Abstract
Objective
Schizophrenia is a severe and heritable brain disorder. Language impairment has been hypothesized to spur its onset and underlie the characteristic symptoms. In this study, we investigate whether altered topological pattern of the language processing brain network exists and could be a potential biomarker of schizophrenia. We hypothesized that both patients with schizophrenia and the genetic high risk population would show significantly weakened efficiencies of the network hubs for normal language processing, especially at left inferior frontal and bilateral temporal lobes.
Method
Language task-based fMRI data from 21 patients with schizophrenia, 22 genetic high risk subjects and 36 controls were analyzed. Graph theoretic and post hoc analyses of the fMRI data, and correlations between the functional network features and scores of language tests were carried out.
Results
Compared to controls, patients with schizophrenia and the high risk subjects showed significantly weakened network hubs in left inferior frontal and right fusiform gyri. A unique topology of super active and intercommunicating network hubs at left fusiform gyrus and right inferior/middle frontal gyri, which were associated with the behavioral language impairment was found in the patient group, compared to the high risk and control groups.
Conclusions
Aberrant systems-level topology of language processing network, especially significantly weakened network hubs in left inferior frontal and right fusiform gyri, may serve as a candidate biomarker of schizophrenia. Supported by existing findings, the hyperactive left fusiform gyrus communicating with right frontal lobe might be the key neurophysiological component causing hallucinations in schizophrenia. These findings provided a new systems-level diagnostic target for the disorder.
Keywords: Schizophrenia, Genetic high risk, Language processing network, fMRI, Graph theoretical techniques
1. Introduction
Schizophrenia is a severe, chronic and heritable brain disorder. First-degree relatives of schizophrenia patients have an almost 10-fold increased risk of developing the disorder (Gottesman and Shields, 1973). According to NIMH report (2012), it affects at least 2.4 million Americans in a given year and more than 24 million people worldwide. The underlying genetic mechanism for the pathogenesis of the disorder is not well understood. Anomalies in language processing have been hypothesized to spur the onset and underlie the characteristic symptoms of schizophrenia (Crow, 1995). Recent functional and structural neuroimaging studies in patients with schizophrenia (Bluhm et al., 2007; Kircher et al., 2001; Sommer et al., 2004; Liemburg et al., 2012), and in those during the prodromal stage prior to illness onset and/or at high genetic risk (Lawrie et al., 1999; Whyte et al., 2006; Rajarethinam et al., 2011), have shown widespread functional and structural abnormalities in the language processing-related brain regions. These may be relevant to the underlying genetic basis for the disorder.
Brain regions work interactively as a network when processing sensory and cognitive information. The anatomical pathways for language processing were first documented by Broca and Wernicke in the 19th Century, and redefined based on results from numerous language task-based functional imaging studies. In this revised model, bilateral superior temporal gyri and occipital cortex serve as auditory and visual input centers (McNealy et al., 2006). The supramarginal gyrus (Brodmann’s area (BA) 40) works as a site for phonological encoding in word production, whereas the angular gyrus (BA 39) for memory of visual word forms (Poeppel and Hickok, 2004). These two regions interface with widely distributed conceptual knowledge systems located primarily at the junction of the left temporal, occipital, and parietal lobes, i.e. inferior temporal region (Wernicke’s area), fusiform and lingual gyri. The sound, or visual-based system, interfaces not only with the conceptual knowledge systems, but also with frontal motor systems (Broca’s area: BA 44, 45) via an auditory-motor interface system in the inferior parietal lobe. This circuit is the primary substrate for phonological and semantic working memory, and likely plays a role in volitional speech production. Structurally, Broca’s area is left lateralized. There is a link between Broca’s area and the large-scale distributed network for conceptual representation (such as BA 9, 10, 23, 24, 28) (Poeppel and Hickok, 2004). Thus, the whole circuit of language processing is a highly distributed multilevel bidirectional network.
Development of the advanced graph theoretic techniques has allowed us to characterize the important topological properties of the functional brain networks (Bullmore and Sporns, 2009). Studies have revealed that the functional brain networks for sensory and cognitive information processing in normal human brain have a small-world property, which is characterized by a high level of local clustering and short path lengths linking all nodes within the network, and produces optimal organization for rapid synchronization and information transferring with minimal wiring cost (He and Evans, 2010; Rubino and Sporns, 2010). Recent resting-state fMRI studies have reported altered default mode network topological properties in patients with schizophrenia (Bluhm et al., 2007; Liu et al., 2008; Yu et al., 2011). The systems-level topological features of the functional brain networks for language processing in patients with schizophrenia and the high risk population have not yet been investigated.
In this study, we investigated whether altered topological patterns of the language processing network, assessed with a lexical discrimination task (Li et al., 2007b), during fMRI in patients with schizophrenia and the genetic high risk subjects, could be a potential biomarker of schizophrenia. We hypothesized that both patients with schizophrenia and the high risk subjects would show significantly weakened efficiencies of the network hubs for normal language processing, especially at left inferior frontal and temporal gyri.
