Abstract
In addition to its role as a toxicological signal mediator, the Aryl Hydrocarbon Receptor (AHR) is also a transcription factor known to regulate cellular responses to oxidative stress and inflammation through transcriptional regulation of molecules involved in the signaling of nucear factor-erythroid 2-related factor-2 (Nrf2), p53 (TRP53), retinoblastoma (RB1), and NFκB. Recent research suggests that AHR activation of these signaling pathways may provide the molecular basis for understanding AHR’s evolving role in endogenous developmental functions during hematopoietic stem-cell maintenance and differentiation. Recent developments into the hematopoietic roles for AHR are reviewed, aiming to reconcile divergent findings as to the endogenous function of AHR in hematopoiesis. Potential mechanistic explanations for AHR’s involvement in hematopoietic differentiation are discussed, focusing on its known role as a cell cycle mediator and its interactions with Hypoxia-inducible transcription factor-1 alpha (HIF1-α). Understanding the physiological mechanisms of AHR activation and signaling have far reaching implications ranging from explaining the action of various toxicological agents to providing novel ways to expand stem cell populations ex vivo for use in transplant therapies.
Keywords: Aryl Hydrocarbon Receptor, Megakaryocyte, Stem Cells, Hematopoietic Stem Cells, Cell cycle, Differentiation, Hematopoiesis, Reactive Oxygen Species, Oxidative Stress, Dioxin, Oxidative DNA Damage, Xenobiotics
Introduction
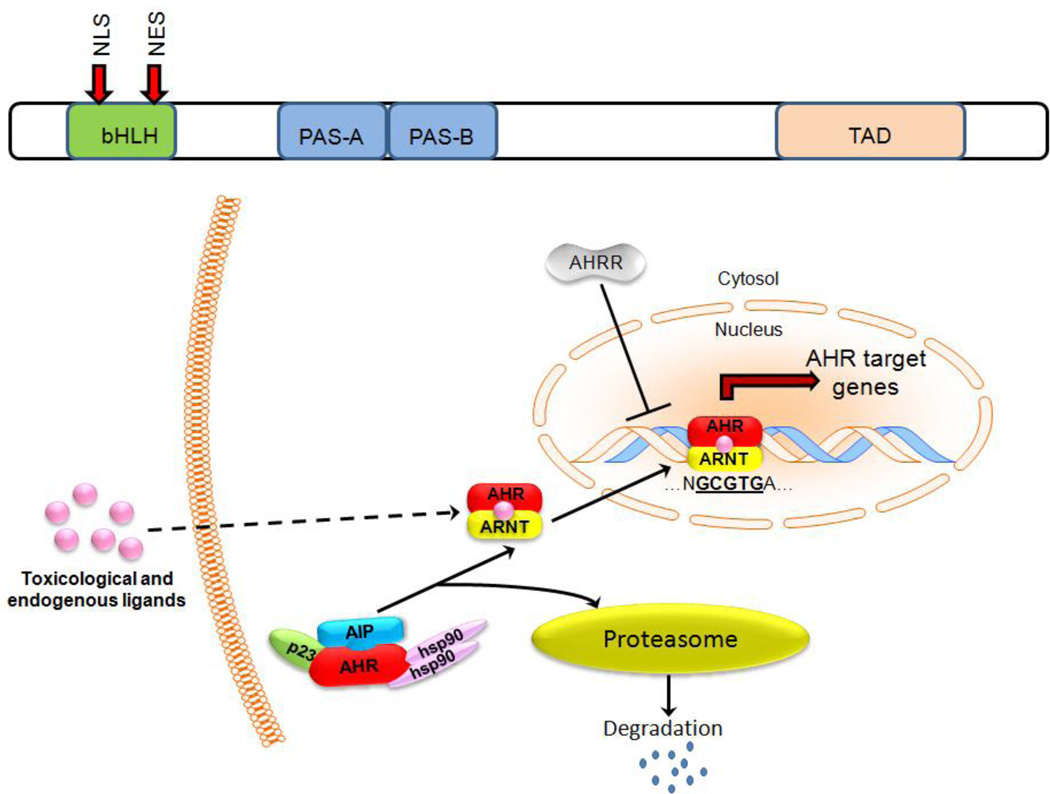
The aryl hydrocarbon receptor (AHR) belongs to the basic helix-loop-helix (bHLH)/PAS (Per/Arnt/Sim) family of transcription factors. Like all bHLH/PAS family members, AHR contains several important structural elements (Fig. 1A) including an N-terminal basic helix-loop-helix (bHLH) domain, a C-terminal transcriptional activation domain, and a central PAS domain containing two degenerate repeats (PAS-A and PAS-B) that mediate DNA binding and are involved in protein-protein interactions. The PAS domains of AHR mediate chaperone interactions, ligand binding, and the formation of heterodimers between AHR and other PAS-domain containing proteins (1). A glutamine-rich transcriptional activation domain (TAD) in the C terminus of AHR plays a role in co-activator recruitment and transactivation (2). Other important members of the bHLH/PAS-transcription factor family include BMAL-Clock, Per, c-MYC, Tal1, and HIF-1α and are involved in normal physiological functions including circadian-rhythm maintenance, organ development, neurogenesis, and responses to hypoxia. This formed the basis for a question that has since become core to AHR biology: does AHR have similar physiologic functions?
Fig. 1.
A) AHR contains important structural motifs that are critical to its function including the basic helix-loop helix-domain (bHLH) that contains both a nuclear localization sequence (NLS) and nuclear export sequence (NES). AHR also contains two PER-ARNT-SIM homolology (PAS) regions and a c-terminal transcriptional activation domain (TAD). B) Activation by either toxicological or endogenous ligands results in the dissociation of several cofactors and allows for the binding of AHR to ARNT, creating an active AHR-ARNT heterodimer. AHR-ARNT heterodimers then translocate to the nucleus, where they bind target gene promoters and initiate gene transcription. AHR = Aryl hydrocarbon receptor, ARNT = aryl hydrocarbon receptor nuclear translocator, AIP = aryl hydrocarbon receptor interacting protein, HSP90 = shock protein 90, AHRR = AHR Repressor protein.
AHR is well known for mediating transcriptional signals originating from environmental toxins such as 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD or dioxin), polycyclic aromatic hydrocarbons (PAHs), benzene, and polychlorinated biphenyls (PCBs). As such, AHR activation has been best studied in the context of xenobiotic signaling (Fig. 1B). In its basal or resting state, AHR remains predominantly cytoplasmic as part of a protein complex with the molecular chaperone heat shock protein 90 (HSP90), p23, and XAP2 (aka ARA9 or AIP) (3). Various cellular stresses activate AHR and lead to a conformational change that results in the exposure of the nuclear localization sequence (NLS) and AHR nuclear translocation. AHR then dissociates from HSP90 and binds to the AHR nuclear translocator ARNT/Hif1-β (4). The AHR/ARNT heterodimer then binds to promoter regions of target genes that contain the AHR DNA binding consensus sequence 5’-T/GNGCGTGA/CG/CA-3’, with the core sequence underlined (5). AHR also contains a nuclear export sequence (NES), which plays a role limiting the amount of AHR present within the nucleus by shuttling AHR from the nucleus to the cytosol, where it is degraded by the proteasome-ubiquitin system (6). Perhaps due to its physiological importance, AHR signaling is fine-tuned with another layer of regulation by direct competition with the AHR Repressor protein (AHRR) for ARNT DNA binding sites (7). AHRR expression is induced by a variety of AHR agonists (8), suggesting that AHRR forms a negative feedback loop with AHR by repressing AHR signaling if AHR becomes too abundant.
Recent studies have focused on emerging roles of AHR in normophysiology and in particular its role or roles as an important regulator of hematopoiesis, which is the focus of this review. These emerging functional roles relate to how AHR senses various stress signals both in the largely hypoxic environment of the hematopoietic stem-cell compartment, and during the differentiation and development of hematopoietic progenitor and mature cells. We first review briefly the control of hematopoietic development, and then the established role of AHR as a mediator of toxicological signals, aiming to set the foundation for discussing AHR’s emerging role in normophysiology and in particular hematopoiesis.
