Abstract
Neonatal seizures are common, often require electroencephalographic (EEG) monitoring for diagnosis and management, may be associated with worse neurodevelopmental outcome, and can often be treated with existing anticonvulsants. A neonatal electrographic seizure is defined as a sudden, repetitive, evolving and stereotyped event of abnormal electrographic pattern with amplitude of at least two microvolts and a minimum duration of ten seconds. The diagnosis of neonatal seizures relies heavily on the neurophysiologist’s interpretation of EEG. Consideration of specific criteria for the definition of a neonatal seizure, including seizure duration, location, morphology, evolution, semiology, and overall seizure burden, have utility for both the clinician and researcher. We review the importance of EEG in the diagnosis and management of neonatal seizures, the electrographic characteristics of neonatal seizures, the impact of neonatal seizures on outcome, and tools to aid in the identification of neonatal seizures.
Keywords: seizures, neonate, electroencephalography, status epilepticus
Why is the electroencephalogram critical in identifying and managing neonatal seizures?
Neonatal critical care is increasingly focused on neurologic monitoring and neuroprotection.(Bonifacio, Glass, Peloquin et al. 2011) Seizures are one of the most common neurologic problems managed by neonatal neurocritical care services,(Glass, Bonifacio, Peloquin et al. 2010) occurring in 1–5 per 1000 live births Neonatal seizures are most often symptomatic of acute neurologic injury, and the most common underlying etiologies are hypoxic-ischemic encephalopathy, infections, cerebral dysgenesis, and stroke.(Scher, Aso, Beggarly et al. 1993; Lanska and Lanska 1996; Ronen, Penney and Andrews 1999; McBride, Laroia and Guillet 2000; Tekgul, Gauvreau, Soul et al. 2006; Ronen, Buckley, Penney et al. 2007) Less common but important etiologies include inborn errors of metabolism(Ficicioglu and Bearden 2011) and neonatal epilepsy syndromes such as benign familial neonatal epilepsy, benign neonatal seizures, early myoclonic epilepsy, and early infantile epileptic encephalopathy (Ohtahara syndrome).(Yamamoto, Okumura and Fukuda 2010) Thus, a high suspicion for neonatal seizures prompts discovery, management and rapid and thorough etiologic evaluation.
Diagnosing neonatal seizures can be quite challenging for two main reasons. First, determining whether abnormal movements are the clinical manifestations of electrographic seizures is difficult. Neonatal seizure descriptors involve determining whether there is a clonic, tonic, or myoclonic element,(Volpe 1973; Mizrahi and Kellaway 1987; Mizrahi 1989; Lombroso 1996; Sheth, Hobbs and Mullett 1999; Ronen, Buckley et al. 2007) whether automatisms occur,(Tharp 2002) and whether subtle signs such as apnea and autonomic signs are present.(Okumura, Hayakawa, Kato et al. 2008) The most recent International League Against Epilepsy classification report suggests classifying neonatal seizures according to the same descriptors as other seizures, rather than as a separate entity.(Berg, Berkovic, Brodie et al. 2010) However, despite great efforts to carefully describe the appearance of neonatal seizures, inter-rater agreement in neonatal seizure identification is suboptimal. A recent study presented twenty cases including clinical data and video clips of abnormal neonatal movements to 137 observers, including 91 physicians from seven neonatal intensive care units. Observers classified the movements as seizure or non-seizure. The average number of correctly classified events was only 50%, as compared to the gold standard of electroencephalographic classification. Inter-observer agreement was poor for both physicians and other healthcare professionals.(Malone, Ryan, Fitzgerald et al. 2009) Thus, to avoid over-treating non-epileptic movements or under-treating true seizures, bedside caregivers often rely on continuous EEG monitoring to determine which signs are, or are not, the clinical manifestations of electroencephalographic seizures. When accurately identified, the clinical semiology does have prognostic value. In one study of preterm and term neonates with seizures, pure clonic seizures without facial involvement in term infants suggested favorable outcome while generalized myoclonic seizures in preterm infants were associated with mortality.(Ronen, Buckley et al. 2007)
Second, about 80–90% of electrographic seizures do not have any associated clinical correlate (Clancy, Legido and Lewis 1988; Bye and Flanagan 1995; McBride, Laroia et al. 2000; Scher, Alvin, Gaus et al. 2003; Clancy 2006; van Rooij, de Vries, Handryastuti et al. 2007; Murray, Boylan, Ali et al. 2008; Lawrence, Mathur, Nguyen The Tich et al. 2009) and would not be identified without continuous EEG. These are referred to as non-convulsive or electrographic-only seizures. Further complicating the matter, anticonvulsant administration may terminate clinically evident seizures while electrographic-only seizures persist.(Connell, Oozeer, de Vries et al. 1989; Scher, Alvin et al. 2003) This is referred to as electroclinical uncoupling or dissociation. For these reasons, neonatal seizure identification and management, especially after anticonvulsants have been administered, is often highly dependent on continuous EEG monitoring. Summary statements focused on clinical care(Silverstein, Jensen, Inder et al. 2008) and neonatal seizure research(Clancy 2006) have both emphasized the importance of continuous EEG monitoring. The recent American Clinical Neurophysiology Society's Guideline on Continuous Electroencephalography Monitoring in Neonates states that “conventional video-EEG monitoring is the gold standard for neonatal seizure detection and quantification and should be used whenever available for seizure detection and differential diagnosis of abnormal appearing, paroxysmal clinical events. It is the ideal tool to measure the exact number and duration of seizures, their site(s) of onset and spatial patterns of migration.”(Shellhaas, Chang, Tsuchida et al.)
What are the electroencephalographic characteristics of neonatal seizures?
A neonatal seizure is often defined as a sudden, repetitive, evolving and stereotyped episode of abnormal electrographic activity with amplitude of at least 2µV (peak to peak) and a minimum duration of ten seconds.(Clancy and Legido 1987; Shellhaas and Clancy 2007; Shah, Boylan and Rennie 2010) Although there is no universally accepted standard definition,(Shewmon 1990) consideration of each of these components can be useful when faced with unclear electrographic patterns. Further, although the clinical team often focuses on seizures in a binary “present” or “absent” manner, from a neurophysiologist’s perspective, identifying seizures as “present” is only the first step in reporting the information contained in the EEG. Description of the seizure’s duration, location, morphology, evolution, and semiology in addition to the record’s overall seizure burden has management implications and facilitates comparison of findings over time. Also, detailed descriptions may help establish whether there are clinically meaningful interval changes. Reductions in seizure number, duration, or spread may indicate anticonvulsants in use are effective.