2. Materials and methods
2.1. Subjects
A total of 93 subjects were involved in the study. Seven subjects were excluded from analyses due to heavy head motion, 3 were excluded due to poor fMRI task response accuracy, 2 with low IQ, and 2 were not able to finish the whole scan protocol. Eventually, a total of 79 subjects were included in analyses (36 normal controls, 22 high risk subjects, and 21 patients with schizophrenia). Basic demographic comparisons among groups were provided in Table 1. The control subjects did not have evidence of any psychotic disorders (schizophrenia, bipolar disorder or psychosis not otherwise specified) upon evaluation, and no history of any psychotic disorder, psychiatric hospitalization or suicide in first or second-degree relatives. Individuals at genetic high risk were those who were within the peak age of risk for schizophrenia, and originated from families in which at least one first-degree relative (parent or sibling) had a diagnosis of schizophrenia and at least one other relative had a psychotic illness (Li et al., 2007b). All subjects underwent an interview using the DIGS (Diagnostic Interview for Genetic Studies; (Nurnberger, Jr. et al., 1994)). Diagnoses were made based on the interview, information from family members, and medical records as appropriate. The normal controls and high-risk subjects were never treated with medication for psychotic symptoms, whereas the patients with were on a combination of conventional and atypical medications. Six patients were siblings of unaffected individuals in the high risk group.
Table 1.
Subject characteristics and cognitive test scores analyzed by one-way ANOVA
| Normal controls (n = 36) | High-risk subjects (n = 22) | Schizophrenia Patients (n = 21) | F | p | |
|---|---|---|---|---|---|
| Age | 22.9±5.4 | 21.4±5.3 | 36.7±9.3 | 46.8 | 0.000 |
| Male/Female | 17/19 | 8/14 | 13/8 | Chi-sq.=9.7 | 0.093 |
| L/R Handed | 2/34 | 3/19 | 2/19 | -- | -- |
| Education(years) | 14.2±2.21 | 12.6±2.75 | 14.5±2.20 | 0.815 | 0.606 |
| Racial composition | 21 Ca, 15 Nc | 15 Ca, 7 Nc | 17 Ca, 4 Nc | Chi-sq.=3.1 | 0.09 |
| WAIS-VCI | 107.9±17.5 | 105.9±12.8 | 108.6±15.0 | 0.168 | 0.846 |
| PPVT-III | 102.9±13.6 | 101.5±12.9 | 102.5±16.6 | 0.7 | 0.932 |
| WRAT | 103.7±11.2 | 106.3±8.5 | 106.6±11.9 | 0.596 | 0.553 |
Ca: Caucasian; Nc: Non-Caucasian
For each subject, select subtests from the age-appropriate Wechesler Intelligence Scale were administered (Wechsler, 2004). The Verbal Comprehension Index (WAIS-VCI) was used as the primary measure of verbal capacity. The Wide Range Achievement Test – Reading (WRAT-Reading) was performed by decoding a list of progressively harder words, as both a measure of reading ability and as an estimate of premorbid IQ (Wilkinson GS, 1993). The Peabody Picture Vocabulary Test (PPVT-III) was also performed as a test of receptive language, where the subject was shown panels of four pictures and asked to point to the one that best describes a word spoken by the examiner (Dunn and Dunn, 1997).
This study received Institutional Review Board Approval for human subjects’ research at the Nathan S. Kline Institute for Psychiatric Research, and at New York University School of Medicine. Participants over the age of 18 gave written informed consent for their participation after being carefully explained the nature of the study and its procedures. Subjects under the age of 18 provided assent, and consent was provided by a parent/guardian for their participation.
2.2. Experimental task
A Visual Word Discrimination task was used in this study. The experimental task has been described in detail in (Li et al., 2007b). Briefly, three types of blocks interleaved in the task. During the Lexical Decision Blocks, English language words (all concrete nouns) were presented on a screen in a random fashion, interspersed with pseudowords matched to the real words for number of letters. Subjects were instructed to use their right hand to press the left button when a word appeared and to press the right button when the letters displayed were not considered to be a real word. During the Non-Linguistic Control Blocks, two kinds of non-linguistic symbols were shown on the screen. The subjects were instructed to press the left button in response to the symbol denoting “left” and the right button in the response to the symbol denoting “right”. The rest blocks contained a blank screen, and subjects were instructed to keep their eyes open, remain relaxed and motionless.
This visual word discrimination task had 10 blocks, where each block comprised 10 stimuli, each presented for 1000 ms with an interstimulus interval of 3000 ms. The task was repeated four times. The stimuli were presented in random order within blocks. The order of blocks within sequences and the order of trials within blocks differed across the 4 runs, but were identical for all subjects.
2.3. MRI Scans
Imaging was performed on a 1.5T MRI system (Siemens Vision systems, Erlangen, Germany). For each experiment 165 functional volumes sensitive to blood oxygen level dependent (BOLD) contrast were acquired with a T2-weighted gradient-echo EPI sequence [TR=2s, TE=50ms, flip angle=85°, Matrix=64×64, FOV=224, pixel size=3.5×3.5]. Each volume was comprised of 22 axial slices of 5mm slice thickness with no gap. A high resolution 3D MPRAGE data was collected for spatial mapping and registration of the fMRI data.
2.4. Data preprocessing
The fMRI data from each subject was preprocessed using the FSL/FEAT tools. Each image data was initially corrected for slice timing, head motion, and spatial intensity, and spatially smoothed with an 8mm full-width and half maximum Gaussian kernel. A high-pass temporal filter of 1/70 Hz was used to remove low-frequency noise. Non-brain structures were extracted using the Brain Extraction Tool. The fMRI data was first aligned to the skull striped T1-weighted image from the same subject using an affine translation, and further registered to the MNI152 template using a linear registration. Nine components were regressed out from each fMRI data, which included the 6 motion correlation parameters and nuisance signals (white matter, cerebrospinal fluid, and global signal). The residual time series of each image data were analyzed to generate the task-related activation map by using FMRIB improved linear regression (FILM) tool. Activation map for each subject and the average map for each group were generated. The Z statistic image was thresholded using clusters determined by Z>2.3 and a cluster corrected significance threshold of p<0.05.
