1. Introduction
Within the framework of the stress-vulnerability model of schizophrenia (Walker & Diforio 1997; Walker et al., 2004), it has been proposed that stress contributes to the development of psychosis through effects of the hypothalamic pituitary adrenal (HPA) axis, and interaction of cortisol with dopaminergic neurotransmission and hippocampal circuitry (Corcoran et al., 2003; Thompson et al., 2004). HPA-axis abnormalities, specifically elevated cortisol levels, have been observed in first-episode drug-naïve patients with schizophrenia (Ryan et al., 2004), in the siblings of patients with a psychotic disorder (Collip et al., 2011; Habets et al., 2012), and in adolescents with schizotypal personality disorder (Weinstein et al., 1999; Walker et al., 2001; Mittal et al., 2007); it has also been associated with the later development of psychotic disorder among adolescents at increased clinical risk (Walker et al., 2010). This increased cortisol secretion is considered to reflect HPA axis hyperactivity in the context of impaired negative feedback (Walker & Diforio, 1997), and may index heightened sensitivity or impaired tolerance to stress.
In support of the premise that increased cortisol levels are related to stress sensitivity in individuals vulnerable to psychosis, there is evidence that siblings of probands with psychotic disorder experience heightened cortisol responses to daily stressors compared to controls (Collip et al., 2011), and that cortisol secretion in response to a laboratory stress challenge in clinical high risk (CHR) patients is intermediate to that of healthy controls and schizophrenia patients (Mizrahi et al., 2012). Furthermore, both daily hassles (Thompson et al., 2007) and impaired stress tolerance (Corcoran et al., 2012) have been associated with cortisol levels in CHR patients. Cortisol secretion has also been associated with positive symptoms in patients with psychotic disorder (Belvederi Murri et al., 2012), in their siblings (Collip et al., 2011), and in individuals at increased clinical risk (Walker et al., 2001; Corcoran et al., 2012), while mood symptoms have been variably associated with cortisol levels in CHR cohorts (Thompson et al., 2007; Corcoran et al., 2012).
Psychotropic medications are known to influence HPA axis function (e.g., Pariante et al., 2012). Given the growing body of research pointing to the effects of antidepressant and antipsychotic medications in modulating symptoms and conversion to psychosis in the prodrome (reviewed in Walker et al., 2009), exploration of the relationship between psychotropic treatments and cortisol secretion in CHR subjects is highly relevant.
In the current study, we examined basal cortisol secretion in both CHR patients and healthy controls. Based on the literature reviewed above, we hypothesized that CHR patients would exhibit higher basal cortisol levels than controls; we also hypothesized that cortisol levels would be significantly related to increased stress sensitivity (Thompson et al., 2007; Corcoran et al., 2012) and positive symptoms (Walker et al., 2001) when accounting for medication status and demographic variables (age, gender) relevant to cortisol secretion and symptoms (Walker et al., 2010). In an exploratory manner, we examined the association of basal salivary cortisol with other symptoms characteristic of CHR patients (negative, depressive and anxiety), as well as with transition to psychotic disorder.
2. Methods
Participants
Clinical high risk patients (ages 12 – 25) were ascertained at the Center of Prevention and Evaluation (COPE), an observational cohort study at the New York State Psychiatric Institute of Columbia University, using the Structured Interview for Prodromal Syndromes / Scale of Prodromal Symptoms (SIPS/SOPS; Miller et al., 1999) to determine CHR status. Patients were treated naturalistically with antidepressants (serotonin reuptake inhibitors, SSRIs), and more rarely, with second generation antipsychotics. Exclusion criteria included history of threshold psychotic symptoms, significant risk of harm to self or others, major medical illness, attenuated psychotic symptoms occurring solely in the context of substance intoxication or withdrawal, and IQ < 70. Data were available for eventual transition to psychotic disorder within 2 years (yes/no), which was defined using the “Presence of Psychosis” criteria of the SIPS/SOPS (Miller et al., 1999). Age-similar healthy controls were recruited from the same source communities as the CHR patients in the New York metropolitan area, using websites, fliers, brochures and educational presentations in the community. Additional exclusion criteria for healthy controls included family history of psychotic disorder, history of adoption, diagnosis of DSM-IV cluster A personality disorder, and any Axis-I DSM-IV diagnosis within the prior two years. Diagnostic status of healthy controls was determined with the Diagnostic Interview for Genetic Studies (DIGS; Nurnberger et al., 1994) for participants age 18 and older, and with the Schedule for Affective Disorders and Schizophrenia for School-age Children — Present and Lifetime version (KSADS-PL; Birmaher et al., 1997) for participants under 18. This study was approved by the Institutional Review Board of the New York State Psychiatric Institute, and all participants provided written informed consent if 18 or older, or assent with written parental consent if 17 or younger.
