Abstract
Background:
Between 2005 and 2010, we treated patients with hydrocephalus related to cerebral metastases, who were not good candidates for surgical resection by either endoscopic third ventriculostomy (ETV) or ventriculoperitoneal shunting (VPS). Patients were excluded from ETV if they had a clinical history suggestive of non-obstructive hydrocephalus, including: (1) history of infection or ventricular hemorrhage and (2) leptomeningeal carcinomatosis. The rest of the patients were treated with VPS.
Methods:
We analyzed the clinical outcome of these patient cohorts, to determine whether the efficacy of VPS was compromised due to a history of infection, ventricular hemorrhage, or leptomeningeal carcinomatosis, and compared these results to those patients who underwent ETV.
Results:
Sixteen patients were treated with ETV and 36 patients were treated with VPS. The overall efficacy of symptomatic palliation was comparable in the ETV and VPS patients (ETV = 69%, VPS = 75%). In both groups, patients with more severe hydrocephalic symptoms such as nausea, vomiting, and lethargy were more likely to benefit from the procedure. The overall complication rate for the two groups was comparable (ETV = 12.6%, VPS = 19.4%), although the spectrum of complications differed. The overall survival, initial Karnofsky performance status (KPS), and three-month KPS, were similarly comparable (median survival: ETV 3 months, VPS 5.5 months; initial KPS: ETV = 66 ± 7, VPS = 69 ± 12; 3 months KPS: ETV = 86 ± 7, KPS = 84 ± 12).
Conclusion:
VPS remains a reasonable option for poor RPA grade metastasis patients with hydrocephalus, even in the setting of a previous infection, hemorrhage, or in those with leptomeningeal disease. Optimal treatment of this population will involve the judicious consideration of the relative merits of VPS and ETV.
Keywords: Cerebral metastasis, endoscopic third ventriculostomy, palliation, Ventriculoperitoneal shunting
INTRODUCTION
Estimates suggest that 1 – 5% of the patients with cerebral metastasis suffer from either obstructive or communicating hydrocephalus.[3,20] Relative to patients with other forms of hydrocephalus, patients who develop hydrocephalus related to cerebral metastases exhibit an extremely poor prognosis. In most series,[15,20,21] approximately 50% of the patient will expire three months after treatment, with only 10% surviving for one year. Systemic disease progression remains the major cause of demise in this population.
In terms of treatment options, good surgical candidates, with reasonable remaining life expectancy, who present with obstructive hydrocephalus, may be treated with surgical resection of the offending lesion. Poor surgical candidates, including patients with poor survival expectations, or lesions not amenable to surgical resection without excess surgical morbidity, have been historically treated with ventriculoperitoneal shunting.
The Recursive Partitioning Analysis (RPA) classification is a scale for patients with cerebral metastasis commonly used to prognosticate survival expectations, and is often used as a guide to therapeutic intervention. This classification was developed by the Radiation Therapy Oncology Group (RTOG), who integrated the variables of age, KPS, and systemic oncological disease status into an ordinal scale.[8,12,19,25] RPA class I is defined by age < 65 and absence of significant neurocognitive dysfunction (KPS > 70), controlled systemic disease, and no extracerebral metastasis; RPA class III is defined by KPS < 70; all other patients are defined as RPA class II. The respective median survival for RPA class I, II, and III are 7.1, 4.2, and 2.3 months, respectively.[7]
We set out to determine whether ETV is advantageous in treating patients with obstructive hydrocephalus from cerebral metastases. The use of ETV is attractive because, relative to VPS, it offers an effective means of palliation, with theoretically fewer associated complications, as no mechanical device is implanted. Between 2005 and 2010, we treated poor RPA grade patients with ETV for obstructive hydrocephalus if they showed the following characteristics: (1) No history of infection or ventricular hemorrhage, and (2) no leptomeningeal carcinomatosis. These criteria were set forth to exclude patients with hydrocephalus that was potentially attributable to non-obstructive causes, as the efficacy of ETV in these settings remained controversial. The remaining patients were treated with VPS. We previously reported the efficacy of ETV in terms of palliating symptoms related to hydrocephalus.[3] Here we analyzed the clinical outcomes of the patients treated with VPS and compared them to those treated with ETV.
