Abstract
Background
While heparin possesses multiple mechanisms of action, enhanced factor Xa inhibition by antithrombin is accepted as the predominant therapeutic mechanism. The contribution of factor IXa inhibition to heparin activity in human plasma remains incompletely defined.
Objectives
To determine the relevance of factor IXa as a therapeutic target for heparins, particularly serpin-independent inhibition of intrinsic tenase (factor IXa-factor VIIIa) activity.
Patient/Methods
Thrombin generation was detected by fluorogenic substrate cleavage. Inhibitory potency (EC50) of low molecular weight heparin (LMWH), super-sulfated LMWH (ssLMWH), Fondaparinux, and unfractionated heparin (UFH) was determined by plotting concentration versus relative velocity index (ratio +/− heparin). Inhibition was compared under factor IX-dependent and independent conditions (0.2 or 4 pM TF, respectively) in normal plasma, and in mock- or antithrombin/factor IX-depleted plasma supplemented with recombinant factor IX.
Results
UFH and Fondaparinux demonstrated similar potency under factor IX-dependent and independent conditions, whereas LMWH (2.9-fold) and ssLMWH (5.1-fold) demonstrated increased potency with limiting TF. UFH (62-fold) and Fondaparinux (42-fold) demonstrated markedly increased EC50 values in antithrombin-depleted plasma, whereas LMWH (9.4-fold) and ssLMWH (2-fold) were less affected, with an EC50 within the therapeutic range for LMWH. The molecular target for LMWH/ssLMWH was confirmed by supplementing factor IX/antithrombin-depleted plasma with 90 nM recombinant factor IX possessing mutations in the heparin-binding exosite. Mutated factor IX demonstrated resistance to inhibition of thrombin generation by LMWH and ssLMWH that paralleled the effect of these mutations on intrinsic tenase inhibition.
Conclusions
Therapeutic LMWH concentrations inhibit plasma thrombin generation via antithrombin-independent interaction with the factor IXa heparin-binding exosite.
Introduction
Unfractionated heparin (UFH), a mixture of polysaccharide chains (avg. M.W. 15–18 kDa) with broad polydispersity, has been a mainstay in the treatment of acute thrombosis since the late 1930’s.[1] UFH has multiple in vitro mechanisms of action, which include antithrombin-dependent inhibition of thrombin, factors Xa, IXa, XIa, and XIIa; heparin cofactor II (HCII)-dependent inhibition of thrombin, and serpin-independent inhibition of factor X activation by the intrinsic tenase complex.[2, 3] Antithrombin-dependent activities of UFH require the high affinity pentasaccharide sequence, which is present in less than 1/3 of heparin chains.[4] Acceleration of thrombin inhibition additionally requires an oligosaccharide chain length of at least 18 saccharide units to act as a “template” for the binding of both inhibitor and protease [2, 5]. Low molecular weight heparin (LMWH), derived by partial chemical or enzymatic depolymerization of UFH (avg. M.W. 5 kDa,) has lower inhibitory activity versus thrombin relative to UFH based on this reduced chain length. LMWH preferentially inhibits factor Xa, demonstrating anti-factor Xa/antithrombin activity ratios of 2:1 to 4:1 (versus 1:1 for UFH). Similarly, Fondaparinux is a semi-synthetic form of the high affinity pentasaccharide that accelerates protease inhibition solely by conformational activation of antithrombin, and has trivial effects on thrombin inhibition.[2]Thus, acceleration of factor Xa inhibition via conformational activation of antithrombin is generally accepted as the predominant therapeutic mechanism for low molecular weight forms of heparin.
The equivalent clinical outcomes obtained with LMWH and UFH in the treatment of venous thromboembolism (VTE) argues that template-mediated thrombin inhibition is not critical to the efficacy of these heterogeneous preparations [6–8]. The efficacy of Fondaparinux in initial therapy of VTE likewise suggests that conformational activation of antithrombin is sufficient for antithrombotic effect.[9, 10] In contrast, heparin depleted of high affinity pentasaccharide (low affinity heparin) retains in vivo antithrombotic efficacy in an animal model, suggesting that antithrombin-independent mechanisms may also contribute to therapeutic efficacy.[11] Therapeutic UFH and LMWH concentrations selectively inhibit intrinsic tenase activity via an antithrombin-independent mechanism in a purified system, however, the complexity of protein-heparin interactions and simultaneous antithrombin-dependent effects in human plasma obscures the potential contribution of this mechanism to antithrombotic effects.[3, 12, 13] Super-sulfated LMWH (ssLMWH), derived from LMWH by sodium periodate oxidation to reduce antithrombin affinity followed by O-sulfation, is an antithrombin-independent inhibitor of the assembled intrinsic tenase complex, and to a lesser extent, prothrombinase in a purified system [14]. This antithrombin-independent LMWH is a valuable tool for evaluating the importance of factor IXa and the intrinsic tenase complex as a potential target for heparin in human plasma.
Factor X activation by the intrinsic tenase complex is the rate-limiting step for thrombin generation [15–17], and the interaction between factor IXa and the factor VIIIa A2 subunit is the critical protein-protein interaction for cofactor activation of the protease within this enzyme complex.[18] Mutagenesis of human factor IXa demonstrates an extensive overlap between the cofactor and heparin-binding sites on the protease domain.[13, 19, 20] Heparin oligosaccharides bound to this protease exosite specifically disrupt interaction with the cofactor A2 subunit, inhibiting factor X activation by the intrinsic tenase complex.[12] This protease exosite is a critical regulator of hemostasis, based on the ability of selected mutations to regulate the rate of plasma thrombin generation and formation of saphenous vein thrombi in response to FeCl3-induced injury in the mouse [21]. To determine the physiologic relevance of serpin-independent inhibition of factor IXa by therapeutic heparins, we employed a modified thrombin generation assay, immuno-depleted plasmas, and a panel of recombinant factor IX proteins with mutations in the heparin-binding exosite to evaluate the contribution of: 1) the intrinsic tenase complex as a therapeutic target, 2) antithrombin-independent inhibition mechanisms, and 3) the heparin binding exosite of factor IXa as a molecular target. The results demonstrate that therapeutic concentrations of LMWH inhibit plasma thrombin generation in an antithrombin-independent manner via interaction with the factor IXa heparin-binding exosite.
Materials and Methods
Materials
Human pooled plasma and factor IX-deficient patient plasmas were purchased from George King (Overland Park, KS). FIX-depleted and FIX/ATIII double depleted human plasmas derived from the same parent plasma, and neutralizing sheep antibody versus human heparin cofactor II (HCII) were purchased from Affinity Biologicals (Ancaster, Ontario, Canada). Corn trypsin inhibitor (CTI), human plasma-derived factor IX, IXa, and thrombin were purchased from Enzyme Research (South Bend, IN). Phosphatidylserine (PS) and phosphatidylcholine (PC) were purchased from Avanti Lipids (Alabaster, AL). Cholesterol was purchased from Calbiochem (San Diego, CA). Phosphatidylcholine: phosphatidlylserine:cholesterol (molar ratio 75:25:1) phospholipid vesicles (PC:PS vesicles) were prepared by extrusion through a 100 nm polycarbonate filter. [22]
The low molecular weight heparin dalteparin was from Eisai, Inc (Woodcliff Lake, New Jersey). Unfractionated porcine intestinal heparin (UFH) with a predominant MW 17,000–19,000 and bovine serum albumin were purchased from Sigma (St. Louis, MO). Fondaparinux was from GlaxoSmithKline (Research Triangle Park, NC). Super-sulfated LMWH (ssLMWH) was generously provided by Dr. Jeffery Weitz (McMaster University, Hamilton, Ontario, Canada). Dimethylsulfoxide (DMSO) was purchased from Mallinckrodt (St. Louis, MO). Lyophilized bovine thrombin-α2-macroglobulin complex was purchased from Diagnostica Stago, Inc. (Parsippany, NJ). Thromborel S, a human thromboplastin from Dade Behring (Deerfield, IL), was used as the source of relipidated human tissue factor. The fluorogenic substrate Z-Gly-Gly-Arg-AMC·HCl was obtained from Bachem (King of Prussia, PA).
Expression and purification of recombinant factor IX
A HEK 293 cell line stably transfected with human factor IX R170A was provided by Darrel Stafford (University of North Carolina-Chapel Hill). [23] Stable HEK 293 cell lines expressing human factor IX wild type (WT), K126A, K132A, R165A, R233A were constructed as described. [19] Recombinant factor IX proteins were purified to homogeneity from conditioned media, and quantified by absorbance at 280 nm.
Fluorogenic assay for detection of plasma thrombin generation
Thrombin generation in human plasma was detected by cleavage of the fluorogenic substrate Z-Gly-Gly-Arg-AMC as previously described, with a 360/40-nm-excitation and 460/40-nm-emission filter set in a Biotek Synergy HT fluorescent plate reader equipped with Gen 5 software (Biotek Instruments, Inc., Winooski, VT).[21] The substrate Z-Gly-Gly-Arg-AMC HCl was reconstituted at 100 mM in DMSO and stored at -20 °C. Prior to each assay, a fresh fluorogenic substrate and calcium solution (Flu·Ca substrate) was prepared by adding 100 µl of 1.0 M CaCl2 to 875 µl of 20 mM HEPES, pH 7.35, 60 g/l BSA at 37°C, followed by 25 µl of 100 mM Z-Gly-Gly-Arg-AMC in DMSO with vigorous mixing to yield a final concentration of 2.5 mM Z-Gly-Gly-Arg-AMC·HCl and 100 mM CaCl2. Plasmas were thawed sequentially on ice for 5 min, at room temperature for 5 min, and in a 37°C water bath for 5 min. Calibration with bovine thrombin-α2-macroglobulin complex calibrator at final plasma concentrations of 500, 250, 50 and 5 nM, was performed as described.[21] The raw data for the first 10 min was imported into Technothrombin TGA evaluation software from Technoclone (Vienna, Austria) to construct calibration curves for each plasma.
