Abstract
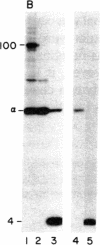
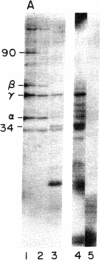
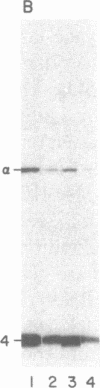
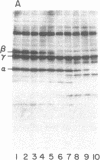
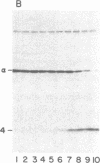
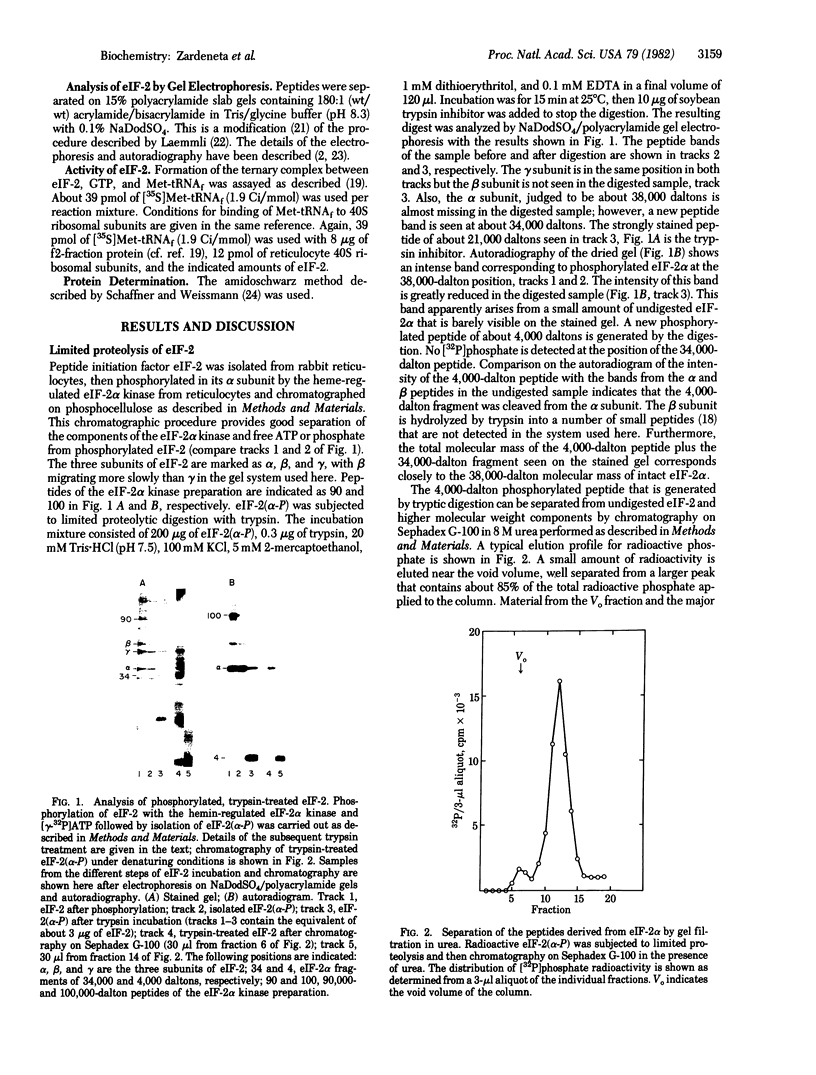
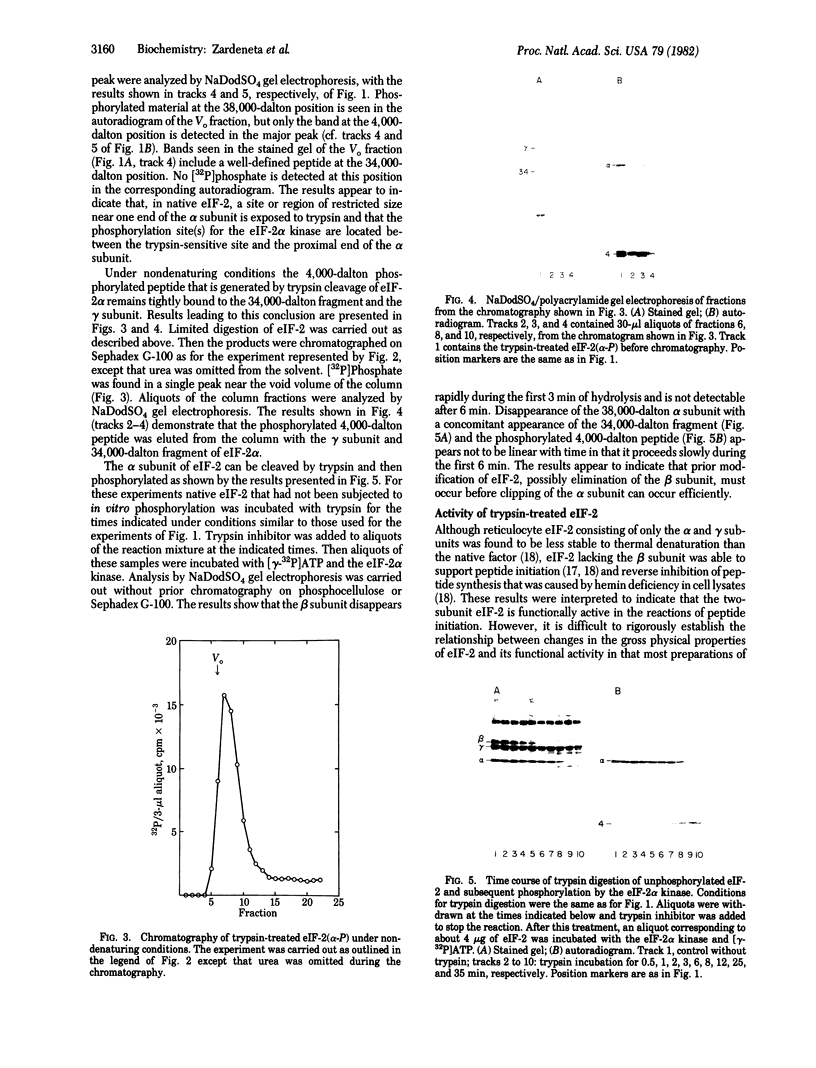
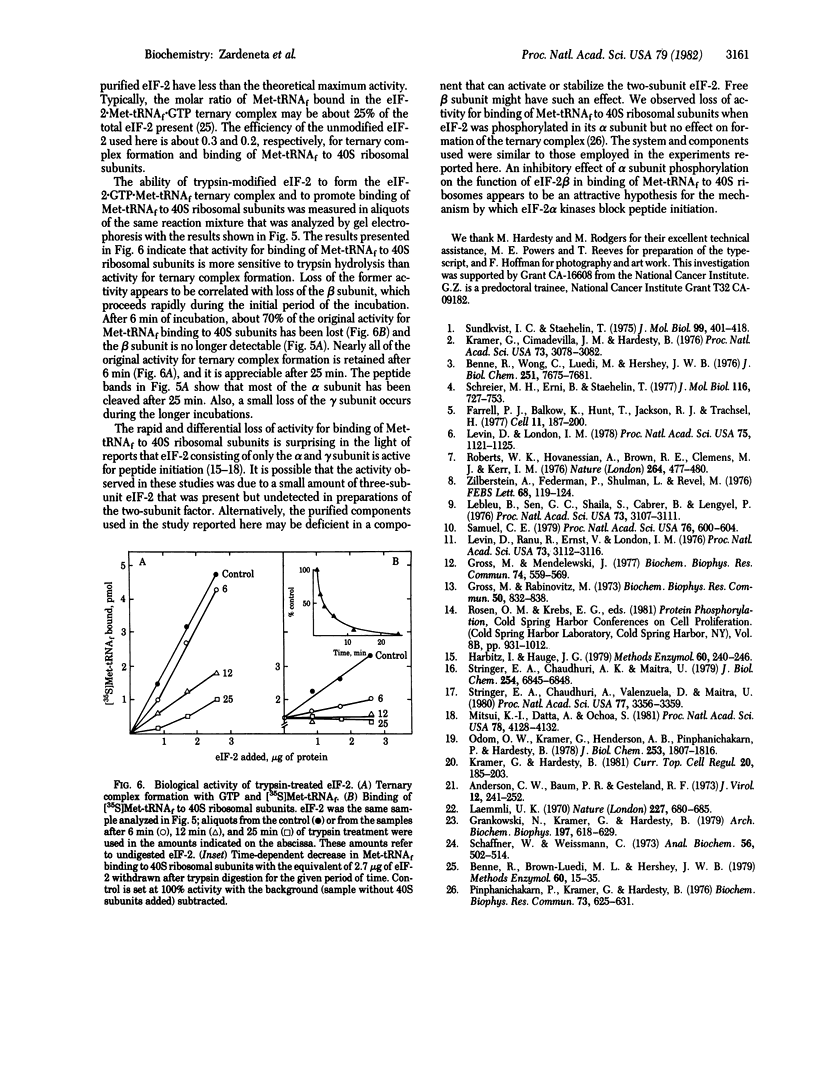
A procedure is described by which the 38,000-dalton alpha subunit of native eukaryotic peptide initiation factor 2 (eIF-2) can be cleaved by trypsin to yield a 34,000-dalton fragment and a peptide of about 4,000 daltons after elimination of the beta subunit. Under nondenaturing conditions the 4,000-dalton peptide remains bound to the modified eIF-2 and still can be phosphorylated by the heme-controlled eIF-2 alpha kinase from reticulocytes. All of the phosphorylation sites for this protein kinase are located on the 4,000-dalton peptide. The ability of eIF-2 to form a ternary complex with GTP and Met-tRNAf and the ability to promote binding of Met-tRNAf to 40S ribosomal subunits are lost differentially during the proteolysis. Loss of te latter activity occurs rapidly and appears to be correlated with loss of the beta subunit. Loss of activity for ternary complex formation is correlated with the appearance of the 4,000-dalton peptide.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne R., Brown-Luedi M. L., Hershey J. W. Protein synthesis initiation factors from rabbit reticulocytes: purification, characterization, and radiochemical labeling. Methods Enzymol. 1979;60:15–35. doi: 10.1016/s0076-6879(79)60005-8. [DOI] [PubMed] [Google Scholar]