MATERIALS AND METHODS
Patient selection and outcome evaluation
Clinical information was obtained after Institutional Review Board approval. We reviewed the records of surgical cases performed at our institution between the years of 2005 and 2010, and identified 52 patients with brain metastasis not amenable to surgical resection, who suffered from hydrocephalus, and who subsequently underwent a cerebrospinal fluid (CSF) diversion procedure. This constituted approximately 8% of the Beth Israel Deaconess Medical Center (BIDMC) cancer patients, who underwent surgery for symptoms related to cerebral metastasis during the five-year period. Patients were first screened to determine whether they met the criteria for the ETV procedure. Patients with (1) a history of infection or ventricular hemorrhage and (2) leptomeningeal carcinomatosis were excluded from ETV, as the efficacy of ETV in the setting of non-obstructive hydrocephalus remained controversial. Patients fulfilling these criteria and whose anatomy was favorable for ETV, underwent this procedure. Patients not meeting these criteria underwent VPS placement.
Assessment of the outcome was determined by a review of medical records documenting the neurological and functional status. Success of VPS was defined on a clinical basis as a partial or complete relief of symptoms. Failure was defined as no change or deterioration in condition regardless of imaging findings. The outcome was assessed in the immediate postoperative period after surgery (n = 36), and at the three- and six-month follow-ups (n = 25, 7, respectively). Postoperative computed tomography (CT) images from all treated patients were reviewed and compared with the preoperative CT images.
Surgery
All VPS procedures were performed under general anesthesia with endotracheal intubation. Preoperative magnetic resonance (MR) or CT imaging of the head was used for surgical planning. The proximal shunt catheter was passed free hand into the lateral ventricle using anatomical landmarks for guidance. Fixed-pressure Medtronic® Delta® Valves, Performance Level 1.0, were used in all cases. The distal catheter was tunneled subcutaneously and placed into the peritoneal space under direct visualization, either through open methods or with laparoscopic assistance, depending on the surgeon's preference. Patients with leptomeningeal disease had an Ommaya reservoir placed through a separate incision, in addition to VPS placement, during the same operation. Postoperative CT scans of the head were performed on all patients, to confirm the appropriate intraventricular positioning of the proximal catheter and to rule out any procedural complications such as hemorrhages.
Endoscopic third ventriculostomy was performed as previously described.[3] All third ventriculostomies were performed under image-guidance, employing frame-based stereotaxy. After induction of anesthesia, a Riechert / Mundinger stereotactic head frame (Inomed GmbH, Emmendingen, Germany) was secured onto the patient's cranium, and 1.25 mm slice-thickness contrast-enhanced computerized tomography (CT) imaging of the head was subsequently acquired using an intraoperative scanner (Ceretom, Neurologica, MA). CT images were processed to yield three-dimensional reconstructions using a software by Inomed (Praezis 3.0, Germany). Using these reconstructions, an optimal trajectory through the Foramen of Monroe, offering an en-face view of the floor of the third ventricle, was planned. The basilar tip was identified, and a target position was selected, to avoid contact with the basilar tip. Care was also taken to avoid trajectory intersection with any visualized vessels. The aiming bow was then mounted onto the stereotactic head frame. A burr hole was then placed as the entry point based on the planned trajectory. A 4.0 mm introducer sheath was inserted along the trajectory into the lateral ventricle under the guidance of the rigid frame. Entry into the lateral ventricle was verified by withdrawal of CSF. An oval Oi HandyPro endoscope (4.0 × 2.6 mm, Storz, Tutlingen, Germany), equipped with three channels for instrument, suction, and irrigation, was then advanced into the lateral ventricle with stereotactic guidance. Under direct vision, the neuroendoscope was then further advanced into the third ventricle. A disconnected monopolar electrode was used to perforate the thinnest portion of the floor of the third ventricle, just anterior to the two mamillary bodies. The perforation was enlarged using a Fogarty No. 4 balloon catheter inflated to a diameter of approximately 5 mm.
