Abstract
The Rho-associated kinases ROCK1 and ROCK2 are critical for cancer cell migration and invasion, suggesting they may be useful therapeutic targets. In this study, we describe the discovery and development of RKI-1447, a potent small molecule inhibitor of ROCK1 and ROCK2. Crystal structures of the RKI-1447/ROCK1 complex revealed that RKI-1447 is a Type I kinase inhibitor that binds the ATP binding site through interactions with the hinge region and the DFG motif. RKI-1447 suppressed phosphorylation of the ROCK substrates MLC-2 and MYPT-1 in human cancer cells, but had no effect on the phosphorylation levels of the AKT, MEK and S6 kinase at concentrations as high as 10 μM. RKI-1447 was also highly selective at inhibiting ROCK-mediated cytoskeleton re-organization (actin stress fiber formation) following LPA stimulation, but does not affect PAK-meditated lamellipodia and filopodia formation following PDGF and Bradykinin stimulation, respectively. RKI-1447 inhibited migration, invasion and anchorage-independent tumor growth of breast cancer cells. In contrast, RKI-1313, a much weaker analog in vitro, had little effect on the phosphorylation levels of ROCK substrates, migration, invasion or anchorage-independent growth. Lastly RKI-1447 was highly effective at inhibiting the outgrowth of mammary tumors in a transgenic mouse model. In summary, our findings establish RKI-1447 as a potent and selective ROCK inhibitor with significant anti-invasive and anti-tumor activities and offer a preclinical proof-of-concept that justify further examination of RKI-1447 suitability as a potential clinical candidate.
Keywords: Rho kinase, Anti-invasive, Anti-tumor, Breast cancer, RKI-1447
INTRODUCTION
The Rho family of small GTPases transduces biological signals from cell surface receptors such as receptor tyrosine kinases to the nucleus. They act as molecular switches that bind GTP (active) and GDP (inactive) to regulate cell proliferation, survival and cytoskeleton organization, thus, affecting cell shape/morphology, adhesion and movement (1-3). Some of these GTPases, particularly RhoA and RhoC, have attracted much attention because of their well documented role in malignant transformation hallmarks including migration, invasion and metastasis. For example, RhoA and RhoC are required for Ras-driven malignant transformation (4), and their overexpression induces migration, invasion and metastasis while their dominant negative forms block these hallmarks of cancer (5, 6). Furthermore, several Rho GTPases are over expressed in a large number of human cancers (7-10), and the expression of RhoA and RhoC has been shown to be much higher in metastatic tumors (11, 12). Several studies have suggested RhoC as a prognostic biomarker for metastasis in melanoma, breast and pancreatic cancer patients (5, 11, 12). The mechanism by which RhoA and RhoC contribute to metastasis is believed to involve the binding of RhoA-GTP and RhoC-GTP to their major effectors, the Rho-associated kinases 1 and 2, together referred to here as ROCKs (13-19). ROCKs control cell shape, adhesion and migration by regulating actin-myosin contractibility. This is accomplished by ROCKs phosphorylating several major substrates such as myosin light chain (MLC), the MLC phosphatase PP1 regulatory subunit MYPT-1 and LIM kinases 1 and 2. Phosphorylation of MLC activates it to drive migration and invasion (14, 20). Phosphorylation of LIMK 1 and 2 activates it to phosphorylate and inactivate cofilin from inhibiting migration/invasion (21). Phosphorylation of MYPT-1 blocks dephosphorylation and inactivation of MLC (16).
The role of ROCKs in migration, invasion and metastasis has been well documented. For example, over expression of ROCKs induces migration and invasion in several tumor types including breast cancer (22-24) while dominant negative ROCKs and ROCKs inhibitors block invasion and metastasis in vitro and in vivo (23, 25-30). Furthermore, the expression levels of ROCKs is 10-fold higher in breast tumor biopsies as compared to their corresponding normal mammary tissue, and these high expression levels correlate with metastasis, poor clinical outcome and shorter survival time of breast cancer patients (26, 31). The overwhelming evidence implicating the Rho GTPases/ROCK pathway in migration, invasion and metastasis prompted the development of inhibitors to interfere with this pathway with the ultimate goal of discovering novel anti-tumor drugs (14, 26, 27, 29). To this end several approaches have been taken. One of these is to develop geranylgeranyltransferase-1 (GGT-1) inhibitors (GGTIs), since GTPases such as RhoA and RhoC require the lipid post-translation modification geranylgeranylation for their ability to induce malignant transformations (32). Though this approach has shown great promise pre-clinically and one compound GGTI-2418 has reached Phase I clinical trials (33), a drawback is that GGTIs inhibit GGT-1, an enzyme that has dozens of substrates (32). A more selective approach to interfering with the Rho GTPase/ROCK pathway is to develop ROCK inhibitors as potential anti-metastatic agents. In this manuscript we describe the discovery and development of RKI-1447 a highly potent ROCK inhibitor. First, using x-ray crystallography we solved the structure of the RKI-1447-ROCK1 complex and determine the mode of action of RKI-1447. Furthermore, we demonstrated that RKI-1447 suppresses ROCK-dependent signaling, cell morphology, anchorage-independent cell growth, migration invasion and in vivo growth of mammary tumors.
MATERIALS AND METHODS
Cell lines
Human breast (MDA-MB-231 and MDA-MB-468) and Lung (H-1299) cell lines were purchased from ATCC. These cells have not been authenticated.
ROCK1 and ROCK2 kinase assays
The Invitrogen Z-Lyte® FRET kinase assay with KKRPQRRYSNVF peptide as substrate (from MLC sequence) was used (Invitrogen, cat. PV3793) exactly as described by us (34).
Enzyme purification and Protein crystallography
Enzyme purification and protein crystallography were performed exactly as described by us previously (34). Data were reduced with XDS (35). The structure was solved by molecular replacement using the MolRep program from CCP4 (36) with the monomer of pdb 3TV7 as a starting model. PHENIX (37) was employed for refinement, and model building was performed using Coot (38). Figures were prepared using PYMOL (39).
Evaluation of the phosphorylation levels of MLC-2 and MYPT-1 by Western blot analysis
Cells were plated and treated the next day with vehicle, RKI-1447 or RKI-1313 for 1 hour. [The chemical synthesis of RKI-1447 and RKI-1313 was described by us (34). The mesylate salts of the compounds were used to enhance solubility.] The cells were then harvested, lysed and processed for western blotting as described by us previously (40). The antibodies that were used for western blotting were MYPT1 (Cat#612164; BD Transduction Laboratories), Phospho-T696-MYPT1 (Cat#ABS45; Millipore), MLC2 (Cat#3672S), Phospho-S19-MLC2 (Cat#3671S), Akt (Cat#9272), Phospho-S473-Akt (Cat#9271), Phospho-S298-MEK(Cat#9128S), MEK (Cat#9122S), Phospho-S240-S244-S6 (Cat#2215S), S6 (Cat#2217), Cleaved PARP (Cat#5625S) (Cell Signaling Technology) and Tubulin (Cat#T5168;Sigma).
