Abstract
Left fronto-cortical hypoactivity, thought to reflect reduced activity in approach-related systems, and right parietal hypoactivity, associated with emotional under-arousal, have been noted in major depressive disorder (MDD). Altered theta activity in the anterior cingulate cortex (ACC) has also been associated with the disorder. We assessed resting frontal and parietal alpha asymmetry and power in non-medicated MDD (N=53; 29 females) and control (N=43; 23 females) individuals. Theta activity was examined using standardized low-resolution electromagnetic tomography (sLORETA) in the ACC [BA24ab and BA32 comprising the rostral ACC and BA25/subgenual (sg) ACC]. The MDD group, and particularly depressed males, displayed increased overall frontal and parietal alpha power and left midfrontal hypoactivity (alpha2-indexed). They also exhibited increased sgACC theta2 activity. MDD females had increased right parietal activity, suggesting increased emotive arousal. Thus, unmedicated depressed adults were characterized by lower activity in regions implicated in approach/positive affective tendencies as well as diffuse cortical hypoarousal, though sex specific modulations emerged. Altered theta in the sgACC may reflect emotion regulation abnormalities in MDD.
Keywords: Major depressive disorder, alpha asymmetry, theta activity, anterior cingulate cortex, sLORETA, sex
Background
Electroencephalographic (EEG) research has revealed that increased relative right fronto-cortical activity (probed with EEG alpha, which is inversely related to cortical activity; Neuper & Pfurtscheller, 2001) tends to emerge during the processing of negative information and emotions, while greater relative left fronto-cortical activity is associated with positive information/affective processing (Davidson, 1998). However, electrocortical asymmetry profiles accompanying the processing of anger or cognitive dissonance have also been associated with increased relative left fronto-cortical activity (Wacker et al., 2003; Harmon-Jones, 2004). To account for this, current frontal asymmetry models posit that approach tendencies and positive information/emotion processing are associated with greater left anterior activity while withdrawal tendencies and negative information/emotion processing are linked with right frontal activity. In support of this, individuals with greater relative left anterior activity report increased positive and decreased negative affect compared to those with the opposite asymmetry (Tomarken et al., 1992). Resting frontal alpha asymmetry has also been found to predict affective responses to emotive stimuli (Tomarken et al., 1990; Wheeler et al., 1993). As such, resting anterior asymmetry may be a trait-like feature biasing affective style (Jacobs & Snyder, 1996), though it is also plausible that these profiles may reflect a motivational abnormality rather than an affective disposition (Pizzagalli et al., 2005).
Individuals with major depressive disorder (MDD) tend to exhibit relative left frontal hypoactivity (Davidson & Slagter, 2000; Deldin & Chiu, 2005; Deslandes et al., 2008; Pössel et al., 2008; Kemp et al., 2010), which, along with right hyperactivity, has been associated with greater depression scores (Saletu et al., 2010). Remitted depressives also exhibit decreased relative left frontal activity (Henriques & Davidson, 1990). Thus, left frontal hypoactivity may be a risk marker for MDD. Although similar frontal asymmetry patterns have also been noted in other psychiatric disorders (e.g. anxiety, ADHD; Hale et al., 2010; Moscovitch et al., 2011), they have been most extensively studied and reliably altered in MDD.
Increased alpha power/amplitude over right parietotemporal regions has also been associated with MDD (Bruder et al., 2005; 2011; Kentgen et al., 2000). Right parietotemporal activity is thought to be involved in modulating emotion-related autonomic arousal (Heller & Nitschke, 1997), thus, decreased right parietotemporal activity may reflect diminished emotional arousal in the disorder. In support of this, depressed patients with co-morbid anxiety, characterized by a hyper-aroused state, were found to display right parietotemporal hyperactivity (Keller et al., 2000; Kentgen et al., 2000) while those without anxiety exhibit decreased activity in this region (Bruder et al., 1997). Accordingly, electrocortical asymmetry models have been expanded to include two dimensions: the valance/motivation dimension involving anterior regions and the arousal dimension involving right parietotemporal aspects.
However, certain caveats regarding the asymmetry model exist. First, when anterior asymmetry is examined in individuals without extreme or stable asymmetry, its relationship with dispositional affect weakens (Debener et al., 2000). Second, methodological differences (e.g. reference, sites assessed, alpha bands) influence the relationship between frontal asymmetry and affective style/motivation (Thibodeau et al., 2006). Factors such as age and sex have also been shown to modulate asymmetry, though the effects of the latter have been mixed and not extensively probed (Stewart et al., 2010). Lastly, several studies have noted no frontal asymmetry alterations in MDD, questioning its reliability as an endophenotype (Carvalho et al., 2011; Segrave et al., 2011).
In addition to alpha asymmetry alterations, enhanced alpha amplitude/power has been noted in MDD (Pollock & Schneider, 1990; Roemer et al., 1992; Baehr et al., 1998; Kemp et al., 2010, but see Knott & Lapierre, 1987; Bruder et al., 1997; Mientus et al., 2002). This increase tends to emerge posteriorly, though anterior alpha power increases have also been documented (Bauer & Hesselbrock, 2002; Ricardo-Garcell et al., 2009). Thus, depression may be associated with overall cortical hypoactivity. Evidence of altered delta, beta and gamma power in MDD is less consistent and sparse, though midfrontal theta modulations have been noted and are discussed below.
In humans, midfrontal theta power has been localized to the frontal lobes, specifically the anterior cingulate cortex (ACC; Ishii et al., 1999). Though tonic theta rhythms are evident areas across the scalp (but are maximal midfrontally), midfrontal theta tends be phasic, as it emerges during working and episodic memory as well as during spatial navigation tasks, for instance. Thus, midfrontal theta has been linked with focused and sustained attention/concentration as well as mental effort. Though the functional correlates of resting midfrontal theta are less characterized, it is nevertheless thought to reflect ACC activity (Mitchell et al., 2008).
The ACC is involved in a range of cognitive and emotive functions such as conflict monitoring, error detection and in evaluating the emotional significance of stimuli (i.e., operations that evoke theta activity; Pizzagalli, 2011). It is a heterogeneous structure that is subdivided into ventral and dorsal aspects. The subgenual (sgACC; BA25) and rostral aspects (BA32 and BA24ab) comprise the ventral ACC; dorsally, the ACC includes BA24′ and BA32′ (Pizzagalli et al., 2011). The latter constitute the dorsal ‘cognitive’ ACC as it is intimately connected with the dorsolateral prefrontal cortex (DLPFC). The ventral ACC comprises the ‘affective’ region as it is connected with limbic and subcortical structures as well as the orbital PFC (Ongür & Price, 2000). The sgACC, in particular, has been implicated in visceral responses to emotive processing, in emotive memory formation and in regulating reward contingencies (Drevets et al., 2008).
A handful of studies have indicated that MDD is associated with increased anterior and right hemisphere scalp theta amplitude/power (Kwon et al., 1996; Knott et al., 2000; Ricardo-Garcell et al., 2009). However, decreased frontal theta activity in MDD has been noted using source localization techniques [magnetoencephalography, low-resolution brain electromagnetic tomography (LORETA); Wienbruch et al., 2003; Coutin-Churchman & Moreno, 2008; Saletu et al., 2010]. Methodological differences may underlie these discrepancies, whereby frontal scalp theta amplitude/power likely stems from several neural generators, while source localization/neuroimaging approaches enable activity assessment in specific regions. Sex may also account for some of the variability, though its influence on theta activity has been under-explored (Morgan et al., 2005). Altered theta may reflect disrupted functional connectivity in fronto-cingulate pathways mediating emotive regulation in MDD (Pizzagalli et al., 2003). This idea is strengthened by evidence that ACC-localized theta is useful in predicting antidepressant treatment response (Pizzagalli et al., 2001, 2005; Mulert et al., 2007; Korb et al., 2011) and is modulated with treatment (Knott et al., 1996; Landolt & Gillin, 2002).
