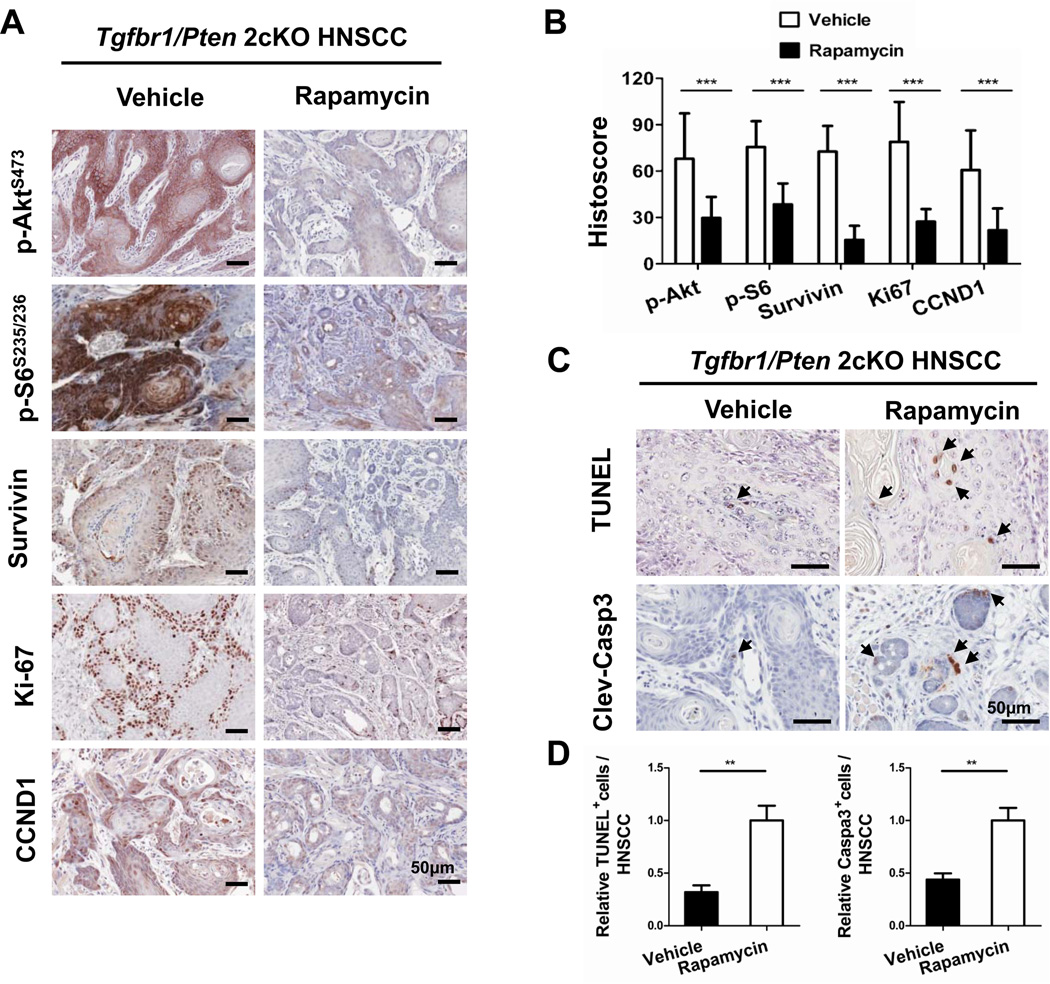
In order to analyze the underlying cellular processes that are affected by Rapamycin treatment, we determined its effects on cell proliferation and apoptosis. Rapamycin treatment resulted in a significant decrease in immunostaining of major indicators of cell proliferation, such as p-Akt levels, p-S6, survivin, and cyclin D1 in HNSCC sections from the treated mice (n=11), as compared with control (n=9) (Figs. 6A and B). In order to assess the effects of Rapamycin on apoptosis, we carried out terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays and immunostaining for cleaved caspase 3. As shown in Figs. 6C and D, we observed a significant increase in apoptosis by both TUNEL and cleaved caspase 3 (P<0.01) assays in the Rapamycin-treated group (n=11), as compared with the control group (n=9). Therefore, these data indicate that Rapamycin treatment results in significantly lower proliferation and increased apoptosis in these HNSCC tumors.
Fig. 6. Decrease in Akt/S6/survivin signaling and proliferation in Tgfbr1/Pten 2cKO mice HNSCC after Rapamycin treatment.