The initiator solution was composed of 0.12 mg/ml CTI, 25 µM PC:PS vesicles, 0.6 pM or 12 pM tissue factor and 0–40 µM heparins in TGA buffer. For comparison purposes, weight-based heparin concentrations were converted to approximate molarity using an average molecular weights of 18,000, 5,000, and 1728 daltons for UFH, LMWH/ssLMWH, and Fondaparinux, respectively. Plasma (60 µl) and initiator solution (20 µl) were added to each well and preheated at 37°C for 10 min. Flu·Ca substrate (20 µl) at 37°C was then added, mixed at medium intensity for 5 sec, and readings were obtained at 30 sec intervals for one hour. Final concentrations (extrapolated to the 60 µl plasma volume) were 0.2 pM (limiting) or 4 pM (excess) tissue factor, 8.3 µM PC:PS vesicles, 40 µg/ml CTI, heparin (0–10 or 0–40 µM) and 90 nM plasma-derived or recombinant factor IX (if present). For experiments assessing the contribution of HCII to inhibition of thrombin generation by LMWH, FIX/AT-depleted plasma was pre-incubated with either neutralizing sheep anti-human HCII IgG or control sheep IgG at a 1:9 (v/v) ratio for 20 min at RT.
Fluorescent signal data were exported to Technothrombin® TGA evaluation software, and thrombin generation over time was determined using the appropriate calibration curve for each plasma. Thrombin generation parameters lag time (start until first burst in thrombin formation), peak thrombin concentration, time to thrombin peak, and velocity index (slope between the end of lag time and peak thrombin) were determined using the software. The potency of heparin inhibition of plasma thrombin generation was estimated by plotting inhibitor concentration versus velocity index and fitting the data to the following equation to obtain the EC50:
B represents the fractional inhibition, I represents the concentration of heparin, m represents the response for an “infinite” dose and the interval was set as [0,1), EC50 represents the concentration of heparin that that causes a 50% reduction in the velocity index for thrombin generation, and n represents the pseudo Hill coefficient [24].
The time course of plasma thrombin generation by Western Blotting
Thrombin generation was initiated in 1.5 ml eppendorf tubes with 0.2 pM human tissue factor, 8.3 µM PC:PS vesicles, and 40 µg/ml CTI in mock-depleted plasma with or without indicated concentrations of heparin at 37°C. Individual reactions were quenched by addition of 100 µl 2X SDS-PAGE loading buffer containing 5 M urea at the indicated time points. Quenched samples were incubated at 37°C for 5 min, boiled for 5 min, diluted 1:20 in non-reducing SDS-PAGE loading buffer, and subjected to 4–12% gradient SDS-PAGE, with overnight transfer to Immobilon-P (Millipore, Billerica, MA). Membranes were blocked in 5% milk/1% BSA and detected with polyclonal sheep anti-human thrombin antibody (Haematologic Technologies Inc., Essex Junction, VT) followed by peroxidase-conjugated affinity purified donkey anti-sheep IgG (Jackson Immunoresearch, West Grove, PA) as described.[21] The washed Immobilon-P membrane was immersed in Supersignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL) for 5 min and exposed to Kodak BioMax Xar film (Kodak, Rochester, New York).
Results
Inhibition of plasma thrombin generation by heparins in the presence of limiting or excess tissue factor concentration
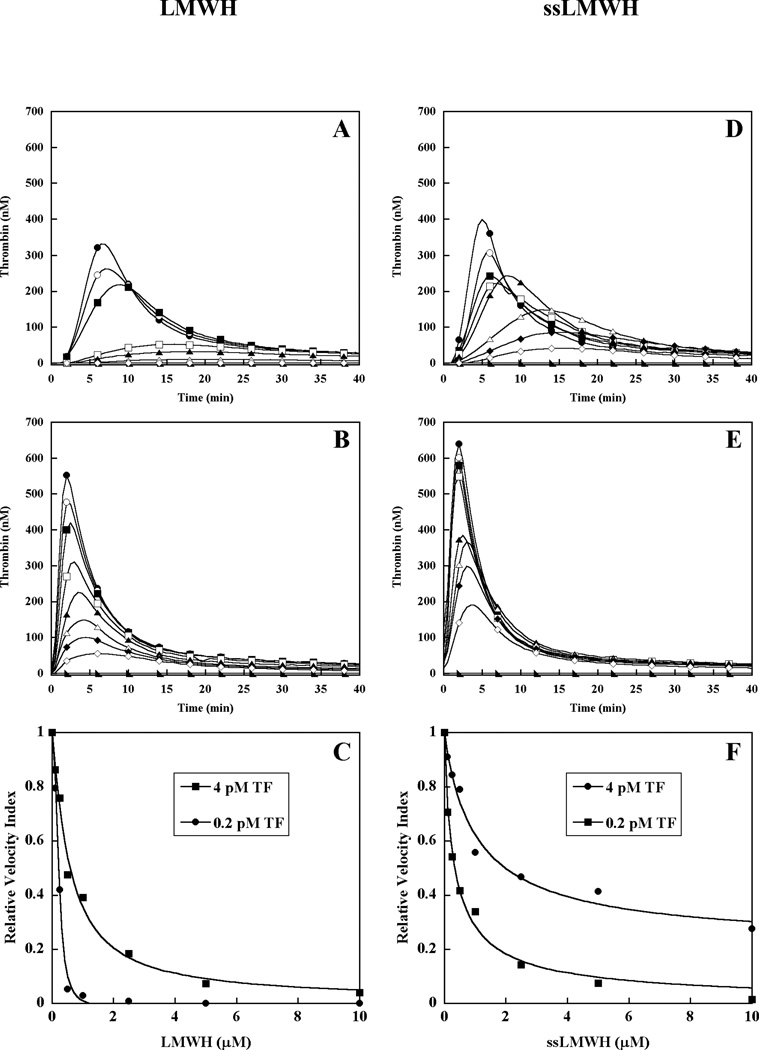
To evaluate the contribution of intrinsic tenase complex as a molecular target for heparin, thrombin generation was triggered by 0.2 or 4 pM tissue factor (TF) in pooled normal human plasma in the presence of increasing concentrations of low molecular weight heparin (LMWH), super-sulfated LMWH (ssLMWH), Fondaparinux, and unfractionated heparin (UFH) (Fig 1). As previously reported in our system, the magnitude of the thrombin response is highly dependent on factor IX when triggered by limiting TF (0.2 pM), but largely independent of factor IX when triggered by excess TF (4 pM).[25] In the absence of TF, pooled plasma alone failed to generate detectable thrombin. Addition of 0.2 pM TF triggered the expected thrombin generation response (Fig 1A, D, G, J) in the absence of heparin, while 4 pM TF triggered a response with moderately enhanced peak thrombin concentration and a shortened lag phase (Fig 1B, E, H, K). Representative dose responses for LMWH, ssLMWH, Fondaparinux, and UFH are shown for each TF condition (Fig 1), and relative heparin potency is expressed as the mean EC50 value for reduction in the velocity index (slope) for thrombin generation (Table I). Our analysis emphasizes the velocity index (slope), as opposed to the “endogenous thrombin potential” (area under the curve), as the former parameter is defined earlier in the time course, and consequently less affected by fluorogenic substrate or prothrombin depletion.[21] LMWH demonstrated a dose-dependent decrease in velocity index (slope) and peak thrombin concentration under both TF conditions, with relatively less prolongation of the time to peak thrombin in the presence of excess TF (Fig 1A-B). Under limiting TF conditions, LMWH completely inhibited plasma thrombin generation, while in the presence of excess TF, modest (5–10%) activity remained at the highest concentration (10 μM) (Fig 1C). Increasing ssLMWH resulted in a similar pattern of inhibition, with dose-dependent reduction in velocity index and peak thrombin concentration, and minimal prolongation of the time to peak thrombin in the presence of excess TF (Fig 1D-E). Partial inhibition of thrombin generation was observed, with residual activity of approximately 5 and 35% for 0.2 and 4 pM TF, respectively (Fig 1F). Finally, Fondaparinux (Fig 1 G-H) and UFH (Fig 1J-K) also demonstrated dose dependent reduction in velocity index and peak thrombin concentration, with modest prolongation of the time to peak thrombin in the presence of either 0.2 or 4 pM TF. Both of these heparins demonstrated complete or near complete inhibition (Fig 1 I & L), with almost identical EC50 values under both TF conditions (Table 1). In contrast, LMWH and ssLMWH demonstrated 2.9- and 5.1-fold lower EC50 values, respectively, in the presence of limiting TF (Table I). The enhanced potency under factor IX-dependent conditions suggests that inhibition of intrinsic tenase activity contributes to the mechanism of action for LMWH and ssLMWH in human plasma.
Figure 1. Effect of heparins on plasma thrombin generation in the presence of limiting or excess tissue factor.
