Abstract
This study sought to determine whether cannabinoid-1 (CB1) receptor binding was altered in the postmortem dorsolateral prefrontal cortex (DLPFC) of individuals with schizophrenia (schizophrenia; n=47) compared to controls (n=43). The CB1 receptor inverse agonist radioligand [3H]MePPEP was used to measure specific binding to CB1 receptors. The specific binding of [3H]MePPEP to CB1 receptors was 20% higher in patients with schizophrenia than in controls. Power analyses suggested that 53 subjects per group would be needed to detect a similar difference in vivo with positron emission tomography (PET) and the structurally related inverse agonist radioligand [18F]FMPEP-d2 (80% statistical power, p<0.05).
Keywords: cannabinoid, CB1, schizophrenia, dorsolateral prefrontal cortex, [3H]MePPEP
1. Introduction
The cannabinoid CB1 receptor binds endogenous and exogenous cannabinoids in the brain and modulates various neurotransmitters (Howlett et al., 2002). CB1 receptors may be altered in schizophrenia, but the direction of this alteration remains unclear. Three of five studies that used in vitro radiography methods found significantly increased CB1 receptor binding in patients with schizophrenia compared with controls; an additional study found no difference in CB1 levels in the DLPFC of subjects with non-paranoid schizophrenia but did find elevated levels (22%) of CB1 binding in individuals with paranoid schizophrenia compared to controls (Dalton et al., 2011;Dean et al., 2001;Deng et al., 2007;Newell et al., 2006;Zavitsanou et al., 2004). In contrast, three of four postmortem studies using immunodetection methods found lower CB1 receptor density (Bmax) in patients with schizophrenia compared to healthy controls (Eggan et al., 2008;Eggan et al., 2010;Koethe et al., 2007;Uriguen et al., 2009). The discrepancies in results could be attributed to methodology.
This study sought to determine whether CB1 receptor binding is altered in the postmortem DLPFC of a large cohort of individuals with schizophrenia and controls using in vitro receptor binding assays, and to use these data to determine the sample size needed to conduct in vivo positron emission tomography (PET) imaging studies. Receptor binding data were also analyzed to assess the potential influence of a common functional variation (rs2023239) in the gene coding for CB1 receptors (CNR1), which was previously shown to be associated with higher CB1 receptor binding in vitro (Hutchison et al. 2008)
2. Materials and Methods
2.1. Human Postmortem Brain Samples
2.1.1 Subjects
Postmortem DLPFC tissue samples from patients with schizophrenia and healthy controls were collected as previously described (Lipska et al., 2006); n=8 schizophrenic and 9 controls for the homologous assay; n=47 schizophrenic and 43 controls for the two-point assay). Tissue samples from the homologous binding assay were not included in the two-point assay cohort. Informed consent was obtained from family members according to established guidelines. Medical, psychiatric, and substance use history, smoking status, and demographic information were collected by telephone interview with next-of-kin within one week of donation (see Table 1).
Table 1.
Summary of subject demographics.
| Controls (n = 43) | Schizophrenia (n = 47) | p | |
|---|---|---|---|
| [3H]MePPEP specific binding* (pmol/mg protein) | 0.471 ± 0.173 | 0.566 ± 0.209 | 0.050 |
| Gender | |||
| Male | 28 | 23 | |
| Female | 15 | 24 | 0.122 |
| Race | |||
| African American | 27 | 27 | |
| Caucasian | 15 | 19 | |
| Hispanic | 1 | 1 | |
| Age at Death (years)* | 43 ± 15 | 55 ± 15 | <0.001 |
| Age of Onset of Illness | NA | 23.1 ± 7.3 | NA |
| Duration of Illness (years) | NA | 31.5 ± 11.8 | NA |
| pH* | 6.5 ± 0.3 | 6.2 ± 0.2 | <0.001 |
| Postmortem Interval (hours) | 36.7 ± 13.3 | 42.0 ± 26.0 | 0.219 |
| Body Mass Index | 30.9 ± 6.59 | 29.4 ± 9.10 | 0.374 |
| Brain Weight (g)* | 1370 ± 175 | 1228 ± 143 | 0.017 |
| Time Frozen (years)* | 10.2 ± 3.60 | 8.11 ± 2.87 | 0.005 |
| Manner of Death | |||
| Natural | 33 | 35 | |
| Accidental | 10 | 6 | |
| Suicide | 0 | 4 | |
| Undetermined | 0 | 2 | |
| Habitual Smoker at TOD* | 11 | 38 | <0.001 |
| Comorbid Alcohol Abuse/ Dependence | 0% | 14 | |
| Comorbid Substance Abuse/ Dependence | 0 | 8 | |
| THC or Metabolites in Toxicology | 0 | 1 | |
| Nicotine* | 8/35 | 18/27 | 0.028 |
| Cotinine* | 10/33 | 21/21 | 0.010 |
| Chlorpromazine Equivalents | |||
| Last Dose (mg qd) | 776 ± 642 | ||
| Average Daily Dose (mg qd) | 619 ± 426 | ||
| Lifetime Dose (mg) | 6227139 ± 4474756 |
p < 0.05;
p<0.001.
