Abstract
OBJECTIVE
Deep vein thrombosis (DVT) resolution instigates an inflammatory response, resulting in vessel wall damage and scarring. Urokinase-plasminogen activator (uPA) and its inhibitor, plasminogen activator inhibitor-1 (PAI-1), are integral components of the fibrinolytic system, essential for VT resolution. This study determined the vein wall response when exposed to increased and decreased plasmin activity.
Methods
A mouse inferior vena cava (IVC) ligation model in uPA −/− or PAI-1 −/− and their genetic wild types (B6/SvEv and C57/BL6, respectively) was used to create stasis thrombi, with tissue harvest at either 8 or 21d. Tissue analysis included gene expression of vascular smooth muscle cells (alpha SMA [αSMA], SM22) and endothelial marker (CD31), by real time PCR, ELISA, matrix metalloproteinase (MMP) -2 and 9 activity by zymography and vein wall collagen by picrosirius red histological analysis. A P < .05 was considered significant.
RESULTS
Thrombi were significantly larger in both 8d and 21d uPA −/− as compared to WT, and were significantly smaller in both 8 and 21d PAI-1 −/− as compared to WT. Correspondingly, 8d plasmin levels were reduced in half in uPA −/− and increased 3 fold in PAI-1 −/− when compared to respective WT thrombi (P < .05, N = 5 – 6). The endothelial marker CD31 was elevated 2 fold in PAI-1 −/− mice at 8d, but reduced 2.5 fold at 21d in uPA −/− as compared with WT (P = .02, N = 5 – 6), suggesting less endothelial preservation. Vein wall VSMC gene expression showed that 8d and 21d PAI-1 −/− mice had 2.3 and 3.8 fold more SM22 and 1.8 and 2.3 fold more αSMA expression than respective WT (P < .05, N = 5 – 7), as well as 1.8 fold increased αSMA (+) cells (N = 3 – 5, P ≤ .05). No significant difference in MMP2 or 9 activity was found in the PAI-1 −/− mice compared with WT, while 5.4 fold more MMP9 was present in 21d WT than 21d uPA −/− (P = .03, N = 5). Lastly, collagen was ~2 fold greater at 8d in PAI-1 −/− IVC as compared to WT (P = .03, N = 6) with no differences observed in uPA −/− mice.
CONCLUSIONS
In stasis DVT, plasmin activity is critical for thrombus resolution. Divergent vein wall responses occur with gain or loss of plasmin activity, and despite smaller VT, greater vein wall fibrosis was associated with lack of PAI-1.
INTRODUCTION
Deep vein thrombosis (DVT) is estimated to affect more than 300,000 people in North America and 460,000 people in the European Union each year.1 Approximately one-third of patients with DVT will develop postthrombotic syndrome (PTS),2, 3 which impairs quality of life and productivity.4 The rate at which DVT resolves has been found to correlate with the development of PTS.5 Little is known about what occurs at the vein wall level after DVT in relation to PTS. Current management is limited to elastic compression stockings, and other approaches such as pneumatic compression units and short-term use of venoactive agents are being explored.6,7
DVT resolution is an inflammatory process that affects vessel wall remodeling.8 Leukocytes, chemokines and hemostatic regulators play a role in both thrombosis and vein wall inflammation.9–11 Vein wall remodeling in the rodent after venous thrombosis (VT) involves profibrotic growth factors, collagen deposition, a change in the vein wall cell milieu, and matrix metalloproteinase (MMP) expression and activation.8 Currently, both the mechanism of thrombus formation and the length of contact time between the thrombus and the vein wall are thought to influence the vessel wall response.12 After vascular injury, there is denudation of the endothelial lining, deposition of new extracellular matrix, increased vascular smooth muscle cell (VSMC) proliferation and migration, and a switch of VSMC to a cellular synthetic state.13, 14 While all of these changes have been characterized in the arterial vasculature, the vessel wall undergoing DVT in the presence of increased or decreased plasmin has not been similarly characterized.
Plasmin, a serine protease formed by the cleavage of its proenzyme plasminogen, is the primary fibrinolytic enzyme responsible for DVT resolution. Plasminogen activators are serine proteases that activate plasminogen to the enzyme plasmin. The major plasminogen activator found in circulation is urokinase-plasminogen activator (uPA) and the major inhibitor of plasminogen activators is plasminogen activator inhibitor 1 (PAI-1). While the studies on the role of PAI-1 and risk of DVT have been inconsistent,15, 16 impaired thrombus resolution has been observed in uPA −/− mice.17 Moreover, the role of PAI-1 in vascular response depends on the model, genetic background and timing of analysis.18 By manipulating uPA and PAI-1 through genetically deleted mice, corresponding larger and smaller VT can also be indirectly examined to assess the vein wall effect.
In this study, we hypothesize that greater plasmin activity (PAI-1 −/−) would be associated with less vein wall fibrosis and less plasmin activity (uPA −/−) would be associated with greater fibrosis in a stasis model.
METHODS
Animal Model
Male mice from B6/SvEv wild type (WT), B6/SvEv uPA −/− (kindly donated by Drs. D. Collen and P. Carmiliet), C57BL6 WT and C57BL6 PAI-1 −/− (kindly donated by Dr. D. Lawrence) were used for all studies. Because of genetic background differences, each genetic deletion group were only compared with their respective genetic background controls. All animal studies were done with University of Michigan Animal Use Committee approval. For all surgical procedures, the mice underwent general anesthesia with isoflorane/O2. Stasis venous thrombosis was induced by inferior vena cava (IVC) ligation.9, 12, 13 A laparotomy with ligation of the IVC below the renal veins and division of all visible side branches was done. At sacrifice, at 8 and 21 days post-ligation, the thrombosed IVC was carefully dissected and removed for histological and biochemical studies. The thrombus is easy to remove at or before 8 days. After this, the thrombus and vein wall form a residual fibrosed remnant through 21 days. Thus, at 8d, the thrombus and vein wall were able to be assessed separately, while at 21d, these were examined together. This time frame was chosen to correlate with mid to late vein wall remodeling after DVT, and when post thrombotic syndrome (PTS) develops.
