Abstract
Background:
Review of intracranial gunshot wounds (GSWs) undergoing emergent neurosurgical intervention despite a very low Glasgow Coma Scale (GCS) score on admission in order to identify predictors of good outcome, with correlates to recent literature.
Methods:
A retrospective review of select cases of GSWs presenting to our trauma center over the past 5 years with poor GCS requiring emergent neurosurgical intervention and a minimum of 1-year follow-up.
Results:
Out of a total of 17 patients who went to the operating room (OR) for GSW to the head during this period, 4 cases with a GCS < 5 on admission were identified. All cases required a hemicraniectomy to alleviate cerebral swelling. Two cases presented with a unilaterally blown pupil due to raised intracranial pressure. The remaining 2 cases had equal and reactive pupils. One patient with a GCS of 3 and a significant bilateral pattern of parenchymal bullet injury was initially assessed in moribund status but rallied and received a delayed hemicraniectomy on day 7. Three out of 4 patients are functionally independent at 1-year follow-up. The fourth patient who received a delayed decompression remains wheelchair dependent.
Conclusion:
Victims of GSWs can have good outcomes despite having a very poor admission GCS score and papillary abnormalities. Factors predicting good outcomes include the following: time from injury to surgical intervention of < 1 h; injury to noneloquent brain; and absence of injury to midbrain, brainstem, and major vessels.
Keywords: Decompressive hemicraniectomy, gunshot wounds, head, intracranial
INTRODUCTION
Intracranial gunshot wound (GSW) injuries are one of the most deadly traumas. Each year in the United States, there are an estimated 70,000 victims of GSWs resulting in 30,000 deaths.[4] The high morbidity and mortality of gunshot injuries to the head impose a staggering burden on hospitals, families, court systems, and society.[6] Intracranial GSWs came to national and international attention last year when American Congresswoman Gabrielle Giffords was shot in the head in an assassination attempt on January 8, 2011. Her dramatic and successful recovery after neurosurgical intervention is a case study in the successful management of GSWs, highlighting the need for evidence-based treatment algorithms to decrease the morbidity and mortality of these injuries.
The prevalence and characteristics of traumatic brain injury (TBI) secondary to GSWs is strikingly variable in societies and reflects both the global and local scenery of violence. Since every gun/projectile combination is associated with a unique pattern of injury, war injuries differ significantly from civilian GSWs.[10,13] Pertaining to the urban location of our trauma center, we have chosen to focus on civilian GSWs, which typically occur in the setting of homicide and suicide attempts or during accidents.
Civilian GSWs are most commonly inflicted by small-caliber (0.22–0.38), low-velocity (less than 304.8 m/s) projectiles, delivered over a range of less than 50 m. These ballistic characteristics are important because the total kinetic energy (KE) imparted to the cranium and brain from the projectile can be estimated from KE = ½mv2 with m and v equal to the mass and velocity of the bullet, respectively. Moreover, GSWs with smaller, lower velocity projectiles cause less damage than that seen with high-velocity projectiles used in warfare.[5] Due to advances in surgical techniques and critical care inpatient management, there has been a marked reduction in mortality and morbidity from patients admitted with TBI over the last 30 years.[15] However, the postoperative mortality rates for GSWs remain well above 20%.[3] Due to a lack of definitive prospective studies, there are no high-grade recommendations for the management of these patients.
Recent research has focused on developing preoperative predictors of survival and functional outcome in patients with GSWs. Most studies use the admission Glasgow Coma Scale (GCS) score as a valuable prognosticator of outcome. Clark and colleagues reported death in all patients with a GCS score of 3 and questioned the value of any surgical intervention in these cases.[5] Other studies have recommended aggressive management for patients with arrival GCS score > 8 due to high mortality despite surgery in patients with GCS score < 8.[6,9] Also, patients with fixed and dilated pupils have higher mortality rates despite surgery when compared with patients with active papillary reflexes.[6,9,13,18]
The routine use of computed tomography (CT) scans during trauma evaluation for patients with GSWs has had a significant impact on management. CT scans provide a quick, noninvasive method of assessing the location and extent of intracranial injury. Numerous studies have attempted to correlate the location of intracranial injury with outcome. The most cited prognostic factors with regard to CT findings are the presence of intracranial hematomas, ventricular injury, posterior fossa involvement, and multilobar injury.[5,14,17,18] Kim and colleagues performed a retrospective analysis of radiographic CT scans from patients with through-and-through gunshot wounds and found, in particular, that bullets passing through a specific midline area of the deep-seated parenchyma of the brain approximately 4 cm above the dorsum sellae was inevitably associated with fatal outcome.[14] Given the attention that has been garnered to Congresswoman Gifford's GSW to the head, we herein present a case series of select patients who sustained intracranial GSWs, detailing their surgical management and clinical outcomes. We then perform a root cause analysis of these patients to explain why they made a good recovery despite a first impression of a devastating injury, and finally, make correlations with current literature regarding predictive factors for outcomes.
MATERIALS AND METHODS
A retrospective chart review was performed of all adult patients admitted to our trauma center over the past 5 years with a GSW to the head. We identified 28 patients who were admitted alive to our center during this interval, 17 of whom qualified for emergent surgical intervention. Out of those 17 patients, only 4 patients were identified, who had a very poor GCS score < 5 on arrival but still received immediate neurosurgical intervention for their injuries, which was performed by the senior author (EMK).
