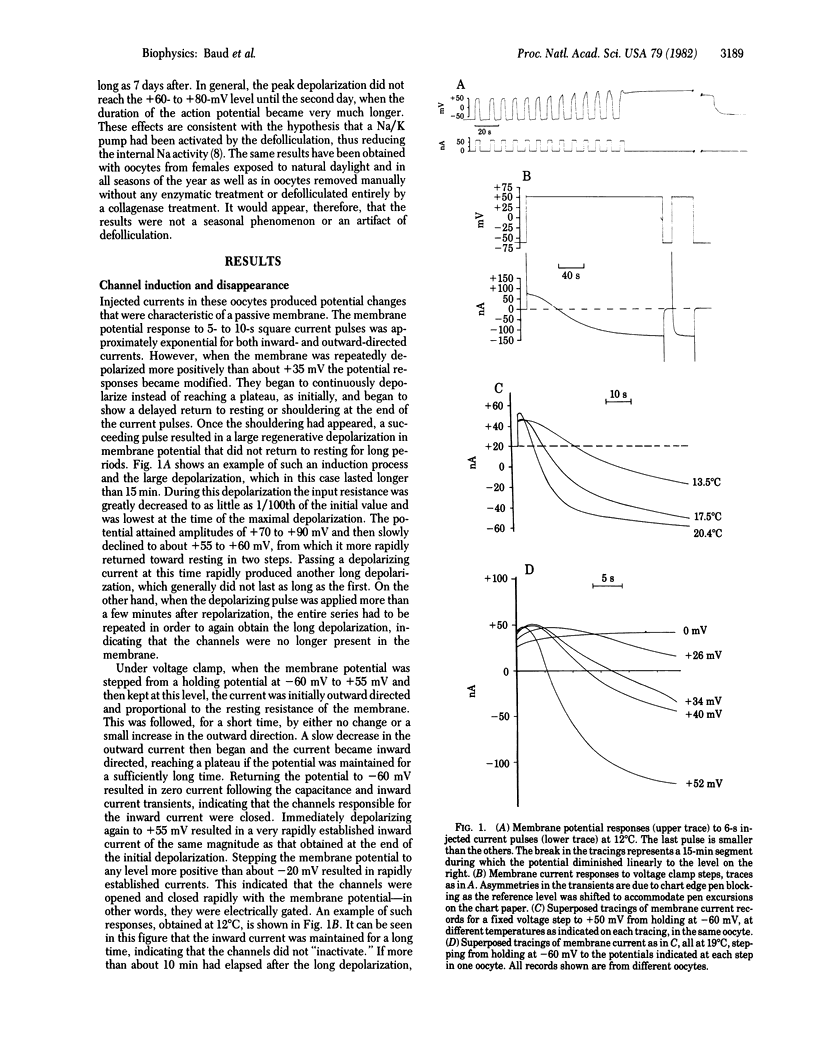
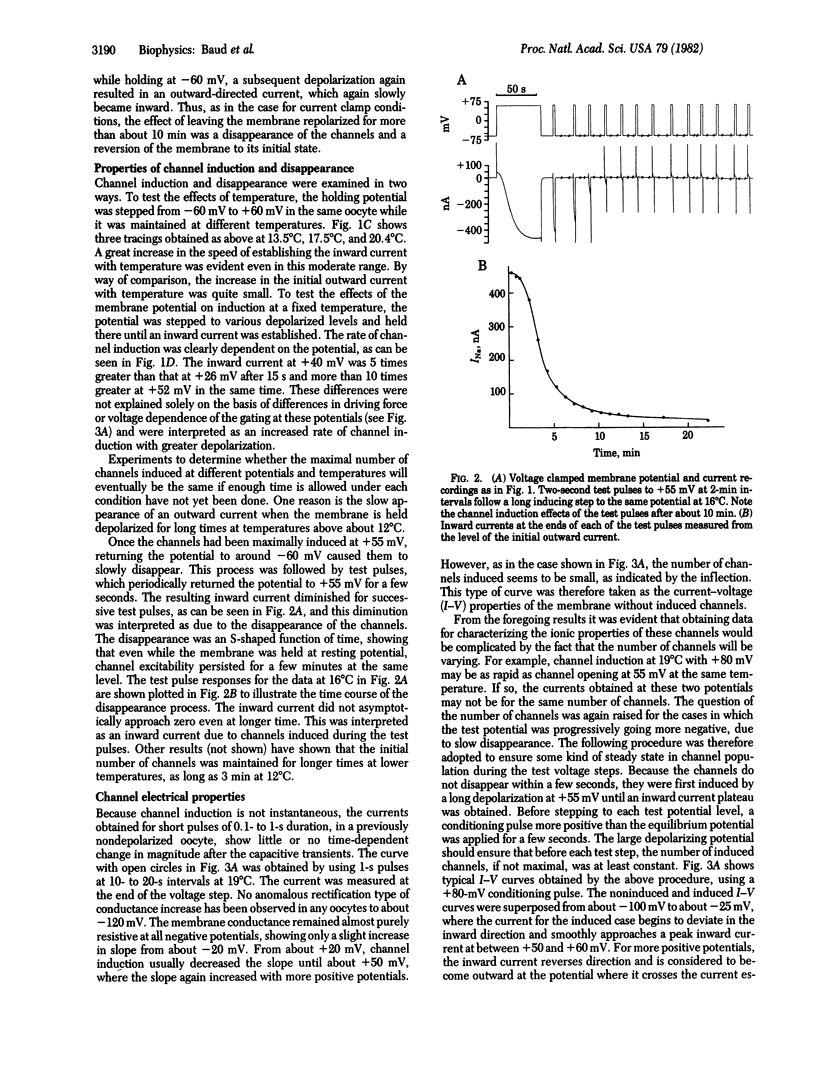
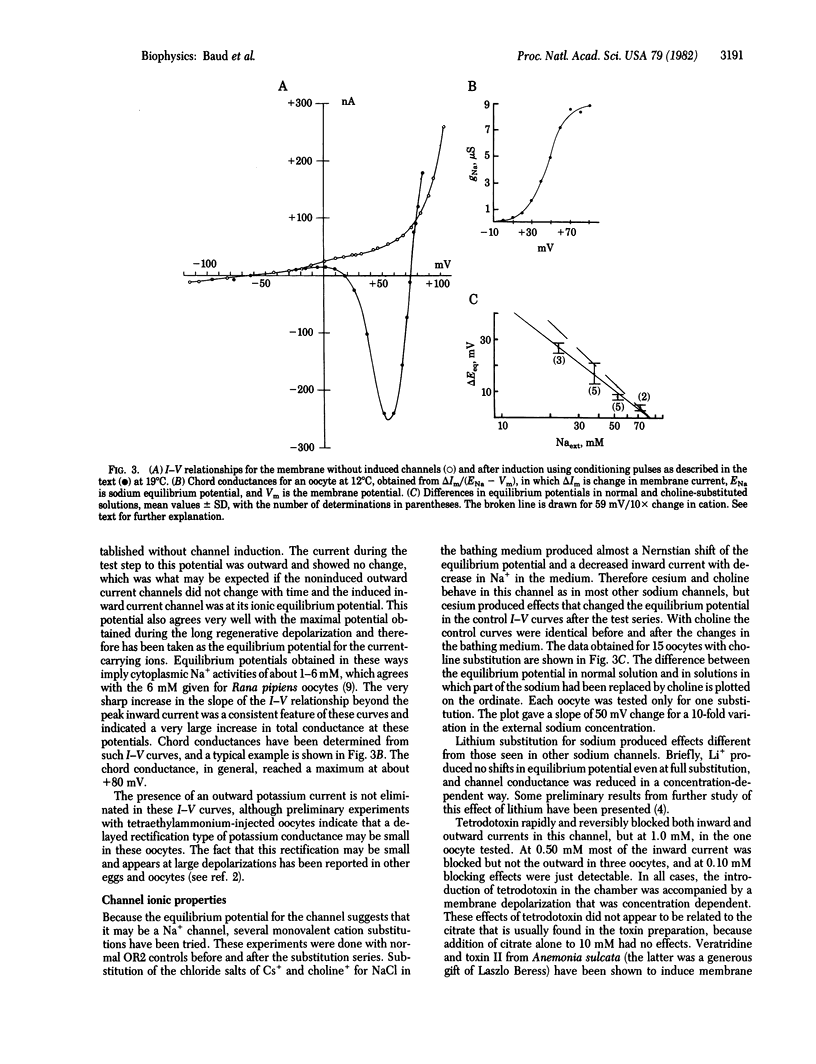
Abstract
An electrically gated Na+ channel can be made to appear in the membrane of the Xenopus laevis oocyte by simple depolarization. This membrane normally responds passively to imposed transmembrane currents with resting potentials around -60 mV, but when it is held depolarized to more than about +30 mV it becomes possible to obtain long-lasting regenerative depolarizations up to +80 mV; these depolarizations can last as long as 20 min. This potential is due to an "induction" of a Na+-dependent channel that is electrically gated open and closed. Its threshold for opening is about -20 mV and it is selective for Na+ over Cs+ and choline+ but is blocked by relatively small quantities of Li+. When a long voltage clamp step to a positive potential under ENa (+70 to +90 mV) is applied, an inward current is observed for many minutes, implying that this channel does not have an inactivation mechanism. The inward Na+ current is blocked by 0.50 mM tetrodotoxin. When the membrane is held at or near resting potential, the excitability