Histology / Immunohistochemical/ Collagen Staining
Tissue samples were formalin fixed, paraffin embedded, and cut into 5 μm sections as described.9, 13 Nonspecific sites were blocked with normal serum, and sections were incubated with primary antibodies to Mac2 (1:200, Cedarlane Laboratories, Burlington, NC), αSMA (1:500; Santa Cruz), and Fibroblast specific protein – 1 (FSP-1; Santa Cruz 1:500). A species-specific ABC peroxidase kit for either rabbit or rat (Vector Laboratories Inc., Burlingame, California) was used according to the manufacturer’s instructions for the corresponding secondary antibody and subsequent steps. Color development was performed with diaminobenzidine (DAB). The slides were counterstained with hematoxylin. In a blinded fashion, positive cells in 5 high power fields (1000x) radially around the IVC were counted and totaled.
Picrosirius red staining to quantify collagen content was performed as described.19, 20 These sections were then analyzed in crossed-plane polarized light from a monochromatic source to assess cross linked collagen. Two images for each were obtained using a Zeiss Axio M1 scope and Zeiss AxioVision software (Carl Zeiss Microimaging GmbH, Göttingen, Germany) at 0 and 90 degrees to the plane of polarization, in order to capture the birefringence of fibers extinguished in one direction. The images were analyzed blindly utilizing NIH Image J software. The area corresponding to the vein wall was selected as a region of interest, and then the image underwent threshold segmentation to differentiate collagen from other (mainly cellular and empty space) components of the vein wall. A vein wall collagen score was assigned by the formula [(%birefringent area) x (measured vein wall area)] / (total specimen area).
To account for non-collagen vein wall changes, intimal thickness scoring was assessed from H and E sections as described.21 A consistent mid-section thrombosed IVC segment was used for all histological analysis.22–24
Real time qPCR
Expression of β-actin, alpha SMA, SM22, CD31, procollagen I (Col1a2) and procollagen III (Col3a), were determined as follows: RNA was isolated by treatment of IVC wall segments with TRIzol reagent and reverse transcribed by incubating with Oligo-(dT) primer and M-MLV Reverse Transcriptase (Life Technologies, Carlsbad, CA) at 94°C for 3 minutes followed by 40°C for 70 minutes. The resultant cDNA was amplified by Taq Polymerase (Promega, Madison, WI) in the Rotogene quantitative polymerase chain reaction system (Qiagen Inc., Valencia, CA). SYBR Intercalating Dye (Roche, Indianapolis, IN) was used to monitor levels of cDNA amplification for each gene. Primers sequences were derived using validated primer sequences from Superarray (Fredrick, MD). Rotogene quantification utilizes the cycle threshold (Ct) for the gene of interest normalized to the housekeeping gene β-actin. Relative mRNA expression is calculated by the formula 2-(Ct target gene- Ct reference gene) and cycle lengths used are within the exponential phase of the PCR.14 These primers include: B-Actin PPM02945A Ref Pos 164–183; ASMA PPMM04483A Ref Pos 1107; SM22 PPM03802C Ref Pos 639–657; CD31 PPM03802C Ref Pos 1844; Col1a2 PPM04448E Ref Pos 4816; Col3a PPM04784B Ref Pos 4380.
ELISA
Tissue homogenate of the thrombus for plasmin and fibrinogen (from Innovative Research, Novi, MI), collagen IV (Exocell, Philadelphia, PA), and Thrombin-Antithrombin Complex (TAT, Enzyme Research Laboratories, South Bend, IN) were determined using a commercial kit according to manufacturer’s instructions. Quantification of peptide mediators was normalized to total protein in the sample as described.9, 13
SDS-PAGE Gelatin Zymography
As described9, 10 homogenized IVC tissue and thrombus were subjected to substrate zymography for MMP-2 and -9 using pre-cast 10% SDS-polyacrylamide gels containing 1 mg/mL of gelatin (unless otherwise stated, all zymography supplies were from Novex, San Diego, CA). Densitometry analysis was performed using a FOTO/Analyst CCD CAMERA (Fotodyne, Hartland, WI) and GEL-Pro Analyzer software version 3.1 (Media Cybernetics, Silver Springs, MD). Pro and active MMP-2 and -9 activity optical densities were summed and normalized to total protein.
Statistical analysis
All data are represented as mean ± SE. Two-tailed unpaired Student’s T-test was used for comparison between control and genetically deleted group (Graph PAD Prism, San Diego, CA), P ≤ .05 was assigned significance.