Determination of anchorage-independent growth using soft agar assays
The soft agar assays were carried out exactly as described by us (41).
Migration assay
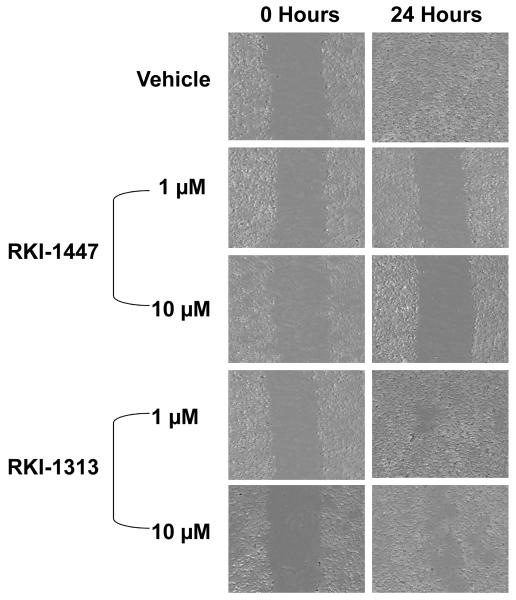
MDA-MB-231 cells were seeded at 4×105 cells per well in a 6-well plate and allowed to grow overnight. The cells were then starved for 24 hours. The media was then aspirated and the cells scratched with a pipette tip, and treated either with vehicle, RKI-1447 or RKI-1313 at the indicated concentrations for 24 hours. Pictures were taken with a Leica Microscope before (time 0) and 24 hours after drug treatments.
Invasion assay
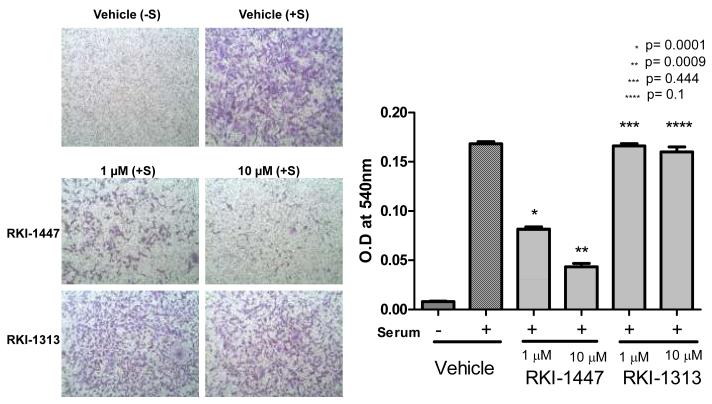
Invasion assay was performed in Corning Transwell inserts coated with Matrigel. MDA-MB-231 cells were seeded at 3.5×105 and allowed to grow overnight in a 6 well-plate. The cells were then treated with either vehicle, RKI-1447 or RKI-1313 for 21 hours and trypsinized, resuspended in DMEM containing 0.1% BSA and plated at 20,000 cells/insert in the top chamber over the Matrigel. The bottom chamber contains 20% FBS as the “chemoattractant”. The cells in the top chamber were carefully removed after 48 hours and the filter membranes containing the invaded cells were fixed with methanol, stained with crystal violet and photographed. The membranes were then dissolved in 10% acetic acid and the absorbance was read at 540 nm.
Effects of RKI-1447 and RKI-1313 on cell morphology
NIH 3T3 cells were plated at 8000 cells/well in 8-chamber slides in serum free media for 24 hours, and treated with vehicle, 1 μM RKI-1447 or 1 μM RKI-1313 for 1 hour. The cells were then stimulated with complete media, 10 μM LPA, 200 ng/mL bradykinin or 30 ng/mL PDGF for 30 mins. After stimulation, the cells were fixed in 4% paraformaldehyde and stained with Texas-Red Phalloidin. Mounting medium containing DAPI (Cat#H-1200; Vector Laboratories) was then added and at least 100 cells per well were observed. The cells were imaged using Zeiss Upright Fluorescence Microscope.
RKI-1447 anti-tumor efficacy studies
MMTV/neu transgenic mice [FVB/N-Tg (MMTVneu) 202 Mul/J] were purchased from the Jackson Laboratory and bred to produce multiple litters to maintain the colony. The anti tumor efficacy studies were performed exactly as described by us previously (41). Briefly, the mice were treated i.p. daily for 14 days with either Vehicle [20%-2-hydroxypropyl-beta-cyclodextrin (HPCD)] or 200 mpk RKI-1447 dissolved in freshly prepared HPCD. The percent change in volume was calculated on the basis of the tumor volume on the last day of treatment (Vf) relative to that on the day of initiation of treatment (V0). The average percent change in tumor volume was then calculated for each treatment group.
RESULTS
Discovery of RKI-1447, a highly potent ROCK1 and ROCK2 inhibitor
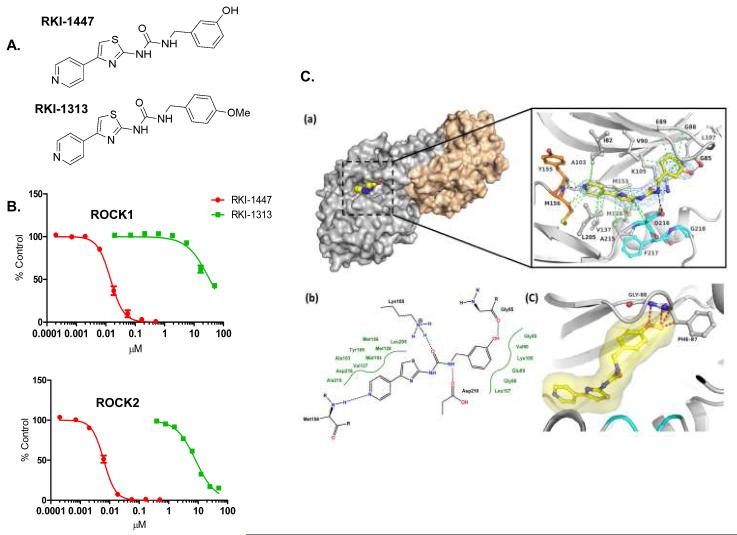
Our chemistry efforts resulted recently in the identification of a family of pyridylthiazole ROCK inhibitors (34). Structure activity relationship studies of this family of ROCK inhibitors led to the discovery of a pair of closely related analogues; RKI-1447 (potent ROCK inhibitor) and RKI-1313 (weak ROCK inhibitor). Figures 1A and 1B show that RKI-1447 inhibits potently ROCK1 and ROCK2 in a dose-dependent manner with IC50 values of 14.5 nM and 6.2 nM, respectively. In contrast, RKI-1313 where the meta-hydroxyl on the phenyl ring of RKI-1447 was replaced with a para-methoxy group was much less active and had IC50 values 34 μM for ROCK 1 and 8 μM for ROCK2 (Figures 1A and 1B).