- Benne R., Wong C., Luedi M., Hershey J. W. Purification and characterization of initiation factor IF-E2 from rabbit reticulocytes. J Biol Chem. 1976 Dec 10;251(23):7675–7681. [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Grankowski N., Kramer G., Hardesty B. Purification and partial characterization of the catalytic subunit of cAMP-dependent protein kinases from reticulocytes. Arch Biochem Biophys. 1979 Oct 15;197(2):618–629. doi: 10.1016/0003-9861(79)90286-8. [DOI] [PubMed] [Google Scholar]
- Gross M., Mendelewski J. Additional evidence that the hemin-controlled translational repressor from rabbit reticulocytes is a protein kinase. Biochem Biophys Res Commun. 1977 Jan 24;74(2):559–569. doi: 10.1016/0006-291x(77)90340-0. [DOI] [PubMed] [Google Scholar]
- Gross M., Rabinovitz M. Partial purification of a translational repressor mediating hemin control of globin synthesis and implication of results on the site of inhibition. Biochem Biophys Res Commun. 1973 Feb 5;50(3):832–838. doi: 10.1016/0006-291x(73)91320-x. [DOI] [PubMed] [Google Scholar]
- Harbitz I., Hauge J. G. Purification and properties of eIF-2 from pig liver. Methods Enzymol. 1979;60:240–246. doi: 10.1016/s0076-6879(79)60021-6. [DOI] [PubMed] [Google Scholar]
- Kramer G., Cimadevilla J. M., Hardesty B. Specificity of the protein kinase activity associated with the hemin-controlled repressor of rabbit reticulocyte. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3078–3082. doi: 10.1073/pnas.73.9.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer G., Hardesty B. Phosphorylation reactions that influence the activity of elF-2. Curr Top Cell Regul. 1981;20:185–203. doi: 10.1016/b978-0-12-152820-1.50009-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lebleu B., Sen G. C., Shaila S., Cabrer B., Lengyel P. Interferon, double-stranded RNA, and protein phosphorylation. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3107–3111. doi: 10.1073/pnas.73.9.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D., London I. M. Regulation of protein synthesis: activation by double-stranded RNA of a protein kinase that phosphorylates eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1121–1125. doi: 10.1073/pnas.75.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D., Ranu R. S., Ernst V., London I. M. Regulation of protein synthesis in reticulocyte lysates: phosphorylation of methionyl-tRNAf binding factor by protein kinase activity of translational inhibitor isolated from hemedeficient lysates. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3112–3116. doi: 10.1073/pnas.73.9.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui K., Datta A., Ochoa S. Removal of beta subunit of the eukaryotic polypeptide chain initiation factor 2 by limited proteolysis. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4128–4132. doi: 10.1073/pnas.78.7.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odon O. W., Kramer G., Henderson A. B., Pinphanichakarn P., Hardesty B. GTP hydrolysis during methionyl-tRNAf binding to 40 S ribosomal subunits and the site of edeine inhibition. J Biol Chem. 1978 Mar 25;253(6):1807–1816. [PubMed] [Google Scholar]
- Pinphanichakarn P., Kramer G., Hardesty B. Partial reaction of peptide initiation inhibited by the reticulocyte hemin-controlled repressor. Biochem Biophys Res Commun. 1976 Dec 6;73(3):625–631. doi: 10.1016/0006-291x(76)90856-1. [DOI] [PubMed] [Google Scholar]
- Roberts W. K., Hovanessian A., Brown R. E., Clemens M. J., Kerr I. M. Interferon-mediated protein kinase and low-molecular-weight inhibitor of protein synthesis. Nature. 1976 Dec 2;264(5585):477–480. doi: 10.1038/264477a0. [DOI] [PubMed] [Google Scholar]