Measures
Salivary Cortisol
Salivary cortisol secretion is a reliable indicator of free cortisol in plasma, and salivary cortisol sampling is a widely validated method of indexing HPA axis function (Kelly et al., 2008). Salivary cortisol levels have been correlated with serum and 24-hour urine cortisol levels in children and adults (Woodside et al., 1991; Putignano et al., 2001). Cortisol secretion follows a daily circadian rhythm: the lowest levels generally occur around midnight and levels then typically begin to rise before waking (Wust et al., 2000), with a daily peak between 5:00 am and 8:00 am. This peak is then followed by a rapid decline for the next few hours, and then a gradual decline over the remainder of the day (Shirtcliff et al., 2011). As repeated sampling of salivary cortisol secretion over the course of a full cycle was not tenable for many CHR patients, we obtained a single salivary cortisol sample. However, due to the diurnal pattern of cortisol levels, we standardized the time of day at which the sample was collected for all participants.
Participants arrived at the clinic at approximately 11 a.m. After sitting quietly for 30 minutes, participants provided a saliva sample for the salivary cortisol measure. Samples were collected using Sarstedt (Germany) polyester swabs without preparation. Participants were instructed to place the swab in their mouth, between their lower lip and gum, and to keep it there until it was wet, a process supervised by research staff. When deemed saturated, the swab was placed in a Salivette plastic vial and capped and labelled. Within hours, the samples were stored at −25 degrees Celsius at the New York State Psychiatric Institute. Samples were then transported to the Nathan Kline Institute, where they were weighed, centrifuged and analysed by the Analytical Psychopharmacology Laboratory under the supervision of Thomas A Cooper. This methodology is similar to that employed in a prior study in another similarly-ascertained CHR cohort (Corcoran et al., 2012).
Symptoms
The Structured Interview for Prodromal Syndromes/Scale of Prodromal Symptoms (SIPS/SOPS; Miller et al., 1999) was used not only for initial case identification and determination of eventual transition to threshold psychosis but also to assess attenuated positive, negative and disorganization symptoms, in addition to “impaired stress tolerance.” Hamilton Rating Scales were used to assess depressive (Hamilton, 1960) and anxiety (Hamilton, 1976) symptoms separately. Details of current use of psychotropic medication (serotonin reuptake inhibitors and/or atypical antipsychotics) were obtained from participants. No patients reported taking any other class of psychotropic medication. For the purpose of analysis, psychotropic medication status was categorized as yes/no.
Statistical Analyses
Data distributions were examined for outliers and deviation from normality. Two outliers were excluded from the analysis (cortisol measures > 3 standard deviations from the mean). The main dependent variables (cortisol and symptom measures) survived normality testing and thus were analysed employing parametric methods. Given evidence of the influence of psychotropic medication on the HPA axis (Pariante et al., 2004; Zhang et al., 2005), we compared the total CHR patient group, as well as the medication-free (i.e. not on antidepressants or antipsychotics) and medicated (i.e. taking an antidepressant and/or antipsychotic) CHR subgroups separately, to the healthy controls. As there are normative developmental increases (Walker et al., 2001) and gender differences (Walder et al., 2012) in cortisol secretion, age and gender were included as covariates in statistical analyses.