Figure 1. RKI-1447 and RKI-1313 Chemical structures, in vitro ROCK inhibitory activity and RKI-1447-ROCK1 co-crystal structure.
1A. Chemical structure of RKI-1447 and RKI-1313. 1B. RKI-1447 is much more potent than RKI-1313 at inhibiting both ROCK1 and ROCK2. 1C. Structure of the RKI-1447-ROCK1 complex. (a) Surface presentation of one of the two ROCK1 dimers in complex with RKI-1447 determined by X-ray crystallography at 2.9 Å resolution and detailed binding interactions in the ATP site. The hinge region is indicated in orange, the DFG motif in cyan, and RKI-1447 in yellow. Displayed in blue is the 2 Fo-Fc electron density, contoured at 1σ around the RKI-1447 inhibitor. The Fo-Fc electron density map with the RKI-1447 inhibitor omitted during refinement is shown in the supplementary data (Supplementary Figure S4). The hydrogen bonding and van der Waal interactions are shown as black and green dotted lines, respectively. (b) Schematic presentation of the binding interactions between RKI-1447 and the ATP site. (c) Model of RKI-1313 bound to the binding site of RKI-1447.
Crystal structure of the RKI-1447-ROCK1 complex reveals that RKI-1447 is a Type 1 inhibitor that binds both the hinge region and the DFG motif of the ROCK ATP binding site
To determine the mode of binding of RKI-1447 to ROCK, we determined the crystal structure of RKI-1447 in complex with the kinase domain of human ROCK1 (residues 6-415). The RKI-1447-ROCK1 complex was crystallized in space group C2221 with two ROCK1 dimers in the asymmetric unit. The structure was refined to 2.9 Å with Rcryst and Rfree values of 22.8 and 29.0%, respectively. Figure 1C shows the overall structure of the dimer and the interactions that RKI-1447 establishes in the ATP binding site. Specifically, RKI-1447 is a Type I inhibitor that binds to the hinge region through a hydrogen bond formed between the pyridine ring nitrogen and the main chain amide NH of Met156. The inhibitor extends from the hinge region along Asp216 of the DFG motif towards the β-turn comprising residues Gly85 – Gly88. The urea moiety interacts with the ε-amino group of Lys105 and the carboxyl group of Asp216. The phenol hydroxyl is in hydrogen bonding distance with the main chain carbonyl oxygen of Gly85. The pyridine, thiazole and phenol moieties of the inhibitor form multiple van der Waals (hydrophobic) interactions with surrounding residues (3.4 Å < d < 4 Å). The network of non-covalent binding interactions between RKI-1447 and ROCK1 together with the extended, low energy conformation of the inhibitor, explain the high inhibitory potency of RKI-1447 towards ROCKs. This binding mode also explains the weak inhibitory activity of the analogue RKI-1313. Introduction of the methoxy group in para position of the phenyl ring is likely to cause substantial steric hindrance with Gly88 and Phe87 (Figure 1C (panel (c)). In addition, elimination of the hydroxyl group from the meta position of the phenyl ring will result in the loss of hydrogen bonding potential with the main chain carbonyl of Gly85.
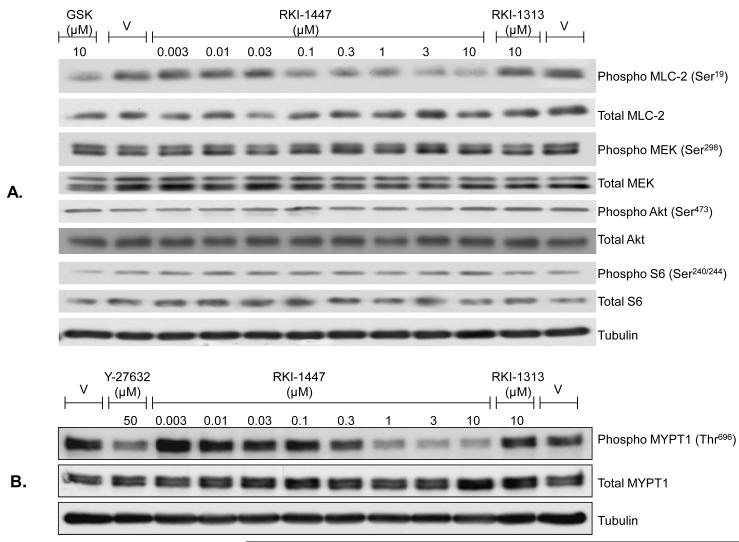
RKI-1447 is much more potent than RKI-1313 at inhibiting the phosphorylation of the ROCK substrates MLC-2 and MYPT-1 in human cancer cells
We next determined the ability of RKI-1447 and RKI-1313 to inhibit ROCKs in intact human cancer cells. To this end, we determined the effects of RKI-1447 and RKI-1313 on the phosphorylation levels of two ROCK substrates: MLC-2 and MYPT-1. This was carried out by treating cells with various concentrations of the compounds and processing the cells for Western immunoblotting to determine their effects on the levels of P-MLC-2, P-MYPT-1, total MLC-2 and total MYPT-1 as described under Materials and Methods. Figure 2A shows that RKI-1447 treatment of MDA-MB-231 human breast cancer cells decreased the levels of P-MLC-2, but not total MLC-2, in a concentration-dependent manner with significant effects starting at 100 nM. RKI-1313 did not decrease P-MLC-2 at 10 μM consistent with its weak inhibitory activity against ROCK1 and ROCK2 in vitro (Figure 1B). RKI-1447 also decreased the levels of P-MYPT-1 in MDA-MB-231 cells in a dose-dependent manner (Supplementary Figure S1). Similar results were obtained with another human breast cancer cell line, MDA-MB-468, where RKI-1447 decreased the levels of both P-MLC-2 and P-MYPT-1 in a dose-dependent manner (Supplementary Figure S1). Furthermore, RK1-1447 but not RKI-1313 inhibited the levels of P-MYPT-1 in a concentration-dependent manner in H1299 human lung cancer cells (Figure 2B). Figure 2A also shows that RKI-1447 had no effects on the phosphorylation levels of Akt, Mek and S6 suggesting that RKI-1447 is selective for ROCK over kinases that phosphorylate Akt (i.e. mTORC2), Mek (i.e. PAK) and S6 (i.e. S6K).