RESULTS
Patient population, overall survival, and karnofsky performance status
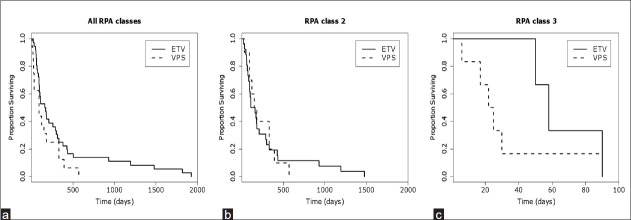
Between 2005 and 2010, we treated 36 patients with VPS for symptomatic hydrocephalus secondary to cerebral metastases. Patient characteristics are shown in Table 1. There were 16 men and 20 women. The age of the patients ranged from 31 to 78 years, with a median age of 59 years. By RPA classification, seven patients were class I (19.4%), twenty-seven patients were class II (75%), and two patients were class III (5.6%). The primary metastatic tumors in this series originated from lung (n = 13), melanoma (n = 10), breast (n = 9), renal (n = 3) cancer, and colon (n = 1). Nine patients had leptomeningeal spread (LMS) at the time of treatment. Eleven patients had previous interventricular hemorrhage from the tumor. The mean KPS score of patients who survived at least three months from the time of initial VPS procedure was 69 ± 12 and improved to 84 ± 12 at the three-month follow-up [Figure 1]. The median overall survival after VPS was 5.5 months [Figure 2a]. These outcomes are comparable to the previously reported ETV outcomes, whose median overall survival was three months and whose three-month KPS improved to 86 ± 7 from an initial KPS of 66 ± 7.[3] Notably, nine patients survived beyond a year (25%) and two patients survived beyond five years (one had metastasis that originated from the breast and the other from the lung). The only patient to survive less than one month had an intraventricular tumor present, with leptomeningeal spread.
Table 1.
Clinical summary of the 36 patients who underwent ventriculoperitoneal shunting
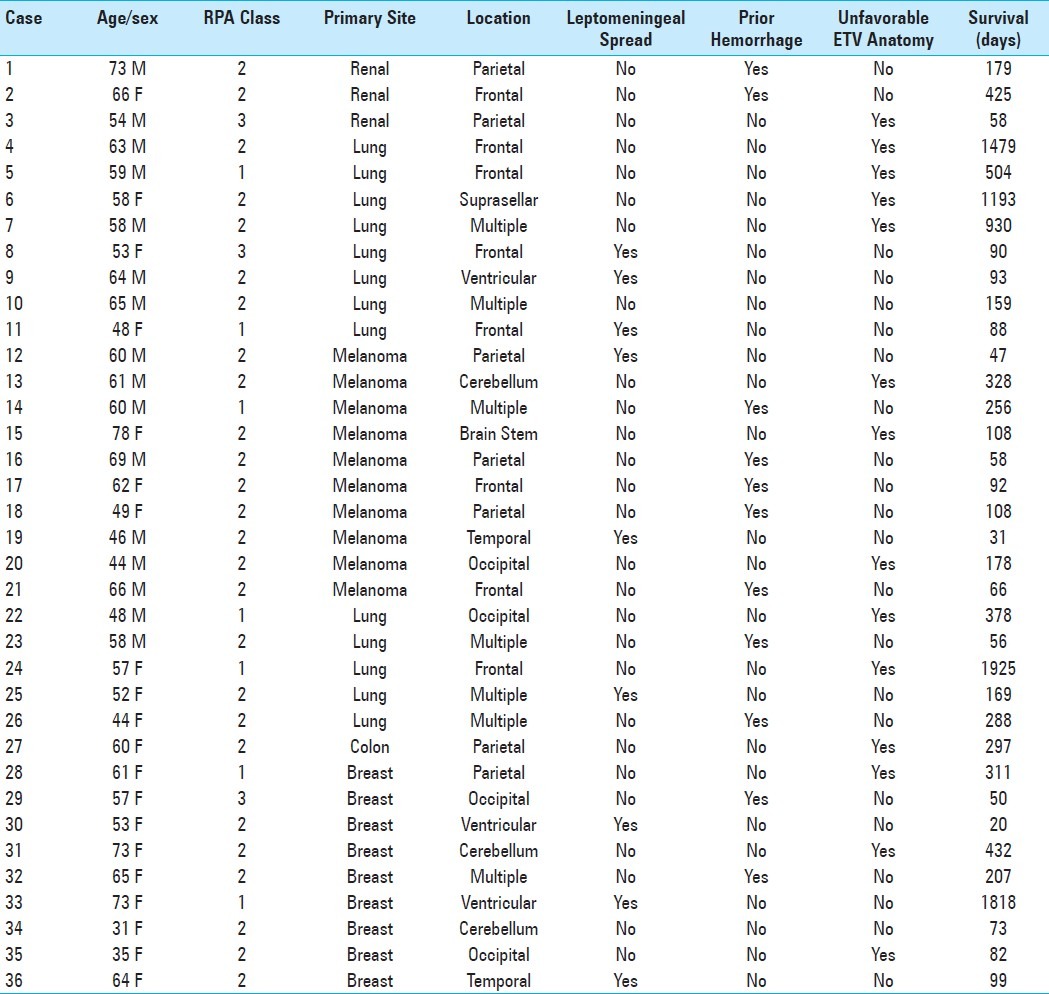
Figure 1.