RESULTS
Thrombus Resolution is altered in uPA −/− and PAI-1 −/− mice
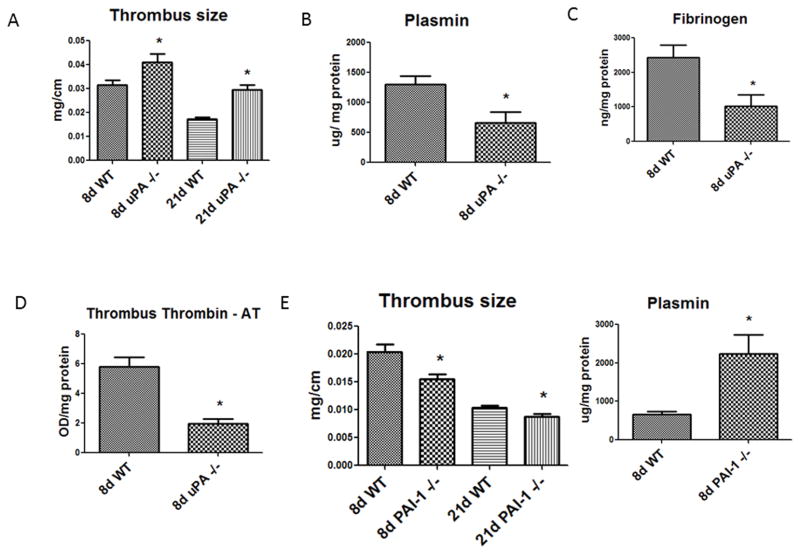
As measured by weight to length of the thrombosed IVC, uPA −/− mice had significantly larger thrombi than WT at both 8d (N = 13; P = .03) and at 21d (N = 17; P < .01) (Figure 1a). This phenotype is consistent with past studies derived from a slightly different model.17 Correspondingly, uPA −/− mice had less plasmin activity, less thrombus fibrinogen and TAT at 8 days than WT (N = 6; P = .02) (Figure 1b – d). Conversely, PAI-1 −/− mice had significantly smaller thrombi than WT at both 8 days (N = 12; P < .01) and at 21 days (N = 17; P < .01) (Figure 1e). The PAI-1 −/− mice had significantly more plasmin activity at 8 days (N = 6; P = .03) (Figure 1f), but no differences in thrombus fibrinogen or TAT (data not shown). However, no significant difference in plasmin in either group was found at 21 days (data not shown). Grossly, the thrombus histo-morphology was different with a large non nucleated cell thrombus in uPA −/− mice and a small cellular thrombus in PAI-1 −/− at 21d (Figures 2a – d). While the early 2 day time point was not a focus of this study, we found the thrombi were larger in uPA−/− as compared with WT (4.0 +/− 0.17 vs. 3.1 +/− 0.2 mg/mm; N = 4–6, P = .009). Conversely, PAI1−/− were not significantly different at the 2 day time point (3.0 +/− .24 vs. 2.6 +/− 0.10 mg/mm; N =7–8, P = .24).
Figure 1.
A) Thrombus size was larger in uPA −/− as compared with WT. B) Plasmin activity was decreased in uPA−/− at 8d as compared with WT; C) Despite larger thrombi, the fibrinogen content was lower, as was TAT at 8d in uPA −/− mice compared with WT. D) At 8 and 21d, VT were smaller in the PAI-1 −/− mice as compared with WT. E) Thrombus size was smaller in the PAI-1−/− as compared with WT. F) Plasmin activity was increased in PAI-1−/− at 8d as compared with WT. * = P < .05.
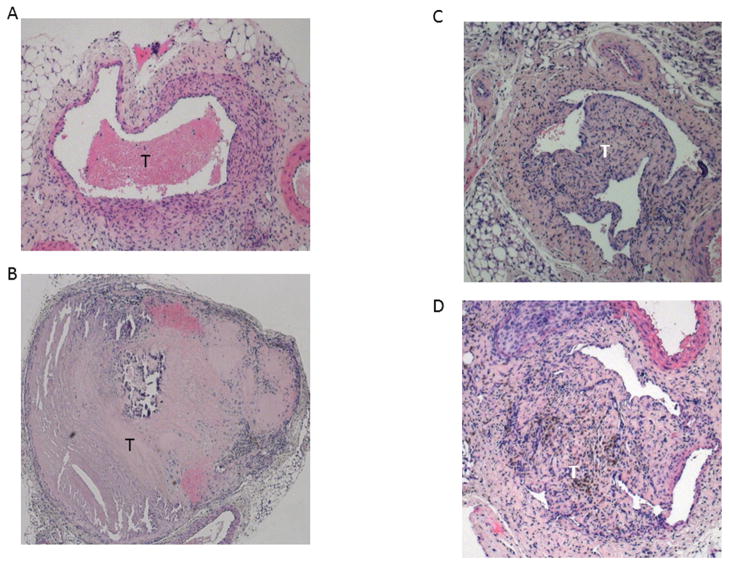
Figure 2.
Hematoxylin and Eosin stained IVC sections of 21d WT (A) and uPA −/− (B) examples. Note large and relative acellularity of the uPA −/− thrombus. The 21d WT (C) and PAI-1 −/− (D) are shown with similar thrombus morphology and cellularity.
Vein wall endothelial gene expression in uPA −/− and PAI-1 −/− mice
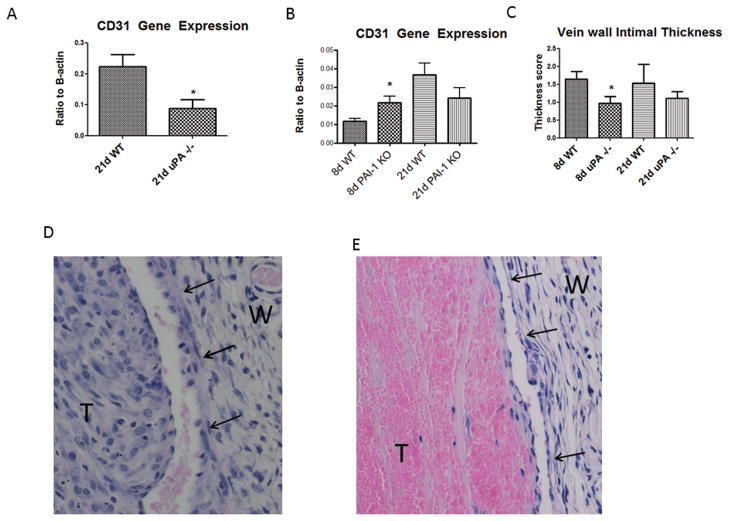
Denudation of the endothelium is associated with the mechanical stretch and inflammatory injury caused by thrombi.10, 22, 25 There was no difference in 8 day CD31 gene expression, an endothelial marker, in uPA−/− as compared to WT (0.20 +/− .14 vs. .08 +/− .4; N = 6, P = .48) However, there was ~2.5 fold greater CD31 gene expression, in 21d WT than in 21d uPA −/− mice (N = 5 – 6; P = .02) (Figure 3a). Conversely, PAI-1 −/− mice had 2 fold greater CD31 gene expression at 8d compared with WT (N = 5 – 7, P = .03) (Figure 3b).