A, Representative immunohistochemical staining of p-Akt, p-S6, survivin, Ki-67, and CyclinD1 in vehicle and Rapamycin treatment group. Brown staining indicates the immunoreactivity (5 mice from each group). B, Shows a histoscore based on quantification of staining for p-Akt using membrane quantification software, histoscore of p-S6 and survivin using pixel quantification, and histoscore of Ki-67 and cyclin D1 based on quantification of nuclear staining. C, Increase in the apoptotic cells was detected using TUNEL in situ analysis (upper panel) and immunohistochemical staining of cleaved caspase 3 (lower panel, arrow shows positive cells, scale bars=50uM). Quantification using Aperio nuclear quantification software, and statistics (D) using Graph Pad Prism 5. (Mean ± SEM, **, P<0.01, Mann –Whitney U test).
Discussion
A newly gained molecular understanding of HNSCC initiation and progression may soon present the opportunity to develop novel drug targets and therapeutic approaches. In this regard, among the multiple aberrant genetic, epigenetic, and signaling events known to occur in HNSCCs, the persistent activation of the Akt/mTOR pathway has emerged as a potential drug target for HNSCC treatment (5). Recent preclinical investigations have indicated that the use of mTOR inhibitors, including Rapamycin and its analogues, can significantly reduce tumor burden and even recurrence of HNSCC in animal models generated by different strategies such as tumor xenografts, carcinogen-induced tumors, and genetic engineering used to recapitulate initiation and progression of HNSCC (7, 9, 10, 16, 17). Our in vitro data suggested that loss of Tgfbr1 activates Akt/mTOR/survivin expression in the human HNSCC cell lines. Furthermore, using a genetically engineered mouse model that spontaneously develops visible HNSCC tumors on the tongue and facial region with consistent full penetrance for conditional deletion of Pten and Tgfbr1, we now show that activation of the Akt/mTOR/survivin axis is the most significant molecular event caused by the deletion of Pten and Tgfbr1. Inhibition of mTOR with Rapamycin can reduce tumoral growth in the head and neck region as well as in the tongue, thereby prolonging animal survival.
Interestingly, we found that knockdown Tgfbr1 could activate phosphorylation of the Akt and mTOR pathways and increase survivin levels in vitro. In line with these observations, although survivin is known as a downstream target of mTOR (11), we noticed that the knockdown of Tgfbr1 increases survivin to a greater extent than knockdown of Pten. This finding is consistent with the previously reported independent finding that the loss of both Tgfbr1 and Tgfbr2 correlated with the increased survivin level (18), and that TGFβ1 down-regulated survivin in prostate cancer through Rb/E2F4 and Smad2/3 pathways (19). Even more interestingly, the knockdown of both Tgfbr1 and Pten increased survivin levels. Additionally, we observed significant activation of the Akt/mTOR/survivin axis in Tgfbr1/Pten 2cKO mice compared with Pten cKO mice. Together, our data suggest that loss of Tgfbr1 will additionally and independently activate the Akt/mTOR/survivin axis. Our observations in the human HNSCC cell line, indicating that a decrease in Tgfbr1 expression alone increases survivin expression, have important clinical implications. In an HNSCC patient with lower expression of Tgfbr1, which potentially activates the Akt /mTOR/survivin axis, further analysis of mTOR and survivin may be needed for confirmation. An HNSCC patient with reduced Tgfbr1 expression may be deemed suitable for an adjunctive chemotherapeutic treatment with the mTOR inhibitor, such as Rapamycin or its analogue. Chemopreventive experiments provided evidence that activation of the mTOR pathway is one of the main molecular drivers for tumorigenesis in Tgfbr1/Pten 2cKO mice. Treatment with the inhibitor of mTORC1 delays the tumorigenesis in this animal model and prolongs its life span, supporting evidence of the anticancer functions of mTOR inhibitors such as Rapamycin, which may be partly explained by the multiple roles of mTOR in cell proliferation, metabolism, transcription, angiogenesis, and immunity (20–23). The experimental therapeutics also provide critical evidence that, even after the onset of tumors, inhibition of mTOR may be a good chemotherapeutic agent for HNSCC in these mice, especially with loss of Pten (24). Interestingly, this animal model is very sensitive to Rapamycin and tumor shrinkage was observed over the course of a week, which is not usually the case in mousce models for mTOR inhibition. On one hand, our observation suggests that deletion of both Tgfbr1 and Pten will activate mTOR. On the other hand, this result may in fact be due to the mouse model’s intact immune system, and inhibition of mTOR may affect tumor microenvironments such as the immune system, chemokine, and angiogenesis (21, 22). Our previous and present findings also suggest that there is a significant activation of the EGFR/PI3K/Akt axis, activation of the stat3 and IKKβ/NF-κB pathways, and overexpression of VEGFA and cylcin D1 in this mouse model, all of which are important molecular events amenable for mTOR inhibition (25). Although Tgfbr1 gene mutation may not be a significant factor in HNSCC (26, 27), the reports on tumor-susceptible polymorphism resulting in hypofunction of TGFBR1 should not been ignored (28, 29). Indeed, we found that almost 40% of HNSCC patients have concurrent low protein levels of Tgfbr1 and Pten (14), and therefore this genetically manipulated mouse model may not fully reflect the clinical spectrum of human HNSCC. While keeping this potential limitation in mind, this mouse model has revealed a potent anticancer function of mTOR inhibitors, which could have multiple beneficial implications for treating HNSCC patients.