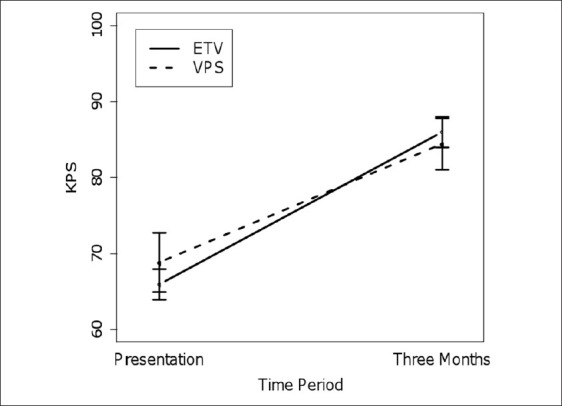
Mean Karnofsky Performance Scores of patients surviving at least three months after treatment
Figure 2.
Kaplan-Meier plots comparing survival of patients undergoing ETV vs VPS for (a) all patients, (b) RPA class 2 patients only, and (c) RPA class 3 patients only.
Indications and outcomes
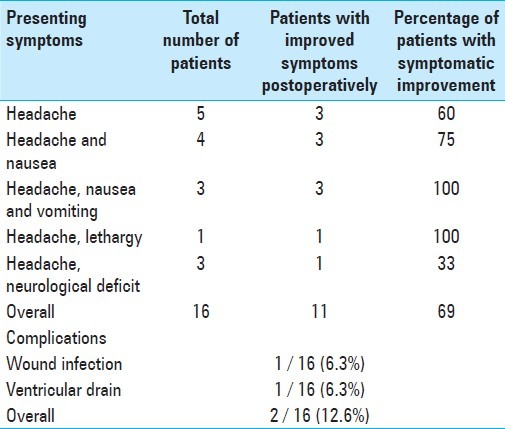
All 36 patients complained of either isolated headache or headache with various other symptoms, including: Nausea, vomiting, and lethargy (visual changes, papillary edema, etc.). All patients had imaging workup demonstrating obstruction of CSF flow tract by cerebral metastases or leptomeningeal disease with ventriculomegaly. Postoperative CT scans showed decreased ventricular size and radiographic resolution of hydrocephalus in 32 of the 36 patients treated with VPS. The four patients without radiographic changes had symptomatic improvement from hydrocephalus postoperatively, despite no measurable change in ventricle size. Symptomatic improvement was observed after palliative VPS in 27 of the 36 patients (77%). A breakdown of the specific symptoms is shown in Table 2. Six procedures were performed for severe headache alone, without associated symptoms. Three of these patients (60%) reported improvement from headache post VPS. Ten procedures were performed for patients who presented with headache and nausea. Eight of these patients (80%) reported improved clinical symptoms post VPS. Fifteen procedures were performed for patients who presented with either headache, nausea, and vomiting or headache with lethargy. All fifteen patients demonstrated symptomatic improvement after VPS (100%). Only one of the five patients (20%) with a focal neurological deficit experienced improvement following the procedure. Overall, patients who presented with headache associated with nausea, vomiting, or lethargy were more likely to benefit from VPS (P < 0.001) than those who presented with isolated headache. These outcomes were comparable to those previously reported for the patients who underwent ETV during the same time period (Table 3 is presented here for comparison).[3] Of the ETV-treated patients, those with symptomatic improvement immediately post operation, and who were symptom-free at three months, remained symptom-free at six months
Table 2.
