Abstract
Ciguatoxins are sodium channel activator toxins that cause ciguatera, the most common form of ichthyosarcotoxism, which presents with peripheral sensory disturbances, including the pathognomonic symptom of cold allodynia which is characterized by intense stabbing and burning pain in response to mild cooling. We show that intraplantar injection of P-CTX-1 elicits cold allodynia in mice by targeting specific unmyelinated and myelinated primary sensory neurons. These include both tetrodotoxin-resistant, TRPA1-expressing peptidergic C-fibres and tetrodotoxin-sensitive A-fibres. P-CTX-1 does not directly open heterologously expressed TRPA1, but when co-expressed with Nav channels, sodium channel activation by P-CTX-1 is sufficient to drive TRPA1-dependent calcium influx that is responsible for the development of cold allodynia, as evidenced by a large reduction of excitatory effect of P-CTX-1 on TRPA1-deficient nociceptive C-fibres and of ciguatoxin-induced cold allodynia in TRPA1-null mutant mice. Functional MRI studies revealed that ciguatoxin-induced cold allodynia enhanced the BOLD (Blood Oxygenation Level Dependent) signal, an effect that was blunted in TRPA1-deficient mice, confirming an important role for TRPA1 in the pathogenesis of cold allodynia.
Keywords: ciguatoxin, cold allodynia, Nav, nociceptor, TRPA1
Introduction
In 1774, when Captain James Cook was exploring the coast of the New Hebrides, sailors aboard the HMS Resolution experienced a peculiar kind of poisoning after eating fish (Beaglehole, 1961). The initial symptoms occurred soon after their meal and consisted of gastrointestinal effects, in particular intense nausea, diarrhoea and abdominal pain. However, subjectively among the most distressing symptoms were neurological disturbances affecting the central nervous system, and also peripheral sensory disturbances including paraesthesias, localized intense pruritus and several painful dysaesthesias. The most prominent of these was a long-lasting sensory disorder reminiscent of cold allodynia, where exposure to cool objects or water induced severe burning pain and electric shock-like sensations (Bagnis et al, 1979). This form of fish poisoning, known as ‘ciguatera’ occurs worldwide in circumtropical regions, with the global incidence estimated to be as high as 50 000–500 000 cases annually (Fleming et al, 1998), making it the most common form of non-bacterial food poisoning. Ciguatera is caused by ciguatoxins, a group of lipophilic, polycyclic polyether toxins that are produced by dinoflagellates of the genus Gambierdiscus and bioaccumulate through the marine food chain (Lewis and Holmes, 1993). Structurally related variants of ciguatoxin exist in the Caribbean, the Indian and Pacific Ocean (C-CTX, I-CTX and P-CTX, respectively). Of these, P-CTX-1 is the most potent and thought to be responsible for the majority of neurological symptoms associated with ciguatera in the Pacific (Lewis, 2001). Ciguatoxins have previously been recognized as potent activators of voltage-gated sodium channels (Nav), however, little is known about the mechanisms by which they produce cold allodynia. In this study, we sought to identify the sensory neuronal populations mediating these symptoms and to elucidate the cellular and molecular basis of ciguatoxin-induced cold allodynia.
Results
A mouse model reproduces ciguatoxin-induced cold allodynia
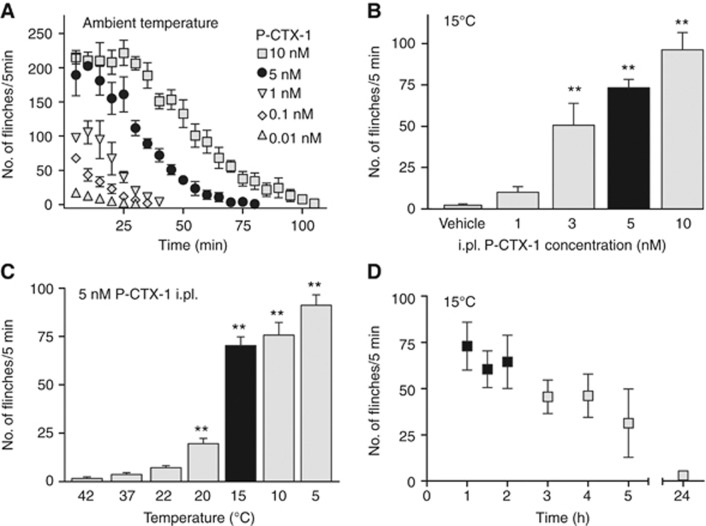
To elucidate the molecular pathways through which P-CTX-1 selectively targets neurons to elicit pain and cold allodynia, we established a new animal model of ciguatoxin-induced peripheral sensory disturbances. Systemic administration of ciguatoxin by the intraperitoneal (i.p.) or oral route in mice is associated with diarrhoea, hypothermia, salivation, lacrimation, muscle weakness, decreased motor activity and cyanosis (Hoffman et al, 1983). Importantly, systemic administration of ciguatoxin also results in a decrease in nerve conduction velocity and blunts the corneal and nociceptive withdrawal reflex. Therefore, in order to avoid systemic effects of ciguatoxin and to isolate the actions of CTX on peripheral sensory neurons, we used administration of low nanomolar solutions of P-CTX-1 (1–10 nM) by shallow intraplantar (i.pl.) injection. P-CTX-1 caused rapid, dose-dependent development of spontaneous pain in C57BL/6 mice, evidenced by flinching, lifting, shaking and licking of the affected hind paw that was accompanied by the development of cold allodynia (Figure 1A–D). Specifically, 45–60 min after i.pl. administration of P-CTX-1 the spontaneous nocifensive behaviour ceased, revealing prominent signs of cold allodynia that comprised paw lifting, licking, flinching and shaking observed at 20°C or cooler (Figure 1C). In contrast, at elevated temperatures up to 42°C, these animals displayed little or no nocifensive behaviour (Figure 1C). In addition, no mechanical allodynia was observed after i.pl. injection of P-CTX-1, consistent with the absence of mechanical sensitization in nociceptive C-fibres recorded from isolated rat saphenous nerve preparations (Supplementary Figure 1).
Figure 1.
A mouse model reproduces ciguatoxin-induced cold allodynia. (A) Intraplantar administration of P-CTX-1 caused dose-dependent spontaneous pain behaviour at room temperature, evidenced by increased number of paw lifts, licks, flinches and shakes. This spontaneous nocifensive behaviour subsided within ∼60–100 min (n=6 each). (B) P-CTX-1-induced cold allodynia—assessed after spontaneous pain had faded—appeared dose-dependent (40 μl volume per i.pl. injection, measured at 15°C; n=5–12 animals/group) and (C) temperature-dependent (5 nM; 40 μl by i.pl. injection; n=6–23 animals/group). (D) Time course of 5 nM P-CTX-1-induced cold allodynia at 15°C: cold allodynia persisted for several hours but subsided within 24 h (n=6). Black bars in (B, C): for all further experiments, 5 nM was chosen and cold allodynia was assessed at 15°C; black squares in (D) indicate the timeframe in which cold allodynia was assessed in (B–D) (and experiments underlying Figures 4L and 5A, 5F). Statistical significance was determined using a one-way ANOVA with Dunnett’s post hoc comparison; **P<0.01 compared to 37°C or vehicle. Data are presented as mean±s.e.m.
This new animal model thus produces behavioural responses that parallel the human symptomatology of ciguatera, and confirms the exquisite sensitivity of peripheral sensory neurons to P-CTX-1. Striking effects were observed upon i.pl. administration of as little as 5–500 pg of P-CTX-1, making this marine polyether toxin one of the most potent pro-algesic compounds known, with much higher doses of capsaicin (2500, ng/paw), bradykinin (300 ng/paw) or NGF (50 ng/paw) required to achieve comparable effects (Amaya et al, 2006).
TRPA1-expressing DRG neurons show exquisite sensitivity to P-CTX-1
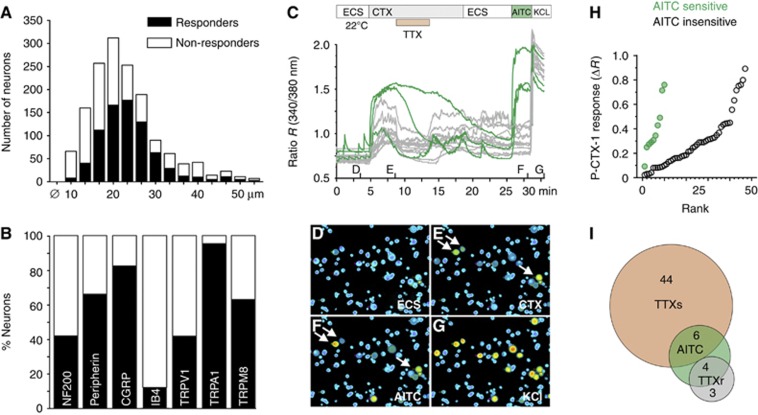
Cold allodynia is a symptom reported by a high proportion (76–94%) of ciguatera sufferers, suggesting that ciguatoxin activates specific nociceptive pathways that are intrinsically linked to the development of cold allodynia. To characterize the sensory neuronal populations targeted by P-CTX-1, we used a high-content bioimaging assay that allows live-cell Ca2+ imaging to be combined with immunohistochemistry at the level of single cells (Figure 2A and B; Supplementary Figure 2). In cultured mouse dorsal root ganglion (DRG) neurons, 51% of neurons responded to stimulation with P-CTX-1 (1 nM) with an increase in intracellular Ca2+ (Figure 2A; Supplementary Figure 2). Immunohistochemical characterization of the ciguatoxin-sensitive population using previously characterized antibodies (Supplementary Table 1) showed that 42% of NF200-positive (a marker of large myelinated A-fibre-associated cells) and 66% of peripherin-positive (a marker of unmyelinated C-fibre and thinly myelinated Aδ-fibre-associated cells) neurons responded to the addition of P-CTX-1, while IB4-positive DRG neurons largely defined the ciguatoxin-insensitive neuronal population (12% were ciguatoxin sensitive). Interestingly, virtually all CGRP- (82%) and TRPA1- (95%) positive cells were activated by low P-CTX-1 concentrations (1 nM), suggesting that TRPA1-expressing peptidergic neurons play a pivotal role in the symptomatology of ciguatera. Higher concentrations of P-CTX-1 were less discriminating and increasingly excited remaining neuronal populations, with 76% of DRG neurons excited by 5 nM P-CTX-1 (Supplementary Figure 3).
Figure 2.
