Abstract
Purpose
Exclusive right hemisphere language lateralization is rarely observed in the Wada angiography results of epilepsy surgery patients. Cortical stimulation mapping (CSM) is infrequently performed with such patients, as most undergo non-dominant left hemisphere resections, which are presumed not to pose any risk to language. Early language reorganization is typically assumed in such individuals, taking left hemisphere epileptiform activity as confirmation of change resulting from a pathological process. We present data from CSM and Wada studies demonstrating that right hemisphere language occurs in the absence of left hemisphere pathology, suggesting it can exist as a normal, but rare variant, in some individuals. Further, these data confirm the Wada test findings of atypical dominance.
Methods
Cortical stimulation mapping data were examined for all right hemisphere surgical patients with right hemisphere speech at our Center between 1974 and 2006. Out of 1209 interpretable Wada procedures, 89 (7.4%) patients had exclusive right hemisphere speech, and 21 (1.7%) of these patients underwent surgery involving the right hemisphere. Language site location was determined by examining intraoperative photographs, and site distribution was statistically compared to published findings from left hemisphere language dominant patients (Ojemann et al., 1989).
Key Findings
Language cortex was identified in the right hemisphere during CSM for all patients with available data. All sites could be classified in superior or middle temporal gyri, inferior parietal lobe, or inferior frontal gyrus; all of which were common zones where language was identified in the left hemisphere dominant comparison sample.
Significance
Results suggest: 1) the Wada procedure is a valid measure for identifying right hemisphere language processing without any false lateralization found in the patients mapped with CSM (i.e., a positive Wada is 100% sensitive for finding RH language sites), and 2) the distribution of language sites is consistent across right hemisphere and left hemisphere language dominant patients, supporting the theory that right hemisphere language can occur as a normal variant of language lateralization.
Keywords: atypical language lateralization, epilepsy surgery, Wada, cortical stimulation mapping
Cortical stimulation mapping (CSM) involves inducing a localized electrical blockage of cortical function, and has been used in neurosurgery to identify areas essential to language functioning since the pioneering work of Penfield and Roberts with epilepsy surgery patients during the mid-1950s (Penfield & Roberts, 1959). Identification of language cortex enables the surgeon to avoid language areas when formulating a surgical strategy and to expand the resection of epileptic regions with increased safety (Kim et al., 2009). CSM results have brought insight to the distribution of language, confirming the presence of the classical Broca’s and Wernicke’s areas and identifying nontraditional sites found elsewhere in the cortex (Ojemann et al 1989). These data have also validated the accuracy of the intracarotid amobarbital (Wada) procedure, confirming that essential language sites are present in patients that are left-hemisphere (LH) dominant for language. The Wada procedure has served as the gold-standard for determining language dominance in presurgical patients since the early days of epilepsy surgery. Nevertheless, little data have been published examining CSM in patients who are determined to be exclusively right-hemisphere (RH) dominant for speech. Such individuals are believed to be rare, and atypical language lateralization is thought to often develop in patients following an early life injury involving their left cerebral hemisphere (Moddel et al., 2009; Rasmussen & Milner, 1977). Surgical patients experiencing such pathological language reorganization may not undergo CSM, as neurosurgical intervention is more likely to involve the LH of these individuals. As the Regional Epilepsy Center at the University of Washington has over 30 years of data involving CSM and the Wada procedure, we sought to examine the frequency of RH language dominance, validate the Wada procedure in these cases, and determine how the CSM results of such patients compare to those of individuals with typical speech lateralization.
Validation of the Wada Procedure
The Wada procedure has traditionally been the primary method for determining language dominance in neurosurgical candidates, as it provides a means to assess each hemisphere’s contribution to language function independently (Loring et al., 1992). This procedure remains in use by most epilepsy centers for the presurgical evaluation, although increasing efforts have been made to employ a noninvasive alternative [e.g., fMRI] (Arora et al., 2009). The principle behind the Wada was demonstrated by W. James Gardner in 1941 (Gardner, 1941), who injected procaine directly into the cortex thought to potentially be involved in language. Juhn Wada more formally established this procedure in the area of epilepsy surgery, making separate injections of amobarbital into each internal carotid artery to produce hemispheric anesthesia (Wada, 1949). During this period of anesthesia, patients are asked to carry out tasks to evaluate language and memory. Anesthetizing the area renders it nonfunctional, causing the hemisphere with language representation to be revealed when language deficits appear during an injection.
The validity of LH language dominance as predicted by the Wada procedure has been demonstrated in several group studies using CSM (Loring et al., 1992; Ojemann et al., 1989), yet validity data for RH language dominance in epilepsy have been restricted primarily to a handful of case studies with one or two subjects (Rosenbaum et al., 1989; Wyllie et al., 1990). Duffau and colleagues (2008) found RH language sites in a small series of tumor patients (n=9) who were all left-handed, but did not have Wada data on any of their patients (some percentage were likely bilateral language cases). One recent study examined CSM results in 15 neurosurgical patients (mostly tumor patients) that had exhibited either RH (n=7) or bilateral (n=8) language on the Wada (Chang et al., 2011). These researchers found language sites in the RH of all 15 patients, thoroughly delineated their neuroanatomical location, and noted that the pattern of language sites was consistent with findings from LH language dominant patients. There are also a few additional studies that demonstrate RH speech in a small number of patients identified as bilateral by the Wada (Jabbour et al., 2005; Loring et al., 1988).