Seed ROIs were selected from a combination (union) of all the clusters that were activated in average activation maps of the three groups. First, this combined activation map was parcellated according to the AAL template (Tzourio-Mazoyer et al., 2002), which carved the cerebral cortex and subcortical structures into 90 anatomical areas bilaterally (note that cerebella regions were not considered in this study). As shown from Table 2 and Figure 1(A), 64 seed ROIs (spheres, R=5mm) were identified from the coordinates at peaks of local maximal activations. In this study, we interrogated the components of the fMRI signals in 0.015 – 0.125 Hz, which has been convinced to contain the majority of the task-related hemodynamic signals by multiple studies in block-design task-based fMRI data (Ginestet and Simmons, 2011; Achard et al., 2006; Bassett et al., 2010). According to existing studies, the hemodynamic response associated signals for different tasks may stay in different sub-bands. Thus, in our study, we first tested three wavelet scales, to assess whether signals from different bands would have different sensitivities to the task design. Since our sampling frequency was 2s (TR = 2s) and wavelet kth scale provided information on the frequency band [2−k−1/TR, 2−k/TR], we obtained 0.015–0.031Hz, 0.031–0.062Hz and 0.062–0.125Hz, for wavelet scales 2, 3, 4, using the maximum-overlap discrete wavelet transform that had been extensively applied to time-frequency analyses of fMRI signals (Percival and Walden, 2000).
Table 2.
Node ROIs for functional brain network construction. Network hubs in groups of normal controls (NC), high-risk (HR) and patients (SCH) are labeled. (L.=left; R.=right)
| Regions | Volume (mm3)a | Peak MNI coordinates [x y z] | Zb | Degree | Between-centrality | ||
|---|---|---|---|---|---|---|---|
| L. Superior frontal gyrus (dorsal) | 2496 | −26 | −8 | 56 | 3.77 | ||
| R. Superior frontal gyrus (dorsal) | 2984 | −28 | −4 | 58 | 3.85 | ||
| L. Superior frontal gyrus (medial) | 1176 | −4 | 16 | 42 | 3.52 | HR | |
| R. Superior frontal gyrus (medial) | 2505 | 4 | 26 | 46 | 3.39 | NC, HR, | |
| R. Orbitofrontal gyrus (superior) | 1736 | 22 | 34 | −24 | 3.44 | ||
| L. Middle frontal gyrus | 10496 | −38 | 38 | 26 | 3.51 | ||
| R. Middle frontal gyrus | 9064 | 28 | −4 | 56 | 3.67 | NC, | |
| L. Orbitofrontal gyrus (middle) | 1176 | −38 | 52 | −10 | 2.66 | ||
| R. Orbitofrontal gyrus (middle) | 992 | 22 | 36 | −22 | 3.32 | ||
| L. Orbitofrontal gyrus (inferior) | 6552 | −36 | 24 | −4 | 3.46 | ||
| R. Orbitofrontal gyrus (inferior) | 3496 | 38 | 30 | −2 | 3.01 | ||
| L. Inferior frontal gyrus (opercular) | 3512 | −44 | 10 | 2 | 4.8 | NC, HR, SCH | NC |
| R. Inferior frontal gyrus (opercular) | 7862 | 44 | 10 | 22 | 4.16 | ||
| L. Inferior frontal gyrus (triangular) | 3592 | −44 | 16 | 2 | 4.04 | NC, HR, SCH | NC, HR, SCH |
| R. Inferior frontal gyrus (triangular) | 7504 | 46 | 12 | 22 | 4.02 | ||
| R. Rectus gyrus | 1040 | 16 | 26 | −12 | 2.92 | SCH | SCH |
| L. Precentral gyrus | 14040 | −46 | 8 | 30 | 5.12 | ||
| R. Precentral gyrus | 6400 | 48 | 10 | 30 | 3.75 | ||
| L. Supplementary motor area | 7056 | −6 | 4 | 46 | 5.17 | ||
| R. Supplementary motor area | 4344 | 4 | 8 | 58 | 4.38 | ||
| L. Postcentral gyrus | 8536 | −38 | −32 | 40 | 4.89 | ||
| R. Postcentral gyrus | 2088 | 48 | −30 | 44 | 3.2 | ||
| R. Anterior gingulate gyrus | 480 | 8 | 6 | 28 | 3.27 | ||
| L. Middle gingulate gyrus | 2408 | −6 | 4 | 44 | 4.98 | ||
| R. Middle gingulate gyrus | 4688 | 12 | 22 | 40 | 3.74 | ||
| L. Superior parietal gyrus | 3072 | −24 | −62 | 50 | 5.26 | ||
| L. Inferior parietal gyrus | 8536 | −40 | −32 | 40 | 5.03 | ||
| R. Inferior parietal gyrus | 1008 | 32 | −54 | 46 | 3.86 | HR, | |
| L. Angular gyrus | 1432 | −32 | −60 | 32 | 3.36 | ||
| R. Angular gyrus | 2496 | 28 | −62 | 44 | 4.15 | HR, | |
| L. Supramarginal gyrus | 944 | −54 | −24 | 42 | 3.65 | SCH | |
| R. Supramarginal gyrus | 1152 | 50 | −30 | 44 | 3.08 | ||
| L. Middle temporal gyrus | 2024 | −42 | −64 | −4 | 4.94 | ||
| R. Middle temporal gyrus | 3168 | 44 | −62 | −4 | 3.25 | ||
| L. Inferior temporal gyrus | 6576 | −42 | −64 | −10 | 5.79 | NC, SCH | NC, |
| R. Inferior temporal gyrus | 6392 | 48 | −60 | −18 | 4.6 | SCH | |
| L. Temporal pole (superior) | 336 | −50 | 10 | −8 | 3.08 | ||
| L. Fusiform gyrus | 7554 | −42 | −66 | −14 | 5.97 | SCH | SCH |
| R. Fusiform gyrus | 6152 | 40 | −68 | −18 | 4.87 | NC, | NC, |
| L. Supperior occipital gyrus | 896 | −26 | −70 | 36 | 4.21 | ||
| R. Supperior occipital gyrus | 1504 | 24 | −98 | 4 | 4.06 | ||
