Abstract
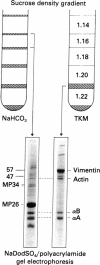
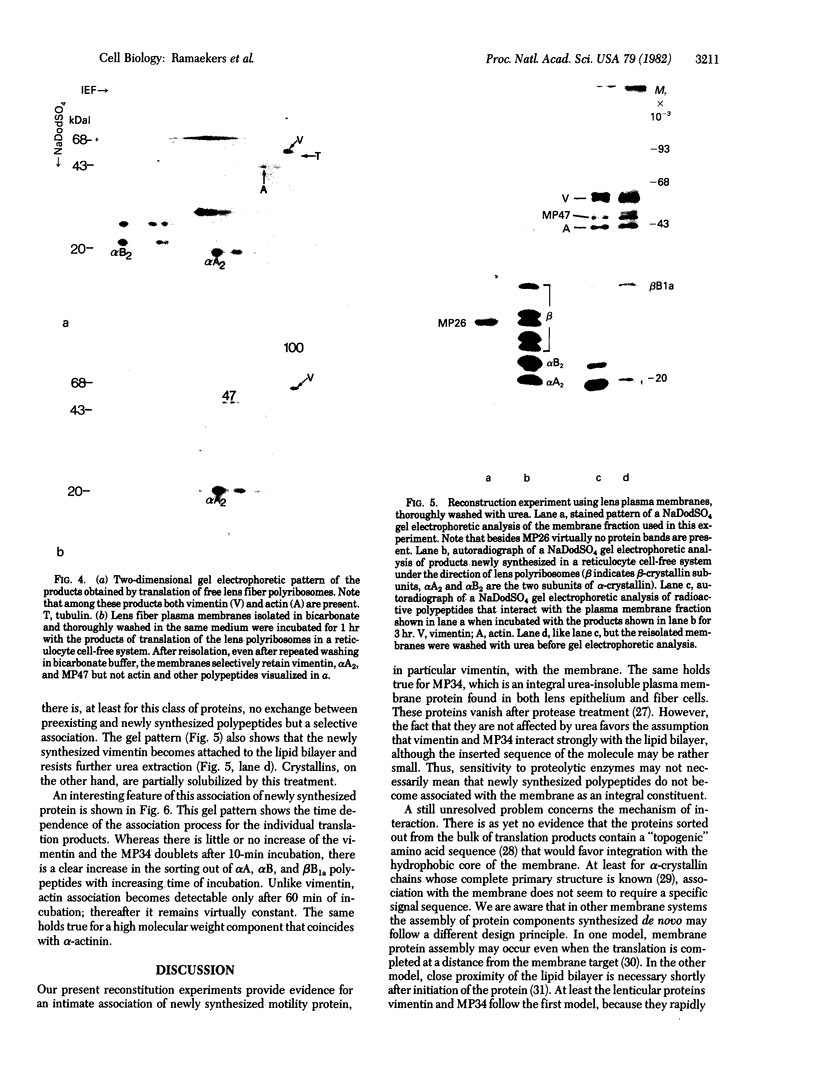
Electron microscopical features of the lens fiber plasma membrane-cytoskeleton complex are suggestive of an intimate association between the intermediate-sized filaments (IF) and the lipid bilayer. Biochemical analysis of this complex reveals the occurrence of an appreciable amount of vimentin as a protein subunit of lenticular IF. Additional evidence for association between IF and membranes is provided by the observation that newly synthesized vimentin is associated with plasma membranes added to a reticulocyte lysate programmed with lens polyribosomes. Concomitantly alpha-crystallin polypeptide chains (alpha A2) are also found associated with the plasma membrane together with a hitherto unidentified 47-kilodalton protein. Once associated with the lipid bilayer, the vimentin polypeptide resists urea treatment, suggesting that it has become an integral constituent associated with part of the membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asselbergs F. A., Koopmans M., Van Venrooij W. J., Bloemendal H. Post-translational assembly of lens alpha-crystallin in the reticulocyte lysate and in Xenopus laevis oocytes. Eur J Biochem. 1978 Nov 2;91(1):65–72. doi: 10.1111/j.1432-1033.1978.tb20937.x. [DOI] [PubMed] [Google Scholar]
- Benedetti E. L., Dunia I., Bloemendal H. Development of junctions during differentiation of lens fibers. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5073–5077. doi: 10.1073/pnas.71.12.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal H., Benedetti E. L., Bont W. S. Preparation and characterization of free and membrane-bound polysomes. Methods Enzymol. 1974;30:313–327. doi: 10.1016/0076-6879(74)30034-1. [DOI] [PubMed] [Google Scholar]
- Bloemendal H., Lenstra J. A., Dodemont H., Ramaekers F. C., Groeneveld A. A., Dunia I., Benedetti E. L. SV40-transformed hamster lens epithelial cells: a novel system for the isolation of cytoskeletal messenger RNAs and their translation products. Exp Eye Res. 1980 Nov;31(5):513–525. doi: 10.1016/s0014-4835(80)80010-8. [DOI] [PubMed] [Google Scholar]
- Bloemendal H., Schoenmakers J., Zweers A., Matze R., Benedetti E. L. Polyribosomes from calf-lens epithelium. Biochim Biophys Acta. 1966 Jul 20;123(1):217–220. doi: 10.1016/0005-2787(66)90179-1. [DOI] [PubMed] [Google Scholar]
- Bloemendal H., Vermorken A. J., Kibbelaar M., Dunia I., Benedetti E. L. Nomenclature for the polypeptide chains of lens plasma membranes. Exp Eye Res. 1977 Apr;24(4):413–415. doi: 10.1016/0014-4835(77)90155-5. [DOI] [PubMed] [Google Scholar]
- Bloemendal H., Zweers A., Vermorken F., Dunia I., Benedetti E. L. The plasma membranes of eye lens fibres. Biochemical and structural characterization. Cell Differ. 1972 Jun;1(2):91–106. doi: 10.1016/0045-6039(72)90032-2. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Devillers-Thiery A., Kindt T., Scheele G., Blobel G. Homology in amino-terminal sequence of precursors to pancreatic secretory proteins. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5016–5020. doi: 10.1073/pnas.72.12.