Summary of the indications, efficacy, and morbidity in patients treated with ventriculoperitoneal shunt
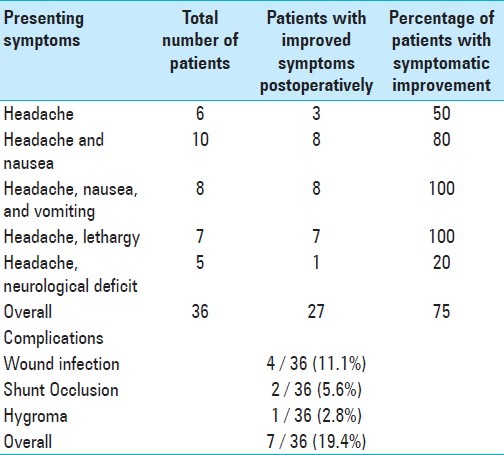
Table 3.
Summary of the indications, efficacy, and morbidity in patients treated with endoscopic third ventriculostomy. Reproduced from a prior publication for purposes of comparison[3]
Complications
There were a total of seven shunt failures or shunt-related complications that required subsequent surgery. One was due to the development of a hygroma. Two shunt failures were due to occlusion of the shunt system (5.6%). Of note, both occlusions occurred in patients who had prior interventricular hemorrhages. Neither of the shunt occlusions were a result of infection. This was confirmed through the microbiological testing of CSF obtained from a shunt tap.
Four VPS patients developed shunt infections following surgery (11.1%). Three of the four infections occurred in patients with leptomeningeal disease, after the ommaya reservoir had been accessed. The remaining infection occurred in a patient, who previous to VPS placement, had an external ventricular drain in place for seven days, due to a prior intraventricular hemorrhage. Of note, there were no incidents of intraperitoneal dissemination of a tumor observed. However, we cannot exclude the possibility of a clinically silent intraperitoneal spread that may have gone undiagnosed, as routine abdominal imaging was not performed.
DISCUSSION
Here we report our experience in the treatment of hydrocephalus from cerebral metastasis by VPS, when the treatment modality is determined by a specified algorithm that selects patients who would likely benefit from ETV. The median survival in patients who underwent CSF-diversion for hydrocephalus was 5.5 months, which is in line with other literature reports for metastasis-related hydrocephalus.[15–17,20] The modern series of palliative CSF diversions for elevated intracranial pressure (ICP) in the adult metastatic population demonstrate symptomatic improvement in approximately 70-80% and a complication rate of 10-30%.[5,9,20,21] It is important to note that our results, unlike prior series, have been obtained despite selecting patients who had greater risk factors for shunt failure, such as, prior infection and hemorrhage.[4,14,24]
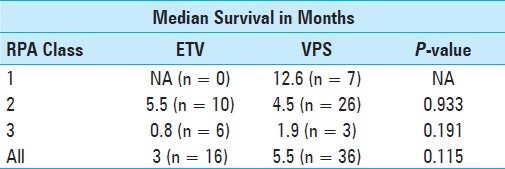
When compared to our previously reported ETV results of patients treated during the same time period, the clinical efficacy and complication rates were similar.[3] The selection criteria were designed to optimize ETV success, by excluding patients with disease processes compromising CSF resorption, and selecting such patients to undergo VPS instead. These included prior intracranial hemorrhages, history of intracranial infections, and those with leptomeningeal spread.[4,14,24] These same criteria could also be risk factors for VPS complications and failure.[16] Despite working with a patient population that was at a higher risk for shunt complications, our VPS success rate was 77% for symptomatic improvement, comparing similarly to the 69% rate of symptomatic improvement in the cohort of patients who underwent ETV.[3] There was no significant difference in the number of VPS patients who required a second surgery for shunt revision (19%, n = 7) and the number of ETV patients (13%, n = 2) who required a second surgery for either wound revision or persistence of symptoms (P = 0.94). The median survival difference between the patients receiving VPS (5.5 mos) and those with ETV (3 mos) was not statistically significant, after correcting for RPA status [Figures 2b–c, Table 4]. Although the focus of this analysis was on the poor grade RPA patients (RPA class II and III), the overall data set (including RPA class I patients) is shown in Figure 2a, to demonstrate that the overall survival of the patients shown here was comparable to those presented in the general literature.