| L. Middle occipital gyrus | 5264 | −26 | −94 | −4 | 6.3 | ||
| R. Middle occipital gyrus | 4216 | 28 | −96 | 0 | 5.55 | NC, HR, | NC, HR, SCH |
| L. Inferior occipital gyrus | 5856 | −28 | −94 | −6 | 6.58 | ||
| R. Inferior occipital gyrus | 7546 | 24 | −96 | −4 | 6.13 | NC, | SCH |
| L. Lingual gyrus | 1848 | −28 | −94 | −14 | 5.68 | HR, | |
| R. Lingual gyrus | 3280 | 22 | −94 | −8 | 5.58 | NC, SCH | NC, SCH |
| L. Calcarine cortex | 1360 | −18 | −99 | −6 | 5.53 | HR, | |
| R. Calcarine cortex | 2112 | 22 | −98 | −4 | 5.53 | NC, SCH | |
| L. Parahippocampal cortex | 496 | −20 | −18 | −20 | 2.65 | ||
| L. Insula | 5608 | −46 | 8 | 2 | 4.68 | NC, HR, SCH | |
| R. Insula | 4376 | 34 | 24 | 2 | 4.33 | ||
| L. Rolandic operculum | 2504 | −48 | 8 | 2 | 4.4 | ||
| R. Rolandic operculum | 728 | 46 | 8 | 14 | 2.93 | ||
| L. Caudate | 952 | −18 | −14 | 20 | 2.67 | ||
| R. Caudate | 4104 | 20 | −16 | 24 | 3.37 | ||
| L. Putemen | 1984 | −32 | −12 | −6 | 3.0 | ||
| R. Putemen | 3136 | 26 | 20 | 6 | 3.14 | ||
| L. Pallidum | 168 | −26 | −14 | −4 | 2.45 | ||
| L. Thalamus | 5024 | −16 | −18 | 4 | 3.3 | ||
| R. Thalamus | 3504 | 22 | −20 | 12 | 3.02 | ||
| L. Amydala | 504 | −28 | −6 | −18 | 2.44 | ||
| L. Hippocampus | 3088 | −20 | −14 | −18 | 3.53 | ||
| R. Hippocampus | 1888 | 28 | −36 | 6 | 2.99 | ||
is the number of activated voxels × 27mm3; the ROIs were R=5 spheres with origins located at the peak Coordinates;
is the z value of the peak.
Figure 1.
Locations of the nodes and the small-world properties of the functional brain networks. The global and local efficiencies (images B and C) were shown as a function of the cost in the networks. The global and local efficiency curves of the functional brain networks of the three diagnostic groups located in between that of the random and regular networks, over a range of 0.1 ≤ cost ≤ 0.3 (between the vertical dash lines), known as a small-world regime. The random networks were generated by 50 random rewirings of the edges while keeping the same number of nodes and degree distribution as brain networks. The regular networks were generated by 50 random rewiring of the edges along the two sides of the main diagonal (NC = normal controls, HR = high-risk subjects, SCH = schizophrenia patients). Image D illustrated the degree distributions of three groups, which were fitted by an exponentially truncated power of the form P(k)~kα−1e−k/kc(NC: α = 1.5667; kc = 4.871; HR: α = 1.525, kc = 5.092; SCH: α = 1.498, kc = 5.246). One-way ANOVA indicated that there were no significant differences in the fitted parameters (α: F(2, 80) = 2.86, p = 0.063; kc: F(2, 80) = 2.65, p = 0.077) among the three groups.
We eventually decided to use the whole band, based on the testing results that did not show significant sensitivity differences among the three sub-bands. Thus averaged wavelet coefficients of the three frequency sub-bands were utilized for functional connectivity analysis. Pearson correlation of the average wavelet coefficients in each pair of the ROIs was calculated. The absolute values of the correlation coefficients were used to construct the functional connectivity matrix.
2.5. Network efficiency
The functional connectivity matrix was then converted into a binary graph, by using the network cost as threshold. The cost CG, of a network (graph), G, was defined as following:
where N and K were the total number of nodes (ROIs) and edges (functional correlations), respectively; N (N −1)/2 was the number of all the possible sub-networks in the graph G (Latora and Marchiori, 2001). We investigated the network properties over a wide range of the cost values from 0.1 to 0.5 using increments of 0.01. According to existing studies, the selected threshold interval would allow the small-world properties to be properly estimated and the sub-networks to be connected with enough discriminatory power in functional connectivity (Achard and Bullmore, 2007). Then we calculated the two global metrics, global efficiency, Eglob(G) and local efficiency, Eloc(G), defined as:
where lij was the shortest path length between nodes i and j; Eglob(Gi) the global efficiency of the sub-network Gi that was constructed by the set of nodes that were immediate neighbors of nodes i (Latora and Marchiori, 2001). The graph was considered to be a small-world network if it met the following criteria: Eglob(Gregular) < Eglob(G) < Eglob(Grandom) and Eloc(Grandom) < Eloc(G) < Eloc(G regular), where Eglob(Gregular), Eglob(G random), Eloc(Gregular) and Eloc(Grandom) were the global and local efficiencies of node-and degree-matched regular and random networks.
