Summary
Impaired consciousness in epilepsy has a major negative impact on quality of life. Prior work suggests that complex partial seizures (CPS) and generalized tonic-clonic seizures (GTCS), which both cause loss of consciousness, affect similar fronto-parietal networks. Milder involvement in CPS than in GTCS may spare some simple behavioral responses, resembling the minimally conscious state. However, this difference in responses has not been rigorously tested previously. During video/EEG monitoring, we administered a standardized prospective testing battery including responses to questions and commands, as well as tests for reaching/grasping a ball and visual tracking in 27 CPS (14 patients) and 7 GTCS (6 patients). Behavioral results were analyzed in the ictal and post-ictal periods based on video review. During both CPS and GTCS, patients were unable to respond to questions or commands. However, during CPS patients often retain minimally conscious ball grasping and visual tracking responses. Patients were able to successfully grasp a ball in 60% or to visually track in 58% of CPS, and could carry out both activities in 52% of CPS. In contrast, during GTCS preserved ball grasp (10%), visual tracking (11%) or both (7%) were all significantly less than in CPS. Post-ictal ball grasping and visual tracking were also somewhat better following CPS than GTCS. These findings suggest that impaired consciousness in CPS is more similar to minimally conscious state than to coma. Further work may elucidate the specific brain networks underlying relatively spared functions in CPS, ultimately leading to improved treatments aimed at preventing impaired consciousness.
Keywords: Consciousness, Epilepsy, Complex Partial Seizures, Generalized Tonic-Clonic Seizures, Visual Tracking, Minimally Conscious State, Vegetative State
Introduction
Impaired consciousness drastically alters normal human quality of life. In coma, patients show no meaningful reaction to their surroundings while in other disorders of consciousness, patients may exhibit some simple responses (Laureys & Tononi 2009). For example, vegetative state patients can open their eyes and turn towards stimuli but do not show visual tracking or respond appropriately to questions or commands; minimally conscious state patients show simple responses such as ball grasping and visual tracking but do not show consistent interactive communication or object use (Giacino, et al. 2002). Epileptic seizures also cause impaired consciousness, which vary in severity. In generalized tonic-clonic seizures (GTCS) there is profound impairment, transiently resembling coma (Blumenfeld, et al. 2009). However, in other seizures milder deficits may be seen. In commonly-used terminology (ILAE 1981), partial seizures without impaired consciousness are called simple partial, while those with impaired consciousness are called complex partial seizures (CPS). Determining which specific behavioral functions are impaired and spared in CPS may help elucidate the anatomical networks underlying impaired consciousness in CPS, leading ultimately to improved treatments. However, the precise nature of impairment during CPS has not been thoroughly investigated. Specifically, it is not known whether CPS more closely resembles a transient vegetative or minimally conscious state. Therefore, we prospectively evaluated patients’ ability to perform simple tasks including ball grasping and visual tracking with a standardized testing battery (Yang, et al. 2012) in CPS as well as GTCS during inpatient video/EEG monitoring.
This objective method of assessing impaired consciousness is significant in that further study could allow for greater understanding of minimally conscious capacities during CPS. Additionally, this could assist in functional mapping of specific neurobiological substrates of consciousness when correlated with seizure localization based on neuroimaging and EEG findings (Blumenfeld 2011).
Methods
All procedures were approved by the institutional review board at Yale University School of Medicine and participants gave written informed consent. We recruited inpatients at Yale-New Haven Hospital undergoing epilepsy video/electroencephalogram (EEG) monitoring from June 2009 through July 2011. Inclusion criteria were: age 7 years or older and the ability to follow simple questions and commands at baseline. Exclusion criterion was the presence of non-epileptic seizures.
As described previously, trained examiners sat with patients during their hospitalization for an average of 27 hours per patient, and immediately after seizure onset commenced a standard behavioral testing battery, the Responsiveness in Epilepsy Scale (RES) (Yang et al., 2012). The initial items of RES test ability to respond to verbal questions and commands. Patients who failed these were tested on ability to grasp a tennis ball placed on the dorsal surface of each hand while being told “take the ball,” and on visual tracking, being told “look at the mirror” as a mirror was moved slowly to the left and right in front of the face. Testing was repeated during the ictal and post-ictal periods until patients returned to their baseline level of behavioral performance (Yang et al., 2012).
