Abstract
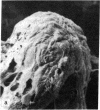
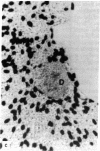
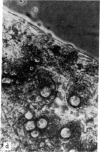
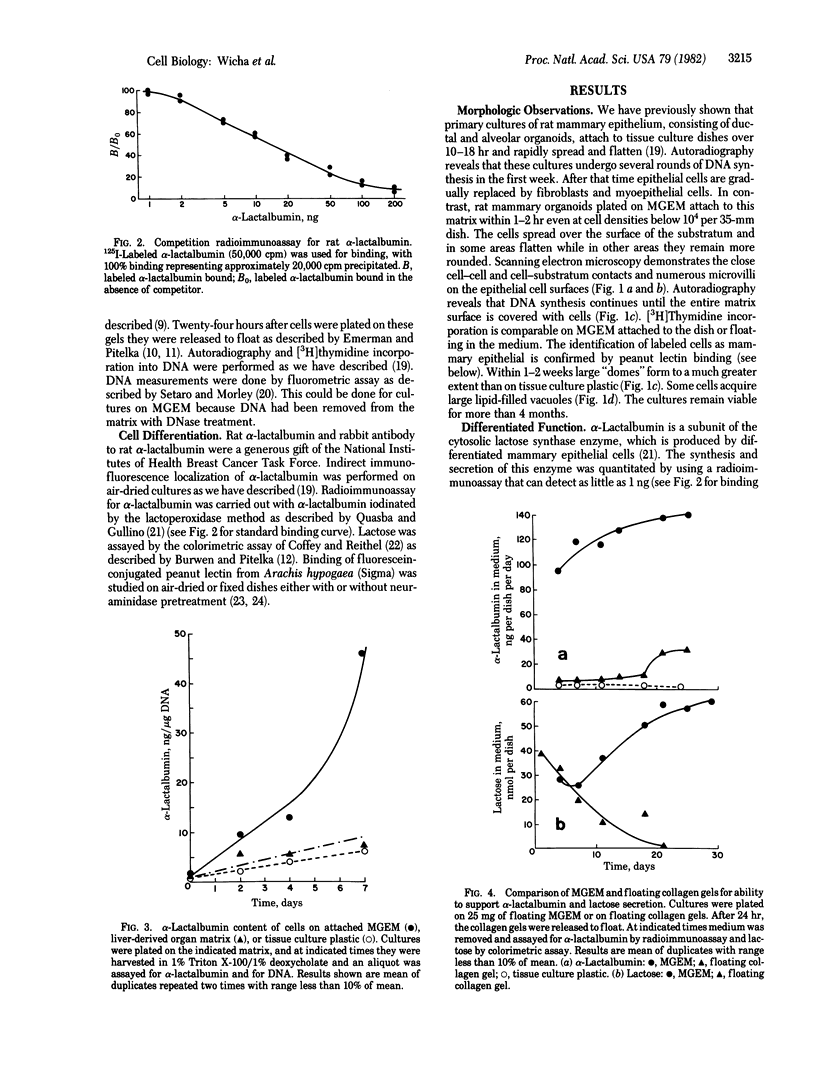
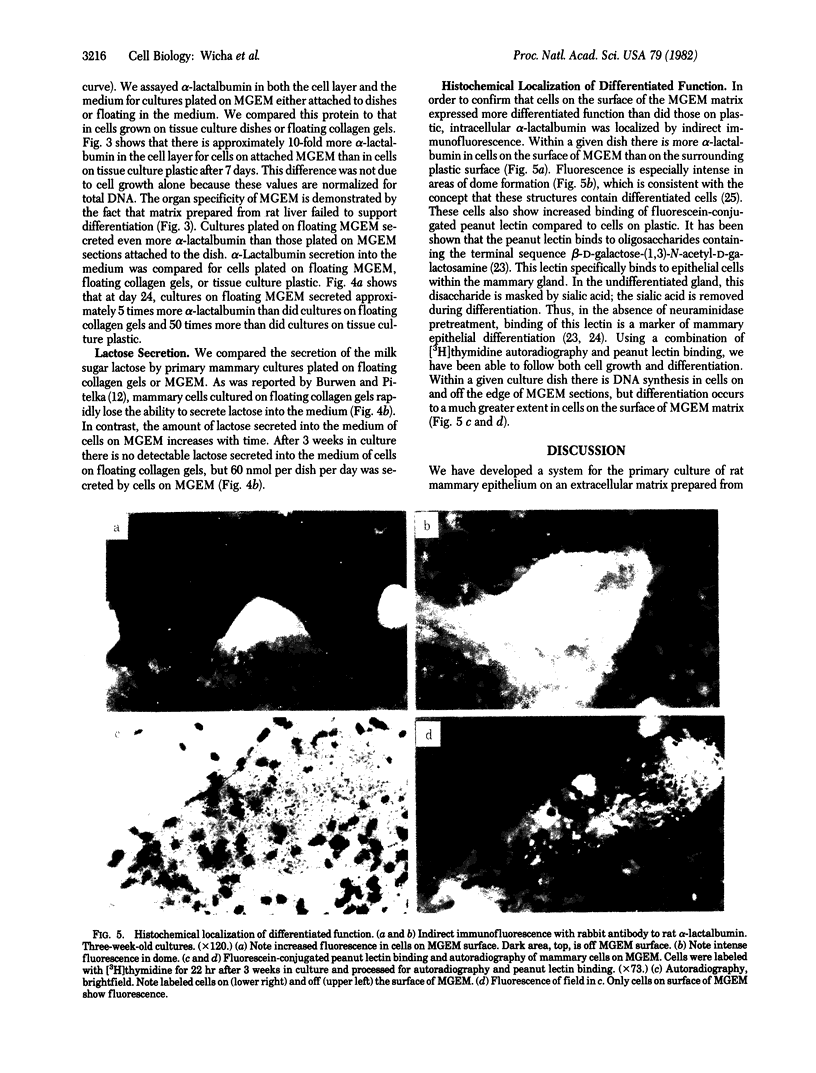
The study of growth and differentiation of mammary epithelium has been hampered by the difficulty of maintaining these functions in vitro. We describe a system for the primary culture of rat mammary epithelium on an acellular matrix derived from whole rat mammary glands that maintains growth and differentiation for months. Cultures plated on this complex substratum produce 50 times the alpha-lactalbumin of those on tissue culture dishes and 5 times the alpha-lactalbumin of those on floating collagen gels as determined by radioimmunoassay. Unlike cultures grown on floating collagen gels, which rapidly lose the ability to secrete the milk sugar lactose, mammary cells on this matrix retain this ability for over 30 days in culture. The organ specificity of this mammary extracellular material is shown by the failure of extracellular matrix prepared from rat liver to support mammary differentiation. Within a given culture dish, cells on the surface of mammary extracellular matrix are more differentiated than those on the adjacent plastic. This is demonstrated by their increased alpha-lactalbumin content as shown by indirect immunofluorescence, and by their increased ability to bind fluorescein-conjugated peanut lectin. Cells on the surface of the matrix continue to synthesize DNA as determined by [3H]thymidine incorporation and autoradiography. Even when mammary epithelial cells are plated at low density, cell division continues until the matrix is covered with a confluent layer. We propose that the limited growth, differentiation, and survival of mammary cells in previously described in vitro systems may have been due to substrate that were inadequate to support these functions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen C. R., Larson B. L. Comparative maintenance of function in dispersed cell and organ cultures of bovine mammary tissue. Exp Cell Res. 1970 Jul;61(1):24–30. doi: 10.1016/0014-4827(70)90253-3. [DOI] [PubMed] [Google Scholar]
- Banerjee S. D., Cohn R. H., Bernfield M. R. Basal lamina of embryonic salivary epithelia. Production by the epithelium and role in maintaining lobular morphology. J Cell Biol. 1977 May;73(2):445–463. doi: 10.1083/jcb.73.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolander F. F., Jr, Topper Y. J. Stimulation of lactose synthetase activity and casein synthesis in mouse mammary explants by estradiol. Endocrinology. 1980 Feb;106(2):490–495. doi: 10.1210/endo-106-2-490. [DOI] [PubMed] [Google Scholar]
- Burwen S. J., Pitelka D. R. Secretory function of lactating mouse mammary epithelial cells cultured on collagen gels. Exp Cell Res. 1980 Apr;126(2):249–262. doi: 10.1016/0014-4827(80)90263-3. [DOI] [PubMed] [Google Scholar]
- Ceriani R. L. Hormone induction of specific protein synthesis in midpregnant mouse mammary cell culture. J Exp Zool. 1976 Apr;196(1):1–12. doi: 10.1002/jez.1401960102. [DOI] [PubMed] [Google Scholar]
