Abstract
Medulloblastomas are malignant brain tumors that arise in the cerebellum in children and disseminate via the cerebrospinal fluid to the leptomeningeal spaces of the brain and spinal cord. Challenged by the poor prognosis for patients with metastatic dissemination, pediatric oncologists have developed aggressive treatment protocols, combining surgery, craniospinal radiation, and high-dose chemotherapy that often cause disabling neurotoxic effects in long-term survivors. Insights into the genetic control of medulloblastoma dissemination have come from transposon insertion mutagenesis studies. Mobilizing the Sleeping Beauty transposon in cerebellar neural progenitor cells caused widespread dissemination of typically nonmetastatic medulloblastomas in Patched+/− mice, in which Sonic Hedgehog (Shh) signaling is hyperactive. Candidate metastasis genes were identified by sequencing the insertion sites and then mapping these sequences back to the mouse genome. To determine whether genes located at transposon insertion sites directly caused medulloblastomas to disseminate, we overexpressed candidate genes in Nestin+ neural progenitors in the cerebella of mice by retroviral transfer in combination with Shh. We show here that ectopic expression of Eras, Lhx1, Ccrk, and Akt shifted the in vivo growth characteristics of Shh-induced medulloblastomas from a localized pattern to a disseminated pattern in which tumor cells seeded the leptomeningeal spaces of the brain and spinal cord.
Keywords: medulloblastoma, metastasis, leptomeningeal dissemination
Introduction
Medulloblastomas are malignant brain tumors that originate from neural progenitor cells in the developing cerebellum. A powerful predictor of short survival times for pediatric patients is the presence of metastasis (1). A defining characteristic of metastasis in medulloblastoma is the proclivity of tumor cells to disseminate via the cerebrospinal fluid (CSF) to the leptomeninges of the brain and spinal cord. This pattern distinguishes medulloblastomas from tumors originating in other organs, which metastasize through the bloodstream or lymphatic channels. The pressing need to reduce the risk of metastasis has driven the development of aggressive treatment protocols, combining maximum surgical resection, craniospinal radiation, and multidrug chemotherapy [reviewed in (2)].
Because the prospect for long-term survival is so poor once leptomeningeal dissemination has occurred, radiation to the entire neuraxis is an indispensable part of medulloblastoma treatment regimens for children older than three years. After treatment, however, children are at high risk for developing cognitive impairment, skeletal growth retardation, endocrine dysfunction, and behavioral disturbances later in life (3, 4). Therefore, therapies that target metastasis specifically will likely protect patients from some of these treatment-related side effects.
An emerging concept in pediatric oncology is that medulloblastomas comprise a diverse set of tumors, in which different subgroups arise by transformation of neural progenitor cells, responding to different molecular signaling pathways. Large-scale gene expression profiling studies of patient tumor specimens have shown that the Sonic Hedgehog (Shh) signaling pathway is activated in 25–30% of medulloblastomas (5–7).
Animal models of medulloblastoma, created using genetically engineered mice, have shown conclusively that activating the Shh signaling pathway in the cerebellum during early postnatal development can induce medulloblastoma formation. Several different methods of activating the Shh pathway have been reported, including (a) targeted deletion of the Patched gene, which encodes the inhibitory receptor for Shh (8), (b) ectopic expression of Shh by retroviral transfer (9, 10), and (c) transgenic overexpression of Smoothened, a positive effector of Shh signaling (11). With the exception of a homozygous version of the ND2:SmoA1 model, in which expression of a constitutively activated allele of the Smoothened gene is driven by the neuroD2 gene promoter (12), leptomeningeal dissemination has not been reported in mouse models of Shh-driven medulloblastoma.
The tight correlation between metastasis and poor prognosis for medulloblastoma patients heightens the need to understand the genetic determinants of leptomeningeal dissemination. Insights into the genetic control of medulloblastoma dissemination have come from experiments in which the Sleeping Beauty (SB) transposon system was used to induce insertional mutations in granule neuron precursors (GNPs) in the developing cerebellum in mice. GNPs are lineage-restricted progenitor cells that can transform into medulloblastomas in response to hyperactive Shh signaling (13–15).
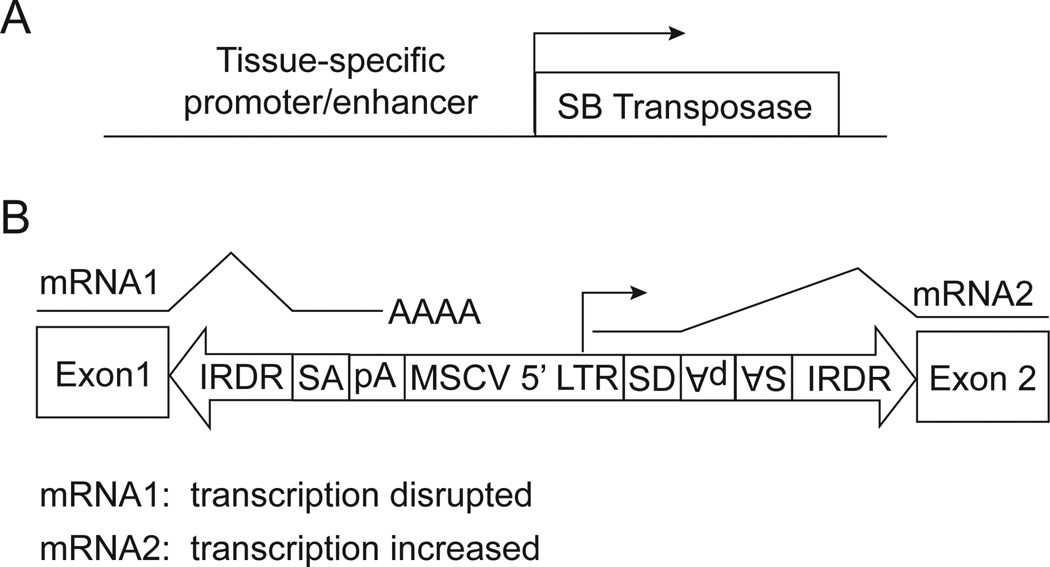
The SB transposon system (Fig. 1) is a powerful tool for cancer gene discovery in mice [reviewed in (16)]. In the presence of a transposase enzyme, the SB transposon is excised from genomic DNA and inserted throughout the genome at sites containing TA dinucleotides. A transposon integration site present in the genome at a significantly high frequency is called a common insertion site (CIS). Tumors can arise if the transposon activates proto-oncogenes or inactivates tumor suppressor genes located at the integration sites (transposon insertion mutagenesis).
Figure 1.
The Sleeping Beauty transposon system. SB consists of two components, the transposable element (transposon) and the transposase enzyme, which catalyzes transposon mobilization. A, Vector for expressing the transposase under control of a tissue-specific promoter/enhancer. B, The SB transposon contains a pair of inverted repeat/direct repeat elements (IRDR), flanking the mobile cargo sequence. For cancer gene identification, the cargo sequence is designed to mimic retroviral insertional mutagenesis. The transposon contains splice acceptor sites (SA) and polyadenylation (pA) sequences to disrupt the expression of genes into which the transposon integrates (mRNA1). The transposon also contains 5’ sequences from the murine stem cell virus (MSCV) long terminal repeat (LTR) to serve as promoter/enhancer elements, which increase expression of adjacent genes (mRNA2). The MSCV LTR is followed by a splice donor (SD). Thus, a transcript initiated in the LTR can splice into downstream exons of endogenous genes. The SB transposon schematized here is the T2/Onc vector [reviewed in (16)].