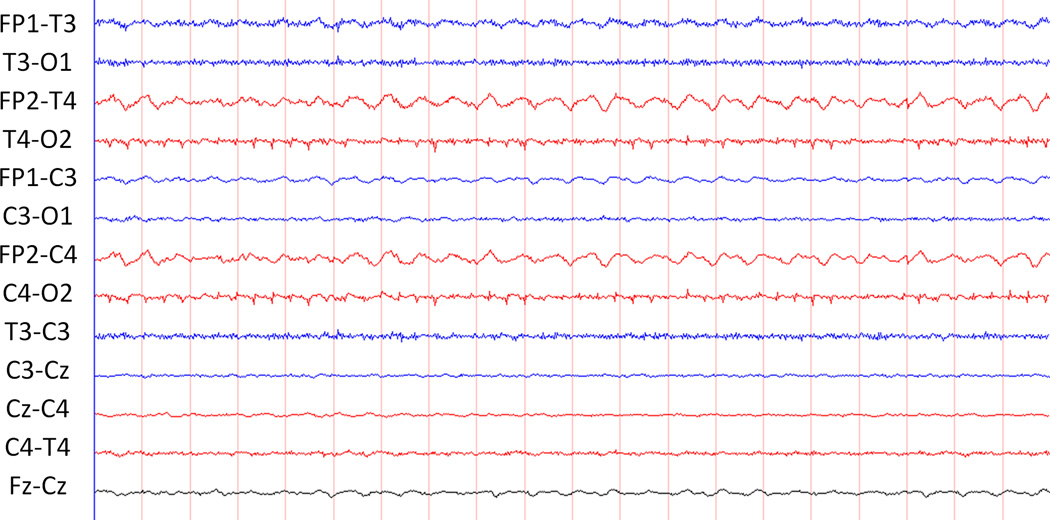
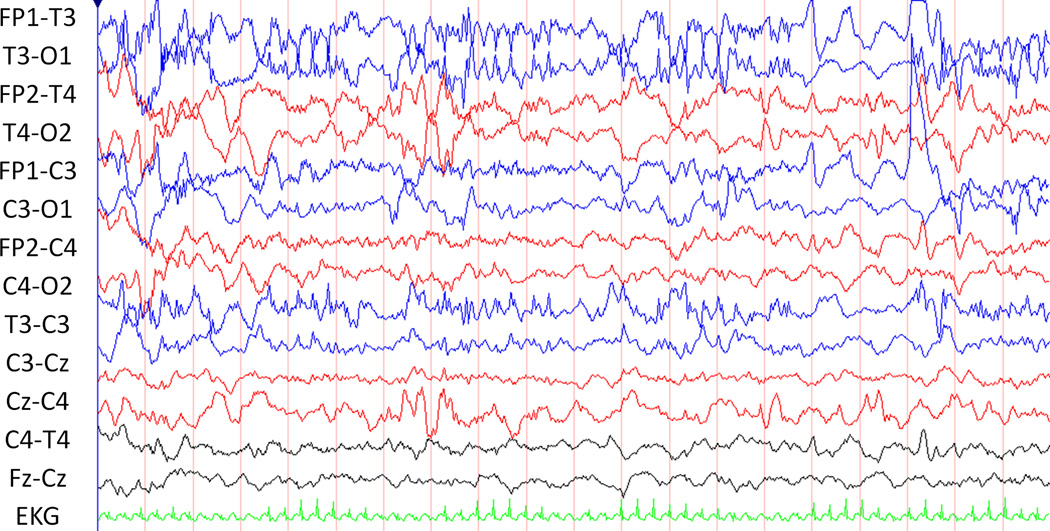
Specifying that seizures must be “sudden” emphasizes that they are paroxysmal and that they must stand out from the background.(Clancy and Legido 1987) Seizures must evolve with a distinct beginning, middle, and end (Clancy and Legido 1987) and describing this evolution is useful in distinguishing seizures from non-ictal rhythmic and periodic discharges. Rhythmic activity consists of repetition of a waveform with relatively uniform morphology and duration, but is not always ictal (Figures 1 and 2). Non-ictal rhythmic activity may be normal (e.g., rhythmic temporal theta) or abnormal (e.g., brief intermittent rhythmic discharges). Periodic discharges are repetitive waveforms with relatively uniform morphology occurring with a definable and quantifiable interval between consecutive waveforms. While rhythmic and periodic patterns may be similar to seizures in morphology, they lack evolution.
Figure 1.
This tracing contains rhythmic, non-evolving activity at Fp2-T4 and FP2-C4.
Figure 2.
This tracing clusters of abnormal, repetitive, non-evolving sharps.
Studies of patients with heterogeneous etiologies indicate that the average duration of neonatal seizures ranges from one to five minutes, with the majority lasting less than three minutes.(Clancy and Legido 1987; Scher, Aso et al. 1993; Shellhaas and Clancy 2007; Stevenson, Mesbah, Boylan et al. 2010; Thomas, Temko, Lightbody et al. 2010) By definition, a neonatal electrographic seizure has a minimum duration of ten seconds and is separated by other distinct seizures by at least ten seconds. This criterion distinguishes neonatal seizure from other types of rhythmic discharges, but it is not based on data relating the duration of an electrographic event to a specific physiologic change or neurodevelopmental impact. Electrographic events which meet seizure criteria but last less than ten seconds are referred to as BRDs (Brief Rhythmic Discharges), BIRDs (Brief Intermittent/Ictal/Inter-ictal Rhythmic Discharges), and BERDs (Brief Electroencephalographic Rhythmic Discharges). BRDs have been shown to occur more frequently in neonates with pathology and to coexist with seizures in some patients. However, the impact of BRDs on outcome and utility in distinguishing them from seizures is unclear.(Shewmon 1990) One study described 340 neonatal sleep EEG recordings from 277 high-risk neonates. Of the 130 subjects with rhythmic discharges, 67 (51%) had only BRDs, whereas the remainder had at least some discharges longer than ten seconds in duration. BRDs in isolation were significantly associated with neonatal hypoglycemia and periventricular leukomalacia, and they were also predictive of an increased risk for abnormal neurodevelopmental outcome.(Oliveira, Nunes, Haertel et al. 2000) A smaller study confirmed that, BRDs, like seizures, were associated with other background abnormalities and with increased morbidity and mortality.(Nagarajan, Palumbo and Ghosh 2011) Thus, while BRDs are considered to be electrographically distinct from neonatal seizures, in practice they indicate pathology and often co-occur with electrographic seizures.
Seizures are often electroencephalographically stereotyped, and this may be helpful in identifying recurrent seizures. While a neonate may have more than one seizure type, generally there is a limited repertoire of electrographic seizures. If a recurring rhythmic abnormal pattern is not due to artifact, it raises the possibility of an electrographic seizure. Similarly, if there is no consistent electrographic correlate to a clinical event of interest, then the events are more likely non-epileptic. Because seizures are stereotyped, clinical events tend to correlate with stereotyped electrographic pattern.