Prevalence of Atypical Language Lateralization
Years of accumulated knowledge from the use of the Wada confirms that most neurosurgical candidates are LH dominant for language, with atypical language lateralization in a minority (Loring et al., 1990; Moddel et al., 2009). The prevalence of exclusive RH speech is believed to be infrequent, and right-sided surgery on such patients is believed to be rare. Most studies have suggested the prevalence of RH language representation to range from approximately 2 to 10% in epilepsy patients undergoing the Wada (Loring et al., 1990; Moddel et al., 2009), with the exception of a study by Rasmussen and Milner (1977) that estimated it to be as high as nearly 20%. These studies have estimated bilateral language representation to range from 5 to 25% in this population. Variability in reported prevalence rates is believed to primarily reflect differences in the implementation of the Wada across epilepsy centers, as many institutions have not routinely administered the Wada procedure to all individuals. Most centers select patients for the Wada based on the presumed clinical likelihood of atypical language occurrence. Variability in reported rates of atypical language may also be influenced by small sample sizes used in these studies, and general variability across Wada methodologies. Finally, while the Wada procedure is invasive and administered only to neurosurgical candidates, functional technologies (fMRI, functional transcranial Doppler sonography, MEG) have been used to estimate rates of atypical language prevalence in healthy subjects (Knecht et al., 2000; Szaflarski et al., 2002). These studies suggest that atypical language lateralization (RH or bilateral) will occur in 4 to 7.5% of right-handers and up to 22% of left-handers in the general population.
Studies of “crossed-aphasia,” a term for the development of language impairment in normal right-handed individuals who sustained damage to the right hemisphere later in life, also provides evidence that atypical language lateralization may be a normal variant of language development (Hecaen & Albert 1978). Such studies suggest that atypical language may occur in .4% to 3% of healthy right handed adults (Marien et al 2004; Zangwill 1979), although most reports have not included the necessary functional imaging studies to determine whether this reflects bilateral or right only language (See Vandervliet et al 2008; Vitali et al 2011 for recent exceptions). More than two hundred cases appear in the research literature (Marien et al 2004); with more recent studies documenting similar aphasia types, lesion-behavior relationships, and recovery patterns as in uncrossed aphasia (Castro-Caldas & Confraria 1984; Coppens et al 2002; Yarnell 1981). The similarity of the resulting syndromes suggests that atypical language representation could parallel the more typical left-hemisphere variety.
Right-hemisphere Speech Patients as Surgical Candidates
Most neurosurgical patients found to have RH speech are undergoing surgical procedures involving their LH. This is because atypical lateralization of the neural language network appears to occur most frequently as a result of early childhood neuronal injuries leading to a shift of language functions from the left to the RH. A previous study from our Center (Miller et al., 2003) had also suggested that atypical speech lateralization may not occur in epilepsy patients with normal neurologic histories through the age of 15 years.
In the current study, we retrospectively examined the CSM and Wada data of all patients found to have RH speech and who also underwent RH surgical procedures. This represents the largest published sample of epilepsy patients undergoing the Wada procedure, and is unique in that it is a near consecutive series requiring the obligatory completion of this study for all surgical candidates during the time span of the study regardless of hemisphere of seizure onset and the routine injection of both hemispheres. Additionally, the Wada procedure remained unchanged throughout the duration of the study. We predicted that CSM results for these RH patients would positively identify one or more essential language sites in each patient, providing validation for the sensitivity of the Wada procedure for detecting RH speech in a large, unselected sample of RH language patients. We also hypothesized that the general distribution of sites would be consistent with the patterns observed in neurosurgical patients with LH language dominance, as we believe that prior research suggests this group will predominantly reflect a sample of patients with an atypical yet normal variant of language organization.
Methods
Subjects
From a database of 1255 consecutive Wada procedures performed between January, 1974 through October, 2006 at the UW Regional Epilepsy Center, 1209 of these were deemed interpretable. All epilepsy surgical patients at UW underwent the Wada procedure unless deemed too cognitively compromised to do so. The procedure was not restricted to those with a suspicion of atypical speech (e.g., left-handedness or RH onset and language dysfunction). Likewise, all Wada procedures completed at UW involved injection of each cerebral hemisphere rather than the practice of only injecting the side of seizure onset, with the rare exception in the case of a patient with the affected hemisphere being too severely compromised to risk injection of the more intact hemisphere. From the total sample of interpretable cases, 89 were shown to have exclusive RH dominant language (7.4% of the interpretable sample), and 21 of these subsequently underwent RH surgery (1.7%). Thirteen of these patients had intraoperative mapping data available for analysis. For four of the older cases, the intraoperative photos or behavioral data from the stimulation paradigm were not available for review. Four of the remaining cases were performed by other UW neurosurgeons, and no CSM results were available for them either. For the total RH language sample (n = 89), all patients were adults, 67% were female, and 33.7% were right-handed. For the 21 RH language patients who underwent RH surgery, all experienced complex partial seizures arising from the RH (predominantly either frontal or temporal lobe onset). Seven of these patients presented with cryptogenic epilepsy, seven with seizures secondary to RH tumors, one with a right occipital arteriovenous malformation (AVM), one with seizures following a RH stroke, and five with seizure onset related to various neurological diseases or injuries that could potentially affect both cerebral hemispheres (e.g., traumatic brain injury, meningitis, encephalitis, tuberous sclerosis). Six of the 21 patients were right-handed (28.6%). Handedness and footedness were determined using the Reitan Handedness Scale (Reitan & Wolfson, 1993). We provide demographic information for the patients with exclusive RH language in Table 1, and also present demographic data separately for the subgroup with CSM results. This subgroup included proportionally fewer right-handed and right-footed subjects than did the total sample, but otherwise did not significantly differ from the broader group in terms of education level, gender, age at assessment, or age at seizure onset.
Table 1.