We also investigated the nodal efficiency, E nodal (G,i), which was a local measure to evaluate the communication efficiency between a node i and all other nodes in the network G (Latora and Marchiori, 2001).
2.6. Network hubs
We also identified the network hubs in each group. In this study, a node was defined as a hub if the value of a measure, either degree (D) or between-centrality (BC), of this node was significantly higher than the average value over all the nodes in the network. The degree (Di) of a node i was the number of edges connected to the node i, and the between-centrality (BCi) of node i was defined as the proportion of all the shortest paths between pairs of other nodes in the network that include node i (Bullmore and Sporns, 2009).
Let , k =1, ···, m; i =1, ···, N be the measure of Di or BCi of subject k in the group. The grand sample mean, , and the grand sample standard deviation, , in each diagnostic group was calculated. The standardized measure of node i in each group was then defined as , where for each node i. These standardized values were then tested with a normal distribution. A node i was a network hub if 1−Φ (zi)< α, where Φ(·) was the standard normal cumulative distribution function, and α = 0.05the level of significance.
2.7. Group statistic analyses
Group statistic analyses were performed using a General Linear Model from the MATLAB Statistics Toolbox (The MathWorks Inc, Natick, MA). We compared the demographic/cognitive measures, the global features (Eglob and Eloc) and the average nodal efficiency at each cost value, among three groups using one-way Analysis of Variance (ANOVA). Sex was added as a fixed-effect factor, and age added as a random-effect covariate. Multiple comparisons were corrected by Bonferroni correction at α=0.05. A significance threshold of P < 0.05 was applied to each test.
A linear regression analysis was utilized between the nodal efficiency measure of each node and the scores for WAIS, PPVT and WRAT in each group. Again, sex and age effects were controled, and multiple comparisons were corrected by Bonferroni correction at a=0.05. A significance threshold of P < 0.05 was applied to each test.
3. Results
3.1. Clinical and behavior data
From Table 1, the ages of high-risk subjects and normal controls did not differ significantly (p=0.320), while the patients were significantly older than the other two groups (p=0.000). There were no significant between-group differences in sex, handedness, education levels, and performance scores of the language related cognitive tests. All the participants performed the fMRI task with a >80% accurate response rate.
3.2. Network efficiencies
Figure 1(B and C) illustrated the global features of functional brain networks, the global and local efficiencies, in the three diagnostic groups, over a range of network costs (0.1 ≤ cost ≤ 0.5). We observed that the locations of the global and local efficiency curves of the three groups were between the corresponding curves of the random and regular graphs in the range 0.1 ≤ cost ≤ 0.3, known as a small-world regime (Achard and Bullmore, 2007). Compared to normal controls, the high-risk subjects and patients showed decreased global efficiencies and increased local efficiencies (not significant though). Figure 1(D) showed that the degree distributions of the three networks followed a truncated power-low of the form (k)~kα−1e−k/kc.
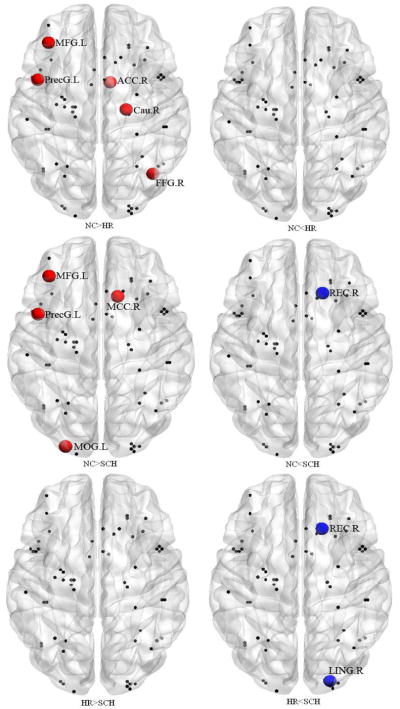
Group comparisons of the nodal efficiencies of all the nodes over the small-world regime showed significant differences in the left inferior frontal gyrus (BA44, p = 0.032), left middle frontal gyrus (BA46, p = 0.03), and right rectus gyrus (BA11, p < 0.0001) among three groups. Results of the Post hoc t tests, as shown in Figure 2, demonstrated that compared to controls, both high-risk and patient groups had significantly decreased nodal efficiencies in the left BA44 and BA46; whereas the patient group had significantly increased nodal efficiency in the right BA11, compared to controls and the high risk subjects.
Figure 2.
Between-group differences in the nodal efficiency of each node in the language processing network. (NC=normal controls, HR=high-risk, and SCH=patients with schizophrenia). MFG: middle frontal gyrus; PrecG: Precentral gyrus; REC: rectus gyrus; ACC: anterior cingulum cortex; MCC: middle cingulum cortex; Cau: caudate; FFG: fusiform gyrus; MOG: middle occipital gyrus; LING: lingual gyrus; L: left hemisphere; R: right hemisphere.