The maintenance and differentiation of hematopoietic stem cells
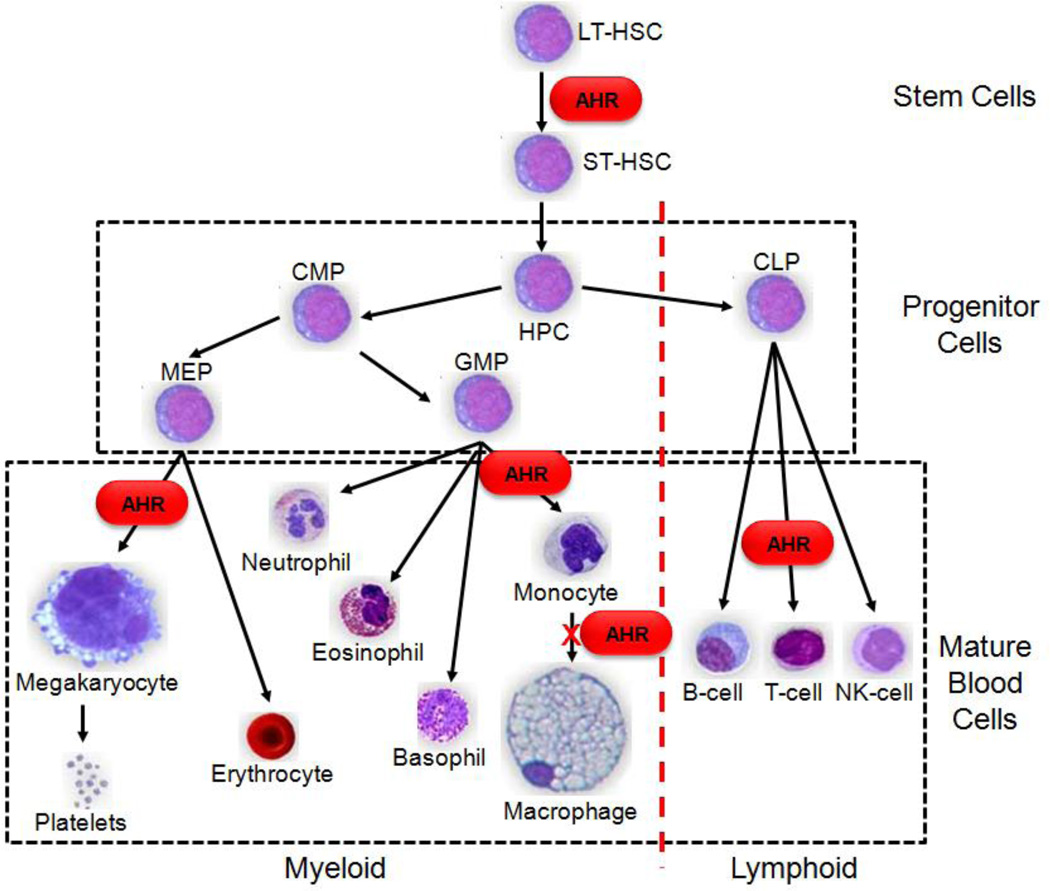
As shown in Figure 2, all hematopoietic lineages originate from a small population of multipotent common hematopoietic stem cells (HSC). Due to the its nature as a stem cell, a single HSC can repopulate the entire hematopoietic system of a lethally irradiated mouse (9). As blood cells mature, they acquire a defined phenotype as the result of coordinated cell-specific gene expression (10). Through a complex regulatory system, stage-specific cytokines and cell-cell interactions result in specific gene-expression patterns that identify each developmental stage and lineage in hematopoiesis. Transcription factors mediate differentiation signals elicited by hematopoietic growth factors, interleukins, environmental cues, and hormones to “direct” an HSC toward various lineages and maturation states. Alterations in these carefully orchestrated signals may result in multiple blood abnormalities, including changes in blood populations (e.g., decreased white blood counts or increased platelet levels), abnormal proliferation of progenitor cells (e.g., myelodysplastic syndromes), and can lead to various forms of leukemia (11). Hematopoietic regulation results from the combined influence of multiple transcription factors working in concert to coordinate and regulate temporal and lineage specific gene transcription. GATA2, for example, is essential for the development, maintenance, and function of HSCs, early progenitor cells, and is important for erythroid differentiation (12), while the closely related GATA1 is involved in the maturation of megakaryocytic cells (13).
Fig. 2.
Hematopoiesis is the step-wise, ordered acquisition of phenotypic traits and is commonly represented as a hierarchal model starting with quiescent LT-HSC cells and ending with mature blood cells. LT-HSC = long-term hematopoietic stem cell, ST-HSC = short-term hematopoietic stem cell, CMP=common myeloid progenitor, CLP = common myeloid progenitor cell, MEP = Megakaryocyte-erythrocyte progenitor, GMP = Granulocyte-macrophage progenitor.
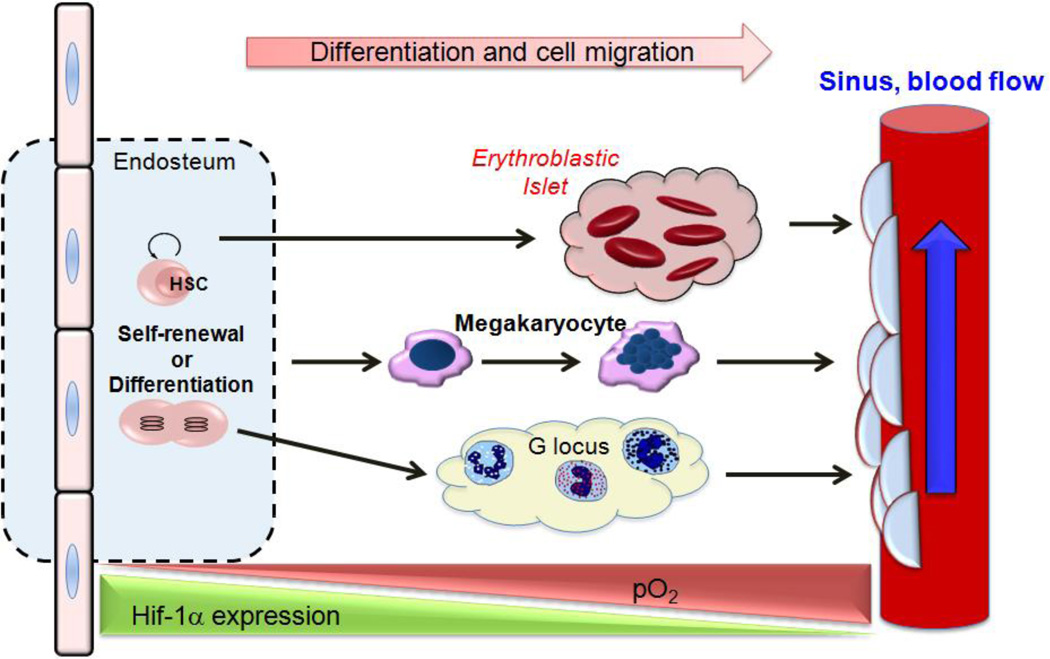
HSCs reside in regions of low oxygen tension (described in more detail below) and the hypoxia-inducible factor-1 alpha (HIF-α) is critical for the ability of HSCs to respond to variations in oxygen tension within the bone marrow microenvironment (14). Anatomical features within bone marrow lead to oxygen and nutrient gradients and establish various niches that cells must adapt to in order to survive and thrive. Bone marrow organization is architecturally complex, and many of its structural features directly or indirectly influence where cells localize and how cells migrate (15). As shown in Figure 3, the most primitive hematopoietic stem and progenitor cells reside in the endosteum, hypoxic extravascular (frequently referred to as perivascular) regions furthest away from the sinuses that favor HSC quiescence (16–18). To cope with the low oxygen conditions, HSCs within the endosteum constitutively express HIF-1α, a transcription factor responsible for cellular adaptation in response to reduced oxygen conditions (19). Simsek et al. (20) recently determined that constitutive HIF-1α expression in HSCs within the endosteal niche regulates the metabolic state of HSCs resulting in quiescence and protection from oxidative stress. Regulation of HIF-1α expression in HSCs is critical for normal stem-cell maintenance, as HSCs isolated from HIF-1α deficient mice lose the ability to remain quiescence, while over-stabilization of HIF-1α by a biallelic loss of the E3 ubiquitin ligase Von Hippel-Lindau (VHL) induces HSC quiescence (21). As HSCs mature, they reside in regions of greater oxygenation that are favorable to differentiation. This paradigm is thought to occur for every blood cell lineage; sites of megakaryocytic, erythroblastic, and granulocytic differentiation (occurring in erythroblastic islets, bone marrow, and G-loci, respectively) are shown in Figure 3.
Fig. 3.
Starting in the hypoxic endosteum and ending in the bloodstream, differentiating hematopoietic cells must adapt to variations in pO2 and pH as they differentiate. One proposed mechanism by which hematopoietic cells can adapt to these changes is through stabilization of Hif-1α. G-locus = granulocytic locus
The established role of AHR as a mediator of toxicological and stress responses
Exposure to AHR toxicological ligands is known to induce liver, eye and ovarian toxicity and results in mutagenesis, birth defects, and multiple forms of cancer (22). While AHR signaling has been implicated as the mechanism for numerous toxicological agents, we will focus on the two best-studied AHR ligands – TCDD and Benzo[a]pyrene (BAP). Because the AHR signaling cascade mediates most, if not all, of the toxic effects of TCDD, AHR is also called the dioxin receptor. Single doses of TCDD alter both T- and B-cell dependent immune responses, impair host resistance to a variety of infectious agents and antigens, and prevent the rejection of transplanted tumors (23). Although AHR activation decreases the overall proteasome activity of cells, increased AHR signaling results in increased ube213 mRNA and protein expression, leading to increased p53 ubiquitination/degradation and increased apoptosis (24). A known carcinogen, BAP is commonly found in coal tar and cigarette smoke and causes genetic damage in lung cells that was identical to the damage observed in the DNA of most malignant lung tumors (25). BAP toxicity results from the cytochrome P450 detoxifying complex converting BAP to the ultimate mutagen, benzo[a]pyrene diol epoxide (BPDE). The resulting diol epoxide reacts and binds to DNA, resulting in mutations, especially on the tumor suppressor p53 (26). Interestingly, habitual smokers have a greater tendency toward platelet aggregation than non-habitual smokers, suggesting that a component of cigarette smoke such as BAP may also impact platelet function (27).