Demographic information for the 21 epilepsy patients with RH language dominance who underwent RH surgery for epilepsy at the University of Washington Regional Epilepsy Center.
| Group Description | Age at Mapping (years) | Age of Seizure Onset (years) | Gender (% Female) | Handedness* (% Right Handed) | Footedness* (% Right Footed) | Education (years) |
|---|---|---|---|---|---|---|
|
| ||||||
| All RH language dominant patients undergoing RH surgery (n =21) | X = 37.4 | X = 24.1 | 66.6% (14/21) | 28.6% (6/21) | 38.1% (8/21) | X = 12.6 |
| SD = 13.5 | SD = 17.4 | SD = 2.3 | ||||
| Range: 18–61 | Range = 5–61 | Range: 8–17 | ||||
|
| ||||||
| Subset of RH language dominant patients with available CSM results (n = 13) | X = 36.2 | X = 21.1 | 61.5% (8/13) | 7.7% (1/13) | 23.1% (3/13) | X = 13.1 |
| SD = 13.40 | SD = 14.6 | SD = 2.7 | ||||
| Range: 20–56 | Range = 5–49 | Range: 8–17 | ||||
Note. RH = right hemisphere; CSM = cortical stimulation mapping; X = mean; SD = standard deviation. No differences were observed between the sample with available CSM data and the total sample using appropriate parametric and nonparametric tests with the exception of the results marked with asterisks.
The sample with available CSM data differed from the total sample on this variable at the p<.01 level.
Table 2 provides a comparison of the total group and the subset with CSM data on presurgical IQ assessment and cognitive testing, which was available for 19 of 21 patients. Assessment of language and memory varied somewhat over the 30 year time span of the study, with a variety of tasks of auditory comprehension, verbal fluency, naming ability, and auditory/verbal and visual memory used over the years (e.g., various versions/subtests of the Wechsler Memory Scales, Rey Auditory Verbal Learning and Complex Figure Tests). Therefore, we classified patients on the basis of test performance compared to normative data, and assigned performance ratings for each subject according to the following classification system: normal (scores <1 SD below the mean), mildly impaired (1 to 1.5 SD below the mean), moderately impaired (1.5 to 2 SD below the mean), or severely impaired (>2 SD below the mean). As can be seen, general intellectual functioning was generally average for the sample, and less than 25% of these patients exhibited moderate or greater deficits in either language or auditory/verbal memory at baseline. Approximately 11% of the sample exhibited at least moderate deficits in visual memory performance. Over half of the total sample performed in the average range on nearly all administered tests from these domains. Table 3 provides a more extensive breakdown of demographic and disease-related variables for the subgroup of patients with CSM results, so that specific variables can be examined in relation to individual CSM results to be presented in Table 4.
Table 2.
Presurgical Neurocognitive Performances for the 21 epilepsy patients with RH language dominance who underwent RH surgery for epilepsy at the University of Washington Regional Epilepsy Center.
| Group Description | FSIQ | VIQ | PIQ | Language Functioning (% with moderate or greater deficits) | Auditory/Verbal Memory Functioning (% with moderate or greater deficits) | Visual Memory Functioning (% with moderate or greater deficits) |
|---|---|---|---|---|---|---|
|
| ||||||
| All RH language dominant patients undergoing RH surgery (n =21) | X = 92.4 | X = 92.6 | X = 93.6 | 21.1% (4/19) | 21.1% (4/19) | 10.5% (2/19) |
| SD = 14.4 | SD = 12.4 | SD = 16.5 | ||||
| Range: 64–125 | Range = 72–128 | Range = 60–128 | ||||
|
| ||||||
| Subset of RH language dominant patients with available CSM results (n = 13) | X = 96.1 | X = 95.8 | X = 97.5 | 25.0% (3/12) | 8.3% (1/12) | 8.3% (1/12) |
| SD = 14.6 | SD = 13.2 | SD = 4.8 | ||||
| Range: 82–125 | Range = 84–128 | Range = 79–128 | ||||
Note. RH = right hemisphere; FSIQ = Full-Scale IQ from the Wechsler Adult Intelligence Scale; VIQ = Verbal IQ; PIQ = Performance IQ; CSM = cortical stimulation mapping; X = mean; SD = standard deviation. No differences were observed on cognitive measures between the sample with available CSM data and the total sample using appropriate parametric and nonparametric tests. Moderate dysfunction was defined as greater than 1.5 SD below the mean of normative test data.
Table 3.
Demographic, IQ, and disease-related information presented for each right hemisphere language dominant epilepsy patient who underwent right hemispheric surgery.