- Coffey R. G., Reithel F. J. An enzymic determination of lactose. Anal Biochem. 1969 Nov;32(2):229–232. doi: 10.1016/0003-2697(69)90079-7. [DOI] [PubMed] [Google Scholar]
- David G., Bernfield M. Type I collagen reduces the degradation of basal lamina proteoglycan by mammary epithelial cells. J Cell Biol. 1981 Oct;91(1):281–286. doi: 10.1083/jcb.91.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman J. T., Enami J., Pitelka D. R., Nandi S. Hormonal effects on intracellular and secreted casein in cultures of mouse mammary epithelial cells on floating collagen membranes. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4466–4470. doi: 10.1073/pnas.74.10.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman J. T., Pitelka D. R. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977 May;13(5):316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Engel L. W., Young N. A. Human breast carcinoma cells in continuous culture: a review. Cancer Res. 1978 Nov;38(11 Pt 2):4327–4339. [PubMed] [Google Scholar]
- Gospodarowicz D., Delgado D., Vlodavsky I. Permissive effect of the extracellular matrix on cell proliferation in vitro. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4094–4098. doi: 10.1073/pnas.77.7.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Ill C. Extracellular matrix and control of proliferation of vascular endothelial cells. J Clin Invest. 1980 Jun;65(6):1351–1364. doi: 10.1172/JCI109799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschka S. D., Konigsberg I. R. The influence of collagen on the development of muscle clones. Proc Natl Acad Sci U S A. 1966 Jan;55(1):119–126. doi: 10.1073/pnas.55.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juergens W. G., Stockdale F. E., Topper Y. J., Elias J. J. Hormone-dependent differentiation of mammary gland in vitro. Proc Natl Acad Sci U S A. 1965 Aug;54(2):629–634. doi: 10.1073/pnas.54.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalides N. A., Alper R., Clark C. C. Biochemistry and metabolism of basement membranes. Int Rev Cytol. 1979;61:167–228. doi: 10.1016/s0074-7696(08)61998-1. [DOI] [PubMed] [Google Scholar]
- Klein P. J., Newman R. A., Müller P., Uhlenbruck G., Schaefer H. E., Lennartz K. J., Fischer R. Histochemical methods for the demonstration of Thomsen-Friedenreich antigen in cell suspensions and tissue sections. Klin Wochenschr. 1978 Aug 1;56(15):761–765. doi: 10.1007/BF01476765. [DOI] [PubMed] [Google Scholar]
- Lever J. E. Regulation of dome formation in differentiated epithelial cell cultures. J Supramol Struct. 1979;12(2):259–272. doi: 10.1002/jss.400120210. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G., Pitot H. C. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Exp Cell Res. 1975 Aug;94(1):70–78. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- Newman R. A., Klein P. J., Rudland P. S. Binding of peanut lectin to breast epithelium, human carcinomas, and a cultured rat mammary stem cell: use of the lectin as a marker of mammary differentiation. J Natl Cancer Inst. 1979 Dec;63(6):1339–1346. [PubMed] [Google Scholar]