Analysis was performed based on review of video/EEG recordings by consensus of two reviewers. Ability to grasp the ball with at least one hand or to visually track in at least one direction (left or right) were considered successful responses. As in prior work (Giacino, et al. 2004), to attain a correct grasp response we required that the wrist must rotate and the fingers extend as the ball is moved along the dorsal surface of the hand, and the ball must be held without dropping it; to attain correct visual tracking, eyes must follow the mirror for 45 degrees in one direction without loss of fixation. Mean success rate per seizure was calculated across repeated administrations of each test and analyzed separately during the ictal and post-ictal periods. Seizures types were classified based on video/EEG review using the 1981 ILAE criteria (ILAE 1981).
Results
Testing was initiated in a total of 84 seizures (24 patients). Patients failed to respond to questions and commands in 34 seizures, of which 27 were CPS (14 patients, 5 male) and 7 were GTCS (6 patients, 4 male) (Table 1). All tested seizures had a focal onset, including the GTCS, which were secondarily generalized. Absence seizures were not observed in this patient sample. Mean patient ages for CPS and GTCS respectively were 38.7 ± 3.7 and 29.1 ± 3.3 years (mean ± SEM). Mean seizure durations respectively were 116 ± 23.2 and 209.7 ± 75.1 s. Ball grasping was tested ictally a mean of 3.6 ± 0.6 times per seizure and visual tracking 3.2 ± 0.5 times per seizure. In the post-ictal period, ball grasping was tested 7.7 ± 2.2 and visual tracking 6.8 ± 2.2 times per seizure.
Table 1.
Clinical information for patients tested.
| Pt | age, sex | # Szs, type | MRI | PET Hypo- metabolism | Ictal SPECT Hyper- metabolism | Scalp EEG Onset* | Intracranial EEG Onset* | Overall localization |
|---|---|---|---|---|---|---|---|---|
| 1 | 51M | 3 CPS | L T atrophy and polymicrogyria | Normal. | L F | L > R H | Mostly R inf F-T, but some Bi or L T | Unlocalized |
| 2 | 27M | 3 CPS | R HC atrophy and L T gray matter abnormal | L T | LT and RO | L T and R T | N/A | RO and LT |
| 3 | 18F | 2 CPS,1 GTCS | Normal | L T | L T | Bi T | R > L mes T | Bi T |
| 4 | 27M | 1 GTCS | Normal | L T | N/A | L H | L F | LF |
| 5 | 21M | 1 GTCS | Normal | N/A | N/A | L F | N/A | LF |
| 6 | 42M | 2 CPS | Previous L T resection | R lat T | R T | R > L T | R mes T, and R T-O | R T, O |
| 7 | 58F | 1 CPS | Increased signal Bi HC and RP | N/A | N/A | Bi T | N/A | Bi T |
| 8 | 30F | 1 CPS | R F cortical thickening | R F | N/A | R F | R F | RF |
| 9 | 24F | 2 CPS | L T heterotopia | N/A | N/A | Bi F | N/A | Unlocalized |
| 10 | 33F | 1 GTCS | Encephalomalacia, R T-P-O | R T-P-O | N/A | R hemisphere | R F, O | R H |
| 11 | 51M | 3 CPS | Normal | Bi T | N/A | L H | Multifocal | Unlocalized |
| 12 | 21F | 2 CPS | Previous R F resection | Normal | N/A | Multifocal | N/A | Unlocalized |
| 13 | 40M | 1 GTCS | Normal | N/A | RT | R T | N/A | R T |
| 14 | 33M | 2 GTCS | Bi R>L HC atrophy | Normal | N/A | Right temporo-parietal | N/A | R H |
| 15 | 24F | 1 CPS | Normal | Bi T | N/A | L F, T | L T | L T |
| 16 | 29F | 2 CPS | R HC atrophy, R O indistinct sulci | R T | N/A | R T | R H diffuse onset | R H |
| 17 | 59M | 1 CPS | R HC atrophy, R F, P infarct | RT, and in area of R F, P infarct | N/A | R T | N/A | RT |
| 18 | 38F | 3 CPS | Normal | mild R T | N/A | L H | N/A | L H |
| 19 | 43F | 1 CPS | Previous L T resection | Normal | N/A | L T | L HC, L fusiform gyrus | L HC, L fusiform gyrus |
Based on overall EEG of all seizures, not just seizure occurring during RES.