RESULTS
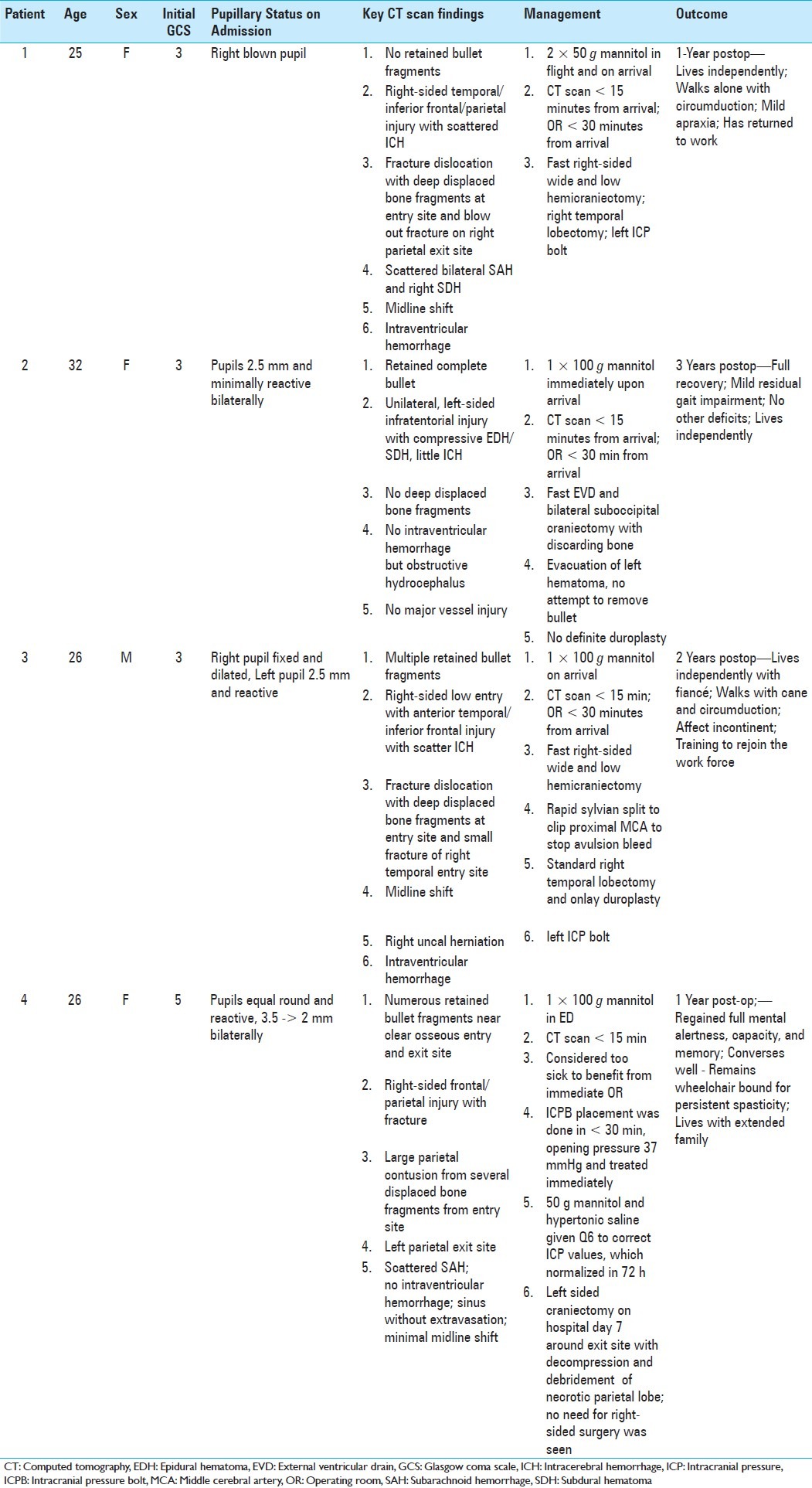
Case 1: A 25 year-old woman with a close range GSW to the right inferior frontal, temporal, and parietal lobes
History – This 25 year-old female sustained a close range GSW to the head. She had a short downtime, was intubated at the scene by the emergency medical response team (EMT) with a GCS of 3 and a right blown pupil. She reached our medical center within 30 minutes in stable condition and was rushed to the CT scanner.
Imaging highlights—Head CT demonstrated a bullet trajectory through the right cheek exiting the right parietal skull with no retained bullet fragments with a blow-out fracture of the right parietal skull [Figure 1a and b]. There was intraparenchymal hemorrhage of the right temporal lobe with some midline shift and blood in the temporal horn of the right lateral ventricle [Figure 1c and d].
Figure 1.
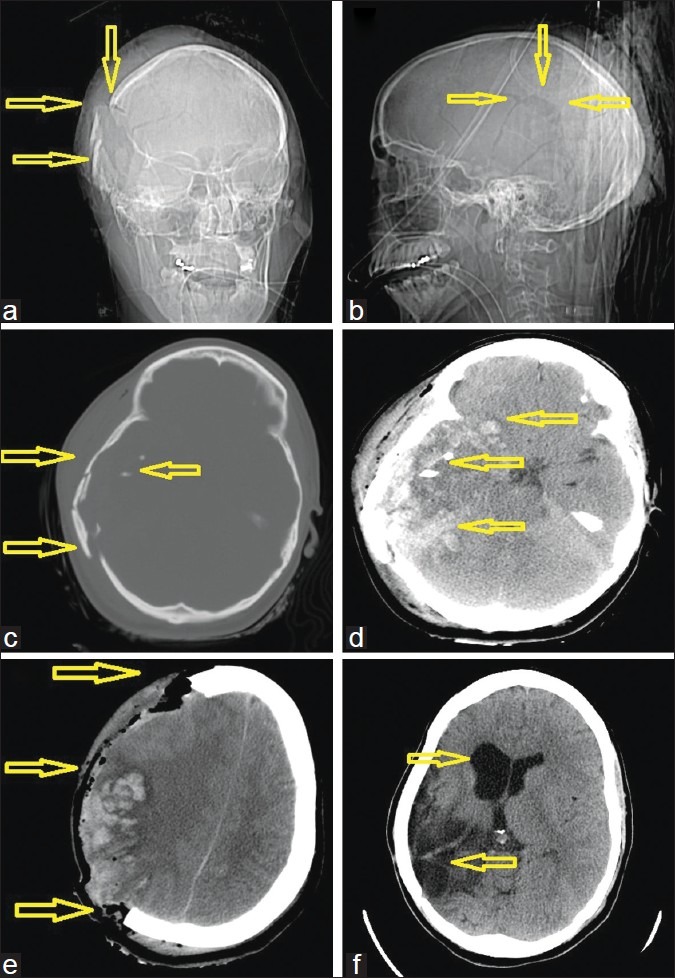
A 25-year-old woman with a gunshot wound to the right cerebral hemisphere. (a) computed tomography (CT)-scout image anterior view and (b) lateral view. Arrows demonstrate the right parietal blowout fracture representing the exit wound. (c) Admission CT, axial views in bone windows, clearly showing the right side shattered calvaria, which was likely acting as a hinge-craniotomy thus accommodating some swelling and at the same level (d) soft tissue windows, demonstrating the extensive multilobar hemorrhagic contusions caused by the pressure wave. (e) Immediate postoperative scan after a wide hemicraniectomy with expansile onlay duroplasty. (f) Postoperative scan after the patient had undergone delayed reconstruction with an allograft cranioplasty as indicated by the arrows
Management—The patient received intravenous mannitol en route to the hospital. Time from arrival to hospital, transport to the CT scanner, and to the OR was less than 30 minutes. Despite a low GCS with a unilateral blown pupil, several factors were in this patient's favor, namely: a low and lateral trajectory of the bullet; an intact right orbit; no injury to the proximal middle cerebral artery (MCA) and its branches; no scatter of displaced bone fragments to cause further parenchymal injury; and the function of the blow out fracture acting as a “hinge hemicraniectomy,” thus effectively decompressing the swollen brain [Figure 1c]. A wide right-sided decompressive hemicraniectomy (>100 cm2) was performed. The bone was discarded due to an open and contaminated wound. A right temporal lobectomy was performed and an intracranial pressure (ICP) monitor was inserted on the left side [Figure 1e].