Figure 3.
Gene expression of the endothelial marker CD31 was reduced in 21d uPA−/− compared with WT (A). Conversely, CD31 gene expression was increased at 8d in PAI-1 −/− (B) as compared with WT. Vein wall intimal thickness was reduced in 8d uPA−/− vein wall as compared with WT (C). Mild neointimal thickening is present in 8d WT vein walls(D), but little in the uPA −/− vein wall (E). Note again of marked cellularity of 8d WT thrombi, but few present in uPA −/− thrombi.
As a measure of the non-collagen component of the vein wall, the intimal thickness score was used.26 A loss of intimal thickness was found at 8d in uPA −/− mice compared with WT (Figure 3c – e). No significant difference was observed in the uPA −/− intimal thickness as compared with WT at 21d. No difference in intimal thickness was found in the PAI −/− as compared with WT at 8 or 21d (data not shown).
Vein Wall Fibrosis is Increased in PAI-1 −/− after VT
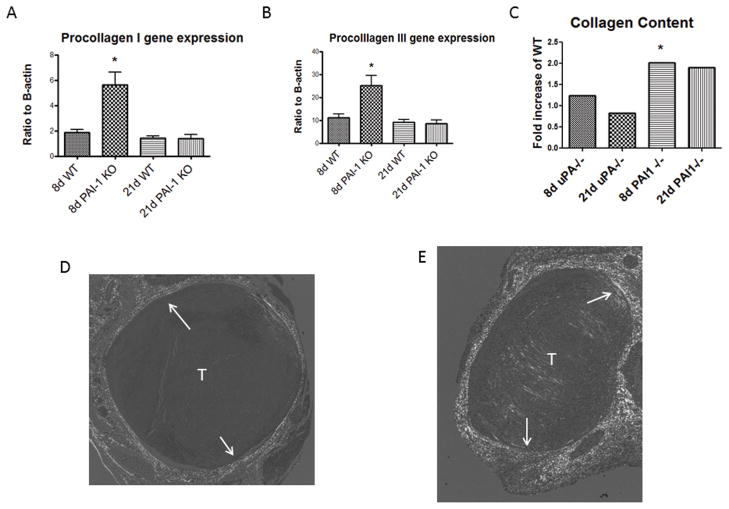
Vein walls become thickened and fibrotic in stasis VT in both mice and in humans.10, 12, 27 We found that procollagen I and III gene expression was elevated 3 fold in PAI-1 −/− as compared with WT (P < .05) (Figure 4a, b). No differences in these gen expressions in procollagen I or III gene expression in uPA −/− as compared with WT was found (not shown). Consistently, vein wall collagen content was elevated 2 fold in 8d PAI-1 −/− as compared with WT (2.2 ± .45 vs. 0.9 ± .16 AU; N = 6, P = .03)), and a trend at 21d (6.2 ± 1.8 vs. 3.1 ± 1 AU, N = 5–6, P = .13) (Figure 4c – e). No significant differences in vein wall collagen content were found at 8 or 21d in uPA −/− as compared to WT.
Figure 4.
Vein wall procollagen I (A) and III (B) gene expression was elevated in 8d PAI-I −/− as compared with WT. By Picosirius red analysis, collagen content was elevated at 8d in PAI-1 −/− compared with WT, and a trend at 21d while noo difference in collagen content was found in uPA −/− at 8 or 21d (C). Birefringence images show a thin vein wall collagen in WT (D), but greater amount in PAI-1 −/− vein wall (E). P < .05. T = thrombus; W = wall.
Vein Wall Cellular and Proteinase Response in uPA −/− and PAI-1 −/− Mice after VT
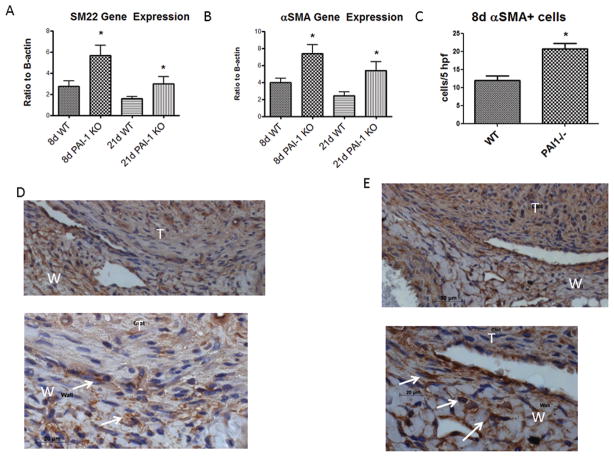
To assess if there were differences in matrix VSMC, we assessed for gene expression and for antigen expression by immunohistochemistry. PAI-1 −/− mice had significantly more vein wall SM22 gene expression, at both 8d (N = 5 – 7; P = .03) and 21d (N = 5 – 7; P = .03) (Figure 5a), than WT. In addition, PAI-1 −/− mice also had significantly more αSMA expression at both 8d (N = 5 – 7; P = .01) and 21d (N = 5 – 7; P = .03) (Figure 5b). We found a corresponding 1.8 fold increase in αSMA (+) cells at 8d in PAI-1 −/− as compared with WT (N = 3 – 5, P = .02)(Figure 5c – e). No difference in fibroblast type cells were found (FSP-1 + cells: 3.7 +/− .3 vs. 3.4 +/− 7, N = 3 – 5, P = .8) at 8d in PAI-1 −/− compared to WT. No significant difference was found in uPA −/− vein wall vSMC marker gene expression as compared with WT at 8 or 21d.