Characterization of ciguatoxin-sensitive sensory neuron populations. (A) DRG neurons responding to P-CTX-1 (1 nM) were predominantly medium to large sized. (B) Immunohistochemical characterization of ciguatoxin-sensitive DRG neurons using antibodies for NF200, peripherin, CGRP, IB4, TRPV1, TRPA1 and TRPM8. Black bars, P-CTX-1 responders; white bars, P-CTX-1 non-responders. (C) Ca2+ responses at 22°C in rat DRG neurons and representative images after stimulation with (D) extracellular solution (ECS), (E) 1 nM P-CTX-1, 300 nM tetrodotoxin (TTX), (F) 25 μM allyl isothiocyanate (AITC) and (G) 60 mM potassium chloride. Arrows in (E, F) indicate P-CTX-1-sensitive neurons marked green in (C). (H) AITC-sensitive neurons (green) showed significantly larger ciguatoxin-induced Ca2+ responses than AITC-insensitive neurons (grey; P=0.005, Wilcoxon-matched pairs test). (I) Venn diagram illustrating pharmacological characteristics of all cells with calcium increase upon P-CTX-1 application. The P-CTX-1-induced calcium increase could be blocked to at least 50% by TTX (300 nM) in the majority of cells.
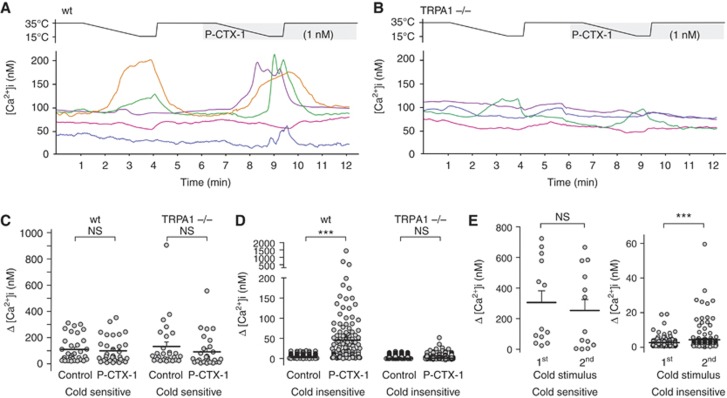
Preferential activation of TRPA1-expressing cells by P-CTX-1 was further confirmed by pharmacological characterization of cultured DRG neurons using ratiometric calcium imaging. Here, cells with functional TRPA1 expression, tested by application of the agonist allyl isothiocyanate (AITC, 25 μM), showed the largest calcium increase in response to P-CTX-1 (Figure 2C–H). Furthermore, the CTX-induced calcium increase was reduced by tetrodotoxin (TTX; 300 nM to at least 50%) in the majority of cells (81%; Figure 2C and I), consistent with the predominant pharmacological action of P-CTX-1 on TTX-sensitive (TTXs) voltage-gated sodium channels (VGSC). Based on these observations, we next assessed the contribution of TRPA1 to neuronal cold responses and cold sensitization induced by ciguatoxin in wild-type (wt) and TRPA1-deficient mice (Figure 3). In cultured mouse DRG neurons from wt and TRPA1−/− animals, lowering the temperature from 35° to 15°C elicited cold-induced Ca2+ responses in 16.5% of wt and 6.9% of TRPA1−/− neurons. These cold responses are most likely mediated by TRPM8 and other putative cold sensors, such as TRPC5 (Bautista et al, 2007; Zimmermann et al, 2011). Interestingly, the Ca2+ responses of these cold-sensitive neurons were not significantly affected by P-CTX-1 (Figure 3). However, P-CTX-1 elicited a striking cold sensitization in 39% of previously cold-insensitive neurons (Figure 3D). This effect was less pronounced in the absence of P-CTX-1 (Figure 3E; 8.9% sensitized to cold) and was dependent on TRPA1, as ciguatoxin-mediated de-novo sensitization to cooling was absent in DRG neurons from TRPA1−/− mice (Figure 3D; 6.6% sensitized to cold).
Figure 3.
Ciguatoxin induces new sensitivity to cold in cultured DRG neurons via TRPA1. (A–D) Cold responses and cold sensitization by P-CTX-1 (1 nM) in cultured DRG neurons from (A) wt and (B) TRPA1−/−mice. (C) Ca2+ responses of cold-sensitive cultured neurons were not significantly affected by P-CTX-1 but (D) P-CTX-1 induced novel sensitivity to cooling in previously cold-insensitive neurons, which was absent in TRPA1−/− neurons. (E) Cold sensitization to a second cold stimulus was less pronounced in wt neurons in the absence of P-CTX-1. Statistical significance was a determined using a paired, two-tailed Student’s t-test; *P<0.05; ***P<0.001; NS, P>0.05. Data are presented as mean±s.d.
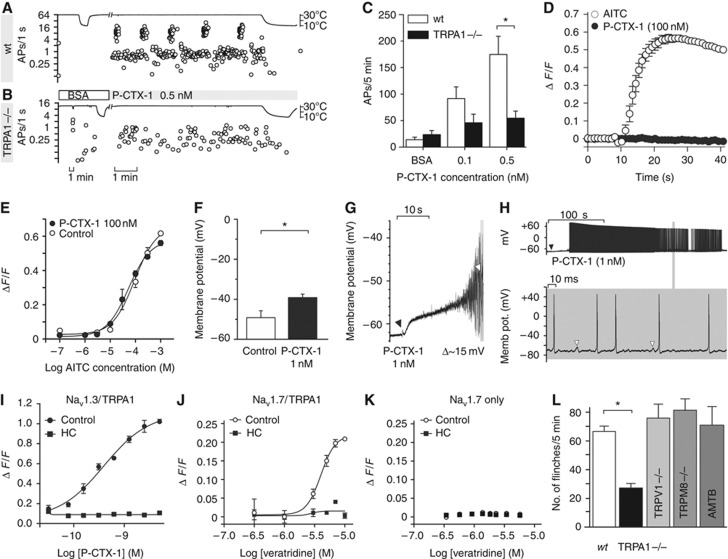
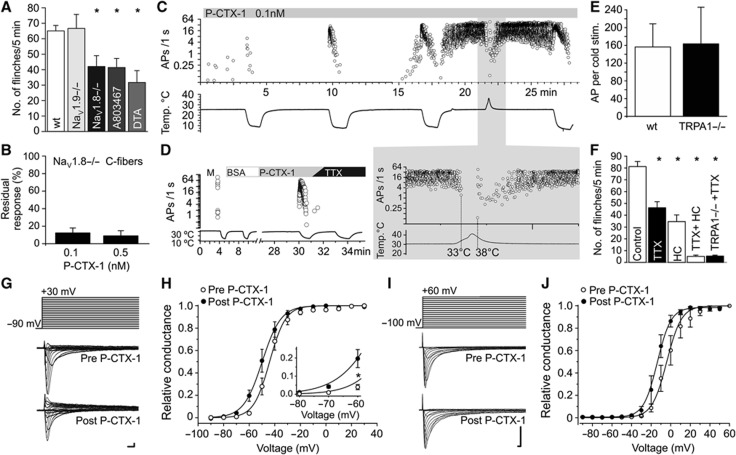
To further characterize the contribution of TRPA1 to ciguatoxin-induced peripheral sensitization, we performed ex-vivo recordings from murine skin-saphenous nerve preparations. This preparation permits single-fibre recordings of propagated action potentials arising from sensory stimulation directly to the receptive fields of C- and A-fibres (Zimmermann et al, 2009). Application of as little as 0.1 nM P-CTX-1 at a bath temperature of 28–30°C induced ongoing activity in the majority of C-fibre nerve endings and at 0.5 nM, strong and long-lasting action potential bursts appeared in some fibres (Figure 4A). This is consistent with a shift of the dynamic range of ciguatoxin-modified C-fibres, and reminiscent of the thermal sensitization observed previously in C-fibres treated with menthol (Zimmermann et al, 2011). The ongoing activity induced by P-CTX-1 at skin temperature ceased upon further cooling (Figure 4A), likely due to inactivation of TTXs VGSC that eventually leads to quiescent C-fibres (Zimmermann et al, 2007). Importantly, these ciguatoxin-mediated effects on C-fibres were greatly reduced in skin-nerve preparations from TRPA1−/− mice (Figure 4A–C; see Supplementary Table 2).
Figure 4.
TRPA1 mediates ciguatoxin-induced cold allodynia. (A–C) P-CTX-1-induced ongoing activity in single C-fibres recorded from murine skin-saphenous nerve preparations is markedly reduced in preparations from TRPA1-deficient mice. Representative recording from (A) a wt and (B) a TRPA1−/− nociceptor. (C) Cumulative ciguatoxin-induced action potentials in 5 min from wt (white bars, n=11) and TRPA1−/− (black bars, n=12) animals. In all, 8/10 (80%) of wt fibres and 5/12 (42%) of TRPA1−/− fibres more than doubled the number of cumulative actions potentials in response to 0.5 nM P-CTX-1. (D, E) P-CTX-1 neither activates nor potentiates heterologously expressed TRPA1. Cos-1 cells heterologously expressing TRPA1 were loaded with Fluo-4 and Ca2+ responses to P-CTX-1 and AITC measured using a FLIPRTETRA plate reader. (D) In TRPA1-expressing cells, P-CTX-1 (100 nM) did not elicit increases in intracellular Ca2+, while AITC (300 μM) caused rapid Ca2+ increases. (E) Pretreatment for 5 min with 100 nM P-CTX-1 did not significantly affect the AITC concentration-response curve. Data represent n=3 wells and are representative of three independent experiments. (F) P-CTX-1 applied at 1 nM depolarized the membrane potential of cultured DRG neurons recorded in current-clamp mode by 10 mV on average (n=6). (G, H) Representative examples of P-CTX-1 induced depolarization. (G) Application of P-CTX-1 (black arrow) caused depolarization of membrane potential followed by action potential firing (white arrow: first action potential). (H) Upper panel: ciguatoxin-induced depolarization rapidly leads to series of action potentials. Detail expanded in lower panel: white arrows: membrane oscillations, frequently followed by action potentials. (I–K) P-CTX-1 and veratridine elicit TRPA1-mediated calcium responses in HEK cells co-expressing Nav1.3 or Nav1.7 and TRPA1. HEK293 cells were loaded with Fluo-4 and Ca2+ responses at 20°C measured using a FLIPRTETRA plate reader. (I) Stimulation with P-CTX1 caused an increase in Ca2+ that was blocked by the TRPA1 inhibitor HC030031 (100 μM) and absent in cells only expressing Nav1.3 or TRPA1. Expression of functional TRPA1 and Nav was verified using AITC or membrane potential dye, respectively; n=6 wells in n=5 independent experiments. (J) Stimulation with veratridine caused a concentration-dependent increase in Ca2+ (open circles) that was blocked by the TRPA1 inhibitor HC030031 (100 μM; black squares). (K) Veratridine did not elicit Ca2+ responses in HEK293 cells expressing only TRPA1 (not shown) or only Nav1.7. Data are presented as mean±s.e.m. with n=15 wells and are representative of two independent experiments. (L) Compared to wt littermates (Control; n=31), ciguatoxin-induced cold allodynia (P-CTX-1, 5 nM; 15°C) is reduced in TRPA1−/− (n=14), but not in TRPM8−/− (n=7) or TRPV1−/− (n=10) animals, or after intraplantar treatment with the TRPM8 antagonist AMTB (100 μM). All data are presented as mean±s.e.m. Statistical significance was determined using a one-way ANOVA with Dunnett’s post hoc comparison; *P<0.05.