This study assessed resting frontal and parietal alpha power and asymmetry in MDD and control males and females. Alpha power and asymmetry were assessed using three reference montages, which have been shown to influence asymmetry (Stewart et al., 2010; Hagemann, 2004). Given evidence that alpha2 has been linked with memory retrieval while alpha1 is broadly associated with attentive processes (Klimesch et al., 2007), and that individual variability characterizes alphaTotal (Segrave et al., 2011), power in alpha sub-bands was assessed. We expected greater relative left frontal alpha power in MDD. Given that past work suggests decreased ACC-localized theta activity in MDD, we probed this and expected similar findings. Theta sub-bands were assessed as previous work suggests somewhat different profiles of theta sub-bands in MDD (Fingelkurts et al., 2006). Finally, correlations were carried out between the electrophysiological indices and clinical scores. To our knowledge, very few studies have assessed alpha asymmetry and power as well as ACC-theta in the same (large) sample of depressed males and females despite known alterations in these indices in MDD.
Methods
Participants
Resting EEG activity was obtained from 53 adults with a primary diagnosis of MDD (Table 1). Patients were diagnosed by psychiatrists using the Structured Clinical Interview for DSM (Diagnostic and Statistical Manual of Mental Disorders) IV-TR Diagnoses, Axis I, Patient Version (SCID-IV-I/P; First et al., 1997); most patients have had previous major depressive episodes. The 17 and 29 item versions of the Hamilton Rating Scale for Depression (HAMD17/29; Hamilton, 1960) and Montgomery-Åsberg Depression Rating Scale (MADRS; Montgomery & Åsberg, 1979) were used to assess symptom severity; MADRS scores were ≥22 (moderate depression) at enrollment. Exclusion criteria included: Bipolar Disorder (BP-I/II or NOS), psychosis history, current (<6 months) drug/alcohol abuse or dependence, history of seizures or known increased seizure risk, unstable (≥3 months) medical condition and history of anorexia/bulimia. Patients at a significant risk for suicide were also excluded. Patients with a secondary diagnosis of some anxiety disorder were included (N=33: no anxiety co-morbidity; N=12: sub-threshold anxiety; N=8: secondary diagnosis of some form of anxiety). At the time of testing, patients were not taking any psychoactive drugs; appropriate drug washout periods were employed prior to testing for any previously medicated patients.
Table 1.
Major Depressive Disorder (MDD) and Control Group Characteristics & Demographics (Means ± S.D.)
| MDD Females (N=29) | MDD Males (N=24) | Control Females (N=23) | Control Males (N=20) | |
|---|---|---|---|---|
| Age | 43.2 ± 11.6* | 37.7 ± 11.6 | 37.2 ± 7.8 | 35.8 ± 11.9 |
| Education (Years) | 15.6 ± 2.4 | 16.5 ± 2.5 | 16.4 ± 2.0 | 16.4 ± 1.9 |
| HAMD17 | 22.4 ± 5.1* | 19.1 ± 4.7 | - | - |
| HAMD29 | 32.5 ± 5.4 | 29.5 ± 6.7 | - | - |
| MADRS | 30.8 ± 5.1 | 30.8 ± 5.4 | - | - |
| BDI-II | - | - | 4.4 ± 5.0 | 3.1 ± 4.8 |
| Ethnicity | 27 Caucasian; 1 Asian; 1 South Asian; | 21 Caucasian; 2 Asian; 1 African | 20 Caucasian; 2 South Asian; 1 African | 19 Caucasian; 1 Asian |
HAMD17/29: Hamilton Rating Scale for Depression, 29 & 17 item versions;
MADRS: Montgomery-Åsberg Depression Rating Scale; BDI-II: Beck Depression Inventory-II
p<.05, MDD females had higher HAMD-17 versus MDD males; MDD females were older than control females
Forty-three adults with no psychiatric, alcohol/drug abuse or dependence history (assessed with non-patient version of the SCID [SCID-IV-I/NP]), and no history of seizures or brain trauma were tested (Table 1). Controls were included only if they scored ≤13 on the Beck Depression Inventory-II (BDI-II; Beck et al., 1996) and if they had no psychiatric history in first-degree relatives (Family Interview for Genetic Studies [FIGS]-assessed; Maxwell, 1992).
Session Procedures
Prior to testing, participants abstained for >3 hr from caffeine and/or nicotine, as well as alcohol and drugs (other than medication for a physical condition) beginning at midnight. Upon arrival, mood evaluations were carried out using the Profile of Mood States (POMS; McNair et al., 1992) questionnaire from which values are aggregated to form seven mood dimensions (tension-anxiety, depression-dejection, anger-hostility, vigor-activity, fatigue-inertia, confusion-bewilderment and total mood disturbance, which is computed from the other dimensions). Electrodes were applied and EEG was recorded. All participants were compensated $30.00 CDN/session (patients participated in multiple sessions as part of a larger study). This study was approved by the Royal Ottawa Health Care Group and the University of Ottawa Social Sciences and Humanities Research Ethics Boards; informed consent was obtained from all participants.
Electrophysiological Recordings
While participants were seated in a sound-and light-attenuated chamber, EEG recordings were obtained during 3 min vigilance-controlled eyes-closed (EC) and 3 min eyes-open (EO) conditions (counterbalanced). EEG was recorded (500 Hz; mastoid referenced) using a cap with 32 Ag/AgCl electrodes (EasyCap, Herrsching-Breitbrunn, Germany) positioned according to the 10-10 system (Chatrian et al., 1985); electrooculographic (EOG) activity was also obtained. An AFz electrode served as the ground. Impedance was maintained at ≤5 KΩ and EEG was recorded with amplifier filters set at 0.1–80 Hz (BrainVision Recorder, Richardson, TX, USA). Acquired signals were stored for subsequent analyses (BrainVision Analyzer, Richardson, TX, USA).
Off-line, EEG data were re-referenced and analyzed using three references: average mastoids (TP9/10), Cz and average references. Signals were filtered (0.1–30 Hz), ocular-corrected (Gratton et al., 1983) and segmented into 2 s epochs (50% overlap). This was followed by artifact rejection, which excluded epochs with EEG activity exceeding +/−75 μV; data were also visually inspected for artifacts and faulty channels. Subsequently, >100 s artifact-free data for each EO/EC condition were subjected to a Fast Fourier Transform algorithm (Hanning window with 5% cosine taper) for computation of absolute, ln-transformed power (μV2) at alpha1 (8–10.5 Hz), alpha2 (10.5–13 Hz), alphaTotal (8–13 Hz), theta1 (4–6 Hz), theta2 (6–8 Hz) and thetaTotal (4–8 Hz). Alpha power was assessed at F4/3, F8/7, P4/3 and P7/8. Asymmetry indices were calculated for each alpha band by subtracting power at left electrodes from homologous right electrodes (i.e., F4-F3, F8-F7, P4-P3; positive values reflect greater right hemisphere alpha power, and thus decreased relative right hemisphere activity; negative values reflect the opposite). Only right-handers (Oldfield et al., 1971) were examined in the alpha asymmetry analyses (MDD females=27, males=22; control females=23, males=18).