| Patient | CSM | Age at Assessment (years) | Age at Seizure Onset (years) | Gender | Handedness/Footedness | FSIQ | VIQ | PIQ | Education (years) | Diagnosis/Likely Etiology for Seizures |
|---|---|---|---|---|---|---|---|---|---|---|
| I | yes | 56 | 13 | Female | LH/RF | 91 | 84 | 102 | 12 | Right TL epilepsy (TBI at age 3) |
| II | yes | 56 | 49 | Female | RH/LF | 89 | 88 | 93 | 8 | Right TL epilepsy (Idiopathic) |
| III | yes | 23 | 18 | Female | LH/LF | 82 | 84 | 81 | 10 | Right TL epilepsy (Idiopathic) |
| IV | yes | 39 | 39 | Female | LH/LF | 116 | 105 | 128 | 16 | Right TL tumor |
| V | yes | 44 | 44 | Male | LH/LF | * | * | * | * | Right FL oligodendroglioma |
| VI | yes | 25 | 16 | Female | LH/LF | 91 | 97 | 86 | 16 | Right TL epilepsy (Idiopathic) |
| VII | yes | 24 | 17 | Female | LH/LF | 88 | 85 | 97 | 12 | Right TL tumor |
| VIII | yes | 24 | 5 | Male | LH/RF | 85 | 90 | 81 | 11 | Right TL low grade glioma |
| IX | yes | 33 | 7 | Male | LH/LF | 87 | 95 | 79 | 17 | Right TL epilepsy (encephalitis) |
| X | yes | 48 | 16 | Male | LH/LF | 90 | 91 | 92 | 12 | Right TL epilepsy (Idiopathic) |
| XI | yes | 48 | 12 | Female | LH/LF | 91 | 91 | 92 | 12 | Right TL epilepsy (tuberous sclerosis) |
| XII | yes | 20 | 8 | Female | LH/RF | 118 | 112 | 123 | 14 | Right TL epilepsy (Idiopathic) |
| XIII | yes | 31 | 30 | Male | LH/LF | 125 | 128 | 116 | 16 | Right anterior TL ganglioglioma |
| XIV | No | 37 | 9 | Female | RH/RF | 87 | 87 | 89 | 12 | Right TL epilepsy (Idiopathic) |
| XV | N/A | 61 | 61 | Female | RH/LF | * | * | * | * | 2 Right TL tumors (1 mesial TL/1 lateral TL) |
| XVI | No | 28 | 11 | Female | RH/RH | 90 | 89 | 93 | 12 | Right TL epilepsy (meningitis) |
| XVII | N/A | 29 | 24 | Male | RH/RF | 78 | 82 | 77 | 12 | Right TL epilepsy (TBI as infant/rubella) |
| XVIII | no | 37 | 18 | Female | LH/RF | 84 | 84 | 85 | 13 | Right TL epilepsy (RH stroke) |
| XIX | N/A | 18 | 9 | Male | LH/LH | 64 | 72 | 60 | 12 | Right TL epilepsy (Idiopathic) |
| XX | N/A | 54 | 54 | Female | RH/RF | 99 | 95 | 107 | 12 | Right posterior TL tumor |
| XXI | No | 52 | 45 | Female | LH/LF | 100 | 101 | 98 | 10 | Right occipital AVM |
Note. CSM = cortical stimulation data; IQ = intelligence quotient; FSIQ = Full-Scale IQ from the Wechsler Adult Intelligence Scale; VIQ = Verbal IQ; PIQ = Performance IQ; LH = left-handed; LF = left-footed; RH = right-handed; RF = right-footed; TBI = traumatic brain injury; TL = temporal lobe; FL = frontal lobe; AVM = arteriovenous malformation.
These patients did not undergo neuropsychological assessment prior to surgery.
Table 4.
Distribution of language sites in right hemisphere language dominant epilepsy patients as compared to left hemisphere language dominant epilepsy patients from larger Ojemann et al. (1989) study.
| Patient | Parietal | Superior Temporal | Middle Temporal | Posterior Inferior Frontal |
|---|---|---|---|---|
| I | 0 | 0 | 1 | 1 |
| II | 1 | 0 | 0 | 1 |
| III | 0 | 1 | 1 | * |
| IV | * | * | * | 1 |
| V | 0 | 1 | 0 | 1 |
| VI | * | 1 | 1 | * |
| VII | * | * | * | 1 (middle frontal) |
| VIII | 0 | 1 | 0 | 1 |
| IX | 0 | 0 | 0 | 1 |
| X | 0 | 0 | 1 | 1 |
| XI | 1 | 0 | 0 | 1 |
| XII | 1 | 0 | 0 | 1 |
| XIII | 0 | 1 | 1 | * |
| Patients with sites/Patients tested | 3/10 (30%) | 5/11 (45.5%) | 5/11 (45.4%) | 9/10 (90%) |
| LH dominant patients with sites/Patients tested (G. Ojemann et al., 1989) | 41/102 (40.1%) | 68/104 (65.3%) | 37/98 (37.8%) | 65/82 (79.3%) |
| Pearson chi square | χ2 = .33 p = .57 | χ2 = 1.21, p = .27 | χ2 = .19, p = .67 | χ2 = 0.65, p = .42 |
Indicates that this area was not mapped.
This study investigated the distribution of left hemispheric language sites in 117 LH language dominant patients. Patients in this study typically had one frontal language sites and one or more temporoparietal language sites.