- Overton J. Response of epithelial and mesenchymal cells to culture on basement lamella observed by scanning microscopy. Exp Cell Res. 1977 Mar 15;105(2):313–323. doi: 10.1016/0014-4827(77)90130-6. [DOI] [PubMed] [Google Scholar]
- Qasba P. K., Gullino P. M. alpha-Lactalbumin content of rat mammary carcinomas and the effect of pituitary stimulation. Cancer Res. 1977 Oct;37(10):3792–3795. [PubMed] [Google Scholar]
- Rojkind M., Gatmaitan Z., Mackensen S., Giambrone M. A., Ponce P., Reid L. M. Connective tissue biomatrix: its isolation and utilization for long-term cultures of normal rat hepatocytes. J Cell Biol. 1980 Oct;87(1):255–263. doi: 10.1083/jcb.87.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakura T., Nishizuka Y., Dawe C. J. Mesenchyme-dependent morphogenesis and epithelium-specific cytodifferentiation in mouse mammary gland. Science. 1976 Dec 24;194(4272):1439–1441. doi: 10.1126/science.827022. [DOI] [PubMed] [Google Scholar]
- Salomon D. S., Liotta L. A., Kidwell W. R. Differential response to growth factor by rat mammary epithelium plated on different collagen substrata in serum-free medium. Proc Natl Acad Sci U S A. 1981 Jan;78(1):382–386. doi: 10.1073/pnas.78.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setaro F., Morley C. G. A modified fluorometric method for the determination of microgram quantities of DNA from cell or tissue cultures. Anal Biochem. 1976 Mar;71(1):313–317. doi: 10.1016/0003-2697(76)90043-9. [DOI] [PubMed] [Google Scholar]
- Timpl R., Martin G. R., Bruckner P., Wick G., Wiedemann H. Nature of the collagenous protein in a tumor basement membrane. Eur J Biochem. 1978 Mar;84(1):43–52. doi: 10.1111/j.1432-1033.1978.tb12139.x. [DOI] [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Garbisa S., Kidwell W. R. Basement membrane collagen requirements for attachment and growth of mammary epithelium. Exp Cell Res. 1979 Nov;124(1):181–190. doi: 10.1016/0014-4827(79)90268-4. [DOI] [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Kidwell W. R. Effects of free fatty acids on the growth of normal and neoplastic rat mammary epithelial cells. Cancer Res. 1979 Feb;39(2 Pt 1):426–435. [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Vonderhaar B. K., Kidwell W. R. Effects of inhibition of basement membrane collagen deposition on rat mammary gland development. Dev Biol. 1980 Dec;80(2):253–256. doi: 10.1016/0012-1606(80)90402-9. [DOI] [PubMed] [Google Scholar]
- Yang J., Richards J., Bowman P., Guzman R., Enami J., McCormick K., Hamamoto S., Pitelka D., Nandi S. Sustained growth and three-dimensional organization of primary mammary tumor epithelial cells embedded in collagen gels. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3401–3405. doi: 10.1073/pnas.76.7.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Richards J., Guzman R., Imagawa W., Nandi S. Sustained growth in primary culture of normal mammary epithelial cells embedded in collagen gels. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2088–2092. doi: 10.1073/pnas.77.4.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]