Clinical outcome—We maintained the patient on mannitol and monitored her ICPs in the intensive care unit (ICU). She did not have any rise in ICP above normal following the operation, was following commands on postoperative day 2, and was subsequently extubated. She initially had a dense left hemiparesis, which improved significantly after a course of rehabilitation. She remains functionally independent 2 years following her injury and is able to walk alone with some circumduction, has some apraxia, but is conversant and able to work.
Case 2—A 30 year-old woman with a close range GSW to the back of the head
History—This 30-year-old woman sustained a solitary close range GSW to the left side of the back of her head [Figure 2a and b]. She had a GCS score of 3 at the scene and her pupils were 2.5 mm and minimally reactive. She had a short downtime, was intubated by the EMT, and transported to hospital within 20 minutes. She was hemodynamically stable and was taken to the CT scanner within 15 minutes upon arrival.
Figure 2.
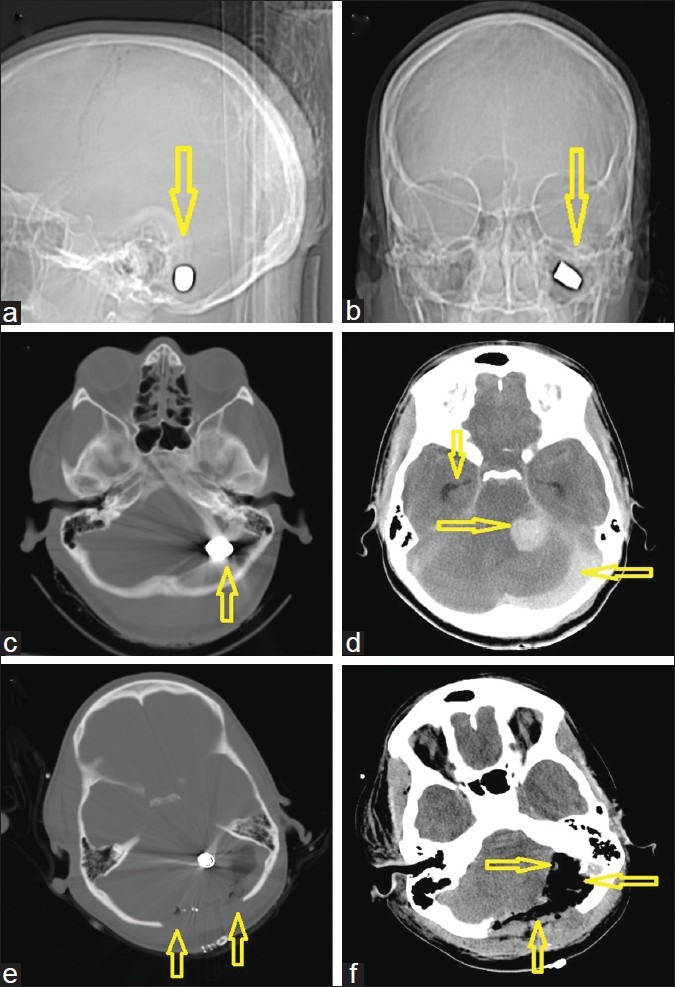
A 30-year-old woman with a gunshot wound to the back of the head. (a) computed tomography (CT)-scout image anterior view and (b) lateral view. Arrows demonstrate the left suboccipital target site with an intact bullet (c) Admission CT, axial views in bone windows, showing the bullet which was lodged behind the petrous bone (d) Soft tissue windows, demonstrating the focal posterior fossa hemorrhage near the cerebellopontine angle, but leaving an intact brain stem. A second significant hemorrhage/collection under the occipital bone is visible possibly originating from the sinus. Note the dilated temporal horns bilaterally indicative of obstructive hydrocephalus prior to external ventricular drain insertion. (e) Immediate postoperative scan after a wide bilateral suboccipital midline craniectomy with expansile onlay duroplasty. (f) This scan shows a postoperative scan of the same slice location in soft tissue windows after decompression as indicated by the arrows
Imaging highlights—Head CT showed a lodged bullet near the left cerebellopontine angle with associated traumatic subarachnoid hemorrhage, an infratentorial epidural hematoma, and an intraparenchymal hematoma with pneumocephalus [Figure 2c and d].
Management—Despite the bullet being lodged in the posterior fossa, we opted for emergent surgical intervention because we felt that several factors were in her favor: (1) the bullet had missed the brainstem; (2) there was no apparent injury to the posterior cerebral circulation; and (3) the fourth ventricle was closed and intact. An intraoperative right-sided external ventricular drain (EVD) was inserted prior to performing a bilateral suboccipital craniectomy with evacuation of the blood clot without disturbing the lodged bullet fragment [Figure 2e and f].
Clinical outcome—The patient had ICP monitoring in the ICU for 48 h. There were no spikes in her ICPs above normal and she was following commands on postoperative day 2. The EVD remained in situ for 6 days. Her postoperative exam was significant for nystagmus, double vision, and scanning speech. She was initially nonambulatory due to balance issues. She was sent to rehabilitation and at 3 years following her injury, she has made a wonderful recovery with almost no gait impairment and no permanent deficits.
Case 3—A 26-year-old man with a distant range GSW to the right ear and orbit
History—This 26-year-old man sustained a GSW to the right side of the head at a distance. The bullet entered through his right ear with no apparent exit wound. He had a GCS score of 3 at the scene, was intubated by EMT, and transported to our center within 20 minutes. His right pupil was fixed and dilated and his left pupil was 2.5 mm and reactive.