To discover genes that promote spinal leptomeningeal dissemination in Shh-driven medulloblastomas, Wu et al (17) expressed the SB transposase selectively in Math1+ GNPs in Patched+/− mice, which develop localized, nonmetastatic medulloblastomas at an incidence of 39% by 8 months of age. In sharp contrast, 97% of Patched+/− mice in which the SB transposon was mobilized by driving expression of the SB transposase from the Math1 gene promoter developed medulloblastomas that metastasized widely throughout the spinal leptomeninges by 10 weeks of age. Candidate metastasis genes were identified by sequencing the insertion sites in tumor-bearing animals and then mapping these sequences back to the mouse genome. In this way, the investigators identified gene-centric common insertion sites (gCISs), in which transposon insertions occurred significantly more frequently than the background rate. In 139 matched pairs of primary/metastatic tumors from Patched+/− mice, 359 gCISs were identified in the primary brain tumors, 285 in the spinal metastases, and 60 in both primary tumor and spinal metastasis from the same animals. To determine whether gCIS–associated genes can promote dissemination of tumor cells from Shh-induced medulloblastomas to the leptomeningeal spaces of the brain and spinal cord, we expressed four candidate genes (Eras, Lhx1, Ccrk, and Akt) in neural progenitor cells in the cerebellum of mice by retroviral transfer in combination with Shh.
Materials and Methods
Transgenic mice
The use of mice in this study was approved by the Institutional Animal Care and Use Committee of the University of Utah. Production of the Ntv-a mouse line, in which expression of the tv-a transgene is driven by promoter/enhancer sequences of the Nestin gene, has been described previously (18). Because of the breeding strategy used to introduce the transgene, Ntv-a mice are hybrids composed of the following genetic strains: C57BL/6, BALB/C, FVB/N, and CD1.
Retroviral vector construction
Construction of RCAS-Shh, which contains an in-frame, carboxy-terminal epitope tag consisting of six repeats of the influenza virus hemagglutinin (HA) epitope, was described previously (10). RCAS-Akt transfers an oncogenic allele of Akt (Akt-Myr-Δ11-60) that contains an amino-terminal myristylation signal, which enhances the affinity of the Akt protein for the plasma membrane (19, 20). The cDNA clones for Eras (mouse), Lhx1 (mouse), and Ccrk (human) were obtained from the American Type Culture Collection (Manassas, VA), where they were deposited by the Integrated Molecular Analysis of Genomes and their Expression (IMAGE) consortium (http://image.hudsonalpha.org). RCAS vectors were prepared by ligating a PCR-generated cDNA corresponding to the complete coding sequence into the parent retroviral vector RCASBP(A) (21). RCAS vectors for Eras, Lhx1, and Ccrk each contained an internal ribosome entry site (IRES), coupled to the Aequorea coerulescens green fluorescent protein (GFP), for tracking the cellular localization of the expressed proteins. To produce live virus, we transfected plasmid versions of RCAS vectors into immortalized chicken fibroblasts (DF-1 cells) and allowed them to replicate in culture.
In vivo somatic cell gene transfer in transgenic mice
To induce medulloblastomas in mice, we used a version of the RCAS/tv-a somatic cell gene transfer system to transfer and express the Shh gene in Nestin+ neural progenitor cells in the cerebellum. This system uses a replication-competent, avian leukosis virus, splice acceptor (RCAS) vector, derived from the subgroup A avian leukosis virus (ALV-A), and a transgenic mouse line (Ntv-a) that produces TVA (the cell surface receptor for ALV-A) under control of the Nestin gene promoter (18). Nestin is an intermediate filament protein that is expressed by multipotent neural progenitor cells. When mammalian cells are transduced with RCAS retrovirus vectors, the newly generated provirus integrates into the host cell genome, and the transferred gene is expressed as a spliced message under control of the constitutive retroviral promoter (long terminal repeat sequence). RCAS-transduced mammalian cells do not produce infectious virus because mRNA splicing eliminates the retroviral genes that are necessary for viral replication.
To transfer genes via RCAS vectors, we injected retrovirus packaging cells (DF-1 cells transfected with and producing recombinant RCAS retrovirus) into the lateral cerebellum from an entry point just posterior to the lambdoid suture of the skull (bilateral injections of 105 cells in 1–2 µl of phosphate buffered saline). For experiments, in which simultaneous transfer of two genes was the goal, cell pellets were prepared by mixing equal numbers of both retrovirus-producing cells. We injected mice within 72 hours after birth because the number of Nestin-expressing neural progenitor cells decreases progressively afterward. The mice were sacrificed as soon as they showed signs of increased intracranial pressure, indicated by enlarging head circumference (a sign of hydrocephalus), gait ataxia, or failure to thrive. Asymptomatic mice were sacrificed 4 months after injection. The brains were fixed in formalin, and quartered by parallel incisions in the coronal plane. To identify dissemination of tumor cells to the spinal leptomeningeal space, we fixed whole spinal column preparations in formalin for 48–72 hr and then removed the spinal cord by microdissection. Brain and spinal cord specimens were embedded in paraffin and sectioned for histochemical analysis.
Immunocytochemistry and microscopy
Tissue sections were cut 4 µm thick, mounted on glass slides, deparaffinized with toluene, hydrated through a descending series of ethanol, autoclaved in a citrate-based antigen retrieval solution (Vector Laboratories, Burlingame, CA) for 5 min, and cooled to room temperature. Sections were then treated with H2O2 (1% v/v) for 10 min to quench endogenous peroxidase activity and washed with phosphate-buffered saline. After immersion in normal horse serum (2%), sections were incubated with primary antibody in a humid chamber at 4°C overnight. Immunoreactive staining was visualized using a biotin-free reporter enzyme staining system (ImmPRESS, Vector Laboratories, Burlingame, CA), which utilizes a micropolymer of peroxidase and affinity-purified secondary antibodies. Diaminobenzidine was used as the chromogenic substrate and toluidine blue as a nuclear counterstain. We used the following antibodies from the indicated commercial sources: mAbF7 (1:50)—HA (Santa Cruz Biotechnology; Santa Cruz, CA); mAb3580 (1:500)—GFP (Chemicon; Temecula, CA). Tissue sections were visualized using a Zeiss Axiovert 200 microscope and photomicrographs were captured using an AxioCam high-resolution CCD camera and Axiovision imaging software (Carl Zeiss International, Germany).
Expression profiling and molecular subgrouping of human medulloblastomas
Human primary medulloblastomas (n=103) were profiled on Affymetrix Genechip Human Exon 1.0ST arrays at The Centre for Applied Genomics (TCAG, www.tcag.ca; Toronto, Canada). Expression analysis was performed, using Affymetrix Expression Console (Version 1.1), as previously described (22). Additional, publically available medulloblastoma expression data sets (n=187) were obtained from NCBI Gene Expression Omnibus and used to validate our findings (6, 23). Subgrouping of tumors was performed using an 84-gene expression classifier (7).