Abbreviations: HC=hippocampal, R=right, L=left, O=occipital, T=temporal, P=parietal, F=frontal, Inf=inferior, Lat=lateral, Mes = mesial, Bi=bilateral, H=hemisphere, GTCS=generalized tonic-clonic seizure, CPS=complex partial seizure, szs=seizures, Pt=patient.
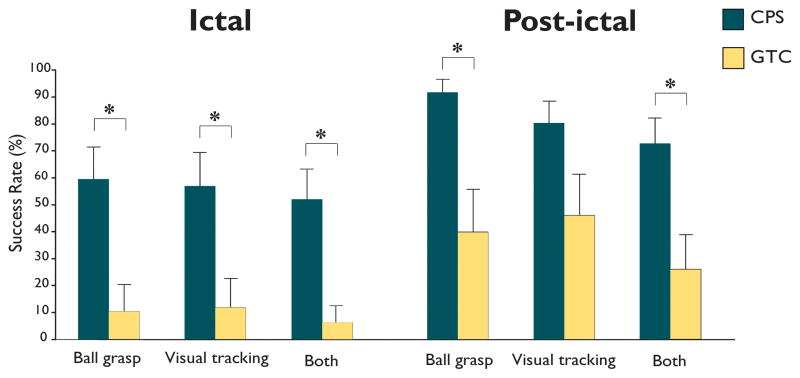
We found that ball grasping and visual tracking were spared over half the time in complex partial seizures, but uncommonly in generalized tonic-clonic seizures. During CPS, the mean success rate for ball grasping was 60 ± 12%, the mean success rate for visual tracking was 58 ± 12 %, and for both 52 ± 11 % (Figure 1). These were significantly better than during GTCS where mean success rate for ball grasping was only 10 ± 10 %, for visual tracking 11 ± 11% and for both 7 ± 7 % (all p<0.05, t-test). In the post-ictal period, ball grasping and visual tracking were also somewhat better following CPS than GTCS (Figure 1).
Figure 1.
Evidence of minimal consciousness during CPS. Ictal mean success rates were over 50% on the ball grasping and visual tracking tasks in complex partial seizures (CPS). This was significantly higher than during generalized tonic-clonic seizures (GTCS). Post-ictal success rates on these tasks were also somewhat higher following CPS than GTCS. Results for success in both tasks in the same testing cycle (Both) were also significantly better in CPS than GTCS. * = p<0.05
In patients who had multiple seizures (Table 1) performance was relatively consistent. Thus, within-patient standard deviation for ictal success rate was 11.7% on average, and a majority of these 10 patients had at least two seizures with identical success rates. Of note, performance did not wax and wane, but rather reached maximal impairment and then progressively recovered in the postictal period. In only a minority of testing items (~5%) did a patient have worse performance than previously during that same postictal period and, among these cases, all lasted only a single testing iteration.
It was recently found that blink to visual threat may be spared in some patients who otherwise meet all criteria for the vegetative state (Vanhaudenhuyse, et al. 2008). We tested ictal blink to visual threat in a limited number of patients and found that it was spared in 3 of 4 CPS, yet spared in no (0 of 7) GTCS (p=0.02, Fisher’s exact test).
Discussion
The results described here demonstrate that patients can often exhibit simple responses such as ball grasping and visual tracking during CPS. In contrast, these responses were usually absent in GTCS. For the single patient in whom these behaviors were preserved during a GTCS, the responses were made as the seizure evolved from generalized to limited unilateral involvement. These findings support prior speculation that CPS more closely resemble a transient minimally conscious state, rather than more severe disorders of consciousness such as coma or vegetative state (Blumenfeld 2011). Patients in coma or vegetative state do not exhibit visual tracking or reaching and grasping, nor do they have automatisms which can be seen in the minimally conscious state and CPS (Giacino et al., 2002).