Imaging highlights—CT scan showed multiple retained bullet fragments primarily on the right extracranial aspect of the skull but penetrated bilaterally into the intracranial compartment [Figure 3a–c]. This was associated with a large right inferotemporal blowout fracture as well as a fracture of the middle cranial fossa. The bullet entry point through the right middle temporal fossa was low and generated ricochet fragments in the left frontal area [Figure 3c]. There was also a right temporal intraparenchymal hematoma with associated subarachnoid hemorrhage [Figure 3d and e].
Figure 3.
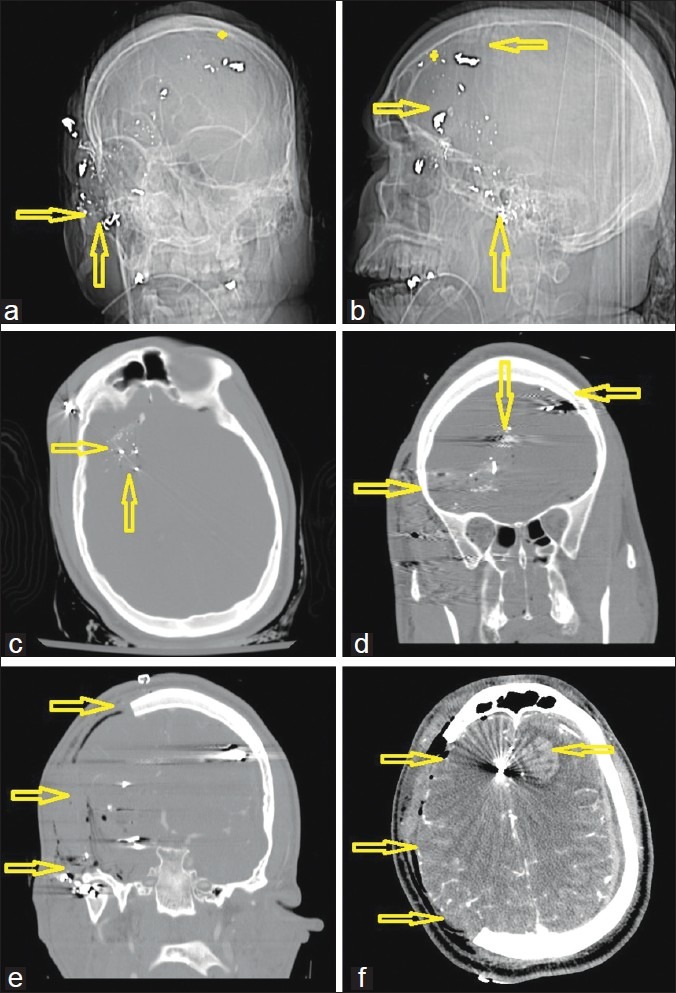
A 26-year-old man with a gunshot wound to the right ear and orbit. (a) Computed tomography (CT)-scout image anterior view and (b) lateral view. Double arrows demonstrate the entry site at the right zygoma with a completely disintegrated bullet. (c) Admission CT, axial views, bone windows, showing the metal artifacts from the bullet case, which was scattered along the sphenoid bone. (d) Preoperative coronal reconstructions of the bone windows, demonstrating the midline crossing bullet trajectory as indicated by the arrows (star represents ricochet point). Note that the path does not cross a sinus or the ventricles. (e) Immediate postoperative result also in coronal reconstructions after a wide right hemicraniectomy with duroplasty. (f) Postoperative CT scan on the same day in soft tissue windows after decompression as indicated by the arrows. Note the new left frontal hemorrhagic contusion from the bullet fragment that was bounced off the inner table at the ricochet point
Management—The patient was brought to the OR within 30 minutes upon arrival to the hospital. Fortunately, the bullet trajectory had missed critical midline structures (i.e. anterior cerebral arteries, the fornix, and ventricles) and the intraparenchymal hemorrhage was confined and limited to the anterior temporal pole. The bullet had severed several branches of the MCA, which led to some persistent active bleeding. A wide and low right-sided hemicraniectomy was emergently performed with splitting of the right sylvian fissure, identification, and clipping of 2 avulsed branches of the proximal MCA [Figure 3e–h]. A standard anterior temporal lobectomy was then performed with onlay duraplasty and placement of a left-sided ICP bolt.
Clinical outcome—The patient was monitored in the ICU and was found to be obeying commands on postoperative day 4. He had a dense right-sided hemiparesis but has made a very significant recovery 2 years following his injury, now living independently with his fiancée, using a cane as a walking aid, and retraining to join the workforce.
Case 4—A 26-year-old woman with a penetrating GSW to the head
History—This 26-year-old woman sustained a single GSW to the right-side of her head. EMT found her with a GCS score of 5. Her pupils were equal, round, and reactive at 3.5 mm bilaterally. She was intubated, brought to the hospital, and rushed to the CT scanner in less than 30 minutes.
Imaging highlights—CT showed a right frontoparietal fracture with several displaced bone fragments and a large parietal intraparenchymal contusion [Figure 4a–c]. The bullet passed through the falx, causing a subdural hematoma and crossed into the left parietal lobe before exiting the left side of the skull [Figure 4d–f]. There was no intraventricular blood and minimal midline shift.
Figure 4.