A direct effect of P-CTX-1 on TRPA1 was ruled out as P-CTX-1 at concentrations up to 100 nM failed to directly activate or sensitize Ca2+ responses mediated through heterologous expressed hTRPA1 (Figure 4D and E), and did not affect TRPA1 currents in whole-cell patch-clamp recordings (Supplementary Figure 4). Based on these results, it appears that ciguatoxin-induced membrane depolarization and membrane oscillations, which was determined to be +10 mV on average in cultured DRG neurons recorded in current-clamp configuration (Figure 4F–H), in combination with a cooling-induced leftward shift of the voltage dependence of TRPA1 activation reported previously (Karashima et al, 2009), are sufficient to activate TRPA1 in cold nociceptors which is then perceived as the burning pain of cold allodynia. In support of this hypothesis, in HEK293 cells co-expressing TRPA1 and the TTXs Nav1.7 or Nav1.3, stimulation by P-CTX-1 or the Nav activator veratridine at 20°C generated TRPA1-mediated Ca2+ responses that were blocked by the TRPA1 antagonist HC030031, the sodium channel blocker tetracaine, and were absent in cells only expressing TTXs Nav or TRPA1 (Figure 4I–K; Supplementary Figure 5). The contribution of TRPA1 to ciguatoxin-induced peripheral sensitization was also critical in vivo, with the development of ciguatoxin-mediated cold allodynia significantly decreased in TRPA1−/− mice. In contrast, cold allodynia was unaltered in mice lacking TRPM8 or TRPV1 (Figure 4L), or the recently identified cold transducer TRPC5 (data not shown). In addition, the TRPM8-specific antagonist AMTB did not affect ciguatoxin-induced cold allodynia, and no reduction in spontaneous pain was apparent in TRPM8 knockout animals (Figure 4L).
Both Nav 1.8 and TTXs Nav subtypes are effectors of ciguatoxin-induced cold allodynia
Based on previous reports that the TTX-resistant (TTXr) Nav1.8 is essential for the detection and perception of noxious cold pain (Zimmermann et al, 2007; Abrahamsen et al, 2008), we assessed the contribution of Nav1.8 to ciguatoxin-induced cold allodynia. In contrast to previous studies reporting negligible responses of Nav1.8−/− animals in response to noxious cold stimuli (Zimmermann et al, 2007), we found only a partial inhibition of ciguatoxin-induced cold allodynia in Nav1.8−/− animals (35.5% reduction of cold allodynia), which was mimicked by i.pl. administration of the Nav1.8 blocker A803467 (10 μM, 36.4% reduction of cold allodynia) and no significant effect in Nav1.9−/− mice (Figure 5A). Similar results were obtained in mice with diphtheria toxin-mediated ablation of Nav1.8-expressing nociceptors (Nav1.8-DTA), where residual ciguatoxin-induced cold allodynia persisted (48.8% remaining cold allodynia; Figure 5A) despite an almost complete loss of noxious cold responses in these animals (Abrahamsen et al, 2008). Consistent with ablation of predominantly Nav1.8-expressing C-fibres in the Nav1.8-DTA mice, ex-vivo recordings from C-fibres devoid of Nav1.8 showed an 85–90% reduction of ciguatoxin-induced activity (Figure 5B; see Supplementary Table 2), with the residual ciguatoxin-induced action potentials now entirely TTXs. This result was congruent with an analogous ∼15% attenuation of the CTX effect by TTX in wt C-fibres. These results reveal that both TTXr Nav1.8 and TTXs Nav contribute to ciguatoxin-induced cold allodynia, and that in addition to Nav1.8-expressing C-fibres, TTXs pathways contribute to ciguatoxin-induced cold allodynia.
Figure 5.
TTXr Nav1.8 and TTXs sodium channels are effectors of ciguatoxin-induced cold allodynia. (A) Compared to wt littermates (Control; n=47), ciguatoxin-induced cold allodynia (P-CTX-1, 5 nM; 15°C) appears unchanged in Nav1.9−/− (n=12), but markedly reduced in Nav1.8−/− (n=16) animals. This was confirmed using the Nav1.8 blocker A803467 injected i.pl. (10 μM in a volume of 40 μl; n=11) and in mice with diphtheria toxin-mediated ablation of Nav1.8-expressing nociceptors (Nav1.8-DTA; n=6) animals. (B) P-CTX-1-induced ongoing activity is reduced by >85% in C-fibres from skin-saphenous nerve preparations from Nav1.8-deficient mice. (C, D) Representative recording from a wt A-fibre. P-CTX-1 (0.1 nM) significantly increased activity of A-fibres in isolated mouse skin-saphenous nerve preparations. (C) Repeated cold stimulation of an A-fibre (C57BL/6, conduction velocity 11.9 m/s, 1.4 mN) after treatment with P-CTX-1 led to increasing action potential discharge until spontaneous activity occurred at 30°C. This activity was suppressed by heating (threshold temperature 33°C) and resumed upon cooling <38°C. (D) P-CTX-1 (0.1 nM) induced effects in A-fibres were entirely sensitive to TTX applied at 300 nM and (E) independent of TRPA1 (bars represent mean of two cold responses; wt: white bars, n=11 and TRPA1−/−: black bars, n=15). (F) Compared to wt littermates (Control; n=13), ciguatoxin-induced cold allodynia was significantly reduced by intraplantar injection of TTX (2 μM, n=13) or the TRPA1 blocker HC030031 (100 μM; n=6). Combination of both compounds (n=6) or application of TTX in TRPA1-deficient animals (n=5) abolishes ciguatoxin-induced cold allodynia. (G) Representative TTXs current traces recorded from large-sized DRG neurons (42.9±1.4 μm). Upper lane: voltage protocol; middle: traces before and lower: traces after perfusion with P-CTX-1 (1 nM). (H) Effect of P-CTX-1 (1 nM) on the voltage–conduction relationship of TTXs channels measured in mouse DRG neurons (n=9). (I) Representative recording of current traces recorded from ND7/23 cells heterologously expressing Nav1.8. Upper lane: voltage protocol; middle: traces before and lower: traces after perfusion with P-CTX-1 (1 nM). (J) Effect of P-CTX-1 (1 nM) on the voltage–conduction relationship of Nav1.8 heterologously expressed in ND7/23 cells (n=5); scale bars in (G, I) represent 1 ms and 1 nA; all data are presented as mean±s.e.m.
Indeed, in A-fibres, treatment with P-CTX-1 caused strong ongoing activity and cold sensitization that was completely blocked by TTX (Figure 5C and D), consistent with previous reports showing that A-fibres are devoid of TTXr Nav1.8 (Akopian et al, 1996; Zimmermann et al, 2007). Ciguatoxin-induced A-fibre activity was suppressed by warming, while re-cooling (to cool temperatures or skin temperature) immediately reactivated action potential firing (Figure 5C). This effect was unaltered in TRPA1−/− mice (Figure 5E). Residual cold sensitivity in sensory neurons, which cannot be attributed to TRPA1 or TRPM8, has been described in the literature, although the molecular identity of the responsible cold transducer remains unknown to date (Bautista et al, 2007; Munns et al, 2007; Knowlton et al, 2010). Since A-fibres arise from large diameter DRG neurons that do not survive culture conditions well, such effects may have escaped detection in our cultured DRG neuron assays (Figures 2 and 3). This suggests that the residual cold allodynia observed in TRPA1-deficient mice arises from the effects of P-CTX-1 on these TTXs fibres. Indeed, the contribution of TTXs channels to ciguatoxin-induced cold allodynia was further confirmed in behavioural experiments, where i.pl. administration of TTX (2 μM) significantly decreased ciguatoxin-induced cold allodynia by 30% (Figure 5F). Furthermore, cold allodynia was completely abolished by simultaneous i.pl. injection of HC030031 and TTX, or in TRPA1−/− mice treated with i.pl. TTX (Figure 5F).
To start to explain these results, we investigated the effect of P-CTX-1 on the biophysical properties of TTXs and TTXr Nav in voltage-clamp recordings (Figure 5G–J). Consistent with previous reports (Strachan et al, 1999), P-CTX-1 shifted the voltage dependence of activation of TTXs channels at 23°C by 7.3±1.0 mV to more negative potentials, inducing significant channel activation at −60 mV (see insert in Figure 5G), a potential close to the supposed resting membrane potential of DRG neurons (Amir et al, 1999). Similarly, P-CTX-1 elicited a 10 mV hyperpolarizing shift of the voltage dependence of Nav1.8 (Figure 5I) that persisted at cooler temperatures (Yamaoka et al, 2009).
In A-fibres, cold sensitization occurred at innocuous cool temperatures (28–20°C), where only few TTXs channels are in a state of slow inactivation (Zimmermann et al, 2007). Thus, the shift in activation elicited by P-CTX-1 results in overactive TTXs channels that are able to fire at high rates during mild cooling. This effect of P-CTX-1 is presumably synergized by the effects of P-CTX-1 on voltage-gated potassium channels (Birinyi-Strachan et al, 2005) and the biophysical effects of cooling. Cooling decreases the activation threshold of the sodium currents, leads to an increase in membrane resistance and results in depolarizing closure of background potassium channels (Griffin and Boulant, 1995; Maingret et al, 2000; Reid and Flonta, 2001; Viana et al, 2002; Zimmermann et al, 2007). These biophysical effects presumably are of major importance in nerve endings due to their high surface area to volume ratio. In contrast, a decrease in membrane resistance and opening of potassium channels during heating may short circuit the sodium current and opposes the ciguatoxin-induced increase in neuronal excitability (Figure 5C, inset), providing a biophysical explanation for the pain-relieving effects of warmth and the ‘temperature reversal’ reported in clinical ciguatera cases.