Figure 2. RKI-1447 but not RKI-1313 inhibits selectively the phosphorylation of MLC-2 and MYPT-1.
MDA-MB-231 (A) and H1299 (B) cells were treated with RKI-1447 or RKI-1313 and processed for western blotting as described under Materials and Methods. GSK-429286 and Y-27632 were used as controls. Data are representative of 2 independent experiments.
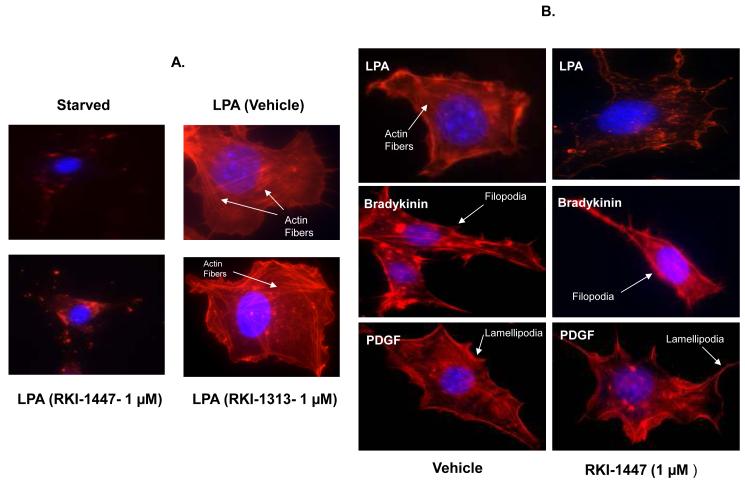
RKI-1447 inhibits LPA-induced actin stress fiber formation but not PDGF-induced lamellipodia formation or bradykinin-induced filopodia formation
The ability of LPA to induce actin stress fiber formation is known to be mediated by activation of the RhoA/ROCK pathway whereas the ability of PDGF and bradykinin to induce lamellipodia and filopodia is known to be mediated by the RAC1/PAK and the CDC42/PAK pathways, respectively. We reasoned that if RKI-1447 is selective for ROCKs, then it should only inhibit LPA-induced actin stress fiber formation but not lamellipodia and filopodia formation by PDGF and Bradykinin. To this end, we starved NIH3T3 cells and treated them with either vehicle or RKI-1447 prior to stimulation with either LPA, PDGF or Bradykinin, stained the cells with phalloidin to evaluate their morphological changes as described under Materials and Methods. Figure 3A shows that starved cells contain no actin stress fibers, filopodia or lamellipodia. Stimulation with LPA resulted in actin stress fiber formation and this was blocked by RKI-1447 but not RKI-1313 treatment. Stimulation with PDGF resulted in the formation of lamellipodia and this was not affected by RKI-1447 treatment (Figure 3B). Similar results were obtained with Bradykinin where RKI-1447 had no effects on bradykinin-induced filopodia formation (Figure 3B). These results further support the selectivity of RKI-1447 as a ROCK inhibitor.
Figure 3. RKI-1447 suppresses LPA-induced actin stress fiber but not PDGF-induced lamellipodia or bradykinin-induced filopodia formation.
(A) Starved NIH-3T3 cells were treated with either vehicle, RKI-1447 or RKI-1313 prior to treatment with LPA, (B) Starved NIH-3T3 cells were treated with either vehicle or RKI-1447 prior to treatment with LPA, bradykinin or PDGF, and then stained with phalloidin as described under Materials and Methods. Data are representative of 2 independent experiments.
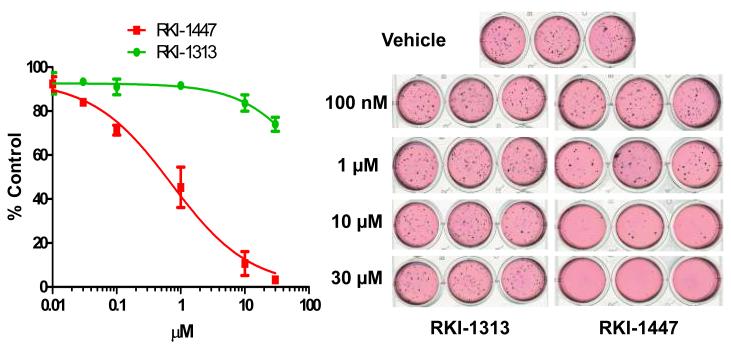
RKI-1447 is much more potent than RKI-1313 at inhibiting anchorage-independent colony formation, migration and invasion of breast cancer cells
The results presented in Figures 1, 2 and 3, and Supplementary Figure S1 clearly demonstrated that RKI-1447 is a potent and selective ROCK1 and ROCK2 inhibitor capable of reaching its target and inhibiting selectively the phosphorylation of the ROCK substrates MLC-2 and MYPT-1. Because of the prominent role that ROCKs play in adhesion, migration and invasion we reasoned that inhibition of ROCKs should result in suppression of these hallmarks of malignant transformation. To this end, MDA-MB-231 cells were treated with various concentrations of RKI-1447 and its less active analogue RKI-1313 and processed for anchorage-independent tumor colony formation in soft agar, migration using wound healing assays and invasion using Boyden Chamber assays as described under Materials and Methods. Figure 4 shows that treatment of MDA-MB-231 cells with RKI-1447 hampered their ability to form soft agar colonies with an IC50 value of 709 nM. In contrast, RKI-1313 up to 30 μM had little effect. Furthermore, in the absence of RKI-1447, MDA-MB-231 cells migrated after being scratched and filled the wound created by the scratch (Figure 5). Treatment with RKI-1447 inhibited this scratch-induced migration at both 1 and 10 μM (Figure 5); whereas RKI-1313 had minimal effect on migration consistent with its much less potent activity against ROCKs in vitro and in intact cells. Finally, treatment of MDA-MB-231 cells with RKI-1447 inhibited their serum-induced invasion by 53% and 85% at 1 and 10 μM, respectively (Figure 6). Consistent with the soft agar and migration assays, RKI-1313 had little effect on inhibiting MDA-MB-231 tumor cell invasion (Figure 6). It is important to point out that RKI-1447 at these concentrations (1 and 10 μM) was more than 10-fold less active at inhibiting anchorage-dependent proliferation/survival of MDA-MB-231 breast cancer cells as measured by MTT assay (see Supplementary Figure S2), indicating that the ability of RKI-1447 to inhibit soft agar growth, migration and invasion is most likely not due to its ability to inhibit anchorage-dependent proliferation/survival. Consistent with this, RKI-1447 concentrations as high as 10 μM did not induce apoptosis (PARP cleavage) and had little effect on cell cycle progression (Supplementary Figure S3).