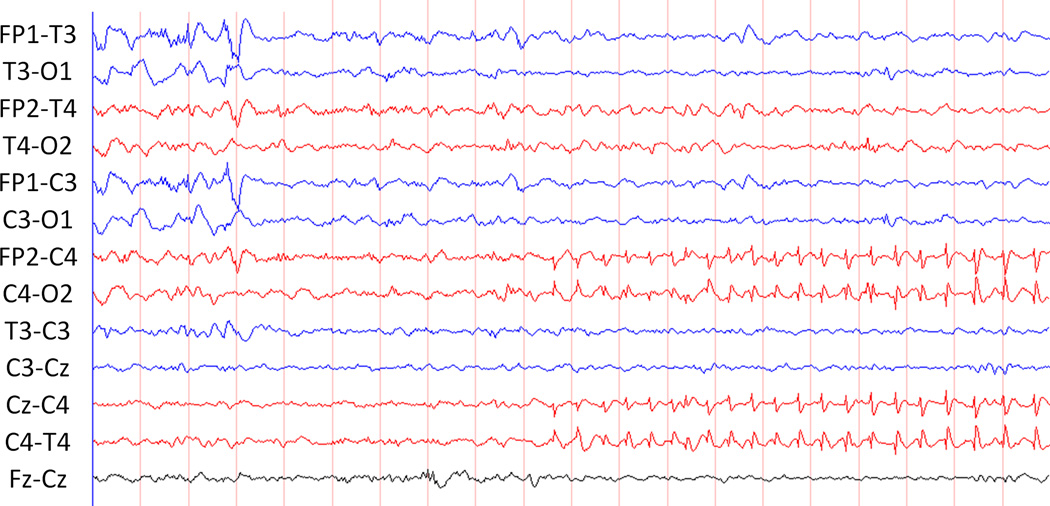
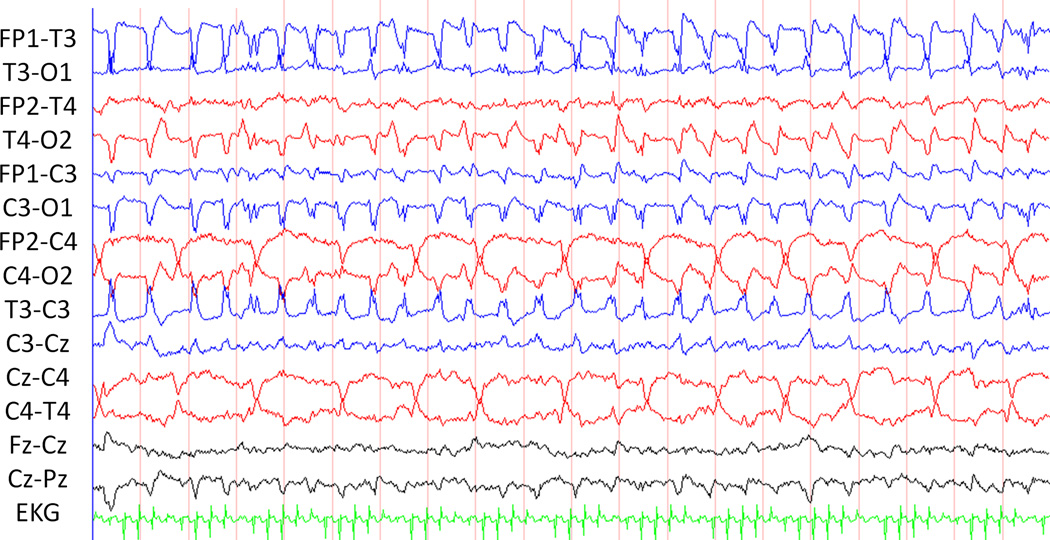
Describing the anatomical location of a seizure may have clinical implications. If neuroimaging is inconclusive or not immediately available, EEG findings may provide the first evidence of a focal lesion. Similarly, seizures originating from a new location may indicate a second focal lesion has developed, or the initial lesion has expanded. Seizure localization has been categorized by various methods. The most precise and time-consuming manner is to identify each electrode involved at various points throughout the seizure. A more practical method may be to identify the seizure onset focus as well as the region involved at the time of maximal spread. This is our typical practice. Seizures may be localized (identified at a few electrodes) (Figure 3), lateralized (present in one hemisphere), or diffuse (asynchronously present in all electrodes). Similarly, seizures may be described as unifocal, multifocal, or bilaterally independent (Figure 4). The majority of neonatal seizures have focal onset; true generalized onset is extremely rare.(Clancy 1996) Up to 81% of neonatal seizures originate from the central-temporal or vertex electrodes.(Shellhaas and Clancy 2007; Nagarajan, Ghosh and Palumbo 2011) Thus, if reduced arrays are used (as with amplitude integrated EEG), they should generally include these electrodes. The American Clinical Neurophysiology Society’s Guideline recommends P3-P4 electrode locations for single channel amplitude integrated EEG and C3-P3 with C4-P4 for two channel amplitude integrated EEG.(Shellhaas, Chang et al.) The presence of multiple independent foci(Bye, Cunningham, Chee et al. 1997; Watanabe, Hayakawa and Okumura 1999; Domenech-Martinez, Castro-Conde, Herraiz-Culebras et al. 2003) and spread from a lateralized onset to the contralateral hemisphere(Pisani, Copioli, Di Gioia et al. 2008) have been associated with worse neurological outcome.
Figure 3.
Localized onset of seizure in the right central region.
Figure 4.
Bilateral independent seizures. The evolving rhythmic discharges in the right central region and left hemisphere occur independently occur independently.
Describing the morphology of neonatal seizures helps optimize communication between readers. Several seizure morphology classification systems have been proposed.(Lombroso 1985; Rowe, Holmes, Hafford et al. 1985; Deburchgraeve, Cherian, De Vos et al. 2008; Pisani, Copioli et al. 2008; Mitra, Glover, Ktonas et al. 2009; Stevenson, Mesbah et al. 2010) Ictal morphology may be categorized as either sinusoidal (focal fast activity and high voltage rhythmic slowing) or sharp (polyspike bursts, sharply contoured rhythmic activity “sawtooth seizures,” repetitive evolving spikes, and spike-wave or sharp-wave discharges). These categories are not mutually exclusive. It is unknown whether seizures of differing morphologies have different impacts on cerebral physiology or neurodevelopmental outcome.
After neonatal seizures are identified and described, the neurophysiologist must consider the seizure burden in order to gauge response to anticonvulsant treatment and overall clinical trajectory. Having a consistent method for assessing seizure burden is essential in providing this information. Additionally, both seizure frequency and overall seizure duration correlate with neurodevelopmental outcomes, adding prognostic importance to seizure burden assessments.(McBride, Laroia et al. 2000; Pisani, Copioli et al. 2008) There is no consensus as to how seizure burden should be defined. Options include (1) counting the total number of seizures in a recording, (2) calculating the number of seizures per hour (or per epoch), or (3) calculating the total seizure duration within an epoch (i.e. the sum of all seizure durations in a specified time). Total duration is sometimes called the “Ictal Fraction,”(Pisani, Copioli et al. 2008) and does not necessarily correlate with the absolute number of seizures.(Shellhaas and Clancy 2007; Murray, Boylan et al. 2008) In a study of 851 electrographic seizures, the mean seizure rate was 7 per hour (range 0.5–21), the mean percent time seizing was 25% (range 0.7–87%), and the correlation between these two measures was only moderate (Spearman coefficient 0.58).(Shellhaas and Clancy 2007)
Status epilepticus refers to a high seizure burden. In the adult, status epilepticus has been defined as a continuous seizure, or multiple seizures without return to baseline resulting in dysfunction for at least thirty minutes.(Kaplan 2005) However, most neonates with seizures have some degree of encephalopathy, making a clear return to baseline very unlikely. In neonates, status epilepticus has been defined as a continuous seizure lasting thirty minutes or a series of seizures whose total duration exceeds 50% of a given epoch or both. (Prasad and Seshia 2006; Pisani, Cerminara, Fusco et al. 2007; Silverstein and Jensen 2007; Nash, Bonifacio, Glass et al. 2011; Wusthoff, Dlugos, Gutierrez-Colina et al. 2011) When defined as greater than 50% of a record, 14% of 125 neonates experiencing seizures met criteria for status epilepticus.(Shellhaas and Clancy 2007) In a second study 43% of 40 neonates with electrographic seizures had status epilepticus, defined as at least 30 minutes in which seizures occurred at least 50% of the time.(McBride, Laroia et al. 2000) Status epilepticus has been associated with worse outcomes among neonates with seizures, even worse than for repetitive individual seizures.(Glass, Nash, Bonifacio et al.; Ortibus, Sum and Hahn 1996; Pisani, Cerminara et al. 2007; Pisani, Sisti and Seri 2009) Thus, given the imperative of prompt full treatment, a looser clinical definition is applied by some clinicians, who treat frequent or prolonged seizures exceeding about five minutes in duration as status epilepticus.
What is the impact of neonatal seizures?