AHR responds to toxicological stress by regulating cell-cycle genes and proliferation
In addition to activating genes involved in xenobiotic metabolism, such as members of the cytochrome P450 superfamily (28), exposure to environmental toxins resulted in numerous AHR-dependent effects, including altered cell-cycle regulation and proliferation. In 5L hepatoma cells, dioxin induced G1 cell-cycle arrest through a mechanism that involves the AHR binding to the retinoblastoma (Rb) tumor suppressor protein (29). However, polycyclic aromatic hydrocarbon (PAH) insults increased the percentage of rat liver epithelial cells entering S-phase and were associated with increased cyclin A expression and cyclin A/Cdk2 activity in an AHR-dependent manner (30). These opposing results may indicate differential responses to various AHR ligands or suggest that the effects of these ligands, and potentially the impact of AHR, is cell-type specific. AHR knockdown in HepG2 human hepatoma cells blocks the G1/S cell-cycle transition by downregulating cyclin D1 and cyclin E (31). However, this study also showed that AHR knockdown in MCF-7 human breast tumor cells promoted the G1/S cell-cycle transition, suggesting that the impact of knocking down AHR by siRNA is cell-context dependent. Both of these interpretations warrant further investigations into the mechanisms of AHR signaling.
AHR serves as an important physiological mediator of multiple forms of cellular stress
While best known for modulating toxicological responses, AHR performs many other functions in cells and can be best described as a general mediator of environmental stresses, especially in terms of mitigating damage from reactive oxygen species (ROS), changes in oxygen levels, and inflammation. In regulating the responses to these stresses, AHR is critical to normal cellular function and affects many important processes in cell proliferation and differentiation. AHR’s role as a mediator of cellular stresses can be partially explained by its interactions with HSP90, a molecular chaperone known to regulate protein function in order to allow cells to adjust to intra- and extracellular stresses (32). Exposure to many AHR-activating environmental pollutants results in the release of AHR from HSP90 and leads to oxidative stress and production of toxic ROS that damages membrane lipids, mutates DNA, and serves as a significant stress for exposed cells (33). Toxicity from benzene, known to activate AHR signaling, is partially due to the formation of reactive intermediates that lead to increased formation of reactive oxygen species (ROS) (34). Similarly, dioxin exposure results in generalized in vivo oxidative stress in multiple tissues and results in increased production of superoxide anions by peritoneal macrophages (35). Exposure of mice to dioxin also results in sustained oxidative stress and oxidative, which persist for over 8 weeks despite no further dioxin exposure (36).
Genes regulated upon AHR activation also provide strong evidence for a role for AHR during cellular responses to various stresses. Genes involved in inflammatory responses such as NFκB and antioxidative responses to oxidative stress and cell survival including Nrf2 (NF-E2 p45-related factor 2) are particularly important, and will described in more detail. Dioxin exposure results in immunotoxicity and induction of inflammatory genes through its interactions with RelB and subsequent modulation of NFκB signaling during chemokine induction of U937 macrophages in response to dioxin exposure (37). AHR also appears to help cells cope with ultraviolet radiation. Exposure of the immortalized HaCaT keratinocyte cell line or dorsal skin of AHR KO mice to ultraviolet radiation results in an AHR-mediated inflammatory response, induction of cyclooxygenase-2 (COX-2), and altered cell cycling (38). Further demonstrating how signaling cascades normally associated with responses to cellular stress can influence differentiation, chromatin immunoprecipitation studies demonstrated that Nrf2 regulated AHR expression during adipocyte differentiation, and that interfering with either of signaling cascades resulted in impaired differentiation (39). In vivo experiments further demonstrated that Nrf2 protects livers of mice against TCDD-mediated oxidative stress and DNA damage induced by sustained activation of AHR (40).
Increasing evidence of a non-toxicological role for AHR
Decades of research focused on the role of AHR in response to toxicological ligands, but a shift has recently occurred as many have begun to question if this functional role is too narrow for a ubiquitous transcription factor such as AHR. Here we present the evidence that supports an important role of AHR in normophysiology. It is important to note that cell-cycle regulation by AHR is not dependent on toxicological ligands, and that cellular context may be the most important aspect of uncovering a physiologic function for AHR. Ma et al. have shown that AHR-defective mouse hepatoma (Hepa 1c1c7) cells proliferate more slowly than wild type cells due to a block during G1 phase of the cell cycle, and that transfection with AHR cDNA reverses the G1 block (41). Compared with wild-type mouse embryonic fibroblasts (MEFs), AHR-null MEFs exhibited a lower proliferation rate with an accumulation of 4N DNA content and increased apoptosis due to down-regulation of Cdc2 and Plk, two kinases important for G(2)/M phase of cell-cycle (42). Because expression of transforming growth factor-beta (TGF-beta), a proliferation inhibitor, was present at high levels in conditioned medium from AHR-null MEFs, the authors concluded that AHR influences TGF-beta production, leading to alterations in cell-cycle control at the G(2)/M phase of cell cycle. While these findings agree with a role for AHR in cell cycle progression, they are in stark contrast to published reports showing that AHR inhibition leads to increased proliferation/expansion of hematopoietic stem and progenitor cells (43). Other hematopoietic studies found that the AHR promoter is hypermethylated in human acute lymphoblastic leukemia (44), suggesting that AHR deactivation contributes to the increased proliferation and decreased differentiation state that characterizes acute lymphoblastic leukemia.
Action without xenobiotic binding: AHR is involved in neuronal development
AHR homologs are conserved throughout nature in species as diverse as mammals, birds, reptiles, amphibians, fish, and invertebrates (45). Because most AHR homologs appear to influence developmental pathways, detoxification of xenobiotics by AHR may have developed recently in evolution and may mask earlier AHR functions as a regulator of developmental processes. Suggesting that that the original function of AHR was not to mediate detoxification of xenobiotics, many of these species including Caenorhabditis elegans do not bind xenobiotics (46). The Drosophila melanogaster AHR ortholog, spineless, is a bHLH-PAS transcription factor that plays a central role in development. Loss-of-function spineless mutations result in multiple developmental defects including legs forming where antennas are normally found, deletion of distal leg structures, and reduced bristle size (47). Like AHR, spineless functions as a heterodimer with the Drosophila ortholog of ARNT, Tango. Spineless also plays an important role in the development of specialized neurons involved in color vision (48). Importantly, although Spineless-Tango heterodimers have very similar binding specificities to AHR-ARNT heterodimers found in mammals, the interaction of spineless with Tango does not require the presence of xenobiotic ligands (49), and as such represents an instance where AHR physiological function is independent of toxicological ligand binding and/or signal progression. Similarly, the C. elegans AHR ortholog, CeAHR, regulates neural development, possibly due to aberrant axonal migration (50). Together, these findings provide a physiological role for AHR during normal development and open the possibility that AHR may influence additional developmental programs.
Multiple developmental defects in AHR-null mice result in the identification of endogenous AHR ligands
In an effort to understand the physiological function of AHR, several labs independently generated AHR-null mice using somewhat different targeting strategies (51–53). Although the different strategies used to generate knockout mice led to phenotypic differences, these strains collectively suggest that AHR has physiological roles independent of xenobiotic metabolism, especially in hepatic development, reproductive health, immunology, and vascular biology. For example, AHR knockout mice have smaller livers, portal fibrosis, and early lipid accumulation (51). AHR also plays a role in reproductive development, as AHR-null mice have difficulty maintaining pregnancy due to aberrant ovarian follicle formation (54). One of the most consistent phenotypes found in AHR-null murine models was an impaired immune system. AHR-null mice consistently show evidence of immunological defects, and these mice are more susceptible to Helicobacter hepaticus infections, an opportunistic infection indicating immunodeficiency (55). Vascular defects present in AHR-null mice include portosystemic shrinking and persistent fetal structures, including the ductus venosus, a fetal portocaval shunt of the developing liver that normally closes immediately after birth. AHR-null mice fail to close this shunt, resulting in aberrant hepatovascular blood flow (56). Experiments using mutant AHR and ARNT hypomorphs suggest that some phenotypes seen in AHR-null mice, such as ductus venosus closure and vascular development, can be rescued by treatment with AHR xenobiotic ligands (57, 58), but caution must be exercised before one assumes that toxicological ligands will mimic or even help identify normal physiologic AHR functions.