Figure 4. RKI-1447 but not RKI-1313 inhibits anchorage-independent growth of breast cancer cells.
MDA-MB-231 cells were treated with various concentrations of RKI-1447 or RKI-1313 and processed for soft agar growth assays as described under Materials and Methods. Data show averages of 3 independent experiments.
Figure 5. RKI-1447 but not RKI-1313 inhibits migration of breast cancer cells.
MDA-MB-231 cells were treated with RKI-1447 or RKI-1313 and processed for scratch-wound healing migration assays as described under Materials and Methods. Data are representative of 3 independent experiments.
Figure 6. RKI-1447 but not RKI-1313 inhibits invasion of breast cancer cells.
MDA-MB-231 cells were treated with RKI-1447 or RKI-1313 and processed for invasion assays as described under Materials and Methods. Data show averages of 3 independent experiments.
RKI-1447 inhibits mammary tumor growth in vivo
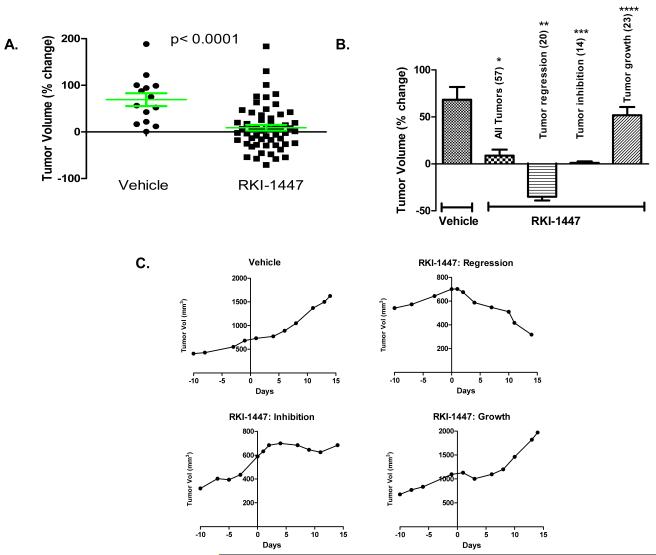
The results described in Figures 1-6 demonstrate that RKI-1447 is a potent and selective ROCK inhibitor that suppresses ROCK-driven cytoskeleton re-organization, signaling, migration, invasion and anchorage-independent tumor cell growth of breast cancer cells. While these results are encouraging they were all performed in cultured cells. We next determined whether RKI-1447 can inhibit tumor growth in an in vivo environment. To this end, we used the ErbB2 mammary cancer transgenic mouse model. In this model, the mice harbor the ErbB2 gene under the control of the MMTV promoter, and spontaneously develop tumors in their mammary tissues. Mammary tumors were measured beginning at the time of tumor onset and treatment (once a day for 14 days) with vehicle (20% HPCD) or RKI-1447 (200 mpk/day) began when tumor volumes reached 75 to 2,300 mm3. A wide range of tumor volumes was used to ensure that responses were not volume dependent. Supplementary Tables S1 and S2 show initial volume before treatment, final volume at the end of treatment and the % change in tumor volume for 57 tumors from 28 mice treated with RKI-1447 and 14 tumors from 13 mice treated with vehicle. The percent change was calculated from the tumor volume on the last day of treatment relative to the volume on the day of initiation of treatment. Figure 7A shows the % change in tumor volume for each individual tumor whereas Figure 7B shows the average percent change for each treatment group. Tumors from mice treated with vehicle increased in size with an average percent change in tumor volume of 68.3% (Figure 7B). In contrast, tumors from mice treated with the RKI-1447 increased in size with an average percent change in tumor volume of only 8.8% (p<0.0001). Thus, RKI-1447 inhibited mammary tumor growth by 87% ([1-(8.8/68.3)] × 100), and on average the mammary tumors from RKI-1447 treated mice were 7.7 fold smaller compared to those tumors from mice treated with the vehicle control. A closer look at the data from Supplementary Table 1 and Figure 7A lead us to divide the RKI-1447 treated group into 3 sub-groups: those tumors that regressed (tumors whose volumes decreased by 10% or greater-see representative growth curve in Figure 7C upper right panel), those tumors that continued to grow (tumors whose volumes increased by 10% or more-see representative growth curve in Figure 7C lower right panel) and those tumors whose growth was inhibited (tumors whose volume decreased or increased by less than 10%-see representative growth curve in Figure 7C lower left panel). Figure 7B shows that RKI-1447 treatment caused 20 tumors (35% of the total of 57 tumors treated) to regress, 14 tumors (25%) to be growth inhibited and had no effect on the growth of 23 tumors (40%). The 20 tumors that regressed did by an average of 35.1%. The 23 tumors that were not affected grew on average by 51.8% similar to the vehicle treated tumors (average of 68.3%) (Figure 7B). Finally, RKI-1447 treatment inhibited the growth of 14 tumors with a change in volume of only 1.04%. Therefore, as compared to the vehicle treated group where tumors grew on average 68.3%, the tumors in this group grew only by 1.04% indicating a 98% ([1-(1.04/68.3)] × 100) growth inhibition. Thus, in the majority (60%) of tumors, RKI-1447 treatment caused either regression (in 35% of tumors) or tumor growth inhibition (in 25% of tumors). RKI-1447 or vehicle did not result in weight loss (see supplementary Tables S3 and S4).
Figure 7. Effects of RKI-1447 on tumor growth in ErbB2 mammary tumor transgenic mouse model.
Mice bearing mammary tumors were treated (once a day for 14 days) with vehicle (20% HPCD) or RKI-1447 (200 mpk/day). (A) Shows the percent change in tumor volume for each individual tumor. (B) Shows the average percent change for each treatment group. (C) Shows representative tumor growth curves from vehicle treated mice (upper left) as well as from RKI-1447 treated mice where tumors regressed (upper right), were growth inhibited (lower left) or continued to grow (lower right). Statistical significance was evaluated by determining the p value for the differences between vehicle and RKI-1447 treated groups: *p= 0.0001 (all tumors), **p< 0.0001 (tumor regression), ***p< 0.0001 (tumor inhibition), ****p= 0.2928 (tumor growth).