Neonates with clinical seizures are at higher risk for morbidity and mortality. In a follow-up study of 82 neonates with clinical seizures, 17 (27%) developed epilepsy, 16 (25%) developed cerebral palsy, 13 (20%) had mental retardation, and 17 (27%) had learning disorders.(Ronen, Buckley et al. 2007) In a second study of 89 term neonates with clinical seizures, mortality was 7%, and 28% of survivors had an unfavorable outcome.(Tekgul, Gauvreau et al. 2006) However, outcome is largely dependent on the etiology of the encephalopathy and seizures (Tekgul, Gauvreau et al. 2006; Ronen, Buckley et al. 2007), and data addressing whether seizures are independently associated with worse neurodevelopmental outcome is conflicting. Animal studies suggest that neonatal seizures may impair brain development and lead to long-term neurodevelopmental deficits including epilepsy.(Silverstein and Jensen 2007; Moreira, de Siqueira, Lague et al. 2011; Rakhade, Klein, Huynh et al. 2011) In human neonates with hypoxic-ischemic encephalopathy, seizures are associated with impaired brain metabolism.(Miller, Weiss, Barnwell et al. 2002)
A study of 68 neonates who underwent continuous EEG monitoring for at least 18 hours compared outcomes in the 40 neonates with seizures and 28 neonates without seizures. Electrographic seizure occurrence was associated with microcephaly, severe cerebral palsy, and failure to thrive. Neonates with a higher number of seizures were at higher risk for these unfavorable outcomes.(McBride, Laroia et al. 2000) A study evaluated 218 term neonates with moderate to severe neonatal encephalopathy who underwent head cooling and amplitude-integrated EEG monitoring. Even in multivariate analysis, the absence of electrographic seizures on amplitude integrated EEG was associated with lower rates of death and severe disability at 18 months.(Wyatt, Gluckman, Liu et al. 2007) One study of 77 term neonates with hypoxic ischemic encephalopathy reported that even after controlling for the degree of injury evident on magnetic resonance imaging, children with clinical seizures had worse motor and cognitive outcome at four years. Further, neonates with severe seizures had lower intelligence quotient scores than those with mild/moderate seizures.(Glass, Glidden, Jeremy et al. 2009) Of 106 preterm and term neonates with electrographic seizures with outcome assessed at 24 months corrected age, 25 of the 26 (96%) with status epilepticus had an adverse outcome, and even in multivariate analysis the presence of status epilepticus was an independent predictor of abnormal outcome and future epilepsy.(Pisani, Cerminara et al. 2007) In a therapeutic hypothermia clinical trial that involved amplitude integrated EEG, the presence of seizures was associated with unfavorable outcome (49% without seizures vs. 67% with seizures).(Gluckman, Wyatt, Azzopardi et al. 2005) Of 247 high-risk term infants, 311 had seizures on amplitude integrated EEG and these constituted status epilepticus in 56 (18%). Of the 56 with status epilepticus, 42 (75%) had a poor outcome. Overall, the background pattern but not status epilepticus predicted outcome. However, among the 48 neonates with hypoxic ischemic encephalopathy, a longer duration of status epilepticus was predictive of worse neurodevelopmental outcome by Griffiths Mental Developmental Scale at postnatal ages of 18 months. Status epilepticus duration was 215 minutes in those with poor outcome versus 85 minutes in those with favorable outcome.(van Rooij, de Vries et al. 2007) In contrast, a secondary analysis of 208 neonates enrolled in a hypothermia study, 127 (61%) of whom had clinical seizures, found that after adjusting for study treatment and severity of encephalopathy, clinical seizures were not associated with death, or moderate or severe disability, or lower Bayley Mental Development Index scores at eighteen months of life.(Kwon, Guillet, Shankaran et al. 2011)
Several studies have explored the relationship between electrographic seizures and brain injury evident on magnetic resonance imaging. A study randomized nineteen term neonates with moderate to severe hypoxic ischemic encephalopathy with seizures on amplitude-integrated quantitative EEG monitoring to management with or without access to the amplitude integrated EEG data. There was a non-significant trend toward shorter median seizure duration in neonates managed with access to the amplitude integrated data (196 versus 503 minutes). Within the entire cohort, a significant correlation existed between seizure duration and the severity of MRI injury.(van Rooij, Toet, van Huffelen et al. 2010) In a second study, 56 neonates treated with hypothermia for hypoxic ischemic encephalopathy underwent EEG monitoring and MRI at five days. Moderate-severe MRI injury occurred more commonly in neonates with seizures and occurred in all neonates who had experienced status epilepticus. Additionally, neonates with clinical and non-convulsive seizures were as likely to have MRI injury.(Glass, Nash et al.)
Data are conflicting in neonates with congenital heart disease. The Boston Circulatory Arrest Trial reported that in 126 neonates with transposition of the great arteries post-operative, EEG monitoring identified seizures in 25 (20%) and this was associated with lower psychomotor scores on the Bayley Scales of Infant Development scores and increased likelihood of MRI abnormalities at one year.(Bellinger, Jonas, Rappaport et al. 1995) In the Children’s Hospital of Philadelphia series of 114 survivors of neonatal and infant congenital heart disease surgery, electrographic seizures occurred in 15 (13%) and were associated with an increased risk of abnormal neuromuscular examination at one year (73% versus 41%). Electrographic seizures were not associated with lower Bayley Scales of Infant Development scores, although seizures originating from the frontal lobe were associated with a lower Psychomotor Development Index.(Gaynor, Jarvik, Bernbaum et al. 2006)
Although no evidence-based guidelines exist, the management of neonatal seizures has been reviewed recently.(Glass and Sullivan 2009; Jensen 2009)
What neurophysiology tools can aid in neonatal seizure identification?
Given the impact of neonatal seizures and the unique dependence on electroencephalography for their diagnosis, efforts increasingly emphasize optimizing neurophysiology techniques for neonatal seizure identification. In current clinical practice, screening tools used in conjunction with EEG may permit rapid identification of some seizures and enhance interpretation.
In particular, amplitude-integrated EEG (aEEG) has gained popularity among neonatologists as a method of seizure identification. aEEG uses a reduced number of electrodes to generate a single or dual-channel EEG tracing. The EEG signal is modified and compressed to generate the final display showing several hours within a single page. The exact algorithm used to generate the aEEG output varies slightly between manufacturers.(Quigg and Leiner 2009)