Results
Shh-induced medulloblastomas are localized (nonmetastatic) tumors
Our objective was to use the RCAS/tv-a somatic cell gene transfer system to determine whether ectopic expression of genes associated with SB gCISs in Patched+/− mice could promote spinal leptomeningeal dissemination in mice bearing Shh-induced medulloblastomas. First, we injected a control group of newborn N/tv-a mice with RCAS-Shh and examined hematoxylin and eosin (H&E)–stained sections of brain and spinal cord during a 4-month observation period. We found tumors in the cerebellum in 27 of 64 mice (42%), an incidence that was consistent with our previous studies, which showed that 15–39% of mice developed brain tumors during 3 months of observation (19, 24–26). Microscopically, the tumors resembled the classic histological subtype of human medulloblastomas, which is characterized by homogeneous sheets of densely packed cells containing carrot-shaped, hyperchromatic nuclei and scant cytoplasm (Fig. 2A). Careful examination of spinal cord sections showed small clusters of tumor cells attached to the leptomeninges of the cord or transiting spinal nerves in only 3 of 64 mice examined (5%) (Fig. 2B). Thus, dissemination of tumor cells to the spinal leptomeningeal space occurred in only 11% of the 27 mice in which tumors had formed in the brain (Table 1). We concluded that the background level of spinal leptomeningeal dissemination in Shh-induced medulloblastomas was sufficiently low to use our mouse model as a platform for identifying more potent metastasis-inducing genes.
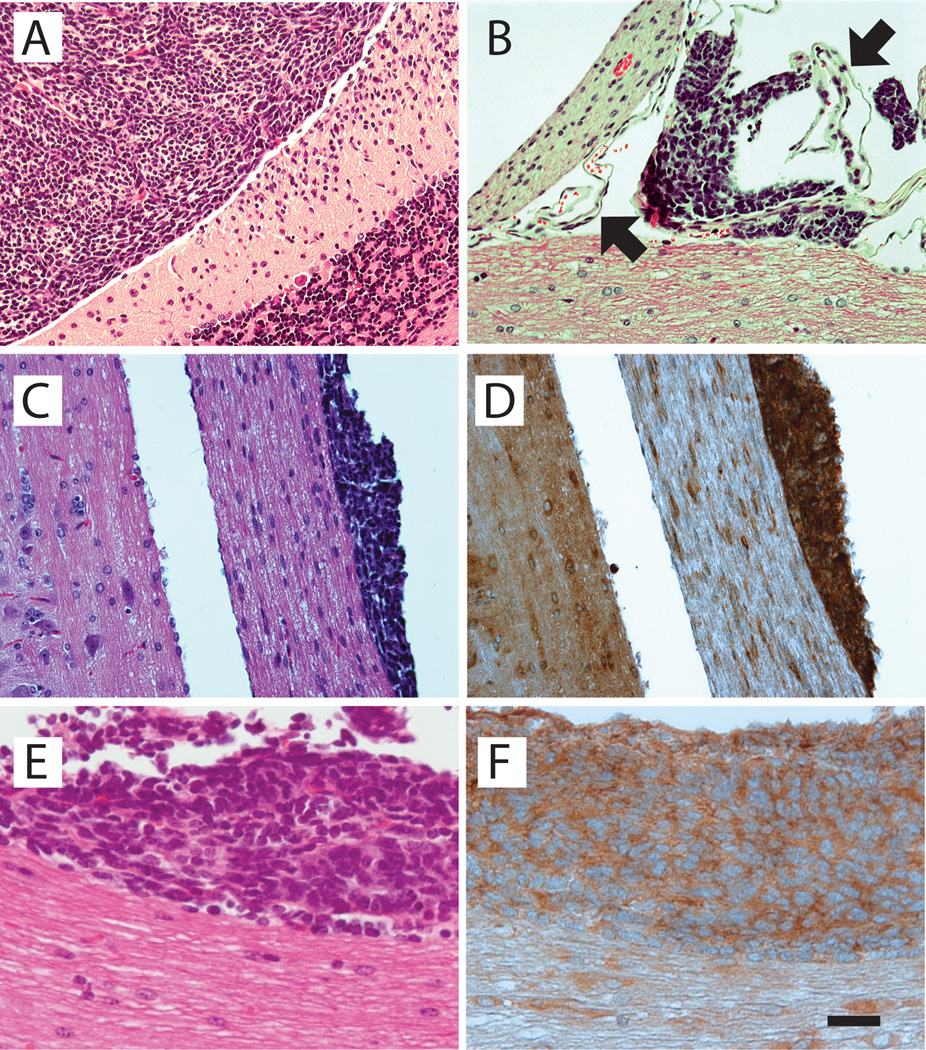
Figure 2.
Histopathology of spinal leptomeningeal dissemination. A, classic cytoarchitecture of Shh-induced medulloblastoma, showing a uniform field of undifferentiated tumor cells (left) abutting the cerebellar cortex (right) (H&E). B, aggregates of Shh-induced medulloblastoma cells attached to the leptomeninges (arrows) of the spinal cord (below) and a spinal nerve (left) (H&E). C, metastasis of Shh+Lhx1–induced medulloblastoma cells to a spinal nerve (right) exiting the spinal cord (left) (H&E). D, immunoperoxidase staining of tissue section adjacent to that shown in C, showing GFP immunoreactivity in tumor cells, thus verifying expression of retrovirus-transferred Lhx1. E, metastasis of Shh+Ccrk–induced medulloblastoma to the surface of the spinal cord (H&E). F, tissue section adjacent to that shown in E, showing GFP immunoreactive staining in Shh+Ccrk–induced medulloblastoma cells attached to the spinal cord. Scale bar, 50 µm (A–D), 25 µm (E–F).
Table 1.
Incidence of medulloblastoma formation in brain and spine leptomeningeal space during a 4-month observation period
| Genes transferred | Brain tumor incidence | Spine tumor incidence* | P value† (spine) |
|---|---|---|---|
| Shh | 27 of 64 (42%) | 3 of 27 (11%) | – |
| Shh + Eras | 34 of 63 (54%) | 10 of 34 (29%) | 0.083 |
| Shh + Lhx1 | 24 of 44 (55%) | 8 of 24 (33%) | 0.054 |
| Shh + Ccrk | 23 of 45 (51%) | 10 of 23 (43%) | 0.007 |
| Shh + Akt | 22 of 39 (56%) | 9 of 22 (41%) | 0.016 |
Spine tumor incidence calculated as a percentage of mice with histologically verified brain tumors.
P values were calculated using χ2 contingency test to compare spine tumor incidence after combined gene transfer versus transfer of Shh alone.
Eras, Lhx1, and Ccrk are metastasis-inducing oncogenes
To identify the strongest driver genes of medulloblastoma leptomeningeal dissemination, we first focused on the 285 gCISs that were present either exclusively in the spinal metastatic tumors or in both the primary tumors and matched metastases. The objective of this criterion was to focus on genes whose expression conferred a growth advantage to tumor cells in the microenvironment of the spinal cord. Conceivably, genetic selection pressure would then lead to the clonal expansion of gCIS–containing cells that were adapted to this new milieu.
