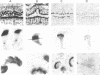
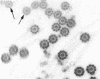
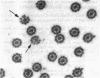
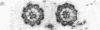Abstract
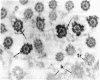
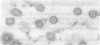
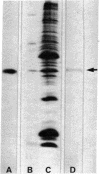
Tubulin, actin, and myosin have been localized in isolated demembranated ciliated cells from quail oviduct by immunocytochemistry in both light and electron microscopy by using purified antibodies. The peripheral doublets and the central tubules are stained by the antitubulin whereas the kinetosomes are poorly stained. Actin antibodies clearly stain the axonemes, but only on the proximal-half portion, whereas myosin antibodies stain a small area of the axonemes just above the ciliary neck region.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G. Isolation of ciliated or unciliated basal bodies from the rabbit oviduct. J Cell Biol. 1974 Feb;60(2):393–404. doi: 10.1083/jcb.60.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. Peroxidase labelled antibody and Fab conjugates with enhanced intracellular penetration. Immunochemistry. 1971 Dec;8(12):1175–1179. doi: 10.1016/0019-2791(71)90395-8. [DOI] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R. E., Lane B. P., Miller F. Electron microscope demonstration of tubulin in cilia and basal bodies of rat tracheal epithelium by the use of an antitubulin antibody. J Cell Biol. 1977 Nov;75(2 Pt 1):586–592. doi: 10.1083/jcb.75.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R. E., Lane B. P., Miller F. Identification of contractile proteins in basal bodies of ciliated tracheal epithelial cells. J Histochem Cytochem. 1980 Nov;28(11):1189–1197. doi: 10.1177/28.11.7000888. [DOI] [PubMed] [Google Scholar]
- Gounon P., Karsenti E. Involvement of contractile proteins in the changes in consistency of oocyte nucleoplasm of the newt Pleurodeles waltlii. J Cell Biol. 1981 Feb;88(2):410–421. doi: 10.1083/jcb.88.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Gröschel-Stewart U., Schreiber J., Mahlmeister C. Production of specific antibodies to contractile proteins, and their use in immunofluorescence microscopy. I. Antibodies to smooth and striated chicken muscle myosins. Histochemistry. 1976 Feb 26;46(3):229–236. doi: 10.1007/BF02462786. [DOI] [PubMed] [Google Scholar]
- Karsenti E., Guilbert B., Bornens M., Avrameas S., Whalen R., Pantaloni D. Detection of tubulin and actin in various cell lines by an immunoperoxidase technique. J Histochem Cytochem. 1978 Nov;26(11):934–947. doi: 10.1177/26.11.363933. [DOI] [PubMed] [Google Scholar]
- Kleve M. G., Clark W. H., Jr Association of actin with sperm centrioles: isolation of centriolar complexes and immunofluorescent localization of actin. J Cell Biol. 1980 Jul;86(1):87–95. doi: 10.1083/jcb.86.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Piperno G., Huang B., Luck D. J. Two-dimensional analysis of flagellar proteins from wild-type and paralyzed mutants of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1600–1604. doi: 10.1073/pnas.74.4.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., Luck D. J. An actin-like protein is a component of axonemes from Chlamydomonas flagella. J Biol Chem. 1979 Apr 10;254(7):2187–2190. [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres L. D., Renaud F. L., Portocarrero C. Studies on reactivated cilia. II. Reactivation of ciliated cortices from the oviduct of Anolis cristatellus. Exp Cell Res. 1977 Sep;108(2):311–320. doi: 10.1016/s0014-4827(77)80038-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]