Source Localization
EC theta1, theta2 and thetaTotal EEG was re-referenced to the average (as in Pizzagalli et al., 2001) and subjected to analysis with standardized low-resolution electromagnetic tomography (sLORETA) software (Pascual-Marqui, 2002; artifact- and ocular-corrected epochs, 60 s of data from 28 electrodes). Practical considerations dictated the use of 60 s of data for sLORETA analyses; others have used substantially shorter recording periods (e.g. Korb et al., 2011). sLORETA analysis estimates neuronal activity as current density based on the Montreal Neurological Institute 152 template creating a low-resolution activation image. The sLORETA solution space consists of 6239 voxels (5×5×5 mm/voxel) restricted to gray matter. Current source density is calculated from a linear, weighted sum of scalp potentials; this value is then squared for each voxel yielding current density power measures (A/m2). Validation of the previous version of sLORETA (i.e., LORETA) has been independently replicated (Phillips et al., 2002) and cross-validated (Pizzagalli et al., 2004; Mulert et al., 2005); cross-validation of sLORETA also exists (Olbrich et al., 2009). sLORETA was used to estimate theta current source density at specific ACC regions of interest (ROIs). Consistent with precedent work (Mulert et al., 2007), ROIs (including both hemispheres) were: BA24ab (16 voxels) and BA32 [54 voxels], comprising the rostral ACC, as well as BA25 [subgenual (sg) ACC; 12 voxels; Supplementary Table 1; Figure 1]. Due to faulty channels, several participants were excluded from the sLORETA analysis (N=50 MDD; 28 females and N=39 controls; 23 females). As hemispheric effects were not assessed, 6 left-handed or ambidextrous individuals (4 MDD; 2 controls) were included in the sLORETA analysis (their inclusion did not alter results, data not shown).
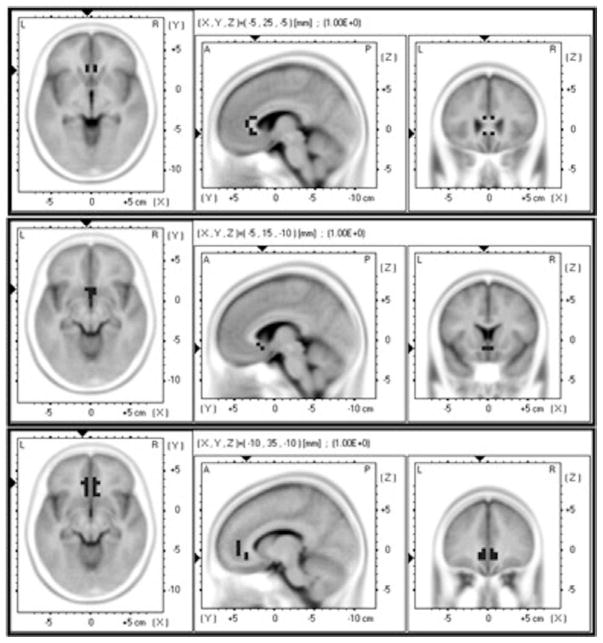
Figure 1.
Anterior cingulate cortex (ACC) regions of interest (ROIs). Top panel: ROI consisting of voxels from BA24. Middle panel: ROI consisting of voxels from BA25 (subgenual ACC). Bottom panel: ROI consisting of voxels in BA32.
Statistical Analyses
Analyses of variances (ANOVAs) were carried out (SPSS Inc., Chicago, IL, USA) on each POMS dimension with group (MDD; controls) and sex (males; females) as between-subject factors. Repeated-measures ANOVAs (rmANOVAs) were carried out on ln-transformed frontal absolute power in each alpha band with four within-subject factors: regional aspect (medial: F3/4; lateral: F7/8), condition (EC, EO), reference (average, Cz, mastoid) and hemisphere [left (L), right (R)]. Sex and group were between-subject factors. Similarly, rmANOVAs were carried out on absolute parietal alpha power in each band, with hemisphere (L: average P3/7power ;R: average P4/8power), condition and reference as within- and sex and group as between-subject factors; parietal aspects (medial/lateral) were not assessed as there was little rationale for doing so. rmANOVAs were carried out on each alpha asymmetry index (F4-F3, F8-F7, P4-P3; P8-P7 asymmetry is not typically probed and was not assessed in the current study) with reference and condition as within- and group and sex as between-subjects factors. Given the known influence of anxiety on parietal alpha asymmetry, its presence/absence (secondary clinical diagnosis, N=8 patients) was used as a covariate in P4-P3 alpha asymmetry assessments. Only significant (p<.05) main effects are reported as well as interactions wherein direct group or sex comparisons revealed significant differences. For the ROI analyses, rmANOVAs were applied for each theta band, with ROI (BA24ab, BA25, BA32) as within-and group and sex as between-subject factors; main effects are reported as are interactions indicating direct group or sex differences. Greenhouse-Geisser corrections were applied to all significant results. To account for multiple comparisons, Bonferroni adjustments (built into SPSS) were applied to all pairwise comparisons. Correlations were carried out between EC F4-F3 asymmetry in the MDD group (sex collapsed; maximal group differences have been reported for F4-F3 alpha; Pössel et al., 2008) for each alpha band at all three reference montages and MADRS, HAMD17/29 and POMS depression-dejection scores (sex collapsed) for the MDD group. Similar correlations were carried out with EC theta activity at the three ROIs (BA25, BA24ab, BA32). To adjust for multiple comparisons, significance was set at p<.005 for the correlations. Unless specified, means ± SEMs (standard error of the mean) are reported.
Results
Profile of Mood States (POMS)
Scores were unavailable for one patient (N=52; controls: N=43). A main group effect was noted for tension [F(1,91)=110.59, p<.001; MDD: 18.2 ± .8, control: 5.2 ± .9], depression [F(1,91)=243.47, p<.001; MDD: 35.4 ± 1.4, control: 3.4 ± 1.5], anger [F(1,91)=71.36, p<.001; MDD: 16.9 ± 1.0; control: 3.9 ± 1.1], fatigue [F(1,91)=216.76, p<.001; MDD: 20.2 ± .7; control: 4.4 ± .8], confusion [F(1,91)=155.18, p<.001; MDD: 16.5 ± .7, control: 4.1 ± .7] and total mood disturbance [F(1,91)=300.20, p<.001; MDD: 102.5 ± 3.9, control: 1.6 ± 4.3]; scores were greater for the MDD versus control group. Vigour [F(1,91) = 169.29, p<.001] scores were lower for the MDD (5.0 ± .7) versus control group (19.4 ± .8).
EEG Results
Alpha Power: Effects of Regional Aspect, Hemisphere, Condition and Reference
A main effect of condition existed for alpha1 [F(1,86)=130.57 (frontal), 190.01 (parietal), p<.001], alpha2 [F(1,86)=199.61 (frontal), 194.08 (parietal) p<.001] and alphaTotal [F(1,86)=213.40 (frontal), 276.44 (parietal), p<.001], with greater power in the EC condition. A main effect of reference was found for alpha1 [F(2,86)=170.97 (frontal), 343.03 (parietal), p<.001], alpha2 [F(2,86)=24.82 (frontal), 24.68 (parietal), p<.001] and alphaTotal [F(2,86)=142.55 (frontal), 379.86 (parietal), p<.001]. For frontal and parietal alpha1, power was different in all reference montages (p<.05), with smallest power in the average and greatest in the mastoid reference montage; the same was true for frontal alphaTotal (p<.001). For frontal alpha2 and parietal alpha2/Total, smaller power values existed in the average versus both the Cz and mastoid references (p<.001). A main effect of regional aspect was found for frontal alpha2 [F(1,86)=31.50, p<.001] and alphaTotal [F(1,86)=11.35, p=.001], with greater power in the lateral (F7/8) versus medial (F3/4) aspect; a similar trend existed for frontal alpha1 [F(1,86)=3.71, p=.056]. A main effect of hemisphere existed for parietal alpha2 [F(1,86)=5.45, p=.022] and alphaTotal [F(1,86)=4.20, p=.043], with greater power in the right hemisphere.
Frontal Alpha Power: Effects of Group and Sex
Alpha1: A main effect of group existed [F(1,86)=5.44, p=.022] with greater alpha1 power in the MDD (1.70 ± .12 μV2) versus control (1.29 ± .13 μV2) group.
Alpha2: A main effect of group [F(1,86)=6.80, p=.011], with greater alpha2 power in the MDD (1.19 ± .08 μV2) versus control (.88 ± .09 μV2) group, was found. A reference×hemisphere×group×sex interaction [F(2,172)=8.36, p=.003] existed. Follow-up comparisons indicated group differences (Cz reference) in the left frontal hemisphere between MDD versus control males (p=.047; same trend in right frontal hemisphere, p=.055). For the mastoid reference, the same difference was found in the left frontal hemisphere (p=.010; same trend in right hemisphere, p=.051). In both cases, greater alpha2 power existed in MDD versus control males.