Second, we selected gCISs in which (a) the SB transposon had integrated 5’ of exon I or into intron I, and (b) the murine stem cell virus (MSV) promoter of the SB transposon pointed to the direction of gCIS-associated gene transcription. We used these criteria to identify genes whose transcription might be activated by the integrated transposon. We selected such genes, which were likely to be metastasis-promoting oncogenes, because the RCAS/tv-a system was designed to transfer and express dominantly activating genes. We did not test medulloblastomas from SB mice for altered expression of the gCIS. Although the correlation between transposon insertion and gCIS expression has not been analyzed comprehensively, studies of SB-induced T-cell lymphomas and squamous cell carcinomas indicate that a close correlation exists (27, 28). We also selected gCISs that occurred in the spine tumors from ≥3 different mice to minimize the effect of random integration. Of the 285 metastasis gCISs, 32 met this second set of criteria.
Third, we selected gCISs (n=20) that had a coding sequence <3000 base pairs to assure efficient transfer and expression of genes via RCAS retroviral vectors in mice. We focused initially on three genes for which there was published literature that strongly supported a role for the encoded protein in cancer biology: Eras (embryonic stem cell–expressed Ras), Lhx1 (LIM-class homeobox gene 1), and Ccrk (cell cycle–related kinase).
To determine whether Eras, Lhx1, and Ccrk could directly cause Shh-induced medulloblastomas to metastasize, we used the RCAS/tv-a system to transfer and express each gene in Nestin+ neural progenitor cells in the cerebella of newborn mice, in combination with Shh. Our results demonstrated that Eras, Lhx1, and Ccrk increased the incidence of spinal leptomeningeal dissemination, as a percentage of mice with histologically verified tumors in the cerebellum, from a baseline of 11% (Shh alone) to 29% (Shh+Eras), 33% (Shh+Lhx1), and 43% (Shh+Ccrk) (Table 1). In addition to increasing the incidence of spinal leptomeningeal dissemination, Eras, Lhx1, and Ccrk increased the thickness of tumor cell nodules that were attached to the spinal cord and nerves five- to eightfold (Figs. 2C, E). The mean cross-sectional area of spinal leptomeningeal nodules in mice bearing Shh-induced medulloblastomas (0.026 mm2) was increased by the addition of Eras (0.157 mm2), Lhx1 (0.207 mm2), and Ccrk (0.134 mm2) (P=0.02 by ANOVA).
Importantly, expression of Eras, Lhx1, or Ccrk did not significantly increase the incidence of tumor formation in the brain compared with that of Shh alone, indicating that these genes were specific drivers of metastasis, not merely initiators of tumor formation (Table 1). To verify that the tumor cells expressed the genes that we transferred by RCAS vectors, we demonstrated specific immunostaining with an antibody directed against GFP, which was transcribed in tandem with the inserted oncogene through an IRES sequence (Figs. 2D, F).
In the clinical setting, metastasis of medulloblastomas to the spinal column is accompanied by spread of tumor cells to the leptomeninges of the cerebellum and forebrain. Accordingly, we observed in mice that medulloblastomas induced by Shh in combination with Eras, Lhx1, or Ccrk showed extensive dissemination of tumor cells to brain leptomeningeal spaces that were not contiguous with the primary tumor site in the cerebellum. Figure 3 shows examples of tumor dissemination to the brain stem (Figs. 3A, B), hippocampal fissure (Figs. C, D), and the subependymal space of the lateral ventricles (Fig. 3E). We scored brain sections from all tumor-bearing mice for invasiveness, which we defined as (a) tumor on brain sections remote from the cerebellum or (b) local extension into the adjacent fourth ventricle (Fig. 3F). The percentage of Shh-induced medulloblastomas showing invasiveness (48%) was increased by the addition of Eras (88%), Lhx1 (83%), and Ccrk (74%) (P=0.003 by χ2 contingency test).
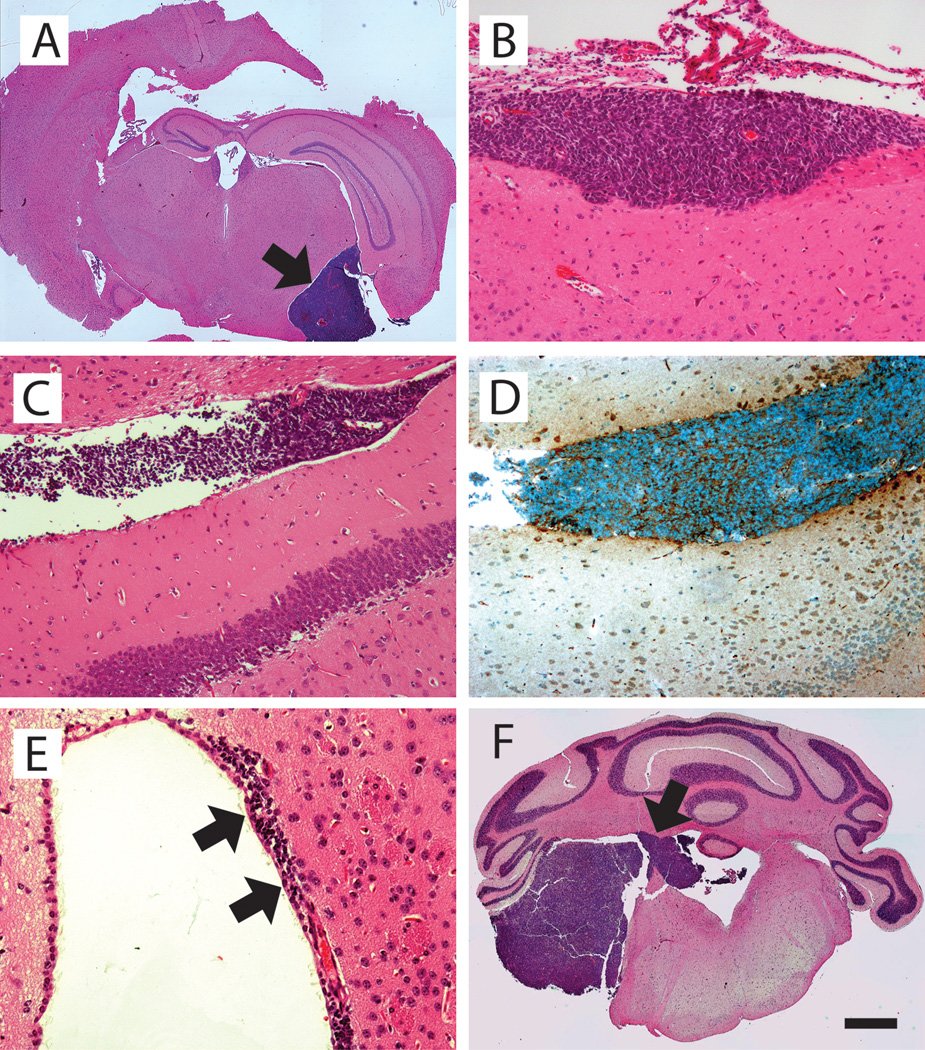
Figure 3.