will disappear with time, but it can be made to reappear by again depolarizing the membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cross N. L., Elinson R. P. A fast block to polyspermy in frogs mediated by changes in the membrane potential. Dev Biol. 1980 Mar;75(1):187–198. doi: 10.1016/0012-1606(80)90154-2. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Jaffe L. A. Electrical properties of egg cell membranes. Annu Rev Biophys Bioeng. 1979;8:385–416. doi: 10.1146/annurev.bb.08.060179.002125. [DOI] [PubMed] [Google Scholar]
- Kado R. T., Marcher K., Ozon R. Mise en évidence d'une dépolarisation de longue durée dans l'ovocyte de Xenopus laevis. C R Seances Acad Sci D. 1979 Apr 23;288(15):1187–1189. [PubMed] [Google Scholar]
- MAENO T. Electrical characteristics and activation potential of Bufo eggs. J Gen Physiol. 1959 Sep;43:139–157. doi: 10.1085/jgp.43.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H., Yoshii M. Surface potential reflected in both gating and permeation mechanisms of sodium and calcium channels of the tunicate egg cell membrane. J Physiol. 1977 May;267(2):429–463. doi: 10.1113/jphysiol.1977.sp011821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. G., Century T. J., Civan M. M. Activity coefficients of intracellular Na+ and K+ during development of frog oocytes. J Membr Biol. 1978 Apr 20;40(1):25–38. doi: 10.1007/BF01909737. [DOI] [PubMed] [Google Scholar]
- Romey G., Jacques Y., Schweitz H., Fosset M., Lazdunski M. The sodium channel in non-impulsive cells. Interaction with specific neurotoxins. Biochim Biophys Acta. 1979 Sep 21;556(2):344–353. doi: 10.1016/0005-2736(79)90053-1. [DOI] [PubMed] [Google Scholar]
- Schlichter L. C., Elinson R. P. Electrical responses of immature and mature Rana pipiens oocytes to sperm and other activating stimuli. Dev Biol. 1981 Apr 15;83(1):33–41. doi: 10.1016/s0012-1606(81)80005-x. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Dumont J. N., Sega M. W. Protein incorporation by isolated amphibian oocytes. 3. Optimum incubation conditions. J Exp Zool. 1973 Jun;184(3):321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Steinhardt R. A. Maturation of Xenopus oocytes. II. Observations on membrane potential. Dev Biol. 1977 Jun;57(2):305–316. doi: 10.1016/0012-1606(77)90217-2. [DOI] [PubMed] [Google Scholar]