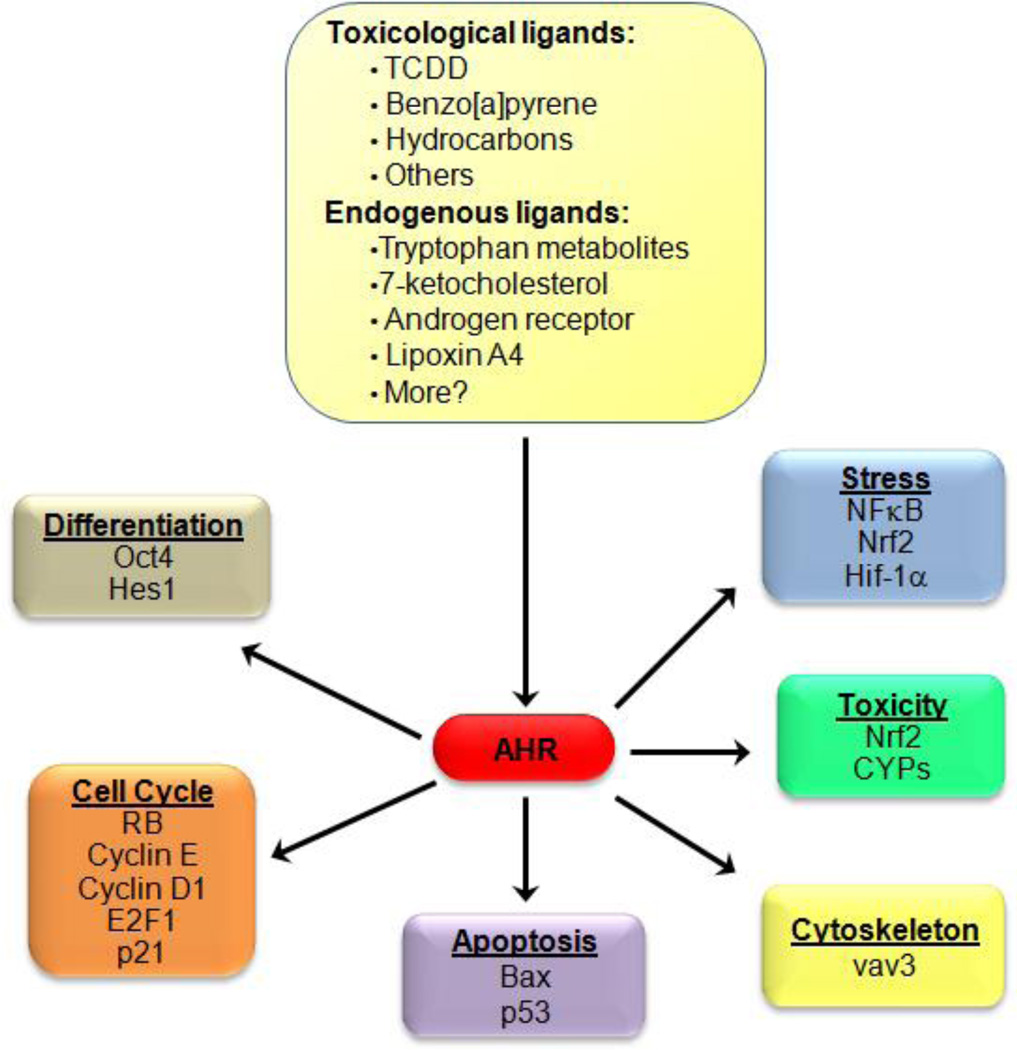
These findings stress the importance of AHR activation as part of normal biology, and contribute to recent enthusiasm in finding endogenous (non-toxicological) AHR ligands. Early support for the presence of endogenous AHR ligands came when mouse hepatoma c37 cells and African green monkey kidney CV-1 cells not previously exposed to exogenous AHR ligands were found to already contain transcriptionaly active AHR-ARNT complexes (59), suggesting that some unknown endogenous factor was activating AHR. These findings attracted the attention of many labs and soon many potential endogenous AHR ligands were discovered, including tryptophan metabolites, indole containing structures, tetrapyroles such as bilirubin and biliverdin, 7-ketocholesterol, equilenin, a form of equine estrogen, several prostaglandins, the arachidonic-acid metabolite lipoxin A4, and cAMP (60). A more complete list of potential AHR endogenous ligands and a discussion of their biological relevance has been recently reviewed (61). Key toxicological and endogenous ligands (shown in Fig. 4) impact a broad range of non-toxicological cellular responses including differentiation, apoptosis, and cell cycle progression.
Fig. 4.
A cartoon representation shows several key ligands known to activate AHR, as well as the molecular mechanisms by which AHR activation impacts various cellular processes.
A role for AHR in hematopoietic development
AHR impacts hematopoietic stem cell expansion and may influence stem cell pluripotency
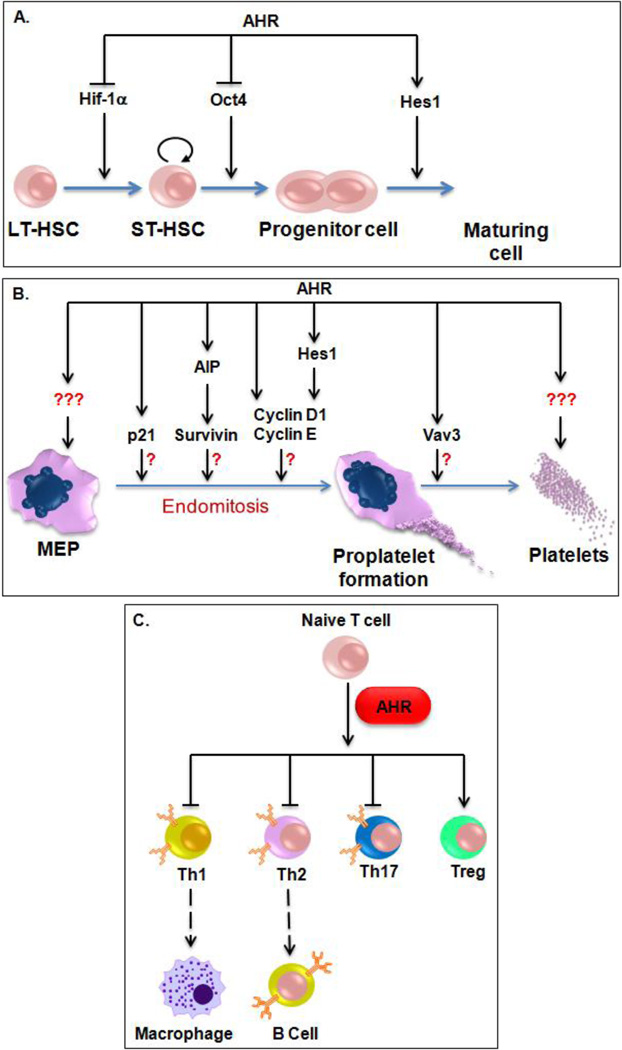
AHR-null mice are fully protected from TCDD-associated and partially protected from benzene-associated hematopoietic defects such as myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) (62), indicating that AHR regulates the physiological responses to these toxic ligands. Murine hematopoietic stem cells treated with TCDD are not able to engraft in lethally irradiated mice (23). The effects of TCDD on hematopoietic stem cell engraftment could be explained based upon the finding that TCDD disrupts the normal cell cycle of hematopoietic stem cells, resulting in fewer quiescent cells (63). The quiescent state is now accepted as a characteristic and indispensable property for the maintenance of HSCs and other primitive stem cells and is known to play an important role in HSC engraftment after bone marrow transplants (64). Using AHR-null mice to distinguish off target TCDD effects from any impacts AHR might have, it was shown that AHR-null mice have increased numbers of the most primitive Lin−Sca+Kit+ (LSK) (65) and that AHR expression initially decreases at the earliest point of hematopoietic stem cell proliferation (66). Interestingly, LSK cells from AHR-null mice proliferate much faster than cells from WT mice (67), suggesting that a lack of AHR leads to hematopoietic stem cell expansion. In agreement with the hypothesis that reduced AHR signaling results in increased hematopoietic stem- and progenitor- cell proliferation, AHR pharmacologic inhibition results in massive HSC expansion, resulting in increased progenitor populations across multiple hematopoietic lineages (43). The molecular mechanism by which AHR regulates HSC biology may involve Oct-4 (octamer-binding transcription factor 4). In vitro models show that AHR regulates Oct-4 in the murine embryonic carcinoma cell line P19 (68), and AHR activation promotes myeloblastic leukemia differentiation by inhibiting Oct-4 expression (69). A critical transcription factor needed for the creation of iPS cells (70), Oct-4 is a member of the POU (Pit-Oct-Unc) family of homeodomain transcription factors and is a key regulator of stem cell pluripotency and differentiation (71). Collectively, these data suggest that AHR may regulate hematopoietic and embryonic stem cell pluripotency, and that AHR is involved in developmental "decisions" leading to mature cell types (Fig. 5A). Clearly, more research is needed to validate these intriguing hypotheses.
Fig. 5.
AHR impacts many stages and lineages of hematopoiesis. A) During the early stages of hematopoiesis, AHR impacts HSC expansion and pluripotency via regulation of Hif-1α, Oct4, and Notch signaling. B) Although still unproven, AHR could influence megakaryocytic polyploidization and platelet function by many molecular mechanisms. C) AHR favors Treg generation at the expense of other T cell populations. LT-HSC = long-term hematopoietic stem cell, ST-HSC = short-term hematopoietic stem cell, Th1 = Type 1 T-helper cell, Th2 = Type 2 T-helper cell, Th17 = T-helper 17 cells, Treg = Regulatory T cells.
Exposure to toxicological ligands suggest that AHR plays a role during normal myeloid differentiation and platelet biogenesis
Highlighting a role for AHR in myeloid biology and development, AHR is upregulated in the absence of exogenous, toxic ligands during monocyte differentiation and activation (72, 73), yet AHR activation appears to inhibit the development of monocytes into functioning macrophages (74). AHR ligands also functionally impact macrophages: macrophages exposed to cigarette smoke condensates are functionally impaired and exhibit impaired phagocytosis, TPA-induced H2O2 production, class II major histocompatibility complex expression, and nitric oxide synthesis (75). Similarly, macrophage exposure to another AHR toxicological agonist, benzo(a)pyrene, resulted in an impaired macrophage antigen presentation to T cells (76). Epidemiological studies suggest that exposure to toxicological AHR ligands such as dioxin may also influence megakaryocytic and/or platelet differentiation. In the early 1980’s, it was found that residents exposed to toxic waste containing TCDD, a known AHR ligand, had significantly higher mean platelet counts than residents that were not exposed (77). Similar studies examining Vietnam veterans who participated in Operation Ranch Hand found that those exposed to the highest levels of TCDD (found in Agent Orange) had increased platelet counts proportional to the dose they had received (78). The AHR signaling pathway also transcriptionaly regulates many important regulators of megakaryopoiesis, including Nrf2, a key activator of stress-responsive genes recently shown to compete with NF-E2 during megakaryocytic differentiation (79, 80). AHR also regulates survivin stability, a gene that is downregulated as an MEP differentiates toward the megakaryocytic, as opposed to erythrocytic, lineage (81). Treating rats with TCDD results in decreased platelet counts, although whether or not TCDD impacts megakaryocytic differentiation and development was less clear (82). In one set of experiments, dioxin treatment reduced platelet counts in rats without affecting megakaryocytic numbers or morphology (83), while further morphological examination of bone marrow smears suggested that megakaryocytes from TCDD-treated rats were reduced in number and degrading (84). In humans, perinatal exposure to dioxin results in decreased platelet counts inversely proportional to the amount of dioxin exposure (85). These contradictory findings suggest either that the effects of toxicological AHR ligands are context and developmental stage dependent, or that there is unknown cross talk between AHR and other signaling pathways. They also highlight the artificial nature of toxicological ligands, and may point to the need to identify endogenous ligands before the physiological role of AHR can be determined.