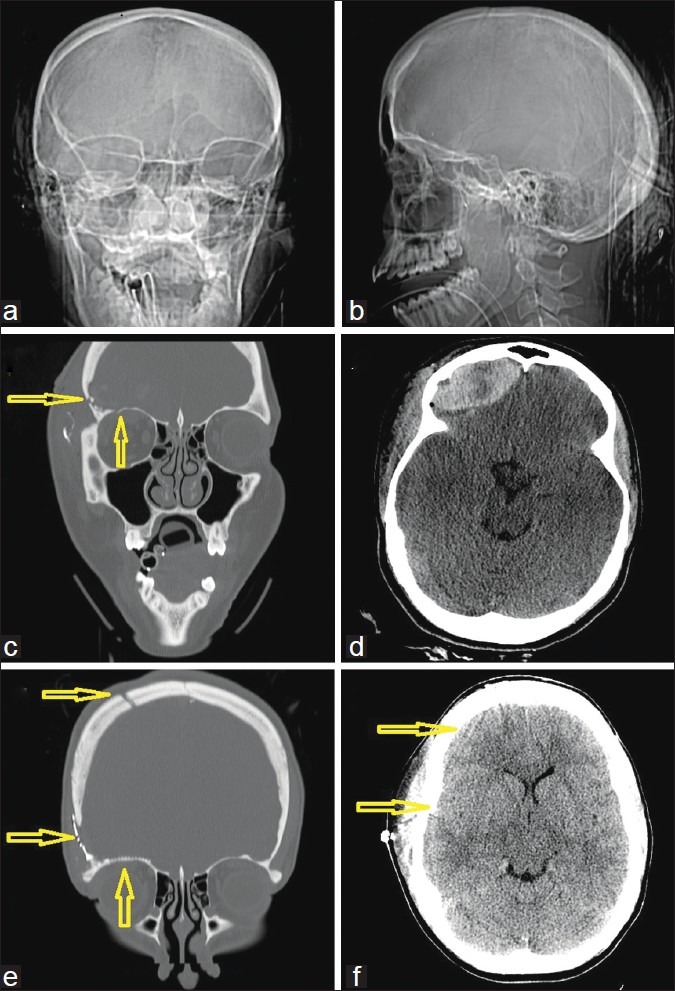
A 26-year-old woman with a penetrating gunshot wound to the head. (a) Computed tomography (CT)-scout image anterior view and (b) lateral view. Several arrows demonstrate the right frontoparietal entry site and a completely disintegrated bullet. (c) Admission CT, axial views, bone windows, showing the metal artifacts from the bullet case, which was scattered along the path. (d) Admission CT, axial views, corresponding soft tissue windows, also showing metal artifacts from the bullet case as well as bone fragments, scattered along the path and some perifocal hypodensity likely indicating edema. (e) Preoperative CT, coronal reconstructions in bone windows, demonstrating the midline crossing bullet trajectory from left to right as indicated by the arrows. Note that the matching CT venogram in (f) shows that the path did not cross the superior sinus or the ventricle
Management—Given her poor GCS score at the scene and bilateral injury through eloquent cortex, it was felt that she would likely have a poor outcome and thus although the OR was ready on standby for emergent neurosurgical intervention, we initially opted for conservative management, with insertion of an ICP bolt, measuring an initial value of 37 mmHg. She was maintained on round the clock doses of mannitol and hypertonic saline and the ICPs normalized over the next 72 h, at which time she was observed to have some motor recovery in her arms. Because the patient demonstrated some meaningful neurologic improvement with normalized ICPs, and further study of the CT angiogram did not show any damage to the superior sagittal sinus, we attributed the persistent edema to injury caused by the bone fragments as opposed to venous congestion, and thus opted to take the patient to the OR in a delayed fashion on admission day 7 for decompression. A left hemicraniectomy was performed with removal of the necrotic parietal lobe and boney debris.
Clinical outcome—The patient had an extended stay in a rehabilitation facility where she regained full mental alertness and cognitive skills, however, she remains wheelchair-bound with persistent spasticity in all 4 limbs.
For ease of reference, we have summarized the above cases in table format [Table 1].
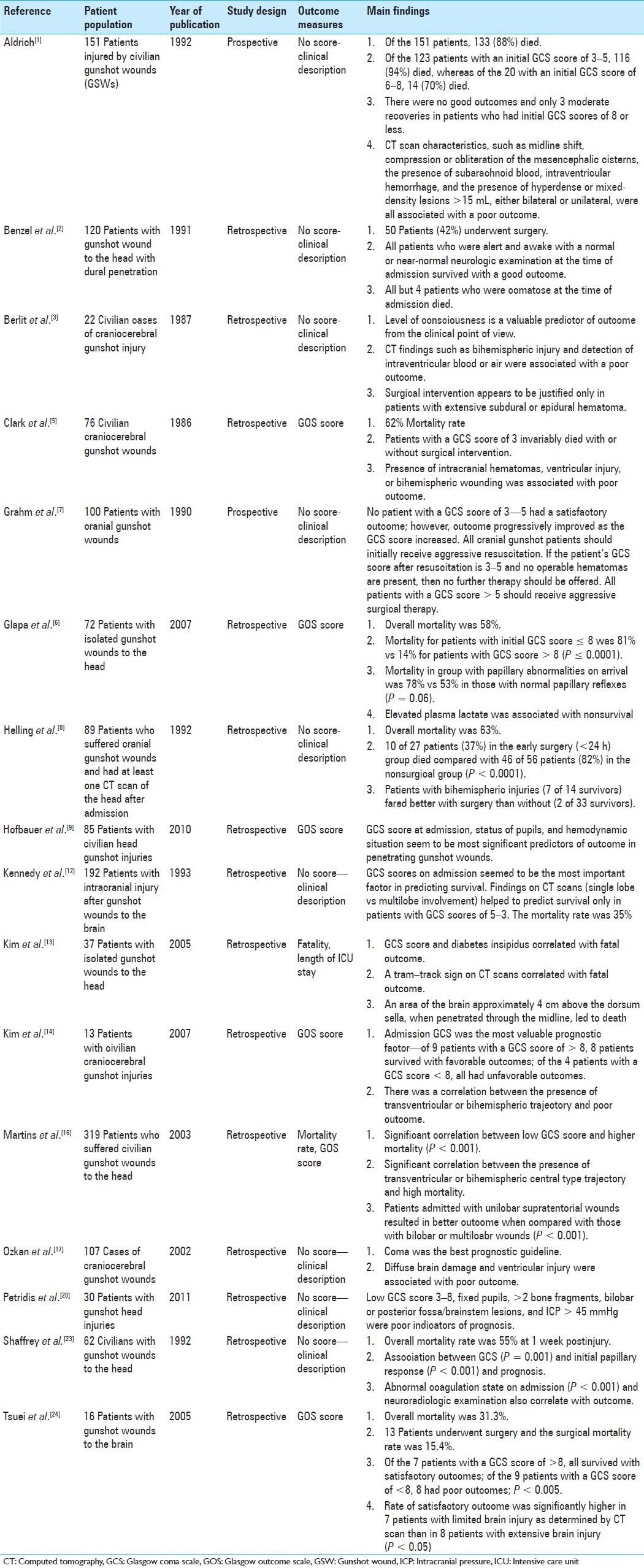
Table 1.