Thrombin generation was initiated with 0.2 pM (panels A, D, G, J) or 4 pM (panels B, E, H, K) human tissue factor (TF), 8.3 µM PC:PS vesicles, and 40 µg/ml CTI (plasma concentrations) in pooled normal human plasma in the presence of increasing heparin concentrations. LMWH, ssLMWH, and Fondaparinux concentrations were 0 (●), 0.1 (○), 0.25 (■), 0.5 (□), 1 (▲), 2.5 (Δ), 5 (◆) and 10 µM (◇). UFH concentrations were: 0 (●), 0.01 (○), 0.1 (■), 0.025 (□), 0.05 (▲), 0.1 (Δ), 0.25 (◆) and 1 µM (◇). Controls included no TF (►) in the absence of heparin. The time course of thrombin generation was measured as described in Material and Methods. Thrombin generation curves represent the mean thrombin concentration over the first 40 min from replicate determinations (n=3), with individual curves identified by representative points. The relative velocity index for thrombin generation was plotted versus heparin concentration for each condition, and fitted as described in Materials and Methods to determine the EC50 for inhibition (panels C, F, I, L). Representative curves are presented.
Table 1.
The EC50 for inhibition of the mean velocity index for thrombin generation by heparin in pooled normal human plasma
| Heparin | 0.2 pM TF EC50 (µM ± SEM) |
4 pM TF EC50 (µM ± SEM) |
Fold-increase |
|---|---|---|---|
| LMWH | 0.20 ± 0.02 | 0.57 ± 0.08 | 2.9 |
| ssLMWH | 0.27 ± 0.03 | 1.4 ± 0.14 | 5.1 |
| UFH | 0.012 ± 0.002 | 0.010 ± 0.001 | 1 |
| Fondaparinux | 0.13 ± 0.003 | 0.12 ± 0.006 | 0.9 |
Thrombin generation was initiated with 0.2 or 4 pM human tissue factor, 8.3 µM PC:PS vesicles, and 40 µg/ml CTI (plasma concentrations) in pooled normal human plasma in the presence of increasing heparin concentrations (LMWH and Fondaparinux 0–10 µM, UFH 0–1 µM). Duplicate wells were averaged to determine individual thrombin generation curves. The relative velocity index was plotted versus heparin concentration for each curve, and the EC50 value determined as described in Material and Methods. The mean EC50 ± S.E. was determined from 3 independent replicates (n=3). Fold-increase describes the ratio between EC50 values obtained in the presence of excess versus limiting TF concentrations.
Effect of heparins on tissue factor triggered plasma thrombin generation as detected by Western Blot
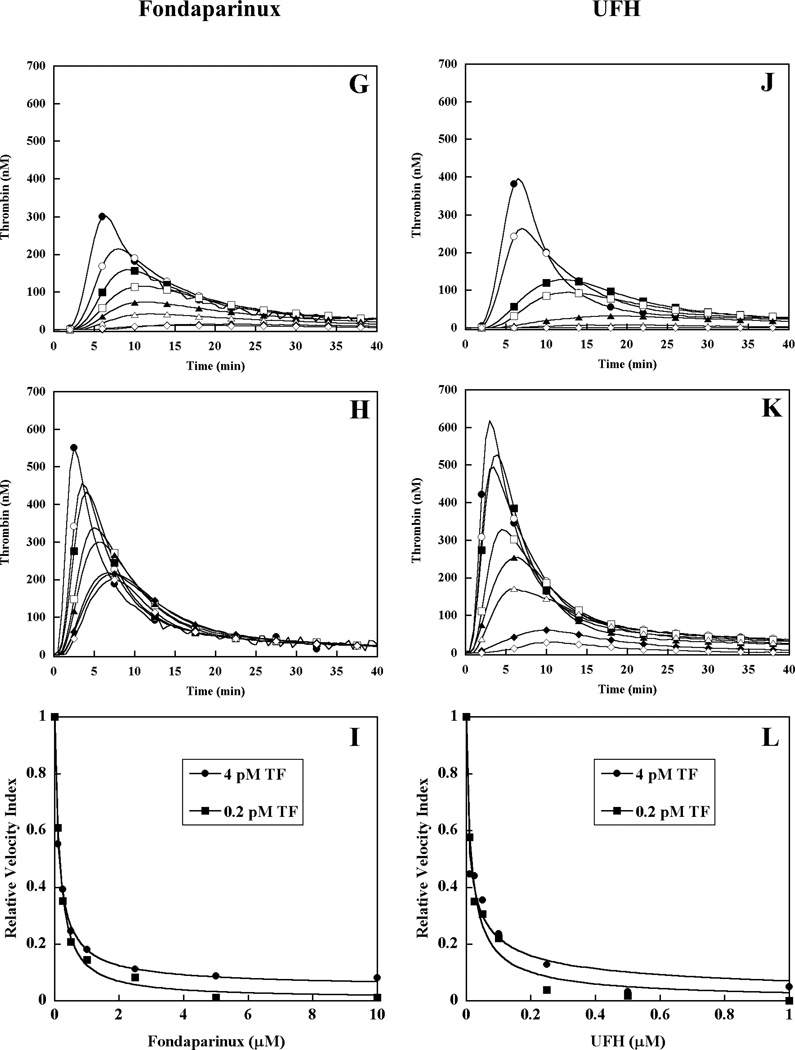
To further assess the relevant mechanism(s) for inhibition of plasma thrombin generation by each heparin, the time course of thrombin activation and inhibition products was monitored by Western blot. Thrombin generation was initiated by 0.2 pM TF in pooled normal human plasma in the absence or presence of each heparin preparation present at their approximate EC50 value (Table I). The reaction products were analyzed by SDS-PAGE under non-reducing conditions using a primary antibody that simultaneously detected prothrombin, thrombin-related cleavage products, and the thrombin-antithrombin complex (TAT) (Fig 2). Pooled normal plasma without heparin demonstrated the initial appearance of free thrombin at 3 min, peak free thrombin at 6 min, initial TAT complex at 6 min with subsequent accumulation over time, and progressive depletion of the prothrombin/meizothrombin band (Fig 2A). In the presence of 0.2 µM LMWH, peak thrombin generation was delayed to 7.5 min, with reduced intensity of the TAT band and less depletion of the prothrombin/meizothrombin band relative to the absence of heparin (Fig 2B). Similarly, addition of ssLMWH (0.25 µM) or Fondaparinux (0.13 µM) resulted in delayed appearance of peak thrombin generation and TAT, and reduced prothrombin/meizothrombin depletion (Fig 2C, 2D). In contrast, addition of UFH (0.012 µM), resulted in delayed peak thrombin but early (3 min) and enhanced appearance of TAT, with depletion of the prothrombin/meizothrombin band that was similar to the absence of heparin (Fig 2E). When UFH was increased to 0.31 µM (1.0 U/ml) there was no discernible free thrombin, TAT accumulation, or prothrombin/meizothrombin depletion (Fig 2F). The effect of heparins on the time course of plasma thrombin generation by Western blotting correlated well with results of the fluorogenic substrate assay. LMWH, ssLMWH and Fondaparinux clearly reduced prothrombin/meizothrombin consumption, while UFH primarily accelerated inhibition of thrombin at their EC50 values in the thrombin generation assay.
Figure 2. Western blot analysis of plasma thrombin generation triggered by limiting tissue factor in the presence of heparins.
Thrombin generation was initiated with 0.2 pM human tissue factor, 8.3 µM PC:PS vesicles, and 40 µg/ml CTI (plasma concentrations) in pooled normal human plasma in the absence (A) and presence of 0.2 µM LMWH (B), 0.25 µM ssLMWH (C), 0.13µM Fondaparinux (D), 0.012 µM and 0.16 µM UFH (E and F). Individual reactions were quenched over time with loading buffer containing 5 M urea, and analyzed by SDS-PAGE under non-reducing conditions as described in Materials and Methods. Proteins transferred to Immobilon-P were detected with a polyclonal sheep anti-human thrombin primary antibody, followed by a peroxidase-conjugated affinity purified donkey anti-sheep IgG, and subsequent development of signal with chemiluminescent substrate.