AHR-mediated Hes1 expression may transcriptionaly regulate cell cycle genes involved in megakaryocytic polyploidization
As part of an investigation of novel regulators of megakaryocytic differentiation, AHR-null mice were found to have ca. 25% fewer high ploidy (≥ 32n) megakaryocytes than WT mice, but more megakaryocytes in the lower ploidy classes of 8n and 16n (86). There are several possible mechanistic explanations for the decreased megakaryocytic polyploidization seen in these cells (Fig. 5B). The first is that reduced AHR expression in CHRF cells led to decreased cyclin D1 and cyclin E, similar to what was seen when AHR was knocked down in HepG2 cells (31). Another possibility is that AHR could regulate the aryl hydrocarbon receptor-interacting protein (AIP), which directly associates with and stabilizes survivin (87), could mediate survivin involvement in megakaryocytic polyploidization (88, 89). AHR may also regulate p21 (90), a cell cycle regulatory gene that has been implicated in megakaryocytic endomitosis (91, 92).
Another possibility involves Hes1, a hematopoietic transcription factor and AHR transcriptional target that is preferentially upregulated during megakaryocytic differentiation of primary CD34+ cells isolated from G-CSF mobilized peripheral blood (86). During megakaryocytic differentiation of the megakaryoblastic CHRF cell line AHR mRNA and protein levels increase, AHR translocates from the cytoplasm to the nucleus, and AHR DNA binding to a putative binding site within the promoter of Hes1 increased. Using RNAi-based approaches to knock down Hes1 in CHRF cells (a human megakaryoblastic leukemia cell line) resulted in ploidy distributions that were shifted towards lower ploidy classes and were incapable of reaching higher ploidy classes (i.e., ≥ 32n), similar to the effects of knocking down AHR (86). In addition to providing further evidence of a physiologic role for AHR during megakaryocytic differentiation, these findings demonstrate that Hes1 is downstream of AHR signaling during this process. Hes1 is a known cell cycle regulator (93, 94), and is expressed higher in erythroid-megakaryocytic progenitor cells than common myeloid progenitors or granulocyte-macrophage progenitor cells (95). Hes1 is also a critical effector of the Notch signaling cascade (96), a signaling pathway believed to regulate the initial endomitotic switch in Drosophila follicle cells by altering cell cycle regulation (97). Suggesting a model where Hes1 inhibits cyclin gene activity to impact cell cycle progression and megakaryocytic polyploidization, the ploidy defect exhibited in Hes1-knockdown CHRF cells is remarkably similar to the impact of knocking down cyclin E and cyclin D3, two genes known to influence megakaryocytic polyploidization (98, 99). Together, these data suggest that AHR-mediated regulation of Hes1 expression and transcriptional activity may influence the degree of polyploidization in differentiating megakaryocytes.
The AHR signaling pathway is necessary for normal immune function
Accumulating evidence also suggests that AHR signaling is important for mammalian immune system function (Fig. 5C). One of the first identifiable phenotypes found in AHR-null murine models was an impaired immune system. AHR-null mice have altered lymphocyte numbers and naïve T cells isolated from AHR-null mice are not as efficient in generating regulatory T cells (Tregs) in vitro as WT mice (100). AHR-activation by various toxicological ligands leads to autoimmune and allergic defects and limits the immune response in infectious disease. AHR signaling appears to suppress autoimmunity via induction of Tregs in response to TCDD (101). As reviewed in (102), TCDD affects immunocompetence, often at doses that do not produce obvious signs of toxicity. AHR may also impact functional immunity, as TCDD exposure results in increased inflammation and inhibits the CD8+ T-cell response to influenza infection in mice (103, 104). In addition, AHR signaling appears to be important to Treg function, as TCDD exposure generated Tregs and prevented graft-versus-host disease (105). AHR activation is also required for primary immunoglobulin-M (IgM) secretion of activated B cells against T cell-dependent and T cell-independent antigens under both in vivo and in vitro conditions (106).
A proposed model of AHR function as a mediator of cellular stress during hematological development
HSCs generally reside in acidic, hypoxic regions of the endosteum and AHR may protect hematopoietic cells from the increasing oxygen levels blood cells face as they differentiate (107, 108). Experimental evidence supports a model by which AHR aids HSCs in coping with the multitude of stresses they face as they differentiate toward the various blood cell lineages. As HSCs emerge from their quiescent state to undergo differentiation, they must adapt to large variations in metabolic activity, undergo dramatic morphological changes (e.g., polyploidization during megakaryocytic differentiation), and respond to (or protect themselves from) changing microenvironmental cues. The latter include changes in oxygen levels and ROS concentrations (16, 17, 107, 109, 110), pH (111–114) and exposure to mechanical forces as they cross endothelial lining of small blood vessels and shear forces when they enter the bloodstream (115–117).
Oxygen availability is a particularly critical component of the endosteal niche where HSCs reside and serves as an important hematopoietic regulator; as HSCs differentiate toward the various hematopoietic lineages, they prefer regions (and are localized within areas of) greater oxygenation (17, 107). The AHR and Hif1-α signaling pathways directly compete for the molecular chaperone Hif1-β (118), so one explanation for the increased proliferation of HSCs when AHR expression is diminished or is absent is that reduced AHR tips the transcriptional balance toward a hypoxic response mediated by Hif1-α. Because Hif1-α is naturally degraded in response to higher oxygen tension (119), one might expect that an external stimulus that induces cellular migration toward levels of higher would result in less Hif1-α bound to its nuclear chaperone, Hif1-β/ARNT. In this model, as more Hif1-α is degraded in response to the physiological oxygen gradient present in the bone marrow, unbound Hif1-β/ARNT accumulates and is accessible for binding with AHR, allowing for AHR signaling and differentiation toward the various blood lineages.
Acknowledgements
This work was supported by Award Numbers F32HL091620 (SL) and R21HL106397 (ETP) from the National Heart, Lung and Blood Institute of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institute or the National Institutes of Health.
Footnotes
Conflict of Interest Declaration:
The authors have no conflicts of interest to disclare.