Individuals with SCZ were older at time of death, had smaller brains, were more likely to smoke cigarettes, and their samples had shorter freezer times and lower pHs. When information about smoking status at the time of death was missing, habitual smoking was verified through toxicological analyses of nicotine and cotinine levels in blood or brain tissue. Not all subjects were tested for nicotine or cotinine, and values are therefore expressed as a ratio of total tested.
For individuals with schizophrenia, each case was reviewed by two board-certified psychiatrists to establish DSM-IV Axis I lifetime psychiatric diagnoses, using psychiatric record reviews and/or family informant interviews (Lipska et al., 2006). Normal controls had no history of significant psychological problems or care, psychiatric admissions, lifetime history of substance abuse or dependence or acute substance intoxication. Toxicology testing was conducted on every case to screen for ethanol and illicit drugs. For individuals with schizophrenia, additional testing was performed by National Medical Services (Willow Grove, PN) to assess antipsychotic medication use at time of death. Whenever possible, use of antipsychotic medications was culled from available medical records and converted to chlorpromazine (CPZ) equivalent (CPZE) doses in milligrams.
2.1.2 Dissection
Gray matter tissue from the crown of the middle frontal gyrus was obtained from the coronal slab midway between the frontal pole and the most anterior extent of the genu of the corpus callosum. DLPFC corresponding to Brodmann's areas 9 and 46 was dissected on dry ice using a hand-held dental drill and immediately stored at −80 °C.
2.2. In vitro [3H]MePPEP Receptor Binding Assay
Tissue was homogenized in buffer (20 mM HEPES, 5 mM MgCl2, 1 mM EDTA, pH 7.4) with a Teflon pestle using a Glas-Col Homogenizing System and centrifuged at 25 000 × g for 25 minutes at 4°C. The pellet was re-suspended, aliquotted, and stored at −80 °C. Protein concentration was determined using the Bradford Protein Assay (Bio-Rad, Hercules, CA).
To determine affinity (KD) and Bmax (n=9 controls, n=8 schizophrenic) of MePPEP for the CB1 receptor, a homologous binding assay was performed in triplicate. One hundred μL [3H]MePPEP (specific activity 83 Ci/mmol; ~ 0.13 nM, diluted in buffer with 0.5% w/v BSA; Amersham GE Healthcare, UK) was added to each assay tube, followed by 100 μL of 10 cold MePPEP (Pharmacore, High Point, NC) concentrations (0.01 nM- 100 nM), buffer (to determine total binding), or 1 μM Rimonibant (Eli Lilly, Indianapolis, IN) (to determine non-specific binding). Eight hundred μL DLPFC homogenate suspension (20 μg/ mL) was added and incubated for 90 minutes in a shaking water bath at 23°C. Samples were filtered with a Brandel cell harvester (Gaithersburg, MD) through Whatman GF/A filter paper, followed by three washes of 3 mL ice-cold 50 mM Tris-HCl buffer (pH=7.4; 4 °C). Radioactivity was measured with liquid scintillation counting for five minutes using 4 mL of Ultima-Gold (Perkin Elmer, Chicago, IL).