DISCUSSION
Breast cancer accounts for 450,000 of cancer deaths and is the leading cause of cancer death in women worldwide (42). As with other cancers, metastasis is the major reason breast cancer patients succumb to this disease. The role of the Rho family of GTPases in malignant transformation, and particularly the role of RhoA and RhoC in migration, invasion and metastasis has been well documented both in pre-clinical models as well as in human patients (4-6, 8-12). The ability of RhoA and RhoC to induce migration and invasion is believed to be mediated primarily through binding and activation of their effectors ROCK1 and ROCK2 (ROCKs) (14-19, 32). The RhoA/C-ROCKs pathway has been shown to be elevated in metastatic tumors as compared to their non-metastatic counter parts, and this has been associated with poor prognosis, resistance to therapy and shorter survival time of breast cancer patients (26, 31). In this manuscript we describe the discovery of RKI-1447 as a potential anti-invasive and anti-tumor agent in breast cancer. As control we have used RKI-1313 a closely related analogue that is a much weaker ROCK inhibitor. RKI-1447 inhibited potently ROCKs and blocked selectively ROCK-dependent signaling and morphological changes. Furthermore, RKI-1447 was much more effective than RKI-1313 at inhibiting breast cancer anchorage-independent growth, migration and invasion. Finally, RKI-1447 significantly inhibited mammary tumor growth in animal models.
The co-crystal structure of the RKI-1447-ROCK1 complex revealed that RKI-1447 belongs to the Type I class of kinase inhibitors. The high potency of RKI-1447 is reflected in an elaborate network of non-covalent interactions in the binding site that involve all inhibitor moieties. This provides a very tight fit of the entire inhibitor molecule and the ATP site over a distance of ~18 Å. Consequently, a slight modification in the phenyl ring, which is opposite to the hinge-binding pyridine, disturbs the binding interactions between the inhibitor and ROCK1 substantially and leads to a significant loss of potency as seen with RKI-1313. Indeed, the hydrogen bond between the phenyl meta-hydroxyl in RKI-1447 is lacking in RKI-1313, and this coupled with the steric hindrance caused by the para-methoxy in RKI-1313 offers a possible explanation for the large difference in potency between RKI-1313 and RKI-1447.
Our studies also showed that RKI-1447 is selective and inhibited the phosphorylation levels of ROCK substrates such as MLC-2 and MYPT-1 but not substrates for PAK (Mek), S6K (S6) and mTORC2 (Akt). This selectively translated into highly specific morphological effects of RKI-1447. Indeed, RKI-1447 inhibited LPA-induced, ROCK-dependent, actin stress fiber formation but not the PAK-dependent, PDGF- and bradykinin-induced lamellipodia and filopodia, respectively. This inhibition of stress fiber formation and the resulting disruption of the cytoskeleton organization suggested that RKI-1447 might have effects on cell motility, invasion and other cancer cell characteristics required for metastasis. Our results clearly demonstrate that the ability of breast cancer cells to form colonies in an anchorage-independent manner in soft agar is hampered by RKI-1447. Similarly, RKI-1447 was very potent at inhibiting breast cancer cell migration in a wound healing assay as well as breast cancer cell invasion through matrigel. In contrast, RKI-1313 was much less effective at inhibiting anchorage-independent growth, migration and invasion, consistent with it its very weak activity to inhibit ROCKs in vitro and in intact cells. The fact that RKI-1313 and RKI-1447 are very close analogues structurally coupled with the fact that their ability to inhibit ROCKs in vitro and in vivo correlates with their biological effects suggests that the ability of RKI-1447 to inhibit anchorage-independent growth, migration and invasion is mediated by its ability to inhibit ROCKs. Furthermore, the ability of RKI-1447 to inhibit anchorage-independent growth, migration and invasion is most likely not due to inhibition of tumor proliferation since the concentrations that inhibited the former had little effects on the later. This is not surprising since several studies have shown that the involvement of ROCKs in oncogenesis is primarily by mediating invasion and metastasis and not proliferation of tumors (22-30).
The receptor tyrosine kinase family plays a major role in breast cancer oncogenesis. In particular, ErbB2 is a significant biomarker that predicts poor prognosis, therapy resistance and poor survival of breast cancer patients. We have used a transgenic mouse model where mammary tumor oncogenesis is driven by ErbB2 to evaluate the effectiveness of RKI-1447 in vivo. This model has extensively and successfully been used to evaluate the effects of a variety of agents on mammary tumorigenesis. For example, this model was used to show that the small molecule tyrosine kinase inhibitors lapatinib that targets both ErbB2 and EGFR (43) and gefitinib that targets EGFR (44) inhibited mammary tumorigenesis. Cytotoxic agents such as the HSP90 inhibitor 17-AAG (45) and carboplatin (46) as well as monoclonal antibodies against ErbB2 (47) were also very effective in this model. In addition, this model was also successfully used by us to demonstrate that the combination of the Akt inhibitor TCN-P and the farnesyltransferase inhibitor Zarnestra is more effective than single agent treatment (41). In the present study, this mammary tumor model was used to demonstrate that RKI-1447 treatment of 28 mice (57 tumors) resulted in an average tumor growth inhibition of 87%; on average tumors from mice treated with RKI-1447 were 7.7 fold smaller than those from mice treated with vehicle. To our knowledge this is the first demonstration that a ROCK inhibitor affects the growth of mammary tumors in an ErbB2-driven breast cancer model. The draw back of this anti-tumor in vivo study is that it did not investigate metastasis directly. However, the growth of these mammary tumors as a 3 dimensional mass depends on their ability to grow in an anchorage-independent manner which is ROCK-dependent, and which was potently inhibited by RKI-1447 in the soft agar studies.
The effect of RKI-1447 in this in vivo model was heterogeneous with 60% of the 57 tumors treated being sensitive and 40% resistant. Among the 34 sensitive tumors, 20 regressed and 14 were growth inhibited. There are several potential mechanisms that could account for these heterogeneous responses. One possibility could be related to expression levels of certain biomarkers known to influence resistant/sensitivity of beast tumors. For example, the expression levels of estrogen receptors (ER) and progesterone receptors (PR) is often associated with sensitivity/resistance to chemotherapeutic agents in both preclinical and clinical settings (48-50). However, this mechanism is unlikely to contribute to the response differences that we have seen as it has been shown that the mammary tumors in this MMTV-Her2 model all express very low levels of ER and are PR negative (51). Another mechanism that could account for the differences in response to RKI-1447 could be related to differences in the epithelial-to-mesenchymal transition (EMT) status. In deed, EMT has been shown to generate cells with properties of stem cells (52), and these can become resistant to treatment (53). Therefore, it would be of great interest to evaluate the EMT status in responders compared to non-responders to RKI-1447. Furthermore, the tumor stroma plays a pivotal role in breast tumor angiogenesis and metastasis (54), and differences in the relative tumor stroma/cancer cell mass could contribute to differences in responses to RKI-1447. It is also possible that tolerance, pharmacokinetics and bioavailability influenced RKI-1447 response, but this is unlikely since these mice are in-bred and should behave similarly with regards to these parameters. Understanding the mechanisms of resistance will pave the way to designing combination therapies of RKIs with other anti-signaling agents including ErbB2 inhibitors and ErbB2 neutralizing antibodies.