AlphaTotal: A main group effect existed [F(1,86)=6.68, p=.011], with greater power in the MDD (2.33 ± .11 μV2) versus control (1.92 ± .12 μV2) group. A reference×aspect×group×sex interaction [F(2,86)=3.99, p=.025] existed, with follow-up comparisons indicating greater alphatotal in MDD versus control females in the lateral aspect (F7/8) in the mastoid reference montage (p=.046). For all references, in both regional aspects, MDD males had greater alphatotal than control males (p<.05).
Frontal Alpha Power Asymmetry (F4-F3)
Alpha1 and AlphaTotal: No main effects or interactions with group or sex were noted.
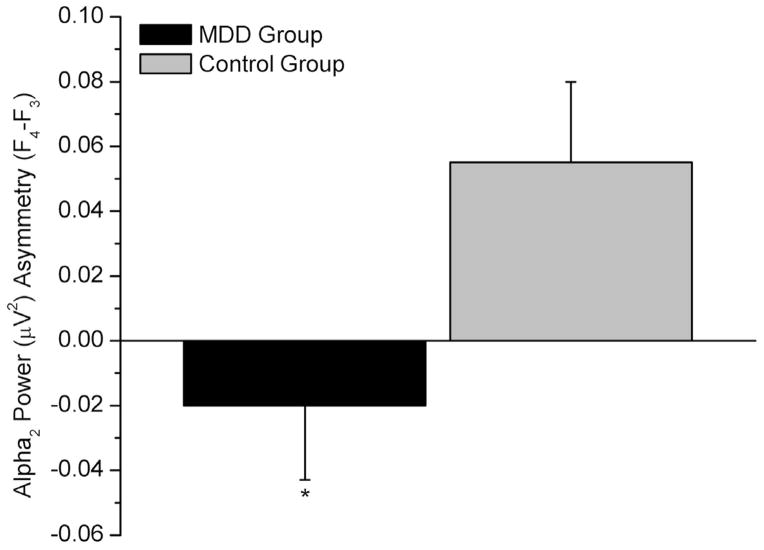
Alpha2: A main effect of group existed [F(1,86)=4.87, p=.030], with a positive index in the control (.055 ± .025 μV2) and a negative one in the MDD group (−.020 ± .023 μV2; Figure 2). A positive index reflects greater right frontal alpha power, thus, relatively decreased right fronto-cortical activity; a negative index reflects greater left frontal alpha power, thus, decreased relative left fronto-cortical activity. A condition×group interaction was noted [F(1,86)=6.14, p=.015], with a group difference in the EO condition (p=.007), with a positive index in controls (.091 ± .031 μV2) and a negative one in the MDD group (−.025 ± .028 μV2). A reference×group×sex interaction existed [F(2,172)=6.58, p=.006], with a group difference in females in the Cz reference montage (p=.010). In males, a group difference was noted in the mastoid reference montage (p=.009). In both cases, the index was negative for the MDD and positive for the control group. In controls, a sex difference was noted in the mastoid reference montage (p=.015), with a positive index in males (.12 ± .041 μV2) and a negative one in females (−.013 ± .036 μV2).
Figure 2.
Midfrontal alpha2 asymmetry in the Major Depressive Disorder (MDD) and control groups (sex collapsed; *p<.05). Negative values reflect greater left alpha2 power (i.e., decreased relative left cortical activity); positive values reflect greater right alpha2 power (i.e., decreased relative right cortical activity).
Frontal Alpha Power Asymmetry (F8-F7)
Alpha1: Only a main effect of reference existed for alpha1 F8-F7 asymmetry [F(2,172)=5.55, p=.012], with a difference between the average (−.077 ± .023 μV2) versus the mastoid (−.008 ± .021 μV2) reference montage (p=.012)
Alpha2: Follow-up comparisons of the reference×group×sex interaction [F(2,172)=6.69, p=.006] indicated a group difference in alpha2 F8-F7 asymmetry in females in the Cz reference (p=.045). MDD females had a more negative asymmetry index (−.095 ± .031 μV2), reflecting greater relative left hypoactivity, than control females (−.001 ± .034 μV2). In the MDD group, a sex difference existed in the Cz reference (p=.036), with a more negative index for females versus males (.004 ± .034 μV2).
AlphaTotal: No main effects of group or sex or interactions were found.
Parietal Alpha Power: Effects of Group and Sex
Alpha1: No effects of group, sex or interactions existed for parietal alpha1 power.
Alpha2: A main effect of group existed [F(1,86)=5.33, p=.023], with greater parietal alpha2 power in the MDD (1.64 ± .10 μV2) versus control (1.31 ± .11 μV2) group. A hemisphere×sex×group interaction [F(1,86)=4.32, p=.041] was noted. Greater parietal alpha2 power exited in MDD (L: 1.67 ± .15 μV2; R: 1.77 ± .15 μV2) versus control (L: 1.15 ± .16 μV2; R: 1.16 ± .16 μV2) males in both hemispheres (L: p=.02; R: p=.007).
AlphaTotal: A main effect of group was found [F(1,86)=4.22, p=.043], with greater parietal power in MDD (2.76 ± .14 μV2) versus controls (2.34 ± .15 μV2).
Parietal Alpha Power Asymmetry (P4-P3)
Alpha1 and AlphaTotal: No effects of interest were noted for parietal asymmetry.
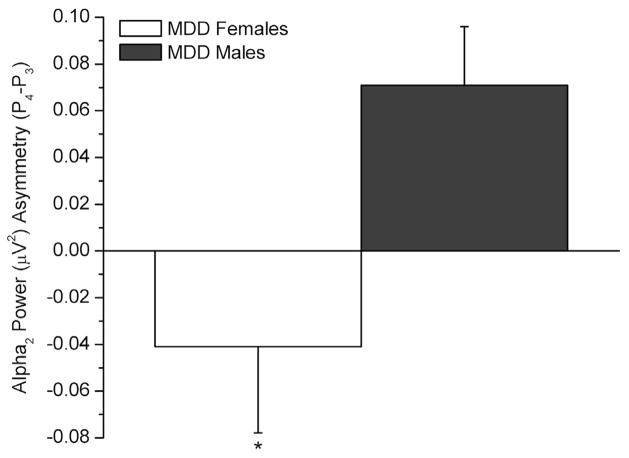
Alpha2: A group×sex interaction existed [F(1,85)=7.06, p=.009], follow-up comparisons indicated a group differences in females (p=.038). MDD females exhibited a negative index (−.053 ± .040), reflecting relative left parietotemporal hypoactivity or relative right parietotemporal hyperactivity, while control females exhibited a positive one (.070 ± .041 μV2), reflecting the opposite. A sex difference existed in the MDD group (p=.032), with a negative parietal asymmetry in MDD females (−.053 ± .04 μV2) and a positive one in males (.074 ± .041 μV2; Figure 3).
Figure 3.
Parietal alpha2 asymmetry in the females and males with Major Depressive Disorder (MDD) (*p<.05). Negative values reflect greater left alpha2 power (i.e., decreased relative left cortical activity or, conversely, increased relative right cortical activity); positive values reflect greater right alpha2 power (i.e., decreased relative right cortical activity).
Anterior Cingulate Cortex Theta Activity
For each theta band, a main effect of region was noted [theta1: F(2,170)=220.36, p<.001; theta2: F(2,170)=58.53, p<.001; thetaTotal: F(2,170)=119.22, p<.001], in all cases theta activity was greatest in BA32, intermediate in BA24ab and smallest in BA25.
Theta1 and ThetaTotal: No sex or group effects or interactions were noted.