Figure 5.
VSMC gene marker showed increased SM22 (A) and αSMA (B) expression in PAI-1 −/− vein wall compared to WT at 8 and 21d. Medial αSMA + cells were increased in PAI-1 −/− at 8d as compared with WT (C). Immunohistology showed the mononuclear medial location of these positive cells in WT (D) and PAI-1 −/− (E) IVC sections. *P < .05; arrows denote (+) cells.
Monocytes direct vascular response to injury, including the vein wall response. At 8d, no significant difference in monocyte (Mac-2+) vein wall counts were observed in any of the groups. However, at 21d increased monocytes were found in both the PAI -1 −/− (19 +/− 5 vs. 5 +/− 1, N = 5, P = .02) and uPA −/− (33 +/− 8 vs. 5 +/− 2, N = 5, P = .01) vein wall as compared with WT controls.
MMPs are present in the vein wall after VT, and metabolize the ECM to allow for VSMC migration, proliferation, and vessel remodeling.12, 28 Despite an increased plasmin activity level in PAI-1 −/− mice, no significant difference in vein wall MMP2 or 9 zymographic activity was found in PAI-1 −/− at 8 or 21d, when compared with WT. A significant decrease in MMP9 activity at 21d in uPA −/− mice as compared to WT was found (40 +/− 15 vs. 10 +/− 5 AU/mg protein; N = 5; P = .03), but no significant differences in MMP2 activity at 8 or 21d were found in uPA −/− or PAI-1 −/− mice (data not shown).
Discussion
Post-thrombotic syndrome affects up to 30% of people after DVT,2, 3 leading to a significant decrease in quality of life.4 Management of PTS pharmacologically has been limited in part by a lack of understanding the underlying pathophysiology of the disease. However, observations suggest that rapid DVT resolution is associated with lower risk of PTS,5 but no specific pharmacological agents are available that directly address this problem.6 The main findings of this study suggest that increased or decreased plasmin results in a divergent injury response at the cellular and genetic level. While this study specifically evaluated the role of increased or decreased plasmin on the vein wall response after VT, the indirect role of thrombus size may also have contributed to the phenotypes herein. Presumably, a larger thrombus should confer greater vein wall damage, both mechanically and due to presence of inflammatory cells and mediators at the thrombus- vein wall interface, but this was not the case.
Zhou and colleagues showed early endothelial injury cracking and denudation after stasis VT, with exposure of prothrombotic elements.29 We also have shown early endothelial loss with recovery at 2 weeks in a rat model of stasis VT, with low molecular weight heparin associated with less endothelial loss at 14 days after thrombosis.22 In the current study, loss of plasmin activity (uPA −/−) was not associated with an increase in fibrotic injury, but primarily affected the endothelial response, with less late CD31 gene expression. Of note, non thrombosed sham CD31 gene expression is ~ 20 fold higher than thrombosed 8d IVC expression (Henke PK, unpublished data). This is also counter to our initial hypothesis that less plasmin would be associated with worsened fibrotic injury. Our data contrasts with the models of arterial injury where endothelial denudation is associated with increased neointimal fibrosis.30, 31 Indeed, not only was no increase in fibrosis observed with loss of uPA, but the neointimal response was significantly less than control WT. Several factors may account for this difference, including the local shear stress, distension, and cellular elements unique in the venous thrombus. Less thrombin (as measured by TAT complex) was also observed in the uPA−/− thrombi, and thrombin has profibrotic effects.32 Further, less fibrin(ogen), per weight of thrombus, was also found in the uPA−/− thrombi, and fibrinogen may stimulate leukocyte proinflammatory and secondary profibrotic activities.33 Moreover, lack of uPA in an arterial model was associated with increased vascular injury, but was predominately luminal occlusion and not the vessel wall. Similar to our findings, no increase in medial fibrosis was observed in uPA −/− arterial walls after balloon injury.34
These observations also suggest that the thrombosis mechanism is likely more important than the size of the thrombus itself in the vein wall injury response. For example, in the rat model, the greatest injury was observed with a stasis compared with non-stasis model.10 Supporting this also is that despite larger VT in the rat due to direct plasmin inhibition, we found less biomechanical evidence of vein wall injury.11 Our data in the late vein wall response (>8d) contrasts with the early independence of uPA on VT resolution. Specifically, early VT resolution in the uPA −/− mouse is independent of uPA, and dependent on MMP2.24 These observations as well as those of others17 highlight the time dependent and phasic nature of VT resolution and subsequent vein wall response.