Table 4.
Comparison of survival between ventriculoperitoneal shunt and endoscopic third ventriculostomy. P-value from Chi square analysis
Review of the four shunt infections revealed that three of the infections occurred after a co-existing Ommaya reservoir had been accessed. Data regarding the risk of infection from routine access of a ventricular reservoir in the existing literature was variable, with rates ranging from 0-20%.[1,2,10,13,22,23] On the one extreme, Lin et al. recently reported a series of 24 patients with leptomeningeal spread, treated with CSF reservoir-on / off valve-peritoneal shunts, for intrathecal chemotherapy administration, without any occurrence of infections.[16] On the other extreme, Richard et al. reported a 21.2% infection rate of Ommaya reservoirs in premature infants treated for post-hemorrhagic ventricular dilatation, some of whom had received intrathecal fibrinolytic therapy, in addition to serial reservoir taps. The general trend suggests that the rates of infection are higher in patients who receive pharmacological agents through the reservoir.[1,13,22] Our results were consistent with the higher range of infection rate for Ommaya access, as these patients had a 33% incidence of infection (three of nine patients). Of note, all three patients underwent intrathecal chemotherapy. This data highlighted the risk of surgery in the oncological population of patients, who might be immunocompromised secondary to a neoplastic process or systemic chemotherapy (or steroid replacement therapy), and the importance of a meticulous sterile technique should be reiterated when accessing the reservoir.
A central question in the treatment of poor RPA grade patients with cerebral metastases and hydrocephalus was, whether the patient should be treated at all. Certainly, any surgical intervention in poor RPA grade patients with cerebral metastases and hydrocephalus warranted judicious clinical judgment. Our clinical experience was that headache related to hydrocephalus was incapacitating, and often resistant to medical management. In this context, we generally found that minimally invasive CSF diversion procedures were suitable and rewarding, as the patient / family greatly appreciated the symptomatic relief and the subsequent decreased narcotic use. In many instances, treatment of the hydrocephalus re-established the opportunity for meaningful social interactions and improved the patient's quality of life. Additionally, there was a group of patients who would go on to survive and derive a long-term benefit from the procedure. In our series, 25% of the patients survived beyond a year, despite poor neurological examination on initial presentation.
The selection criteria for ETV in our study were designed to exclude patients with non-obstructive hydrocephalus (e.g., prior history of hemorrhage and infection). In this context, it is important to note the recent documentation of the efficacy for ETV, even in cases of communicating hydrocephalus, where CSF resorption is thought to be impaired.[6,11,18] Siomin et al. described the collective results from seven international medical centers where ETV was performed in 46 pediatric patients with hydrocephalus, after intraventricular hemorrhage, and another 42 pediatric patients with hydrocephalus after CSF infection.[24] The authors reported successful outcomes in 60.9 and 64.3% of these populations, respectively, with a complication rate of 14.9%.[24] Whether such results are applicable to the adult oncological patient population remains an open question.
There are several limitations to this study. A major limitation of this study is that it is a retrospective study from a single institution, and thus, the patient selection and their treatments are subject to selection bias. Additionally, treatments have been selected based on criteria that will maximize the likelihood of success in terms of ETV. As such, the study must not be interpreted as a direct comparison of ETV and VPS. Another limitation of this study involves the small sample size of the patient population. Most patients with cerebral metastasis either do not develop hydrocephalus or can be reasonably treated with surgical resection. CSF diversion as a sole treatment for hydrocephalus, in these patients, has constituted approximately 8% of the cerebral metastasis patients undergoing surgical intervention. Realizing the small sample size, we have nevertheless performed an analysis of the patients accumulated over the past five years, with the goal of assessing the efficacy of ETV and VPS in a timely manner. This said, our study is the first to rationally triage cerebral metastasis patients with hydrocephalus to ETV or VPS and compare the efficacy of these procedures using quantitative measures of functional outcome (e.g., KPS) and clinical outcome (overall survival).