Wada Procedure
The Seattle version of the Wada procedure was performed on all patients as part of their presurgical evaluation (Dodrill, 1997). The average dose of sodium amobarbital used for these evaluations was approximately 112.5 mg for men and 100.0 mg for women injected over a period of 4 to 6 seconds. Patients were then asked to perform a three-part repetitive task (object naming, reading, and object recall) from injection through return of function. Speech blockages and secondary linguistic changes were documented. The ability to follow commands can be inferred in the Seattle version of the Wada from the patient’s ability to comply with task instructions, providing a measure of auditory language comprehension as well. All patients completed a thorough pre-Wada baseline assessment that simulated the procedure used in the Wada, and established a baseline level of performance across tasks. Language behavior was scored over a time range beginning with initial drug injection, pending resolution of any initial drug obtundation, and continued until language returned to baseline. The repetitive task initially includes only baseline stimuli (there are 20 baseline objects), which allows a direct comparison to the patient’s nondrug performance. After approximately 1 minute post-injection of the amobarbital, 8 novel items are substituted for the items to be named and recalled, to provide novel items for the memory paradigm (these items are tested by free recall and recognition recall after the effects of the amobarbital have worn off). For patients unable to name objects accurately at this point, they are told the name of the object (e.g., “this is a fork”), and then asked to repeat the phrase, “this is a object name.” This is intended to allow equivalent opportunity for memory encoding afforded those that can perform the task spontaneously, and an opportunity to evaluate simple repetition of phrases. Once the 8 novel items have each been presented one time, we switch back to the original set of 20 baseline objects, and cycle through them until the patient reaches their baseline accuracy and response speed (items per minute). Patients were classified as having unilateral speech if language performance during a single injection differed from their baseline nondrug performance. All of the patients in the current sample experienced naming errors, but also often exhibited speech arrest and an inability to follow the simple instructions built into the repetitive tasks (auditory comprehension), reading errors, and positive signs of aphasia (e.g., paraphasic errors). Just like our CSM paradigm, naming is centrally positioned in the Seattle version of the Wada, as we feel this is the most at risk language function in the context of ATL surgery. Nevertheless, we are able to assess multiple language behaviors for all subjects. A patient would be said to have bilateral language if disruption occurred in any language behavior on both the left and right injections or if performance remained normal following both injections. Of note, the latter pattern of performance was never observed in the sample described, but instead reflects our theoretical position. As with any Wada behavioral data, the veracity of the findings have to be determined in light of other relevant factors (e.g., severe visual neglect may lead to problems with reading that are perceptual in nature rather than language-based, patients obtunded by drug administration cannot engage in the task at all initially). Exclusive right hemisphere language was determined by the presence of language dysfunction which occurred with the right injection but was absent with the injection on the left (i.e., the left injection did not produce any significant changes from nondrug baseline). Of note, the core Wada procedure remained invariant through the entire 30 year time course examined with the exception of minimal changes in object stimuli used for naming over the course of time and the addition of secondary memory paradigms that only involved modification of object recall after the effects of the amobarbital had resolved. Likewise, despite amobarbital supply shortages in the US during more recent years, our Center always kept an abundant supply of amobarbital on hand and has never encountered a time where drug was unavailable for this procedure. These are unique characteristics of the UW dataset, as most epilepsy centers have frequently altered and replaced their Wada approach over time and have been affected by various clinical issues such as decreased access to a particular barbiturate.
Intraoperative Cortical Stimulation Mapping
After lateralization of language was determined presurgically with the Wada procedure, cortical stimulation mapping was used for localizing language function within a cerebral hemisphere. Intraoperative CSM used a standard clinical protocol of naming pictures presented on a screen at three second intervals (Ojemann et al., 1989). Presentation of stimuli was originally completed using a slide projector but later shifted to a computer screen. Once a craniotomy was performed using local anesthetic field block and neuroleptoanalgesia (Silbergeld et al, 1992), patients were awakened for language mapping. This procedure started with the determination of an afterdischarge threshold using electrocorticography. Language mapping was performed with the largest bipolar current that did not evoke afterdischarges (typically between 1.5 and 10 mA). This was done to avoid propagation of depolarization to nearby cortex, which can lead to false positive results. Patients were shown slides of line drawings of common man-made objects at three-second intervals, and were trained to name each object as it appeared. While patients performed this task, bipolar current was applied to the cortical surface. Each site was typically stimulated two to three times, though never twice in succession, and at least one slide without stimulation separated each stimulation. A site was considered essential to language function if stimulation of that site produced consistent speech arrest or anomia. The language mapping paradigm included in this study is commonly employed in the neurosurgical setting, and is the same procedure used in the original study demonstrating LH speech sites (Ojemann et al., 1989).
Data Analysis
The accuracy of naming during the large number of slides presented in the absence of stimulation was compared to the accuracy of naming during stimulation of a given site, using Fisher’s exact test. A site was determined to be related to language function if the number of errors across naming trials at that site exceeded the number expected given the baseline error rate (p < .05). The location of the sites was ascertained by examining the intraoperative photographs and determining the relationship of the sites to rolandic cortex, sylvian fissure, and sulci separating the major gyri (See Figure 1 for an example). The cortical map was then divided into four basic areas: superior or middle temporal gyri, inferior parietal lobe, and inferior frontal gyrus. The language site(s) of each patient was placed into one of these four categories. The distribution of sites was then compared to our previously published findings with 117 LH language dominant patients (Ojemann et al., 1989) using Pearson chi square tests of proportion.
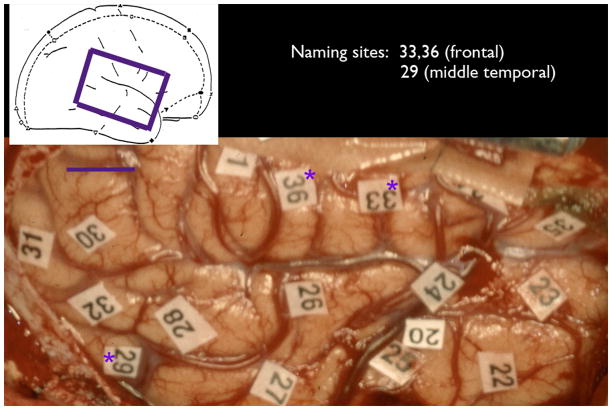
Figure 1.
Intraoperative picture showing the cortical surface of the brain of patient 1, with naming sites indentified as listed in the figure key.
Results
All 13 patients determined to have exclusive RH speech identified by the Wada and complete CSM data were found to have at least one essential language site (results for each patient are presented in Table 4). Typically, patients were found to have one site in the frontal lobe and at least one more posterior site (i.e., temporal or parietal). A posterior frontal lobe site was found in all 10 patients in whom this region was mapped. Seven of eleven patients exhibited at least one TL language site involving either the middle or superior temporal gyrus. Three of the four patients without a TL language site had one site identified in the parietal lobe.
When compared to previously published distribution of language sites in patients with LH language lateralization, these RH language patients showed no significant difference in the regional representation of language sites. Frontal sites were quite common in both groups and posterior sites had a similar distribution across parietal, superior temporal and middle temporal gyri.