In one survey of neonatologists and neurologists in the United States and Europe, 51% reported using both aEEG and EEG in their units, with an additional 22% reporting that only aEEG is used.(Boylan, Burgoyne, Moore et al. 2010) In the UK, approximately 40% of neonatal intensive care units report using aEEG.(Ponnusamy, Nath, Bissett et al. 2010) The primary advantages of aEEG relate to its relative ease of use. The limited electrode array is intended for application by those without specialized training, including doctors, nurses and respiratory therapists. Similarly, because aEEG provides much less detail, it is designed for real-time interpretation at the bedside by caregivers. In the majority of neonatal units, aEEG is interpreted by a neonatologist without neurophysiologist input.(Boylan, Burgoyne et al. 2010) aEEG has a sensitivity and specificity for seizure detection that is largely dependent on the experience of the user. While new users identify seizures with specificity below 50%, experienced users can achieve sensitivity and specificity of almost 85%.(Shellhaas, Soaita and Clancy 2007; Lawrence, Mathur et al. 2009; Evans, Koh, Lerner et al. 2010; Frenkel, Friger, Meledin et al. 2011) Overall, aEEG has greatest utility as a screening tool, correctly identifying 60–90% of patients with seizures. However, aEEG has greater variability for identification of individual seizures, with studies reporting a range of sensitivity from 12–96%.(Shah, Mackay, Lavery et al. 2008; Lawrence, Mathur et al. 2009; Frenkel, Friger et al. 2011) Seizures are more likely to go unrecognized if the duration is short or if located in regions further from the limited electrode array. Electrode placement also affects aEEG sensitivity, with frontal electrode placement decreasing sensitivity by up to one-third compared to bi-central electrode placement.(Wusthoff, Shellhaas and Clancy 2009) aEEG alone may have low specificity for seizure detection, with an almost 50% false-positive rate in some subgroups.(Evans, Koh et al. 2010) The American Clinical Neurophysiology Society’s Guidelines on EEG Monitoring in Neonates states that aEEG can be a “useful, initial complementary tool” to EEG monitoring, which remains the gold-standard.(Shellhaas, Chang, Tsuchida et al. 2011)
At the same time, some studies indicate that aEEG use confers improved management decision-making and better outcomes. In one blinded, randomized study of 33 infants, neonatologists were allowed to view aEEG in their routine clinical care for 19 patients, but not for 14. Among those patients in whom aEEG was used, there was a trend towards overall reduced seizure duration and less severe brain injury on MRI.(van Rooij, Toet et al. 2010) Similarly, in a more recent study of 202 neonates, those who underwent aEEG monitoring had greater precision in the diagnosis of neonatal seizures than contemporary controls, with fewer seizures diagnosed based on clinical signs alone.(Shellhaas and Barks 2012)
There is also work which explores how to balance the ease of use and real-time interpretation benefits of aEEG with the detailed data available from full array EEG. While early aEEG systems generally used only two electrodes (plus one ground) to produce a single EEG channel output, newer systems allow for two channels, and this may improve the ability to identify seizures related to focal lesions, such as stroke.(Bourez-Swart, van Rooij, Rizzo et al. 2009; van Rooij, de Vries, van Huffelen et al. 2010) Similarly, the use of aEEG in combination with review of source EEG, particularly from multiple channels, has been shown to improve sensitivity and specificity in seizure identification.(Shah, Mackay et al. 2008; Zimbric, Sharpe, Albright et al. 2011) One study compared 3 and 21 channel montages in 35 neonatal EEGs and demonstrated that seizure burden quantification was strongly correlated between the montages.(Zimbric, Sharpe et al. 2011)
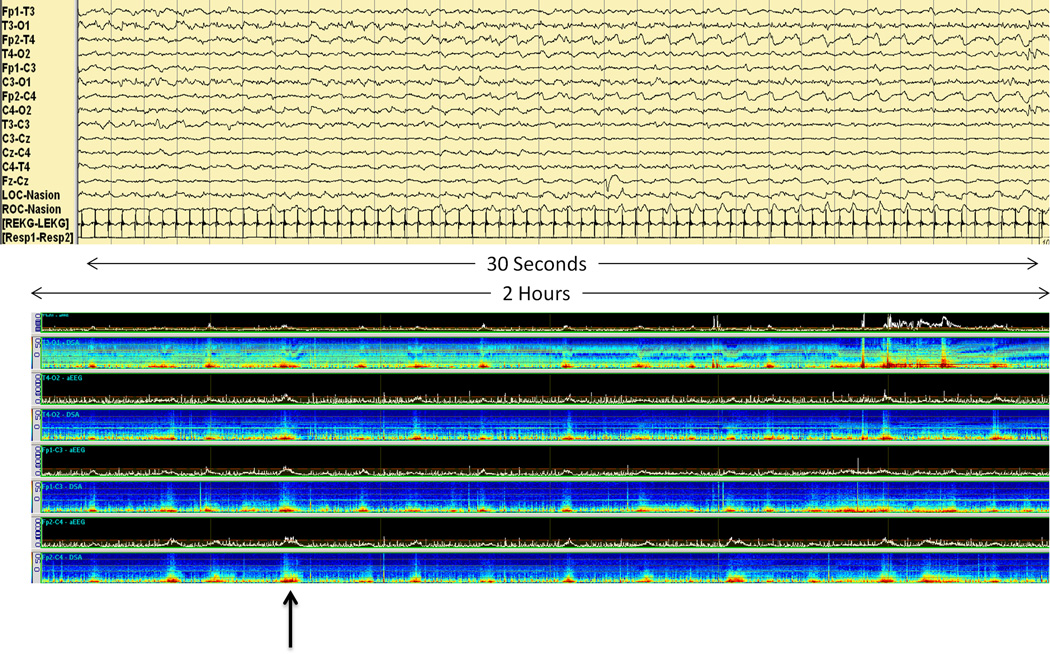
Other approaches to neonatal seizure detection have emphasized alternate methods of display of the conventional EEG signal. These techniques, similar to those used for EEG monitoring in the pediatric and adult populations, rely on EEG data recorded in the traditional manner but displayed in a manner that facilitates rapid review for presence or absence of seizures. Examples include envelope trend or density spectral array trends (Figure 5). For identifying prolonged seizures, these techniques have been shown to have a sensitivity approaching that of aEEG, although this decreases with less user experience and for shorter seizure duration.(Abend, Dlugos and Herman 2008) When multiple methods are used in combination, accuracy improves.(Kobayashi, Mimaki, Endoh et al. 2011)
Figure 5.
Thirty seconds of EEG demonstrates an electrographic seizure. Two hours of quantitative EEG demonstrates seizures on amplitude-integrated EEG and density spectral array EEG.
While not yet in wide clinical use, development of automated seizure detection algorithms remains a major research objective, and they have been used clinically by some centers.(Mitra, Glover et al. 2009; Cherian, Deburchgraeve, Swarte et al. 2011; Temko, Thomas, Marnane et al. 2011) One study described a machine learning algorithm to discriminate between seizure and non-seizure epochs on 267 hours of EEG acquired from 17 term neonates with electrographic seizures. The detection rate depended on the permitted number of false detections per hour. It achieved a detection rates of about 89% with one false seizure detection per hour, 96% with two false detections per hour, or 100% with four false detections per hour.(Temko, Thomas et al. 2011) A second study assessed a neonatal seizure detection algorithm applied to 756 hours of EEG from 24 neonates with electrographic seizures. Backgrounds were categorized as mild-moderate or severely abnormal, and seizures were classified as definite or dubious. Seizures were detected with a total sensitivity of 62% and sensitivity per patient was 66%. After excluding four patients with severely abnormal EEG background and predominantly having dubious seizures, the algorithm showed a median sensitivity per patient of 87%. Sensitivity tended to be better for patients with mild-moderately abnormal backgrounds.(Cherian, Deburchgraeve et al. 2011) Algorithms that remove physiologic artifacts (EKG, pulse, and respiration) may reduce false detections.(De Vos, Deburchgraeve, Cherian et al. 2011) The finding that seizure and background characteristics influence accuracy of detection algorithms is certainly consistent with most neurophysiologists experience that slowly evolving low amplitude seizures may be difficult to identify visually. However, these promising techniques may eventually allow for easier and more rapid seizure detection, combining the ease of use of limited-array and quantitative EEG with the full detail of conventional full-array EEG. Further research is needed to develop improved detection algorithms and establish their optimal clinical role.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nicholas S. Abend, Division of Neurology, Children’s Hospital of Philadelphia and the University of Pennsylvania School Medicine, abend@email.chop.edu.
Courtney J. Wusthoff, Division of Neonatology, Imperial College Healthcare NHS Trust, Courtney.wusthoff@imperial.nhs.uk.