P-CTX-1 and cool temperatures synergistically activate TRPA1-expressing nociceptors to mediate the perception of burning pain
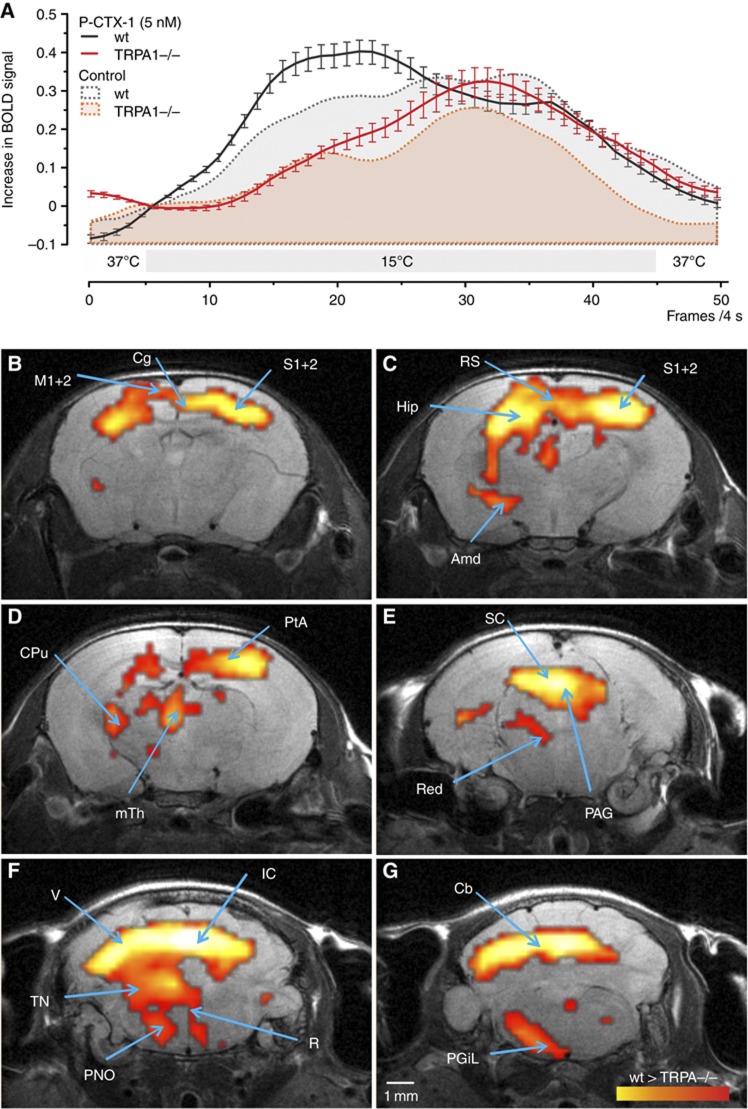
Our results from both behavioural experiments and single-fibre recordings clearly demonstrate a contribution of both TTXs and TTXr VGSCs, in addition to a major role of TRPA1-expressing C-fibres as mediators of ciguatoxin-induced cold allodynia. Thus, while TRPA1 has been established to be activated by temperatures in the noxious range, our results clearly demonstrate that TRPA1-expressing nociceptors are activated at higher temperatures in the presence of ciguatoxin. To explore the role of TRPA1 in temperature-induced nociception further and to analyse to which extent the central processing of cold stimuli and cold allodynia is affected by a lack of TRPA1, we used non-invasive functional MRI (fMRI) measurements (Figure 6A–G; Supplementary Figure 6). Here, changes in BOLD (Blood Oxygenation Level Dependent) signal were measured as a surrogate parameter for changes in neural activity (Hess et al, 2011) caused by repetitive cool stimulation (15°C) of the paw after ciguatoxin application. P-CTX-1 treatment elicited a marked increase in the cool stimulus-evoked BOLD signal, an effect which was blunted in TRPA1−/− animals (Figure 6A), consistent with the significant reduction in ciguatoxin-induced cold allodynia we observed in behavioural experiments on these knockout mice. Brain structures which showed differentially stronger activation by cool stimulation in wt compared to TRPA1−/− mice after P-CTX-1 injection included cerebral targets of the spino-mesencephalic and spino-reticular tract, the medial thalamus, ventral striatum, cingulum and periaqueductal grey (Figure 6), supporting our behavioural observation that TRPA1 crucially contributes to ciguatoxin-induced cold allodynia.
Figure 6.
Cool temperatures and P-CTX-1 activate TRPA1-dependent nociceptive pathways resulting in the perception of burning pain. (A) Compared to wt animals (grey area under the curve), TRPA1−/− mice (red area under the curve) showed an altered hemodynamic response function (HRF) in response to stimulation of the paw with 15°C after i.pl. injection of saline, but also in the presence of P-CTX-1 (black and red timelines). Changes in BOLD signal from n=9–10 animals were averaged over six repetitive cold stimuli. Error bars represent s.e.m. (B–G) Second order statistical parameter maps showing differential brain activity (wt>TRPA1−/−) assessed by BOLD-fMRI in response to cold stimulation following i.pl. P-CTX-1 treatment (5 nM i.pl.) in wt and TRPA1−/− animals, superimposed on the corresponding anatomical image. The yellow-red scale indicates increased activity in wt compared to TRPA1−/− animals. Arrows indicate regions with increased differential activity. For cold stimulation after i.pl. injection of P-CTX-1 (5 nM), (B) the somatosensory (S1/S2) cortex, the cingulate (Cg) cortex and the motor (M1/M2) cortex, (C) the retrosplenial cortex (Rs), hippocampus (Hip), amygdala (Amd), (D) striatum (CPu), parietal association cortex (PtA), medial thalamus (mTh), (E) superior colliculus (SC), red nucleus (Red), periaqueductal grey (PAG), (F) visual cortex (V), inferior colliculus (IC), raphe nucleus (R) tegmental nuclei (TN), pontine reticular nucleus (PNO) (G) cerebellum (Cb) and lateral paragigantocellular nucleus (PGiL) show significantly higher activity in wt than in TRPA1−/− mice.
Surprisingly, we also observed differences in the central processing of the cold (15°C) stimulus under control conditions, where wt mice expressed stronger responses mainly in cortical areas in response to cooling of the paw (Supplementary Figure 6). Moreover, we found that the TRPA1−/− mice showed an altered kinetic of the hemodynamic response function (HRF) in response to cold stimuli, either with or without P-CTX-1 treatment (Supplementary Figure 6), providing the first evidence that the HRF can also be modulated by genetic influence. These results demonstrate that TRPA1 contributes not only to cold allodynia, but also that TRPA1-expressing fibres are activated at much higher temperatures than previously observed. TRPA1 is generally considered to be a sensor of noxious cold temperatures, with behavioural differences in knockout animals occurring at temperatures close to freezing (Karashima et al, 2009). However, based on our results, previous behavioural studies in TRPA1−/− mice may have underestimated the contribution of TRPA1 to cold nociception, as small differences in perception may not lead to frank behavioural changes.
Discussion
Humans are able to detect a broad range of temperatures through activation of thermosensitive nerve endings in the skin. In recent years, significant advances have been made with the identification of several thermosensitive ion channels, in particular the TRP class of thermoreceptors that function as the molecular transducers of innocuous and noxious temperatures. Nevertheless, our understanding of the physiological and pathophysiological role of these channels remains incomplete and moreover, it remains unclear how temperature detection overlaps with the perception of pain. A particularly puzzling and poorly understood phenomenon is cold allodynia, where non-noxious and normally non-painful cool stimuli are perceived as painful. In this context, ciguatera is of particular interest as it is associated with the pathognomonic symptom of cold allodynia and thus may be useful to further dissect the pathways involved. Although it is known that ciguatoxin increases neuronal excitability through activation of VGSCs, the neuronal pathways leading to ciguatoxin-induced cold allodynia are unknown.
Peripheral administration of P-CTX-1 elicits cold allodynia
This study is the first to identify the sensory neuronal populations involved in mediating the symptomatology of ciguatera, and to elucidate the cellular and molecular basis of ciguatoxin-induced cold allodynia. The toxicokinetics of ciguatoxin are complex and likely contribute to the clinical presentation of ciguatera (Bottein et al, 2011). After oral administration in mice, ciguatoxin is rapidly absorbed from the gastrointestinal tract, where it exerts local action to elicit gastrointestinal symptoms such as diarrhoea and abdominal pain (Bottein et al, 2011). The clearance of ciguatoxin involves a bi-exponential elimination best fit using a two-compartment model. This probably occurs due to accumulation of P-CTX-1 in adipose tissue or even lipophilic neuronal membranes, which contributes to a long terminal elimination half-life of ∼4 days. The slow elimination of P-CTX-1 likely contributes to the prolonged duration of neurological effects, and renal excretion of ciguatoxin may contribute to urinary symptoms such as dysuria, although the majority of ciguatoxin is excreted in the faeces. We postulate that the peripheral sensory effects of ciguatoxin after oral consumption arise from a direct excitatory action of the toxin on peripheral sensory neurons. In unpublished observations, intradermal administration of P-CTX-1 in humans elicits symptoms including cold allodynia, consistent with the symptomatology of ciguatera, providing direct support for our new animal model of cold allodynia. This mouse model reveals for the first time that altered excitability of peripheral sensory neurons is sufficient for the development of cold allodynia. The temperature dependence of allodynia observed in this mouse model was similar to the reported threshold of cold allodynia in human ciguatera patients (24–26°C) (Cameron and Capra, 1993). Interestingly, heat allodynia is neither observed clinically (Cameron and Capra, 1993), nor was it observed in our animal model. The precise reasons for the lack of heat hyperalgesia remain to be determined; however, it is likely that differential co-expression with CTX-sensitive Nav and Kv channels contribute to this effect. In addition, mechanical allodynia is neither observed in ciguatera patients, nor was it apparent in our animal model. Thus, cold allodynia is the dominant thermal sensory abnormality present both clinically and in our animal model and is likely to be mediated by specific thermotransducer proteins.