Histopathology of forebrain leptomeningeal dissemination. A, infiltration of Shh+Ccrk–induced medulloblastoma (arrow) into the brain stem and hippocampal fissure (H&E). B, Shh+Lhx1–induced medulloblastoma cells attached to the leptomeninges of the brain stem (H&E). C, Shh+Lhx1–induced medulloblastoma cells in the hippocampal fissure. Granule neurons of the dentate gyrus are visible below (H&E). D, GFP immunoreactive staining in metastasizing cells from Shh+Lhx1–induced medulloblastoma. Tissue section is adjacent to that shown in C. E, Shh+Ccrk–induced medulloblastoma cells in the subependymal space of the lateral ventricle (arrows) (H&E). F, Shh-induced medulloblastoma infiltrating the fourth ventricle (H&E). Scale bar, 500 µm (A, F), 100 µm (B–D), 50 µm (E).
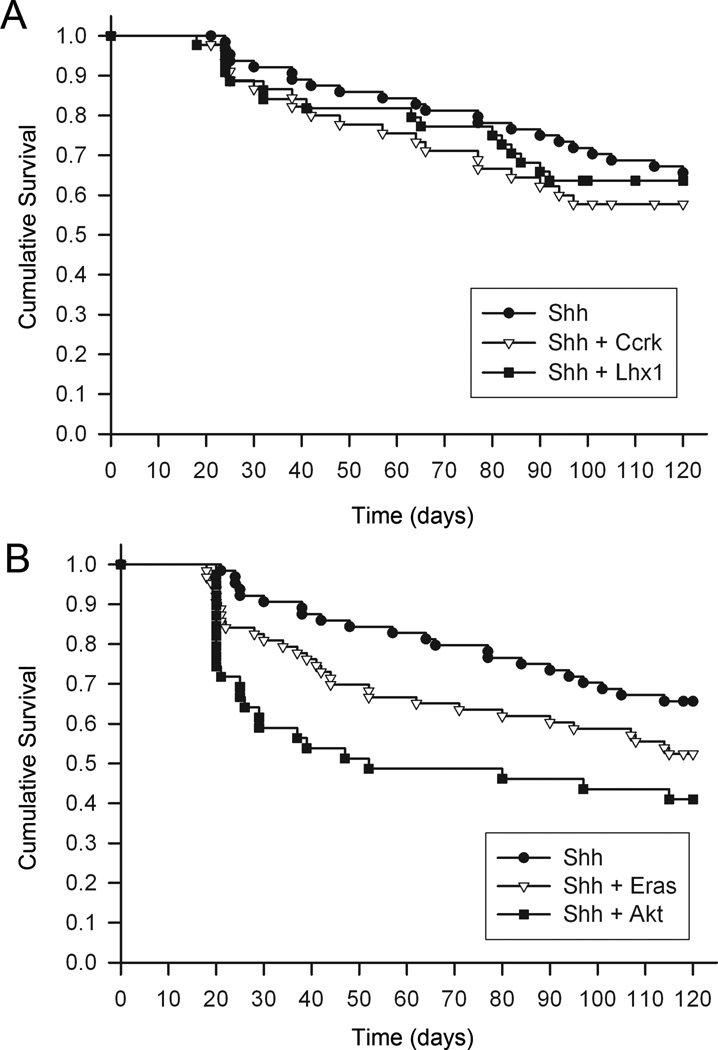
Despite the marked effect on promoting spinal leptomeningeal dissemination, ectopic expression of Lhx1 and Ccrk did not reduce overall survival of mice compared with the Shh control group (Fig. 4A). Eras showed a trend toward shorter survival time (P=0.086 by log-rank test). We attribute this to the fact that almost all of the tumor-bearing mice were sacrificed because of symptoms due to brain compression by the primary tumor or to obstructive hydrocephalus, which we did not attempt to treat. Therefore, the mice did not live long enough to succumb to metastatic disease. This experimental scheme contrasts sharply with current clinical practice, in which aggressive surgical resection of the primary tumor and decompression of hydrocephalus are essential prerequisites for progression-free survival in patients.
Figure 4.
Survival analysis of mice following retroviral transfer of Shh and metastasis-inducing oncogenes. Kaplan-Meier survival analysis of mice injected with RCAS-Shh, alone or in combination with RCAS-Ccrk and RCAS-Lhx1 (A) or RCAS-Eras and RCAS-Akt (B). The mice were injected on day zero and sacrificed at the indicated time points.
Expression of ERAS, LHX1, and CCRK is increased in aggressive subgroups of human medulloblastomas
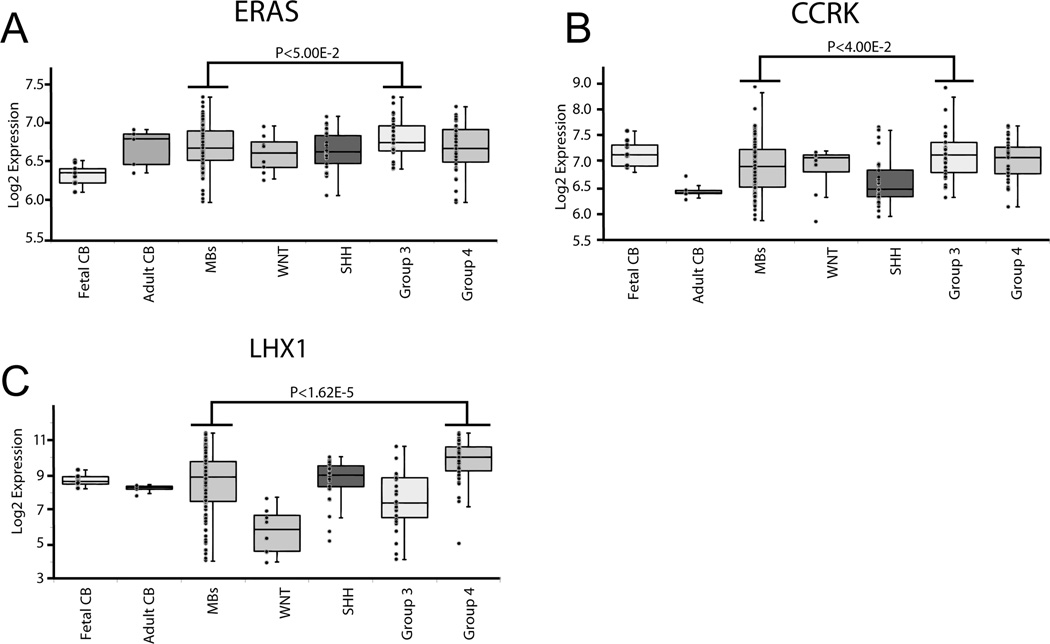
Given the identification of Eras, Lhx1, and Ccrk in a large, unbiased genetic screen for metastasis genes using the SB transposon system and considering our supportive observation of Shh-induced medulloblastomas transitioning from a localized to a disseminated growth pattern, we next focused on the expression of our candidate genes in a large cohort of primary human tumors. We compared relative expression levels of ERAS, LHX1, and CCRK in 103 human medulloblastoma specimens, which had each been assigned to one of the four distinct, nonoverlapping medulloblastoma subgroups (WNT, SHH, Group 3, and Group 4) based on their gene expression profiles (7, 29). Our analysis demonstrated that expression of ERAS and CCRK was higher in Group 3 tumors (Fig. 5A–B) and expression of LHX1 was higher in Group 4 (Fig. 5C), compared with the undivided set of 103 tumors. The association between CCRK and LHX1 expression and tumor subgroup was recapitulated in a second, independently generated data set from 187 medulloblastomas in which ERAS had not been assessed (Supplementary Fig. 1).