Effect of heparins on thrombin generation triggered by limiting tissue factor in antithrombin-depleted plasma
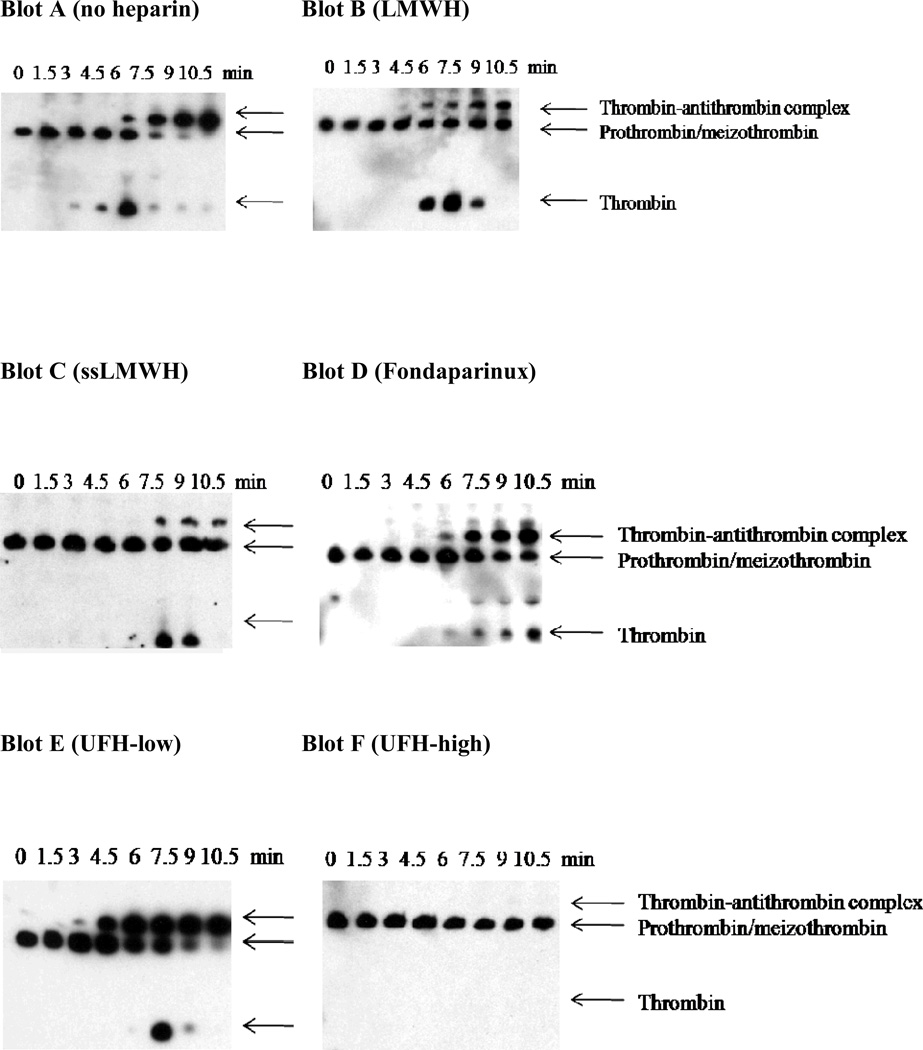
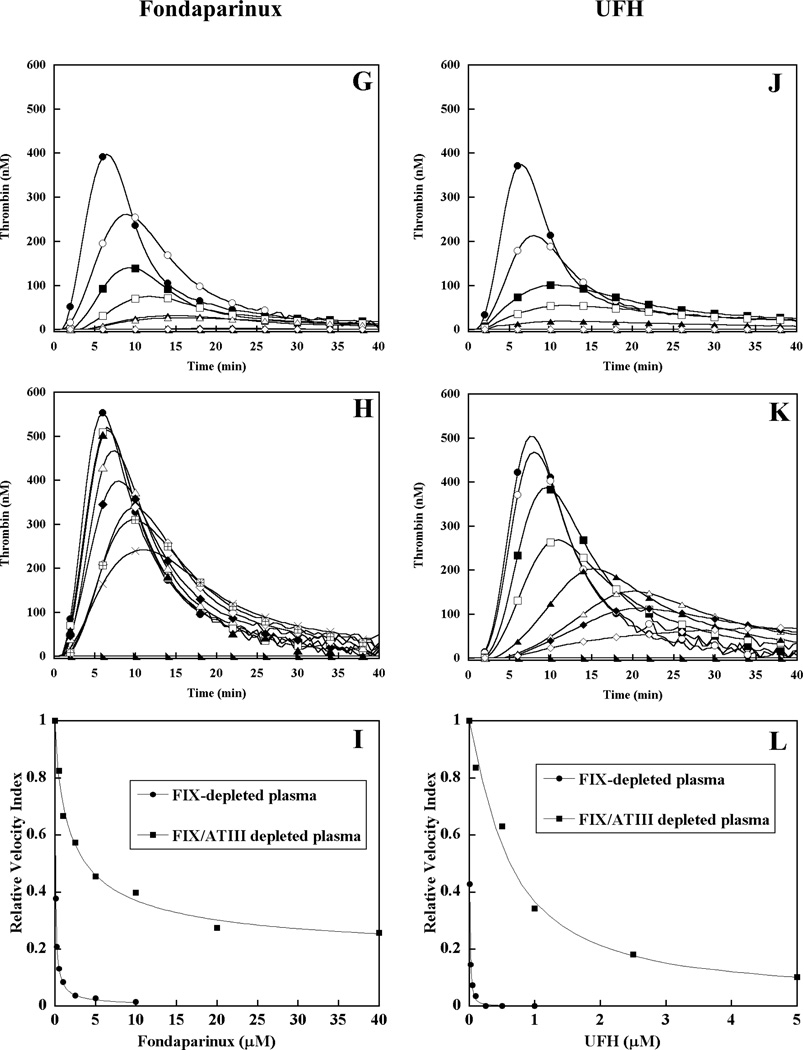
To evaluate the contribution of antithrombin to the heparin inhibition mechanism, thrombin generation triggered by limiting TF (0.2 pM) was compared in factor IX- or factor IX/antithrombin- double depleted plasma supplemented with 100% (90 nM) plasma-derived factor IX (Fig 3). These plasmas underwent identical immune depletion from the same parent plasma to minimize pre-analytical variables in the comparison of control and antithrombin-depleted plasma. In the absence of TF, no detectable thrombin was generated in either factor IX supplemented, immuno-depleted plasma. In the presence of TF, peak thrombin level in the absence of heparin was significantly enhanced in the antithrombin-depleted plasma (Fig 3B, E, H, K) relative to the antithrombin-replete control plasma (Fig 3A, D, G, J). The effect of LMWH on thrombin generation in control plasma was similar to that observed in pooled plasma, with dose-dependent reduction in velocity index and peak thrombin concentration, minimal prolongation of the time to peak thrombin, and complete inhibition (Fig 3A). In antithrombin-depleted plasma, LMWH demonstrated a more gradual reduction in velocity index and peak thrombin, with progressive delay in the time to peak thrombin (Fig 3B). LMWH exhibited partial inhibition in antithrombin-depleted plasma, with residual activity appearing to reach a plateau beyond ~20 µM. Antithrombin-depletion resulted in a 9.4-fold increase in the EC50 for inhibition of thrombin generation by LMWH (Fig 3C, Table 2). The potential contribution of heparin cofactor II (HCII) to inhibition by LMWH was addressed by pre-incubation of the antithrombin-depleted plasma with neutralizing antibodies versus HCII. Neutralization of HCII resulted in a less than 1.4 fold increase in the EC50 for LMWH, suggesting only a modest contribution of this serpin to the inhibition of thrombin generation in the absence of antithrombin (data not shown). ssLMWH demonstrated dose dependent reduction in velocity index and peak thrombin concentration, with progressive delay in the time to peak thrombin in both plasmas (Fig 3D-E). Nearly complete inhibition was observed for ssLMWH in both plasmas. Antithrombin-depletion resulted in only a 2-fold increase in the EC50 for thrombin inhibition by ssLMWH (Fig 3F, Table 2). In contrast, Fondaparinux demonstrated dose dependent reduction in velocity index and peak thrombin concentration with only modest prolongation of the time to peak thrombin, and complete inhibition in control plasma (Fig 3G). This inhibition pattern was greatly blunted with only partial inhibition (~30% residual) observed in antithrombin-depleted plasma (Fig 3H), and only modest delay in peak thrombin activity in either plasma. Antithrombin-depletion resulted in a 42-fold increase in the EC50 for thrombin inhibition by Fondaparinux (Fig 3I, Table 2). Similarly, UFH demonstrated complete inhibition in control plasma without a marked delay in the time to peak thrombin activity (Fig 3J), but only partial inhibition (~25% residual) with progressive delay time to peak thrombin in the antithrombin-depleted plasma (Fig 3K). Antithrombin-depletion resulted in a 63-fold increase in the EC50 for thrombin inhibition by UFH (Fig 3L, Table 2).
Figure 3. Effect of heparins on thrombin generation triggered by limiting tissue factor in factor IX- or factor IX/antithrombin-depleted plasma supplemented with 100% plasma-derived factor IX.
Thrombin generation was initiated with 0.2 pM human tissue factor (TF), 8.3 µM PC:PS vesicles, and 40 µg/ml CTI (plasma concentrations) in FIX- (panels A, D, G, J) or FIX/ATIII- (panels B, E, H, K) depleted plasma supplemented with 90 nM plasma-derived factor IX in the presence of increasing heparin concentrations. LMWH, ssLMWH, and Fondaparinux concentrations were 0 (●), 0.1 (○), 0.25 (■), 0.5 (□), 1 (▲), 2.5 (Δ), 5 (◆), 10 (◇), 20 ( ), and 40 µM (X). UFH concentrations were 0 (●), 0.01 (○), 0.1 (■), 0.025 (□), 0.05 (▲), 0.1 (Δ), 0.25 (◆) and 1 µM (◇) in factor IX-depleted plasma; and 0.1 (○), 0.5 (■), 1 (□), 2.5 (▲), 5 (Δ), 10 (◆) and 20 µM (◇) in factor IX/antithrombin-depleted plasma. Controls included no TF/plasma-derived FIX (►) in the absence of heparin. The time course of thrombin generation was measured as described in Material and Methods. Thrombin generation curves represent the mean thrombin concentration over the first 40 min from replicate determinations (n=3), with individual curves identified by representative points. The relative velocity index for thrombin generation was plotted versus heparin concentration for each condition, and fitted as described in Materials and Methods to determine the EC50 for inhibition (panels C, F, I, L). Representative curves are presented, with the UFH curve truncated to allow better comparison.