CONCLUSION
Endoscopic third ventriculostomy and VPS can serve as complementary strategies for the treatment of poor RPA–class cancer patients with hydrocephalus related to cerebral metastasis. The observation that similar clinical efficacy is attained in selected ETV and VPS patients (and that this efficacy is comparable to a prior series where patients uniformly underwent VPS) suggests two key principles: First, a subset of patients can benefit from ETV, a procedure without implantation of a device or the need for intraperitoneal access. Second, the comparable clinical efficacy between VPS in patients with a prior history of infection, hemorrhage, and leptomeningeal disease, and ETV, suggests that reasonable efficacy can be achieved with VPS in this patient population. The results presented here must lay the foundation for future investigations that aim to refine the indications for ETV and VPS in the metastatic population.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2012/3/1/97/100185
Contributor Information
David D. Gonda, Email: dgonda@ucsd.edu.
Teddy E. Kim, Email: tekim@ucsd.edu.
Peter C. Warnke, Email: pwarnke@partners.org.
Ekkehard M. Kasper, Email: ekasper@bidmc.harvard.edu.
Bob S. Carter, Email: bobcarter@ucsd.edu.
Clark C. Chen, Email: clarkchen@ucsd.edu.
REFERENCES
- 1.Brouwer AJ, Groenendaal F, van den Hoogen A, Verboon-Maciolek M, Hanlo P, Rademaker KJ, et al. Incidence of infections of ventricular reservoirs in the treatment of post-haemorrhagic ventricular dilatation: A retrospective study (1992-2003) Arch Dis Child Fetal Neonatal Ed. 2007;92:F41–3. doi: 10.1136/adc.2006.096339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruinsma N, Stobberingh EE, Herpers MJ, Vles JS, Weber BJ, Gavilanes DA. Subcutaneous ventricular catheter reservoir and ventriculoperitoneal drain-related infections in preterm infants and young children. Clin Microbiol Infect. 2000;6:202–6. doi: 10.1046/j.1469-0691.2000.00052.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen CC, Kasper E, Warnke P. Palliative stereotactic-endoscopic third ventriculostomy for the treatment of obstructive hydrocephalus from cerebral metastasis. Surg Neurol Int. 2011;2:76.4. doi: 10.4103/2152-7806.82083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drake JM, Kulkarni AV, Kestle J. Endoscopic third ventriculostomy versus ventriculoperitoneal shunt in pediatric patients: A decision analysis. Childs Nerv Syst. 2009;25:467–72. doi: 10.1007/s00381-008-0761-y. [DOI] [PubMed] [Google Scholar]
- 5.Farahmand D, Hilmarsson H, Hogfeldt M, Tisell M. Perioperative risk factors for short term shunt revisions in adult hydrocephalus patients. J Neurol Neurosurg Psychiatry. 2009;80:1248–53. doi: 10.1136/jnnp.2007.141416. [DOI] [PubMed] [Google Scholar]
- 6.Gangemi M, Maiuri F, Buonamassa S, Colella G, de Divitiis E. Endoscopic third ventriculostomy in idiopathic normal pressure hydrocephalus. Neurosurgery. 2004;55:129–34. doi: 10.1227/01.neu.0000126938.12817.dc. discussion 134. [DOI] [PubMed] [Google Scholar]
- 7.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–51. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 8.Golden DW, Lamborn KR, McDermott MW, Kunwar S, Wara WM, Nakamura JL, et al. Prognostic factors and grading systems for overall survival in patients treated with radiosurgery for brain metastases: Variation by primary site. J Neurosurg. 2008;109(Suppl):77–86. doi: 10.3171/JNS/2008/109/12/S13. [DOI] [PubMed] [Google Scholar]
- 9.Hoh BL, Lang SS, Ortiz MV, Chi YY, Lewis SB, Pincus DW. Lower incidence of reoperation with longer shunt survival with adult ventriculoperitoneal shunts placed for hemorrhage-related hydrocephalus. Neurosurgery. 2008;63:70–4. doi: 10.1227/01.NEU.0000335072.32105.38. discussion 74-5.10. [DOI] [PubMed] [Google Scholar]