3.3. Network hubs
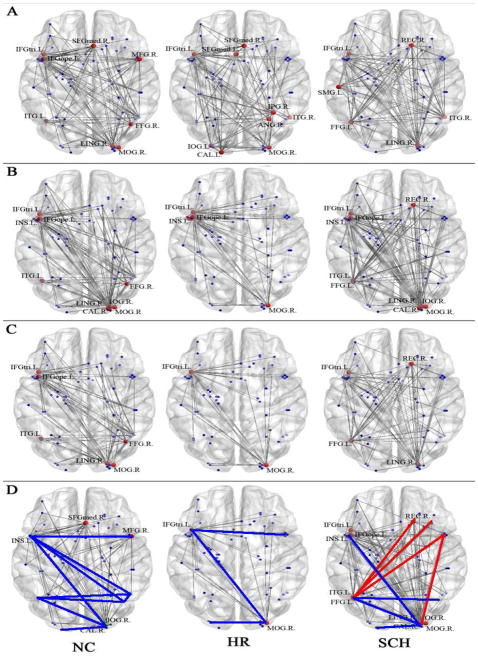
Table 2 and Figure 3(A–C) detailed the anatomical regions and locations of the network hubs in each group. As a summary, Figure 3D demonstrated that compared to the controls, both the high risk cohort and patients with schizophrenia lost communications among the middle occipital gyrus, angular gyrus, and inferior temporal and fusiform gyri within the right hemisphere, as well as showed significantly reduced between-hemisphere interactions between the left side inferior frontal area (Broca’s area) and the right side angular gyrus and the junction of temporal and parietal lobes, i.e. inferior temporal and fusiform gyri. The language processing network in the patient group showed some unique features, i.e. the significantly increased between-hemisphere interactions between the right side middle frontal gyrus and the left side fusiform gyrus, compared to that in the controls and high risk subjects.
Figure 3.
Network hubs in normal controls (NC), high-rish group (HR) and schizophenia patients (SCH) (regions were detailed in Table 2). A: Hubs identified with only between-centrality measure; B: Hubs identified with only degree measure. C: Hubs that satisfy both between-centrality and degree criteria; D: The sketch maps of the major pathways of the language processing networks in three groups. The brain networks were constructed at the cost threshold of 0.2 in each group. The colored lines depict the edges connecting the hubs and other brain regions. Specifically, the red lines demonstrated the unique hub connections in the patient group. IFGtri: inferior frontal gyrus (triangular); IFGope: inferior frontal gyrus (opercular); SFGmed: superior forntal gyrus (medial); REC: rectus gyrus; INS; insula; IPG: inferior parietal gyrus; SMG: supermarginal gyrus; ANG: angular gurus; ITG: inferior temporal gyrus; FFG: fusiform gyrus; LING: lingual gyrus; CAL: calcarine cortex; MOG: middle occipital gyrus; IOG: inferior occipital gyrus; L: left hemisphere; R: right hemisphere.
3.4. Correlations between network features and language measures
We found both the global and local efficiencies, over the small-world regime (0.1 ≤ cost ≤ 0.3), were positively (but not significantly) correlated with the PPVT and WRAT scores in the whole study population. Significant negative correlations between the PPVT scores and the nodal efficiency of the right side BA 44/45 were present in the high-risk group (r=−0.51, p=0.02). In the patient group, significant negative correlations between the WRAT scores and the nodal efficiencies were observed in right sides BA44/45 (r=−0.48, p=0.031 for right side), and in left side BA37 (r=−0.53, p=0.008).
4. Discussion
To our knowledge, this is the first study to exhibit atypical topological features of the functional brain network for visual language processing in both patients with schizophrenia and the unaffected people with high genetic risk, and it identifies a potential neural intermediate phenotype of the disorder. Specifically, we observed significantly decreased nodal efficiencies in the left side inferior frontal region (left BA44/45/46) in both high-risk and patient groups compared to healthy comparison subjects, and significantly increased nodal efficiency in the right side middle frontal gyrus (BA11) in patients with schizophrenia compared to controls and high risk subjects. Moreover, significant negative correlations between the language testing performance scores and the nodal efficiency of the right side BA 44/45 were presented in both the high-risk and patient groups. The left side inferior frontal region (BA44/45), also referred to as the Broca’s area, has been recognized to be a key component for language recognition, comprehension and speech production (Price et al., 2001). Neuroimaging studies of this brain area in patients with schizophrenia and the genetic high risk subjects have consistently reported abnormal gray matter and white matter morphology (Jou et al., 2005; Pantelis et al., 2003), disrupted local white matter network organizations (van den Heuvel et al., 2011), and significantly less leftward functional activations during language tasks (Li et al., 2007a; Whyte et al., 2006). In line with the existing studies, the findings in the present study suggest that the significantly weakened function of the left Broca’s area and its right-shifting pattern greatly contributes to the pathology of language impairment in schizophrenia, and such unique topological feature of the language processing brain network can be a potential biomarker of the disorder. Such similar findings in the unmedicated high risk cohort and a mixture of medicated and unmedicated schizophrenia patients are of particular importance, because they also suggest that the results are unlikely to be affected by medication.
Mapping of the central hubs of the language processing brain networks in the three groups depicted group-specific topological features of the functional brain networks (especially shown in Figure 3D). It demonstrated that compared to the controls, the high risk and patient groups showed significant lack of communications among the hubs in the primary visual region, and the phonological encoding, memory of visual word forms and conceptual knowledge systems (BA 22/37/39/40) within the right hemisphere, as well as significant lack of between-hemisphere interactions between the left side Broca’s area and and the right side conceptual knowledge systems, i.e. inferior temporal and fusiform gyri. Very interestingly, the language processing network in the patient group showed some unique features of super active between-hemisphere interactions between the right side middle frontal gyrus and the left fusiform gyrus, and significant negative correlations between the WRAT scores and the nodal efficiencies of left fusiform gyrus.