TRPA1 is a critical determinant in ciguatoxin-induced cold allodynia
The involvement of two likely candidates, TRPM8 and TRPA1, in thermosensing is contentious. TRPM8 is activated by innocuous cool temperatures <28°C, although activity is maintained in the noxious cold range (<10°C) (McKemy et al, 2002; Peier et al, 2002). Accordingly, TRPM8 has been reported to be involved in temperature preference and environmental as well as noxious cold sensing (Peier et al, 2002; Bautista et al, 2007; Dhaka et al, 2007). In contrast, the cold receptor TRPA1 is thought to be activated by noxious cold temperatures in heterologous expression systems (Story et al, 2003; Karashima et al, 2009). However, a lack of cold-evoked TRPA1 responses, or only indirect activation through cold-induced Ca2+ release, has been reported by several groups (Jordt et al, 2004; Nagata et al, 2005; Zurborg et al, 2007). Similarly, differences in behaviour in TRPA1−/− animals were only reported at noxious temperatures or in pathological models of cold hypersensitivity, or not at all (Bautista et al, 2006; Karashima et al, 2009; del Camino et al, 2010; Gentry et al, 2010; Knowlton et al, 2010; Nassini et al, 2011). In the present study, we provide fMRI evidence that TRPA1 is activated by temperatures well above the noxious range and that TRPA1 is a critical determinant in ciguatoxin-induced cold allodynia. In cultured DRG neurons, Ca2+ responses to cooling, which appeared after P-CTX-1 treatment in previously cold-insensitive neurons, were completely dependent on TRPA1. In contrast, Ca2+ responses in cold-sensitive DRG neurons were neither significantly affected by P-CTX-1 nor affected in TRPA1-deficient mice (Figure 3). These cold responses are likely to be mediated through TRPM8, as both the temperature threshold of activation and the proportion of DRG neurons responding to cooling with increases in Ca2+ are in accordance with the literature values for TRPM8-mediated calcium increases (Bautista et al, 2007).
While some TRPM8-expressing neurons were activated by P-CTX-1, it is likely that these neurons are involved in ciguatoxin-induced symptoms other than cold allodynia, such as increased tear production (lachrymation) observed in rodents (Lewis and Sellin, 1993), a response that has recently been shown to depend on TRPM8 in mice (Parra et al, 2010). In contrast, studies in human ciguatera patients (Cameron and Capra, 1993) and intracutaneous injection of P-CTX-1 in humans (unpublished observations) have revealed that temperature discrimination and cool sensitivity, which are painless sensations mediated through TRPM8 (Bautista et al, 2007; Parra et al, 2010), are not altered by P-CTX-1. Given the main effect of P-CTX-1 is the emergence of the sensation of pain in response to mild cooling, it appears that the cold sensing TRPA1 rather than TRPM8 couples to pain sensing centrally. This was confirmed in behavioural experiments, where ciguatoxin-induced cold allodynia was significantly decreased in TRPA1−/− mice, but not affected in TRPM8−/− animals.
TRPA1 contributes to cold sensing at higher temperatures than previously recognized
Lastly, we demonstrated altered central processing of cold allodynia after P-CTX-1 treatment in TRPA1−/− versus wt mice. In the process, we also revealed for the first time that the TRPA1-dependent cold nociceptive pathway is activated at temperatures well above the noxious cold range and that this signal is processed in the brain well before it results in measurable aversive behaviour in the noxious cold range (e.g., <10°C), suggesting it may play a broader role in protecting the body from damaging cold than previously recognized. TRPA1-mediated effects were observed in brain areas associated with emotion, pain and cold processing, in particular the amygdala, cingulate cortex, and the S1 and S2 somatosensory cortices, illustrating subtle differences in cool perception in TRPA1−/− mice. Furthermore, C-fibres share input to spinal laminae I–III and build projections into the spino-mesencephalic tract, an area which appeared in the fMRI experiment among those with differentially stronger activation during cold allodynia in wt mice. Interestingly, previous fMRI studies in human subjects found analogous brain areas to be activated during heat and cold hypersensitivity (Lorenz et al, 2002; Seifert and Maihöfner, 2007), including projections within the spino-mesencephalic tract, corroborating an important role of TRPA1 in cold sensing in the non-noxious temperature range. This was also observed in behavioural experiments utilizing pharmacological modulators administered by the i.pl. route, and are strongly supported by ex-vivo findings in the isolated skin-nerve preparation. Thus, these findings provide clear evidence for a role of TRPA1 in cold sensing at higher temperatures than previously recognized.
This study shows that i.pl. injection of P-CTX-1 elicits cold, but no heat or mechanical allodynia. While TRPA1 has been implicated as a mechanosensor, it is not absolutely required for mechanical sensitivity of afferent nerve terminals per se, as mechanically sensitive cutaneous fibres are present in normal numbers in TRPA1-deficient mice and deficits of TRAP1-deficient mice to mechanical force were only observed in response to intense mechanical stimulation (Kwan et al, 2009). However, slowly adapting low-threshold Aβ mechanoreceptors from TRPA1-deficient mice have been shown to have reduced action potential firing, suggesting a role of TRPA1 in mechanosensation in these fibres. It is plausible that TRPA1-mediated mechanosensation in these fibres contributes to ciguatoxin-induced sensory disturbances other than mechanical allodynia, such as the tingling and pricking sensations which are commonly described by ciguatera victims, but which are difficult to assess in this murine model.
Ciguatera is a special form of acquired channelopathy
Despite the dominant role of TRPA1 in ciguatoxin-induced cold allodynia, this effect does not appear to be mediated by a direct action of P-CTX-1 on thermosensitive TRP channels. Instead, TRPA1-mediated cold allodynia occurs indirectly through changes in neuronal excitability and membrane potential elicited by P-CTX-1 (Carr et al, 2002). P-CTX-1 activates Nav and inhibits KV channels, resulting in sustained membrane depolarization of several mV (Strachan et al, 1999; Birinyi-Strachan et al, 2005). Under these conditions, a cooling-induced change in the voltage dependence of TRPA1 activation appears sufficient for TRPA1-mediated responses to reach the threshold for action potential generation and to signal burning pain at innocuous cool temperatures. Indeed, depolarization-mediated activation of thermo-TRP channels has been reported recently in heterologous expression systems, further supporting our findings. The fact that voltage-dependent gating is tightly linked to temperature sensing in TRPs is not a new concept (Voets et al, 2004), but in the case of ciguatoxin it becomes pathologic. Differences in resting membrane potential could also provide an explanation for the observation that TRPA1-mediated cold responses can be elicited in heterologous expression systems with relatively positive membrane potentials (Chemin et al, 2000; Kim et al, 2004), but not in DRG cell somata with their relatively hyperpolarized resting membrane potential (Reid, 2005; Zurborg et al, 2007; Caspani and Heppenstall, 2009). Thus, the ciguatoxin-mediated increase in the excitability of a subset of neurons—in the absence of direct effects on thermo-TRP channels—is sufficient to elicit profound sensitization to cooling. This increase in excitability is driven in part by Nav1.8, consistent with its role in cold pain and previous reports of effects of ciguatoxins on Nav1.8 (Strachan et al, 1999; Yamaoka et al, 2009). However, a significant contribution of TTXs channels to ciguatoxin-mediated cold sensitization was also observed. In contrast to noxious cold temperatures (<10°C), where TTXs channels have entered into a state of slow inactivation (Zimmermann et al, 2007), these channels contribute to cold allodynia at innocuous cool temperatures, where they are still able to fire at high rates. This effect is potentiated by the closure of temperature-sensitive potassium channels and the biophysical effects of cooling, which decrease the activation threshold of the sodium currents, lead to an increase in membrane resistance and result in depolarizing closure of background potassium channels (Griffin and Boulant, 1995; Maingret et al, 2000; Reid and Flonta, 2001; Viana et al, 2002; Zimmermann et al, 2007). Taken together, modulation of biophysical membrane properties by cooling in combination with altered membrane potential through Nav activation by P-CTX-1 can be sufficient to elicit action potential bursts and strong and long-lasting spontaneous activity consistent with the prolonged neurological effects of P-CTX-1 observed in ciguatera victims. Thus, while it has been established that both Nav1.8 and TRPA1 are required for noxious cold sensing (Zimmermann et al, 2007; Karashima et al, 2009), our findings show that TRPA1-dependent nociception is activated at innocuous cold temperatures under circumstances where activation of Nav increases membrane excitability, as is the case for ciguatoxin and potentially in neuropathic pain states associated with cold allodynia.
In conclusion, ciguatera can be described as a special form of acquired channelopathy that provides unique insight into the mechanisms of cold transduction and sensitization. P-CTX-1 elicits striking peripheral activation in C-fibres as well as de-novo sensitization and activation in A-fibres, with P-CTX-1 preferentially targeting peptidergic, IB4-negative TRPA1-expressing nociceptors. While P-CTX-1 does not directly activate thermo-TRP channels, TRPA1 is crucial to the development of ciguatoxin-induced cold allodynia, where membrane depolarization through activation of sodium channels in TRPA1-containing neurons leads to peripheral sensitization and consequently to activation of a specific subset of brain structures relevant for allodynia. In addition to Nav activation through P-CTX-1, cold temperatures further enhance neuronal excitability by increasing membrane resistance to produce the pathognomonic symptom of cold allodynia. The recent report that TRPA1-expressing fibres also mediate itch (Wilson et al, 2011) may explain the intense pruritus observed clinically, and further supports the notion that TRPA1-expressing nociceptors are particularly susceptible to the effects of ciguatoxin.
Materials and methods
Materials
P-CTX-1 (>90% purity) was isolated from moray eel (Gymnothorax javanicus) liver as previously described (Lewis et al, 1991), stored as a concentrated stock in 50% methanol/50% H2O and routinely diluted in the presence of 0.1–0.3% bovine serum albumin (BSA) to avoid loss to plastic. All other reagents were from Sigma-Aldrich (Castle Hill, NSW, Australia or Taufkirchen, Germany) unless otherwise stated.
Ethics for animal experiments
The protocol for in-vivo experiments in animals was reviewed by the local animal ethics committee (University of Erlangen) and approved by the local district government. At the University of Queensland, ethical approval for experiments involving animals was obtained from the local animal ethics committee. Experiments involving animals were conducted in accordance with the Animal Care and Protection Act Qld (2002), the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, 7th edition (2004) and the International Association for the Study of Pain Guidelines for the Use of Animals in Research.
Origin of mice
We used adult male C57BL/6J mice, TRPA1−/− (D Corey, Harvard Medical School, Boston, MA, USA; backcrossed for nine generations on C57BL/6), TRPV1−/− (J Davis, formerly GlaxoSmithKline, Harlow, UK; congenic to C57BL/6), TRPM8−/− (A Patapoutian; backcrossed for five generation on C57BL/6 background), Nav1.8−/−, Nav1.9−/−, Nav1.8-DTA mice (John N Wood, University College London, London, UK; congenic to C57BL/6 or backcrossed for at least six generations on the C57BL/6 background, respectively) knockout mice and their age-matched litter controls were used or matched to C57BL/6J when congenic. All animals were genotyped using previously reported primers.