Evidentiary table summarizing a review of the literature on gunshot wounds to the head
DISCUSSION
GSWs to the head are challenging to manage. Prehospital mortality remains very high and the inhospital mortality is as high as 95%.[21] There have been numerous studies in the past 30 years analyzing predictive factors for outcomes in patients with GSWs. Based on our case series, we present a discussion of the current literature in the context of our cases as well as what we feel are important factors to help decrease morbidity and mortality in our patients. We have also formulated an evidentiary table for ease of reference [Table 2].
Table 2.
Summary of the four cases
Clinical status on arrival
Many studies have determined that a low GCS score and pupillary abnormalities portend poor outcome despite neurosurgical intervention. Rosenfeld and colleagues reported 8.1%, 35.5%, and 90.5% survival rates in patients presenting with GCS scores between 3 and 5, 6 and 8, and >9, respectively.[21] Kennedy et al. reported a mortality rate of 91.4% and 61.2% in patients with GCS scores of 3–4, and of 5–8.[12] This has led to guidelines suggesting that the most significant prognostic factor of outcome and mortality is the GCS score on admission.[19]
There is controversy concerning the GCS score as a sole measure for deciding whether aggressive surgical management is warranted. Clark and colleagues reported that there were no survivors among patients who underwent surgery with a preoperative score less than 5.[5] Grahm and colleagues recommended that all cranial gunshot patients initially receive aggressive resuscitation, but that if the patient's GCS score after resuscitation is 3 to 5 and no operable hematomas are present, then no further therapy should be offered. On the other hand, all patients with a GCS score > 5 should receive aggressive surgical therapy.[7] More recent studies have recommended aggressive surgical management in patients with GCS scores ≥ 8.[6,14] Our results differ greatly from those reported by Clark et al., as 3 out of 4 of our patients had a GSC of 3, received emergent surgical decompression and survived. We believe that our results were due to a combination of a short downtime between injury to resuscitation by the EMT, prompt and efficient management by the trauma team shuffling the patient from the trauma bay to the CT scan and up to the OR, minimizing the time that the brain has spent under pressure.
Numerous studies have also attempted to use pupillary status upon admission as a prognostic indicator. Kaufman and colleagues in their study of 480 patients showed a strong correlation of dilated, nonreactive, and unequal pupils with poor outcome.[11] In a retrospective study of 62 patients, Shaffrey and colleagues showed a strong correlation between nonreactive pupils and mortality.[23]
In our cases series, we had patients with GCS ranging from 3 to 5, and our patients had surprisingly good clinical outcomes, regaining a meaningful functional status with the exception of 1 patient, whom we took to the OR in a delayed fashion. We feel that other factors need to be taken into consideration apart from a uniform GCS cutoff value for not performing aggressive surgical intervention, including the following: considering whether the injury pattern involves eloquent cortex and/or midbrain structures; involvement of multiple and/or bilateral lobar regions of the brain; injury to neurovasculature; hence, the pattern of injury is an important predictor of clinical outcome. With respect to pupillary abnormalities, we agree that bilaterally fixed and dilated pupils are a sign of poor prognosis, however, our case series demonstrated that in the case of a unilateral fixed pupil, that timely treatment at the scene with mannitol, in correlation with neuroimaging showing cerebral edema or hematoma, and prompt surgical evacuation and decompression, can still lead to good functional outcomes. Thus, each patient should be considered on a case-by-case basis taking into account clinical picture on admission with relevant findings on neuroimaging.
Correlating surgical intervention based on neuroimaging
It is now standard treatment that a patient with a GSW to the head receives a head CT scan in a timely fashion. The acquisition of CT scans must never be postponed even in spite of a high presenting GCS because approximately 10% of patients with nonpenetrating injury (without breach of the neurocranium) may still suffer a significant intracranial injury and will benefit from neurosurgical intervention.[6,22] The reverse is also true: even in the setting of a GSC as low as 3–5, a young patient deserves surgical intervention if a defined space-occupying lesion is identified on admission CT.[14] CT scanning offers valuable information about the extent and location of penetrating injury that can be used for prognosis. Previous studies have suggested that patients with bi- or multilobar injury and intraventricular injury have worse outcome and increased mortality.[3,5,7] Kim and colleagues conducted a retrospective analysis of radiographic imaging findings in 32 patients with isolated gunshot wounds to the head. By centering a cartesian coordinate system on the dorsum sellae, the authors were able to perform vector measurements of bullet trajectories from entrance to exit points. They found that a recurrent pattern defined by a dark center outlined on either side by dense or hyperdense tracks, coined tram–track sign, was associated with fatal injury (P = 0.005). In addition, proximity of bullet passage through a particular area of the brain, which localized from the skull base to approximately 4 cm above the dorsum sella and was termed the zona fatalis, correlated with fatality. In fact, several patients who received transventricular bicortical injuries survived as long as this particular zone was not violated, as was found in our patients with the bilateral cortical injury (Cases 2 and 4).
Recommendations for management of gunshot injuries to the head
Workup leading to operative management
Our mantra in managing these patients is “Time is brain” as only a fraction of the neurologic harm arises at the moment of impact. The prognostically relevant damage most frequently evolves in the time span after the incident and any achieved outcome correlates to the time between injury and the time of intervention and postoperative management.[13] Clear thinking and an efficient course of action is crucial and it requires a well preinstructed and well-trained team. If the patient presents in a comatose condition with a GCS score of 3–5, we initiate ICP treatment en route or in the trauma bay prior to acquisition of any imaging adhering to ATLS protocols or equivalent algorithms. Isotonic volume resuscitation, normotonia, normorhythmia, and normothermia should be achieved. The patient should be stabilized before the patient is transported to the CT scanner to avoid decompensation in the CT scanner. A standardized CT scan (5-mm cuts parallel to the skull base in brain/bone windows with immediate reformats in coronal and sagittal planes) is invaluable in assisting the neurosurgeon in their decision for surgical intervention.
Operative management
Here are the 15 most important bullet points we consider a recipe for success:
Transport the patient yourself from the CT scanner straight to the OR.
Transfer him or her from the stretcher onto the prepositioned OR bed (call from the scanner about the side!) and position the patient in pins.
Apply only the utmost necessary padding to save time.
Pin the patient in a Mayfield headrest at defined angles (if possible straight angles: either supine or fully lateral). This helps to keep your orientation once you are inside the head and landmarks are no longer visible.
Do not waste time being fancy: shave the entire hemiconvexity (be generous!).
Scratch the skin for landmarks and pay attention to especially the midline!
Use a quick prep-solution: for example, soaking beta-iodine sponges followed by Prevail.