References
- 1.Fukunaga BN, Probst MR, Reisz-Porszasz S, Hankinson O. Identification of functional domains of the aryl hydrocarbon receptor. J Biol Chem. 1995;270(49):29270–29278. doi: 10.1074/jbc.270.49.29270. [DOI] [PubMed] [Google Scholar]
- 2.Kumar MB, Ramadoss P, Reen RK, Vanden Heuvel JP, Perdew GH. The Q-rich subdomain of the human Ah receptor transactivation domain is required for dioxin-mediated transcriptional activity. J Biol Chem. 2001;276(45):42302–42310. doi: 10.1074/jbc.M104798200. [DOI] [PubMed] [Google Scholar]
- 3.Perdew GH. Association of the Ah receptor with the 90-kDa heat shock protein. J Biol Chem. 1988;263(27):13802–13805. [PubMed] [Google Scholar]
- 4.McGuire J, Whitelaw ML, Pongratz I, Gustafsson JA, Poellinger L. A cellular factor stimulates ligand-dependent release of hsp90 from the basic helix-loop-helix dioxin receptor. Mol Cell Biol. 1994;14(4):2438–2446. doi: 10.1128/mcb.14.4.2438. PMCID: 358611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denison MS, Fisher JM, Whitlock JP., Jr. The DNA recognition site for the dioxin-Ah receptor complex. Nucleotide sequence and functional analysis. J Biol Chem. 1988;263(33):17221–17224. [PubMed] [Google Scholar]
- 6.Davarinos NA, Pollenz RS. Aryl hydrocarbon receptor imported into the nucleus following ligand binding is rapidly degraded via the cytosplasmic proteasome following nuclear export. J Biol Chem. 1999;274(40):28708–28715. doi: 10.1074/jbc.274.40.28708. [DOI] [PubMed] [Google Scholar]
- 7.Evans BR, Karchner SI, Allan LL, Pollenz RS, Tanguay RL, Jenny MJ, et al. Repression of aryl hydrocarbon receptor (AHR) signaling by AHR repressor: role of DNA binding and competition for AHR nuclear translocator. Mol Pharmacol. 2008;73(2):387–398. doi: 10.1124/mol.107.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karchner SI, Franks DG, Powell WH, Hahn ME. Regulatory interactions among three members of the vertebrate aryl hydrocarbon receptor family: AHR repressor, AHR1, and AHR2. J Biol Chem. 2002;277(9):6949–6959. doi: 10.1074/jbc.M110779200. [DOI] [PubMed] [Google Scholar]
- 9.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273(5272):242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 10.Tenen DG, Hromas R, Licht JD, Zhang DE. Transcription factors, normal myeloid development, and leukemia. Blood. 1997;90(2):489–519. [PubMed] [Google Scholar]
- 11.Hromas R, Zon L, Friedman AD. Hematopoietic transcription regulators and the origins of leukemia. Crit Rev Oncol Hematol. 1992;12(2):167–190. doi: 10.1016/1040-8428(92)90088-8. [DOI] [PubMed] [Google Scholar]
- 12.Kumano K, Chiba S, Shimizu K, Yamagata T, Hosoya N, Saito T, et al. Notch1 inhibits differentiation of hematopoietic cells by sustaining GATA-2 expression. Blood. 2001;98(12):3283–3289. doi: 10.1182/blood.v98.12.3283. [DOI] [PubMed] [Google Scholar]
- 13.Shivdasani RA, Fujiwara Y, McDevitt MA, Orkin SH. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. Embo J. 1997;16(13):3965–3973. doi: 10.1093/emboj/16.13.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levesque JP, Winkler IG, Hendy J, Williams B, Helwani F, Barbier V, et al. Hematopoietic progenitor cell mobilization results in hypoxia with increased hypoxia-inducible transcription factor-1 alpha and vascular endothelial growth factor A in bone marrow. Stem Cells. 2007;25(8):1954–1965. doi: 10.1634/stemcells.2006-0688. [DOI] [PubMed] [Google Scholar]
- 15.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294(5548):1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 16.Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO(2) distributions in the bone marrow hematopoietic compartment. I. Krogh's model. Biophysical Journal. 2001;81(2):675–684. doi: 10.1016/S0006-3495(01)75732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO(2) distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophysical Journal. 2001;81(2):685–696. doi: 10.1016/S0006-3495(01)75733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jing D, Wobus M, Poitz DM, Bornhauser M, Ehninger G, Ordemann R. Oxygen tension plays a critical role in the hematopoietic microenvironment in vitro. Haematologica. 2011 doi: 10.3324/haematol.2011.050815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levesque JP, Winkler IG, Hendy J, Williams B, Helwani F, Barbier V, et al. Hematopoietic progenitor cell mobilization results in hypoxia with increased hypoxia-inducible transcription factor-1 alpha and vascular endothelial growth factor A in bone marrow. Stem Cells. 2007;25(8):1954–1965. doi: 10.1634/stemcells.2006-0688. [DOI] [PubMed] [Google Scholar]
- 20.Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7(3):380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7(3):391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Nebert DW. The Ah locus: genetic differences in toxicity, cancer, mutation, and birth defects. Crit Rev Toxicol. 1989;20(3):153–174. doi: 10.3109/10408448909017908. [DOI] [PubMed] [Google Scholar]
- 23.Singh KP, Wyman A, Casado FL, Garrett RW, Gasiewicz TA. Treatment of mice with the Ah receptor agonist and human carcinogen dioxin results in altered numbers and function of hematopoietic stem cells. Carcinogenesis. 2009;30(1):11–19. doi: 10.1093/carcin/bgn224. PMCID: 2639033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reyes-Hernandez OD, Mejia-Garcia A, Sanchez-Ocampo EM, Cabanas-Cortes MA, Ramirez P, Chavez-Gonzalez L, et al. Ube2l3 gene expression is modulated by activation of the aryl hydrocarbon receptor: implications for p53 ubiquitination. Biochem Pharmacol. 2010;80(6):932–940. doi: 10.1016/j.bcp.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denissenko MF, Pao A, Tang M, Pfeifer GP. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science. 1996;274(5286):430–432. doi: 10.1126/science.274.5286.430. [DOI] [PubMed] [Google Scholar]
- 26.Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21(48):7435–7451. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- 27.Glynn MF, Mustard JF, Buchanan MR, Murphy EA. Cigarette smoking and platelet aggregation. Can Med Assoc J. 1966;95(11):549–553. [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez FJ, Tukey RH, Nebert DW. Structural gene products of the Ah locus. Transcriptional regulation of cytochrome P1-450 and P3-450 mRNA levels by 3-methylcholanthrene. Mol Pharmacol. 1984;26(1):117–121. [PubMed] [Google Scholar]
- 29.Ge NL, Elferink CJ. A direct interaction between the aryl hydrocarbon receptor and retinoblastoma protein. Linking dioxin signaling to the cell cycle. J Biol Chem. 1998;273(35):22708–22713. doi: 10.1074/jbc.273.35.22708. [DOI] [PubMed] [Google Scholar]
- 30.Andrysik Z, Vondracek J, Machala M, Krcmar P, Svihalkova-Sindlerova L, Kranz A, et al. The aryl hydrocarbon receptor-dependent deregulation of cell cycle control induced by polycyclic aromatic hydrocarbons in rat liver epithelial cells. Mutat Res. 2007;615(1–2):87–97. doi: 10.1016/j.mrfmmm.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Abdelrahim M, Smith R, 3rd, Safe S. Aryl hydrocarbon receptor gene silencing with small inhibitory RNA differentially modulates Ah-responsiveness in MCF-7 and HepG2 cancer cells. Mol Pharmacol. 2003;63(6):1373–1381. doi: 10.1124/mol.63.6.1373. [DOI] [PubMed] [Google Scholar]
- 32.Hendrick JP, Hartl FU. Molecular chaperone functions of heat-shock proteins. Annual review of biochemistry. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- 33.Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf. 2006;64(2):178–189. doi: 10.1016/j.ecoenv.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 34.McHale CM, Zhang L, Smith MT. Current understanding of the mechanism of benzene-induced leukemia in humans: implications for risk assessment. Carcinogenesis. 2012;33(2):240–252. doi: 10.1093/carcin/bgr297. PMCID: 3271273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stohs SJ. Oxidative stress induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Free Radic Biol Med. 1990;9(1):79–90. doi: 10.1016/0891-5849(90)90052-k. [DOI] [PubMed] [Google Scholar]
- 36.Shertzer HG, Nebert DW, Puga A, Ary M, Sonntag D, Dixon K, et al. Dioxin causes a sustained oxidative stress response in the mouse. Biochem Biophys Res Commun. 1998;253(1):44–48. doi: 10.1006/bbrc.1998.9753. [DOI] [PubMed] [Google Scholar]
- 37.Vogel CF, Sciullo E, Matsumura F. Involvement of RelB in aryl hydrocarbon receptor-mediated induction of chemokines. Biochem Biophys Res Commun. 2007;363(3):722–726. doi: 10.1016/j.bbrc.2007.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fritsche E, Schafer C, Calles C, Bernsmann T, Bernshausen T, Wurm M, et al. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci U S A. 2007;104(21):8851–8856. doi: 10.1073/pnas.0701764104. PMCID: 1885591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin S, Wakabayashi N, Misra V, Biswal S, Lee GH, Agoston ES, et al. NRF2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis. Mol Cell Biol. 2007;27(20):7188–7197. doi: 10.1128/MCB.00915-07. PMCID: 2168916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu H, Cui W, Klaassen CD. Nrf2 protects against 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced oxidative injury and steatohepatitis. Toxicol Appl Pharmacol. 2011;256(2):122–135. doi: 10.1016/j.taap.2011.07.019. PMCID: 3183285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma Q, Whitlock JP., Jr. The aromatic hydrocarbon receptor modulates the Hepa 1c1c7 cell cycle and differentiated state independently of dioxin. Mol Cell Biol. 1996;16(5):2144–2150. doi: 10.1128/mcb.16.5.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elizondo G, Fernandez-Salguero P, Sheikh MS, Kim GY, Fornace AJ, Lee KS, et al. Altered cell cycle control at the G(2)/M phases in aryl hydrocarbon receptor-null embryo fibroblast. Mol Pharmacol. 2000;57(5):1056–1063. [PubMed] [Google Scholar]