Animal model of ciguatoxin-evoked nocifensive behaviour and cold allodynia
To assess ciguatoxin-evoked effects in wt C57BL/6 and in age-matched TRPV1−/−, TRPA1−/− and TRPM8−/− mice, a single dose of P-CTX-1 was administered by subcutaneous injection to the hind paw (i.pl.) and nocifensive behaviour was rated by a blinded observer. P-CTX-1 was prepared in concentrations of 0.01–10 nM (final dose of 0.5–50 pg/paw) in sterile Ringer’s solution and injected i.pl. in a volume of 40 μl under light diethyl ether, methoxyflurane or isoflurane anaesthesia. To assess the effects of pharmacological modulators on the development of spontaneous pain and cold allodynia, compounds were either administered by i.p. injection 30 min prior to i.pl. injection of P-CTX-1 (HC030031, 100 mg/kg i.p.) or were co-administered with P-CTX-1 by i.pl. injection as appropriately concentrated solutions (HC030031, 100 μM; TTX, 2 μM; A803467, 10 μM).
After a brief recovery period, spontaneous pain was rated at room temperature by counting the number of paw shakes, lifts, licks or flinches over a 5-min period. To assess heat and cold allodynia in ciguatoxin-treated animals, mice were placed on a temperature-controlled Peltier plate (Ugo Basile, Comerio, Italy or a custom-designed Peltier-based plate with components purchased from Melcor, Trenton, NJ, USA) once spontaneous pain behaviour subsided to <10 counts/5 min and the number of paw shakes, lifts, licks or flinches was quantified over a 5-min period.
To assess contribution of Nav and thermo-TRP channels to the development of ciguatoxin-induced cold allodynia, nocifensive behaviour in response to i.pl. injection of 5 nM P-CTX-1 was determined over a 5-min period at 15°C, 60–90 min after treatment with P-CTX-1.
No systemic effects, including no diarrhoea, ataxia, altered gait or motor paralysis were apparent in any mice and all animals completely recovered within several hours. In addition, no sustained hind paw favouring, inflammation, swelling or ulceration of the ciguatoxin-injected paw was visible. Injection of equal volumes of Ringer’s solution did not elicit any nocifensive behaviour (Figure 1B).
DRG cell culture
DRGs were isolated and cultured as previously described (Zimmermann et al, 2007). In brief, DRGs from T1-L6 were isolated from adult male Wistar rats, male C57/BL6 mice, male Nav1.8−/− mice or male TRPA1−/− mice euthanized by CO2 inhalation and collected in DMEM supplemented with 50 μg/ml gentamicin (Sigma), 100 U/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml amphotericin B (Invitrogen). DRGs were then incubated for 30 min at 37°C and 5% CO2 in dissociation media containing 1 mg/ml collagenase (Sigma) and 0.1 mg/ml protease (Sigma). After three wash steps, cells were triturated through a flame-polished glass Pasteur pipette and cultured for 24 h in TNB 100 solution supplemented by TNB 100 lipid–protein complex, 1 nM NGF, 100 μg/ml streptomycin and penicillin (all from Biochrom, Berlin, Germany) and 200 μg/ml glutamine (Invitrogen, Carlsbad, USA) or Neurobasal medium supplemented with B27, 1 nM NGF and 100 μM glutamine (all from Invitrogen, Mulgrave, VIC, Australia) for high content imaging.
High content imaging and immunochemical characterization of ciguatoxin-sensitive DRG neurons
Mouse DRG neurons were cultured on PDL-coated 96-well half area imaging plates (Corning) for 24 h and loaded with 5 μM Fura-2 for 30 min at 37°C. After two washes with physiological salt solution (PSS; composition: NaCl 140 mM, glucose 11.5 mM, KCl 5.9 mM, MgCl2 1.4 mM, NaH2PO4 1.2 mM, NaHCO3 5 mM, CaCl2 1.8 mM, HEPES 10 mM), Ca2+ responses to stimulation with P-CTX-1 (1 nM) or AITC (100 μM) were measured using the high content imager BD Pathway 855 (BD Biosciences, San Jose, CA, USA) for 60 s with a Hamamatsu Orca ER cooled CCD camera and × 10 objective at 22°C. Cells were then fixed with Histochoice (Amresco, Solon, OH, USA) for 1 h, blocked with 0.3% BSA/0.1% gelatin in PBS at room temperature for 30 min, followed by staining with DAPI (1 μg/ml) and the primary antibodies characterized in Supplementary Table S1 for 1 h.
In addition, for visualization of neuron-specific β3-tubulin, cultures were stained with mouse anti-β3 tubulin antibody (TUJ1, R&D Systems, Minneapolis, MN, USA, 1:1000) and to verify specificity of the rabbit TRPA1 antibody, neurons were additionally stained with mouse monoclonal anti-TRPA1 (Sigma, WH0008989M3, 1:1000). After three washes with PBS, DRG cultures were then incubated for 1 h with the following secondary antibodies as appropriate: anti-rabbit Alexa Fluor® 488 (Invitrogen, 1:1000); anti-sheep Alexa Fluor® 647 (Invitrogen, 1:1000); anti-guinea pig Alexa Fluor® 647 (Invitrogen, 1:1000); anti-mouse Alexa Fluor® 488. DRG cultures were washed three times with PBS and immunofluorescence visualized using the BD Pathway 855 with × 10 objective.
ROIs corresponding to antibody-positive DRG neurons were identified and Ca2+ responses, expressed as ΔR/R, were plotted using GraphPad Prism 4. Only cells with clearly defined ROI corresponding to single DRG neurons were used for analysis. DRG neurons were classified as responders if stimulation with 1 nM P-CTX-1 elicited a ΔR/R increase of at least 0.15 over 60 s (n=771/1524 neurons classified as responders).
Pharmacological characterization of ciguatoxin-sensitive DRG neurons
Rat DRG neurons were plated on PDL-coated glass coverslips and after 24 h culture loaded with 5 μM Fura-2 and 0.02% pluronic F-127 for 30 min at 37°C and 5% CO2. After a brief wash to allow for deesterification, coverslips were transferred to the recording chamber of an Olympus IX71 inverse microscope with an × 20 objective and Ca2+ responses were measured at 22°C. Fura-2 was excited at 340 and 380 nm with a Lambda DG-4 (Sutter Instruments Co.). Images were exposed for 100 ms and acquired at a rate of 3 Hz with a CCD camera (Orca-ER; Hamamatsu Photonics). Data were recorded and further analysed using Metamorph (MDS Analytical Technologies). Background was subtracted before calculation of ratios. After a baseline read with extracellular solution (ECS, composition: NaCl 145 mM, KCl 5 mM, CaCl2 1.25 mM, MgCl2 1 mM, glucose 10 mM, HEPES 10 mM), DRG neurons were stimulated with 1 nM P-CTX-1, followed by perfusion with 300 nM TTX. To identify TRPA1-expressing neurons, DRGs were stimulated with AITC (25 μM), while stimulation with 50 mM KCl was used to identify viable neurons. AITC-sensitive neurons were defined as cells exhibiting an increase of the ratio R of at least 15% over baseline fluorescence upon stimulation with AITC; TTXs cells were defined as cells exhibiting a decrease in intracellular Ca2+ of at least 50%. Ca2+ responses were presented as the fluorescence ratio R F340/380 or converted to intracellular Ca2+ concentration after calibration of Fura-2 fluorescence, using an experimentally determined Kd of 135 nM and a value of 0.2 and 2.6 for R0 and R1, respectively.
P-CTX-1 effects on cold-induced Ca2+ responses in DRG neurons
Dissociated DRG neurons from wt and TRPA1−/− mice were plated on PDL-coated glass coverslips as described above. Recordings were performed on an Olympus IX71 inverse microscope with a × 20 objective and Ca2+ responses were measured at 35°C. Fura-2 was excited at 340 and 380 nm with a Polychrome V monochromator (Till Photonics). Images were exposed for 200 μs and acquired at a rate of 1 Hz with a 12-bit CCD camera (Imago Sensicam QE, Till Photonics). Data were recorded and further analysed using TILLvisION 4.0.1.3 software (Till Photonics). To assess Ca2+ responses to cold stimulation and at different temperatures, a system for fast superfusion of the cultured cells was used (Dittert et al, 2006). Experimental solutions were driven by gravity from six barrels through electronically controlled valves into a manifold that consisted of fused silica tubes connected to an outlet glass capillary. The temperature of the solutions was preconditioned in a heat exchanger by a miniature Peltier device. The final temperature was adjusted by densely coiled copper wire, which wrapped the lower part of the capillary. The temperature was measured by a miniature thermocouple inserted into the outlet capillary near to its orifice. After the baseline was established at 35°C, DRG neurons were cooled in a ramp-shaped manner to 15°C over a period of 180 s, followed by heating back to 35°C within 1 s. After 60 s, 1 nM P-CTX-1 was applied for 360 s, during which a second cold stimulus was delivered. For quantitative comparison of cold sensitization, the maximum increase in intracellular Calcium after cold stimulation, relative to the 10 baseline reads prior to the cold stimulus, was determined for control and ciguatoxin-treated cold responses. Neurons responding to the control cold response with an increase in intracellular calcium of at least 20 nM were classified as cold sensitive (34/204 neurons from wt and 28/406 neurons from TRPA1−/− mice), while neurons with an increase in [Ca2+] of <20 nM were classified as cold insensitive. Cold sensitization was defined as cold responses from previously cold-insensitive neurons that were at least five times larger after treatment with P-CTX-1 compared to control (67/170 neurons sensitized to cold in wt neurons and 25/378 sensitized to cold in TRPA1−/− neurons).
Cell culture and transfection procedures
Cos-1, HEK293 and ND7/23 cells (all from American Tissue Culture Collection, Manassas, VA, USA) were routinely maintained in DMEM containing 10% fetal bovine serum, 2 mM L-glutamine, pyridoxine and 110 mg/ml sodium pyruvate. Cells were split every 3–6 days in a ratio of 1:5 using 0.25% trypsin/EDTA. Cells were plated on T75 tissue culture flasks (Nunc) 24 h prior to transfection and transfected with plasmid DNA of hTRPV1 (J Davis, formerly GlaxoSmithKline, Harlow, UK), mTRPA1 (A Patapoutian, The Scripps Research Institute, La Jolla, CA, USA), rNav1.8 (C Nau, Department of Anesthesiology, Friedrich-Alexander-University Erlangen-Nuremberg, Erlangen, Germany), hTRPA1 (OriGene Technologies, Rockville, MD, USA) and hNav1.7 (N Klugbauer and F Hofmann, Technische Universität Munich, Germany) using Fugene (Roche) or Nanofectin (Paa, Austria) according to manufacturer’s instructions. Twenty-four hours after transfection, cells were plated on 96-well plates, 384-well plates or glass coverslips as required and used 24 h after plating.