), and 40 µM (X). UFH concentrations were 0 (●), 0.01 (○), 0.1 (■), 0.025 (□), 0.05 (▲), 0.1 (Δ), 0.25 (◆) and 1 µM (◇) in factor IX-depleted plasma; and 0.1 (○), 0.5 (■), 1 (□), 2.5 (▲), 5 (Δ), 10 (◆) and 20 µM (◇) in factor IX/antithrombin-depleted plasma. Controls included no TF/plasma-derived FIX (►) in the absence of heparin. The time course of thrombin generation was measured as described in Material and Methods. Thrombin generation curves represent the mean thrombin concentration over the first 40 min from replicate determinations (n=3), with individual curves identified by representative points. The relative velocity index for thrombin generation was plotted versus heparin concentration for each condition, and fitted as described in Materials and Methods to determine the EC50 for inhibition (panels C, F, I, L). Representative curves are presented, with the UFH curve truncated to allow better comparison.
Table 2.
The EC50 for heparin inhibition of the mean velocity index for thrombin generation in FIX or FIX/AT-depleted plasma supplemented with plasma-derived FIX
| Heparin | FIX-depleted plasma EC50(µM ± SEM) |
FIX/AT-depleted plasma EC50(µM ± SEM) |
Fold- increase |
Therapeutic Range |
|---|---|---|---|---|
| LMWH | 0.12 ± 0.01 | 1.1 ± 0.18 | 9.4 | 0.76 – 2.31 µM 0.5 – 1.5 U/ml* |
| ssLMWH | 0.21 ± 0.01 | 0.41 ± 0.03 | 2 | N/A |
| UFH | 0.0092 ± 0.0014 | 0.57 ± 0.03 | 62 | 0.09 – 0.22 µM 0.3 – 0.7 U/ml [2] |
| Fondaparinux | 0.062 ± 0.003 | 2.6 ± 0.36 | 42.4 | 0.31–0.71 µM* |
Thrombin generation was initiated with 0.2 pM human tissue factor, 8.3 µM PC:PS vesicles, and 40 µg/ml CTI (plasma concentrations) in FIX or FIX/AT-depleted human plasma supplemented with 100% plasma-derived factor IX in the presence of increasing heparin concentrations (LMWH and Fondaparinux 0–10 µM, UFH 0–1 µM). Duplicate wells were averaged to determine individual thrombin generation curves. The relative velocity index was plotted versus heparin concentration for each curve, and the EC50 value determined as described in Material and Methods. The mean EC50 ± S.E. was determined from 3 independent replicates (n=3). Fold-increase describes the ratio between EC50 values obtained in antithrombin(AT)-depleted versus control (mock depleted) plasma.
Therapeutic ranges for dalteparin and fondaparinux were obtained from the Micromedex and Physician’s Desk Reference, respectively (accessed online January 15, 2011)
Effect of mutations in the heparin-binding exosite of recombinant factor IX on the ability of LMWH to inhibit tissue factor triggered plasma thrombin generation in the absence of antithrombin
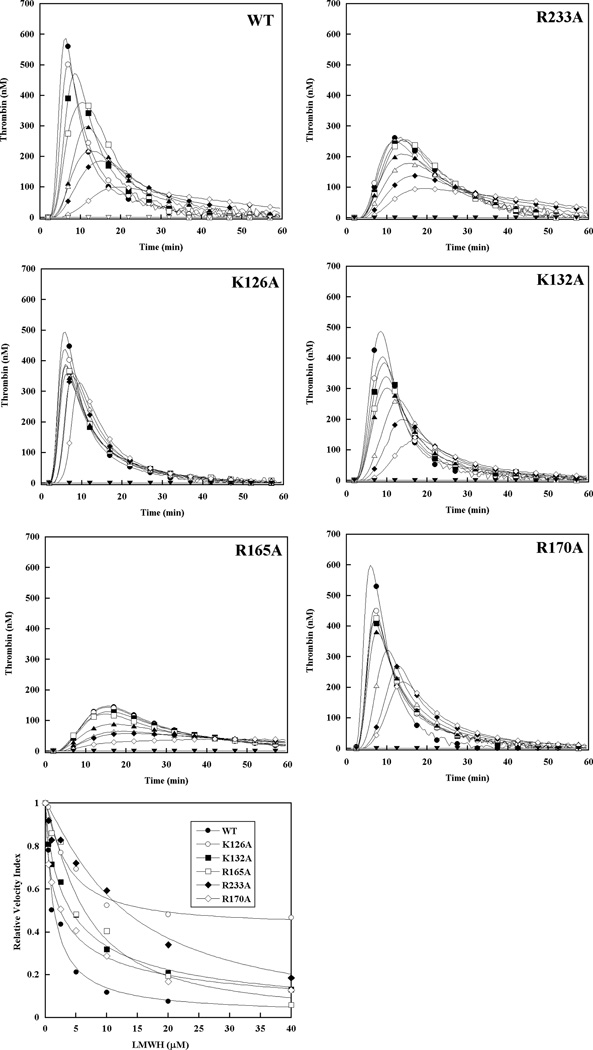
The molecular target for antithrombin-independent inhibition of thrombin generation by LMWH was assessed by supplementing factor IX/antithrombin-depleted plasma with 90 nM recombinant factor IX possessing mutations in the protease heparin binding exosite.[13, 19] When incorporated into the intrinsic tenase complex using purified protein components, the proteases derived from these zymogens demonstrate increased resistance to inhibition by LMWH (R233A>R126A>R165A/K132A/R170A>WT).[20] In the absence of LMWH, these recombinant factor IX mutants also showed different baseline thrombin generation in human plasma: peak thrombin levels were similar for factor IX WT and R170A (or modestly enhanced), modestly reduced for factor IX K126A and K132A, and significantly blunted and delayed for factor IX R233A and R165A (Fig 4), consistent with the effect of these mutations on the protease-cofactor interaction.[13, 19] Minimal thrombin response was noted in the absence of factor IX. Increasing LMWH concentrations resulted in a progressive reduction in velocity index (slope) and peak thrombin, and prolongation of the time to peak thrombin concentration (Fig 4). Similar to plasma-derived factor IX, LMWH partially inhibited thrombin generation in the presence of factor IX WT, with residual activity (~5–10%) even at 40 µM. Recombinant factor IX with reduced heparin affinity demonstrated relative resistance to inhibition of thrombin generation, similar to that observed for inhibition of the intrinsic tenase complex with the purified protease forms.[20] In particular, factor IX R233A was highly resistant to LMWH inhibition of thrombin generation (Fig 4), with an 11-fold increase in EC50 value relative to factor IX WT (Table 3). Factor IX K126A demonstrated a 4.3-fold increase in EC50 for LMWH with a marked increase in residual activity (~45%) relative to wild type protease at high concentrations (Fig 4Table 3). Based on both relative EC50 values for reduction in the velocity index and the maximal degree of inhibition, plasma supplemented with mutant factor IX demonstrated relative resistance to inhibition of thrombin generation by LMWH as follows: R233A> (K126A/R165A/K132A) > R170A > WT (Table 3).
Figure 4. Effect of recombinant factor IX on the ability of LMWH to inhibit plasma thrombin generation triggered by tissue factor in antithrombin-depleted plasma.
Thrombin generation was initiated with 0.2 pM human tissue factor (TF), 8.3 µM PC:PS vesicles, and 40 µg/ml CTI (plasma concentrations) in FIX/AT-depleted plasma supplemented with 100% recombinant FIX (90 nM) in the presence of increasing LMWH: 0 (●), 0.5 (○), 1 (■), 2.5 (□), 5 (▲), 10 (Δ), 20 (◆) and 40 µM (◇). Controls included no TF/recombinant FIX (▼) and 0.2 pM TF only (∇) in the absence of LMWH. The time course of thrombin generation was measured as described in Material and Methods. Thrombin generation curves represent the mean thrombin concentration over the first 40 min of assay from replicate determinations (n=3), and are identified by representative points. The relative velocity index for thrombin generation was plotted versus LMWH concentration and fitted as described in Materials and Methods to determine the EC50 for inhibition. Representative curves are presented.
Table 3.
The EC50 for inhibition of the mean velocity index for thrombin generation by heparin in FIX/AT-depleted plasma supplemented with 100% recombinant factor IX
| rFIX | LMWH (µM ± SEM) |
Fold-increase (relative to WT) |
ssLMWH (µM ± SEM) |
Fold-increase (relative to WT) |
|---|---|---|---|---|
| WT | 1.00 ± 0.07 | 1 | 0.41 ± 0.03 | 1 |
| R233A | 11.4 ± 1.02 | 11.4 | 2.16 ± 0.12 | 5.23 |
| K126A | 4.34 ± 0.09 | 4.34 | 1.43 ± 0.05 | 3.46 |
| R165A | 5.80 ± 0.24 | 5.80 | 1.39 ± 0.09 | 3.37 |
| R170A | 2.26 ± 0.14 | 2.26 | 0.73 ± 0.06 | 1.76 |
| K132A | 4.08 ± 0.22 | 4.08 | 1.29 ± 0.14 | 3.11 |
Thrombin generation was initiated with 0.2 pM human tissue factor, 8.3 µM PC:PS vesicles, and 40 µg/ml CTI (plasma concentrations) in FIX/AT-depleted plasma supplemented with 100% recombinant factor IX in the presence of increasing heparin concentrations (ssLMWH 0–10 µM, LMWH 0–40 µM). Duplicate wells were averaged to determine individual thrombin generation curves. The relative velocity index was plotted versus heparin concentration for each curve, and the EC50 value determined as described in Material and Methods. The mean EC50 ± S.E. was determined from 3 independent replicates (n=3).