The angular, inferior/superior temporal and fusiform gyri are key regions for language and semantic memory processing, visual perception, multimodal sensory integration (Ahmad et al., 2003). Neuroimaging studies have reported functional and structural anomalies of the inferior/superior temporal area in patients with schizophrenia and the genetic high risk populations (Rajarethinam et al., 2004; Rajarethinam et al., 2011; Jacobsen et al., 1998). The fusiform gyrus also involves in face/body recognition and within-category identification (Minnebusch et al., 2009). Studies have concluded that the bilateral fusiform gyri play different roles from one another, but are subsequently interlinked: the left fusiform gyrus plays the role of recognizing key features in objects; whereas the right fusiform gyrus plays the role in determining what the recognized object is, and have suggested that increased neurophysiological activity in the fusiform gyrus may produce hallucinations (Jan Dirk Blom, 2010). Postmortem and in vivo imaging studies have reported reduced gray matter volumes in bilateral fusiform gyri in patients with schizophrenia compared with controls (Onitsuka et al., 2004), and more severe hallucinations significantly correlated with smaller left hemisphere volumes of fusiform gyrus in schizophrenia patients (Onitsuka et al., 2004). Functional abnormality in the right side fusiform gyrus during emotion discrimination was found in a sample at high risk for psychosis (Seiferth et al., 2008). In agreement with these existing studies, we suggest that impaired central hubs in the right fusiform gyrus directly cause the lack of within- and between-hemisphere communications among the visual cortex, angular gyrus, and inferior/superior temporal gyri in the right hemisphere, as well as the lack of communications between Broca’s areas and right side parietal/temporal areas, in both patient and the high risk groups. Such impaired topological organization of the language processing network could be a remarkable intermediate phenotype, and early sign for the onset of the disorder.
Also seen from Figure 3D, a hyperactive network hub in the left fusiform gyrus was uniquely existed in the schizophrenia patient group. It strongly interacted with the abnormal hubs in the right prefrontal lobe, and was significantly negatively correlated with the language testing scores. Based on the numerous findings from existing studies showing the structural and functional impairments of the left fusiform gyrus and its strong correlations with hallucinations in patients with schizophrenia, we suggest that the unique pattern of abnormal between-hemisphere interactions originated from the left fusiform gyrus might be the key neuronal substrates of the symptomology of the disorder.
The present study has some limitations. First, the sample included both male and female subjects. Although a meta-analysis reported no significant difference in language lateralization between men and women (Sommer et al., 2004), sex was still added as a fixed-effect factor in this study to remove the potential confounders. Second, all the patients were medicated. Medication effects to functional brain networks for cognitive information processes have rarely been investigated in patients with chronic schizophrenia, mainly because washing-out periods (times for patients to stop taking medications until the mental and physical effects of the medications are removed) prior to the MRI examinations are not recommended by the physicians of patients with chronic schizophrenia. In addition, the sample size of this study was relatively small. Future work will need a much larger cohort of high-risk individuals, followed longitudinally, to determine who eventually develops schizophrenia and whether the abnormal language processing brain netowrk would have been predictive of the illness.
Supplementary Material
Acknowledgments
Funding Sources
This project was partially supported by a grant from NIMH, R21 MH071720.
Footnotes
Conflict of Interests
All authors reported no actual and potential conflict of interests.
Contributors
Dr. Xiaobo Li designed the neuroimaging study, managed the literature searches, and wrote the first draft of the manuscript.
Dr. Shugao Xia processed the neuroimaging data and statistical analyses.
Dr. Hilary Bertisch administered the neurocognitive assessments.
Dr. Craig Branch designed the MRI scan protocols.
Dr. Lynn Delisi provided the general hypotheses and administered the clinical interviews.
All authors contributed to and have approved the final manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- NIMH. The numbers count-Mental disorders in America. 2012. [Google Scholar]
- Achard R, Bullmore E. Efficiency and Cost of Economical Brain Functional Networks. PLoS Comput Biol. 2007;3:e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci. 2006;26:63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad Z, Balsamo LM, Sachs BC, Xu B, Gaillard WD. Auditory comprehension of language in young children: neural networks identified with fMRI. Neurology. 2003;60:1598–1605. doi: 10.1212/01.wnl.0000059865.32155.86. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Wymbs NF, Porter MA, Mucha PJ, Carlson JM, Grafton ST. Dynamic reconfiguration of human brain networks during learning. Proc Natl Acad Sci U S A. 2010;108(18):7641–6. doi: 10.1073/pnas.1018985108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, Theberge J, Schaefer B, Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Constraints on concepts of pathogenesis. Language and the speciation process as the key to the etiology of schizophrenia. Arch Gen Psychiatry. 1995;52:1011–4. doi: 10.1001/archpsyc.1995.03950240029006. discussion 1019–2. [DOI] [PubMed] [Google Scholar]
- Dunn M, Dunn M. Peabody Picture Vocabulary Test. 3. American Guidance Service; 1997. [Google Scholar]
- Ginestet CE, Simmons A. Statistical parametric network analysis of functional connectivity dynamics during a working memory task. Neuroimage. 2011;55:688–704. doi: 10.1016/j.neuroimage.2010.11.030. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122:15–30. doi: 10.1192/bjp.122.1.15. [DOI] [PubMed] [Google Scholar]