Electrophysiological recordings from skin nerve preparations
The effects of P-CTX-1 on C- and A-fibres were assessed using single fibre recordings from isolated rat and murine skin-saphenous nerve preparations as previously described (Zimmermann et al, 2009).
The hairy skin of the dorsal hind paw and lower leg of male adult C57BL/6, TRPA1−/−, Nav1.8−/− mice was removed together with the saphenous nerve, secured to the bottom of an organ chamber with the epithelial side facing down and continuously perfused with carbogenated SIF. Single A- and C-fibres were isolated from split single fibres of the desheathed saphenous nerve placed in a separate recording chamber under paraffin oil immersion. The receptive fields of the single fibres were identified using mechanical probing. For classification of fibres, the conduction velocity was determined by electrical stimulation of the receptive field with a concentric bipolar Platinum/Iridium steel microelectrode and mechanical sensitivity was assessed using a customized set of von Frey filaments.
The single fibre receptive fields were then isolated using a thin-walled glass ring (volume 500 μl) and continuously perfused at a rate of 10 ml/min for temperature control and superfusion with P-CTX-1 and TTX. In contrast to previous studies, the fibres were not subjected to heat stimulation in order to avoid induction of ongoing activity. This entailed classification of the fibres in two subtypes, CM and CMC. Since only very few CMC fibres were encountered the present results only summarize cold-insensitive CM-fibres. The cold stimulus (60 s) was applied using a countercurrent heat exchange application system as previously described (Zimmermann et al, 2009). DAPSYS V7 ( http://www.dapsys.net) was used to record and analyse the data.
Patch-clamp recordings
Effects of P-CTX-1 on the voltage dependence of activation were assessed in DRG neurons from Nav1.8−/− mice to eliminate the major TTXr component.
Whole-cell voltage-clamp recordings were performed with an EPC-10USB amplifier operated by PatchMaster software (HEKA Electronics, Lambrecht/Pfalz, Germany), using fire polished 1–1.5 MΩ borosilicate glass electrodes (Biomedical Instruments, Jena, Germany) containing (in mM): 140 CsF, 2 NaCl, 1 EGTA, 10 HEPES and 5 TEA-Cl; 308 mOsmol (pH 7.4, adjusted with CsOH), and the extracellular bath contained (in mM): 10 NaCl, 105 Cholin Cl, 3 KCl, 1 MgCl2, 1 CaCl2, 20 TEA-Cl, 0.1 CdCl2, 10 glucose, 10 HEPES; 310 mOsmol (pH 7.4, adjusted with NaOH). For patch-clamp recordings of rNav1.8 in ND7/23 cells, the pipette solution contained (in mM): 140 CsF, 10 NaCl, 1 EGTA, 10 HEPES and 5 TEA-Cl; 308 mOsmol (pH 7.4, adjusted with CsOH) and the extracellular bath contained (in mM): 140 NaCl, 3 KCl, 1 MgCl2, 1 CaCl2, 20 TEA-Cl, 0.1 CdCl2, 10 glucose, 10 HEPES; 326 mOsmol (pH 7.4, adjusted with NaOH). For current-clamp recordings of cultured DRGs, the pipette solution contained (in mM): 135 K-Gluconate, 4 NaCl, 3 MgCl2, 0.3 Na-GTP, 2 Na2-ATP, 5 EGTA, 5 HEPES; 280 mOsmol (pH 7.25, adjusted with KOH). All recordings were conducted at room temperature (∼21°C). Approximately 5 min after establishing cell access, voltage dependence of activation was assessed using 100 ms step depolarizations from a holding potential of −90 mV or −100, respectively. Between each voltage step (ΔV=10 mV, ranging from −90 to +30 mV, or from −90 to +60 mV, respectively) cells were held at Vhold for 5 s. Conductances were calculated from peak currents and normalized conductances were fitted using a Boltzmann equation using OriginPro 8.1 (OriginLab Corp., Northampton, MA, USA).
FLIPR Ca2+ Assays
To assess Ca2+ responses using the FLIPRTETRA (Molecular Devices, Sunnyvale, CA, USA) plate reader, cells were plated at a density of 50 000 cells/well on 96-well black-walled imaging plates (Corning) or a density of 15 000 cells/well on 384-well black-walled imaging plates (Corning) 24 h prior to the assay. Cells were loaded with 5 μM Fluo-4 AM (Invitrogen) in PSS/0.3% BSA for 30 min at 37°C and 5% CO2, washed twice with PSS and transferred the recording chamber of the FLIPRTETRA. Ca2+ responses were measured using a cooled CCD camera (excitation, 470–495 nm; emission, 515–575 nm), with camera gain and intensity adjusted to yield a baseline fluorescence of 1000 arbitrary fluorescence units (AFUs). A two-addition protocol was used, consisting of 10 baseline fluorescence reads, addition of antagonists or P-CTX-1 with fluorescence reads every second for 300 s, followed by addition of agonists and a further 300 fluorescence reads in 1 s intervals. Raw fluorescence data were converted to ΔF/F values as described below.
fMRI in mice
Wt (n=9) and TRPA1−/− (n=10) male mice were anaesthetized with isoflurane and placed on a cradle inside the MR scanner (Bruker BioSpec 47/40; 200 mT/m) with a free bore of 40 cm, equipped with an actively RF-decoupled coil system and a 4-channel head array coil under careful physiological monitoring (respiration, temperature). The temperature of the ciguatoxin-treated or saline-treated paw was controlled by contact using a miniature peltier device (6 mm2 contact surface). In general, the temperature of the hind paw was kept at 35°C, and, during six repetitive stimulations, cooled down to 15°C for 120 s each (presented at 8 min intervals). The Peltier temperature was remote controlled using custom-made software as previously described (Neely et al, 2010). In short, a set of 750 functional EPI images were acquired with two times k-space averaging resulting in a TR of 4000, ms and a total measuring time of 50 min. Finally, 22 corresponding anatomical T2 reference images (field of view 15 × 15 mm, matrix 256 × 128, TR=2000, ms, slice thickness 0.5 mm, TEef=56 ms, RARE) were assembled. Functional analysis was performed using Brain Voyager QX and our custom-designed software, MagNan (Knabl et al, 2008). In brief, the following preprocessing was performed: Motion correction algorithm as implemented in BrainVoyager was applied (trilinear interpolation, motion detected always below 1 pixel). Slice time correction was performed with a Cubic Spline, followed by a high pass temporal filtering (nine cycles) and a 2D Gaussian smoothing of the data (FWHM kernel: 2 pixel, in-plane direction). Next, a single contrast-based GLM analysis (cold stimulus) for each animal was calculated. To detect significantly activated voxels, statistical parametric maps (SPMs) of these contrasts were generated for individual animals and FDR thresholded (q=0.05, two-sided). Different groups of significantly activated voxels (n>4) were labelled belonging to certain brain structures based on the mouse atlas from Paxinos (second edition). After registering the individual SPM’s based on the manual segmented brains by a 12 degree affine transformation scheme they were averaged over all animals per group. Next, the resulting group average TRPA1−/− map was subtracted from the wt map in order to reveal brain areas expressing higher BOLD signals in wt compared to TRPA1−/− mice: these are the areas which are expected to be relevant for cold allodynia. Moreover, in a second, independent approach we characterized all structures of our pain matrix mouse atlas (Neely et al, 2010) by their temporal behaviour over the six stimulus repetitions. First, we calculated the group average BOLD peak response per repetition and brain structure based on the stimulus-specific GLM predictor. Next, these amplitudes of each brain structure were fitted by a linear model. All structures with a slope greater than 0.1 and an r2 greater than 0.5 were assumed to be directly coupled to the increasing sensitization observed in response to consecutive cold stimuli as demonstrated in Figure 6 and Supplementary Figure 6.
Data analysis
Fluorescence values from Ca2+ imaging experiments were converted to ΔR/R (Fura-2) or ΔF/F (Fluo-4) values by subtracting baseline fluorescence readings from subsequent values and normalizing to baseline fluorescence as previously described (Minta et al, 1989). For concentration-response curves, maximum values from the response after addition of agonist were plotted against agonist concentration and a 4-parameter logistic Hill equation was fitted to the data using GraphPad Prism Version 4.00 (San Diego, CA, USA).
Statistical analysis
Statistical significance was defined as P<0.05 and was determined using paired or unpaired Student’s t-tests and one-way ANOVA analysis with Dunnett’s post test as indicated. Statistical analysis was performed using GraphPad Prism Version 4.00 or Origin software (OriginLab Corp., Northampton, USA) and SPSS 10.0 (SPSS Inc., USA).
Supplementary Material
Acknowledgments
We would like to thank Iwona Izydorzyk and Jana Schramm for expert technical assistance, Mark Baker for insightful discussions and David Clapham for helpful comments on the manuscript and for financial support. Funding for this project was obtained through the Australian Research Council ARC LIEF grants for the BD Pathway 855 and FLIPRTETRA, a National Health and Medical Research Council Fellowship and Program Grant (RJL), a National Health and Medical Research Council Postdoctoral Fellowship (IV), an International Association for the Study of Pain Early Career Research Grant (IV and KZ) and the Go8 Australia-Germany Joint Research Co-operation Scheme (IV and KZ). Additional support was obtained from the German Research Council (DFG): LA2740-2/1 (AL), Zi1172/1-1 (KZ), Re704/2-1 and KFO130/TP7 (PWR), 661/TP4 (AH); the Federal Ministry of Education and Research (BMBF): 01EM0514, 01GQ0731, 0314102 (AH); the Dr Ernst und Anita Bauer-Foundation (SS) and the STAEDTLER Foundation (KZ, PWR).