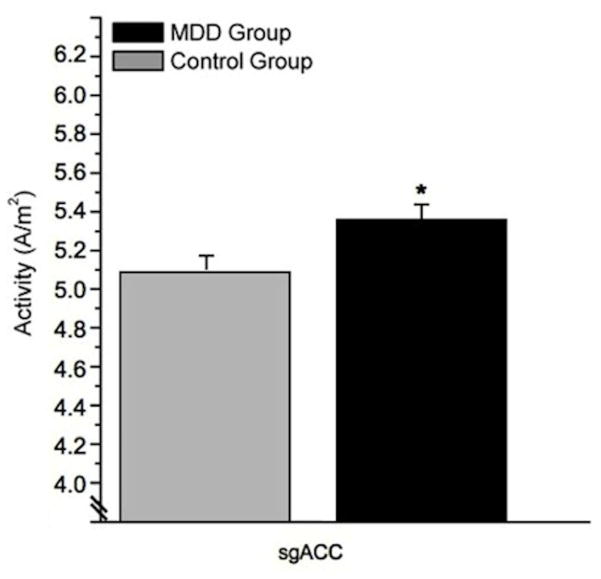
Theta2: A trend for group effect existed [F(1,85)=3.48, p=.066], with greater theta2 activity in the MDD (5.42 ± .076 A/m2) versus control group (5.20 ± .09 A/m2). A region×group interaction existed [F(2,170)=3.22, p=.042], with greater BA25 theta2 activity in MDD (5.36 ± .09 A/m2) versus controls (5.07 ± .1 A/m2, p=.03; Figure 4).
Figure 4.
Theta2 activity in BA25/subgenual anterior cingulate cortex (sgACC) in the Major Depressive Disorder (MDD) and control groups (sex collapsed; *p<.05).
Correlations
F4-F3 alpha1 asymmetry (mastoid reference) tended to correlate negatively with POMS depression-dejection scores [r=−.37, N=53, p=.006].
Discussion
This study assessed frontal and parietal alpha power and asymmetry as well as ACC theta activity in MDD versus control individuals. Depressed individuals were characterized by disturbances in all mood dimensions (POMS-assessed). These findings indicate that while MDD is primarily characterized by depressed affect, it is also associated with disturbances in several mood dimensions, which may contribute to the specific resting electrocortical and neuroimagaing profiles that have been associated with the disorder. Greater anterior alpha power (i.e. frontal hypoactivity) existed in MDD, which was especially evident in males; similar findings were noted for parietal alpha power. Group differences emerged in midfrontal alpha2 asymmetry indicating left frontal hypoactivity in MDD. MDD females were characterized by right parietal hyperactivity relative to MDD males. Finally, greater sgACC theta2 activity existed in MDD. These results and their significance are discussed below.
A main effect of reference was noted for alpha power, but had a less substantial influence on alpha asymmetry. Nevertheless, group effects on alpha power and asymmetry indices emerged only with certain reference montages, highlighting the utility of assessing several montages in comparable future work (Stewart et al., 2010; Hagemann et al., 2004). Specifically, group differences in alpha asymmetry and power differences emerged with the Cz, and to a lesser extent mastoid, reference montages; none emerged with the average reference. However, the 32 channels used in the current study likely lacked the spatial sampling recommended for average referencing (Junghöfer et al., 1999). Given that the Cz is cephalically active, its use as a reference has been discouraged, thought it has been frequently used in frontal asymmetry research. Although the mastoid reference is also problematic (Hagemann et al., 2004), out of the three reference montages used in the current study, it may represent the least biased EEG asymmetry measures.
A main effect of regional aspect existed for alpha2/Total, with a similar trend for alpha1, with greater power in lateral versus medial aspects, which differs from what others have found (Deslandes et al., 2008). Nevertheless, these results suggest that different generators likely contribute to alpha power measured at various frontal sites. Future work, perhaps combining EEG and fMRI, which has superior spatial resolution than sLORETA, should also explore the neurofunctional correlates of scalp-localized frontal alpha asymmetry, as research on this is sparse.
Our finding of increased alpha power in MDD (i.e., cortical hypoactivity) is consistent with precedent work (Pollock & Schneider, 1990; Roemer et al., 1992; Baehr et al., 1998; Kemp et al., 2010; Grin-Yatsenko et al., 2011; Köhler et al., 2011), though notable exceptions exist (Knott & Lapierre, 1987; Bruder et al., 1997; Mientus et al., 2002). Given that enhanced alpha existed in both frontal and parietal regions, this points toward generalized cortical hypoactivity in MDD, which did not appear to be directly related to increased fatigue (POMS-indexed; exploratory correlations, data not shown).
When alpha power results was broken down by sex, we found that although MDD males exhibited increased anterior alpha2, this was more pronounced in the left frontal region, consistent with reports of left frontal hypoactivity in depression (Davidson & Slagter, 2000; Deldin & Chiu, 2005; Kemp et al., 2010). The enhanced posterior alpha2 and frontal alphatotal power in MDD appeared to be driven largely by depressed males, though no direct sex differences emerged. However, the significance and reliability of these sex-sensitive results is unknown, as limited work has explored the influence of sex on resting EEG in MDD adults.
Alpha sub-bands (i.e., alpha1 and alpha2) were examined because alphatotal is susceptible to inter-individual variations due to factors such as genotype and age (Segrave et al., 2011) and alpha sub-bands have been associated with varying cognitive processes (Klimesch et al., 2007). Though the functional correlates of power in alpha sub-bands at rest are unclear, and both are thought to reflect decreased cortical activity, their inspection enables greater specification regarding the electrocortical correlates of MDD. We noted that anterior power in both alpha sub-bands was greater in MDD. However, only alpha2 power was specifically increased in the left frontal hemisphere and enhanced parietally in MDD versus control males, consistent with precedent work also documenting specific alpha2 modulations in MDD (Lubar et al., 2003).
No anterior alpha1 or alphatotal asymmetry differences were noted between the groups, in line with others’ findings (Mathersul et al., 2008; Carvalho et al., 2011; Segrave et al., 2011). However, a tendency for a negative correlation between F4-F3 alpha1 asymmetry and POMS depression-dejection scores in MDD was observed, suggesting that left frontal hypoactivity is associated with greater state depression. A group difference, regardless of reference, existed for midfrontal alpha2 asymmetry. Consistent with the purported involvement of left fronto-cortical activity in approach-related tendencies/motives and positive processing, a positive index was noted in controls indicating increased relative left frontal activity; a negative index existed in the MDD group indicating the opposite. Further breakdown by sex indicated a group difference in midfrontal alpha2 asymmetry in females (Cz reference); in males, a group difference in midfrontal asymmetry emerged (mastoid reference), with the expected asymmetry in both cases. These findings further highlight the influential role of reference in frontal alpha asymmetry assessments and strengthen the idea that altered alpha2 power may be most pronounced in MDD.
Consistent with precedent work (Pössel et al., 2008), mid (F4-F3) versus lateral (F8-F7) frontal asymmetry better differentiated MDD and control individuals. By extension, this suggests that frontolateral asymmetry may be the less reliable index in the valence/motivation frontal alpha asymmetry model. Few main effects, in general, emerged for frontolateral alpha asymmetry, with the exception of a more negative alpha2 asymmetry index (Cz reference) in MDD females versus males (i.e., greater left frontal hypoactivity in MDD females), consistent with precedent work (Stewart et al., 2010). However, this finding failed to reach statistical significance (p=.09) when HAMD17 scores were controlled for (data not shown). In general, little evidence for direct sex differences in frontal alpha asymmetry emerged.