Although the VT were smaller in PAI-1 −/− mice, the vein wall fibrotic injury at 8 days was greater than in WT controls.. However, these observations are consistent with the vessel response from experimental arterial injury models. For example, in both flow restriction and FeCI3 arterial injury models, as well as myocardial injury, PAI-1 deletion or exogenous PAI-1 inhibition were associated with increased neointimal hyperplasia.35–37 While we found no difference in intimal thickness in WT or PAI-1 −/− mice after VT, a thickened and collagen rich vein wall was found with deletion of PAI-1−/−. This phenotype was associated with increased VSMC gene expression and cell number, suggesting either proliferation or influx of VSMC. Our data do not allow us to determine which mechanism occurred, but others have shown in an arterial injury model that increased VSMC proliferation occurs with lack of PAI-1 and may be due to PAI-1’s thrombin inhibitory activity.36 We speculate that since TAT levels were not increased in PAI-1−/− thrombi, the role of vitronectin37 or other migratory processes are more important than the proliferative effect in our stasis model. Specifically, PAI-1 may be protective from fibrotic injury by both its vitronectin binding role (decreased VSMC proliferation and migration) and by its anti-proteolytic activity.37 Moreover, this is in part driven by bone marrow derived progenitor cells that express PAI-1, as PAI-1 competent cells rescued the neointimal hyperplastic injury phenotype.38 Lastly, others have shown TGFB may be elevated in PAI-1 −/− mice after vascular injury, as this cytokine has well documented profibrotic effects.39
The differential role of the monocyte in vein wall response and thrombus resolution after stasis VT has been recently reviewed.8, 40 Counterintuitively, a greater monocyte number is not necessarily associated with greater vein wall damage. For example, in a rat model of stasis VT, P-selectin inhibition was associated with increased vein wall monocyte influx, but less fibrosis and stiffness.21 Monocyte subtypes may confer a pro or anti-inflammatory state depending on the local environment.41 As this was a late response after stasis thrombus injury, and little significant difference of phenotypic injury response was observed in either genotype at 21 days, the mechanistic relevance is not clear. However, neither PAI-1 −/− nor uPA −/− affected monocyte influx at 8d when the phenotypic differences were greatest.
Despite an increase in plasmin in the PAI-1 −/− vein wall, we did not observe an increase in either MMP2 or MMP9 by zymographic analysis. While plasmin can activate these MMPs, other mechanism such as MMP14 or MMP3 also play a major role after vascular injury.42 Our findings suggest that the thrombus size itself does not affect MMP activity outside of the elevation associated with the stasis thrombus model itself.12 Consistent with the known action of plasmin, less MMP9 activity was present late in uPA −/− mice, but this was not associated with a phenotypic difference in vein wall fibrosis. Interestingly, direct plasmin inhibition in a rat stasis model was associated with increased MMP9 and associated with increased biomechanical injury.11 Although less plasmin was present in the uPA −/− mice and no difference in late vein wall injury was found, some activity remained and may account for the difference in vein wall response between the rat and the mouse. Lastly, after day 8, our model does not allow for thrombus separation from the vein wall as they are densely adherent. The decrease in MMP9 activity may also be due to fewer thrombus mononuclear cells,17 which are a prime source of MMP9.
In humans, the longer the thrombus is in contact with the vein wall the greater the damage.5 While this in vivo study shows promise for PAI-1 as a pharmacologic target for VT resolution, whether or not exogenous PAI-1 inhibition increases vein wall fibrosis awaits further study, once exogenous agents become more readily available. Moreover, our model did not allow to assess if an increased fibotic response affects the thrombus adherence and could lead to decreasesd embolization to the pulmonary circulation. Nonetheless, a potential translational therapeutic combination might be a PAI-1 inhibitor in conjunction with a thrombin specific inhibitor, such as hirudin, that abolishes the profibrotic effect of PAI-1 inhibitors.36
Acknowledgments
Supported by HL 083918 and HL092129
Footnotes
Presented in part at the 23rd Annual American Venous Forum Meeting, February 25, 2011
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, Greer IA, Heit JA, Hutchinson JL, Kakkar AK, Mottier D, Oger E, Samama MM, Spannagl M. Venous thromboembolism (vte) in europe. The number of vte events and associated morbidity and mortality. Thromb Haemost. 2007;98:756–764. doi: 10.1160/TH07-03-0212. [DOI] [PubMed] [Google Scholar]
- 2.Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, Cattelan AM, Polistena P, Bernardi E, Prins MH. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- 3.Kahn SR, Shrier I, Julian JA, Ducruet T, Arsenault L, Miron MJ, Roussin A, Desmarais S, Joyal F, Kassis J, Solymoss S, Desjardins L, Lamping DL, Johri M, Ginsberg JS. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698–707. doi: 10.7326/0003-4819-149-10-200811180-00004. [DOI] [PubMed] [Google Scholar]