Author contributions: IV, RH, SS, AS, LC and KZ contributed to animal experiments; AH, MS and KZ performed fMRI experiments; IV, FT, AL, SS, MEb, MEn and KZ performed in-vitro experiments and/or data analysis; IV and KZ wrote the manuscript; and PC, JW, VV, PWR and RL provided intellectual guidance and financial support.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN (2008) The cell and molecular basis of mechanical, cold, and inflammatory pain. Science 321: 702–705 [DOI] [PubMed] [Google Scholar]
- Akopian AN, Sivilotti L, Wood JN (1996) A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 379: 257–262 [DOI] [PubMed] [Google Scholar]
- Amaya F, Wang H, Costigan M, Allchorne AJ, Hatcher JP, Egerton J, Stean T, Morisset V, Grose D, Gunthorpe MJ, Chessell IP, Tate S, Green PJ, Woolf CJ (2006) The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci 26: 12852–12860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir R, Michaelis M, Devor M (1999) Membrane potential oscillations in dorsal root ganglion neurons: role in normal electrogenesis and neuropathic pain. J Neurosci 19: 8589–8596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnis R, Kuberski T, Laugier S (1979) Clinical observations on 3,009 cases of ciguatera (fish poisoning) in the South Pacific. Am J Trop Med Hyg 28: 1067–1073 [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124: 1269–1282 [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D (2007) The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448: 204–208 [DOI] [PubMed] [Google Scholar]
- Beaglehole JC (ed) (1961) The Journals of Captain James Cook on His Voyages of Discovery: The Voyage of the Resolution and Adventure 1772–1775 pp469–470Cambridge, England: Cambridge University Press, [Google Scholar]
- Birinyi-Strachan LC, Gunning SJ, Lewis RJ, Nicholson GM (2005) Block of voltage-gated potassium channels by Pacific ciguatoxin-1 contributes to increased neuronal excitability in rat sensory neurons. Toxicol Appl Pharmacol 204: 175–186 [DOI] [PubMed] [Google Scholar]
- Bottein MY, Wang Z, Ramsdell JS (2011) Toxicokinetics of the ciguatoxin P-CTX-1 in rats after intraperitoneal or oral administration. Toxicology 284: 1–6 [DOI] [PubMed] [Google Scholar]
- Cameron J, Capra MF (1993) The basis of the paradoxical disturbance of temperature perception in ciguatera poisoning. J Toxicol Clin Toxicol 31: 571–579 [DOI] [PubMed] [Google Scholar]
- Carr RW, Pianova S, Brock JA (2002) The effects of polarizing current on nerve terminal impulses recorded from polymodal and cold receptors in the guinea-pig cornea. J Gen Physiol 120: 395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspani O, Heppenstall PA (2009) TRPA1 and cold transduction: an unresolved issue? J Gen Physiol 133: 245–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin J, Monteil A, Briquaire C, Richard S, Perez-Reyes E, Nargeot J, Lory P (2000) Overexpression of T-type calcium channels in HEK-293 cells increases intracellular calcium without affecting cellular proliferation. FEBS Lett 478: 166–172 [DOI] [PubMed] [Google Scholar]
- del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, Cook CA, Petrus MJ, Zhao M, D'Amours M, Deering N, Brenner GJ, Costigan M, Hayward NJ, Chong JA, Fanger CM, Woolf CJ, Patapoutian A, Moran MM (2010) TRPA1 contributes to cold hypersensitivity. J Neurosci 30: 15165–15174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A (2007) TRPM8 is required for cold sensation in mice. Neuron 54: 371–378 [DOI] [PubMed] [Google Scholar]
- Dittert I, Benedikt J, Vyklicky L, Zimmermann K, Reeh PW, Vlachova V (2006) Improved superfusion technique for rapid cooling or heating of cultured cells under patch-clamp conditions. J Neurosci Methods 151: 178–185 [DOI] [PubMed] [Google Scholar]
- Fleming LE, Baden DG, Bean JA, Weisman R, Blythe DG (1998) Seafood toxin diseases: issues in epidemiology and community outreach. InHarmful Algae Reguera B, Blanco J, Fernandez ML, Wyatt T (eds), pp 245–248. Zunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO [Google Scholar]
- Gentry C, Stoakley N, Andersson DA, Bevan S (2010) The roles of iPLA2, TRPM8 and TRPA1 in chemically induced cold hypersensitivity. Mol Pain 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JD, Boulant JA (1995) Temperature effects on membrane potential and input resistance in rat hypothalamic neurones. J Physiol 488: Pt 2 407–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A, Axmann R, Rech J, Finzel S, Heindl C, Kreitz S, Sergeeva M, Saake M, Garcia M, Kollias G, Straub RH, Sporns O, Doerfler A, Brune K, Schett G (2011) Blockade of TNF-alpha rapidly inhibits pain responses in the central nervous system. Proc Natl Acad Sci USA 108: 3731–3736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman PA, Granade HR, McMillan JP (1983) The mouse ciguatoxin bioassay: a dose-response curve and symptomatology analysis. Toxicon 21: 363–369 [DOI] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427: 260–265 [DOI] [PubMed] [Google Scholar]
- Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T (2009) TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci USA 106: 1273–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Choi J, Kim S, Kwon O, Nah SY, Han YS, Rhim H (2004) The biochemical activation of T-type Ca2+ channels in HEK293 cells stably expressing alpha1G and Kir2.1 subunits. Biochem Biophys Res Commun 324: 401–408 [DOI] [PubMed] [Google Scholar]
- Knabl J, Witschi R, Hosl K, Reinold H, Zeilhofer UB, Ahmadi S, Brockhaus J, Sergejeva M, Hess A, Brune K, Fritschy JM, Rudolph U, Mohler H, Zeilhofer HU (2008) Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature 451: 330–334 [DOI] [PubMed] [Google Scholar]
- Knowlton WM, Bifolck-Fisher A, Bautista DM, McKemy DD (2010) TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 150: 340–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL (2009) TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci 29: 4808–4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RJ (2001) The changing face of ciguatera. Toxicon 39: 97–106 [DOI] [PubMed] [Google Scholar]
- Lewis RJ, Holmes MJ (1993) Origin and transfer of toxins involved in ciguatera. Comp Biochem Physiol C 106: 615–628 [DOI] [PubMed] [Google Scholar]
- Lewis RJ, Sellin M (1993) Recovery of ciguatoxin from fish flesh. Toxicon 31: 1333–1336 [DOI] [PubMed] [Google Scholar]
- Lewis RJ, Sellin M, Poli MA, Norton RS, MacLeod JK, Sheil MM (1991) Purification and characterization of ciguatoxins from moray eel (Lycodontis javanicus, Muraenidae). Toxicon 29: 1115–1127 [DOI] [PubMed] [Google Scholar]
- Lorenz J, Cross DJ, Minoshima S, Morrow TJ, Paulson PE, Casey KL (2002) A unique representation of heat allodynia in the human brain. Neuron 35: 383–393 [DOI] [PubMed] [Google Scholar]
- Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, Lazdunski M, Honore E (2000) TREK-1 is a heat-activated background K(+) channel. EMBO J 19: 2483–2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52–58 [DOI] [PubMed] [Google Scholar]
- Minta A, Kao JP, Tsien RY (1989) Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem 264: 8171–8178 [PubMed] [Google Scholar]
- Munns C, AlQatari M, Koltzenburg M (2007) Many cold sensitive peripheral neurons of the mouse do not express TRPM8 or TRPA1. Cell Calcium 41: 331–342 [DOI] [PubMed] [Google Scholar]
- Nagata K, Duggan A, Kumar G, Garcia-Anoveros J (2005) Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci 25: 4052–4061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassini R, Gees M, Harrison S, De Siena G, Materazzi S, Moretto N, Failli P, Preti D, Marchetti N, Cavazzini A, Mancini F, Pedretti P, Nilius B, Patacchini R, Geppetti P (2011) Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain 152: 1621–1631 [DOI] [PubMed] [Google Scholar]
- Neely GG, Hess A, Costigan M, Keene AC, Goulas S, Langeslag M, Griffin RS, Belfer I, Dai F, Smith SB, Diatchenko L, Gupta V, Xia CP, Amann S, Kreitz S, Heindl-Erdmann C, Wolz S, Ly CV, Arora S, Sarangi R et al. (2010) A genome-wide Drosophila screen for heat nociception identifies alpha2delta3 as an evolutionarily conserved pain gene. Cell 143: 628–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra A, Madrid R, Echevarria D, del Olmo S, Morenilla-Palao C, Acosta MC, Gallar J, Dhaka A, Viana F, Belmonte C (2010) Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med 16: 1396–1399 [DOI] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A (2002) A TRP channel that senses cold stimuli and menthol. Cell 108: 705–715 [DOI] [PubMed] [Google Scholar]
- Reid G (2005) ThermoTRP channels and cold sensing: what are they really up to? Pflugers Arch 451: 250–263 [DOI] [PubMed] [Google Scholar]
- Reid G, Flonta M (2001) Cold transduction by inhibition of a background potassium conductance in rat primary sensory neurones. Neurosci Lett 297: 171–174 [DOI] [PubMed] [Google Scholar]
- Seifert F, Maihöfner C (2007) Representation of cold allodynia in the human brain - a functional MRI study. Neuroimage 35: 1168–1180 [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819–829 [DOI] [PubMed] [Google Scholar]
- Strachan LC, Lewis RJ, Nicholson GM (1999) Differential actions of pacific ciguatoxin-1 on sodium channel subtypes in mammalian sensory neurons. J Pharmacol Exp Ther 288: 379–388 [PubMed] [Google Scholar]
- Viana F, de la Pena E, Belmonte C (2002) Specificity of cold thermotransduction is determined by differential ionic channel expression. Nat Neurosci 5: 254–260 [DOI] [PubMed] [Google Scholar]
- Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B (2004) The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430: 748–754 [DOI] [PubMed] [Google Scholar]
- Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM (2011) TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci 14: 595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka K, Inoue M, Miyazaki K, Hirama M, Kondo C, Kinoshita E, Miyoshi H, Seyama I (2009) Synthetic ciguatoxins selectively activate Nav1.8-derived chimeric sodium channels expressed in HEK293 cells. J Biol Chem 284: 7597–7605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K, Hein A, Hager U, Kaczmarek JS, Turnquist BP, Clapham DE, Reeh PW (2009) Phenotyping sensory nerve endings in vitro in the mouse. Nat Protoc 4: 174–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, Kobayashi J, Nau C, Wood JN, Reeh PW (2007) Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature 447: 855–858 [DOI] [PubMed] [Google Scholar]
- Zimmermann K, Lennerz JK, Hein A, Link AS, Kaczmarek JS, Delling M, Uysal S, Pfeifer JD, Riccio A, Clapham DE (2011) Transient receptor potential cation channel, subfamily C, member 5 (TRPC5) is a cold-transducer in the peripheral nervous system. Proc Natl Acad Sci USA 108: 18114–18119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA (2007) Direct activation of the ion channel TRPA1 by Ca2+. Nat Neurosci 10: 277–279 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.