Assessments of parietal alpha2 power asymmetry indicated greater relative right parietal activity in MDD females versus both control females and MDD males. Activity in the right parietotemporal cortex has been linked with emotional arousal, suggesting emotional hyperarousal in depressed females (Kentgen et al., 2000; Manna et al., 2010). While it is tempting to associate increased emotional arousal with anxiety (i.e., excessive emotive and physiological arousal) in MDD females (7 MDD females and 1 MDD male had a secondary anxiety diagnosis), this linear relationship is too simplistic as the incidence of anxiety was co-varied for in the analyses. Including sub-threshold anxiety as a covariate also did not alter the results, though it diminished significance (data not shown). Nevertheless, it is feasible that right parietal hyperactivity may be related to specific features of anxiety (e.g. somatic features; Heller & Nitschke, 1997) rather than anxiety in general, which were not specifically probed. Though our findings of relative right parietal hyperactivity in MDD females contradict some reports (Bruder et al., 2005; 2011; Kentgen et al., 2000), no associations between right parietal hypoactivity and MDD have also been noted (Henriques & Davidson, 1991; Debener et al., 2000; Deslandes et al., 2008, Mathersul et al., 2008) while others, consistent with our results, have reported the opposite (Pössel et al., 2008). Stewart et al., (2011) also found that currently depressed females exhibited greater right parietal activity than those with an MDD history. This was moderated by caffeine consumption, which may have induced greater anxious arousal in currently depressed females. However, this explanation is unlikely in our study given that participants were asked to refrain from caffeine prior to testing, though compliance cannot be assured.
Few studies have examined baseline ACC theta activity in depressed non-medicated individuals versus controls, though some groups have probed frontal scalp-derived theta, and noted both theta power/amplitude reductions (Ohashi et al., 1994; Wienbruch et al., 2003; Saletu et al., 2010) and increases (Roemer et al., 1992; Knott et al., 2000; Köhler et al., 2011) in MDD. In the current study, greater sgACC theta2 activity was found in the MDD group; a similar trend was noted in BA25, part of the rostral ACC (p=.11). Though this runs counter to some findings (Mientus et al., 2002; Coutin-Churchman & Moreno, 2008), our results are consistent with the idea that the ACC, particularly the rostral ACC, is implicated in emotive processing and cognitive control, and that elevated rostral ACC theta in MDD may reflect compensatory activity in fronto-cingulate networks in the disorder (Pizzagalli et al., 2011). In support of this, elevated baseline sgACC theta (Narushima et al., 2010) and rostral ACC theta has been shown to predict a positive antidepressant response (Pizzagalli et al., 2001; Mulert et al., 2007; Korb et al., 2011).
Our findings of increased ACC theta in MDD were confined to theta2 activity, somewhat consistent with previous work indicating that antidepressant-associated modulations emerged only in theta sub-bands (Pizzagalli et al., 2001; Narushima et al., 2010), thus, alterations in specific theta bands in MDD seem feasible. However, the functional significance of resting activity in theta sub-bands should be further explored. Additionally, though the spatial limitations of sLORETA are acknowledged, significant differences existed in theta activity in the examined ROIs suggesting that theta activity was likely elicited by various aspects ACC aspects (though regional overlap is feasible). The sgACC, where theta activity group differences emerged, and midline brain structures have been implicated in regulating emotional behaviors and the stress response, which tend to be disturbed in MDD (Northoff et al., 2011). Previous research has noted glial loss (Drevets et al., 2008) in the sgACC and metabolic activity alterations (Drevets et al., 2007; Monkul et al., 2012) in this region in MDD; these alterations could be associated with increased theta activity. However, given that no correlations between depression scores and sgACC theta activity were noted, the increased theta does not appear to be directly related to illness severity.
Limitations & Conclusions
Certain study limitations must be pointed out. First, current source density (CSD) analysis was not used, though recent work has highlighted its purported superiority given that it is a “reference-independent measure of the strength of extracellular current generators” underlying the EEG (Tenke & Kayser, 2005) and seems the least likely to bias asymmetry measures. Second, alpha activity source localization should be carried out in future investigations, as relatively scant literature exists on the generators of anterior alpha power and asymmetry (Lubar et al., 2003). Third, it is feasible that assessment of electrocortical activity during an emotional challenge may have resulted in more pronounced group differences (Stewart et al., 2011). However, the utility of assessing resting brain activity is particularly advantageous from a clinical/diagnostic perspective. Additionally, theta activity in the dorsal ACC (BA 25′/32′) should be examined in comparable future work as previous neuroimaging research suggests that MDD is associated with decreased activity in this region (Pizzagalli et al., 2011). Finally, though exploratory covariate analyses did not indicate that smoking status/abstinence may have influenced the alpha asymmetry results (data not shown), this, along with factors such as depression severity, co-morbid anxiety and age, should be more carefully controlled for in comparable research.
In confirmation and extension of previous literature, MDD was characterized by a general reduction in cortical activity. Midfrontal alpha2 power asymmetry indices, regardless of sex or reference, indicated increased relative left frontal hypoactivity in MDD, consistent with indications that left frontal hypoactivity is associated with decreased approach-related motivations and positive affective dispositions. MDD females exhibited right parietal hyperactivity perhaps reflecting enhanced baseline emotive arousal states. Finally, the MDD group had increased theta activity in the sgACC, a region implicated in emotion and stress response regulation and which has been also associated with morphological and functional alterations in the disorder.
Supplementary Material
Acknowledgments
We would like to thank C. Hebert for her assistance in patient screening and recruitment, as well as Drs. P. Tessier and S. Norris for their assistance in patient assessments. Finally, we would like to thank D. Shah and J. Choueiry for their contribution to patient testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baehr E, Rosenfeld JP, Baehr R, et al. Comparison of two EEG asymmetry indices in depressed patients vs. normal controls. Int J Psychophysiol. 1998;31(1):89–92. doi: 10.1016/s0167-8760(98)00041-5. [DOI] [PubMed] [Google Scholar]
- 2.Bauer LO, Hesselbrock VM. Lateral asymmetries in the frontal brain: effects of depression and a family history of alcoholism in female adolescents. Alcohol Clin Exp Res. 2002;26(11):1662–8. doi: 10.1097/01.ALC.0000036283.60525.B3. [DOI] [PubMed] [Google Scholar]
- 3.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio (TX): Psychological Corporation; 1996. [Google Scholar]
- 4.Bruder GE, Bansal R, Tenke CE, et al. Relationship of resting EEG with anatomical MRI measures in individuals at high and low risk for depression. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21284. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruder GE, Fong R, Tenke CE, et al. Regional brain asymmetries in major depression with or without an anxiety disorder: a quantitative electroencephalographic study. Biol Psychiatry. 1997;41(9):939–48. doi: 10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- 6.Bruder GE, Tenke CE, Warner V, et al. Electroencephalographic measures of regional hemispheric activity in offspring at risk for depressive disorders. Biol Psychiatry. 2005;57(4):328–35. doi: 10.1016/j.biopsych.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho A, Moraes H, Silveira H, et al. EEG frontal asymmetry in the depressed and remitted elderly: is it related to the trait or to the state of depression? J Affect Disord. 2011;129(1–3):143–8. doi: 10.1016/j.jad.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Chatrian GE, Lettich E, Nelson PL. Ten percent electrode system for topographic studies of spontaneous and evoked EEG activity. Am J EEG Technol. 1985;25:83–92. [Google Scholar]