Figure 5.
Expression of gCIS–associated genes in human medulloblastoma subgroups. Box plots showing relative expression of ERAS (A), CCRK (B), and LHX1 (C) in normal cerebella (CB; fetal n=9, adult n=5) and medulloblastoma samples (MB; n=103) profiled on Affymetrix exon arrays. The 103 medulloblastomas were divided into subgroups (WNT, SHH, Group 3, Group 4) and analyzed separately. Log2 expression is a measure of the luminosity of the gene probe signal, corrected for the background luminosity of each array and normalized using control probes across different arrays.
Previously published work showed that metastasis (defined by the presence of microscopic tumor cells in the CSF, radiographically detected leptomeningeal dissemination, or metastasis outside of the central nervous system) was significantly more common in Group 3 (46.5%) and Group 4 (29.7%) than in the WNT (17.9%) and SHH (19.1%) subgroups (7). In keeping with the above differences in metastasis prevalence, analysis of large cohorts of patients assigned to these subgroups has shown that mean survival times decline progressively in the following order: WNT>SHH>Group 4>Group 3. The fact that mRNA levels of ERAS, LHX1, and CCRK were elevated in tumor subgroups that show a high rate of metastasis and short patient survival times indicates that these genes promote aggressive growth in human medulloblastomas, as they do in experimentally induced tumors in mice. Group 3 and Group 4 medulloblastomas might originate from a completely different precursor cell population than SHH tumors.
PI3K signaling promotes spinal leptomeningeal dissemination in Shh-induced medulloblastomas
The SB transposon mutagenesis study showed that Pten, Akt2, Igf2, and Pik3r were metastasis gCIS–associated genes, thus implicating the PI3K signaling pathway in medulloblastoma metastasis (17). Among these genes, Akt2 and Pik3r met the previously described selection criteria for metastasis-inducing oncogenes. The SB insertions most likely disrupted transcription of the Pten tumor suppressor gene. Several lines of evidence support the idea that PI3K signaling cooperates with Shh signaling to stimulate medulloblastoma growth. Derepression of the PI3K pathway by loss of Pten increased tumor formation in mice carrying an oncogenic allele of Smoothened (30). We reported previously that activation of PI3K signaling by insulin-like growth factor-II increased the incidence of Shh-induced medulloblastoma formation and promoted dissemination of tumor cells to the leptomeninges of the brain (17, 19). To determine whether activating the PI3K pathway could directly cause medulloblastoma cells to seed the spinal leptomeninges, we used an RCAS retroviral vector to transfer an activated, transforming allele of Akt (Akt-Myr-Δ11-60) to Nestin+ cerebellar progenitors in Ntv-a mice and examined spinal cord sections using the methods described above. Mice injected with RCAS-Shh+RCAS-Akt developed tumors in the cerebellum in 56% of cases (P=0.16 compared with RCAS-Shh). The enhancing effect of Akt on primary brain tumor induction was less than that which we reported previously (19). This difference is likely due to the longer observation time in the current study (4 vs. 3 months), during which accumulation of secondary mutations can accelerate the growth of Shh-induced tumors. Nevertheless, the incidence of spinal leptomeningeal dissemination was fourfold higher in medulloblastomas induced by Shh+Akt compared with Shh alone (P=0.016) (Table 1). The fact that overall survival was significantly reduced in mice bearing tumors induced by Shh+Akt compared with Shh alone (P=0.0024; Fig. 4B) indicated that PI3K pathway activation promoted not only the dissemination of tumor cells to the spinal leptomeningeal space, but also more aggressive tumor growth in the brain. No association was found, however, between mRNA levels of any one of the three human AKT genes and aggressive tumor subgroup.
Discussion
Using a genetically engineered mouse model of Shh-induced medulloblastoma, we show here that ectopic expression of Eras, Lhx1, and Ccrk shifted the in vivo growth characteristics from a localized to a disseminated pattern, in which tumor cells seeded the leptomeninges of the brain and spinal cord. Expression of these genes did not increase the incidence of tumor formation in the brain, rather resulted in the acquisition of metastatic traits that enabled medulloblastoma cells to detach from the tumor mass, intravasate into the CSF, and reimplant in the leptomeningeal space. Additionally, we explored the role of PI3K signaling in medulloblastoma pathogenesis and report a previously unknown link between PI3K pathway activation by Akt and spinal leptomeningeal dissemination.
While the genes investigated in this study have previously been implicated in cancer biology, the specific mechanisms through which they contribute to metastasis remain uncertain. A growing body of research provides clues to their function. Eras, a membrane-localized, GTP-binding protein, is believed to mimic the constitutively active Ras oncoprotein, based on structural similarities. Furthermore, Eras binds the catalytic subunit of PI3K and activates the PI3K signal transduction pathway, mediating tumor cell invasiveness (31). Importantly, Eras is frequently expressed in human gastric carcinomas, and tumor immunoreactivity is associated with an increased risk of metastasis (32).
Lhx1 is a transcription factor that is crucial for normal kidney morphogenesis and a positive regulator of tumor cell motility, migration, and invasiveness (33). Evidence from renal cell carcinoma cells suggests a positive feedback mechanism through which Shh signaling increases Lhx1 levels, supporting its oncogenic role in medulloblastoma (34).
Ccrk is a serine-threonine kinase that promotes cell cycle progression in mammalian cells (35). Overexpression of Ccrk transforms human immortalized liver cells, and this process is dependent upon β-catenin signaling (36). Ccrk has oncogenic properties beyond promoting cell cycle progression, insofar as depletion of Ccrk by RNA interference fails to cause the expected cell cycle arrest (37).
The epithelial-mesenchymal transition (EMT) is a process by which carcinoma cells lose their epithelial growth characteristics to become detached and invasive, thus acquiring metastatic properties [reviewed in (38)]. A large body of literature has implicated PI3K signaling as paramount to EMT, with oncogenic growth factors, like hepatocyte growth factor and insulin-like growth factors, triggering EMT through PI3K pathway activation [reviewed in (39)].
While the contributions of an EMT-like process to medulloblastoma leptomeningeal dissemination remain speculative, the antiapoptotic effect of PI3K signaling could confer to tumor cells the survival traits necessary to ultimately colonize the spinal leptomeninges. Specifically, PI3K signaling is a key component of the protective response of cells against anoikis, a version of apoptosis triggered when cells become detached from a solid surface (40). PI3K pathway activation is emerging as an essential adaptive response in metastasizing cancer cells as they are shed from the central tumor mass [reviewed in (41)]. PI3K signaling might also promote aggressive growth of medulloblastomas by directly stimulating the Shh signaling pathway through a cross-talk mechanism similar to that reported recently in esophageal adenocarcinoma (42).