Effect of mutations in the heparin-binding exosite of recombinant factor IX on the ability of ssLMWH to inhibit tissue factor triggered plasma thrombin generation in the absence of antithrombin
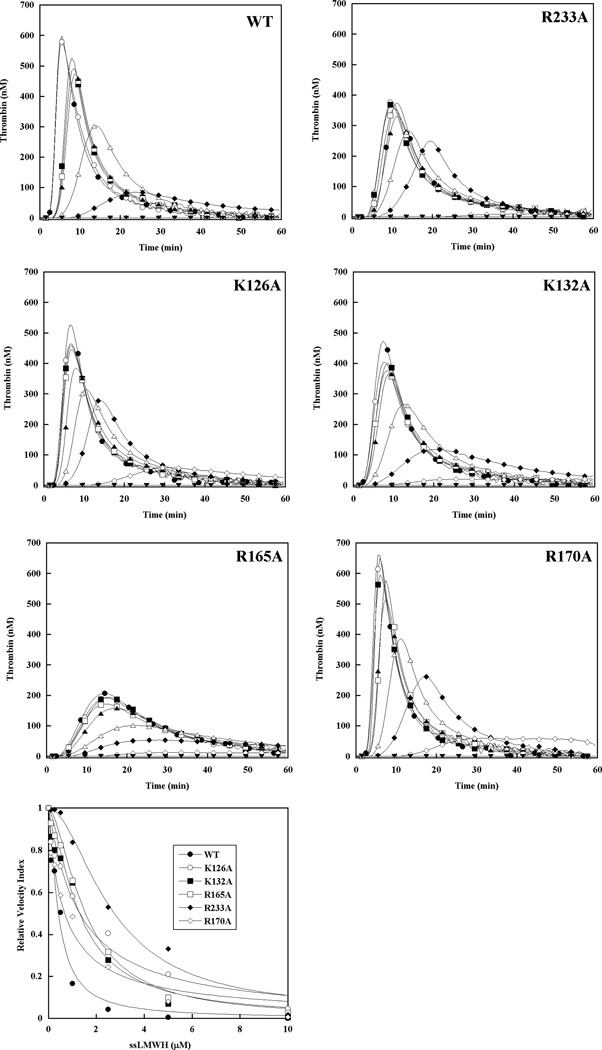
The molecular target for antithrombin-independent inhibition of thrombin generation by ssLMWH was similarly assessed in factor IX/antithrombin-depleted plasma supplemented with 90 nM recombinant factor IX (Fig 5). Increasing ssLMWH resulted in a progressive reduction in velocity index (slope) and peak thrombin, and prolongation of the time to peak thrombin concentration similar to LMWH (Fig 5). In contrast, ssLMWH demonstrated near complete inhibition of plasma thrombin generation in the presence of factor IX WT, and enhanced potency relative to LMWH for all recombinant factor IX proteins based on relative EC50 values (Table 3). Recombinant factor IX with reduced heparin affinity demonstrated relative resistance to inhibition of thrombin generation compared with the wild type protein, similar to that observed for inhibition of the intrinsic tenase complex with purified components.[20] The magnitude of the differences between mutant and wild type proteins was reduced for ssLMWH relative to LMWH (Table 3), with less pronounced tendency towards partial inhibition among the mutant factor IX proteins (Fig 5). However, the rank order of relative resistance to inhibition of thrombin generation based on the EC50 values for reduction in the velocity index for thrombin generation remained similar: R233A>(K126A/R165A/K132A)> R170A>WT (Table 3).
Figure 5. Effect of recombinant factor IX on the ability of ssLMWH to inhibit plasma thrombin generation triggered by tissue factor in antithrombin-depleted plasma.
Thrombin generation was initiated with 0.2 pM human tissue factor (TF), 8.3 µM PC:PS vesicles, and 40 µg/ml CTI (plasma concentrations) in FIX/AT-depleted plasma supplemented with 100% recombinant FIX (90 nM) in the presence of increasing ssLMWH: 0 (●), 0.5 (○), 1 (■), 2.5 (□), 5 (▲), 10 (Δ), 20 (◆) and 40 µM (◇). Controls included no TF/recombinant FIX (▼) and 0.2 pM TF only (∇) in the absence of ssLMWH. The time course of thrombin generation was measured as described in Material and Methods. Thrombin generation curves represent the mean thrombin concentration over the first 40 min of assay from replicate determinations (n=3), and are identified by representative points. The relative velocity index for thrombin generation was plotted versus LMWH concentration and fitted as described in Materials and Methods to determine the EC50 for inhibition. Representative curves are presented.
Discussion
Heparin is a complex, heterogeneous mixture of oligosaccharide chains capable of inhibiting multiple steps in blood coagulation via both antithrombin-dependent and independent mechanisms. LMWH demonstrates more predictable pharmacokinetics and reduced ability to accelerate thrombin inhibition via a “template” mechanism, but retains the heterogeneity of UFH.[2] Accelerated inhibition of factor Xa via conformational activation of antithrombin is generally accepted as the predominant therapeutic mechanism for LMWH. We examined the potential contribution of antithrombin-independent intrinsic tenase inhibition to the anticoagulant activity of LMWH in a physiologically relevant system. The biologic significance of tissue factor-triggered plasma thrombin generation measurements is supported by the association of enhanced thrombin generation with increased risk of recurrent VTE [26, 27], and the ability to discern the clinical severity of bleeding phenotypes in hemophilia [28]. Our results demonstrate the potential relevance of antithrombin-independent inhibition of factor IXa as a therapeutic target for LMWH. While UFH and Fondaparinux demonstrated similar potency for inhibition of plasma thrombin generation under both factor IX-dependent (limiting TF) and independent (excess TF) conditions [25], LMWH and ssLMWH demonstrated increased potency with limiting TF (Fig 1Table 1). Western blot analysis of thrombin generation suggests that ssLMWH, LMWH, Fondaparinux, and UFH (1.0 U/ml) act predominantly to reduce prothrombin activation, rather than accelerating thrombin inhibition by antithrombin (Fig 2). Based on the equivalent clinical outcomes for UFH and LMWH in the treatment of VTE [6–8], these results suggest that thrombin inhibition is not critical to the therapeutic action of these heterogeneous heparin preparations. In contrast, the enhanced potency of LMWH/ssLMWH under factor IX-dependent conditions (Table I) suggests that inhibition of the intrinsic tenase complex contributes to LMWH activity in human plasma.
The contribution of antithrombin-independent mechanisms to the inhibition of thrombin generation by LMWH was demonstrated in antithrombin-depleted plasma. Fondaparinux and UFH demonstrated markedly reduced potency (Fig 3 I & L) with EC50 values approximately 3–8 fold higher than their therapeutic ranges (Table 2), confirming dependence on antithrombin-dependent mechanisms. In contrast, LMWH and ssLMWH were substantially less affected by antithrombin depeletion (Figure 3 B & E, Table 2). Remarkably, the EC50 (1.13 ± 0.18 µM) for LMWH remained within the therapeutic range (0.76–2.31 µM or 0.5–1.5 U/ml), suggesting that antithrombin-independent mechanisms may contribute to LMWH activity in human plasma (Table 2). The LMWH dose response in antithrombin-depleted plasma demonstrated partial inhibition of thrombin generation with a plateau of ~ 8–10% starting activity (Fig 3 A-C), similar to inhibition of intrinsic tenase activity by LMWH with purified components.[12] Likewise, recombinant factor IX possessing mutations that reduce protease-heparin affinity (R233A, K126A, R165A, K132A) and/or increase cofactor affinity (R170A) demonstrated resistance to LMWH inhibition of thrombin generation in antithrombin-depleted plasma (Figs 4 & 5), with the rank order of EC50 values (Table 3) roughly correlating with the KI values for intrinsic tenase inhibition by LMWH with purified components.[13, 19, 20] These results suggest that therapeutic LMWH concentrations inhibit plasma thrombin generation via an antithrombin-independent interaction with the factor IXa heparin-binding exosite. Additionally, the dose response demonstrates partial inhibition at high LMWH concentrations, which may broaden the therapeutic range and limit bleeding risk associated with this anticoagulant mechanism.
This mechanism for plasma thrombin inhibition is supported by studies performed with purified protein components and antithrombin-independent glycosaminoglycans. These studies demonstrate that: 1) LMWH inhibits factor X activation by factor IXa (in the presence or absence of cofactor) via interaction with a protease exosite that overlaps with a critical factor VIIIa binding site [12, 19, 20], 2) mutations in the protease domain which reduce factor IXa-heparin affinity result in resistance to LMWH inhibition of factor X activation by factor IXa-phospholipid or the intrinsic tenase complex [13, 20], 3) LMWH and fucosylated chondroitin sulfate (a.k.a. depolymerized holothurian glycosaminoglycan or DHG) bind to overlapping sites on the factor IXa protease domain, and 4) protease mutations that reduce factor IXa-heparin affinity also result in resistance to inhibition by DHG.[29] Likewise, studies in human plasma (and the murine model of hemophilia B) demonstrate that: 1) mutations within the factor IXa heparin binding site regulate plasma thrombin generation and in vivo thrombosis[21], 2) DHG inhibits plasma thrombin generation via interaction with the factor IXa heparin-binding exosite [25], and 3) LMWH inhibits plasma thrombin generation via an antithrombin-independent interaction with the same protease exosite (this manuscript). The purified system employs a two step assay in which cofactor is activated by excess thrombin, neutralized by hirudin, and the thrombin-activated factor VIIIa immediately added to the tenase reaction at ten-fold molar excess over factor IXa. Under these conditions, a significant contribution from “feedback” activation of factor VIII by factor Xa is highly unlikely based on near complete cofactor activation by excess thrombin in the first step, and extensive saturation of the protease with factor VIIIa in the second step. Clearly, multiple mechanisms are possible in plasma, but the primacy of intrinsic tenase inhibition via interaction with the protease heparin-binding exosite is supported by the common antithrombin-independent inhibition mechanism established for DHG and LMWH, the absence of a significant effect of LMWH on factor IX activation (data not shown), and the dominant effect of mutations in the heparin-binding exosite of factor IXa on the inhibition of thrombin generation. Addition of either 700 pM factor VIII or thrombin-activated factor VIIIa to factor VIII deficient plasma, does not significantly affect inhibition of tissue factor-triggered thrombin generation by DHG, indicating that effects on cofactor activation do not contribute significantly to this glycosaminoglycan-mediated, antithrombin-independent inhibition mechanism.[25] The inability to bypass this inhibition with activated cofactor (or activated protease), along with the dominant effect of the factor IXa heparin-binding site mutations, provides strong support for direct interaction with the protease as the dominant inhibition mechanism.