Discussion
Consistent with our hypotheses, the results of CSM were concordant with the findings of the Wada procedure, demonstrating one or more essential language sites in each of 13 RH language dominant patients undergoing RH neurosurgical intervention. All of the patients who underwent CSM of the frontal lobe were found to have at least one language site in this region, with 90% of assessed patients exhibiting a posterior inferior frontal lobe site that appeared consistent with Broca’s area. Most patients also exhibited one to two additional posterior language sites involving either the superior or middle temporal gyrus or the inferior parietal lobe. As with previous studies examining LH language dominant patients (Ojemann et al., 1989; Ojemann, 1979), a great deal of variability was observed in the specific regional locations of the posterior language sites of these RH language dominant individuals. Nevertheless, the proportion of patients exhibiting essential language sites by region was highly consistent between these LH and RH language dominant samples, and there was no evidence of statistical differences between groups. Taken together, these results provide further validation of the Wada procedure in patients with RH language dominance and suggest that the majority of these patients likely reflect cases of normal variation of language lateralization rather than instances of pathological reorganization of function.
Supporting evidence for the conclusion that RH language representation in the majority of our sample likely reflects a normal variation in speech lateralization includes the presence of pathology in the language dominant RH that is to be resected in these patients, and a late onset of disease in nearly all cases (including the eight for whom CSM data were not available). In the absence of early life injury or disease, there would seem to be no obvious pathological reason for a shift from the left to the right cerebral hemisphere. An additional piece of evidence which is present for approximately half of the individuals in this sample, included strong baseline neurocognitive performances in terms of language (particularly naming ability), memory (both auditory/verbal and visual), visual-spatial processing, and general intellectual functioning. In cases of pathological shift of language, one often sees at least mild decrements in the functions that shift as well as significant compromise of functions that are typically located in the RH of individuals with LH language lateralization. For example, the “crowding hypothesis” argues that visual-spatial functions are often diminished in patients with pathological reorganization of language, as such functions are compromised in order to provide space for more essential language functions (Satz, 1994).
Prior research from our Center had suggested that RH language most often results from a pathological process and may not occur in individuals with adult onset of disease (Miller et al., 2003). However, this prior report excluded all tumor patients, as it was argued that it can be difficult to accurately determine disease onset in such cases as tumors may go unrecognized for a number of years. Therefore, if someone had a left TL tumor of apparent adult onset, it might be possible that the tumor had been present for an unspecified time, and had contributed to a pathological shift of language to the RH. Nevertheless, in the current study, all of the patients with tumors (approximately 1/3 of the sample), had tumors involving the RH, so if any switch had occurred it would have been to the LH.
Essentially normal presurgical neurocognitive performances in several of the RH language dominant patients, a lack of early life injury, as well as a number of right-handers in this sample (33.7% of the total sample) raises the possibility that many epilepsy surgical programs might have forgone both the Wada procedure and CSM in some of these patients. Several of these patients might not have been considered to be at risk for decline with RH surgery. More specifically, right-handers would be presumed to have a very small chance of having atypical language representation, particularly in those cases that lacked early life injuries. Given these findings, it would likely be wise to confirm language lateralization in virtually all “elective” neurosurgical patients, particularly if noninvasive methods are eventually established as the gold standard.
While the current data definitively establish that RH language findings on the Wada correspond with CSM results, we cannot completely rule out the possibility that some of the patients classified as RH dominant for language by the Wada were actually bilateral cases, as we did not complete language mapping involving the LH in these patients. This possibility is raised by a report of two epilepsy patients with RH language dominance as determined by the Wada procedure who exhibited LH language sites with CSM (Wyllie et al., 1990). Nevertheless, our Wada results gave no indication whatsoever that these patients might have been bilateral cases, as none of these patients performed in a manner that differed from their baseline language functioning when their LH was anesthesized. For clinical purposes, the important message is that CSM reliably identifies essential language sites in patients determined to be RH dominant for language by the Wada, allowing the surgeon to spare such sites whenever possible in an effort to preserve language, and that a Wada result implicating RH language dominance is likely to be associated with a positive finding of RH CSM sites.
One limitation of the current study is that neuroimaging techniques have changed drastically over the period examined. Therefore, we could have missed subtle left-hemisphere pathology in some of the patients (particularly the earliest patients). However, our suggestion that the occurrence of RH language in this patient cohort is a normal variant rather than a pathological shift in function rests as much on behavioral patterns and evidence of structural/functional pathology in the RH. Of note, we had 3T MRI data on three of our right-handed patients with RH speech (half of our cryptogenic cases), and no structural abnormalities were observed on any of these scans. Approximately one third of our total RH language sample (33.7%) was right-handed, suggesting that crossed language lateralization occurred in approximately 2.5% of our valid cases, which is in the range estimated by research involving the occurrence of crossed aphasia (i.e., .4 to 3%) (Marien et al 2004).
A second limitation involves our tendency to restrict our CSM paradigm to the assessment of naming. While this is a common practice in the neurosurgical setting, and consistent with our earlier published works in this regard (Ojemann et al 1989), assessment of a broader range of language behaviors may have led to additional identified language sites. For example, it is possible that an assessment of auditory comprehension would have led to the identification of a posterior language site in patient 9 in the current study.