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunia I., Sen Ghosh C., Benedetti E. L., Zweers A., Bloemendal H. Isolation and protein pattern of eye lens fiber junctions. FEBS Lett. 1974 Sep 1;45(1):139–144. doi: 10.1016/0014-5793(74)80831-8. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Lingrel J. B. Hemoglobin messenger ribonucleic acid. Distribution of the 9S ribonucleic acid in polysomes of different sizes. Biochemistry. 1969 Mar;8(3):829–831. doi: 10.1021/bi00831a010. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Weber K., Osborn M., Schmid E., Freudenstein C. Antibody to prekeratin. Decoration of tonofilament like arrays in various cells of epithelial character. Exp Cell Res. 1978 Oct 15;116(2):429–445. doi: 10.1016/0014-4827(78)90466-4. [DOI] [PubMed] [Google Scholar]
- Granger B. L., Lazarides E. Desmin and vimentin coexist at the periphery of the myofibril Z disc. Cell. 1979 Dec;18(4):1053–1063. doi: 10.1016/0092-8674(79)90218-6. [DOI] [PubMed] [Google Scholar]
- Kibbelaar M. A., Selten-Versteegen A. M., Dunia I., Benedetti E. L., Bloemendal H. Actin in mammalian lens. Eur J Biochem. 1979 Apr;95(3):543–549. doi: 10.1111/j.1432-1033.1979.tb12995.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ramaekers F. C., Osborn M., Schimid E., Weber K., Bloemendal H., Franke W. W. Identification of the cytoskeletal proteins in lens-forming cells, a special epitheloid cell type. Exp Cell Res. 1980 Jun;127(2):309–327. doi: 10.1016/0014-4827(80)90437-1. [DOI] [PubMed] [Google Scholar]
- Ramaekers F. C., Selten-Versteegen A. M., Bloemendal H. Interaction of newly synthesized alpha-crystallin with isolated lens plasma membranes. Biochim Biophys Acta. 1980 Feb 15;596(1):57–63. doi: 10.1016/0005-2736(80)90170-4. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Mooseker M. S. Actin filament-membrane attachment: are membrane particles involved? J Cell Biol. 1976 Nov;71(2):402–416. doi: 10.1083/jcb.71.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E., Bennett P. M., Calvert R., Ohanian V., Gratzer W. B. In vitro formation of a complex between cytoskeletal proteins of the human erythrocyte. Nature. 1979 Aug 30;280(5725):811–814. doi: 10.1038/280811a0. [DOI] [PubMed] [Google Scholar]
- Van Der Ouderaa F. J., De Jong W. W., Hilderink A., Bloemendal H. The amino-acids sequence of the alphaB2 chain of bovine alpha-crystallin. Eur J Biochem. 1974 Nov 1;49(1):157–168. doi: 10.1111/j.1432-1033.1974.tb03821.x. [DOI] [PubMed] [Google Scholar]
- Vermorken A. J., Kibbelaar M. A., Hilderink J. M., Bloemendal H. Incorporation in vitro of lens membrane protein into reticulocyte membranes. Biochem Biophys Res Commun. 1979 May 28;88(2):597–604. doi: 10.1016/0006-291x(79)92090-4. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wickner W. The assembly of proteins into biological membranes: The membrane trigger hypothesis. Annu Rev Biochem. 1979;48:23–45. doi: 10.1146/annurev.bi.48.070179.000323. [DOI] [PubMed] [Google Scholar]
- Woodcock C. L. Nucleus-associated intermediate filaments from chicken erythrocytes. J Cell Biol. 1980 Jun;85(3):881–889. doi: 10.1083/jcb.85.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]