To determine CB1 receptor binding, total and non-specific binding was determined (n=43 controls, n=47 schizophrenic). Specific binding was calculated by subtracting non-specific binding from total binding.
2.3. Genotyping of CNR1 single nucleotide polymorphism (SNP)
Genotyping was performed with Illumina Human 1M duo v3 chip via standard procedures (Illumina, Inc. San Diego, CA). The target CNR1 gene SNP (rs2023239) was not directly determined, and was therefore imputed by Impute2 software (Howie et al., 2009). Data from Hapmap 3 r2 (http://hapmap.ncbi.nlm.nih.gov/cgi-perl/gbrowse/hapmap3r2_B36/) plus the Human genome 1000 project (http://www.1000genomes.org/) were used as reference, including CEU and YRI populations. The Human genome build 36 map (http://www.ncbi.nlm.nih.gov/projects/genome/assembly/grc/human/) was used.
2.4. Statistical Analysis of Two-Point Assay Data
Statistical analyses were calculated using SPSS software (version 19, SPSS Inc., Chicago, IL). We used linear regression with group status and confounding variables as independent variables to examine group differences in [3H]MePPEP specific binding, and Pearson's Chi-square test, t-test, and Pearson's correlation coefficient to examine differences between groups in clinical and demographic variables, CNR1 gene variation, and to test for correlations between radioligand binding and these variables. The potential influence of the rs2023239 SNP was assessed by including C allele carrier status as a dichotomous independent variable in a regression model.
Effect size was calculated as the group difference in specific binding divided by standard deviation (SD) in the control group. This effect size was used to estimate how many subjects would be needed to observe a similar effect in vivo using PET and the radioligand [18F]FMPEP-d2 (Kb=0.19 nM) (Donohue et al., 2008;Terry et al., 2010). For this power analysis, mean and SD of prefrontal cortical [18F]FMPEP-d2 distribution volume (VT) were assumed to be 18.7 and 5.2, respectively (actual values from 39 healthy male subjects scanned to date in our lab). VT reflects specific binding and was calculated from brain and plasma radioactivity using compartmental modeling (Terry et al., 2010). Sample sizes for two-tailed t-tests were calculated using freely available software (Dupont and Plummer, 1990), and assumed α=0.05 (two-tailed) and β=0.20 (power of 80%).
3. Results
3.1 [3H]MePPEP Binding to DLPFC
KD and Bmax were first determined via homologous binding assays in a small cohort of DLPFC. The KD of MePPEP was 0.31±0.11 nM in controls and 0.31±0.14 nM in patients with schizophrenia. The Bmax was 0.10±0.08 pmol/mg protein in controls and 0.08 ± 0.04 pmol/mg protein in subjects with schizophrenia. Although KD and Bmax were similar between controls and patients with schizophrenia in this preliminary analysis, large variation (%COV>35%) may have prevented the detection of biologically meaningful effect sizes, suggesting that a significantly larger sample size might be necessary to identify differences in Bmax between the two groups.
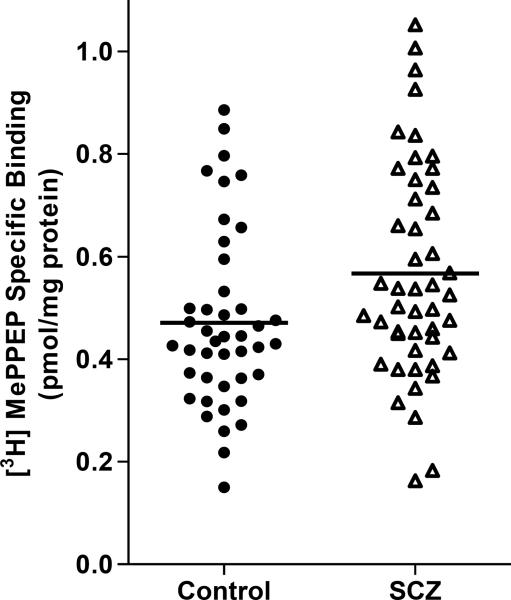
In the subsequent analysis of a larger cohort, specific binding—determined by two-point assays—of [3H]MePPEP to CB1 receptors was about 20% higher in patients with schizophrenia (0.566±0.208 pmol/mg protein) than in controls (0.471±0.173 pmol/mg protein) (Figure 1). The linear regression model showed a statistically significant effect of group on CB1 receptor binding (t=−1.99, p=0.050), but none of the confounding variables were statistically significant. When confounding variables were omitted from the statistical model, the group difference was statistically more significant (t=−2.35, p=0.021). Among patients with schizophrenia, no statistically significant correlations were observed between specific [3H]MePPEP binding and any of the potentially confounding variables (Table 2). In hindsight, the lack of even a trend-level difference between KD and Bmax may have been caused by the relatively small effect of disease status (i.e., schizophrenia) and the large variation of specific binding.