The rationale for factor IXa as an important antithrombotic target is provided by the markedly enhanced potency of this protease for triggering thrombin generation relative to factor Xa, and the significantly higher in vivo thrombogenicity of this protease relative to both factor Xa and thrombin.[15, 30] Factor IXa-induced thrombus formation also demonstrates enhanced sensitivity to heparin inhibition relative to factor Xa and thrombin.[30] Targeting this rate-limiting step interferes with amplification of the coagulation response and provides the broadest dynamic range of inhibition.[31] Furthermore, an intact tissue factor (TF) pathway facilitates the use of bypass agents (ex: factor VIIa) for bleeding or emergent procedures. Targeting intrinsic tenase activity with active-site blocked factor IXa, monoclonal antibody versus the factor IX(a) Gla domain, or a fucosylated chondroitin sulfate (a.k.a. depolymerized holothurian glycosaminoglycan or DHG) demonstrates reduced bleeding risk relative to equitherapeutic doses of UFH/LMWH in animal models of thrombosis or cardiac bypass. [32–37] Thus, both theoretical considerations and animal models suggest that selective factor IX(a) inhibition will reduce the bleeding risk associated with antithrombotic therapy.
The need for antithrombotic agents with reduced bleeding risk is particularly important for patients with malignancy, based on their significantly higher rates of both VTE recurrence and bleeding.[38] LMWH demonstrates superior efficacy in preventing VTE recurrence versus standard therapy with vitamin K antagonists in this patient population.[39] Furthermore, the anti-inflammatory, anti-metastatic, and anti-tumor effects of “non-anticoagulant” heparins in animal models may help explain the potential favorable impact of LMWH on overall mortality in cancer patients.[40, 41] Although hypersulfation of LMWH enhances in vitro affinity for factor IXa [14], in vivo use of oversulfated glycosaminoglycans may be problematic, as exemplified by the life-threatening anaphylactic reactions triggered by oversulfated chondroitin sulfate contaminants in heparin preparations.[42, 43] Furthermore, oversulfated glycosaminoglycans may demonstrate reduced potency and bioavailability due to increased nonspecific binding interactions. For example, supersulfated LMWH and depolymerized fucosylated chondroitin sulfate demonstrate significant disparity between their relative inhibitory potencies for in vitro intrinsic tenase activity (KI) versus ex vivo plasma thrombin generation (EC50) relative to LMWH. While >300-fold differences in apparent inhibitor affinity for factor IXa exist in the purified enzyme assays (DHG > ssLMWH > LMWH), the maximal differences are <3-fold for inhibition of plasma thrombin generation.[3, 20, 25, 29] The clearest comparison is between ssLMWH and LMWH, with the former demonstrating 45-fold higher potency in assays with purified proteins, but <2.5-fold higher potency in antithrombin-depleted plasma. These differences are likely explained by increased plasma protein binding, as previously demonstrated in the comparison between UFH and LMWH.[44] Thus, a LMWH retaining only the chain modifications required for factor IXa binding should maximize tenase inhibitory potency by reducing plasma protein binding and antithrombin-dependent activity (elimination of 3-O sulfation). Despite the favorable risk profile of antithrombin-independent glycosaminoglycans in animal models of thrombosis[36, 37], only a very limited clinical evaluation of chemically modified LMWH has been reported.[45] Chemoenzymatic synthesis of homogenous LMWH preparations potentially allows for specific targeting of factor IXa and/or other important therapeutic targets.[46] Targeting of factor IXa with a defined LMWH preparation would represent a novel antithrombotic approach for high-risk populations.
Acknowledgements
This research was supported by National Institutes of Health grant HL080452 (J.P.S). We would like to thank Darrell Stafford for providing the factor IX R170A cell line, Andreas Mueller-Beckhaus of Bayer HealthCare, LLC for recombinant factor VIII (Kogenate FS); Jeff Weitz for super-sulfated low molecular weight heparin, Hugh Hoogendoorn of Affinity Biologicals for providing immunodepleted plasmas, and Technoclone, Ltd. for the Technothrombin TGA software for analysis of plasma thrombin generation.
References
- 1.Murray GD. Heparin in Thrombosis and Embolism. British Journal of Surgery. 1939:567–598. [Google Scholar]
- 2.Hirsh J, Bauer KA, Donati MB, Gould M, Samama MM, Weitz JI. Parenteral anticoagulants: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:141S–159S. doi: 10.1378/chest.08-0689. [DOI] [PubMed] [Google Scholar]
- 3.Barrow RT, Parker ET, Krishnaswamy S, Lollar P. Inhibition by heparin of the human blood coagulation intrinsic pathway factor X activator. Journal of Biological Chemistry. 1994;269:26796–26800. [PubMed] [Google Scholar]
- 4.Lam LH, Silbert JE, Rosenberg RD. The separation of active and inactive forms of heparin. Biochem Biophys Res Commun. 1976;69:570–577. doi: 10.1016/0006-291x(76)90558-1. [DOI] [PubMed] [Google Scholar]
- 5.Petitou M, Herault JP, Bernat A, Driguez PA, Duchaussoy P, Lormeau JC, Herbert JM. Synthesis of thrombin-inhibiting heparin mimetics without side effects. Nature. 1999;398:417–422. doi: 10.1038/18877. [DOI] [PubMed] [Google Scholar]
- 6.Low-molecular-weight heparin in the treatment of patients with venous thromboembolism. The Columbus Investigators. N Engl J Med. 1997;337:657–662. doi: 10.1056/NEJM199709043371001. [DOI] [PubMed] [Google Scholar]
- 7.Koopman MM, Prandoni P, Piovella F, Ockelford PA, Brandjes DP, van der Meer J, Gallus AS, Simonneau G, Chesterman CH, Prins MH. Treatment of venous thrombosis with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous low-molecular-weight heparin administered at home. The Tasman Study Group. N Engl J Med. 1996;334:682–687. doi: 10.1056/NEJM199603143341102. [DOI] [PubMed] [Google Scholar]
- 8.Levine M, Gent M, Hirsh J, Leclerc J, Anderson D, Weitz J, Ginsberg J, Turpie AG, Demers C, Kovacs M. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med. 1996;334:677–681. doi: 10.1056/NEJM199603143341101. [DOI] [PubMed] [Google Scholar]
- 9.Buller HR, Davidson BL, Decousus H, Gallus A, Gent M, Piovella F, Prins MH, Raskob G, Segers AE, Cariou R, Leeuwenkamp O, Lensing AW. Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med. 2004;140:867–873. doi: 10.7326/0003-4819-140-11-200406010-00007. [DOI] [PubMed] [Google Scholar]
- 10.Buller HR, Davidson BL, Decousus H, Gallus A, Gent M, Piovella F, Prins MH, Raskob G, van den Berg-Segers AE, Cariou R, Leeuwenkamp O, Lensing AW. Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med. 2003;349:1695–1702. doi: 10.1056/NEJMoa035451. [DOI] [PubMed] [Google Scholar]
- 11.Gray E, Cesmeli S, Lormeau JC, Davies AB, Lane DA. Low affinity heparin is an antithrombotic agent. Thrombosis & Haemostasis. 1994;71:203–207. [PubMed] [Google Scholar]
- 12.Sheehan JP, Kobbervig CE, Kirkpatrick HM. Heparin inhibits the intrinsic tenase complex by interacting with an exosite on factor IXa. Biochemistry. 2003;42:11316–11325. doi: 10.1021/bi0342923. [DOI] [PubMed] [Google Scholar]
- 13.Yuan QP, Walke EN, Sheehan JP. The factor IXa heparin-binding exosite is a cofactor interactive site: mechanism for antithrombin-independent inhibition of intrinsic tenase by heparin. Biochemistry. 2005;44:3615–3625. doi: 10.1021/bi047934a. [DOI] [PubMed] [Google Scholar]
- 14.Anderson JA, Fredenburgh JC, Stafford AR, Guo YS, Hirsh J, Ghazarossian V, Weitz JI. Hypersulfated low molecular weight heparin with reduced affinity for antithrombin acts as an anticoagulant by inhibiting intrinsic tenase and prothrombinase. J Biol Chem. 2001;276:9755–9761. doi: 10.1074/jbc.M010048200. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman M, Monroe DM, Oliver JA, Roberts HR. Factors IXa and Xa play distinct roles in tissue factor-dependent initiation of coagulation. Blood. 1995;86:1794–1801. [PubMed] [Google Scholar]
- 16.Lawson JH, Kalafatis M, Stram S, Mann KG. A model for the tissue factor pathway to thrombin. I. An empirical study. J Biol Chem. 1994;269:23357–23366. [PubMed] [Google Scholar]