Do not waste time using local anesthesia/epinephrine for better hemostasis.
Incise with the goal of creating a generous flap to accommodate postoperative swelling.
Perform a large hemicraniectomy (>100 cm2) for optimal decompression and do not forget to prepare a Frazier bur-hole in all posterior fossa lesions, so you can place an EVD intraoperative or postoperatively, as needed.
Save the bone flap in the bone bank if possible, so you do not waste time on a second abdominal incision to minimize your time from the OR to the ICU.
Always irrigate copiously with antibiotic solution: for example, bacitracin-enriched saline.
Perform a wide durotomy and decompress before you place any dural tenting stitches because this achieves effective decompression earlier and you spare the brain some more vital minutes under pressure.
Close the dura provisionally, for example, with an onlay allograft (eg, Duragen) to prevent adhesion via scarring of the brain surface to the undersurface of the muscle flap. A subgaleal cerebrospinal fluid (CSF) collection is of no concern here, since you will be back for a cranioplasty at a later date.
Close the muscle flap in 3 layers only as to save some time: (1) muscle and fascia with 1–0, (2) galea with inverted 2–0, and (3) skin with a running stitch.
Perioperative management
An adequate cerebral perfusion pressure is critical to keeping brain tissue alive. We advocate a low threshold for transferring the patient to an experienced center with an ICU setting for ICP monitoring. The critical cerebral perfusion pressure threshold for ischemia lies around 50–60 mmHg. If you notice a rapid decline in the neurologic examination, you should initiate therapy immediately with hyperventilation, elevation of head of the bed to 30 degrees, administration of mannitol (ca. 1 g/kg), and transport the patient to the CT scanner to evaluate for evolving intracranial pathology.
Do not over-resuscitate with IV fluids and do not use fluids, such as half normal saline (0.45%), which act as hypoosmolar volume expanders and may create significant further edema. Keep ICP low with osmotic diuresis (eg, furosemide 20 mg IV once). In addition, initiate continuous blood pressure (BP) monitoring with an arterial line, and avoid systolic drops of BP < 90 mmHg. Do not be hesitant to use blood products early to support hemostasis and to avoid coagulopathy or disseminated intravascular coagulation as observed with thromboplastin release from brain injury or massive blood loss.
We support the use of broad-spectrum antibiotic prophylaxis for 48–72 h for prevention of meningitis secondary to a CSF leak. Vancomycin 1 gm Q12 h, gentamycin 80 mg Q 8 h; and flagyl 500 mg Q6 h will suffice. We do not maintain this regimen beyond day 3 because most CSF leaks will close spontaneously within 48 h or will be taken care of during surgery. However, if there is a significant amount of bony debris translocated into the parenchyma, 7–10 days of antibiotic coverage is reasonable.
We also recommend the use of antiepileptic medications for a minimum of the first 7 days of injury for subarachnoid hemorrhage. If significant parenchymal damage has incurred, we continue these medications until the patient is assessed by a rehabilitation team or at time of follow-up at 3 months. Phenytoin is the drug of choice and clearly decreases the incidence of early posttraumatic seizures and associated morbidity.
Postoperative considerations
All basic postoperative prophylactic strategies in trauma care apply for GSW victims as well. A comprehensive discussion of postoperative strategies is beyond the scope of this discussion. Briefly, most patients have a rough course during the first 3–7 days since swelling seems to peak during postoperative days 3–4 and raises in ICP should be managed early and aggressively. Wean all patients from the ventilator as soon as possible. If the patient does not regain consciousness soon, opt for an early tracheostomy and gastrostomy tube placement in anticipation of a long postoperative course. Ensure full caloric intake by day 7 postinjury to support wound healing. Combine mechanical DVT prophylaxis via compression stockings or intermittent pneumatic compression stockings with low-molecular weight heparin or low-dose unfractionated heparin as early as postoperative day 2. Provide a bowel regimen and supply adequate pain medications. Support the patient with anxiolytics and sedation in the setting of ICU care. Mobilize the patient early to prevent deep vein thromboses and pulmonary embolisms, as well as formation of pressure sores.
Final considerations
Congresswoman Gabrielle Gifford's near-fatal shooting and her remarkable recovery to her current condition has shed some light on the incredible tasks a neurosurgeon faces in the setting of a GSW to the head. Most of these injuries leave the victims either dead or with devastating neurologic injuries; however, there are several factors that can help push for a favorable outcome. First, the patients that beat the odds usually receive expert care very quickly. Congresswoman Gifford was brought to the hospital within minutes after the incident and was operated at about 38 min from the arrival at the hospital. Second, if the patient is young, in good health, and the injury is “limited,” the chances of a meaningful survival sometimes increase dramatically. If the trauma is isolated to the head, and the patient remains hemodynamically stable with good oxygenation, the odds for survival also increase. Finally, the bullet trajectory will define precise anatomic conditions that can increase the likelihood of survival, thus an ad hoc interpretation of the bullet trajectory and its impact on vital and eloquent brain regions can aid the clinician in predicting prognosis and is a vital skill set. For trauma neurosurgeons, a competent analysis of these predictors for outcome sets the scene for the often heroic attempts at saving these lives.
CONCLUSION
In summary, we present a case series of select patients with GSWs to the head, 4 of whom presented with very low GCS scores and pupillary abnormalities, but still had favorable functional outcomes. We cannot overstress the importance of neuroimaging in helping the trauma team decide which patients would benefit from timely neurosurgical intervention, and also offer our perioperative recommendations in decreasing the morbidity and mortality of these often tragic injuries.[24]
Contributor Information
David J. Lin, Email: dlin4@bidmc.harvard.edu.
Fred C. Lam, Email: fclam@bidmc.harvard.edu.
Jeffrey J. Siracuse, Email: jsiracus@bidmc.harvard.edu.
Ajith Thomas, Email: athomas6@bidmc.harvard.edu.
Ekkehard M. Kasper, Email: ekasper@bidmc.harvard.edu.