Fi1 1.
Cannabinoid CB1 specific binding of [3H]MePPEP (pmol/mg protein) in postmortem dorsolateral prefrontal cortex (DLPFC) of controls and patients with schizophrenia (SCZ). CB1 binding potential was 20% higher in patients with SCZ; analyses were controlled for categorical and continuous variables.
Table 2.
Correlations between CB1 receptor binding in the dorsolateral prefrontal cortex (DLPFC) of individuals with schizophrenia (SCZ) and controls.
| SCZ |
Controls |
|||
|---|---|---|---|---|
| Variable | R | p | R | p |
| pH | −0.034 | 0.818 | 0.024 | 0.879 |
| PMI | 0.163 | 0.273 | 0.023 | 0.884 |
| BMI | −0.036 | 0.818 | 0.243 | 0.121 |
| Age of Onset | −0.01 | 0.989 | N.A. | N.A. |
| Duration of Illness | 0.11 | 0.499 | N.A. | N.A. |
| Age at Death | 0.035 | 0.817 | −0.273 | 0.076 |
| Brain Weight | 0.204 | 0.169 | 0.114 | 0.467 |
| Freezer Storage Time | 0 | 0.998 | −0.121 | 0.465 |
| Age at First Hospitalization | 0.02 | 0.915 | N.A. | N.A. |
| Median CPZE | −0.15 | 0.341 | N.A. | N.A. |
| Cumulative Lifetime CPZE | −0.3 | 0.051 | N.A. | N.A. |
| Last Known CPZE | −0.25 | 0.147 | N.A. | N.A. |
| t |
p |
t |
p |
|
| Smoking Status | 0.94 | 0.352 | 0.422 | 0.675 |
| Gender | 0.108 | 0.915 | 0.154 | 0.879 |
| Comorbid Alcohol Abuse/ Dependence | 0.28 | 0.780 | N.A. | N.A. |
| Comorbid Substance Abuse/ Dependence | 0.47 | 0.642 | N.A. | N.A. |
| Antipsychotic drugs in brain at autopsy | −0.46 | 0.646 | N.A. | N.A. |
| Nicotine in brain at autopsy | −0.23 | 0.823 | 0.70 | 0.489 |
| Cotinine in brain at autopsy | −1.30 | 0.200 | 0.56 | 0.581 |
| Gender | 0.108 | 0.915 | 0.154 | 0.879 |
BMI: body mass index; CPZE: chlorpromazine equivalents; PMI: postmortem interval
3.2 Power Analysis
Power analysis suggested that 53 subjects per group would be needed to detect a difference of this magnitude in vivo with 80% statistical power and p<0.05 using PET and [18F]FMPEP-d2.
3.3 Genotype of CNR1 SNP
Among patients with schizophrenia and controls, 45% and 49% carried the rs2023239C allele, respectively (χ2=0.16; p=0.69). C allele carrier status did not significantly predict CB1 receptor binding when included in a regression model with group status and potential confounding variables (F=0.63, p=0.43). Among both healthy controls and patients with schizophrenia, C allele carriers had higher specific binding than non-carriers (6%, n=38 for healthy controls and 8%, n=42 for patients with schizophrenia), but this difference was not statistically significant.