- 4.Kahn SR, Shbaklo H, Lamping DL, Holcroft CA, Shrier I, Miron MJ, Roussin A, Desmarais S, Joyal F, Kassis J, Solymoss S, Desjardins L, Johri M, Ginsberg JS. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6:1105–1112. doi: 10.1111/j.1538-7836.2008.03002.x. [DOI] [PubMed] [Google Scholar]
- 5.Meissner MH, Caps MT, Zierler BK, Polissar N, Bergelin RO, Manzo RA, Strandness DE., Jr Determinants of chronic venous disease after acute deep venous thrombosis. J Vasc Surg. 1998;28:826–833. doi: 10.1016/s0741-5214(98)70057-6. [DOI] [PubMed] [Google Scholar]
- 6.Kahn SR. The post-thrombotic syndrome. Hematology Am Soc Hematol Educ Program. 2010:216–220. doi: 10.1182/asheducation-2010.1.216. [DOI] [PubMed] [Google Scholar]
- 7.Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg. 53:500–509. doi: 10.1016/j.jvs.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 8.Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol. 2008;28:387–391. doi: 10.1161/ATVBAHA.108.162289. [DOI] [PubMed] [Google Scholar]
- 9.Henke PK, Pearce CG, Moaveni DM, Moore AJ, Lynch EM, Longo C, Varma M, Dewyer NA, Deatrick KB, Upchurch GR, Jr, Wakefield TW, Hogaboam C, Kunkel SL. Targeted deletion of ccr2 impairs deep vein thombosis resolution in a mouse model. J Immunol. 2006;177:3388–3397. doi: 10.4049/jimmunol.177.5.3388. [DOI] [PubMed] [Google Scholar]
- 10.Henke PK, Varma MR, Moaveni DK, Dewyer NA, Moore AJ, Lynch EM, Longo C, Deatrick CB, Kunkel SL, Upchurch GR, Jr, Wakefield TW. Fibrotic injury after experimental deep vein thrombosis is determined by the mechanism of thrombogenesis. Thromb Haemost. 2007;98:1045–1055. [PubMed] [Google Scholar]
- 11.Dewyer NA, Sood V, Lynch EM, Luke CE, Upchurch GR, Jr, Wakefield TW, Kunkel S, Henke PK. Plasmin inhibition increases mmp-9 activity and decreases vein wall stiffness during venous thrombosis resolution. J Surg Res. 2007;142:357–363. doi: 10.1016/j.jss.2007.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deatrick KB, Eliason JL, Lynch EM, Moore AJ, Dewyer NA, Varma MR, Pearce CG, Upchurch GR, Wakefield TW, Henke PK. Vein wall remodeling after deep vein thrombosis involves matrix metalloproteinases and late fibrosis in a mouse model. J Vasc Surg. 2005;42:140–148. doi: 10.1016/j.jvs.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Henke PK, Varga A, De S, Deatrick CB, Eliason J, Arenberg DA, Sukheepod P, Thanaporn P, Kunkel SL, Upchurch GR, Jr, Wakefield TW. Deep vein thrombosis resolution is modulated by monocyte cxcr2-mediated activity in a mouse model. Arterioscler Thromb Vasc Biol. 2004;24:1130–1137. doi: 10.1161/01.ATV.0000129537.72553.73. [DOI] [PubMed] [Google Scholar]
- 14.Ginzinger DG. Gene quantification using real-time quantitative pcr: An emerging technology hits the mainstream. Exp Hematol. 2002;30:503–512. doi: 10.1016/s0301-472x(02)00806-8. [DOI] [PubMed] [Google Scholar]
- 15.Schulman S, Wiman B. The significance of hypofibrinolysis for the risk of recurrence of venous thromboembolism. Duration of anticoagulation (durac) trial study group. Thromb Haemost. 1996;75:607–611. [PubMed] [Google Scholar]
- 16.Crowther MA, Roberts J, Roberts R, Johnston M, Stevens P, Skingley P, Patrassi GM, Sartori MT, Hirsh J, Prandoni P, Weitz JI, Gent M, Ginsberg JS. Fibrinolytic variables in patients with recurrent venous thrombosis: A prospective cohort study. Thromb Haemost. 2001;85:390–394. [PubMed] [Google Scholar]
- 17.Singh I, Burnand KG, Collins M, Luttun A, Collen D, Boelhouwer B, Smith A. Failure of thrombus to resolve in urokinase-type plasminogen activator gene-knockout mice: Rescue by normal bone marrow-derived cells. Circulation. 2003;107:869–875. doi: 10.1161/01.cir.0000050149.22928.39. [DOI] [PubMed] [Google Scholar]
- 18.Fay WP, Garg N, Sunkar M. Vascular functions of the plasminogen activation system. Arterioscler Thromb Vasc Biol. 2007;27:1231–1237. doi: 10.1161/ATVBAHA.107.140046. [DOI] [PubMed] [Google Scholar]
- 19.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 20.Cuttle L, Nataatmadja M, Fraser JF, Kempf M, Kimble RM, Hayes MT. Collagen in the scarless fetal skin wound: Detection with picrosirius-polarization. Wound Repair Regen. 2005;13:198–204. doi: 10.1111/j.1067-1927.2005.130211.x. [DOI] [PubMed] [Google Scholar]
- 21.Myers DD, Jr, Henke PK, Bedard PW, Wrobleski SK, Kaila N, Shaw G, Meier TR, Hawley AE, Schaub RG, Wakefield TW. Treatment with an oral small molecule inhibitor of p selectin (psi-697) decreases vein wall injury in a rat stenosis model of venous thrombosis. J Vasc Surg. 2006;44:625–632. doi: 10.1016/j.jvs.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 22.Moaveni DK, Lynch EM, Luke C, Sood V, Upchurch GR, Wakefield TW, Henke PK. Vein wall re-endothelialization after deep vein thrombosis is improved with lowmolecular-weight heparin. J Vasc Surg. 2008;47:616–624. doi: 10.1016/j.jvs.2007.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henke PK, Mitsuya M, Luke CE, Elfline MA, Baldwin JF, Deatrick KB, Diaz JA, Sood V, Upchurch GR, Wakefield TW, Hogaboam C, Kunkel SL. Toll-like receptor 9 signaling is critical for early experimental deep vein thrombosis resolution. Arterioscler Thromb Vasc Biol. 31:43–49. doi: 10.1161/ATVBAHA.110.216317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sood V, Luke CE, Deatrick KB, Baldwin J, Miller EM, Elfline M, Upchurch GR, Jr, Wakefield TW, Henke PK. Urokinase plasminogen activator independent early experimental thrombus resolution: Mmp2 as an alternative mechanism. Thromb Haemost. 104:1174–1183. doi: 10.1160/TH10-03-0184. [DOI] [PubMed] [Google Scholar]