Analyses of covariance (ANCOVA), with age and gender added as covariates, were used to evaluate differences in cortisol levels and symptom measures between CHR patients and healthy controls, with further analyses stratifying CHR patients by medication status. Partial correlations were conducted for cortisol measures and clinical symptoms within the CHR group, controlling for age and medication status. Exploratory analyses were conducted to determine associations of cortisol measures with transition to psychosis within two years.
Alpha was set at .05 for hypothesized differences between CHR subjects and controls, and for hypothesized associations of salivary cortisol secretion with impaired stress tolerance and positive symptoms. All other analyses have an alpha of .005, adjusting for multiple (i.e. 11) comparisons (group differences for six symptoms, correlations with cortisol for four symptoms, and comparison of converters vs. non-converters).
3. Results
Demographic and clinical characteristics
CHR patients and controls did not differ significantly in age or ethnicity (dichotomized to “Caucasian” and “non-Caucasian”; see Table 1 for details). The patient group, however, had a significantly larger proportion of males than did the control group (p=.002; Table 1). Nine of the CHR patients were taking antidepressants (SSRIs), of which five were also taking a second generation antipsychotic, and 3 patients were taking a second-generation antipsychotic only; thus 12 (36%) were in the medicated subgroup, whereas 21 (64%) were medication-free. There were no significant differences in age (t32=−.04, p=.96), gender (χ21=.013, p=.91), or ethnicity (χ21=3.09, p=.08) between CHR patients who were taking medications and those who were not. All of the healthy controls were medication-free.
Table 1.
Demographics, salivary cortisol and symptoms in clinical high risk patients and healthy controls.
| Total CHR (n=33) | Medication Free CHRa (n=21) | Healthy Controls (n=13) | |
|---|---|---|---|
|
|
|||
| Demographics | |||
| Gender (% males) | 91%** | 90.5%** | 46% |
| Race/Ethnicity (% Caucasian) | 56%b | 40% | 85% |
| Age (M(SD)) | 18.6 (3.1) | 18.6 (2.9) | 20.3 (4.2) |
| Endocrine measure | |||
| Baseline salivary cortisol (M(SD)) | 2.05 (0.93)c | 2.31 (.99)* | 1.44 (0.67) |
| Scale of Prodromal Symptoms (M(SD)) | |||
| Positive symptoms*** | 11.5 (4.9) | 12.2 (5.1) | 0.7 (.85) |
| Negative symptoms*** | 11.3 (5.2) | 12.5 (5.2) | 1.9 (1.9) |
| Disorganization symptoms*** | 6.0 (2.9) | 6.4 (3.0) | 0.8 (1.2) |
| Impaired stress tolerance** | 2.0 (1.8) | 2.2 (1.9) | 0.1 (0.3) |
| Hamilton Scales (M(SD)) | |||
| Anxiety* | 8.5 (6.3) | 7.9 (5.5) | 1.9 (1.9) |
| Depressive symptoms** | 9.5 (5.8) | 9.9 (6.1) | 1.5 (1.7) |
Note. CHR=Clinical high risk; M=mean; SD=Standard deviation. Baseline salivary cortisol sampled at 11:30am, measured in ng/mL.
The subgroup of CHR patients who were not receiving psychotropic medications.
Among the total CHR group, 8.7% were African-American, 26.1% Hispanic, and 8.7% were more than one race. Among medication-free CHR patients, 6.7% were African-American, 40% were Hispanic and 13.3% were more than one race. All non-Caucasian healthy controls were African-American.
Compared to controls, p=.07.
Asterisks correspond to p values related to comparisons between patient groups and healthy controls; age and gender were added as covariates for the between-group comparisons of cortisol and symptom measures.
p<0.05;
p<0.01;
p<0.001.