4. Discussion
This study found that the specific binding of [3H]MePPEP to CB1 was 20% higher in the postmortem DLPFC of patients with schizophrenia than in healthy controls. These results are consistent with most published findings that used in vitro radiography methods (Dalton et al., 2011;Dean et al., 2001;Newell et al., 2006;Zavitsanou et al., 2004). Similarly, the lone in-vivo PET study in humans investigating this issue found increased CB1 receptor binding in individuals with schizophrenia, although this increase was only statistically significant in the pons (Wong et al., 2010). Our results suggest that this observed lack of statistical significance may be due to the small sample size of that cohort. Conversely, our results are not consistent with published findings using immunodetection (Eggan et al., 2008;Eggan et al., 2010;Koethe et al., 2007;Uriguen et al., 2009). Several factors may have led to the disparate results obtained via the different methods, including condition of the protein, location of the receptor, or specificity of the antibody or radioligand for the receptor.
Although medication effects cannot be discounted as a possible factor in binding in schizophrenia, no association was detected between binding and antipsychotic use at time of death, as measured by either toxicology or CPZE estimates.
Another issue of interest is whether the in vitro results obtained at 23°C could reasonably be used to estimate the sample size of an in vivo PET study, which would, of course, measure binding at 37°C. First, we performed our assays at 23°C because most published studies of CB1 receptor binding have used this temperature. This allowed our results to be more accurately compared to prior publications. Second, we expect that our primary finding of elevated CB1 receptor binding at 23°C will be replicated if performed at 37°C. The effect of temperature on MePPEP binding to CB1 is, to our knowledge, unknown; however, for most G-protein coupled receptors, KD increases as temperature increases. Unless temperature disproportionately affects KD in patients compared to controls, we expect that our result at 23°C would be replicated at 37°C and, therefore, that these results are appropriate for the power calculation of a future PET study.
Power analysis suggested that 53 subjects per group would be needed to detect a similar difference in vivo using PET and [18F]FMPEP-d2. While this large sample size may seem initially discouraging, a clinical imaging study comparing patients with schizophrenia and healthy controls would have several advantages over in vitro studies. Specifically, in vivo imaging would measure multiple brain regions simultaneously and could include patients in different phases of the illness. It would also obviate many of the confounding factors inevitably associated with in vitro methods.
Finally, genetic variation at rs2023239 in the CNR1 gene has been associated with substance abuse, and increased CB1 receptor binding has been found in post-mortem samples from patients with alcohol dependence who carry the C allele of this locus (Hutchison et al., 2008). The present study found that this SNP had no main effect on specific binding. The study therefore failed to replicate the previous findings obtained in patients with alcohol dependence; however, we did find a non-significant trend towards increased binding in C allele carriers from both groups compared with non-carriers.
Taken together, the results presented here support previous findings of increased CB1 specific binding in patients with schizophrenia, and lend further credence to the hypothesis that the cannabinoid system is altered in the brain of individuals with schizophrenia.
Acknowledgements
The authors gratefully acknowledge the support of the Intramural Program of the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH).
Role of Funding Source This study was supported in part by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH). The NIMH had no further role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest All authors have no conflict of interest to report, financial or otherwise.
Contributors KJ designed the study; wrote the protocol; managed literature searches; collected, analyzed, and interpreted the data; and drafted and revised the manuscript. JH designed the study; wrote the protocol; managed literature searches; conducted the statistical analysis; interpreted the data; and drafted and revised the manuscript. IDH managed literature searches; and drafted and critically revised the manuscript. KBA designed the study; wrote the protocol; and collected and analyzed data. SZ designed the study; wrote the protocol; helped interpret the data; and critically revised the manuscript. TMH dissected brain tissues; and drafted and critically reviewed the manuscript. AD-S organized and provided demographic data on brain tissues; managed literature searches and data interpretation; and drafted and critically revised the manuscript. RBI designed the study; wrote the protocol; analyzed and interpreted the data; and critically revised the manuscript. JEK provided the tissues; provided the genotyping and demographic data; and critically revised the manuscript. All authors contributed to and have approved the final manuscript.