In summary, we have designed a highly potent and selective ROCK Type 1 inhibitor that binds to the ATP site of ROCK 1 at the hinge region and the DFG motif. RKI-1447 but not its closely related analogue RKI-1313 was highly effective at inhibiting ROCK-dependent signaling, cytoskeletal changes, anchorage-independent colony formation, migration and invasion. Finally, RKI-1447 inhibited tumor growth and caused tumor regression in animal models with little side effects. Our studies warrant further advanced pre-clinical studies to determine the suitability of RKI-1447 as a potential clinical candidate.
Supplementary Material
Acknowledgements
The authors would like to thank the Moffitt Cancer Center Chemical Biology Core, the Analytical Microscopy core and the animal facility.
Grant Support: This work was supported by NCI grant U19 CA 067771.
Footnotes
Conflict of Interests
The authors have no conflict of interests.
REFERENCES
- 1.Bernards A. GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim Biophys Acta. 2003 Mar 17;1603(2):47–82. doi: 10.1016/s0304-419x(02)00082-3. [DOI] [PubMed] [Google Scholar]
- 2.DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005 Jul;15(7):356–63. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005 Feb;6(2):167–80. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 4.Izawa I, Amano M, Chihara K, Yamamoto T, Kaibuchi K. Possible involvement of the inactivation of the Rho-Rho-kinase pathway in oncogenic Ras-induced transformation. Oncogene. 1998 Dec 3;17(22):2863–71. doi: 10.1038/sj.onc.1202213. [DOI] [PubMed] [Google Scholar]
- 5.Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000 Aug 3;406(6795):532–5. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 6.Yoshioka K, Nakamori S, Itoh K. Overexpression of small GTP-binding protein RhoA promotes invasion of tumor cells. Cancer Res. 1999 Apr 15;59(8):2004–10. [PubMed] [Google Scholar]
- 7.Burbelo P, Wellstein A, Pestell RG. Altered Rho GTPase signaling pathways in breast cancer cells. Breast Cancer Res Treat. 2004 Mar;84(1):43–8. doi: 10.1023/B:BREA.0000018422.02237.f9. [DOI] [PubMed] [Google Scholar]
- 8.Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int J Cancer. 1999 May 31;81(5):682–7. doi: 10.1002/(sici)1097-0215(19990531)81:5<682::aid-ijc2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 9.Ridley AJ. Rho proteins and cancer. Breast Cancer Res Treat. 2004 Mar;84(1):13–9. doi: 10.1023/B:BREA.0000018423.47497.c6. [DOI] [PubMed] [Google Scholar]
- 10.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002 Feb;2(2):133–42. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 11.Kleer CG, van Golen KL, Zhang Y, Wu ZF, Rubin MA, Merajver SD. Characterization of RhoC expression in benign and malignant breast disease: a potential new marker for small breast carcinomas with metastatic ability. Am J Pathol. 2002 Feb;160(2):579–84. doi: 10.1016/S0002-9440(10)64877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suwa H, Ohshio G, Imamura T, Watanabe G, Arii S, Imamura M, et al. Overexpression of the rhoC gene correlates with progression of ductal adenocarcinoma of the pancreas. Br J Cancer. 1998;77(1):147–52. doi: 10.1038/bjc.1998.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp Cell Res. 2000 Nov 25;261(1):44–51. doi: 10.1006/excr.2000.5046. [DOI] [PubMed] [Google Scholar]
- 14.Olson MF. Applications for ROCK kinase inhibition. Curr Opin Cell Biol. 2008 Apr;20(2):242–8. doi: 10.1016/j.ceb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redowicz MJ. Rho-associated kinase: involvement in the cytoskeleton regulation. Arch Biochem Biophys. 1999 Apr 1;364(1):122–4. doi: 10.1006/abbi.1999.1112. [DOI] [PubMed] [Google Scholar]
- 16.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003 Jun;4(6):446–56. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 17.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003 Oct;83(4):1325–58. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 18.Tang Y, Olufemi L, Wang MT, Nie D. Role of Rho GTPases in breast cancer. Front Biosci. 2008;13:759–76. doi: 10.2741/2718. [DOI] [PubMed] [Google Scholar]
- 19.Zicha D, Dobbie IM, Holt MR, Monypenny J, Soong DY, Gray C, et al. Rapid actin transport during cell protrusion. Science. 2003 Apr 4;300(5616):142–5. doi: 10.1126/science.1082026. [DOI] [PubMed] [Google Scholar]
- 20.Olson MF, Sahai E. The actin cytoskeleton in cancer cell motility. Clin Exp Metastasis. 2009;26(4):273–87. doi: 10.1007/s10585-008-9174-2. [DOI] [PubMed] [Google Scholar]
- 21.Scott RW, Olson MF. LIM kinases: function, regulation and association with human disease. J Mol Med (Berl) 2007 Jun;85(6):555–68. doi: 10.1007/s00109-007-0165-6. [DOI] [PubMed] [Google Scholar]
- 22.Bourguignon LY, Zhu H, Shao L, Zhu D, Chen YW. Rho-kinase (ROK) promotes CD44v(3,8-10)-ankyrin interaction and tumor cell migration in metastatic breast cancer cells. Cell Motil Cytoskeleton. 1999;43(4):269–87. doi: 10.1002/(SICI)1097-0169(1999)43:4<269::AID-CM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Itoh K, Yoshioka K, Akedo H, Uehata M, Ishizaki T, Narumiya S. An essential part for Rho-associated kinase in the transcellular invasion of tumor cells. Nat Med. 1999 Feb;5(2):221–5. doi: 10.1038/5587. [DOI] [PubMed] [Google Scholar]
- 24.Li B, Zhao WD, Tan ZM, Fang WG, Zhu L, Chen YH. Involvement of Rho/ROCK signalling in small cell lung cancer migration through human brain microvascular endothelial cells. FEBS Lett. 2006 Jul 24;580(17):4252–60. doi: 10.1016/j.febslet.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 25.Imamura F, Mukai M, Ayaki M, Akedo H. Y-27632, an inhibitor of rho-associated protein kinase, suppresses tumor cell invasion via regulation of focal adhesion and focal adhesion kinase. Jpn J Cancer Res. 2000 Aug;91(8):811–6. doi: 10.1111/j.1349-7006.2000.tb01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu S, Goldstein RH, Scepansky EM, Rosenblatt M. Inhibition of rho-associated kinase signaling prevents breast cancer metastasis to human bone. Cancer Res. 2009 Nov 15;69(22):8742–51. doi: 10.1158/0008-5472.CAN-09-1541. [DOI] [PubMed] [Google Scholar]