There are reports in the literature demonstrating that LH language dominant patients requiring resections that either included or encroached upon sites identified by CSM have experienced post-operative dysphasia with persisting language deficits observed at long-term follow-up (Iimberger et al., 2008; Ojemann et al., 1989). However, due to the low occurrence of RH language dominant patients undergoing RH surgery, there is little published outcome data related to post-operative language functioning in these patients. In the recent paper by Chang et al. (2011), only one of the patients had any persisting language deficits after undergoing speech mapping. Of note, however, we recently published extensive postsurgical findings for patient 13 from our current sample (Drane et al., 2009), as this patient experienced significant declines in both category-related naming and recognition of objects and persons (a finding which we typically observe only in patients who have experienced dysfunction of both anterior temporal lobes) (Damasio et al., 1996; Drane et al., 2008; Tranel et al., 1997). This patient exhibited a normal postsurgical performance on naming and recognition tests involving man-made objects and animals (including the Boston Naming Test and some specially designed measures used in the category-specific research of Damasio and colleagues) (Damasio et al., 1996; Tranel et al 1997; Tranel et al., 2005). However, he exhibited severe deficits in naming and recognition of unique objects, such as famous persons and landmarks. We are finding that similar deficits often occur in recognition of these specific object categories following nondominant TL resection and for naming of these objects following dominant TL resection despite the conduction of standard CSM (Drane et al., 2008; Drane et al., 2009).
In conclusion, we find that patients who had RH speech, identified by the Wada test, uniformly had sites of naming disruption by CSM, demonstrating the accuracy of the finding of atypical dominance in all patients who underwent such mapping. It appears that the presence of RH language, albeit a rare phenomena (7.4% of 1209 Wada patients in the current study had RH language), occurs more frequently than one encounters complications related to the Wada (major complications are estimated to occur in less than 1% of cases undergoing cerebral angiography) (Kaufmann et al., 2007), supporting the need for language lateralization testing in the preoperative setting. Further, the relatively similar cognitive profile between these patients and LH language dominant epilepsy patients, lack of reorganization of language away from the side of pathology, and similarity of the language maps in RH language dominant patients to prior results in LH dominant patients, all suggest that RH language dominance can exist as a normal variant even in right-handed individuals.
Acknowledgments
This research was supported in part by the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institute of Health (NIH) [Grant Numbers: K23 NSO49100 and K02 NS070960]. This study was approved by the Institutional Review Board (application #05-5738-G 02) of the University of Washington Medical School, where all data were collected. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
None of the authors has any financial conflict of interest to disclose.
References
- Arora J, Pugh K, Westerveld M, Spencer S, Spencer DD, Todd Constable R. Language lateralization in epilepsy patients: fMRI validated with the Wada procedure. Epilepsia. 2009;50:2225–41. doi: 10.1111/j.1528-1167.2009.02136.x. [DOI] [PubMed] [Google Scholar]
- Castro-Caldas A, Confraria A. Age and type of crossed aphasia in dextrals due to stroke. Brain Lang. 1984;23:126–33. doi: 10.1016/0093-934x(84)90011-7. [DOI] [PubMed] [Google Scholar]
- Chang EF, Wang DD, Perry DW, Barbaro NM, Berger MS. Homotopic organization of essential language sites in right and bilateral cerebral hemispheric dominance. J Neurosurg. 2011;114:893–902. doi: 10.3171/2010.11.JNS10888. [DOI] [PubMed] [Google Scholar]
- Coppens P, Hungerford S, Yamaguchi S, Yamadori A. Crossed aphasia: An analysis of the symptoms, their frequency, and a comparison with left-hemisphere aphasia symptomatology. Brain Lang. 2002;83:425–63. doi: 10.1016/s0093-934x(02)00510-2. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio A. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- Dodrill CB, Ojemann GA. An exploratory comparison of three methods of memory assessment with the intracarotid amobarbital procedure. Brain and Cogn. 1997;33:210–33. doi: 10.1006/brcg.1997.0893. [DOI] [PubMed] [Google Scholar]
- Drane DL, Ojemann GA, Aylward E, Ojemann JG, Johnson LC, Silbergeld DL, Miller JW, Tranel D. Category-specific naming and recognition deficits in temporal lobe epilepsy. Neuropsychologia. 2008;46:1242–55. doi: 10.1016/j.neuropsychologia.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane DL, Ojemann GA, Ojemann JG, Aylward E, Silbergeld DL, Miller JW, Tranel D. Category-specific recognition and naming deficits following resection of a right anterior temporal lobe tumor in a patient with atypical language lateralization. Cortex. 2009;450:630–40. doi: 10.1016/j.cortex.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Capelle L, Taillandier L. Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with Grade II glioma in the left dominant hemisphere. J Neurosurg. 2008;109:461–71. doi: 10.3171/JNS/2008/109/9/0461. [DOI] [PubMed] [Google Scholar]
- Gardner WJ. Injection of procaine into the brain to locate speech area in left-handed persons. Arch Neurol Psychiatry. 1941;46:1035–8. [Google Scholar]