- 43.Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329(5997):1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mulero-Navarro S, Carvajal-Gonzalez JM, Herranz M, Ballestar E, Fraga MF, Ropero S, et al. The dioxin receptor is silenced by promoter hypermethylation in human acute lymphoblastic leukemia through inhibition of Sp1 binding. Carcinogenesis. 2006;27(5):1099–1104. doi: 10.1093/carcin/bgi344. [DOI] [PubMed] [Google Scholar]
- 45.Hahn ME. Aryl hydrocarbon receptors: diversity and evolution. Chem Biol Interact. 2002;141(1–2):131–160. doi: 10.1016/s0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 46.Powell-Coffman JA, Bradfield CA, Wood WB. Caenorhabditis elegans orthologs of the aryl hydrocarbon receptor and its heterodimerization partner the aryl hydrocarbon receptor nuclear translocator. Proc Natl Acad Sci U S A. 1998;95(6):2844–2849. doi: 10.1073/pnas.95.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duncan DM, Burgess EA, Duncan I. Control of distal antennal identity and tarsal development in Drosophila by spineless-aristapedia, a homolog of the mammalian dioxin receptor. Genes Dev. 1998;12(9):1290–1303. doi: 10.1101/gad.12.9.1290. PMCID: 316766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wernet MF, Mazzoni EO, Celik A, Duncan DM, Duncan I, Desplan C. Stochastic spineless expression creates the retinal mosaic for colour vision. Nature. 2006;440(7081):174–180. doi: 10.1038/nature04615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emmons RB, Duncan D, Duncan I. Regulation of the Drosophila distal antennal determinant spineless. Dev Biol. 2007;302(2):412–426. doi: 10.1016/j.ydbio.2006.09.044. PMCID: 1876787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qin H, Powell-Coffman JA. The Caenorhabditis elegans aryl hydrocarbon receptor, AHR-1, regulates neuronal development. Dev Biol. 2004;270(1):64–75. doi: 10.1016/j.ydbio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93(13):6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268(5211):722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 53.Mimura J, Yamashita K, Nakamura K, Morita M, Takagi TN, Nakao K, et al. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells. 1997;2(10):645–654. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- 54.Benedict JC, Lin TM, Loeffler IK, Peterson RE, Flaws JA. Physiological role of the aryl hydrocarbon receptor in mouse ovary development. Toxicol Sci. 2000;56(2):382–388. doi: 10.1093/toxsci/56.2.382. [DOI] [PubMed] [Google Scholar]
- 55.Fernandez-Salguero PM, Ward JM, Sundberg JP, Gonzalez FJ. Lesions of arylhydrocarbon receptor-deficient mice. Vet Pathol. 1997;34(6):605–614. doi: 10.1177/030098589703400609. [DOI] [PubMed] [Google Scholar]
- 56.Lahvis GP, Lindell SL, Thomas RS, McCuskey RS, Murphy C, Glover E, et al. Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice. Proc Natl Acad Sci U S A. 2000;97(19):10442–10447. doi: 10.1073/pnas.190256997. PMCID: 27043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walisser JA, Bunger MK, Glover E, Bradfield CA. Gestational exposure of Ahr and Arnt hypomorphs to dioxin rescues vascular development. Proc Natl Acad Sci U S A. 2004;101(47):16677–16682. doi: 10.1073/pnas.0404379101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walisser JA, Bunger MK, Glover E, Harstad EB, Bradfield CA. Patent ductus venosus and dioxin resistance in mice harboring a hypomorphic Arnt allele. J Biol Chem. 2004;279(16):16326–16331. doi: 10.1074/jbc.M400784200. [DOI] [PubMed] [Google Scholar]
- 59.Chang CY, Puga A. Constitutive activation of the aromatic hydrocarbon receptor. Mol Cell Biol. 1998;18(1):525–535. doi: 10.1128/mcb.18.1.525. PMCID: 121520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okey AB. An aryl hydrocarbon receptor odyssey to the shores of toxicology: the Deichmann Lecture, International Congress of Toxicology-XI. Toxicol Sci. 2007;98(1):5–38. doi: 10.1093/toxsci/kfm096. [DOI] [PubMed] [Google Scholar]
- 61.Stejskalova L, Dvorak Z, Pavek P. Endogenous and exogenous ligands of aryl hydrocarbon receptor: current state of art. Curr Drug Metab. 2011;12(2):198–212. doi: 10.2174/138920011795016818. [DOI] [PubMed] [Google Scholar]
- 62.Yoon BI, Hirabayashi Y, Kawasaki Y, Kodama Y, Kaneko T, Kanno J, et al. Aryl hydrocarbon receptor mediates benzene-induced hematotoxicity. Toxicol Sci. 2002;70(1):150–156. doi: 10.1093/toxsci/70.1.150. [DOI] [PubMed] [Google Scholar]
- 63.Garrett RW, Gasiewicz TA. The aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin alters the circadian rhythms, quiescence, and expression of clock genes in murine hematopoietic stem and progenitor cells. Mol Pharmacol. 2006;69(6):2076–2083. doi: 10.1124/mol.105.021006. [DOI] [PubMed] [Google Scholar]
- 64.Jetmore A, Plett PA, Tong X, Wolber FM, Breese R, Abonour R, et al. Homing efficiency, cell cycle kinetics, and survival of quiescent and cycling human CD34(+) cells transplanted into conditioned NOD/SCID recipients. Blood. 2002;99(5):1585–1593. doi: 10.1182/blood.v99.5.1585. [DOI] [PubMed] [Google Scholar]
- 65.Gasiewicz TA, Singh KP, Casado FL. The aryl hydrocarbon receptor has an important role in the regulation of hematopoiesis: implications for benzene-induced hematopoietic toxicity. Chem Biol Interact. 2010;184(1–2):246–251. doi: 10.1016/j.cbi.2009.10.019. PMCID: 2846208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh KP, Casado FL, Opanashuk LA, Gasiewicz TA. The aryl hydrocarbon receptor has a normal function in the regulation of hematopoietic and other stem/progenitor cell populations. Biochem Pharmacol. 2008 doi: 10.1016/j.bcp.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh KP, Garrett RW, Casado FL, Gasiewicz TA. Aryl hydrocarbon receptor-null allele mice have hematopoietic stem/progenitor cells with abnormal characteristics and functions. Stem Cells Dev. 2011;20(5):769–784. doi: 10.1089/scd.2010.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benisek M, Kubincova P, Blaha L, Hilscherova K. The effects of PAHs and N-PAHs on retinoid signaling and Oct-4 expression in vitro. Toxicol Lett. 2011;200(3):169–175. doi: 10.1016/j.toxlet.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 69.Bunaciu RP, Yen A. Activation of the aryl hydrocarbon receptor AhR Promotes retinoic acid-induced differentiation of myeloblastic leukemia cells by restricting expression of the stem cell transcription factor Oct4. Cancer Res. 2011;71(6):2371–2380. doi: 10.1158/0008-5472.CAN-10-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol. 2007;25(10):1177–1181. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- 71.Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95(3):379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 72.Hayashi S, Okabe-Kado J, Honma Y, Kawajiri K. Expression of Ah receptor (TCDD receptor) during human monocytic differentiation. Carcinogenesis. 1995;16(6):1403–1409. doi: 10.1093/carcin/16.6.1403. [DOI] [PubMed] [Google Scholar]
- 73.Crawford RB, Holsapple MP, Kaminski NE. Leukocyte activation induces aryl hydrocarbon receptor up-regulation, DNA binding, and increased Cyp1a1 expression in the absence of exogenous ligand. Mol Pharmacol. 1997;52(6):921–927. doi: 10.1124/mol.52.6.921. [DOI] [PubMed] [Google Scholar]
- 74.van Grevenynghe J, Rion S, Le Ferrec E, Le Vee M, Amiot L, Fauchet R, et al. Polycyclic aromatic hydrocarbons inhibit differentiation of human monocytes into macrophages. J Immunol. 2003;170(5):2374–2381. doi: 10.4049/jimmunol.170.5.2374. [DOI] [PubMed] [Google Scholar]
- 75.Braun KM, Cornish T, Valm A, Cundiff J, Pauly JL, Fan S. Immunotoxicology of cigarette smoke condensates: suppression of macrophage responsiveness to interferon gamma. Toxicol Appl Pharmacol. 1998;149(2):136–143. doi: 10.1006/taap.1997.8346. [DOI] [PubMed] [Google Scholar]
- 76.Myers MJ, Schook LB, Bick PH. Mechanisms of benzo(a)pyrene-induced modulation of antigen presentation. J Pharmacol Exp Ther. 1987;242(2):399–404. [PubMed] [Google Scholar]
- 77.Webb K, Evans RG, Stehr P, Ayres SM. Pilot study on health effects of environmental 2,3,7,8-TCDD in Missouri. American journal of industrial medicine. 1987;11(6):685–691. doi: 10.1002/ajim.4700110609. [DOI] [PubMed] [Google Scholar]
- 78.Michalek JE, Akhtar FZ, Longnecker MP, Burton JE. Relation of serum 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) level to hematological examination results in veterans of Operation Ranch Hand. Arch Environ Health. 2001;56(5):396–405. doi: 10.1080/00039890109604474. [DOI] [PubMed] [Google Scholar]
- 79.Motohashi H, Kimura M, Fujita R, Inoue A, Pan X, Takayama M, et al. NF-E2 domination over Nrf2 promotes ROS accumulation and megakaryocytic maturation. Blood. 2010;115(3):677–686. doi: 10.1182/blood-2009-05-223107. PMCID: 2810977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miao W, Hu L, Scrivens PJ, Batist G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: direct cross-talk between phase I and II drug-metabolizing enzymes. J Biol Chem. 2005;280(21):20340–20348. doi: 10.1074/jbc.M412081200. [DOI] [PubMed] [Google Scholar]