Salivary cortisol secretion
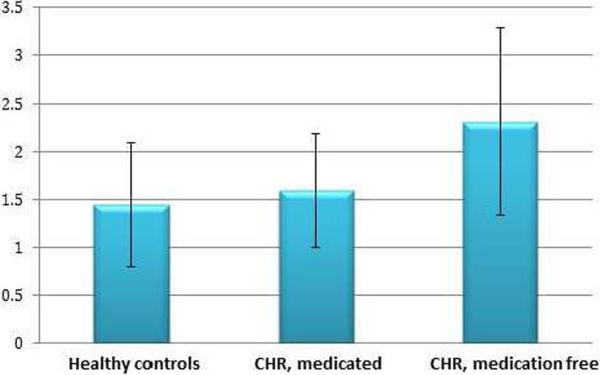
Salivary cortisol secretion (ng/mL) was not significantly related to demographic characteristics (age, gender, or ethnicity) in the total sample or in the CHR or healthy control groups separately (data not shown). Compared to healthy controls (n=13), the entire group of CHR patients (n=33) exhibited a trend for increased basal salivary cortisol secretion (F1,44=3.34, p=.07;Table 1). However, the subgroup of CHR patients who were medication-free (n=21) had significantly higher cortisol secretion than both the CHR subgroup taking psychotropic medication (mean (SD): medication-free CHR= 2.31(.99); medicated CHR = 1.59 (.62); F1,31= 1.81, p = 0.04) and healthy controls (healthy controls = 1.44 (.67); F1,32= 5.41, p = 0.03); see Table 1 and Figure 1.
Figure 1. Mean cortisol levels displayed by group.
Figure representing mean cortisol by group, including bars representing standard deviation. The “y” axis represents cortisol levels measured in ng/mL. Baseline cortisol was sampled at 11.30 am (see text). Healthy controls, n=13; CHR, medicated: clinical high risk subjects taking SSRIs and/or atypical antispsychotics, n=12; CHR, medication-free: n=21.
Salivary cortisol secretion and symptoms
As expected, CHR patients had significantly greater symptom severity than normal controls in all symptom domains assessed by the SIPS/SOPS (Table 1; all p values≤0.004), as well as more severe depressive (p=.001) and anxiety symptoms (p=0.01); however, the difference in anxiety symptoms was not considered significant after correction for multiple comparisons. Among patients, symptoms were not significantly related to medication status or demographic characteristics (gender, ethnicity or age), with the exception that negative symptoms were negatively related to age (r=−.30, p=.04). In the 23 CHR patients for whom symptoms and salivary cortisol were measured within the same 30 days (mean (SD)=12(8) days), salivary cortisol secretion was not significantly related to any symptoms; however, there was a trend association between cortisol and impaired stress tolerance (r=.53, p=.06), uncorrected for multiple comparisons (Table 2). Among the 33 CHR patients, 9 (27.3%) made the transition to psychosis during the two-year follow-up period. This converter subgroup had relatively less basal salivary cortisol secretion (mean (SD) = 1.74 (.66) ng/ml) than those CHR patients who did not develop psychosis (2.16 (1.0) ng/ml); this difference did not reach statistical significance in this small cohort (t31 = 1.15, p =.26). Forty-four percent of patients who later transitioned to psychosis were taking psychotropic medications at baseline (4 out of 9), in contrast to 33.3% of the non-converter group.
Table 2.
Partial correlation coefficients for salivary cortisol and symptoms in clinical high risk patients, controlling for age and medication status.
| Baseline Salivary Cortisol (ng/ml) | ||
|---|---|---|
| r | p-value | |
|
|
||
| Scale of Prodromal Symptoms | ||
| Positive Symptoms | −.22 | .36 |
| Negative Symptoms | −.06 | .78 |
| Disorganization Symptoms | −.44 | .19 |
| Impaired Stress Tolerance | .53 | .06 |
| Hamilton | ||
| Anxiety Symptoms | −.09 | .62 |
| Depressive Symptoms | −.08 | .85 |
Note. Hypothesized associations of salivary cortisol with positive symptoms and impaired stress tolerance have an alpha of .05. For the other correlations, we used an alpha of .005, correcting for multiple comparisons.