- 27.Routhier A, Astuccio M, Lahey D, Monfredo N, Johnson A, Callahan W, et al. Pharmacological inhibition of Rho-kinase signaling with Y-27632 blocks melanoma tumor growth. Oncol Rep. 2010 Mar;23(3):861–7. [PubMed] [Google Scholar]
- 28.Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol. 2006 Aug 8;16(15):1515–23. doi: 10.1016/j.cub.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 29.Ying H, Biroc SL, Li WW, Alicke B, Xuan JA, Pagila R, et al. The Rho kinase inhibitor fasudil inhibits tumor progression in human and rat tumor models. Mol Cancer Ther. 2006 Sep;5(9):2158–64. doi: 10.1158/1535-7163.MCT-05-0440. [DOI] [PubMed] [Google Scholar]
- 30.Yoshioka K, Foletta V, Bernard O, Itoh K. A role for LIM kinase in cancer invasion. Proc Natl Acad Sci U S A. 2003 Jun 10;100(12):7247–52. doi: 10.1073/pnas.1232344100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lane J, Martin TA, Watkins G, Mansel RE, Jiang WG. The expression and prognostic value of ROCK I and ROCK II and their role in human breast cancer. Int J Oncol. 2008 Sep;33(3):585–93. [PubMed] [Google Scholar]
- 32.Berndt N, Hamilton AD, Sebti SM. Targeting protein prenylation for cancer therapy. Nat Rev Cancer. 2011 doi: 10.1038/nrc3151. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Dwyer PJ, Gallagher M, Nguyen B, Waddell MJ, Chiorean EG. Phase I accelerated dose-escalating safety and pharmacokinetic (PK) study of GGTI-2418, a novel geranylgeranyltransferase I inhibitor in patients with refractory solid tumors. Ann Oncol. 2010;21(Suppl 2):ii42. [Google Scholar]
- 34.Pireddu R, Forinash KD, Sun NN, Martin MP, Sung SS, Alexander B, et al. Pyridylthiazole-Based Ureas as Inhibitors of Rho Associated Protein Kinases (ROCK1 and 2) Med Chem Commun. 2012;3(6):699–709. doi: 10.1039/C2MD00320A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kabsch W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J Appl Cryst. 1993;26:795–800. [Google Scholar]
- 36.The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994 Sep 1;50(Pt 5):760–3. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 37.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010 Feb;66(Pt 2):213–21. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004 Dec;60(Pt 12 Pt 1):2126–32. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 39.Delano WL. PyMOL Molecular Graphics System. DeLano Scientific LLC; Palo Alto, CA, USA: 2008. [Google Scholar]
- 40.Kazi A, Sun J, Doi K, Sung SS, Takahashi Y, Yin H, et al. The BH3 alpha-helical mimic BH3-M6 disrupts Bcl-X(L), Bcl-2, and MCL-1 protein-protein interactions with Bax, Bak, Bad, or Bim and induces apoptosis in a Bax- and Bim-dependent manner. J Biol Chem. 2011 Mar 18;286(11):9382–92. doi: 10.1074/jbc.M110.203638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balasis ME, Forinash KD, Chen YA, Fulp WJ, Coppola D, Hamilton AD, et al. Combination of farnesyltransferase and Akt inhibitors is synergistic in breast cancer cells and causes significant breast tumor regression in ErbB2 transgenic mice. Clin Cancer Res. 2011 May 1;17(9):2852–62. doi: 10.1158/1078-0432.CCR-10-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011 Mar-Apr;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 43.Strecker TE, Shen Q, Zhang Y, Hill JL, Li Y, Wang C, et al. Effect of lapatinib on the development of estrogen receptor-negative mammary tumors in mice. J Natl Cancer Inst. 2009 Jan 21;101(2):107–13. doi: 10.1093/jnci/djn436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu C, Speers C, Zhang Y, Xu X, Hill J, Steinbis E, et al. Effect of epidermal growth factor receptor inhibitor on development of estrogen receptor-negative mammary tumors. J Natl Cancer Inst. 2003 Dec 17;95(24):1825–33. doi: 10.1093/jnci/djg117. [DOI] [PubMed] [Google Scholar]
- 45.Rodrigues LM, Chung YL, Al Saffar NM, Sharp SY, Jackson LE, Banerji U, et al. Effects of HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin (17-AAG) on NEU/HER2 overexpressing mammary tumours in MMTV-NEUNT mice monitored by Magnetic Resonance Spectroscopy. BMC Res Notes. 2012 May 23;5(1):250. doi: 10.1186/1756-0500-5-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts PJ, Bisi JE, Strum JC, Combest AJ, Darr DB, Usary JE, et al. Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapy. J Natl Cancer Inst. 2012 Mar 21;104(6):476–87. doi: 10.1093/jnci/djs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Junutula JR, Flagella KM, Graham RA, Parsons KL, Ha E, Raab H, et al. Engineered thio-trastuzumab-DM1 conjugate with an improved therapeutic index to target human epidermal growth factor receptor 2-positive breast cancer. Clin Cancer Res. 2010 Oct 1;16(19):4769–78. doi: 10.1158/1078-0432.CCR-10-0987. [DOI] [PubMed] [Google Scholar]
- 48.Arpino G, Weiss H, Lee AV, Schiff R, De Placido S, Osborne CK, et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005 Sep 7;97(17):1254–61. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- 49.Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009 Jun;7(1-2):4–13. doi: 10.3121/cmr.2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cui X, Schiff R, Arpino G, Osborne CK, Lee AV. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol. 2005 Oct 20;23(30):7721–35. doi: 10.1200/JCO.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Bershtein LM, Alimova IN, Tsyrlina EV, Anisimov VN. Mammary tumors in HER-2/NEU mice are characterized by low content of estrogen receptors-alpha and absence of progesterone receptors. Bull Exp Biol Med. 2003 Jun;135(6):580–1. doi: 10.1023/a:1025437620749. [DOI] [PubMed] [Google Scholar]
- 52.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008 May 16;133(4):704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010 Aug 26;29(34):4741–51. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khamis ZI, Sahab ZJ, Sang QX. Active roles of tumor stroma in breast cancer metastasis. Int J Breast Cancer. 2012;2012:574025. doi: 10.1155/2012/574025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.