- 10.Hudgins RJ, Boydston WR, Gilreath CL. Treatment of posthemorrhagic hydrocephalus in the preterm infant with a ventricular access device 1998. Pediatric Neurosurg. 1998;29:309–13.11. doi: 10.1159/000028744. [DOI] [PubMed] [Google Scholar]
- 11.Kehler U, Gliemroth J. Extraventricular intracisternal obstructive hydrocephalus--A hypothesis to explain successful 3rd ventriculostomy in communicating hydrocephalus. Pediatr Neurosurg. 2003;38:98–101. doi: 10.1159/000068053. [DOI] [PubMed] [Google Scholar]
- 12.Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24:1295–304. doi: 10.1200/JCO.2005.04.6185. [DOI] [PubMed] [Google Scholar]
- 13.Kormanik K, Praca J, Garton HJ, Sarkar S. Repeated tapping of ventricular reservoir in preterm infants with post-hemorrhagic ventricular dilatation does not increase the risk of reservoir infection. J Perinatol. 2010;30:218–21. doi: 10.1038/jp.2009.154. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni AV, Drake JM, Mallucci CL, Sgouros S, Roth J, Constantini S. Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. J Pediatr. 2009;155:254–9 e1. doi: 10.1016/j.jpeds.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 15.Lee SH, Kong DS, Seol HJ, Nam DH, Lee JI. Ventriculoperitoneal shunt for hydrocephalus caused by central nervous system metastasis. J Neurooncol. 2011;104:545–51. doi: 10.1007/s11060-010-0512-2. [DOI] [PubMed] [Google Scholar]
- 16.Lin N, Dunn IF, Glantz M, Allison DL, Jensen R, Johnson MD, et al. Benefit of ventriculoperitoneal cerebrospinal fluid shunting and intrathecal chemotherapy in neoplastic meningitis: A retrospective, case-controlled study. J Neurosurg. 2011;115:730–6. doi: 10.3171/2011.5.JNS101768. [DOI] [PubMed] [Google Scholar]
- 17.Lokich J, Levine H, Nasser I. Malignancy-related hydrocephalus: Clinical features and results of ventricular peritoneal shunt procedure in three patients. Am J Clin Oncol. 1998;21:366–8. doi: 10.1097/00000421-199808000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell P, Mathew B. Third ventriculostomy in normal pressure hydrocephalus. Br J Neurosurg. 1999;13:382–5. doi: 10.1080/02688699943484. [DOI] [PubMed] [Google Scholar]
- 19.Niwinska A, Murawska M. New Breast Cancer Recursive Partitioning Analysis Prognostic Index in Patients with Newly Diagnosed Brain Metastases. Int J Radiat Oncol Biol Phys. 2011;82:2065–71. doi: 10.1016/j.ijrobp.2010.10.077. [DOI] [PubMed] [Google Scholar]
- 20.Omuro AM, Lallana EC, Bilsky MH, DeAngelis LM. Ventriculoperitoneal shunt in patients with leptomeningeal metastasis. Neurology. 2005;64:1625–7. doi: 10.1212/01.WNL.0000160396.69050.DC. [DOI] [PubMed] [Google Scholar]
- 21.Reddy GK, Bollam P, Caldito G, Willis B, Guthikonda B, Nanda A. Ventriculoperitoneal shunt complications in hydrocephalus patients with intracranial tumors: An analysis of relevant risk factors. J Neurooncol. 2011;103:333–42. doi: 10.1007/s11060-010-0393-4. [DOI] [PubMed] [Google Scholar]
- 22.Richard E, Cinalli G, Assis D, Pierre-Kahn A, Lacaze-Masmonteil T. Treatment of post-haemorrhage ventricular dilatation with an Ommaya's reservoir: Management and outcome of 64 preterm infants. Childs Nerv Syst. 2001;17:334–40. doi: 10.1007/s003810000418. [DOI] [PubMed] [Google Scholar]
- 23.Sandberg DI, Bilsky MH, Souweidane MM, Bzdil J, Gutin PH. Ommaya reservoirs for the treatment of leptomeningeal metastases. Neurosurgery. 2000;47:49–54. doi: 10.1097/00006123-200007000-00011. discussion 54-45. [DOI] [PubMed] [Google Scholar]
- 24.Siomin V, Cinalli G, Grotenhuis A, Golash A, Oi S, Kothbauer K, et al. Endoscopic third ventriculostomy in patients with cerebrospinal fluid infection and/or hemorrhage. J Neurosurg. 2002;97:519–24. doi: 10.3171/jns.2002.97.3.0519. [DOI] [PubMed] [Google Scholar]
- 25.Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: An analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510–4. doi: 10.1016/j.ijrobp.2007.06.074. [DOI] [PubMed] [Google Scholar]