4. Discussion
Our study extends work by Walker and colleagues (Mittal et al., 2007; Walker et al., 2001; Weinstein et al., 1999), showing that adolescents at heightened clinical risk for psychosis have elevated baseline salivary cortisol secretion as compared with healthy controls; this finding was especially clear among CHR patients who were medication-free. These results support the proposal that excess activation of the HPA axis and/or neuroendocrine abnormalities characterize the psychosis risk state for at least a subset of patients. Additionally, cortisol secretion was related to impaired stress tolerance at a trend level (.06), which is consistent with findings obtained with a separate but similarly-ascertained CHR cohort who were assessed for basal cortisol secretion using a similar design (Corcoran et al., 2012). However, basal salivary cortisol secretion was not significantly related to other symptoms, in contrast to other studies that have reported associations with positive (Walker et al., 2001) and mood symptoms (Thompson et al., 2007).
Of interest is our finding that CHR patients who were medication-free had significantly greater basal salivary cortisol secretion than both healthy controls and CHR patients who reported taking psychotropic medication. Antidepressant and antipsychotic medications have been reported to dampen HPA axis activity through effects on central nervous system glucocorticoid receptors (Walker et al., 2008; Pariante et al., 2012). Both antipsychotic (Mondelli et al., 2010; Zhang et al., 2005; Scheepers et al., 2001) and antidepressant (Pariante et al., 2004) treatment can lead to decreased baseline and stress-reactive cortisol levels. Research to date on pharmacological treatment (both second-generation antipsychotics and SSRIs) in CHR patients provides some evidence for treatment-associated reductions in symptoms and in transition to psychosis (reviewed in Walker et al., 2009). It is plausible that the efficacy of these medications is at least in part mediated by “normalizing” HPA axis functioning. The potential efficacy of cognitive behavioural therapy in CHR patients might also be mediated by effects on stress sensitivity and the HPA axis. It may be useful then to include simple salivary assay of cortisol secretion as a biomarker in clinical trials in CHR patients, for both pharmacological and non-pharmacological treatment strategies.
In exploratory analysis, we observed non-significantly lower baseline cortisol levels in patients who later transitioned to psychosis, in contrast to the findings of Walker et al. (2010). This may be related to the fact that a higher proportion of patients in the converter group were taking psychotropic medications, and thus suggests that characterization of HPA axis function in psychosis and the high risk state may benefit from further examination in medication-free samples. However, these results must be interpreted tentatively given the reduced power of this analysis.
Limitations of the study include its small sample size (with the risk for Type 2 error), the gender and ethnicity imbalance between CHR patients and healthy controls (Kudielka & Kirschbaum 2005; Fuller-Rowell et al., 2012), no documentation of phase of menstrual cycle for females, which may be relevant to HPA axis activity (Walder et al., 2012), a predominantly male sample of CHR patients (limiting generalizability) and shortcomings relative to the methodology of cortisol sampling (single measure), detailed in Corcoran et al., 2012. Furthermore, although we endeavoured to minimize the effect of contextual stress (e.g., that associated with participating in an assessment session) by adding a 30 minute “resting period” prior to obtaining the salivary sample, it is possible that the effects of novelty or anticipation (Weinstein et al., 1999) influenced the level of cortisol measured in some patients. An experimental study with repeated cortisol sampling would be needed in order to fully tease apart stress-related from basal cortisol secretion.
Despite its limitations, this study contributes to the growing literature on HPA axis activity in clinical high risk patients, adding further evidence that baseline cortisol secretion is increased during the at-risk state in at least a subset of patients, and that it is related to stress sensitivity; our findings also suggest that the use of antipsychotics and antidepressants may have a “normalizing” effect on HPA axis activity in high risk states.