- He Y, Evans A. Graph theoretical modeling of brain connectivity. Curr Opin Neurol. 2010;23(4):341–50. doi: 10.1097/WCO.0b013e32833aa567. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Giedd JN, Castellanos FX, Vaituzis AC, Hamburger SD, Kumra S, Lenane M, Rapoport JL. Progressive reduction of temporal lobe structures in childhood onset schizophrenia. Am J Psychiatry. 1998;155:678–685. doi: 10.1176/ajp.155.5.678. [DOI] [PubMed] [Google Scholar]
- Blom Jan Dirk. A Dictionary of Hallucinations. Springer; 2010. [Google Scholar]
- Jou RJ, Hardan AY, Keshavan MS. Reduced cortical folding in individuals at high risk for schizophrenia: a pilot study. Schizophr Res. 2005;75(2–3):309–13. doi: 10.1016/j.schres.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Bulimore ET, Brammer MJ, Williams SC, Broome MR, Murray RM, McGuire PK. Differential activation of temporal cortex during sentence completion in schizophrenic patients with and without formal thought disorder. Schizophr Res. 2001;50(1–2):27–40. doi: 10.1016/s0920-9964(00)00042-6. [DOI] [PubMed] [Google Scholar]
- Latora V, Marchiori M. Efficient behavior of small-world networks. Physical Review Letters. 2001;87:198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Whalley H, Kestelman JN, Abukmeil SS, Byrne M, Hodges A, Rimmington JE, Best JJ, Owens DG, Johnstone EC. Magnetic resonance imaging of brain in people at high risk of developing schizophrenia. Lancet. 1999;353:30–33. doi: 10.1016/S0140-6736(98)06244-8. [DOI] [PubMed] [Google Scholar]
- Li X, Branch CA, Ardekani BA, Bertisch H, Hicks C, DeLisi LE. fMRI study of language activation in schizophrenia, schizoaffective disorder and in individuals genetically at high risk. Schizophr Res. 2007a;96:14–24. doi: 10.1016/j.schres.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Branch CA, Bertisch HC, Brown K, Szulc KU, Ardekani BA, DeLisi LE. An fMRI study of language processing in people at high genetic risk for schizophrenia. Schizophr Res. 2007b;91:62–72. doi: 10.1016/j.schres.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liemburg EJ, Vercammen A, Ter Horst GJ, Curcic-Blake B, Knegtering H, Aleman A. Abnormal connectivity between attentional, language and auditory networks in schizophrenia. Schizophr Res. 2012;135(1–3):15–22. doi: 10.1016/j.schres.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, Yu C, Liu H, Liu Z, Jiang T. Disrupted small-world networks in schizophrenia. Brain. 2008;131:945–961. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- McNealy K, Mazziotta JC, Dapretto M. Cracking the language code: neural mechanisms underlying speech parsing. J Neurosci. 2006;19;26(29):7629–39. doi: 10.1523/JNEUROSCI.5501-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnebusch DA, Suchan B, Koster O, Daum I. A bilateral occipitotemporal network mediates face perception. Behav Brain Res. 2009;198(1):179–85. doi: 10.1016/j.bbr.2008.10.041. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–59. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863–4. [DOI] [PubMed] [Google Scholar]
- Onitsuka T, Shenton ME, Salisbury DF, Dickey CC, Kasai K, Toner SK, Frumin M, Kikinis R, Jolesz FA, McCarley RW. Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an MRI study. Am J Psychiatry. 2004;161(9):1603–11. doi: 10.1176/appi.ajp.161.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361(9354):281–8. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Percival DB, Walden AT. Wavelet methods for time series analysis. Cambridge University Press; 2000. [Google Scholar]
- Poeppel D, Hickok G. Towards a new functional anatomy of language. Cognition. 2004;92(1–2):1–12. doi: 10.1016/j.cognition.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Price CJ, Warburton EA, Moore CJ, Frackowiak RS, Friston KJ. Dynamic diaschisis: anatomically remote and context-sensitive human brain lesions. J Cogn Neurosci. 2001;13:419–429. doi: 10.1162/08989290152001853. [DOI] [PubMed] [Google Scholar]
- Rajarethinam R, Sahni S, Rosenberg DR, Keshavan MS. Reduced superior temporal gyrus volume in young offspring of patients with schizophrenia. Am J Psychiatry. 2004;161:1121–1124. doi: 10.1176/appi.ajp.161.6.1121. [DOI] [PubMed] [Google Scholar]
- Rajarethinam R, Venkatesh BK, Peethala R, Phan KL, Keshavan M. Reduced activation of superior temporal gyrus during auditory comprehension in young offspring of patients with schizophrenia. Schizophr Res. 2011;130(1–3):101–5. doi: 10.1016/j.schres.2011.05.025. [DOI] [PubMed] [Google Scholar]
- Rubino M, Sporns O. Complex network measures of brain connectivity: Uses and interpratations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Seiferth NY, Pauly K, Habel U, Kellermann T, Shah NJ, Ruhrmann S, Klosterkotter J, Schneider F, Kircher T. Increased neural response related to neutral faces in individuals at risk for psychosis. Neuroimage. 2008;40(1):289–97. doi: 10.1016/j.neuroimage.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Ramsey NF, Mandl RC, van Oel CJ, Kahn RS. Language activation in monozygotic twins discordant for schizophrenia. Br J Psychiatry. 2004;184:128–135. doi: 10.1192/bjp.184.2.128. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Stam CJ, Kahn RS, Hulshoff Pol HE. Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. J Neurosci. 2011;30(47):15915–26. doi: 10.1523/JNEUROSCI.2874-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. The Psychological Corporation; San Antonio, TX: 2004. [Google Scholar]
- Whyte MC, Whalley HC, Simonotto E, Flett S, Shillcock R, Marshall I, Goddard NH, Johnstone EC, Lawrie SM. Event-related fMRI of word classification and successful word recognition in subjects at genetically enhanced risk of schizophrenia. Psychol Med. 2006;36(10):1427–39. doi: 10.1017/S0033291706008178. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test. 3. Wide Range, Inc; Wilmington, DE: 1993. [Google Scholar]
- Yu Q, Sui J, Rachakonda S, He H, Gruner W, Pearlson G, Kiehl KA, Calhoun VD. Altered topological properties of functional network connectivity in schizophrenia during resting state: a small-world brain network study. PLoS One. 2011;6(9):e25423. doi: 10.1371/journal.pone.0025423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.