- 9.Coutin-Churchman P, Moreno R. Intracranial current density (LORETA) differences in QEEG frequency bands between depressed and non-depressed alcoholic patients. Clin Neurophysiol. 2008;119(4):948–58. doi: 10.1016/j.clinph.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Davidson R. Anterior electrophysiological asymmetrics, emotion, and depression: Conceptual and methodological conundrums. Psychophysiology. 1998;35:607–14. doi: 10.1017/s0048577298000134. [DOI] [PubMed] [Google Scholar]
- 11.Davidson RJ, Slagter HA. Probing emotion in the developing brain: functional neuroimaging in the assessment of the neural substrates of emotion in normal and disordered children and adolescents. Ment Retard Dev Disabil Res Rev. 2000;6(3):166–70. doi: 10.1002/1098-2779(2000)6:3<166::AID-MRDD3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 12.Debener S, Beauducel A, Nessler D, et al. Is resting anterior EEG alpha asymmetry a trait marker for depression? Findings for healthy adults and clinically depressed patients. Neuropsychobiology. 2000;41:31–7. doi: 10.1159/000026630. [DOI] [PubMed] [Google Scholar]
- 13.Deldin PJ, Chiu P. Cognitive restructuring and EEG in major depression. Biol Psychol. 2005;70(3):141–51. doi: 10.1016/j.biopsycho.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Deslandes AC, de Moraes H, Pompeu FA, et al. Electroencephalographic frontal asymmetry and depressive symptoms in the elderly. Biol Psychol. 2008;79(3):317–22. doi: 10.1016/j.biopsycho.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Drevets WC, Price JL, Simpson JR, Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 16.Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol. 2002;12(6):527–44. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- 17.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13(8):663–81. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fingelkurts AA, Fingelkurts AA, Rytsälä H, et al. Composition of brain oscillations in ongoing EEG during major depression disorder. Neurosci Research. 2006;56(2):133–44. doi: 10.1016/j.neures.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 19.First MB, Spitzer RL, Williams JBW, et al. Structured Clinical Interview for DSM-IV (SCID) American Psychiatric Association; Washington (DC): 1997. [Google Scholar]
- 20.Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55(4):468–84. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- 21.Grin-Yatsenko VA, Baas I, Ponomarev VA, et al. Independent component approach to the analysis of EEG recordings at early stages of depressive disorders. Clin Neurophysiol. 2010;121(3):281–9. doi: 10.1016/j.clinph.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Hagemann D. Individual differences in anterior EEG asymmetry: methodological problems and solutions. Biol Psychol. 2004;67(1–2):157–82. doi: 10.1016/j.biopsycho.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Hale TS, Smalley SL, Dang J, et al. ADHD familial loading and abnormal EEG alpha asymmetry in children with ADHD. J Psychiatr Res. 2010;44(9):605–15. doi: 10.1016/j.jpsychires.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harmon-Jones E. Contributions from research on anger and cognitive dissonance to understanding the motivational functions of asymmetrical frontal brain activity. Biol Psychol. 2004;67(1–2):51–76. doi: 10.1016/j.biopsycho.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Heller W, Nitschke JB. Regional brain activity in emotion: A framework for understanding cognition in depression. Cogn Emot. 1997;11:637–61. [Google Scholar]
- 27.Henriques JB, Davidson RJ. Regional brain electrical asymmetries discriate between previously depressed and healthy control subjects. J Abnorm Psychol. 1990;99(1):22–31. doi: 10.1037//0021-843x.99.1.22. [DOI] [PubMed] [Google Scholar]
- 28.Hosokawa T, Momose T, Kasai K. Brain glucose metabolism difference between bipolar and unipolar mood disorders in depressed and euthymic states. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2):243–50. doi: 10.1016/j.pnpbp.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Ishii R, Shinosaki K, Ukai S, et al. Medial prefrontal cortex generates frontal midline theta rhythm. Neuroreport. 1999;10(4):675–9. doi: 10.1097/00001756-199903170-00003. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs GD, Snyder D. Frontal brain asymmetry predicts affective style in men. Behav Neurosci. 1996;110(1):3–6. doi: 10.1037//0735-7044.110.1.3. [DOI] [PubMed] [Google Scholar]
- 31.Junghöfer M, Elbert T, Tucker DM, et al. The polar average reference effect: a bias in estimating the head surface integral in EEG recording. Clin Neurophysiol. 1999;110(6):1149–55. doi: 10.1016/s1388-2457(99)00044-9. [DOI] [PubMed] [Google Scholar]
- 32.Keller J, Nitschke JB, Bhargava T, et al. Neuropsychological differentiation of depression and anxiety. J Abnorm Psychol. 2000;109(1):3–10. [PubMed] [Google Scholar]
- 33.Kemp AH, Griffiths K, Felgham KL, et al. Disorder specificity despite comorbidity: resting EEG alpha asymmetry in major depressive disorder and post-traumatic stress disorder. Biol Psych. 2010;85(2):350–4. doi: 10.1016/j.biopsycho.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Kentgen LM, Tenke CE, Pine DS, et al. Electroencephalographic asymmetries in adolescents with major depression: influence of comorbidity with anxiety disorders. J Abnorm Psychol. 2000;109(4):797–802. doi: 10.1037//0021-843x.109.4.797. [DOI] [PubMed] [Google Scholar]
- 35.Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-tig hypothesis. Brain Res Rev. 2007;53(1):63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Knott V, Mahoney C, Kennedy S, et al. Pre-treatment EEG and its relationship to depression severity and paroxetine treatment outcome. Pharmacopsychiatry. 2000;33(6):201–5. doi: 10.1055/s-2000-8356. [DOI] [PubMed] [Google Scholar]
- 37.Knott VJ, Lapierre YD. Computerized EEG correlates of depression and antidepressant treatment. Prog Neuropsychopharmacol Biol Psychiatry. 1987;11:213–21. doi: 10.1016/0278-5846(87)90063-7. [DOI] [PubMed] [Google Scholar]
- 38.Knott VJ, Telner JI, Lapierre YD, et al. Quantitative EEG in the prediction of antidepressant response to imiprae. J Affect Disord. 1996;39:175–84. doi: 10.1016/0165-0327(96)00003-1. [DOI] [PubMed] [Google Scholar]
- 39.Köhler S, Ashton CH, Marsh R, et al. Electrophysiological changes in late life depression and their relation to structural brain changes. Int Psychogeriatr. 2011;23(1):141–8. doi: 10.1017/S1041610210001250. [DOI] [PubMed] [Google Scholar]
- 40.Korb AS, Hunter AM, Cook IA, et al. Rostral anterior cingulate cortex activity and early symptom improvement during treatment for major depressive disorder. Psychiatry Res. 2011;192(3):188–94. doi: 10.1016/j.pscychresns.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwon JS, Youn T, Jung HY. Right hemisphere abnormalities in major depression: quantitative electroencephalographic findings before and after treatment. J Affect Disord. 1996;40(3):169–73. doi: 10.1016/0165-0327(96)00057-2. [DOI] [PubMed] [Google Scholar]
- 42.Landolt HP, Gillin JC. Different effects of phenelzine treatment on EEG topography in waking and sleep in depressed patients. Neuropsychopharmacology. 2002;27(3):462–9. doi: 10.1016/S0893-133X(02)00322-6. [DOI] [PubMed] [Google Scholar]
- 43.Lubar JF, Congedo M, Askew JH. Low-resolution electromagnetic tomography (LORETA) of cerebral activity in chronic depressive disorder. Int J Psychophysiol. 2003;49(3):175–85. doi: 10.1016/s0167-8760(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 44.Manna CB, Tenke CE, Gates NA, et al. EEG hemispheric asymmetries during cognitive tasks in depressed patients with high versus low trait anxiety. Clin EEG Neurosci. 2010;41(4):196–202. doi: 10.1177/155005941004100406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathersul D, Williams LM, Hopkinson, et al. Investigating models of affect: relationships among EEG alpha asymmetry, depression, and anxiety. Emotion. 2008;8(4):560–72. doi: 10.1037/a0012811. [DOI] [PubMed] [Google Scholar]
- 46.Maxwell E. The Family Interview for Genetic Studies Manual. Washington (DC): 1992. [Google Scholar]