Acknowledgement
None.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest All authors declare that they have no conflicts of interest
References
- 1.Belvederi Murri M, Pariante CM, Dazzan P, Hepgul N, Papadopoulos AS, Zunszain P, Di Forti M, Murray RM, Mondelli V. Hypothalamic-pituitary-adrenal axis and clinical symptoms in first-episode psychosis. Psychoneuroendocrinology. 2012;37(5):629–44. doi: 10.1016/j.psyneuen.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (k-sads-pl): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Collip D, Nicolson NA, Lardinois M, Lataster T, van Os J, Myin-Germeys I, G.R.O.U.P. Daily cortisol, stress reactivity and psychotic experiences in individuals at above average genetic risk for psychosis. Psychol Med. 2011;41(11):2305–15. doi: 10.1017/S0033291711000602. [DOI] [PubMed] [Google Scholar]
- 4.Corcoran CM, Smith C, McLaughlin D, Auther AM, Malaspina D, Cornblatt BA. HPA axis function and symptoms in adolescents at clinical high risk for schizophrenia. Schizophrenia Research. 2012 Mar;135(1–3):170–4. doi: 10.1016/j.schres.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corcoran C, Walker E, Huot R, Mittal V, Tessner K, Kestler L, Malaspina D. The stress cascade and schizophrenia: etiology and onset. Schizophrenia Bulletin. 2003;29(4):671–692. doi: 10.1093/oxfordjournals.schbul.a007038. [DOI] [PubMed] [Google Scholar]
- 6.Fuller-Rowell TE, Doan SN, Eccles JS. Differential effects of perceived discrimination on the diurnal cortisol rhythm of African Americans and Whites. Psychoneuroendocrinology. 2012;37(1):107–18. doi: 10.1016/j.psyneuen.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habets P, Collip D, Myin-Germeys I, Gronenschild E, van Bronswijk S, Hofman P, Lataster T, Lardinois M, Nicolson NA, van Os J, Marcelis M. Pituitary volume, stress reactivity and genetic risk for psychotic disorder. Psychol Med. 2012 Jul;42(7):1523–33. doi: 10.1017/S0033291711002728. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamilton M. Hamilton Anxiety Scale. In: W G, editor. ECDEU Assessment Manual for Psychopharmacology. NIMH; Rockville, MD: 1976. [Google Scholar]
- 10.Kelly SJ, Young R, Sweeting H, Fischer JE, West P. Levels and confounders of morning cortisol collected from adolescents in a naturalistic (school) setting. Psychoneuroendocrinology. 2008;33(9):1257–68. doi: 10.1016/j.psyneuen.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69(1):113–32. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Hoffman R, Davidson L. Symptom assessment in schizophrenic prodromal states. The Psychiatric Quarterly. 1999;70(4):273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- 13.Mittal VA, Dhruv S, Tessner KD, Walder DJ, Walker EF. The relations among putative biorisk markers in schizotypal adolescents: minor physical anomalies, movement abnormalities, and salivary cortisol. Biological Psychiatry. 2007;61:1179–86. doi: 10.1016/j.biopsych.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 14.Mizrahi R, Addington J, Rusjan PM, Suridjan I, Ng A, Boileau I, Pruessner JC, Remington G, Houle S, Wilson AA. Increased stress-induced dopamine release in psychosis. Biol Psychiatry. 2012;71(6):561–7. doi: 10.1016/j.biopsych.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Mondelli V, Dazzan P, Hepgul N, Di Forti M, Aas M, D'Albenzio A, Di Nicola M, Fisher H, Handley R, Marques TR, Morgan C, Navari S, Taylor H, Papadopoulos A, Aitchison KJ, Murray RM, Pariante CM. Abnormal cortisol levels during the day and cortisol awakening response in first-episode psychosis: the role of stress and of antipsychotic treatment. Schizophr Res. 2010;116(2–3):234–42. doi: 10.1016/j.schres.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic-studies — rationale, unique features, and training. Archives of General Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- 17.Pariante CM, Thomas SA, Lovestone S, Makoff A, Kerwin RW. Do antidepressants regulate how cortisol affects the brain? Psychoneuroendocrinology. 2004;29(4):423–47. doi: 10.1016/j.psyneuen.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Pariante CM, Alhaj HA, Arulnathan VE, Gallagher P, Hanson A, Massey E, McAllister-Williams RH. Central glucocorticoid receptor-mediated effects of the antidepressant, citalopram, in humans: A study using EEG and cognitive testing. Psychoneuroendocrinology. 2012;37(5):618–28. doi: 10.1016/j.psyneuen.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Putignano P, Dubini A, Toja P, Invitti C, Bonfanti S, Redaelli G, Zappulli D, Cavagnini F. Salivary cortisol measurement in normal-weight, obese and anorexic women: comparison with plasma cortisol. Eur J Endocrinol. 2001;145(2):165–71. doi: 10.1530/eje.0.1450165. [DOI] [PubMed] [Google Scholar]