- 25.Deroo S, Deatrick KB, Henke PK. The vessel wall: A forgotten player in post thrombotic syndrome. Thromb Haemost. 2010;104:681–692. doi: 10.1160/TH10-03-0183. [DOI] [PubMed] [Google Scholar]
- 26.Sood V, Luke C, Miller E, Mitsuya M, Upchurch GR, Jr, Wakefield TW, Myers DD, Henke PK. Vein wall remodeling after deep vein thrombosis: Differential effects of low molecular weight heparin and doxycycline. Ann Vasc Surg. 2010;24:233–241. doi: 10.1016/j.avsg.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deatrick KB, Elfline M, Baker N, Luke CE, Blackburn S, Stabler C, Wakefield TW, Henke PK. Postthrombotic vein wall remodeling: Preliminary observations. J Vasc Surg. 2011;53:139–146. doi: 10.1016/j.jvs.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raffetto JD, Khalil RA. Matrix metalloproteinases in venous tissue remodeling and varicose vein formation. Curr Vasc Pharmacol. 2008;6:158–172. doi: 10.2174/157016108784911957. [DOI] [PubMed] [Google Scholar]
- 29.Zhou J, May L, Liao P, Gross PL, Weitz JI. Inferior vena cava ligation rapidly induces tissue factor expression and venous thrombosis in rats. Arterioscler Thromb Vasc Biol. 2009 doi: 10.1161/ATVBAHA.109.185678. [DOI] [PubMed] [Google Scholar]
- 30.Walter DH, MD, Rittig K, MD, Bahlmann FH, MD, Kirchmair R, MD, Silver M, BS, Murayama T, MD, PhD, Nishimura H, MD PhD, Losordo DW, MD, Asahara T, MD, PhD, Isner JM., MD Statin therapy accelerates reendothelialization - a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circ. 2002;105:3017–3024. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 31.Kong D, Melo LG, Mangi AA, Zhang L, Lopez-Ilasaca M, Perrella MA, Liew CC, Pratt RE, Dzau VJ. Enhanced inhibition of neointimal hyperplasia by genetically engineered endothelial progenitor cells. Circulation. 2004;109:1769–1775. doi: 10.1161/01.CIR.0000121732.85572.6F. [DOI] [PubMed] [Google Scholar]
- 32.McNamara CA, Sarembock IJ, Gimple LW, Fenton JW, 2nd, Coughlin SR, Owens GK. Thrombin stimulates proliferation of cultured rat aortic smooth muscle cells by a proteolytically activated receptor. J Clin Invest. 1993;91:94–98. doi: 10.1172/JCI116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flick MJ, LaJeunesse CM, Talmage KE, Witte DP, Palumbo JS, Pinkerton MD, Thornton S, Degen JL. Fibrin(ogen) exacerbates inflammatory joint disease through a mechanism linked to the integrin alphambeta2 binding motif. J Clin Invest. 2007;117:3224–3235. doi: 10.1172/JCI30134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schafer K, Konstantinides S, Riedel C, Thinnes T, Muller K, Dellas C, Hasenfuss G, Loskutoff DJ. Different mechanisms of increased luminal stenosis after arterial injury in mice deficient for urokinase- or tissue-type plasminogen activator. Circ. 2002;106:1847–1852. doi: 10.1161/01.cir.0000031162.80988.2b. [DOI] [PubMed] [Google Scholar]
- 35.Moriwaki H, Stempien-Otero A, Kremen M, Cozen AE, Dichek DA. Overexpression of urokinase by macrophages or deficiency of plasminogen activator inhibitor type 1 causes cardiac fibrosis in mice. Circ Res. 2004;95:637–644. doi: 10.1161/01.RES.0000141427.61023.f4. [DOI] [PubMed] [Google Scholar]
- 36.de Waard V, Arkenbout EK, Carmeliet P, Lindner V, Pannekoek H. Plasminogen activator inhibitor 1 and vitronectin protect against stenosis in a murine carotid artery ligation model. Arterioscler Thromb Vasc Biol. 2002;22:1978–1983. doi: 10.1161/01.atv.0000042231.04318.e6. [DOI] [PubMed] [Google Scholar]
- 37.Wu J, Peng L, McMahon GA, Lawrence DA, Fay WP. Recombinant plasminogen activator inhibitor-1 inhibits intimal hyperplasia. Arterioscler Thromb Vasc Biol. 2009;29:1565–1570. doi: 10.1161/ATVBAHA.109.189514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schafer K, Schroeter MR, Dellas C, Puls M, Nitsche M, Weiss E, Hasenfuss G, Konstantinides SV. Plasminogen activator inhibitor-1 from bone marrow-derived cells suppresses neointimal formation after vascular injury in mice. Arterioscler Thromb Vasc Biol. 2006;26:1254–1259. doi: 10.1161/01.ATV.0000215982.14003.b7. [DOI] [PubMed] [Google Scholar]
- 39.Luttun A, Lupu F, Storkebaum E, Hoylaerts MF, Moons L, Crawley J, Bono F, Poole AR, Tipping P, Herbert J-M, Collen D, Carmeliet P. Lack of plasminogen activator inhibitor-1 promotes growth and abnormal matrix remodeling of advanced atherosclerotic plaques in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2002;22:499–505. doi: 10.1161/hq0302.104529. [DOI] [PubMed] [Google Scholar]
- 40.Saha P, Humphries J, Modarai B, Mattock K, Waltham M, Evans CE, Ahmad A, Patel AS, Premaratne S, Lyons OT, Smith A. Leukocytes and the natural history of deep vein thrombosis: Current concepts and future directions. Arterioscler Thromb Vasc Biol. 2011;31:506–512. doi: 10.1161/ATVBAHA.110.213405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wynn TA, Barron L. Macrophages: Master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson JL, Dwivedi A, Somerville M, George SJ, Newby AC. Matrix metalloproteinase (mmp)-3 activates mmp-9 mediated vascular smooth muscle cell migration and neointima formation in mice. Arterioscler Thromb Vasc Biol. 2011;31:e35–44. doi: 10.1161/ATVBAHA.111.225623. [DOI] [PubMed] [Google Scholar]