References
- Abend NS, Dlugos D, Herman S. Neonatal seizure detection using multichannel display of envelope trend. Epilepsia. 2008;49:349–352. doi: 10.1111/j.1528-1167.2007.01425.x. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Jonas RA, Rappaport LA, Wypij D, Wernovsky G, Kuban KC, Barnes PD, Holmes GL, Hickey PR, Strand RD, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med. 1995;332:549–555. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- Berg A, Berkovic S, Brodie M, Buchhalter J, Cross J, Boas W, Engel J, French J, Glauser T, Mathern G, Moshe S, Nordli D, Plouin P, Scheffer I. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Bonifacio SL, Glass HC, Peloquin S, Ferriero DM. A new neurological focus in neonatal intensive care. Nat Rev Neurol. 2011;7:485–494. doi: 10.1038/nrneurol.2011.119. [DOI] [PubMed] [Google Scholar]
- Bourez-Swart MD, van Rooij L, Rizzo C, de Vries LS, Toet MC, Gebbink TA, Ezendam AG, van Huffelen AC. Detection of subclinical electroencephalographic seizure patterns with multichannel amplitude-integrated EEG in full-term neonates. Clin Neurophysiol. 2009;120:1916–1922. doi: 10.1016/j.clinph.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Boylan G, Burgoyne L, Moore C, O'Flaherty B, Rennie J. An international survey of EEG use in the neonatal intensive care unit. Acta Paediatr. 2010;99:1150–1155. doi: 10.1111/j.1651-2227.2010.01809.x. [DOI] [PubMed] [Google Scholar]
- Bye AM, Cunningham CA, Chee KY, Flanagan D. Outcome of neonates with electrographically identified seizures, or at risk of seizures. Pediatr Neurol. 1997;16:225–231. doi: 10.1016/s0887-8994(97)00019-2. [DOI] [PubMed] [Google Scholar]
- Bye AM, Flanagan D. Spatial and temporal characteristics of neonatal seizures. Epilepsia. 1995;36:1009–1016. doi: 10.1111/j.1528-1157.1995.tb00960.x. [DOI] [PubMed] [Google Scholar]
- Cherian PJ, Deburchgraeve W, Swarte RM, De Vos M, Govaert P, Van Huffel S, Visser GH. Validation of a new automated neonatal seizure detection system: A clinician's perspective. Clin Neurophysiol. 2011;122:1490–1499. doi: 10.1016/j.clinph.2011.01.043. [DOI] [PubMed] [Google Scholar]
- Clancy RR. The contribution of EEG to the understanding of neonatal seizures. Epilepsia. 1996;37(Suppl 1):S52–S59. doi: 10.1111/j.1528-1157.1996.tb06022.x. [DOI] [PubMed] [Google Scholar]
- Clancy RR. Prolonged electroencephalogram monitoring for seizures and their treatment. Clin Perinatol. 2006;33:649–665. doi: 10.1016/j.clp.2006.06.004. vi. [DOI] [PubMed] [Google Scholar]
- Clancy RR. Summary proceedings from the neurology group on neonatal seizures. Pediatrics. 2006;117:S23–S27. doi: 10.1542/peds.2005-0620D. [DOI] [PubMed] [Google Scholar]
- Clancy RR, Legido A. The exact ictal and interictal duration of electroencephalographic neonatal seizures. Epilepsia. 1987;28:537–541. doi: 10.1111/j.1528-1157.1987.tb03685.x. [DOI] [PubMed] [Google Scholar]
- Clancy RR, Legido A, Lewis D. Occult neonatal seizures. Epilepsia. 1988;29:256–261. doi: 10.1111/j.1528-1157.1988.tb03715.x. [DOI] [PubMed] [Google Scholar]
- Connell J, Oozeer R, de Vries L, Dubowitz L, Dubowitz V. Clinical and EEG response to anticonvulsants in neonatal seizures. Archives of Disease in Childhood. 1989;64:459–464. doi: 10.1136/adc.64.4_spec_no.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos M, Deburchgraeve W, Cherian PJ, Matic V, Swarte RM, Govaert P, Visser GH, Van Huffel S. Automated artifact removal as preprocessing refines neonatal seizure detection. Clin Neurophysiol. 2011 doi: 10.1016/j.clinph.2011.04.026. [DOI] [PubMed] [Google Scholar]
- Deburchgraeve W, Cherian PJ, De Vos M, Swarte RM, Blok JH, Visser GH, Govaert P, Van Huffel S. Automated neonatal seizure detection mimicking a human observer reading EEG. Clin Neurophysiol. 2008;119:2447–2454. doi: 10.1016/j.clinph.2008.07.281. [DOI] [PubMed] [Google Scholar]
- Domenech-Martinez E, Castro-Conde JR, Herraiz-Culebras T, Gonzalez-Campo C, Mendez-Perez A. [Neonatal convulsions: influence of the electroencephalographic pattern and the response to treatment on the outcome] Rev Neurol. 2003;37:413–420. [PubMed] [Google Scholar]
- Evans E, Koh S, Lerner J, Sankar R, Garg M. Accuracy of amplitude integrated EEG in a neonatal cohort. Arch Dis Child Fetal Neonatal Ed. 2010;95:F169–F173. doi: 10.1136/adc.2009.165969. [DOI] [PubMed] [Google Scholar]
- Ficicioglu C, Bearden D. Isolated neonatal seizures: when to suspect inborn errors of metabolism. Pediatr Neurol. 2011;45:283–291. doi: 10.1016/j.pediatrneurol.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Frenkel N, Friger M, Meledin I, Berger I, Marks K, Bassan H, Shany E. Neonatal seizure recognition - Comparative study of continuous-amplitude integrated EEG versus short conventional EEG recordings. Clin Neurophysiol. 2011;122:1091–1097. doi: 10.1016/j.clinph.2010.09.028. [DOI] [PubMed] [Google Scholar]
- Gaynor JW, Jarvik GP, Bernbaum J, Gerdes M, Wernovsky G, Burnham NB, D'Agostino JA, Zackai E, McDonald-McGinn DM, Nicolson SC, Spray TL, Clancy RR. The relationship of postoperative electrographic seizures to neurodevelopmental outcome at 1 year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. 2006;131:181–189. doi: 10.1016/j.jtcvs.2005.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass HC, Bonifacio SL, Peloquin S, Shimotake T, Sehring S, Sun Y, Sullivan J, Rogers E, Barkovich AJ, Rowitch D, Ferriero DM. Neurocritical Care for Neonates. Neurocrit Care. 2010;12:421–429. doi: 10.1007/s12028-009-9324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, Miller SP. Clinical Neonatal Seizures are Independently Associated with Outcome in Infants at Risk for Hypoxic-Ischemic Brain Injury. J Pediatr. 2009;155:318–323. doi: 10.1016/j.jpeds.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass HC, Nash KB, Bonifacio SL, Barkovich AJ, Ferriero DM, Sullivan JE, Cilio MR. Seizures and magnetic resonance imaging-detected brain injury in newborns cooled for hypoxic-ischemic encephalopathy. J Pediatr. 159:731–735 e1. doi: 10.1016/j.jpeds.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass HC, Sullivan JE. Neonatal seizures. Curr Treat Options Neurol. 2009;11:405–413. doi: 10.1007/s11940-009-0045-1. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- Jensen FE. Neonatal seizures: an update on mechanisms and management. Clin Perinatol. 2009;36:881–900, vii. doi: 10.1016/j.clp.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan PW. The clinical features, diagnosis, and prognosis of nonconvulsive status epilepticus. Neurologist. 2005;11:348–361. doi: 10.1097/01.nrl.0000162954.76053.d2. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Mimaki N, Endoh F, Inoue T, Yoshinaga H, Ohtsuka Y. Amplitude-integrated EEG colored according to spectral edge frequency. Epilepsy Res. 2011;96:276–282. doi: 10.1016/j.eplepsyres.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Kwon JM, Guillet R, Shankaran S, Laptook AR, McDonald SA, Ehrenkranz RA, Tyson JE, O'Shea TM, Goldberg RN, Donovan EF, Fanaroff AA, Poole WK, Higgins RD, Walsh MC. Clinical Seizures in Neonatal Hypoxic-Ischemic Encephalopathy Have No Independent Impact on Neurodevelopmental Outcome: Secondary Analyses of Data From the Neonatal Research Network Hypothermia Trial. J Child Neurol. 2011;26:322–328. doi: 10.1177/0883073810380915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanska MJ, Lanska DJ. Neonatal seizures in the United States: results of the National Hospital Discharge Survey 1980–1991. Neuroepidemiology. 1996;15:117–125. doi: 10.1159/000109898. [DOI] [PubMed] [Google Scholar]