Although medulloblastomas can metastasize outside of the nervous system, their usual mode is to disseminate along CSF channels to spinal and intracranial leptomeninges. Our knowledge of leptomeningeal metastasis is rudimentary. The fact that SB transposon insertions were found in Lhx1 and Ccrk in spinal metastatic tumors, but not in the primary brain tumors, suggests that misexpression of these genes conferred a selective growth advantage to tumor cells in the spinal microenvironment. For that reason, Lhx1 and Ccrk are analogous to metastasis virulence genes in the invasion–metastasis cascade of epithelial cancers [reviewed in (43)]. Eras and the PI3K pathway genes Pten, Akt2, Igf2, and Pik3r had transposon insertions in both spine tumors and brain tumors, indicating, by analogy to metastasis initiation and progression genes in the invasion–metastasis model, possible roles in tumor cell invasiveness, detachment, and resistance to anoikis.
By quantifying SB transposon insertions in medulloblastomas and corresponding spinal metastases, Wu et al (17) showed that metastatic tumors can originate from cells that make up only a minor subclone in the primary tumor in the cerebellum. This concept has important therapeutic implications, insofar as treatments aimed at molecular targets in the primary tumor might not be effective against genetically divergent metastatic tumors. We detected no apparent difference in the percentage of transgene-expressing cells in the cerebellar tumors compared with the spinal metastases in our mouse model system, probably because we expressed the metastasis-driving transgenes concurrently with the tumor-initiating Shh gene in the cerebellum. The fact that the levels of ERAS, LHX1, and CCRK mRNA are increased in aggressive subgroups of human medulloblastomas supports the idea that some of the genetic events that drive leptomeningeal dissemination are present in the original tumors.
We used a highly stringent set of criteria for selecting testable metastasis genes. This approach was taken to design a tractable experimental plan. The fact that hundreds of gCISs were found in SB transposon–induced metastases shows that the genetic landscape of medulloblastoma dissemination is very complex. Nevertheless, our results indicate that Shh-induced medulloblastomas can start down a path of disseminated growth by addition of only a single gene. It is not known in individual patients how many different genes initiate and maintain metastasis. The answer to this question will require genomic analysis of medulloblastoma cells that have metastasized to the spinal leptomeningeal space, a project that is hindered by the fact that surgical excision of metastatic tumors is rarely indicated in patient treatment plans.
Supplementary Material
Acknowledgements
The authors thank Kristin Kraus (University of Utah) for editorial assistance and Peter K. Vogt (The Scripps Research Institute, La Jolla, CA) for the Akt-Myr-Δ11-60 expression vector.
Grant Support
This work was supported by grants from the NIH (CA108622 to D.W.F. and CA148699 to M.D.T.) and the Pediatric Brain Tumor Foundation. X.W. was supported by a fellowship from the American Brain Tumor Association in tribute to Tracy Greenwood. A.D. received the Vanier Scholarship from the Canadian Institute of Health Research.
ABBREVIATIONS
- Shh
Sonic Hedgehog
- ALV
avian leukosis virus
- RCAS
replication-competent, avian leukosis virus, splice acceptor
- PI3K
phosphatidylinositol 3–kinase
- gCIS
gene-centric common insertion site
- GNP
granule neuron precursor
- GFP
green fluorescent protein
- Eras
embryonic stem cell–expressed Ras
- Lhx1
LIM class homeobox-1
- Ccrk
cell cycle–related kinase
- EMT
epithelial–mesenchymal transition
Footnotes
Conflict of interest statement: The authors disclose no potential conflicts of interest.
References
- 1.Ellison DW, Kocak M, Dalton J, Megahed H, Lusher ME, Ryan SL, et al. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol. 2011;29:1400–1407. doi: 10.1200/JCO.2010.30.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollack IF. Multidisciplinary management of childhood brain tumors: a review of outcomes, recent advances, and challenges. J Neurosurg Pediatr. 2011;8:135–148. doi: 10.3171/2011.5.PEDS1178. [DOI] [PubMed] [Google Scholar]
- 3.Radcliffe J, Packer RJ, Atkins TE, Bunin GR, Schut L, Goldwein JW, et al. Three- and four-year cognitive outcome in children with noncortical brain tumors treated with whole-brain radiotherapy. Ann Neurol. 1992;32:551–554. doi: 10.1002/ana.410320411. [DOI] [PubMed] [Google Scholar]
- 4.Silber JH, Littman PS, Meadows AT. Stature loss following skeletal irradiation for childhood cancer. J Clin Oncol. 1990;8:304–312. doi: 10.1200/JCO.1990.8.2.304. [DOI] [PubMed] [Google Scholar]
- 5.Thompson MC, Fuller C, Hogg TL, Dalton J, Finkelstein D, Lau CC, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24:1924–1931. doi: 10.1200/JCO.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- 6.Kool M, Koster J, Bunt J, Hasselt NE, Lakeman A, van Sluis P, et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One. 2008;3:e3088. doi: 10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2010;29:1408–1414. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodrich LV, Scott MP. Hedgehog and patched in neural development and disease. Neuron. 1998;21:1243–1257. doi: 10.1016/s0896-6273(00)80645-5. [DOI] [PubMed] [Google Scholar]
- 9.Weiner HL, Bakst R, Hurlbert MS, Ruggiero J, Ahn E, Lee WS, et al. Induction of medulloblastomas in mice by sonic hedgehog, independent of Gli1. Cancer Res. 2002;62:6385–6389. [PubMed] [Google Scholar]
- 10.Rao G, Pedone CA, Coffin CM, Holland EC, Fults DW. c-Myc enhances Sonic hedgehog-induced medulloblastoma formation from nestin-expressing neural progenitors in mice. Neoplasia. 2003;5:198–204. doi: 10.1016/S1476-5586(03)80052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallahan AR, Pritchard JI, Hansen S, Benson M, Stoeck J, Hatton BA, et al. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res. 2004;64:7794–7800. doi: 10.1158/0008-5472.CAN-04-1813. [DOI] [PubMed] [Google Scholar]
- 12.Hatton BA, Villavicencio EH, Tsuchiya KD, Pritchard JI, Ditzler S, Pullar B, et al. The Smo/Smo model: hedgehog-induced medulloblastoma with 90% incidence and leptomeningeal spread. Cancer Res. 2008;68:1768–1776. doi: 10.1158/0008-5472.CAN-07-5092. [DOI] [PubMed] [Google Scholar]
- 13.Schüller U, Heine VM, Mao J, Kho AT, Dillon AK, Han YG, et al. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell. 2008;14:123–134. doi: 10.1016/j.ccr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang ZJ, Ellis T, Markant SL, Read TA, Kessler JD, Bourboulas M, et al. Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells. Cancer Cell. 2008;14:135–145. doi: 10.1016/j.ccr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben-Arie N, Bellen HJ, Armstrong DL, McCall AE, Gordadze PR, Guo Q, et al. Math 1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390:169–172. doi: 10.1038/36579. [DOI] [PubMed] [Google Scholar]