The inhibition of mTOR in experimental and clinical HNSCC lesions leads to a rapid decrease in the phosphorylated state of S6, a downstream target of mTORC1 (20) that also serves as a biomarker for the molecular response to mTOR inhibitors in their target tissues. In 2cKO mouse HNSCCs, Rapamycin also causes a rapid decrease in the phosphorylation of Akt at serine 473, a downstream target of mTORC2 (9, 10, 17), suggesting a sensitivity to Rapamycin (25). As shown in the human HNSCC xenograft animal model (7), our data also suggested that inhibition of mTOR significantly decreases expression of survivin and cyclin D1, inhibits proliferation, and promotes apoptosis in this mouse HNSCC. This effect could contribute to the antitumor activity of Rapamycin. Thus, the inhibition of mTORC1 in mouse HNSCCs may result in a reduced cell cycle and may induce apoptosis.
Among the risk factors influencing a patient’s outcome, the presence of an increase in survivin levels and active Akt/mTOR signaling at the time of diagnosis represents the most important factor predicting a poor prognosis (3, 12, 30, 31). Unfortunately, high expression of survivin is often accompanied by early metastatic disease and recurrence (12). In this regard, the emerging preclinical and clinical information about the promising beneficial effects of mTOR inhibitors in HNSCCs, together with our present findings, demonstrate a potentially successful outcome of such therapy for the HNSCC patients. We believe that the Tgfbr1/Pten 2cKO mice provide a good animal model for preclinical evaluations of Akt/mTOR and survivin inhibitors, as part of targeted strategies for chemopreventive and chemotherapeutics treatment of HNSCC.
Supplementary Material
Translational Relevance.
Our concurrent analysis of the genomic alterations of the transforming growth factor-β receptor I (Tgfbr1) and PIP3 phosphatase PTEN (Pten) defines the compound mutations of these genes resulting in head and neck squamous cell carcinoma (HNSCC). These mutations in human HNSCC cell lines and the conditional mutant mice are associated with activation of mTORC1 and increased levels of survivin. The successful outcome of our strategy to treat HNSCC tumors in these mice with the mTOR inhibitor Rapamycin highlights the promising use of Rapamycin in chemopreventive and chemotherapeutic treatment of HNSCC.
Acknowledgement
We thank Dr. Alfredo Molinolo for helpful discussion, Ms. Shelagh Johnson for expert editorial assistance, and Mr. Papa Milton for his veterinary assistance.
Grant Support
Grant support was provided by the Intramural Research Programs of the National Institute of Dental and Craniofacial Research, NIH (A.B.Kulkarni, ZIA-DE-000698), National Natural Science Foundation of China (81072203).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4–5):309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11(1):9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 4.Molinolo AA, Amornphimoltham P, Squarize CH, Castilho RM, Patel V, Gutkind JS. Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncol. 2009;45(4–5):324–334. doi: 10.1016/j.oraloncology.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molinolo AA, Hewitt SM, Amornphimoltham P, Keelawat S, Rangdaeng S, Meneses Garcia A, et al. Dissecting the Akt/mammalian target of rapamycin signaling network: emerging results from the head and neck cancer tissue array initiative. Clin Cancer Res. 2007;13(17):4964–4973. doi: 10.1158/1078-0432.CCR-07-1041. [DOI] [PubMed] [Google Scholar]
- 6.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18(16):1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 7.Patel V, Marsh CA, Dorsam RT, Mikelis CM, Masedunskas A, Amornphimoltham P, et al. Decreased lymphangiogenesis and lymph node metastasis by mTOR inhibition in head and neck cancer. Cancer Res. 2011;71(22):7103–7112. doi: 10.1158/0008-5472.CAN-10-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan CO, Amirghahari N, Abreo F, Rong X, Caldito G, Jones ML, et al. Overexpressed eIF4E is functionally active in surgical margins of head and neck cancer patients via activation of the Akt/mammalian target of rapamycin pathway. Clin Cancer Res. 2004;10(17):5820–5827. doi: 10.1158/1078-0432.CCR-03-0483. [DOI] [PubMed] [Google Scholar]
- 9.Nathan CO, Amirghahari N, Rong X, Giordano T, Sibley D, Nordberg M, et al. Mammalian target of rapamycin inhibitors as possible adjuvant therapy for microscopic residual disease in head and neck squamous cell cancer. Cancer Res. 2007;67(5):2160–2168. doi: 10.1158/0008-5472.CAN-06-2449. [DOI] [PubMed] [Google Scholar]