- Lawrence R, Mathur A, Nguyen The Tich S, Zempel J, Inder T. A pilot study of continuous limited-channel aEEG in term infants with encephalopathy. J Pediatr. 2009;154:835–841 e1. doi: 10.1016/j.jpeds.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Lombroso CT. Neonatal polygraphy in full-term and premature infants: a review of normal and abnormal findings. J Clin Neurophysiol. 1985;2:105–155. doi: 10.1097/00004691-198504000-00002. [DOI] [PubMed] [Google Scholar]
- Lombroso CT. Neonatal seizures: historic note and present controversies. Epilepsia. 1996;37 Suppl 3:5–13. doi: 10.1111/j.1528-1157.1996.tb01814.x. [DOI] [PubMed] [Google Scholar]
- Malone A, Ryan C, Fitzgerald A, Burgoyne L, Connolly S, Boylan G. Interobserver agreement in neonatal seizure identification. Epilepsia. 2009;50:2097–2101. doi: 10.1111/j.1528-1167.2009.02132.x. [DOI] [PubMed] [Google Scholar]
- McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology. 2000;55:506–513. doi: 10.1212/wnl.55.4.506. [DOI] [PubMed] [Google Scholar]
- Miller SP, Weiss J, Barnwell A, Ferriero DM, Latal-Hajnal B, Ferrer-Rogers A, Newton N, Partridge JC, Glidden DV, Vigneron DB, Barkovich AJ. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology. 2002;58:542–548. doi: 10.1212/wnl.58.4.542. [DOI] [PubMed] [Google Scholar]
- Mitra J, Glover JR, Ktonas PY, Thitai Kumar A, Mukherjee A, Karayiannis NB, Frost JD, Jr, Hrachovy RA, Mizrahi EM. A multistage system for the automated detection of epileptic seizures in neonatal electroencephalography. J Clin Neurophysiol. 2009;26:218–226. doi: 10.1097/WNP.0b013e3181b2f29d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi EM. Consensus and controversy in the clinical management of neonatal seizures. Clin Perinatol. 1989;16:485–500. [PubMed] [Google Scholar]
- Mizrahi EM, Kellaway P. Characterization and classification of neonatal seizures. Neurology. 1987;37:1837–1844. doi: 10.1212/wnl.37.12.1837. [DOI] [PubMed] [Google Scholar]
- Moreira JD, de Siqueira LV, Lague VM, Porciuncula LO, Vinade L, Souza DO. Short-term alterations in hippocampal glutamate transport system caused by one-single neonatal seizure episode: implications on behavioral performance in adulthood. Neurochem Int. 2011;59:217–223. doi: 10.1016/j.neuint.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Murray DM, Boylan GB, Ali I, Ryan CA, Murphy BP, Connolly S. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch Dis Child Fetal Neonatal Ed. 2008;93:F187–F191. doi: 10.1136/adc.2005.086314. [DOI] [PubMed] [Google Scholar]
- Nagarajan L, Ghosh S, Palumbo L. Ictal electroencephalograms in neonatal seizures: characteristics and associations. Pediatr Neurol. 2011;45:11–16. doi: 10.1016/j.pediatrneurol.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Nagarajan L, Palumbo L, Ghosh S. Brief Electroencephalography Rhythmic Discharges (BERDs) in the Neonate With Seizures: Their Significance and Prognostic Implications. J Child Neurol. 2011 doi: 10.1177/0883073811409750. [DOI] [PubMed] [Google Scholar]
- Nash KB, Bonifacio SL, Glass HC, Sullivan JE, Barkovich AJ, Ferriero DM, Cilio MR. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neurology. 2011;76:556–562. doi: 10.1212/WNL.0b013e31820af91a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura A, Hayakawa F, Kato T, Itomi K, Maruyama K, Kubota T, Suzuki M, Kidokoro H, Watanabe K. Ictal electroencephalographic findings of neonatal seizures in preterm infants. Brain Dev. 2008;30:261–268. doi: 10.1016/j.braindev.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Oliveira AJ, Nunes ML, Haertel LM, Reis FM, da Costa JC. Duration of rhythmic EEG patterns in neonates: new evidence for clinical and prognostic significance of brief rhythmic discharges. Clin Neurophysiol. 2000;111:1646–653. doi: 10.1016/s1388-2457(00)00380-1. [DOI] [PubMed] [Google Scholar]
- Ortibus EL, Sum JM, Hahn JS. Predictive value of EEG for outcome and epilepsy following neonatal seizures. Electroencephalogr Clin Neurophysiol. 1996;98:175–185. doi: 10.1016/0013-4694(95)00245-6. [DOI] [PubMed] [Google Scholar]
- Pisani F, Cerminara C, Fusco C, Sisti L. Neonatal status epilepticus vs recurrent neonatal seizures: clinical findings and outcome. Neurology. 2007;69:2177–2185. doi: 10.1212/01.wnl.0000295674.34193.9e. [DOI] [PubMed] [Google Scholar]
- Pisani F, Copioli C, Di Gioia C, Turco E, Sisti L. Neonatal seizures: relation of ictal video-electroencephalography (EEG) findings with neurodevelopmental outcome. J Child Neurol. 2008;23:394–398. doi: 10.1177/0883073807309253. [DOI] [PubMed] [Google Scholar]
- Pisani F, Sisti L, Seri S. A scoring system for early prognostic assessment after neonatal seizures. Pediatrics. 2009;124:e580–e587. doi: 10.1542/peds.2008-2087. [DOI] [PubMed] [Google Scholar]
- Ponnusamy V, Nath P, Bissett L, Willis K, Clarke P. Current availability of cerebral function monitoring and hypothermia therapy in UK neonatal units. Arch Dis Child Fetal Neonatal Ed. 2010;95:F383–F384. doi: 10.1136/adc.2009.181578. [DOI] [PubMed] [Google Scholar]
- Prasad AN, Seshia SS. Status epilepticus in pediatric practice: neonate to adolescent. Adv Neurol. 2006;97:229–243. [PubMed] [Google Scholar]
- Quigg M, Leiner D. Engineering aspects of the quantified amplitude-integrated electroencephalogram in neonatal cerebral monitoring. J Clin Neurophysiol. 2009;26:145–149. doi: 10.1097/WNP.0b013e3181a18711. [DOI] [PubMed] [Google Scholar]
- Rakhade SN, Klein PM, Huynh T, Hilario-Gomez C, Kosaras B, Rotenberg A, Jensen FE. Development of later life spontaneous seizures in a rodent model of hypoxia-induced neonatal seizures. Epilepsia. 2011;52:753–765. doi: 10.1111/j.1528-1167.2011.02992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronen GM, Buckley D, Penney S, Streiner DL. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology. 2007;69:1816–1822. doi: 10.1212/01.wnl.0000279335.85797.2c. [DOI] [PubMed] [Google Scholar]
- Ronen GM, Penney S, Andrews W. The epidemiology of clinical neonatal seizures in Newfoundland: a population-based study. J Pediatr. 1999;134:71–75. doi: 10.1016/s0022-3476(99)70374-4. [DOI] [PubMed] [Google Scholar]