- 16.Collier LS, Largaespada DA. Hopping around the tumor genome: transposons for cancer gene discovery. Cancer Res. 2005;65:9607–9610. doi: 10.1158/0008-5472.CAN-05-3085. [DOI] [PubMed] [Google Scholar]
- 17.Wu X, Northcott PA, Dubuc A, Dupuy AJ, Shih DJ, Witt H, et al. Clonal selection drives genetic divergence of metastatic medulloblastoma. Nature. 2012;482:529–533. doi: 10.1038/nature10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holland EC, Hively WP, DePinho RA, Varmus HE. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 1998;12:3675–3685. doi: 10.1101/gad.12.23.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao G, Pedone CA, Del Valle L, Reiss K, Holland EC, Fults DW. Sonic hedgehog and insulin-like growth factor signaling synergize to induce medulloblastoma formation from nestin-expressing neural progenitors in mice. Oncogene. 2004;23:6156–6162. doi: 10.1038/sj.onc.1207818. [DOI] [PubMed] [Google Scholar]
- 20.Aoki M, Batista O, Bellacosa A, Tsichlis P, Vogt PK. The Akt kinase: molecular determinants of oncogenicity. Proc Natl Acad Sci U S A. 1998;95:14950–14955. doi: 10.1073/pnas.95.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Federspiel MJ, Bates P, Young JAT, Varmus HE, Hughes SH. A system for tissue-specific gene targeting: transgenic mice susceptible to subgroup A avian leukosis virus-based retroviral vectors. Proc Natl Acad Sci U S A. 1994;91:11241–11245. doi: 10.1073/pnas.91.23.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Northcott PA, Fernandez LA, Hagan JP, Ellison DW, Grajkowska W, Gillespie Y, et al. The miR-17/92 polycistron is up-regulated in sonic hedgehog-driven medulloblastomas and induced by N-myc in sonic hedgehog-treated cerebellar neural precursors. Cancer Res. 2009;69:3249–3255. doi: 10.1158/0008-5472.CAN-08-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fattet S, Haberler C, Legoix P, Varlet P, Lellouch-Tubiana A, Lair S, et al. Beta-catenin status in paediatric medulloblastomas: correlation of immunohistochemical expression with mutational status, genetic profiles, and clinical characteristics. J Pathol. 2009;218:86–94. doi: 10.1002/path.2514. [DOI] [PubMed] [Google Scholar]
- 24.McCall TD, Pedone CA, Fults DW. Apoptosis suppression by somatic cell transfer of Bcl-2 promotes Sonic hedgehog-dependent medulloblastoma formation in mice. Cancer Res. 2007;67:5179–5185. doi: 10.1158/0008-5472.CAN-06-4177. [DOI] [PubMed] [Google Scholar]
- 25.Browd SR, Kenney AM, Gottfried ON, Yoon JW, Walterhouse D, Pedone CA, et al. N-myc can substitute for insulin-like growth factor signaling in a mouse model of sonic hedgehog-induced medulloblastoma. Cancer Res. 2006;66:2666–2672. doi: 10.1158/0008-5472.CAN-05-2198. [DOI] [PubMed] [Google Scholar]
- 26.Binning MJ, Niazi T, Pedone CA, Lal B, Eberhart CG, Kim KJ, et al. Hepatocyte Growth Factor and Sonic Hedgehog Expression in Cerebellar Neural Progenitor Cells Costimulate Medulloblastoma Initiation and Growth. Cancer Res. 2008;68:7838–7845. doi: 10.1158/0008-5472.CAN-08-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dupuy AJ, Akagi K, Largaespada DA, Copeland NG, Jenkins NA. Mammalian mutagenesis using a highly mobile somatic Sleeping Beauty transposon system. Nature. 2005;436:221–226. doi: 10.1038/nature03691. [DOI] [PubMed] [Google Scholar]
- 28.Dupuy AJ, Rogers LM, Kim J, Nannapaneni K, Starr TK, Liu P, et al. A modified sleeping beauty transposon system that can be used to model a wide variety of human cancers in mice. Cancer Res. 2009;69:8150–8156. doi: 10.1158/0008-5472.CAN-09-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2011;123:465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castellino RC, Barwick BG, Schniederjan M, Buss MC, Becher O, Hambardzumyan D, et al. Heterozygosity for Pten promotes tumorigenesis in a mouse model of medulloblastoma. PLoS One. 2010;5:e10849. doi: 10.1371/journal.pone.0010849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi K, Mitsui K, Yamanaka S. Role of ERas in promoting tumour-like properties in mouse embryonic stem cells. Nature. 2003;423:541–545. doi: 10.1038/nature01646. [DOI] [PubMed] [Google Scholar]
- 32.Kubota E, Kataoka H, Aoyama M, Mizoshita T, Mori Y, Shimura T, et al. Role of ES cell-expressed Ras (ERas) in tumorigenicity of gastric cancer. Am J Pathol. 2010;177:955–963. doi: 10.2353/ajpath.2010.091056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dormoy V, Beraud C, Lindner V, Thomas L, Coquard C, Barthelmebs M, et al. LIM-class homeobox gene Lim1, a novel oncogene in human renal cell carcinoma. Oncogene. 2011;30:1753–1763. doi: 10.1038/onc.2010.557. [DOI] [PubMed] [Google Scholar]
- 34.Dormoy V, Danilin S, Lindner V, Thomas L, Rothhut S, Coquard C, et al. The sonic hedgehog signaling pathway is reactivated in human renal cell carcinoma and plays orchestral role in tumor growth. Mol Cancer. 2009;8:123. doi: 10.1186/1476-4598-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Wu C, Galaktionov K. p42, a novel cyclin-dependent kinase-activating kinase in mammalian cells. J Biol Chem. 2004;279:4507–4514. doi: 10.1074/jbc.M309995200. [DOI] [PubMed] [Google Scholar]
- 36.Feng H, Cheng AS, Tsang DP, Li MS, Go MY, Cheung YS, et al. Cell cycle-related kinase is a direct androgen receptor-regulated gene that drives beta-catenin/T cell factor-dependent hepatocarcinogenesis. J Clin Invest. 2006;121:3159–3175. doi: 10.1172/JCI45967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wohlbold L, Larochelle S, Liao JC, Livshits G, Singer J, Shokat KM, et al. The cyclin-dependent kinase (CDK) family member PNQALRE/CCRK supports cell proliferation but has no intrinsic CDK-activating kinase (CAK) activity. Cell Cycle. 2006;5:546–554. doi: 10.4161/cc.5.5.2541. [DOI] [PubMed] [Google Scholar]
- 38.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 39.Qiao M, Sheng S, Pardee AB. Metastasis and AKT activation. Cell Cycle. 2008;7:2991–2996. doi: 10.4161/cc.7.19.6784. [DOI] [PubMed] [Google Scholar]
- 40.Moro L, Arbini AA, Yao JL, di Sant'Agnese PA, Marra E, Greco M. Mitochondrial DNA depletion in prostate epithelial cells promotes anoikis resistance and invasion through activation of PI3K/Akt2. Cell Death Differ. 2009;16:571–583. doi: 10.1038/cdd.2008.178. [DOI] [PubMed] [Google Scholar]
- 41.Rennebeck G, Martelli M, Kyprianou N. Anoikis and survival connections in the tumor microenvironment: is there a role in prostate cancer metastasis? Cancer Res. 2005;65:11230–11235. doi: 10.1158/0008-5472.CAN-05-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Ding Q, Yen CJ, Xia W, Izzo JG, Lang JY, et al. The crosstalk of mTOR/S6K1 and Hedgehog pathways. Cancer Cell. 2012;21:374–387. doi: 10.1016/j.ccr.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen DX, Massague J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8:341–352. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.