- Samuel C. E. Mechanism of interferon action: phosphorylation of protein synthesis initiation factor eIF-2 in interferon-treated human cells by a ribosome-associated kinase processing site specificity similar to hemin-regulated rabbit reticulocyte kinase. Proc Natl Acad Sci U S A. 1979 Feb;76(2):600–604. doi: 10.1073/pnas.76.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Erni B., Staehelin T. Initiation of mammalian protein synthesis. I. Purification and characterization of seven initiation factors. J Mol Biol. 1977 Nov;116(4):727–753. doi: 10.1016/0022-2836(77)90268-6. [DOI] [PubMed] [Google Scholar]
- Stringer E. A., Chaudhuri A., Maitra U. Purified eukaryotic initiation factor 2 from calf liver consists of two polypeptide chains of 48,000 and 38,000 daltons. J Biol Chem. 1979 Aug 10;254(15):6845–6848. [PubMed] [Google Scholar]
- Stringer E. A., Chaudhuri A., Valenzuela D., Maitra U. Rabbit reticulocyte initiation factor 2 contains two polypeptide chains of molecular weights 48,000 and 38,000. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3356–3359. doi: 10.1073/pnas.77.6.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundkvist I. C., Staehelin T. Structure and function of free 40 S ribosome subunits: Characterization of initiation factors. J Mol Biol. 1975 Dec 15;99(3):401–418. doi: 10.1016/s0022-2836(75)80135-5. [DOI] [PubMed] [Google Scholar]
- Zilberstein A., Federman P., Shulman L., Revel M. Specific phosphorylation in vitro of a protein associated with ribosomes of interferon-treated mouse L cells. FEBS Lett. 1976 Sep 15;68(1):119–124. doi: 10.1016/0014-5793(76)80418-8. [DOI] [PubMed] [Google Scholar]