- Hecaen H, Albert MA. Human neuropsychology. New York: John Wiley & Sons; 1978. [Google Scholar]
- Iimberger J, Ruge M, Kreth FW, Briegel J, Reulen HJ, Tonn JC. Intraoperative mapping of language functions: A longitudinal neurolinguistic analysis. J Neurosurg. 2008;109:583–92. doi: 10.3171/JNS/2008/109/10/0583. [DOI] [PubMed] [Google Scholar]
- Jabbour RA, Hempel A, Gates JR, Zhang W, Risse GL. Right hemisphere language mapping in patients with bilateral language. Epilepsy and Behav. 2005;6:587–92. doi: 10.1016/j.yebeh.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Kaufmann TJ, Huston J, III, Mandrekar JN, Schleck CD, Thielen KR, Kallmes DF. Complications of Diagnostic Cerebral Angiography: Evaluation of 19,826 Consecutive Patients. Radiology. 2007;243:812–9. doi: 10.1148/radiol.2433060536. [DOI] [PubMed] [Google Scholar]
- Kim SS, McCutceon IE, Suki D, Weinberg JS, Sawaya R, Lang FF, Ferson D, Heimberger AB, Demonte F, Prabhu SS. Awake craniotomy for brain tumors near eloquent cortex: Correlation of intraoperative cortical mapping with neurological outcomes in 309 consecutive patients. Neurosurgery. 2009;64:836–46. doi: 10.1227/01.NEU.0000342405.80881.81. [DOI] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, Ringelstein E, Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123:2512–8. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Loring DW, Flanigin H, Meador KJ, Lee GP, Smith JR. Right hemisphere language representation determined by intracarotid sodium amytal testing and functional cortical speech mapping. Epilepsia. 1988;29:686. [Google Scholar]
- Loring DW, Meador KJ, Lee GP, Murro AM, Smith JR, Flanigin HF, Gallagher BB, King DW. Cerebral language lateralization: Evidence from intracarotid amobarbital testing. Neuropsychologia. 1990;28:831–8. doi: 10.1016/0028-3932(90)90007-b. [DOI] [PubMed] [Google Scholar]
- Loring DW, Meador KJ, Lee GP, King DW. Amobarbital effects and lateralized brain function: The Wada test. New York: Spring-Verlag; 1992. [Google Scholar]
- Marien P, Paghera B, De Deyn PP, Vignolo LA. Adult crossed aphasia in dextrals revisited. Cortex. 2004;40:41–74. doi: 10.1016/s0010-9452(08)70920-1. [DOI] [PubMed] [Google Scholar]
- Miller JW, Dodrill CB, Born DE, Ojemann GA. Atypical speech is rare in individuals with a normal developmental histories. Neurology. 2003;60:1042–4. doi: 10.1212/01.wnl.0000052692.14990.78. [DOI] [PubMed] [Google Scholar]
- Moddel G, Lineweaver T, Schuele SU, Reinholz J, Loddenkemper T. Atypical language lateralization in epilepsy patients. Epilepsia. 2009;50:1505–16. doi: 10.1111/j.1528-1167.2008.02000.x. [DOI] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J of Neurosurg. 1989;71:316–26. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- Ojemann GA. Individual variability in cortical localization of language. J of Neurosurg. 1979;50:164–9. doi: 10.3171/jns.1979.50.2.0164. [DOI] [PubMed] [Google Scholar]
- Penfield W, Roberts L. Speech and brain mechanisms. Princeton, N. J: Princeton University Press; 1959. [Google Scholar]
- Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann N Y Acad Sci. 1977;299:355–69. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Tucson: Neuropsychology Press; 1993. [Google Scholar]
- Rosenbaum T, DeToledo J, Smith DB, Kramer RA, Stanulis RG, Kennedy RM. Preoperative assessment of language laterality is necessary in all epilepsy surgical candidates: A case report. Epilepsia. 1989;30:712. [Google Scholar]
- Satz P, Strauss E, Hunter M, Wada J. Re-examination of the crowding hypothesis: Effects of age of onset. Neuropsychology. 1994;8:255–62. [Google Scholar]
- Silbergeld DL, Mueller WM, Colley PS, Ojemann GA, Lettich E. Use of propofol (Diprivan) for awake craniotomies: technical note. Surg Neurol. 1992;38:271–2. doi: 10.1016/0090-3019(92)90038-o. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Binder JR, Possing MS, Mckiernan KA, Ward BD, Hammeke TA. Language lateralization in left-handed and ambidextrous people. Neurology. 2002;59:238–44. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997;35:1319–27. doi: 10.1016/s0028-3932(97)00085-7. [DOI] [PubMed] [Google Scholar]
- Tranel D, Enekwechi N, Manzel K. A test for measuring recognition and naming of landmarks. J Clin Exp Neuropsychol. 2005;27:102–26. doi: 10.1080/138033990513663. [DOI] [PubMed] [Google Scholar]
- Vandervliet EJM, Verhoeven J, Engelborghs S, De Dyn PP, Parizel PM, Marien P. fMRI findings in an aphasic patient with reversed cerebral dominance for language. Acta Neurol Belg. 2008;108:161–6. [PubMed] [Google Scholar]
- Vitali P, Dronkers NF, Pincherle A, Giovagnoli AR, Marras C, D’Incerti LD, Ghielmetti F, Spreafico R, Villani F. Accuracy of pre-surgical fMRI confirmed by subsequent crossed aphasia. Neurol Sci. 2011;32:175–80. doi: 10.1007/s10072-010-0426-y. [DOI] [PubMed] [Google Scholar]
- Wada J. A new method for the determination of the side of cerebral speech dominance: A preliminary report on the intracarotid injection of Amytal in man. Igaku Seibutsugaku. 1949;14:221–2. [Google Scholar]
- Wyllie E, Lüders H, Murphy D, Morris H, 3rd, Dinner D, Lesser R, Godov J, Kotagal P, Kanner A. Intracarotid amobarbital (Wada) test for language dominance: correlation with results of cortical stimulation. Epilepsia. 1990;31:156–61. doi: 10.1111/j.1528-1167.1990.tb06300.x. [DOI] [PubMed] [Google Scholar]
- Yarnell PR. Crossed dextral aphasia: A clinical radiological correlation. Brain Lang. 1981;12:128–39. doi: 10.1016/0093-934x(81)90009-2. [DOI] [PubMed] [Google Scholar]
- Zangwill OL. Two cases of crossed aphasias in dextrals. Neuropsychologia. 1979;17:167–72. doi: 10.1016/0028-3932(79)90007-1. [DOI] [PubMed] [Google Scholar]