5. References
- Dalton VS, Long LE, Weickert CS, Zavitsanou K. Paranoid Schizophrenia is Characterized by Increased CB1 Receptor Binding in the Dorsolateral Prefrontal Cortex. Neuropsychopharmacology. 2011;36(8):1620–1630. doi: 10.1038/npp.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B, Sundram S, Bradbury R, Scarr E, Copolov D. Studies on [3H]CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience. 2001;103(1):9–15. doi: 10.1016/s0306-4522(00)00552-2. [DOI] [PubMed] [Google Scholar]
- Deng C, Han M, Huang XF. No changes in densities of cannabinoid receptors in the superior temporal gyrus in schizophrenia. Neurosci Bull. 2007;23(6):341–347. doi: 10.1007/s12264-007-0051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue SR, Krushinski JH, Pike VW, Chernet E, Phebus L, Chesterfield AK, Felder CC, Halldin C, Schaus JM. Synthesis, ex vivo evaluation, and radiolabeling of potent 1,5-diphenylpyrrolidin-2-one cannabinoid subtype-1 receptor ligands as candidates for in vivo imaging. J Med Chem. 2008;51(18):5833–5842. doi: 10.1021/jm800416m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont WD, Plummer WD., Jr. Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11(2):116–128. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Hashimoto T, Lewis DA. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch Gen Psychiatry. 2008;65(7):772–784. doi: 10.1001/archpsyc.65.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan SM, Stoyak SR, Verrico CD, Lewis DA. Cannabinoid CB1 receptor immunoreactivity in the prefrontal cortex: Comparison of schizophrenia and major depressive disorder. Neuropsychopharmacology. 2010;35(10):2060–2071. doi: 10.1038/npp.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5(6):e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54(2):161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Haughey H, Niculescu M, Schacht J, Kaiser A, Stitzel J, Horton WJ, Filbey F. The incentive salience of alcohol: translating the effects of genetic variant in CNR1. Arch Gen Psychiatry. 2008;65(7):841–850. doi: 10.1001/archpsyc.65.7.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koethe D, Llenos IC, Dulay JR, Hoyer C, Torrey EF, Leweke FM, Weis S. Expression of CB1 cannabinoid receptor in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression. J Neural Transm. 2007;114(8):1055–1063. doi: 10.1007/s00702-007-0660-5. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Deep-Soboslay A, Weickert CS, Hyde TM, Martin CE, Herman MM, Kleinman JE. Critical factors in gene expression in postmortem human brain: Focus on studies in schizophrenia. Biol Psychiatry. 2006;60(6):650–658. doi: 10.1016/j.biopsych.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Newell KA, Deng C, Huang XF. Increased cannabinoid receptor density in the posterior cingulate cortex in schizophrenia. Exp Brain Res. 2006;172(4):556–560. doi: 10.1007/s00221-006-0503-x. [DOI] [PubMed] [Google Scholar]
- Terry GE, Hirvonen J, Liow JS, Seneca N, Tauscher JT, Schaus JM, Phebus L, Felder CC, Morse CL, Pike VW, Halldin C, Innis RB. Biodistribution and dosimetry in humans of two inverse agonists to image cannabinoid CB1 receptors using positron emission tomography. Eur J Nucl Med Mol Imaging. 2010;37(8):1499–1506. doi: 10.1007/s00259-010-1411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriguen L, Garcia-Fuster MJ, Callado LF, Morentin B, La Harpe R, Casado V, Lluis C, Franco R, Garcia-Sevilla JA, Meana JJ. Immunodensity and mRNA expression of A2A adenosine, D2 dopamine, and CB1 cannabinoid receptors in postmortem frontal cortex of subjects with schizophrenia: effect of antipsychotic treatment. Psychopharmacology (Berl) 2009;206(2):313–324. doi: 10.1007/s00213-009-1608-2. [DOI] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Horti AG, Raymont V, Brasic J, Guevara M, Ye W, Dannals RF, Ravert HT, Nandi A, Rahmim A, Ming JE, Grachev I, Roy C, Cascella N. Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. Neuroimage. 2010;52(4):1505–1513. doi: 10.1016/j.neuroimage.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavitsanou K, Garrick T, Huang XF. Selective antagonist [3H]SR141716A binding to cannabinoid CB1 receptors is increased in the anterior cingulate cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(2):355–360. doi: 10.1016/j.pnpbp.2003.11.005. [DOI] [PubMed] [Google Scholar]