- 81.Vargiolu M, Fusco D, Kurelac I, Dirnberger D, Baumeister R, Morra I, et al. The tyrosine kinase receptor RET interacts in vivo with aryl hydrocarbon receptor-interacting protein to alter survivin availability. J Clin Endocrinol Metab. 2009;94(7):2571–2578. doi: 10.1210/jc.2008-1980. [DOI] [PubMed] [Google Scholar]
- 82.Zinkl JG, Vos JG, Moore JA, Gupta BN. Hematologic and clinical chemistry effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in laboratory animals. Environ Health Perspect. 1973;5:111–118. doi: 10.1289/ehp.7305111. PMCID: 1474973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weissberg JB, Zinkl JG. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin upon hemostasis and hematologic function in the rat. Environ Health Perspect. 1973;5:119–123. doi: 10.1289/ehp.7305119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gupta BN, Vos JG, Moore JA, Zinkl JG, Bullock BC. Pathologic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in laboratory animals. Environ Health Perspect. 1973;5:125–140. doi: 10.1289/ehp.7305125. PMCID: 1474959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pluim HJ, Koppe JG, Olie K, van der Slikke JW, Slot PC, van Boxtel CJ. Clinical laboratory manifestations of exposure to background levels of dioxins in the perinatal period. Acta Paediatr. 1994;83(6):583–587. doi: 10.1111/j.1651-2227.1994.tb13086.x. [DOI] [PubMed] [Google Scholar]
- 86.Lindsey S, Papoutsakis ET. The aryl hydrocarbon receptor (AHR) transcription factor regulates megakaryocytic polyploidization. British journal of haematology. 2011;152(4):469–484. doi: 10.1111/j.1365-2141.2010.08548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kang BH, Altieri DC. Regulation of survivin stability by the aryl hydrocarbon receptor-interacting protein. J Biol Chem. 2006;281(34):24721–24727. doi: 10.1074/jbc.M603175200. [DOI] [PubMed] [Google Scholar]
- 88.Wen Q, Leung C, Huang Z, Small S, Reddi AL, Licht JD, et al. Survivin is not required for the endomitotic cell cycle of megakaryocytes. Blood. 2009;114(1):153–156. doi: 10.1182/blood-2008-11-190801. PMCID: 2710943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McCrann DJ, Ravid K. Survivin localization during endomitosis of high ploidy mouse megakaryocytes. Blood. 2010;116(12):2192–2193. doi: 10.1182/blood-2010-04-280420. PMCID: 2951860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pang PH, Lin YH, Lee YH, Hou HH, Hsu SP, Juan SH. Molecular mechanisms of p21 and p27 induction by 3-methylcholanthrene, an aryl-hydrocarbon receptor agonist, involved in antiproliferation of human umbilical vascular endothelial cells. J Cell Physiol. 2008;215(1):161–171. doi: 10.1002/jcp.21299. [DOI] [PubMed] [Google Scholar]
- 91.Baccini V, Roy L, Vitrat N, Chagraoui H, Sabri S, Le Couedic JP, et al. Role of p21(Cip1/Waf1) in cell-cycle exit of endomitotic megakaryocytes. Blood. 2001;98(12):3274–3282. doi: 10.1182/blood.v98.12.3274. [DOI] [PubMed] [Google Scholar]
- 92.Albanese P, Chagraoui J, Charon M, Cocault L, Dusanter-Fourt I, Romeo PH, et al. Forced expression of p21 in GPIIb-p21 transgenic mice induces abnormalities in the proliferation of erythroid and megakaryocyte progenitors and primitive hematopoietic cells. Exp Hematol. 2002;30(11):1263–1272. doi: 10.1016/s0301-472x(02)00933-5. [DOI] [PubMed] [Google Scholar]
- 93.Murata K, Hattori M, Hirai N, Shinozuka Y, Hirata H, Kageyama R, et al. Hes1 directly controls cell proliferation through the transcriptional repression of p27Kip1. Mol Cell Biol. 2005;25(10):4262–4271. doi: 10.1128/MCB.25.10.4262-4271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kabos P, Kabosova A, Neuman T. Blocking HES1 expression initiates GABAergic differentiation and induces the expression of p21(CIP1/WAF1) in human neural stem cells. J Biol Chem. 2002;277(11):8763–8766. doi: 10.1074/jbc.C100758200. [DOI] [PubMed] [Google Scholar]
- 95.Mercher T, Cornejo MG, Sears C, Kindler T, Moore SA, Maillard I, et al. Notch signaling specifies megakaryocyte development from hematopoietic stem cells. Cell Stem Cell. 2008;3(3):314–326. doi: 10.1016/j.stem.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Delaney C, Varnum-Finney B, Aoyama K, Brashem-Stein C, Bernstein ID. Dose-dependent effects of the Notch ligand Delta1 on ex vivo differentiation and in vivo marrow repopulating ability of cord blood cells. Blood. 2005;106(8):2693–2699. doi: 10.1182/blood-2005-03-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deng WM, Althauser C, Ruohola-Baker H. Notch-Delta signaling induces a transition from mitotic cell cycle to endocycle in Drosophila follicle cells. Development. 2001;128(23):4737–4746. doi: 10.1242/dev.128.23.4737. [DOI] [PubMed] [Google Scholar]
- 98.Wang Z, Zhang Y, Kamen D, Lees E, Ravid K. Cyclin D3 is essential for megakaryocytopoiesis. Blood. 1995;86(10):3783–3788. [PubMed] [Google Scholar]
- 99.Geng Y, Yu Q, Sicinska E, Das M, Schneider JE, Bhattacharya S, et al. Cyclin E ablation in the mouse. Cell. 2003;114(4):431–443. doi: 10.1016/s0092-8674(03)00645-7. [DOI] [PubMed] [Google Scholar]
- 100.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci U S A. 2008;105(28):9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 102.Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology. 2009;127(3):299–311. doi: 10.1111/j.1365-2567.2009.03054.x. PMCID: 2712099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Neff-LaFord H, Teske S, Bushnell TP, Lawrence BP. Aryl hydrocarbon receptor activation during influenza virus infection unveils a novel pathway of IFN-gamma production by phagocytic cells. J Immunol. 2007;179(1):247–255. doi: 10.4049/jimmunol.179.1.247. [DOI] [PubMed] [Google Scholar]
- 104.Mitchell KA, Lawrence BP. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) renders influenza virus-specific CD8+ T cells hyporesponsive to antigen. Toxicol Sci. 2003;74(1):74–84. doi: 10.1093/toxsci/kfg110. [DOI] [PubMed] [Google Scholar]
- 105.Marshall NB, Vorachek WR, Steppan LB, Mourich DV, Kerkvliet NI. Functional characterization and gene expression analysis of CD4+ CD25+ regulatory T cells generated in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Immunol. 2008;181(4):2382–2391. doi: 10.4049/jimmunol.181.4.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sulentic CE, Holsapple MP, Kaminski NE. Aryl hydrocarbon receptor-dependent suppression by 2,3,7, 8-tetrachlorodibenzo-p-dioxin of IgM secretion in activated B cells. Mol Pharmacol. 1998;53(4):623–629. [PubMed] [Google Scholar]
- 107.Mostafa SS, Miller WM, Papoutsakis ET. Oxygen tension influences the differentiation, maturation and apoptosis of human megakaryocytes. British journal of haematology. 2000;111(3):879–889. [PubMed] [Google Scholar]
- 108.Levesque JP, Helwani FM, Winkler IG. The endosteal 'osteoblastic' niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010;24(12):1979–1992. doi: 10.1038/leu.2010.214. [DOI] [PubMed] [Google Scholar]
- 109.Mostafa SS, Papoutsakis ET, Miller WM. Oxygen tension modulates the expression of cytokine receptors, transcription factors, and lineage-specific markers in cultured human megakaryocytes. Exp Hematol. 2001;29(7):873–883. doi: 10.1016/s0301-472x(01)00658-0. [DOI] [PubMed] [Google Scholar]
- 110.Ishikawa Y, Ito T. Kinetics of hemopoietic stem cells in a hypoxic culture. Eur J Haematol. 1988;40(2):126–129. doi: 10.1111/j.1600-0609.1988.tb00808.x. [DOI] [PubMed] [Google Scholar]
- 111.Hevehan DL, Papoutsakis ET, Miller WM. Physiologically significant effects of pH and oxygen tension on granulopoiesis. Exp Hematol. 2000;28(3):267–275. doi: 10.1016/s0301-472x(99)00150-2. [DOI] [PubMed] [Google Scholar]
- 112.McAdams TA, Miller WM, Papoutsakis ET. Variations in culture pH affect the cloning efficiency and differentiation of progenitor cells in ex vivo haemopoiesis. British journal of haematology. 1997;97(4):889–895. doi: 10.1046/j.1365-2141.1997.1372951.x. [DOI] [PubMed] [Google Scholar]
- 113.McAdams TA, Miller WM, Papoutsakis ET. pH is a potent modulator of erythroid differentiation. British journal of haematology. 1998;103(2):317–325. doi: 10.1046/j.1365-2141.1998.00975.x. [DOI] [PubMed] [Google Scholar]
- 114.Yang H, Miller WM, Papoutsakis ET. Higher pH promotes megakaryocytic maturation and apoptosis. Stem Cells. 2002;20(4):320–328. doi: 10.1634/stemcells.20-4-320. [DOI] [PubMed] [Google Scholar]
- 115.Dunois-Larde C, Capron C, Fichelson S, Bauer T, Cramer-Borde E, Baruch D. Exposure of human megakaryocytes to high shear rates accelerates platelet production. Blood. 2009;114(9):1875–1883. doi: 10.1182/blood-2009-03-209205. [DOI] [PubMed] [Google Scholar]
- 116.Collins PC, Nielsen LK, Patel SD, Papoutsakis ET, Miller WM. Characterization of hematopoietic cell expansion, oxygen uptake, and glycolysis in a controlled, stirred-tank bioreactor system. Biotechnol Prog. 1998;14(3):466–472. doi: 10.1021/bp980032e. [DOI] [PubMed] [Google Scholar]
- 117.Konstantopoulos K, Kukreti S, McIntire LV. Biomechanics of cell interactions in shear fields. Adv Drug Deliv Rev. 1998;33(1–2):141–164. doi: 10.1016/s0169-409x(98)00024-6. [DOI] [PubMed] [Google Scholar]
- 118.Chan WK, Yao G, Gu YZ, Bradfield CA. Cross-talk between the aryl hydrocarbon receptor and hypoxia inducible factor signaling pathways. Demonstration of competition and compensation. J Biol Chem. 1999;274(17):12115–12123. doi: 10.1074/jbc.274.17.12115. [DOI] [PubMed] [Google Scholar]
- 119.Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272(36):22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]