- 10.Czerninski R, Amornphimoltham P, Patel V, Molinolo AA, Gutkind JS. Targeting mammalian target of rapamycin by rapamycin prevents tumor progression in an oral-specific chemical carcinogenesis model. Cancer Prev Res (Phila) 2009;2(1):27–36. doi: 10.1158/1940-6207.CAPR-08-0147. [DOI] [PubMed] [Google Scholar]
- 11.Vaira V, Lee CW, Goel HL, Bosari S, Languino LR, Altieri DC. Regulation of survivin expression by IGF-1/mTOR signaling. Oncogene. 2007;26(19):2678–2684. doi: 10.1038/sj.onc.1210094. [DOI] [PubMed] [Google Scholar]
- 12.Lo Muzio L, Pannone G, Staibano S, Mignogna MD, Rubini C, Mariggio MA, et al. Survivin expression in oral squamous cell carcinoma. Br J Cancer. 2003;89(12):2244–2248. doi: 10.1038/sj.bjc.6601402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bian Y, Terse A, Du J, Hall B, Molinolo A, Zhang P, et al. Progressive tumor formation in mice with conditional deletion of TGF-beta signaling in head and neck epithelia is associated with activation of the PI3K/Akt pathway. Cancer Res. 2009;69(14):5918–5926. doi: 10.1158/0008-5472.CAN-08-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bian Y, Hall B, Sun ZJ, Molinolo A, Chen W, Gutkind JS, et al. Loss of TGF-beta signaling and PTEN promotes head and neck squamous cell carcinoma through cellular senescence evasion and cancer-related inflammation. Oncogene. 2011 Oct 31; doi: 10.1038/onc.2011.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawakami K, Kawakami M, Joshi BH, Puri RK. Interleukin-13 receptor-targeted cancer therapy in an immunodeficient animal model of human head and neck cancer. Cancer Res. 2001;61(16):6194–6200. [PubMed] [Google Scholar]
- 16.Amornphimoltham P, Leelahavanichkul K, Molinolo A, Patel V, Gutkind JS. Inhibition of Mammalian target of rapamycin by rapamycin causes the regression of carcinogen-induced skin tumor lesions. Clin Cancer Res. 2008;14(24):8094–8101. doi: 10.1158/1078-0432.CCR-08-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amornphimoltham P, Patel V, Sodhi A, Nikitakis NG, Sauk JJ, Sausville EA, et al. Mammalian target of rapamycin, a molecular target in squamous cell carcinomas of the head and neck. Cancer Res. 2005;65(21):9953–9961. doi: 10.1158/0008-5472.CAN-05-0921. [DOI] [PubMed] [Google Scholar]
- 18.Shariat SF, Lotan Y, Saboorian H, Khoddami SM, Roehrborn CG, Slawin KM, et al. Survivin expression is associated with features of biologically aggressive prostate carcinoma. Cancer. 2004;100(4):751–757. doi: 10.1002/cncr.20039. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Song K, Krebs TL, Jackson MW, Danielpour D. Rb/E2F4 and Smad2/3 link survivin to TGF-beta-induced apoptosis and tumor progression. Oncogene. 2008;27(40):5326–5338. doi: 10.1038/onc.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12(1):9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Karar J, Maity A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front Mol Neurosci. 2011;4:51. doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weichhart T, Saemann MD. The multiple facets of mTOR in immunity. Trends Immunol. 2009;30(5):218–226. doi: 10.1016/j.it.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Squarize CH, Castilho RM, Gutkind JS. Chemoprevention and treatment of experimental Cowden's disease by mTOR inhibition with rapamycin. Cancer Res. 2008;68(17):7066–7072. doi: 10.1158/0008-5472.CAN-08-0922. [DOI] [PubMed] [Google Scholar]
- 25.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6(9):729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 26.Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333(6046):1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bian Y, Knobloch TJ, Sadim M, Kaklamani V, Raji A, Yang GY, et al. Somatic acquisition of TGFBR1*6A by epithelial and stromal cells during head and neck and colon cancer development. Hum Mol Genet. 2007;16(24):3128–3135. doi: 10.1093/hmg/ddm274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasche B, Knobloch TJ, Bian Y, Liu J, Phukan S, Rosman D, et al. Somatic acquisition and signaling of TGFBR1*6A in cancer. JAMA. 2005;294(13):1634–1646. doi: 10.1001/jama.294.13.1634. [DOI] [PubMed] [Google Scholar]
- 30.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345(26):1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 31.Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359(11):1143–1154. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.