REFERENCES
- 1.Aldrich EF, Eisenberg HM, Saydjari C, Foulkes MA, Jane JA, Marshall LF, et al. Predictors of mortality in severely head-injured patients with civilian gunshot wounds: A report from the NIH Traumatic Coma Data Bank. Surg Neurol. 1992;38:418–23. doi: 10.1016/0090-3019(92)90109-z. [DOI] [PubMed] [Google Scholar]
- 2.Benzel EC, Day WT, Kesterson L, Willis BK, Kessler CW, Modling D, et al. Civilian craniocerebral gunshot wounds. Neurosurgery. 1991;29:67–71. doi: 10.1097/00006123-199107000-00011. discussion 71-2. [DOI] [PubMed] [Google Scholar]
- 3.Berlit P, Jaschke W, Tornow K. [Gunshot injuries of the skull.Computerized tomography findings and clinical course] Nervenarzt. 1987;58:300–4. [PubMed] [Google Scholar]
- 4.(CDC) CfDCaP. Nonfatal and fatal firearm-related injuries - United States. MMWR Morb Mortal Wkly Rep. 1999;48:1029–34. [PubMed] [Google Scholar]
- 5.Clark WC, Muhlbauer MS, Watridge CB, Ray MW. Analysis of 76 civilian craniocerebral gunshot wounds. J Neurosurg. 1986;65:9–14. doi: 10.3171/jns.1986.65.1.0009. [DOI] [PubMed] [Google Scholar]
- 6.Glapa M, Kourie JF, Doll D, Degiannis E. Early management of gunshot injuries to the face in civilian practice. World J Surg. 2007;31:2104–10. doi: 10.1007/s00268-007-9220-2. [DOI] [PubMed] [Google Scholar]
- 7.Grahm TW, Williams FC, Jr, Harrington T, Spetzler RF. Civilian gunshot wounds to the head: A prospective study. Neurosurgery. 1990;27:696–700. doi: 10.1097/00006123-199011000-00005. discussion 700. [DOI] [PubMed] [Google Scholar]
- 8.Helling TS, McNabney WK, Whittaker CK, Schultz CC, Watkins M. The role of early surgical intervention in civilian gunshot wounds to the head. J Trauma. 1992;32:398–400. doi: 10.1097/00005373-199203000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Hofbauer M, Kdolsky R, Figl M, Grunauer J, Aldrian S, Ostermann RC, et al. Predictive factors influencing the outcome after gunshot injuries to the head-A retrospective cohort study. J Trauma. 2010;69:770–5. doi: 10.1097/TA.0b013e3181c81d7d. [DOI] [PubMed] [Google Scholar]
- 10.Jandial R, Reichwage B, Levy M, Duenas V, Sturdivan L. Ballistics for the neurosurgeon. Neurosurgery. 2008;62:472–80. doi: 10.1227/01.neu.0000316015.05550.7a. discussion 480. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman HH, Levy ML, Stone JL, Masri LS, Lichtor T, Lavine SD, et al. Patients with Glasgow Coma Scale scores 3, 4, 5 after gunshot wounds to the brain. Neurosurg Clin N Am. 1995;6:701–14. [PubMed] [Google Scholar]
- 12.Kennedy F, Gonzalez P, Dang C, Fleming A, Sterling-Scott R. The Glasgow Coma Scale and prognosis in gunshot wounds to the brain. J Trauma. 1993;35:75–7. doi: 10.1097/00005373-199307000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Kim KA, Wang MY, McNatt SA, Pinsky G, Liu CY, Giannotta SL, et al. Vector analysis correlating bullet trajectory to outcome after civilian through-and-through gunshot wound to the head: Using imaging cues to predict fatal outcome. Neurosurgery. 2005;57:737–47. discussion 737-47. [PubMed] [Google Scholar]
- 14.Kim TW, Lee JK, Moon KS, Kwak HJ, Joo SP, Kim JH, et al. Penetrating gunshot injuries to the brain. J Trauma. 2007;62:1446–51. doi: 10.1097/01.ta.0000222909.31666.db. [DOI] [PubMed] [Google Scholar]
- 15.Lu J, Marmarou A, Choi S, Maas A, Murray G, Steyerberg EW. Mortality from traumatic brain injury. Acta Neurochir Suppl. 2005;95:281–5. doi: 10.1007/3-211-32318-x_58. [DOI] [PubMed] [Google Scholar]
- 16.Martins RS, Siqueira MG, Santos MT, Zanon-Collange N, Moraes OJ. Prognostic factors and treatment of penetrating gunshot wounds to the head. Surg Neurol. 2003;60:98–104. doi: 10.1016/s0090-3019(03)00302-1. discussion 104. [DOI] [PubMed] [Google Scholar]
- 17.Ozkan U, Kemaloglu S, Ozates M, Aydin MD. Analysis of 107 civilian craniocerebral gunshot wounds. Neurosurg Rev. 2002;25:231–6. doi: 10.1007/s101430100173. [DOI] [PubMed] [Google Scholar]
- 18.Pabuscu Y, Bulakbasi N, Kocaoglu M, Ustunsoz B, Tayfun C. A different approach to missile induced head injuries. Comput Med Imaging Graph. 2003;27:397–409. doi: 10.1016/s0895-6111(03)00015-6. [DOI] [PubMed] [Google Scholar]
- 19.Part 1: Guidelines for the management of penetrating brain injury. Introduction and methodology. J Trauma. 2001;51(Suppl 2):S3–6. [PubMed] [Google Scholar]
- 20.Petridis AK, Doukas A, Barth H, Mehdorn M. Outcome of craniocerebral gunshot injuries in the civilian population. Prognostic factors and treatment options. Cen Eur Neurosurg. 2011;72:5–14. doi: 10.1055/s-0029-1241850. [DOI] [PubMed] [Google Scholar]
- 21.Rosenfeld JV. Gunshot injury to the head and spine. J Clin Neurosci. 2002;9:9–16. doi: 10.1054/jocn.2001.0949. [DOI] [PubMed] [Google Scholar]
- 22.Semple PL, Domingo Z. Craniocerebral gunshot injuries in South Africa--A suggested management strategy. S Afr Med J. 2001;91:141–5. [PubMed] [Google Scholar]
- 23.Shaffrey ME, Polin RS, Phillips CD, Germanson T, Shaffrey CI, Jane JA. Classification of civilian craniocerebral gunshot wounds: A multivariate analysis predictive of mortality. J Neurotrauma. 1992;9(Suppl 1):S279–85. [PubMed] [Google Scholar]
- 24.Tsuei YS, Sun MH, Lee HD, Chiang MZ, Leu CH, Cheng WY, et al. Civilian gunshot wounds to the brain. J Chin Med Assoc. 2005;68:126–30. doi: 10.1016/S1726-4901(09)70233-3. [DOI] [PubMed] [Google Scholar]