- 47.McNair DM, Lorr M, Droppleman LF. POMS manual: Profile of mood states. Educational and Industrial Testing Service; San Diego (CA): 1992. [Google Scholar]
- 48.Mientus S, Gallinat J, Wuebben Y, et al. Cortical hypoactivation during resting EEG in schizophrenics but not in depressives and schizotypal subjects as revealed by low resolution electromagnetic tomography (LORETA) Psychiatry Research. 2002;116(1–2):95–111. doi: 10.1016/s0925-4927(02)00043-4. [DOI] [PubMed] [Google Scholar]
- 49.Mitchell DJ, McNaughton N, Flanagan D, et al. Frontal-midline theta from the perspective of hippocampal “theta”. Prog Neurobiol. 2008;86(3):156–85. doi: 10.1016/j.pneurobio.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Monkul ES, Silva LA, Narayana S, et al. Abnormal resting state corticolimbic blood flow in depressed unmedicated patients with major depression: a (15)O-H(2)O PET study. Hum Brain Mapp. 2012;33(2):272–9. doi: 10.1002/hbm.21212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montgomery SA, Åsberg S. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 52.Morgan ML, Witte EA, Cook IA, et al. Influence of age, gender, health status, and depression on quantitative EEG. Neuropsychobiology. 2005;52(2):71–6. doi: 10.1159/000086608. [DOI] [PubMed] [Google Scholar]
- 53.Moscovitch DA, Santesso DL, Miskovic V, et al. Frontal EEG asymmetry and symptom response to cognitive behavioral therapy in patients with social anxiety disorder. Biol Psychol. 2011;87(3):379–85. doi: 10.1016/j.biopsycho.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 54.Mulert C, Jäger L, Propp S, et al. Sound level dependence of the primary auditory cortex: Simultaneous measurement with 61-channel EEG and fMRI. Neuroimage. 2005;28(1):49–58. doi: 10.1016/j.neuroimage.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 55.Mulert C, Juckel G, Brunnmeier M, et al. Rostral anterior cingulate cortex activity in the theta band predicts response to antidepressive medication. Clin EEG Neurosci. 2007;38(2):78–81. doi: 10.1177/155005940703800209. [DOI] [PubMed] [Google Scholar]
- 56.Narushima K, McCormick LM, Yamada T, et al. Subgenual cingulate theta activity predicts treatment response of repetitive transcranial magnetic stimulation in participants with vascular depression. J Neuropsychiatry Clin Neurosci. 2010;22(1):75–84. doi: 10.1176/appi.neuropsych.22.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neuper C, Pfurtscheller G. Event-related dynamics of cortical rhythms: frequency-specific features and functional correlates. Int J Psychophysiol. 2001;43(1):41–58. doi: 10.1016/s0167-8760(01)00178-7. [DOI] [PubMed] [Google Scholar]
- 58.Northoff G, Wiebking C, Feinberg T, et al. The ‘resting-state hypothesis’ of major depressive disorder-A translational subcortical-cortical framework for a system disorder. Neurosci Biobehav Rev. 2011;35(9):1929–45. doi: 10.1016/j.neubiorev.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 59.Ohashi Y. [The baseline EEG traits and the induced EEG changes by chronic antidepressant medication in patients with major depression. Early prediction of clinical outcomes solely based on quantification and mapping of EEG] Seishin Shinkeigaku Zasshi. 1994;96(6):444–60. [PubMed] [Google Scholar]
- 60.Olbrich SC, Mulert S, Karch M, et al. EEG-vigilance and BOLD effect during simultaneous EEG/fMRI measurement. NeuroImage. 2009;45(2):319–32. doi: 10.1016/j.neuroimage.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 61.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychololgia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 62.Pascual-Marqui RD. The sLORETA method: Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Method Find Exp Clin. 2002;24(Suppl D):5–12. [PubMed] [Google Scholar]
- 63.Phillips C, Rugg M, Fristont K. Systematic regularization of linear inverse solutions of the EEG source localization problem. Neuroimage. 2002;17:287–301. doi: 10.1006/nimg.2002.1175. [DOI] [PubMed] [Google Scholar]
- 64.Pizzagalli D, Oakes T, Fox A, et al. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol Psychiatry. 2004;9:393–405. doi: 10.1038/sj.mp.4001501. [DOI] [PubMed] [Google Scholar]
- 65.Pizzagalli D, Pascual-Marqui RD, Nitschke JB, et al. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;158(3):405–15. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- 66.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36(1):183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pizzagalli DA, Oakes TR, Davidson RJ. Coupling of theta activity and glucose metabolism in the human rostral anterior cingulate cortex: an EEG/PET study of normal and depressed subjects. Psychophysiology. 2003;40(6):939–49. doi: 10.1111/1469-8986.00112. [DOI] [PubMed] [Google Scholar]
- 68.Pizzagalli DA, Sherwood RJ, Henriques JB, et al. Frontal brain asymmetry and reward responsiveness: a source-localization study. Psychol Sci. 2005;16(10):805–13. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- 69.Pollock VE, Schneider LS. Quantitative, waking EEG research on depression. Biol Psychiatry. 1990;27(7):757–80. doi: 10.1016/0006-3223(90)90591-o. [DOI] [PubMed] [Google Scholar]
- 70.Pössel P, Lo H, Frit A, et al. A longitudinal study of cortical EEG activity in adolescents. Biol Psychol. 2008;78(2):173–8. doi: 10.1016/j.biopsycho.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 71.Ricardo-Garcell J, González-Olvera JJ, Miranda E, et al. EEG sources in a group of patients with major depressive disorders. Int J Psychophysiol. 2009;71(1):70–4. doi: 10.1016/j.ijpsycho.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 72.Roemer RA, Shagass C, Dubin W, et al. Quantitative EEG in elderly depressives. Brain Topogr. 1992;4(4):285–90. doi: 10.1007/BF01135566. [DOI] [PubMed] [Google Scholar]
- 73.Saletu B, Anderer P, Saletu-Zyhlarz GM. EEG topography and tomography (LORETA) in diagnosis and pharmacotherapy of depression. Clin EEG Neurosci. 2010;41(4):203–10. doi: 10.1177/155005941004100407. [DOI] [PubMed] [Google Scholar]
- 74.Segrave RA, Cooper NR, Thomson RH, et al. Individualized alpha activity and frontal asymmetry in major depression. Clin EEG Neurosci. 2011;42(1):45–52. doi: 10.1177/155005941104200110. [DOI] [PubMed] [Google Scholar]
- 75.Stewart JL, Bismark AW, Towers DN, et al. Resting frontal EEG asymmetry as an endophenotype for depression risk: sex-specific patterns of frontal brain asymmetry. J Abnorm Psychol. 2010;119(3):502–12. doi: 10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stewart JL, Coan JA, Towers DN, Allen JJ. Frontal EEG asymmetry during emotional challenge differentiates individuals with and without lifetime major depressive disorder. J Affect Disord. 2011;129(1–3):167–74. doi: 10.1016/j.jad.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tenke CE, Kayser J. Reference-free quantification of EEG spectra: combining current source density (CSD) and frequency principal components analysis (fPCA) Clin Neurophysiol. 2005;116(12):2826–46. doi: 10.1016/j.clinph.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 78.Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: a meta-analytic review. J Abnorm Psychol. 2006;115(4):715–29. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- 79.Tomarken AJ, Davidson RJ, Henriques JB. Resting frontal brain asymmetry predicts affective responses to films. J Pers Soc Psychol. 1990;59(4):791–801. doi: 10.1037//0022-3514.59.4.791. [DOI] [PubMed] [Google Scholar]
- 80.Tomarken AJ, Davidson RJ, Wheeler RE, et al. Individual differences in anterior brain asymmetry and fundamental dimensions of emotion. J Pers Soc Psychol. 1992;62(4):676–87. doi: 10.1037//0022-3514.62.4.676. [DOI] [PubMed] [Google Scholar]
- 81.Wacker J, Heldmann M, Stemmler G. Separating emotion and motivational direction in fear and anger: effects on frontal asymmetry. Emotion. 2003;3(2):167–93. doi: 10.1037/1528-3542.3.2.167. [DOI] [PubMed] [Google Scholar]
- 82.Wheeler RE, Davidson RJ, Tomarken AJ. Frontal brain asymmetry and emotional reactivity: a biological substrate of affective style. Psychophysiology. 1993;30(1):82–9. doi: 10.1111/j.1469-8986.1993.tb03207.x. [DOI] [PubMed] [Google Scholar]
- 83.Wienbruch C, Moratti S, Elbert T, et al. Source distribution of neuromagnetic slow wave activity in schizophrenic and depressive patients. Clin Neurophysiol. 2003;114(11):2052–60. doi: 10.1016/s1388-2457(03)00210-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.