- Rowe JC, Holmes GL, Hafford J, Baboval D, Robinson S, Philipps A, Rosenkrantz T, Raye J. Prognostic value of the electroencephalogram in term and preterm infants following neonatal seizures. Electroencephalogr Clin Neurophysiol. 1985;60:183–196. doi: 10.1016/0013-4694(85)90030-6. [DOI] [PubMed] [Google Scholar]
- Scher MS, Alvin J, Gaus L, Minnigh B, Painter MJ. Uncoupling of EEG-clinical neonatal seizures after antiepileptic drug use. Pediatr Neurol. 2003;28:277–280. doi: 10.1016/s0887-8994(02)00621-5. [DOI] [PubMed] [Google Scholar]
- Scher MS, Aso K, Beggarly ME, Hamid MY, Steppe DA, Painter MJ. Electrographic seizures in preterm and full-term neonates: clinical correlates, associated brain lesions, and risk for neurologic sequelae. Pediatrics. 1993;91:128–134. [PubMed] [Google Scholar]
- Shah DK, Boylan GB, Rennie JM. Monitoring of seizures in the newborn. Arch Dis Child Fetal Neonatal Ed. 2010 doi: 10.1136/adc.2009.169508. [DOI] [PubMed] [Google Scholar]
- Shah DK, Mackay MT, Lavery S, Watson S, Harvey AS, Zempel J, Mathur A, Inder TE. Accuracy of bedside electroencephalographic monitoring in comparison with simultaneous continuous conventional electroencephalography for seizure detection in term infants. Pediatrics. 2008;121:1146–1154. doi: 10.1542/peds.2007-1839. [DOI] [PubMed] [Google Scholar]
- Shellhaas RA, Barks AK. Impact of amplitude-integrated electroencephalograms on clinical care for neonates with seizures. Pediatr Neurol. 2012;46:32–35. doi: 10.1016/j.pediatrneurol.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellhaas RA, Chang T, Tsuchida T, Scher MS, Riviello JJ, Abend NS, Nguyen S, Wusthoff CJ, Clancy RR. The American Clinical Neurophysiology Society's Guideline on Continuous Electroencephalography Monitoring in Neonates. J Clin Neurophysiol. doi: 10.1097/WNP.0b013e31823e96d7. [DOI] [PubMed] [Google Scholar]
- Shellhaas RA, Chang T, Tsuchida T, Scher MS, Riviello JJ, Abend NS, Nguyen S, Wusthoff CJ, Clancy RR. The American Clinical Neurophysiology Society's Guideline on Continuous Electroencephalography Monitoring in Neonates. J Clin Neurophysiol. 2011 doi: 10.1097/WNP.0b013e31823e96d7. [DOI] [PubMed] [Google Scholar]
- Shellhaas RA, Clancy RR. Characterization of neonatal seizures by conventional EEG and single-channel EEG. Clin Neurophysiol. 2007;118:2156–2161. doi: 10.1016/j.clinph.2007.06.061. [DOI] [PubMed] [Google Scholar]
- Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics. 2007;120:770–777. doi: 10.1542/peds.2007-0514. [DOI] [PubMed] [Google Scholar]
- Sheth RD, Hobbs GR, Mullett M. Neonatal seizures: incidence, onset, and etiology by gestational age. J Perinatol. 1999;19:40–43. doi: 10.1038/sj.jp.7200107. [DOI] [PubMed] [Google Scholar]
- Shewmon DA. What is a neonatal seizure? Problems in definition and quantification for investigative and clinical purposes. J Clin Neurophysiol. 1990;7:315–368. doi: 10.1097/00004691-199007000-00003. [DOI] [PubMed] [Google Scholar]
- Silverstein FS, Jensen FE. Neonatal seizures. Ann Neurol. 2007;62:112–120. doi: 10.1002/ana.21167. [DOI] [PubMed] [Google Scholar]
- Silverstein FS, Jensen FE, Inder T, Hellstrom-Westas L, Hirtz D, Ferriero DM. Improving the treatment of neonatal seizures: National Institute of Neurological Disorders and Stroke workshop report. J Pediatr. 2008;153:12–15. doi: 10.1016/j.jpeds.2008.01.041. [DOI] [PubMed] [Google Scholar]
- Stevenson NJ, Mesbah M, Boylan GB, Colditz PB, Boashash B. A nonlinear model of newborn EEG with nonstationary inputs. Ann Biomed Eng. 2010;38:3010–3021. doi: 10.1007/s10439-010-0041-3. [DOI] [PubMed] [Google Scholar]
- Tekgul H, Gauvreau K, Soul J, Murphy L, Robertson R, Stewart J, Volpe J, Bourgeois B, du Plessis AJ. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics. 2006;117:1270–1280. doi: 10.1542/peds.2005-1178. [DOI] [PubMed] [Google Scholar]
- Temko A, Thomas E, Marnane W, Lightbody G, Boylan G. EEG-based neonatal seizure detection with Support Vector Machines. Clin Neurophysiol. 2011;122:464–473. doi: 10.1016/j.clinph.2010.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharp BR. Neonatal seizures and syndromes. Epilepsia. 2002;43 Suppl 3:2–10. doi: 10.1046/j.1528-1157.43.s.3.11.x. [DOI] [PubMed] [Google Scholar]
- Thomas EM, Temko A, Lightbody G, Marnane WP, Boylan GB. Gaussian mixture models for classification of neonatal seizures using EEG. Physiol Meas. 2010;31:1047–1064. doi: 10.1088/0967-3334/31/7/013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij LG, de Vries LS, Handryastuti S, Hawani D, Groenendaal F, van Huffelen AC, Toet MC. Neurodevelopmental outcome in term infants with status epilepticus detected with amplitude-integrated electroencephalography. Pediatrics. 2007;120:e354–e363. doi: 10.1542/peds.2006-3007. [DOI] [PubMed] [Google Scholar]
- van Rooij LG, de Vries LS, van Huffelen AC, Toet MC. Additional value of two-channel amplitude integrated EEG recording in full-term infants with unilateral brain injury. Arch Dis Child Fetal Neonatal Ed. 2010;95:F160–F168. doi: 10.1136/adc.2008.156711. [DOI] [PubMed] [Google Scholar]
- van Rooij LG, Toet MC, van Huffelen AC, Groenendaal F, Laan W, Zecic A, de Haan T, van Straaten IL, Vrancken S, van Wezel G, van der Sluijs J, Ter Horst H, Gavilanes D, Laroche S, Naulaers G, de Vries LS. Effect of treatment of subclinical neonatal seizures detected with aEEG: randomized, controlled trial. Pediatrics. 2010;125:e358–e366. doi: 10.1542/peds.2009-0136. [DOI] [PubMed] [Google Scholar]
- Volpe J. Neonatal Seizures. N Engl J Med. 1973;289:413–416. doi: 10.1056/NEJM197308232890807. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Hayakawa F, Okumura A. Neonatal EEG: a powerful tool in the assessment of brain damage in preterm infants. Brain Dev. 1999;21:361–372. doi: 10.1016/s0387-7604(99)00034-0. [DOI] [PubMed] [Google Scholar]
- Wusthoff CJ, Dlugos D, Gutierrez-Colina AM, Wang A, Cook N, Donnelly M, Clancy RR, Abend NS. Incidence of electrographic seizures during therapeutic hypothermia for neonatal encephalopathy. J Child Neurol. 2011;26:724–728. doi: 10.1177/0883073810390036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wusthoff CJ, Shellhaas RA, Clancy RR. Limitations of single-channel EEG on the forehead for neonatal seizure detection. J Perinatol. 2009;29:237–242. doi: 10.1038/jp.2008.195. [DOI] [PubMed] [Google Scholar]
- Wyatt JS, Gluckman PD, Liu PY, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912–921. doi: 10.1542/peds.2006-2839. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Okumura A, Fukuda M. Epilepsies and epileptic syndromes starting in the neonatal period. Brain Dev. 2010 doi: 10.1016/j.braindev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Zimbric MR, Sharpe CM, Albright KC, Nespeca MP. Three-channel electroencephalogram montage in neonatal seizure detection and quantification. Pediatr Neurol. 2011;44:31–44. doi: 10.1016/j.pediatrneurol.2010.08.014. [DOI] [PubMed] [Google Scholar]