- 17.Rand MD, Lock JB, van t Veer C, Gaffney DP, Mann KG. Blood clotting in minimally altered whole blood. Blood. 1996;88:3432–3445. [PubMed] [Google Scholar]
- 18.Fay PJ, Koshibu K. The A2 subunit of factor VIIIa modulates the active site of factor IXa. J Biol Chem. 1998;273:19049–19054. doi: 10.1074/jbc.273.30.19049. [DOI] [PubMed] [Google Scholar]
- 19.Misenheimer TM, Buyue Y, Sheehan JP. The heparin-binding exosite is critical to allosteric activation of factor IXa in the intrinsic tenase complex: the role of arginine 165 and factor X. Biochemistry. 2007;46:7886–7895. doi: 10.1021/bi7004703. [DOI] [PubMed] [Google Scholar]
- 20.Misenheimer TM, Sheehan JP. The regulation of factor IXa by supersulfated low molecular weight heparin. Biochemistry. 2010 doi: 10.1021/bi100906q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buyue Y, Whinna HC, Sheehan JP. The heparin-binding exosite of factor IXa is a critical regulator of plasma thrombin generation and venous thrombosis. Blood. 2008;112:3234–3241. doi: 10.1182/blood-2008-01-136820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacDonald RC, MacDonald RI, Menco BP, Takeshita K, Subbarao NK, Hu LR. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochimica et Biophysica Acta. 1991;1061:297–303. doi: 10.1016/0005-2736(91)90295-j. [DOI] [PubMed] [Google Scholar]
- 23.Chang J, Jin J, Lollar P, Bode W, Brandstetter H, Hamaguchi N, Straight DL, Stafford DW. Changing residue 338 in human factor IX from arginine to alanine causes an increase in catalytic activity. J Biol Chem. 1998;273:12089–12094. doi: 10.1074/jbc.273.20.12089. [DOI] [PubMed] [Google Scholar]
- 24.Limbird L. Cell Surface Receptors: A Short Course on Theory and Methods. Boston: Kluwer Academic Publishers; 1995. [Google Scholar]
- 25.Buyue Y, Sheehan JP. Fucosylated chondroitin sulfate inhibits plasma thrombin generation via targeting of the factor IXa heparin-binding exosite. Blood. 2009;114:3092–3100. doi: 10.1182/blood-2009-02-203661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutsey PL, Folsom AR, Heckbert SR, Cushman M. Peak thrombin generation and subsequent venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology (LITE) study. J Thromb Haemost. 2009;7:1639–1648. doi: 10.1111/j.1538-7836.2009.03561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hron G, Kollars M, Binder BR, Eichinger S, Kyrle PA. Identification of patients at low risk for recurrent venous thromboembolism by measuring thrombin generation. Jama. 2006;296:397–402. doi: 10.1001/jama.296.4.397. [DOI] [PubMed] [Google Scholar]
- 28.Brummel-Ziedins KE, Whelihan MF, Gissel M, Mann KG, Rivard GE. Thrombin generation and bleeding in haemophilia A. Haemophilia. 2009;15:1118–1125. doi: 10.1111/j.1365-2516.2009.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheehan JP, Walke EN. Depolymerized holothurian glycosaminoglycan and heparin inhibit the intrinsic tenase complex by a common antithrombin-independent mechanism. Blood. 2006;107:3876–3882. doi: 10.1182/blood-2005-07-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gitel SN, Stephenson RC, Wessler S. In vitro and in vivo correlation of clotting protease activity: effect of heparin. Proc Natl Acad Sci U S A. 1977;74:3028–3032. doi: 10.1073/pnas.74.7.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eikelboom JW, Zelenkofske SL, Rusconi CP. Coagulation factor IXa as a target for treatment and prophylaxis of venous thromboembolism. Arterioscler Thromb Vasc Biol. 2010;30:382–387. doi: 10.1161/ATVBAHA.110.203117. [DOI] [PubMed] [Google Scholar]
- 32.Benedict CR, Ryan J, Wolitzky B, Ramos R, Gerlach M, Tijburg P, Stern D. Active site-blocked factor IXa prevents intravascular thrombus formation in the coronary vasculature without inhibiting extravascular coagulation in a canine thrombosis model. J Clin Invest. 1991;88:1760–1765. doi: 10.1172/JCI115495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choudhri TF, Hoh BL, Prestigiacomo CJ, Huang J, Kim LJ, Schmidt AM, Kisiel W, Connolly ES, Jr, Pinsky DJ. Targeted inhibition of intrinsic coagulation limits cerebral injury in stroke without increasing intracerebral hemorrhage. J Exp Med. 1999;190:91–99. doi: 10.1084/jem.190.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feuerstein GZ, Patel A, Toomey JR, Bugelski P, Nichols AJ, Church WR, Valocik R, Koster P, Baker A, Blackburn MN. Antithrombotic efficacy of a novel murine antihuman factor IX antibody in rats. Arterioscler Thromb Vasc Biol. 1999;19:2554–2562. doi: 10.1161/01.atv.19.10.2554. [DOI] [PubMed] [Google Scholar]
- 35.Refino CJ, Jeet S, DeGuzman L, Bunting S, Kirchhofer D. A human antibody that inhibits factor IX/IXa function potently inhibits arterial thrombosis without increasing bleeding. Arterioscler Thromb Vasc Biol. 2002;22:517–522. doi: 10.1161/hq0302.105375. [DOI] [PubMed] [Google Scholar]
- 36.Nagase H, Kitazato KT, Sasaki E, Hattori M, Kitazato K, Saito H. Antithrombin III-independent effect of depolymerized holothurian glycosaminoglycan (DHG) on acute thromboembolism in mice. Thrombosis & Haemostasis. 1997;77:399–402. [PubMed] [Google Scholar]
- 37.Kitazato K, Kitazato KT, Nagase H, Minamiguchi K. DHG, a new depolymerized holothurian glycosaminoglycan, exerts an antithrombotic effect with less bleeding than unfractionated or low molecular weight heparin, in rats. Thromb Res. 1996;84:111–120. doi: 10.1016/0049-3848(96)00166-1. [DOI] [PubMed] [Google Scholar]
- 38.Prandoni P, Lensing AW, Piccioli A, Bernardi E, Simioni P, Girolami B, Marchiori A, Sabbion P, Prins MH, Noventa F, Girolami A. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 39.Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, Rickles FR, Julian JA, Haley S, Kovacs MJ, Gent M. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 40.Casu B, Vlodavsky I, Sanderson RD. Non-anticoagulant heparins and inhibition of cancer. Pathophysiology of haemostasis and thrombosis. 2008;36:195–203. doi: 10.1159/000175157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuderer NM, Ortel TL, Francis CW. Impact of venous thromboembolism and anticoagulation on cancer and cancer survival. J Clin Oncol. 2009;27:4902–4911. doi: 10.1200/JCO.2009.22.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guerrini M, Beccati D, Shriver Z, Naggi A, Viswanathan K, Bisio A, Capila I, Lansing JC, Guglieri S, Fraser B, Al-Hakim A, Gunay NS, Zhang Z, Robinson L, Buhse L, Nasr M, Woodcock J, Langer R, Venkataraman G, Linhardt RJ, Casu B, Torri G, Sasisekharan R. Oversulfated chondroitin sulfate is a contaminant in heparin associated with adverse clinical events. Nature biotechnology. 2008;26:669–675. doi: 10.1038/nbt1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kishimoto TK, Viswanathan K, Ganguly T, Elankumaran S, Smith S, Pelzer K, Lansing JC, Sriranganathan N, Zhao G, Galcheva-Gargova Z, Al-Hakim A, Bailey GS, Fraser B, Roy S, Rogers-Cotrone T, Buhse L, Whary M, Fox J, Nasr M, Dal Pan GJ, Shriver Z, Langer RS, Venkataraman G, Austen KF, Woodcock J, Sasisekharan R. Contaminated heparin associated with adverse clinical events and activation of the contact system. N Engl J Med. 2008;358:2457–2467. doi: 10.1056/NEJMoa0803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young E, Cosmi B, Weitz J, Hirsh J. Comparison of the non-specific binding of unfractionated heparin and low molecular weight heparin (Enoxaparin) to plasma proteins. Thromb Haemost. 1993;70:625–630. [PubMed] [Google Scholar]
- 45.Peters RJ, Spickler W, Theroux P, White H, Gibson M, Molhoek PG, Anderson HV, Weitz JI, Hirsh J, Weaver WD. Randomized comparison of a novel anticoagulant, vasoflux, and heparin as adjunctive therapy to streptokinase for acute myocardial infarction: results of the VITAL study (Vasoflux International Trial for Acute Myocardial Infarction Lysis) Am Heart J. 2001;142:237–243. doi: 10.1067/mhj.2001.116759. [DOI] [PubMed] [Google Scholar]
- 46.Chen J, Jones CL, Liu J. Using an enzymatic combinatorial approach to identify anticoagulant heparan sulfate structures. Chemistry & biology. 2007;14:986–993. doi: 10.1016/j.chembiol.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]