Recent advances in neuroimaging have identified important anatomical networks underlying disorders of consciousness (Laureys & Tononi 2009). Interestingly, the same regions of fronto-parietal association cortex and subcortical arousal circuits which are dysfunctional in coma, vegetative state and minimally conscious state are often transiently impaired in epileptic seizures (Blumenfeld 2011). When patients improve from vegetative to minimally conscious state, increased activity has been observed in these same networks. (Laureys, et al. 2008) With further work, it may be possible to determine if spared minimal conscious responses in CPS are associated with less severely impaired fronto-parietal function in comparison with GTCS. Indeed, prior work suggests that while neocortical blood flow and electrical activity shows significant abnormal increases in GTCS, neocortical activity is moderately decreased in CPS (Blumenfeld et al., 2009; Englot, et al., 2010; Blumenfeld, 2011). Other factors including unilateral vs. bilateral or dominant vs. non-dominant hemispheric involvement could also participate in the degree of impairment and should be investigated further.
In previous studies, “reactive automatisms” have been described in CPS in which patients display simple automatic responses to the environment (Escueta, et al. 1982). The present work adds to these observations by using standardized testing to prospectively quantify the frequency of preserved minimal consciousness in CPS. Further work is needed to identify the anatomical and physiological basis of spared and impaired cognitive function during different seizure types. Hopefully, better understanding of these mechanisms will ultimately lead to new improved treatments for preventing impaired consciousness and its consequences.
Acknowledgments
We thank Abhijeet Gummadavelli and Asht Mishra for the useful comments on the manuscript, and all of the undergraduate volunteers that performed RES examinations. This project was supported by The Patrick and Catherine Weldon Donaghue Medical Research Foundation and the Betsy and Jonathan Blattmachr family. Alison McPherson was supported by the Connecticut College CELS Program, Leticia Rojas by the National Heart Lung and Blood Institute of the NIH, Joshua Matelow by NIH TG T32GM07205, and Andrew Bauerschmidt by the Doris Duke Charitable Foundation.
Footnotes
Financial Disclosure Statement:
None of the authors have financial disclosures to make.
Disclosure of Conflicts of Interest
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. None of the authors has any conflict of interest to disclose.
References
- Blumenfeld H. Epilepsy and the consciousness system: transient vegetative state? Neurol Clin. 2011;29:801–823. doi: 10.1016/j.ncl.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H, Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, Levin AR, Hirsch LJ, Tikofsky R, Zubal IG, Paige AL, Spencer SS. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain. 2009;132:999–1012. doi: 10.1093/brain/awp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escueta AV, Bacsal FE, Treiman DM. Complex partial seizures on closed-circuit television and EEG: a study of 691 attacks in 79 patients. Ann Neurol. 1982;11:292–300. doi: 10.1002/ana.410110310. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Ashwal S, Childs N, Cranford R, Jennett B, Katz DI, Kelly JP, Rosenberg JH, Whyte J, Zafonte RD, Zasler ND. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58:349–353. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85:2020–2029. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- ILAE. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- Laureys S, Boly M, Schnakers C, Vanhaudenhuyse A, Bruno MA, Noirhomme Q, Ledoux D, Faymonville ME, Lamy M, Franck G, Luxen A, Maquet P, Moonen G. Revelations from the unconscious: studying residual brain function in coma and related states. Bull Mem Acad R Med Belg. 2008;163:381–388. discussion 388–390. [PubMed] [Google Scholar]
- Laureys S, Tononi G. The Neurology of Consciousness: Cognitive Neuroscience and Neuropathology. Academic Press; London: 2009. [Google Scholar]
- Vanhaudenhuyse A, Giacino J, Schnakers C, Kalmar K, Smart C, Bruno MA, Gosseries O, Moonen G, Laureys S. Blink to visual threat does not herald consciousness in the vegetative state. Neurology. 2008;71:1374–1375. doi: 10.1212/01.wnl.0000320110.70134.60. [DOI] [PubMed] [Google Scholar]
- Yang L, Shklyar I, Lee HW, Ezeani CC, Anaya J, Balakirsky S, Han X, Enamandram S, Men C, Cheng JY, Nunn A, Mayer T, Francois C, Albrecht M, Hutchison AL, Yap EL, Ing K, Didebulidze G, Xiao B, Hamid H, Farooque P, Detyniecki K, Giacino JT, Blumenfeld H. Impaired consciousness in epilepsy investigated by a prospective responsiveness in epilepsy scale (RES) Epilepsia. 2012;53:437–447. doi: 10.1111/j.1528-1167.2011.03341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]