- 20.Ryan MC, Sharifi N, Condren R, Thakore JH. Evidence of basal pituitary-adrenal overactivity in first episode, drug naïve patients with schizophrenia. Psychoneuroendocrinology. 2004;29(8):1065–70. doi: 10.1016/j.psyneuen.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Scheepers FE, Gespen de Wied CC, Kahn RS. The effect of olanzapine treatment on mchlorophenylpiperazine-induced hormone release in schizophrenia. Journal of Clinical Psychopharmacology. 2001;21(6):575–82. doi: 10.1097/00004714-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Dev Psychobiol. 2011 Sep 27; doi: 10.1002/dev.20607. doi: 10.1002/dev.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson KN, Phillips LJ, Komesaroff P, Yuen HP, Wood SJ, Pantelis C, Velakoulis D, Young AR, McGorry PD. Stress and the HPA-axis functioning in young people at ultra high risk for psychosis. Journal of Psychiatric Research. 2007;41:561–569. doi: 10.1016/j.jpsychires.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Thompson JL, Pogue-Geile MF, Grace AA. Developmental pathology, dopamine, and stress: a model for the age of onset of schizophrenia symptoms. Schizophrenia Bulletin. 2004;30(4):875–900. doi: 10.1093/oxfordjournals.schbul.a007139. [DOI] [PubMed] [Google Scholar]
- 25.Walder DJ, Statucka M, Daly MP, Axen K, Haber M. Biological sex and menstrual cycle phase modulation of cortisol levels and psychiatric symptoms in a non-clinical sample of young adults. Psychiatry Res. 2012 Feb 24; doi: 10.1016/j.psychres.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Walker EF, Brennan PA, Esterberg M, Brasfield J, Pearce B, Compton MT. Longitudinal changes in cortisol secretion and conversion to psychosis in at-risk youth. Journal of Abnormal Psychology. 2010;119(2):401–8. doi: 10.1037/a0018399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker EF, Cornblatt BA, Addington J, Cadenhead KS, Cannon TD, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Woods SW, Heinssen R. The relation of antipsychotic and antidepressant medication with baseline symptoms and symptom progression: a naturalistic study of the North American Prodrome Longitudinal Sample. Schizophr Res. 2009;115(1):50–7. doi: 10.1016/j.schres.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annual Review of Clinical Psychology. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- 29.Walker EF, Walder DJ, Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Developmental Psychopathology. 2001;13(3):721–32. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- 30.Walker EF, Bollini A, Hochman KM. Schizophrenia: etiology and course. Annual Review of Psychology. 2004;55:401–30. doi: 10.1146/annurev.psych.55.090902.141950. [DOI] [PubMed] [Google Scholar]
- 31.Walker EF, Diforio D. Schizophrenia: A Neural Diathesis-Stress Model. Psychological Review. 1997;104(4):667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- 32.Weinstein DD, Diforio D, Schiffman J, Walker E, Bonsall R. Minor physical anomalies, dermatoglyphic asymmetries and cortisol levels in adolescents with schizotypal personality disorder. American Journal of Psychiatry. 1999;156:617–623. doi: 10.1176/ajp.156.4.617. [DOI] [PubMed] [Google Scholar]
- 33.Woodside DB, Winter K, Fisman S. Salivary cortisol in children: correlations with serum values and effect of psychotropic drug administration. Can J Psychiatry. 1991;36(10):746–748. doi: 10.1177/070674379103601011. [DOI] [PubMed] [Google Scholar]
- 34.Wüst S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response - normal values and confounds. Noise Health. 2000;2(7):79–88. [PubMed] [Google Scholar]
- 35.Zhang XY, Zhou DF, Cao LY, Wu GY, Shen YC. Neuropsychopharmacology. 2005;30(8):1532–8. doi: 10.1038/sj.npp.1300756. [DOI] [PubMed] [Google Scholar]